Abstract
The effect of gamma interferon (IFN-γ), tumor necrosis factor alpha (TNF-α), and lipopolysaccharide (LPS) on nitric oxide (NO) production in the mouse vascular aortic endothelial cell line END-D was examined. LPS, TNF-α, and a low concentration of IFN-γ inhibited NO production in END-D cells, while a high concentration of IFN-γ definitely enhanced it. The NO production induced by a high concentration of IFN-γ was further augmented by using IFN-γ in combination with LPS or TNF-α. In sequential incubations of LPS and IFN-γ, the enhancement of NO production required prior treatment with IFN-γ. Stimulation of END-D cells with a high concentration of IFN-γ led to the expression of inducible NO synthase (iNOS). The augmentation of NO production by IFN-γ alone or in combination with LPS or TNF-α was completely blocked by several inhibitors of iNOS. It was strongly suggested that a high concentration of IFN-γ itself enhanced NO production in END-D cells through inducing the expression of iNOS. LPS and TNF-α exclusively modulated the activity of iNOS once its expression was triggered by IFN-γ. On the other hand, a low concentration of IFN-γ, LPS, and TNF-α reduced NO production through down-regulating constitutive NOS (cNOS). The differential regulation of cNOS- and iNOS-mediated NO production by IFN-γ, TNF-α, and LPS is discussed.
Nitric oxide (NO) exhibits a wide range of important functions in vivo, acting as a releasing factor mediating vasodilation, a neuronal messenger molecule, and a major regulatory molecule and principal cytotoxic mediator of the immune system (3, 9, 17). NO is synthesized by constitutively expressed NO synthase (cNOS) for short periods of time. On the other hand, it is also synthesized by an inducible isoform of NOS (iNOS) that, once expressed, produces NO for long periods of time (17, 22). NO production with cNOS and iNOS is regulated in a complicated fashion by various stimuli. The best-studied example of the regulation of NO production almost certainly involves murine macrophages (21). It has been established that NO production is enhanced by gamma interferon (IFN-γ), tumor necrosis factor alpha (TNF-α), and lipopolysaccharide (LPS) in vitro (20, 26). Further, their combination augments NO production markedly in murine macrophages (20, 26). Several cytokines (interleukin 4, interleukin 10, and transforming growth factor β) are also known to modulate NO production directly (10, 22, 31). The extent of their effect, however, seems to vary dramatically under various experimental conditions.
With the discovery of endothelial cell-derived NO, it was found that vascular endothelial cells are capable of producing NO and play an active role in the regulation of vascular tone (11, 16). In addition to its role in the regulation of vasomotor function, NO is also important in the progression of a wide variety of diseases. The activity of cNOS allows for constitutive low-level production of NO by unstimulated vascular endothelial cells and is thought to be fundamental for the maintenance of a nonthrombogenic surface and the inhibition of cell adhesion to the endothelium (22). Vascular endothelial cells stimulated with various agents (LPS, cytokines, and growth factors) begin to accumulate mRNA encoding iNOS several hours following agonist stimulation (4, 25, 29, 34, 35). NO produced by both cNOS and iNOS increases endothelial cell permeability, and the increased permeability allows the accumulation of growth factors necessary for stimulation of mitogenesis and tissue repair (22). However, we have recently used endotoxin-induced hepatic injury as an experimental endotoxic shock model (23) to demonstrate that the expression of iNOS and peroxynitrite-induced nitrotyrosine is detected mainly around blood vessels. NO produced by iNOS in vascular endothelial cells might play a critical role in endotoxin-induced tissue injury. The regulation of NO production by cNOS and iNOS in vascular endothelial cells remains a complex issue. In the present study, we examined the effect of IFN-γ, TNF-α, and LPS on NO production by using the mouse vascular aortic endothelial cell line END-D (24) because of the difficulty of obtaining normal vascular endothelial cells from mice and demonstrating the expression of iNOS in human cells.
MATERIALS AND METHODS
Reagents.
Murine recombinant IFN-γ and TNF-α were purchased from R&D Systems (Minneapolis, Minn.) and Wako Pure Chemicals (Osaka, Japan), respectively. LPS from Escherichia coli O55:B5 was purchased from Difco Laboratories, Detroit, Mich. Polyclonal rabbit antibody against iNOS was obtained from Affinity Bioreagents, Neshanic Station, N.J. l-N6-(1-Lminoethyl)-lysine (l-NIL) and NG-monomethyl-l-arginine (l-NMMA) were obtained from Alexis, San Diego, Calif., and Dojindo, Kumamoto, Japan, respectively. Hydrocortisone and SB203580 were purchased from Sigma, St. Louis, Mo., and Calbiochem, San Diego, Calif., respectively.
Cell culture.
The murine aortic endothelial cell line END-D, kindly provided by K. Kimata, Institute for Molecular Science of Medicine, Aichi Medical University, was maintained in Dulbecco's minimal essential medium (Sigma) containing 10% heat-inactivated fetal calf serum and antibiotics. Heat inactivation was performed at 60°C for 30 min. END-D cells were positive for vascular cell adhesion molecule 1 and intercellular adhesion molecule 1 and negative for E-selectin. They were reported to be suitable for vascular development and diseases (24). The cells were treated with trypsin-EDTA solution (Gibco BRL, Grand Island, N.Y.) to prepare the cell suspension. The cells were cultured in 24-well (5 × 104 cells per 0.5-ml well) or 96-well (2 × 104 cells per 200-μl well) plastic plates overnight and used for various experiments. For all experiments, Dulbecco's minimal essential medium supplemented with 10% heat-inactivated fetal calf serum and antibiotics was used as the culture medium for END-D cells.
Estimation of nitrite concentration.
Nitrite in the supernatant was measured in a microplate reader by the Griess method, as described elsewhere (32). Potassium nitrite diluted in culture medium was used as a standard. The concentration of nitrite was expressed as the mean value of triplicate readings ± the standard deviation.
Immunoblotting.
Cell pellets were suspended at a concentration of 2 × 107 cells/ml in a lysis buffer containing 0.5% Nonidet P-40, 0.15 M NaCl, 0.05 M Tris, and 5 mM EDTA at pH 8.0 for 30 min at 4°C. The insoluble debris was removed by microcentrifugation at 10,000 × g for 10 min at 4°C. Cell lysates were diluted with an equal volume of sample buffer containing 0.5 M Tris, 10% glycerol, 2% sodium dodecyl sulfate, 2% 2-mercaptoethanol, and 0.05% bromophenol blue and boiled for 2 min. Samples were separated under reducing conditions by electrophoresis in a 6% polyacrylamide gel. Proteins separated by gel electrophoresis were transferred to a membrane by electroblotting (33). The membranes were blocked with 5% skim milk in phosphate-buffered saline. After washing in phosphate-buffered saline containing 0.05% Tween 20, they were treated with a 1:2,000 dilution of anti-iNOS antibody and then washed three times. The resulting immune complexes were reacted with a 1:2,000 dilution of horseradish peroxidase-conjugated goat anti-rabbit immunoglobulin antibody (Bio Source International, Camarillo, Calif.). Finally, labeled antigen bands were detected by an ECL reagent (Amersham, London, United Kingdom). A prestained low-molecular-weight standard kit from Nippon Bio-Rad Laboratories, Tokyo, Japan, was used as a reference.
Statistical analysis.
Data were compared and analyzed by Student's t test, and P values less than 0.001 were considered significant.
RESULTS
NO production in END-D cells stimulated with IFN-γ, TNF-α, and LPS alone or in combination.
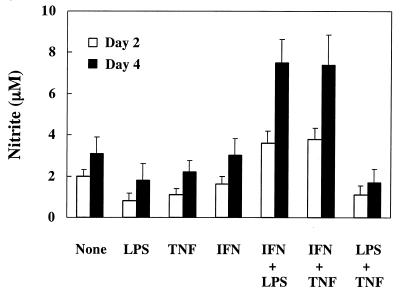
Nitrite in culture supernatants was measured to determine the amount of NO production in END-D cells stimulated with LPS (10 μg/ml), IFN-γ (20 ng/ml, 200 U, and TNF-α (10 ng/ml) alone or in combination for 2 or 4 days (Fig. 1). LPS and TNF-α inhibited NO production of END-D cells at 2 days. Treatment with combined LPS and TNF-α also inhibited NO production of END-D cells. The possibility that reduced NO production was due to injury of END-D cells was excluded, since there was no significant difference in the morphology and growth rates of untreated END-D cells and those stimulated with LPS and/or TNF-α. On the other hand, stimulation with combined IFN-γ and LPS or IFN-γ and TNF-α markedly enhanced NO production of END-D cells at 2 and 4 days, although IFN-γ alone did not affect NO production.
FIG. 1.
NO production in END-D cells stimulated with IFN-γ, TNF-α, and LPS alone or in combination. Nitrite was measured in the culture supernatants from cells stimulated with LPS (10 μg/ml), IFN-γ (20 ng/ml), and TNF-α (10 ng/ml) alone or in combination for 2 or 4 days.
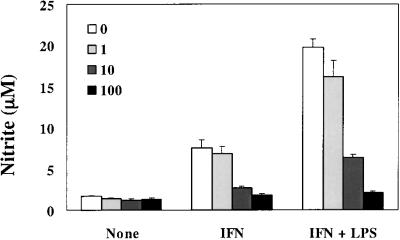
NO production in END-D cells stimulated with various concentrations of IFN-γ, TNF-α, and LPS.
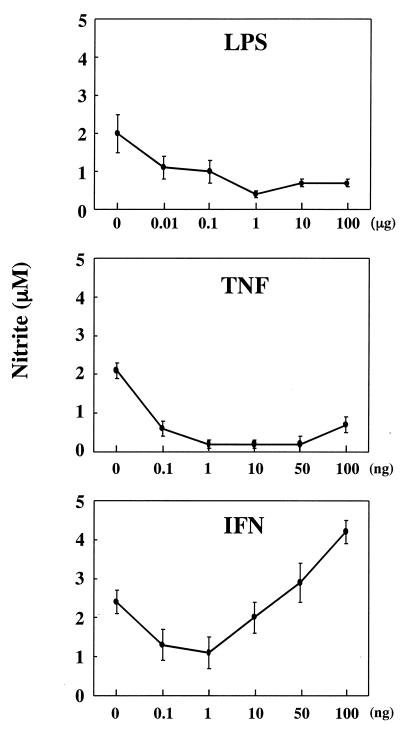
NO production was determined for cultures of END-D cells stimulated with various concentrations of LPS, IFN-γ, and TNF-α for 4 days (Fig. 2). LPS and TNF-α definitely reduced NO production of END-D cells at all concentrations tested. On the other hand, IFN-γ exhibited contrary effects on NO production, depending on its concentration. A relatively low concentration (0.1 or 1 ng/ml) of IFN-γ significantly reduced NO production of END-D cells, whereas a relatively high concentration (50 or 100 ng/ml) of IFN-γ enhanced NO production. IFN-γ at the intermediate concentration (10 ng/ml) did not significantly alter NO production. This was consistent with the result shown in Fig. 1.
FIG. 2.
NO production in END-D cells stimulated with various concentrations of IFN-γ, TNF-α, and LPS. Nitrite was measured in the culture supernatants from cells stimulated with various concentrations of LPS, IFN-γ, and TNF-α for 4 days. Concentrations are given as mass of substance per milliliter.
Time course of NO production in END-D cells stimulated with IFN-γ, TNF-α, and LPS.
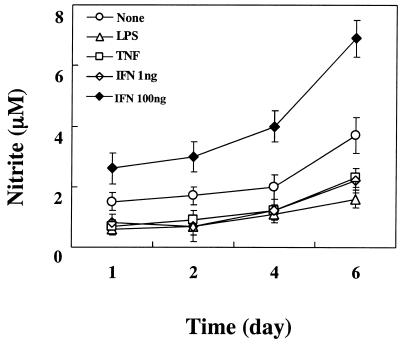
A time course of NO production in END-D cells stimulated with LPS (10 μg/ml), IFN-γ (1 and 100 ng/ml), or TNF-α (10 ng/ml) was followed for 6 days (Fig. 3). Treatment of END-D cells with a high concentration of IFN-γ (100 ng/ml) induced an increase in NO production in a time-dependent fashion. NO production in untreated END-D cells also increased gradually, although its intensity was much lower than that of END-D cells stimulated with IFN-γ. END-D cells stimulated with LPS, TNF-α, and a low concentration of IFN-γ (1 ng/ml) produced less NO than untreated END-D cells did, and there was no significant difference in reduced NO production among them.
FIG. 3.
Time course of NO production in END-D cells stimulated with IFN-γ, TNF-α, and LPS. Nitrite was measured in the culture supernatants from cells stimulated with LPS (10 μg/ml), IFN-γ (100 ng/ml), or TNF-α (10 ng/ml) at 1, 2, 4, and 6 days after the addition of the substance.
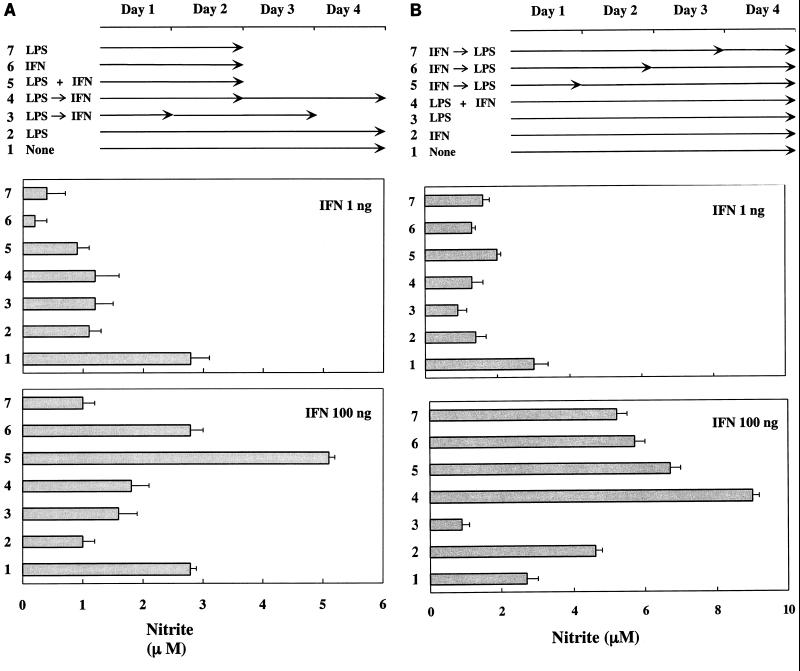
NO production in END-D cells stimulated by sequential incubation with LPS and IFN-γ.
We demonstrated above that IFN-γ and LPS synergistically enhanced NO production in END-D cells. To verify the synergism between LPS and IFN-γ, the effect of sequential incubations with LPS (10 μg/ml) and a low (1-ng/ml) or high (100-ng/ml) concentration of IFN-γ on NO production was examined (Fig. 4). Exposure of END-D cells to LPS for 1 or 2 days followed by the addition of a high concentration of IFN-γ did not exhibit a synergistic effect on NO production (Fig. 4A). Rather, it gave lower values than treatment with IFN-γ alone. The pretreatment with LPS seemed to counteract the enhancement of NO production by IFN-γ. In addition, the sequential treatment with LPS and a low concentration of IFN-γ suppressed NO production in END-D cells, like the individual treatments did.
FIG. 4.
NO production in END-D cells stimulated with sequential incubations of LPS and IFN-γ. Nitrite was measured in the culture supernatants from cells stimulated sequentially with LPS (10 μg/ml) and a low (1-ng/ml) or high (100-ng/ml) concentration of IFN-γ. (A) The cells were exposed to LPS for 1 or 2 days followed by the addition of a low or high concentration of IFN-γ. (B) The cells were exposed to a low or high concentration of IFN-γ for 1, 2, or 3 days followed by the addition of LPS.
Next, the effect of the reversed order of sequential incubations with a low or high concentration of IFN-γ and LPS on NO production was examined (Fig. 4B). The exposure of END-D cells to IFN-γ (100 ng/ml) for 1, 2, or 3 days followed by the addition of LPS further augmented IFN-γ-triggered NO production. LPS, which by itself exhibited an inhibitory action, definitely enhanced NO production in IFN-γ-pretreated END-D cells. However, the enhancing effect of LPS was not seen in END-D cells pretreated with a low concentration (1 ng/ml) of IFN-γ. It was concluded that a high concentration of IFN-γ provided a critical signal to trigger NO production to END-D cells, and LPS exhibited an enhancing action on the cells once NO production was triggered by IFN-γ.
Detection of iNOS expression in END-D cells stimulated with IFN-γ, TNF-α, and LPS.
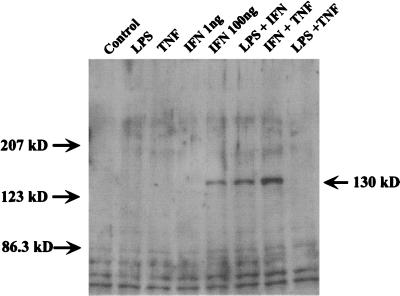
Two different NOSs, cNOS and iNOS, are known to participate in the NO production of vascular endothelial cells (22). Therefore, we studied the expression of iNOS in END-D cells stimulated with IFN-γ, TNF-α, and LPS alone or in combination by an immunoblotting method in order to verify the participation of iNOS in the augmentation of NO production. As shown in Fig. 5, the immunoblotting analysis clearly demonstrated the expression of iNOS (with a molecular mass of 130 kDa) in END-D cells stimulated with a high concentration (100 ng/ml) of IFN-γ or combined IFN-γ and LPS or IFN-γ and TNF-α. On the other hand, no expression of iNOS was detected in untreated END-D cells or those treated with LPS, TNF-α, or a low concentration of IFN-γ (1 ng/ml). Therefore, we concluded that a high concentration of IFN-γ induced the expression of iNOS and enhanced NO production through iNOS and that LPS and TNF-α augmented NO production via IFN-γ-induced iNOS expression.
FIG. 5.
Detection of iNOS expression in END-D cells stimulated with IFN-γ, TNF-α, and LPS alone or in combination. Cell lysates extracted from cells stimulated with IFN-γ (100 ng/ml), TNF-α (10 ng/ml), LPS (10 μg/ml), or a combination were analyzed by an immunoblotting method using anti-iNOS antibody. Note the iNOS band with a molecular mass of 130 kDa in the samples from the cells treated with IFN-γ, IFN-γ and LPS, or IFN-γ and TNF-α.
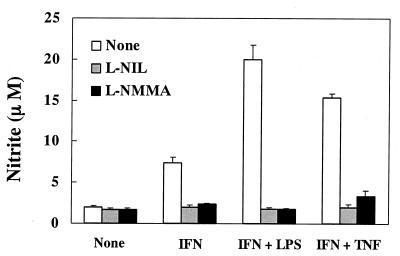
Inhibitory effect of l-NIL and l-NMMA on NO production in END-D cells stimulated with IFN-γ, TNF-α, and LPS.
It was suggested that a high concentration of IFN-γ triggered the expression of iNOS and that LPS and TNF-α further enhanced NO production through IFN-γ-induced iNOS expression. It was of interest to determine whether or not the specific inhibitors of iNOS prevented NO production in END-D cells treated with IFN-γ alone or with combined IFN-γ and LPS or IFN-γ and TNF-α. The addition of either l-NIL (500 μM) or l-NMMA (100 μM) definitely reduced NO production in stimulated END-D cells but did not inhibit NO production in untreated END-D cells (Fig. 6). Once again, the finding strongly suggested that the enhanced NO production in stimulated END-D cells was due to iNOS expression triggered by IFN-γ.
FIG. 6.
Effect of l-NIL and l-NMMA on NO production in END-D cells stimulated with IFN-γ alone or in combination with LPS or TNF-α. Nitrite was measured in the culture supernatants from cells stimulated with IFN-γ (100 ng/ml) alone, IFN-γ and LPS (10 μg/ml), or IFN-γ and TNF-α (10 μg/ml) in the presence of l-NIL (500 μM) or l-NMMA (100 μM) for 4 days.
Inhibitory effect of hydrocortisone on NO production in END-D cells stimulated with IFN-γ alone or with combined IFN-γ and LPS.
Based on the inhibitory effect of hydrocortisone on the activity of iNOS (2, 28), the effect of hydrocortisone on NO production in END-D cells stimulated with IFN-γ alone or with combined IFN-γ and LPS was studied (Fig. 7). Hydrocortisone markedly reduced NO production of END-D cells in a dose-dependent manner. The addition of hydrocortisone at 100 ng/ml completely blocked the enhancement of NO production in stimulated END-D cells.
FIG. 7.
Effect of hydrocortisone on NO production in END-D cells stimulated with IFN-γ alone or combined IFN-γ and LPS. Hydrocortisone was added to the cultures at concentrations of 1, 10 and 100 ng/ml. Nitrite was measured in the culture supernatants from cells stimulated with IFN-γ (100 ng/ml) alone or combined IFN-γ and LPS (10 μg/ml) for 4 days.
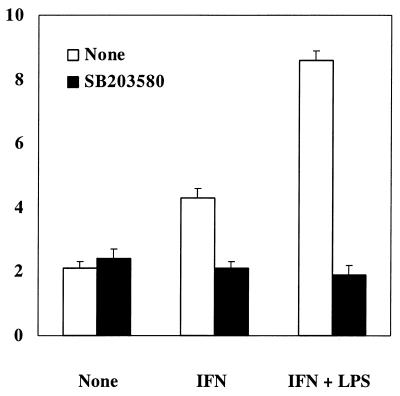
Inhibitory effect of SB203580, an inhibitor of p38 MAPK, on NO production in END-D cells stimulated with IFN-γ.
We studied the signaling pathway involved in NO production of END-D cells by IFN-γ. SB203580, PD98059, and genistein were used as the inhibitors of p38 mitogen-activated protein kinase (MAPK), extracellular signal-regulated kinase 1/2 MAPK, and tyrosine kinase, respectively. As shown in Fig. 8, the addition of SB203580 to END-D cells stimulated with IFN-γ or with combined IFN-γ and LPS completely blocked the enhancement of NO production. SB203580 exhibited no toxic effect on the cell viability of END-D cells. However, the effect of PD98059 and genistein on NO production was not determined since their treatment was deleterious to END-D cells.
FIG. 8.
Effect of SB203580, an inhibitor of p38 MAPK, on NO production in END-D cells stimulated with IFN-γ. SB203580 (25 μM) was added to the cultures of the cells stimulated with IFN-γ (100 ng/ml) or IFN-γ and LPS (10 μg/ml) for 4 days. Nitrite was measured in the culture supernatants.
DISCUSSION
The present study demonstrated that high concentrations of IFN-γ triggered high-level production of NO in END-D cells through the induction of iNOS and that LPS and TNF-α further augmented NO production once iNOS was induced by IFN-γ. Therefore, we concluded that iNOS might play a pivotal role in high NO production of vascular endothelial cells stimulated by high concentrations of IFN-γ alone, combined IFN-γ and LPS, or combined IFN-γ and TNF-α. This conclusion was suggested by several lines of evidence: first, the immunoblotting analysis demonstrated the expression of iNOS only in END-D cells stimulated by high concentrations of IFN-γ alone, combined IFN-γ and LPS, or combined IFN-γ and TNF-α; second, l-NIL and l-NMMA, inhibitors specific for iNOS, completely blocked the augmentation of NO production in stimulated END-D cells; and third, hydrocortisone, which is also known to inhibit the activity of iNOS, reduced NO production in a dose-dependent fashion. It was strongly suggested that the enhanced NO production in END-D cells stimulated with high concentrations of IFN-γ alone or in combination with LPS and TNF-α was caused by the activity of iNOS but not cNOS. In addition, iNOS from vascular endothelial cells appeared to produce a lower level of NO than that from macrophages.
It has been reported that IFN-γ in combination with LPS or TNF-α enhanced NO production in mouse vascular endothelial cells (4, 25, 29, 34, 35). However, the individual actions of IFN-γ, LPS, and TNF-α are not well-documented. Walter et al. (34) reported that treatment with IFN-γ or LPS alone suppresses cNOS-mediated NO production in a mouse vascular endothelial cell line and does not induce the expression of iNOS. This indicates that IFN-γ and LPS, which are unable to trigger iNOS by themselves, can activate iNOS only when they are combined. This is inconsistent with our finding in the present study. We demonstrated for the first time that IFN-γ at a high concentration might trigger the expression of iNOS in vascular endothelial cells by itself. Considering that IFN-γ itself initiates the activation of iNOS in vascular endothelial cells, IFN-γ might play a central role in switching from cNOS to iNOS induction of NO production in vascular endothelial cells. LPS and TNF-α exclusively modulate the activity of iNOS as the amplifier.
Treatment with LPS or TNF-α alone reduced NO production in END-D cells. The reduction in NO production was not due to damages to END-D cells by LPS and TNF-α. Therefore, we suggested that LPS and TNF-α down-regulated the activity of cNOS and reduced NO production, because unstimulated END-D cells did not express iNOS. Furthermore, LPS appeared to inhibit the induction of iNOS by IFN-γ, since prior treatment of END-D cells with LPS abolished IFN-γ-mediated enhancement of NO production. Normal endothelium secretes a low level of NO that may inhibit cellular adhesion and contribute to the maintenance of an antithrombogenic surface (22). Reduced NO production by LPS, TNF-α, and low concentrations of IFN-γ might result in the adhesion of inflammatory cells, including monocytes and neutrophils, to vascular endothelial cells. On the other hand, LPS and TNF-α can induce the expression of iNOS in those inflammatory cells and enhance NO production markedly (20, 26). It is possible that LPS shifts NO production from vascular endothelial cells to inflammatory cells in such conditions.
SB203580, a highly specific p38 MAPK inhibitor, completely blocked the iNOS-mediated NO production by IFN-γ and combined IFN-γ and LPS. This indicated that p38 MAPK might be involved in the induction of iNOS by IFN-γ. The involvement of ERK1/2 MAPK or tyrosine kinase was unclear since treatment with PD98059 or genistein suspended in dimethyl sulfoxide was harmful to END-D cells. Recently, SB203580 has been reported to inhibit the induction of iNOS by LPS in a macrophage cell line (1, 5, 6, 8). The p38 MAPK and c-Jun NH2-terminal kinase/stress-activated protein kinase are known to be key molecules in the signaling of LPS (13, 14, 18) and IFN-γ (7, 15, 30), respectively. In the present study, we demonstrated for the first time that p38 MAPK might be involved in the signaling of IFN-γ-mediated iNOS expression in vascular endothelial cells. Further studies are needed to clarify the exact relationship between IFN-γ-induced expression of iNOS and p38 MAPK.
The present study suggested the presence of a complicated regulation of iNOS expression in vascular endothelial cells by proinflammatory cytokines and LPS. However, it was unclear whether the in vitro findings in the present study could be applied to the in vivo phenomenon or not. Killer (cytotoxic) NO is synthesized by iNOS, whereas signal (messenger) NO is synthesized by cNOS. Recently, we have reported the participation of NO and peroxynitrite in LPS-induced hepatic injury in d-galactosamine-sensitized mice (23). The expression of iNOS was detected in vascular endothelial cells and hepatocytes of the livers. Further, NO also plays an important role in the killing of pathogenic microorganisms (12, 19, 27). The exact role of NO produced in vivo by iNOS in vascular endothelial cells stimulated with IFN-γ, TNF-α, and LPS alone or in combination is still a matter for speculation.
ACKNOWLEDGMENTS
This work was supported in part by a Grant-in-Aid for Scientific Research from the Ministry of Education, Science, Sports, and Culture of Japan.
We are grateful to G. Eguchi, Kumamoto University, for the distribution of the END-D cell line.
REFERENCES
- 1.Ajizian S J, English B K, Meals E A. Specific inhibitors of p38 and extracellular signal-regulated kinase mitogen-activated protein kinase pathways block inducible nitric oxide synthase and tumor necrosis factor accumulation in murine macrophages stimulated with lipopolysaccharide and interferon-gamma. J Infect Dis. 1999;179:939–944. doi: 10.1086/314659. [DOI] [PubMed] [Google Scholar]
- 2.Amin A R, Vyas P, Attur M, Leszczynska-Piziak J, Patel I R, Weissmann G, Abramson S B. The mode of action of aspirin-like drugs: effect on inducible nitric oxide synthase. Proc Natl Acad Sci USA. 1995;92:7926–7930. doi: 10.1073/pnas.92.17.7926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beckman J S, Koppenol W H. Nitric oxide, super oxide, and peroxynitrite: the good, the bad, and the ugly. Am J Physiol. 1996;271:C1424–C1437. doi: 10.1152/ajpcell.1996.271.5.C1424. [DOI] [PubMed] [Google Scholar]
- 4.Bereta J, Bereta M, Allison A C, Kruger P B, Koj A. Inhibitory effect of di-catechol rooperol on VCAM-1 and iNOS expression in cytokine-stimulated endothelium. Life Sci. 1997;60:325–334. doi: 10.1016/s0024-3205(96)00633-9. [DOI] [PubMed] [Google Scholar]
- 5.Chan E D, Winston B W, Uh S T, Wynes M W, Rose D M, Riches D W. Evaluation of the role of mitogen-activated protein kinases in the expression of inducible nitric oxide synthase by IFN-gamma and TNF-alpha in mouse macrophages. J Immunol. 1999;162:415–422. [PubMed] [Google Scholar]
- 6.Chen B C, Chen Y H, Lin W W. Involvement of p38 mitogen-activated protein kinase in lipopolysaccharide-induced iNOS and COX-2 expression in J774 macrophages. Immunology. 1999;97:124–129. doi: 10.1046/j.1365-2567.1999.00747.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Darnell J E, Jr, Kerr I M, Stark G R. Jak-STAT pathways and transcriptional activation in response to IFNs and other extracellular signaling proteins. Science. 1994;264:1415–1421. doi: 10.1126/science.8197455. [DOI] [PubMed] [Google Scholar]
- 8.Da Silva J, Pierrat B, Mary J L, Lesslauer W. Blockade of p38 mitogen-activated protein kinase pathway inhibits inducible nitric-oxide synthase expression in mouse astrocytes. J Biol Chem. 1997;272:28373–28380. doi: 10.1074/jbc.272.45.28373. [DOI] [PubMed] [Google Scholar]
- 9.Dimmeler S, Zeiher A M. Nitric oxide and apoptosis: another paradigm for the double-edged role of nitric oxide. Nitric Oxide Biol Chem. 1997;1:275–281. doi: 10.1006/niox.1997.0133. [DOI] [PubMed] [Google Scholar]
- 10.Ding A, Nathan C F, Graycar J, Derynck R, Stuehr D J, Srimal S. Macrophage deactivating factor and transforming growth factors-beta 1, -beta 2, and -beta 3 inhibit induction of macrophage nitrogen oxide synthesis by IFN-gamma. J Immunol. 1990;145:940–944. [PubMed] [Google Scholar]
- 11.Furchgott R F, Zawadzki J V. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980;288:373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- 12.Green S J, Scheller L F, Marletta M A, Seguin M C, Klotz F W, Slayter M, Nelson B J, Nacy C A. Nitric oxide: cytokine-regulation of nitric oxide in host resistance to intracellular pathogens. Immunol Lett. 1994;43:87–94. doi: 10.1016/0165-2478(94)00158-8. [DOI] [PubMed] [Google Scholar]
- 13.Han J, Lee J D, Bibbs L, Ulevitch R J. A MAP kinase targeted by endotoxin and hyperosmolarity in mammalian cells. Science. 1994;265:808–811. doi: 10.1126/science.7914033. [DOI] [PubMed] [Google Scholar]
- 14.Han J, Lee J D, Tobias P S, Ulevitch R J. Endotoxin induces rapid protein tyrosine phosphorylation in 70Z/3 cells expressing CD14. J Biol Chem. 1993;268:25009–25014. [PubMed] [Google Scholar]
- 15.Ihle J N. The Janus protein tyrosine kinase family and its role in cytokine signaling. Adv Immunol. 1995;60:1–35. doi: 10.1016/s0065-2776(08)60582-9. [DOI] [PubMed] [Google Scholar]
- 16.Kilbourn R G, Belloni P. Endothelial cell production of nitrogen oxides in response to interferon gamma in combination with tumor necrosis factor, interleukin-1, or endotoxin. J Natl Cancer Inst. 1990;82:772–776. doi: 10.1093/jnci/82.9.772. [DOI] [PubMed] [Google Scholar]
- 17.Kroncke K D, Fehsel K, Kolb-Bachofen V. Nitric oxide: cytotoxicity versus cytoprotection—how, why, when and where? Nitric Oxide Biol Chem. 1997;1:107–120. doi: 10.1006/niox.1997.0118. [DOI] [PubMed] [Google Scholar]
- 18.Lee J C, Young P R. Role of CSB/p38/RK stress response kinase in LPS and cytokine signaling mechanisms. J Leukoc Biol. 1996;59:152–157. doi: 10.1002/jlb.59.2.152. [DOI] [PubMed] [Google Scholar]
- 19.Liew F Y. Regulation of nitric oxide synthesis in infectious and autoimmune diseases. Immunol Lett. 1994;43:95–98. doi: 10.1016/0165-2478(94)00157-x. [DOI] [PubMed] [Google Scholar]
- 20.Lorsbach R B, Murphy W J, Lowenstein C J, Snyder S H, Russell S W. Expression of the nitric oxide synthase gene in mouse macrophages activated for tumor cell killing. Molecular basis for the synergy between interferon-gamma and lipopolysaccharide. J Biol Chem. 1993;268:1908–1913. [PubMed] [Google Scholar]
- 21.MacMicking J, Xie Q W, Nathan C. Nitric oxide and macrophage function. Annu Rev Immunol. 1997;15:323–350. doi: 10.1146/annurev.immunol.15.1.323. [DOI] [PubMed] [Google Scholar]
- 22.Magazine H I. Detection of endothelial cell-derived nitric oxide: current trends and future directions. Adv Neuroimmunol. 1995;5:479–490. doi: 10.1016/0960-5428(95)00030-5. [DOI] [PubMed] [Google Scholar]
- 23.Morikawa A, Kato Y, Sugiyama T, Koide N, Chakravortty D, Yoshida T, Yokochi T. Role of nitric oxide in lipopolysaccharide-induced hepatic injury in d-galactosamine-sensitized mice as an experimental endotoxic shock. Infect Immun. 1999;67:1018–1024. doi: 10.1128/iai.67.3.1018-1024.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morita H, Takeuchi T, Suzuki S, Maeda K, Yamada K, Eguchi G, Kimata K. Aortic endothelial cells synthesize a large chondroitin sulphate proteoglycan capable of binding to hyaluronate. Biochem J. 1990;265:61–68. doi: 10.1042/bj2650061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Murata J, Corradin S B, Felley-Bosco E, Juillerat-Jeanneret L. Involvement of a transforming-growth-factor-beta-like molecule in tumor-cell-derived inhibition of nitric-oxide synthesis in cerebral endothelial cells. Int J Cancer. 1995;62:743–748. doi: 10.1002/ijc.2910620616. [DOI] [PubMed] [Google Scholar]
- 26.Nathan C. Nitric oxide as a secretory product of mammalian cells. FASEB J. 1992;6:3051–3064. [PubMed] [Google Scholar]
- 27.Oswald I P, Wynn T A, Sher A, James S L. NO as an effector molecule of parasite killing: modulation of its synthesis by cytokines. Comp Biochem Physiol C. 1994;108:11–18. doi: 10.1016/1367-8280(94)90083-3. [DOI] [PubMed] [Google Scholar]
- 28.Palmer R M, Hickery M S, Charles I G, Moncada S, Bayliss M T. Induction of nitric oxide synthase in human chondrocytes. Biochem Biophys Res Commun. 1993;193:398–405. doi: 10.1006/bbrc.1993.1637. [DOI] [PubMed] [Google Scholar]
- 29.Peng H B, Spiecker M, Liao J K. Inducible nitric oxide: an autoregulatory feedback inhibitor of vascular inflammation. J Immunol. 1998;161:1970–1976. [PubMed] [Google Scholar]
- 30.Schindler C, Darnell J E., Jr Transcriptional responses to polypeptide ligands: the JAK-STAT pathway. Annu Rev Biochem. 1995;64:621–651. doi: 10.1146/annurev.bi.64.070195.003201. [DOI] [PubMed] [Google Scholar]
- 31.Schneemann M, Schoedon G, Frei K, Schaffner A. Immunovascular communication: activation and deactivation of murine endothelial cell nitric oxide synthase by cytokines. Immunol Lett. 1993;35:159–162. doi: 10.1016/0165-2478(93)90085-g. [DOI] [PubMed] [Google Scholar]
- 32.Schoedon G, Blau N, Schneemann M, Flury G, Schaffner A. Nitric oxide production depends on preceding tetrahydrobiopterin synthesis by endothelial cells: selective suppression of induced nitric oxide production by sepiapterin reductase inhibitors. Biochem Biophys Res Commun. 1994;199:504–510. doi: 10.1006/bbrc.1994.1257. [DOI] [PubMed] [Google Scholar]
- 33.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Walter R, Schaffner A, Schoedon G. Differential regulation of constitutive and inducible nitric oxide production by inflammatory stimuli in murine endothelial cells. Biochem Biophys Res Commun. 1994;202:450–455. doi: 10.1006/bbrc.1994.1949. [DOI] [PubMed] [Google Scholar]
- 35.Webb D J, Wen J, Lysiak J J, Umans L, van Leuven F, Gonias S L. Murine alpha-macroglobulins demonstrate divergent activities as neutralizers of transforming growth factor-beta and as inducers of nitric oxide synthesis. A possible mechanism for the endotoxin insensitivity of the alpha2-macroglobulin gene knock-out mouse. J Biol Chem. 1996;271:24982–24988. doi: 10.1074/jbc.271.40.24982. [DOI] [PubMed] [Google Scholar]