Background:
Autoimmunity is increasingly recognized as a key contributing factor in heart muscle diseases. The functional features of cardiac autoimmunity in humans remain undefined because of the challenge of studying immune responses in situ. We previously described a subset of c-mesenchymal epithelial transition factor (c-Met)–expressing (c-Met+) memory T lymphocytes that preferentially migrate to cardiac tissue in mice and humans.
Methods:
In-depth phenotyping of peripheral blood T cells, including c-Met+ T cells, was undertaken in groups of patients with inflammatory and noninflammatory cardiomyopathies, patients with noncardiac autoimmunity, and healthy controls. Validation studies were carried out using human cardiac tissue and in an experimental model of cardiac inflammation.
Results:
We show that c-Met+ T cells are selectively increased in the circulation and in the myocardium of patients with inflammatory cardiomyopathies. The phenotype and function of c-Met+ T cells are distinct from those of c-Met–negative (c-Met−) T cells, including preferential proliferation to cardiac myosin and coproduction of multiple cytokines (interleukin-4, interleukin-17, and interleukin-22). Furthermore, circulating c-Met+ T cell subpopulations in different heart muscle diseases identify distinct and overlapping mechanisms of heart inflammation. In experimental autoimmune myocarditis, elevations in autoantigen-specific c-Met+ T cells in peripheral blood mark the loss of immune tolerance to the heart. Disease development can be halted by pharmacologic c-Met inhibition, indicating a causative role for c-Met+ T cells.
Conclusions:
Our study demonstrates that the detection of circulating c-Met+ T cells may have use in the diagnosis and monitoring of adaptive cardiac inflammation and definition of new targets for therapeutic intervention when cardiac autoimmunity causes or contributes to progressive cardiac injury.
Keywords: cardiac myosins, cardiomyopathies, heart, hepatocyte growth factor, humans, inflammation, mice, myocarditis, T-lymphocytes, therapeutics
Clinical Perspective.
What Is New?
Evidence of active autoimmune myocardial inflammation can be detected through assays of peripheral blood that identify and quantify circulating heart-homing c-Met+ memory T cells.
Phenotyping these T cells provides insights into the mechanisms through which the adaptive immunologic system contributes to myocardial injury in heart muscle diseases where there is an inflammatory component.
What Are the Clinical Implications?
Assays for circulating c-Met+ T cells are readily obtained and may have use as a noninvasive diagnostic marker for inflammatory heart muscle disease.
The persistence, phenotype, and magnitude of a circulating c-Met+ T cell response may also indicate prognostic risk and identify individuals at risk of developing persistent myocardial inflammation and chronic myocarditis.
The c-Met+ T cell response provides a new therapeutic target for the treatment of myocardial inflammation; we demonstrate that blockade of c-Met signaling will abrogate myocardial inflammation in an experimental model of autoimmune myocarditis.
Editorial, see p 1946
T cell–mediated immunity has been linked to a variety of heart diseases, from classic inflammatory cardiac conditions, such as myocarditis, to diseases without a readily evident pathogenic inflammatory component, such as hypertensive cardiomyopathy.1 T cell activation in cardiac inflammation often results from the interaction of an external environmental trigger (viral infection) or an endogenous stimulus (mechanical or oxidative stress) with the immune system of the host.2 Persistence of proinflammatory stimuli or development of autoimmunity leads to chronic myocardial inflammation and ultimately cardiac dysfunction.
Acute myocarditis (AM) is mediated by T cell myocardial inflammation after infectious and noninfectious triggers.3 The acute myocardial injury associated with AM is also the most common cause of acute presentations with features of acute myocardial infarction but with angiographically normal coronary arteries.4 AM is a relatively frequent cause of sudden cardiac death in young adults and athletes5,6 and in older populations.7
In some of these patients, autoimmunity develops, and progressive cardiomyocyte damage leads to systolic impairment and dilated cardiomyopathy (DCM).8,9 AM is considered the most common cause of DCM,10 with some reports suggesting that nearly 50% of patients with a clinical diagnosis of idiopathic dilated cardiomyopathy (iDCM) have immunohistochemically detectable features of lymphocytic myocarditis,11,12 suggesting that chronic myocarditis can be subclinical.
Despite the many experimental models available, it has been difficult to define autoimmunity in inflammatory cardiomyopathy in humans, largely because of the technical challenges of studying the immune response in situ. Whereas there is consistency in reports implicating interleukin (IL)–17–producing T cells in myocarditis, the contribution of Th1 and Th2 responses in human disease remains controversial.13,14 In addition, the identification of an immunophenotype linked with clinical disease progression in human myocarditis has remained elusive.
We previously described a hepatocyte growth factor–induced memory T cell subset that preferentially migrates to the heart.15 These T cells are characterized by expression of the hepatocyte growth factor receptor c-mesenchymal epithelial transition factor (c-Met) and chemokine receptors CXCR3 and CCR4.
In this study, we detect and analyze the presence of c-Met–expressing T cells in the blood of patients with heart muscle disorders in which an inflammatory etiology is suspected (AM in particular). We show that circulating c-Met+ T cells mark the presence of autoreactive inflammation of the heart in humans and mice and define the features of this heart-selective immune response in acute and chronic disease.
Methods
The data that support the findings of this study are available from the corresponding author upon reasonable request. Raw data CEL files have been deposited to Gene Expression Omnibus under accession number GSE186270.
Detailed information on methods not covered in the main article is provided in the Supplemental Material, and specific details on all the reagents used in this study are provided in Table S1.
Study Populations
This study was approved as a component study (substudy 34) of the Barts BioResource (research ethics committee references 14/EE/0007 and 17/WS/0172). All patients provided written consent according to the principles of the Declaration of Helsinki. Study participants were recruited into the following patient groups: AM, iDCM, acute ST-segment–elevation myocardial infarction (STEMI), cardiac surgery, ischemic heart failure (IHF), or active Sjögren syndrome. A group of individuals with known familial heart muscle disease (fHMD) was also studied. Healthy controls (HCs) were recruited locally (Queen Mary, University of London), with 2 ethics research committee approvals (QMERC 2014/61 and QMERC 2014/61).
Sample sizes were estimated from pilot data and studies reporting quantitative changes in peripherally circulating T cells.16–19
For immunohistology, postmortem tissue samples from 5 patients with AM and 5 patients with DCM were obtained.
Details of each population can be found in the Expanded Methods in the Supplemental Material and Tables S2 through S4.
Experimental Autoimmune Myocarditis
The experimental autoimmune myocarditis (EAM) model has been described previously20 and is summarized in the Expanded Methods in the Supplemental Material.
Statistical Analysis
All data are presented as median (interquartile range) or mean ± SD as indicated.
Power calculations were performed in GPower on the basis of Wilcoxon Mann-Whitney test α=0.05, power=0.8, and predicted effect size=0.87. The effect size was defined as the mean standardized difference of the c-Met+ CD45RO+ CD4+% between patients with AM and those with DCM.
Standard paired and unpaired t tests were performed on normally distributed data.
All non–normally distributed data were assessed with Wilcoxon signed-rank and Mann-Whitney U tests for paired and unpaired data, as appropriate, and with a Kruskal-Wallis test if a comparison between >2 groups was performed using a standard Dunn post hoc analysis for multiple comparisons when the Kruskal-Wallis test was statistically significant. Upon assessment of 2 independent variables on a dependent variable, a 2-way analysis of variance was used. Repeated-measures 2-way analysis of variance was used as a statistical method for measuring paired dependent variables. If the 2-way analysis of variance was statistically significant, a Tukey post hoc test for multiple comparisons was used. Grouped, ordinal data were compared with χ2 tests.
The receiver operating characteristic analysis was performed in GraphPad V8 software using default settings. The list of thresholds was estimated by sorting all the values in all groups and averaging adjacent values in the sorted list. Each threshold value is midway between 2 values in the data. Sensitivity is the fraction of values in the patient group above the threshold. Specificity is the fraction of values in the control group below the threshold. CIs were computed from the observed proportion by the Clopper method without correction for multiple comparisons. Significance is defined at a 2-tailed level of 0.05.
Cell proliferation was assessed using the cell proliferation modeling module on FlowJo flow cytometry software according to manufacturer instructions. The proportion of cells dividing was determined after automatic identification of the resting, peak 0, and T cells.
Statistical analysis was performed in Prism (version 8.3.0, GraphPad Software) and a 2-tailed P value of <0.05 was considered statistically significant.
Results
Adaptive Cardiac Inflammation Is Marked by an Increase in Circulating c-Met+ Memory T Cells
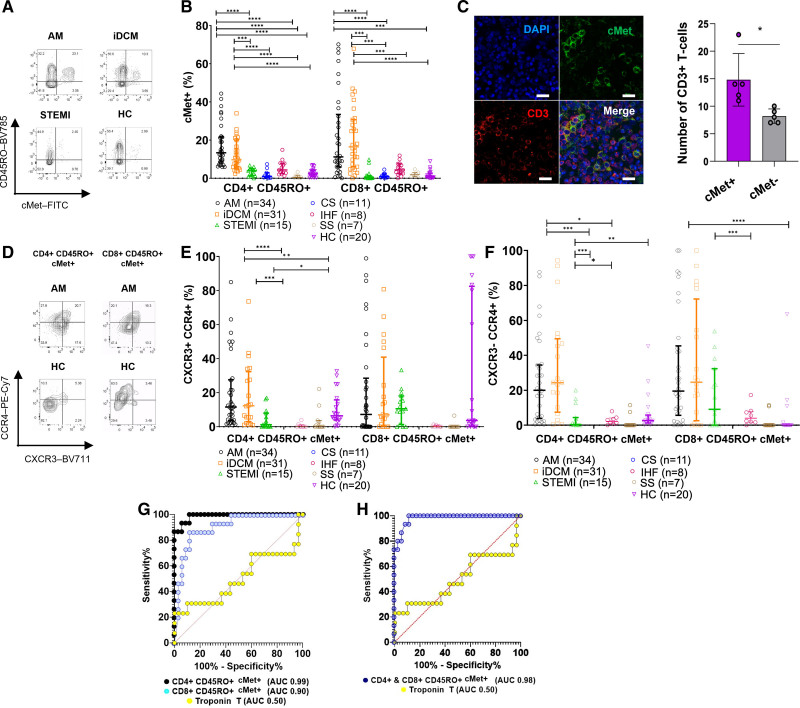
To test the hypothesis that circulating c-Met+ memory T cells mark the presence of myocardial inflammation, we first assessed their presence in the peripheral blood of patients presenting with presumed myocardial infarction but were subsequently diagnosed with AM as a cause of their acute myocardial injury. Additional groups included patients with apparently idiopathic DCM, often associated with cardiac inflammation21: patients with first-time STEMI for whom acute myocardial injury is a manifestation of coronary atherosclerosis in which inflammation localizes to the arteries but not the heart22; patients undergoing elective cardiac surgery but with no evidence of inflammation; patients with IHF; and HCs. A cohort of patients with active Sjögren syndrome, a noncardiac autoimmune condition, was also included as a control.23 Cohort demographics and diagnostic criteria are shown in Tables S2 through S4. Memory (CD45RO+) c-Met+ CD4+ and c-Met+ CD8+ T cells were significantly elevated in the blood of patients with AM or iDCM compared with those with STEMI, cardiac surgery, Sjögren syndrome, or IHF and HCs (Figure 1A and 1B; Figure S1 for gating and Figure S2A for absolute numbers). CD3+ c-Met+ T cells were identified within AM cardiac tissue, in which they represent the majority of infiltrating T cells (Figure 1C and 1D).
Figure 1.
Circulating c-Met–expressing T cells are increased in inflammatory cardiomyopathies. Peripheral blood CD4+ and CD8+ memory T cells were analyzed by flow cytometry for the expression of c-Met. A, Representative dot plots from the patient groups. B, Grouped data for patient and healthy control (HC) groups with significant Kruskal-Wallis for CD4+ and CD8+ T cells with post hoc Dunn multiple comparisons test. C, Confocal analysis of CD3+ (red) and c-Met+ (green) cells in paraffin-embedded postmortem acute myocarditis (AM) samples. Scale bar, 20 μm. The column graph shows the mean number of c-Met+ and c-Met− CD3+ T cells in four 20× fields from each of 5 patient samples (±SEM; paired Student t test). P values are highlighted as follows: ****P<0.0001, ***P<0.0005, **P<0.005, *P<0.05. Data are represented as median ± interquartile range. D through F, c-Met+ memory cells were also analyzed for coexpression of CXCR3 and CCR4 (E) as well as CCR4 single expression (F) within the patient groups indicated under the x axis. A representative dot plot is shown in D. Kruskal-Wallis for CD4+ T cells with post hoc Dunn multiple comparisons test. G, Receiver operating characteristic (ROC) curves for measurement of c-Met+ CD4+ (black) and CD8+ (blue) memory T cells and peak troponin T (yellow) for patients with AM versus patients with ST-segment–elevation myocardial infarction (STEMI). The ROC analysis was performed in GraphPad using the default settings. The list of thresholds was estimated by sorting all the values in all groups and averaging adjacent values in that sorted list. Each threshold value is midway between 2 values in the data. Each sensitivity is the fraction of values above the threshold in the patient group. The specificity is the fraction of values below the threshold in the control group. Each CI is computed from the observed proportion by the Clopper method without any correction for multiple comparisons. Significance is defined at 2-tailed level of 0.05. CD4+ area under the curve (AUC) 0.99, P<0.0001; CD8+ AUC 0.90, P<0.0001; troponin T AUC 0.50, P=0.98. H, ROC of CD4 and CD8 combined: blue indicates CD4 and CD8 CD45RO+ c-Met+ and yellow indicates troponin T. AUC 0.98, P<0.00001. CS indicates cardiac surgery; iDCM, idiopathic dilated cardiomyopathy; IHF, ischemic heart failure; and SS, Sjögren syndrome.
As previously described,24 an increased proportion of c-Met+ CD4+ memory T cells coexpressing the CXCR3 and CCR4 chemokine receptors was detectable in patients with AM or iDCM compared with the other groups (Figure 1E). In contrast, no significant difference was detected in memory c-Met+ CD8+ CXCR3+ CCR4+ T cells. However, both CD4+ and CD8+ c-Met+ CCR4+ T cells were significantly increased (Figure 1F).
STEMI and AM can have almost identical clinical presentations, with similar ECG and serum troponin concentrations, and differential diagnosis often requires emergency coronary angiography and cardiac magnetic resonance imaging. To address the sensitivity and specificity of circulating c-Met+ memory T cells, we performed receiver operating characteristic analyses limited to patients with AM or STEMI (Figure 1G and 1H). For memory c-Met+ CD4+ T cells, the area under the curve for AM was 0.99 (P<0.0001); a threshold of <6.1% c-Met expression had a sensitivity and specificity of 93.3% and 94.1%, respectively. For memory c-Met+ CD8+ T cells, the area under the curve was 0.90 (P<0.0001), and <2.7% threshold had a sensitivity of 86.7% and a specificity of 88.2%.
Phenotypic and Functional Characterization of Circulating c-Met+ T Cells in AM
A phenotypic and functional analysis of total circulating memory T cell populations of participant groups detected differences in T cell subsets and in markers of T cell activation, detailed in Figure S2.25,26
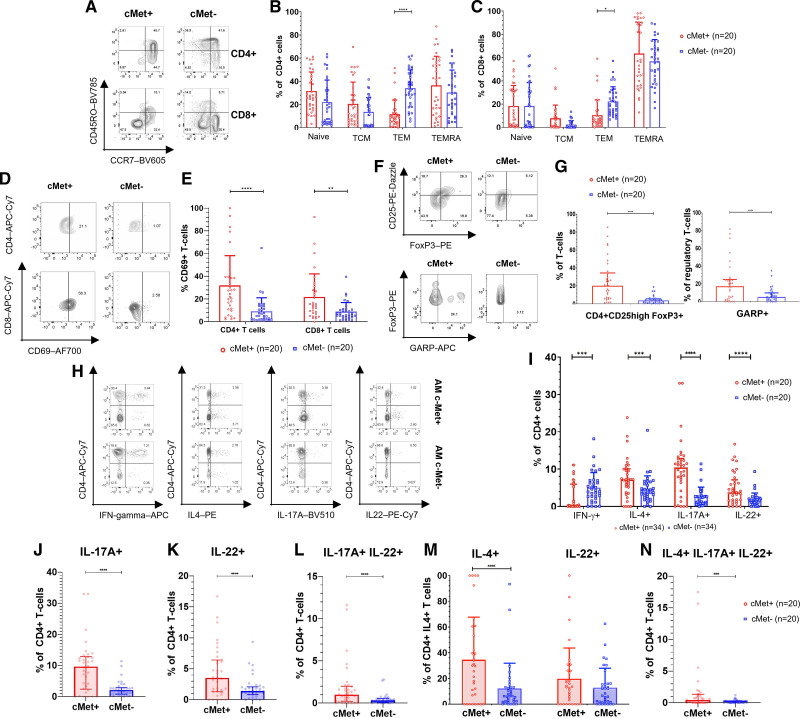
To identify selective functional features of circulating c-Met+ T cells, we applied pairwise comparisons of c-Met+ and c-Met− T cells in patients with AM. First, despite an overall increase in circulating c-Met+ T cells, we observed a significant reduction in the proportion of c-Met+ T cells with an effector phenotype in both CD4+ and CD8+ T cell subsets compared with c-Met− T lymphocytes. In contrast, naive, central memory, and effector memory RA+ T cell (TEMRA) phenotypes in CD4+ and CD8+ T cell subsets were similarly represented between c-Met+ and c-Met− T cells (Figure 2A through 2C).
Figure 2.
Phenotypic and functional characterization of c-Met+ T cells in AM. A, Representative flow cytometry plots showing expression of CD45RO and CCR7 by c-Met+ CD4+ T cells from a patient with acute myocarditis (AM). Summary flow cytometry data from patients are shown in B (CD4+) and C (CD8+). The dots in the graphs represent single individuals. The numbers refer to the number of patients in each respective group in each panel. G and I through N present results from the Wilcoxon signed rank test because paired patient data are compared. For B, C, and E, we used a 2-way analysis of variance because the samples are independent and we were testing whether the cells were c-Met+ or c-Met− and their expression of CD45RO and CCR7. D, Representative dot plots of CD69 expression by c-Met+ and c-Met− peripheral blood CD4+ and CD8+ T cells in a patient with AM. Summary data are shown in E, with a significant 2-way analysis of variance and post hoc Sidak multiple comparisons test. F shows representative dot plots of FoxP3 and CD25 expression (upper dot plots) and expression of GARP (glycoprotein A repetitions predominant) by FoxP3+CD25+ cells (lower dot plots) by c-Met+ and c-Met− peripheral blood CD4+ T cells from a patient with AM. Summary data for GARP are shown in G with a significant Wilcoxon signed rank test. In E and G, the red dots represent c-Met+ T cells and the blue dots represent c-Met− T cells. H and I, The production of the indicated cytokines by peripheral blood c-Met+ and c-Met− CD4+ T cells from patients with AM was assessed by intracellular staining and flow cytometry. Representative dot plots are shown in H. In the data summary in I, the red dots represent c-Met+ cells and the blue dots represent c-Met− cells. Statistical analysis was performed by Wilcoxon signed rank tests. J through N, Analysis of multiple cytokine producer c-Met+ and c-Met− T cells. J, Single-positive interleukin (IL)-17A–producing c-Met+ and c-Met− T cells. K, Single-positive IL-22+ c-Met+ and c-Met− cells. L, IL-17+ IL-22+ coproducing circulating c-Met+ and c-Met− memory T cells. M, c-Met+ and c-Met− IL-4+ IL-17+ T cells and IL-4+ IL-22+ T cells. N, IL-4+ IL-22+ IL-17A+ triple-positive c-Met+ and c-Met− memory T cell populations. Wilcoxon signed rank was used for statistical significance. P values are highlighted as follows: ****P<0.0001, ***P<0.0005, **P<0.005, *P<0.05. Data are represented as median ± interquartile range. APC indicates allophycocyanin; IFN, interferon; PE, phycoerythrin; TCM, central memory T cells; TEM, effector memory T cells; and TEMRA, effector memory RA+ T cells.
We then investigated the activation status of c-Met+ T cells and found that they expressed the early activation marker CD69 at significantly higher levels than c-Met− T cells, both in the CD4+ and CD8+ T cell subsets (Figure 2D and 2E). In patients with AM, circulating c-Met+ T cells included a substantial increase in the regulatory T cell (Treg) subset, and within this subset, of Tregs expressing the activation marker GARP (glycoprotein A repetitions predominant; Figure 2F and 2G).
We profiled cytokine production by c-Met+ T cells. As shown in Figure 2H and 2I, memory c-Met+ CD4+ T cells were significantly more likely to produce IL-4, IL-17A, and IL-22 compared with c-Met− T cells, but were significantly less likely to produce interferon (IFN)–γ. The dominant production of IL-4, IL-17, and IL-22 by c-Met+ CD4+ T cells led us to investigate whether individual cells in this T cell subset could coproduce combinations of these cytokines. As shown in Figure 2J, we observed a significant increase in single-positive IL-17A+ in c-Met+ T cells compared with c-Met− T cells as well as single-positive IL-22+ c-Met+ T cells compared with c-Met− T cells (Figure 2K). We also observed an increase in IL-17+ IL-22+ coexpressing c-Met+ T cells compared with c-Met− T cells (Figure 2L). In addition, the c-Met+ T cell population was significantly enriched in IL-4+ IL-17+ but not IL-4+ IL-22+ coproducing T cells compared with their c-Met− counterparts (Figure 2M).
A subset of IL-4+ IL-17+ IL-22+ coproducing T cells was also significantly increased in the c-Met+ but not c-Met− T cell population (Figure 2N), suggesting extreme plasticity in c-Met+ T cell differentiation.
In keeping with these data, c-Met+ CD4+-enriched T cell populations displayed increased levels of the transcription factors RORγT (RAR-related orphan receptor gamma isoform t), GATA3 (GATA family of conserved zinc-finger transcription factors 3), and AHR (aryl hydrocarbon receptor), respectively, associated with the development of Th17, Th2, and Th22 responses, whereas the c-Met− population showed increased transcription of Th1 inducer T-bet (T-box transcription factor TBX21) associated with Th1 development (Figure S3A).27 We also used bulk RNA transcriptomics to compare the transcriptional activity between c-Met+/− T cells (CD4+ and CD8+), which detected significantly increased transcripts of the olfactory transduction pathway.28 This pathway, together with c-Met, has been implicated in induction of motility in neuronal cells29 but never in T cells, and was subsequently confirmed by reverse transcription polymerase chain reaction (Figure S3B through S3H and Table S5).
A recent study of single-cell RNA sequencing and single T cell receptor sequencing in cardiac tissue from patients with DCM has described the presence of T cells expressing STAT3, known to be involved in c-Met signaling, as well as the chemokine receptor CXCR3.30 We performed an in silico reanalysis of the gene expression in T cell populations (Figure S3) and compared a T cell cluster resembling the c-Met+ population (T cell A) with the rest of the T cell populations (T cell B; Expanded Methods in the Supplemental Material). The T cell A cluster showed upregulation of genes positively regulating IL-4 and IL-13 (MAF, PARP1, NELL2, ID2) and IL-17 (TIGIT, IL32, CREM) and downregulation of genes positively associated with IFN-γ gene transcription (AIF1, PLAC1, S100A4, IFIM3, ZNF683) compared with the rest of T cell populations (T cell B; Figure S3I).
The c-Met+ Memory T Cell Subset Contains Autoreactive T Cells Specific for Cardiac Myosin
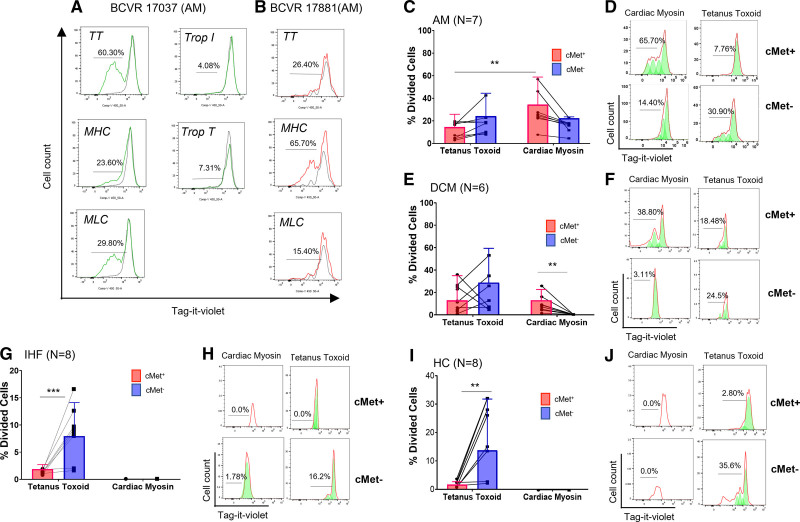
Irrespective of the initial trigger, the pathogenic immune response in AM can develop autoimmune specificity for self-antigen.3 We therefore evaluated the antigen specificity of c-Met+ T cells in patients with AM, iDCM, or IHF and HCs by assaying proliferation of Tag-it Violet–labeled peripheral blood mononuclear cells (PBMCs) from patients with AM exposed to selected cardiac sarcomeric antigens and the recall antigen tetanus toxoid (TT) after 7 days of culture. As shown in Figure 3A through 3D, proliferative responses to cardiac myosin and TT were detected in cell cultures from all patients with AM tested. Responses to the autoantigen cardiac myosin segregated with the c-Met+ T cell population (Figure 3C and 3D). Most of the TT-responding T cells were in the c-Met− fraction. We did not detect T cell proliferation in response to troponin antigens in any of the patients (Figure 3A and 3B).
Figure 3.
c-Met+ T cells proliferate to cardiac myosin. Peripheral blood T cells from patients with acute myocarditis (AM) were labeled with an intravital fluorescent dye (Tag-it Violet) and co-cultured with the indicated antigens. For controls, no antigen was added to the cultures. A and B, Example proliferative responses (Tag-it Violet dilution) in 2 patients with AM (BCVR 17037 and 17881) depicted histographically by total CD3+ live lymphocytes in response to antigen. Unstimulated T cells (no-antigen controls) are shown by the black lines. C, Flow cytometry of postproliferation sample using c-MET antibody to determine c-Met+/− status of proliferating cells. Cell replication is indicated by a left shift on the x axis. Red line indicates raw flow cytometry data (cell count). Green peaks indicate derived exemplar cell replication (eg, cardiac myosin/c-Met+ sample: from right–left division [d] 0, d1, d2, and d3). D shows a summary of the responses to cardiac myosin and tetanus toxoid (TT) by c-Met+ and c-Met− T cells from 7 patients with AM. Each dot represents c-Met+ and c-Met− T cell responses and the lines linking dots identify the same individual’s c-Met+ and c-Met− T cell responses to either TT or myosin. Identical assays were performed using peripheral blood mononuclear cells from 6 patients with idiopathic dilated cardiomyopathy (DCM; E and F), 8 patients with ischemic heart failure (IHF; G and H), and 8 healthy controls (HC; I and J). Data were analyzed using repeated measures 2-way analysis of variance followed by a significant Tukey multiple comparison test for c-Met+ response to TT compared with c-Met+ response to cardiac myosin. P values are highlighted as follows: **P<0.005. Data are represented as median ± interquartile range. MHC indicates myosin heavy chain; MLC, myosin light chain; and Trop, troponin.
Similar antigen-specific responses were detected in PBMCs from patients with DCM. T cells responding to cardiac myosin were detected in the c-Met+ T cell subset (Figure 3E and 3F). In contrast, although T cells proliferated in response to TT, no responses to cardiac antigens by c-Met− T cells were detectable in IHF (Figure 3G and 3H) or HC (Figure 3I and 3J) samples. Because of their minute proportion, it was not possible to detect proliferative responses in c-Met+ T cells from the latter cohorts.
In summary, autoreactive responses mediated by c-Met+ T cells are selectively detectable in individuals with cardiac inflammation. In line with these data, autoantibodies to MHC (myosin heavy chain) have been detected in patients with AM or DCM.31
Circulating c-Met+ T Cell Subpopulations in Different Cardiac Diseases Identify Distinct and Overlapping Mechanisms of Cardiac Inflammation
Myocardial inflammation may contribute to myocardial injury in up to half of iDCM cases, in which unresolved chronic inflammation can lead to progressive myocardial injury and poorer clinical outcomes.2 However, in clinical practice, the causes of myocardial injury in any individual case are often unclear after extensive diagnostic workup and, in many cases, are likely to be multifactorial.32,33,37,38 In this imprecise context, we sought to investigate whether phenotypic or functional differences in circulating c-Met+ T cells could provide evidence of an evolving chronic autoimmune response in iDCM.
As shown previously (Figure 1A through 1D), both CD4+ and CD8+ memory c-Met+ T cells were increased in patients with iDCM, which shared most features with those described for AM, including specificity (Figure 3E and 3F), cytokine production, gene transcription, and phenotype (Figure S4 and S5).
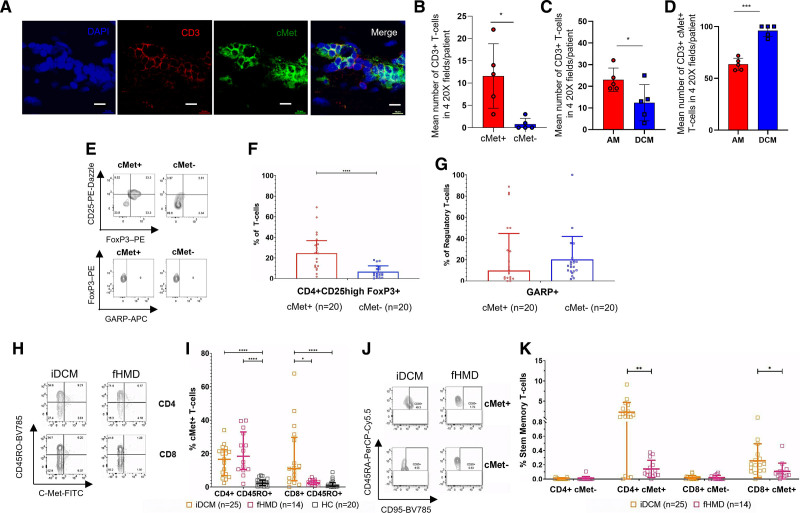
c-Met+ T cells were also present in iDCM cardiac tissue (Figure 4A and 4B). However, despite the decreased number of infiltrating T lymphocytes compared with AM (Figure 4C), almost all T cells in tissue from iDCM expressed c-Met (Figure 4D). The proportion of infiltrating CD4+ and CD8+ T cell subsets was similar in AM and iDCM, with c-Met+ CD4+ and CD8+ T cells representing the dominant phenotype, with a further significant enrichment in iDCM infiltrates (Figure S6).
Figure 4.
Disease-specific features of c-Met+ memory T cells. A, Confocal analysis of CD3+ (red) c-Met+ (green) cells in postmortem paraffin-embedded idiopathic dilated cardiomyopathy (iDCM) samples. Scale bar, 20 μm. B, Mean number of c-Met+ and c-Met− CD3+ T cells in four 20× fields from each of 5 unique iDCM myocardial tissue samples. C, Mean number of c-Met+ and c-Met− CD3+ T cells in four 20× fields from each of 5 independent samples of acute myocarditis (AM) and 5 samples of iDCM myocardium. D, Mean percentage of c-Met+ T cells in CD3+ T cell infiltrates in four 20× fields from each of 5 independent AM or iDCM samples (mean ± SEM, unpaired Student t test). E through G, FoxP3 and CD25 expression (upper dot plots) and expression of GARP (glycoprotein A repetitions predominant) by FoxP3+ CD25+ cells (lower dot plots) by peripheral blood c-Met+ and c-Met− CD4+ T cells from patients with iDCM. E includes representative dot plots and summary data are shown in F and G. H shows representative dot plots for c-Met–expressing CD4+ and CD8+ T cells from peripheral blood samples from iDCM and genetically confirmed familial heart muscle disease (fHMD). A summary of the data, including healthy controls (HC), is shown in I, with a significant Kruskal-Wallis test for both CD4+ and CD8+ T cells. For CD4+ T cells, there was a significant Dunn multiple comparison test for iDCM versus HC and fHMD versus HC, but not iDCM versus fHMD. For CD8+ T cells, there was a significant Dunn multiple comparison test for iDCM versus HC and iDCM versus fHMD but not between fHMD and HC. J and K, Peripheral blood T cells from AM, iDCM, and fHMD were stained for markers of stem memory T cells (CD3+ CD4+ CCR7+ CD45RA+ CD95+). Representative dot plots obtained after gating on CD3+ CD4+ CCR7+ T cells from nonfamilial iDCM and fHMD are shown in J. Grouped data displaying the proportion of CD4+ and CD8+ stem memory T cells in the peripheral blood of iDCM and fHMD patient groups are shown in K. Statistical analysis was performed with Mann-Whitney tests that were significant for the CD4+ c-Met+ T cells and CD8+ c-Met+ T cells but not c-Met− CD4+ or CD8+ T cells. P values are highlighted as follows: ****P<0.0001, ***P<0.001, **P<0.01, *P<0.05. Data are represented as median ± interquartile range. APC indicates allophycocyanin.
Alterations in Treg status was the most striking difference between circulating c-Met+ T cells in AM and iDCM. Despite a similar increase in c-Met+ Treg cells in iDCM, unlike in AM (Figure 2G), c-Met+ Treg cells from patients with iDCM failed to upregulate the activation marker GARP (Figure 4E through 4G). This difference in Treg status may indicate a mechanism for progression to and maintenance of chronic inflammation in iDCM.
Other than potential deficiencies of c-Met+ T cell regulation, the absence of other distinctive features between T cells in AM and iDCM and the further enrichment in cardiac c-Met+ T cell infiltrates point to a spectrum of heart muscle inflammatory diseases in which myocardial injury develops as a result of similar or shared autoimmune pathways.
We next sought to investigate the hypothesis that circulating T cells might provide clues to the pathogenic process underlying cardiac inflammation. To this aim, we compared c-Met+ T cells in the blood of patients with AM, iDCM, or proven fHMD (Tables S2 and S4). Myocarditis-like hot-phase clinical episodes and inflammatory cell infiltrates are well recognized in the natural history of several genetic heart muscle diseases that are conventionally considered distinct from each other and from acquired myocarditis.34 In some cases, particularly when fHMD is caused by mutations in genes encoding desmosome components or filamin, the initial presentation may be clinically indistinguishable from AM.34–36 We therefore investigated whether c-Met+ T cells could be detected in PBMCs from 14 patients with genetic mutations known to be causative for fHMD. There were no significant age differences between fHMD and iDCM groups. Table S4 shows the genetic variants present in the fHMD group.
We found that c-Met+ CD4+ CD45RO+ T cells were present in similar proportions in PBMCs from patients with iDCM and patients with fHMD, and significantly higher than in HCs (Figure 4H and 4I). In striking contrast, c-Met+ CD8+ CD45RO+ T cells were much higher in iDCM than in fHMD compared with iDCM, and proportions of c-Met+ CD8+ CD45RO+ T cells in fHMD and HCs were similar.
This is consistent with the hypothesis that although memory CD4+ c-Met+ T cells are similarly elevated in fHMD and iDCM, the mechanisms of cardiac injury (ie, cell- or antibody-mediated) may be different in these 2 clinical states or at different disease stages. CD4+ T cell–dependent autoantibody responses are thought to play a pathogenic role in several forms of fHMD.37,38,43
This hypothesis was investigated further by assessing the presence of CD45RA+ CCR7+ CD95+ stem memory T cells. This long-lived, self-renewing T cell subset has been detected in CD4+ and CD8+ T cell populations of mice and humans,39 and has been suggested to provide a reservoir of self-reactive T cells in autoimmune diseases.39,40 We observed increased proportions of both CD4+ c-Met+ stem memory T cells in iDCM compared with fHMD (Figure 4J and 4K). These data imply that whereas in iDCM, autoimmunity has the potential to support itself, additional triggers (eg, exercise and other environmental factors) may be needed to precipitate autoimmunity episodes (hot phases) in fHMD.
c-Met+ T Cells Play a Key Role in EAM, and Their Rise in Peripheral Blood Marks the Loss of Immune Tolerance to the Heart
Defining the causative role of c-Met+ T cells in the development of the autoimmune phase of inflammatory cardiomyopathies is logistically difficult to address in human studies. We therefore modeled the role of c-Met+ T cells in EAM, a murine model of progressive cardiac autoimmune inflammation leading from AM to DCM.20 EAM is a T cell–dependent model sharing key features with human AM and DCM (T cell–mediated, autoantigen, disease course, male sex bias, and more).
Male BALB/cAnN mice were immunized subcutaneously with the MHCα peptide (RSLKLMATLFSTYASADR). Control mice received adjuvant alone with the same schedule (Figure 5A). We have previously shown that pharmacologic c-Met inhibition during T cell priming prevents the upregulation of this receptor and cardiac allograft rejection in mice.15 A third group of mice received the c-Met–selective small molecule inhibitor PHA-665752 for 10 days after the initial immunization, a time frame when topographic memory is thought to occur.15
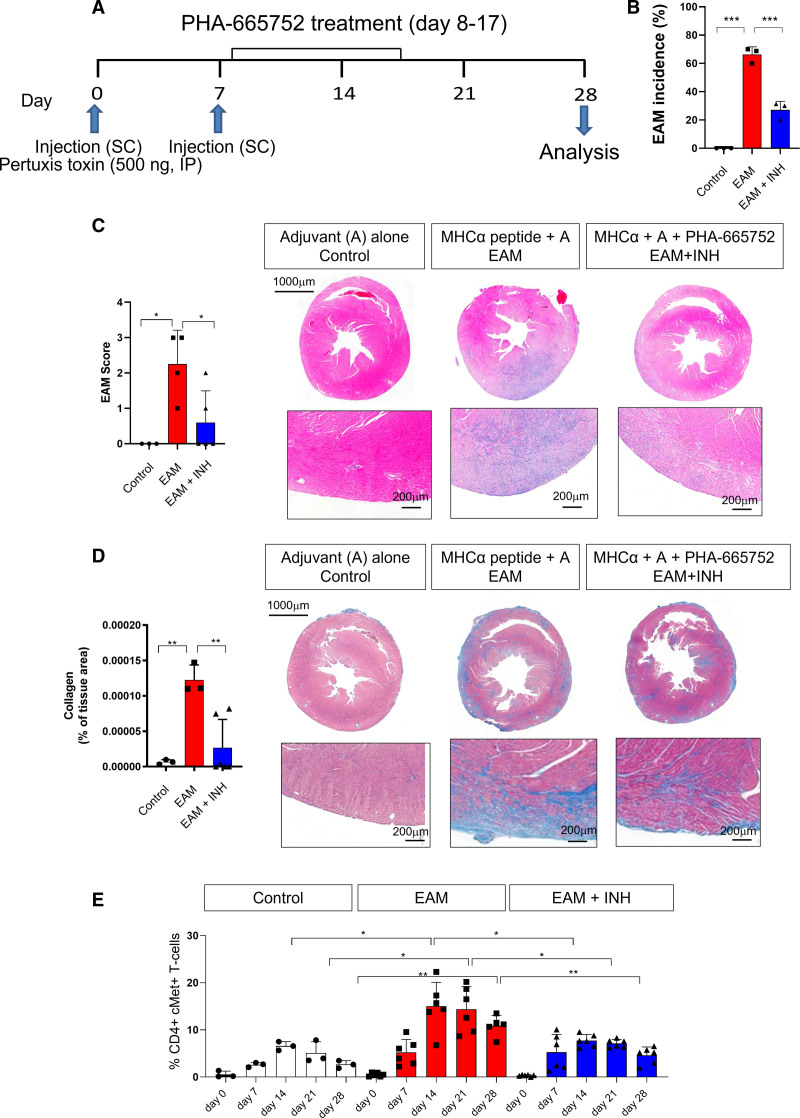
Figure 5.
c-Met+ T cells mediate inflammation in experimental autoimmune myocarditis. To induce autoimmune myocarditis, BALB/cAnN male mice were immunized with murine cardiac MHCα (myosin heavy chain) peptide (RSLKLMATLFSTYASADR) as described in the Methods. As shown in the protocol summarized in A, some mice received intraperitoneal injections of the c-Met inhibitor PHA-665752 (500 µg/mL; experimental autoimmune myocarditis [EAM] + inhibitor [INH]) from day 8 to day 17 after immunization. As a control, a group of mice received an adjuvant alone (control). B, EAM incidence 28 days after the first immunization. The development of inflammatory infiltrates and collagen deposition were assessed by hematoxylin & eosin (C) and Masson trichrome staining (D) of the heart 28 days after immunization. Column graphs show disease scores obtained as described in Methods. Representative images at 20× and 40× magnification are shown on the right side of each panel. Statistical analysis was performed with 1-way analysis of variance. Total n=3. E, Tail vein blood was sampled on the same mouse on the indicated time points. The measurement on different days were taken from the same mouse. The % of CD44 high, CD4+ c-Met+ T cells was determined by flow cytometry. Statistical analysis was performed with repeated measures 2-way analysis of variance test. P values are highlighted as follows: ***P<0.0005, **P<0.005, *P<0.05. Data are represented as median ± interquartile range.
Immunization of mice with MHCα led to AM, assessed as EAM incidence (Figure 5B), area of mononuclear cell infiltrate (Figure 5C), and collagen deposition (Figure 5D), measured by histology on day 28 after the first immunization. Echocardiography revealed functional alterations consistent with AM (Figure S7A). Pharmacologic inhibition of c-Met (EAM + inhibitor) significantly reduced EAM incidence, mononuclear cell infiltrates, and collagen deposition (Figure 5B through 5D), as well as the echocardiographic signs of AM mentioned previously.
The proportion of circulating memory CD44+ c-Met+ T cells was monitored by flow cytometry for the duration of the experiment (see Figure S8 for gating strategy). Development of disease was marked by a progressive increase of circulating CD44+ c-Met+ T cells in immunized mice (Figure 5E; Figure S7B for absolute numbers). In mice treated with the c-Met inhibitor, reduction of disease severity was mirrored by a reduction in circulating c-Met+ T cells.
We further analyzed the distribution and functional characteristics of c-Met+ T cells ex vivo at day 28 of EAM. Increased proportions of both CD4+ and CD8+ c-Met+ memory T cells were found in heart tissue, heart-draining lymph nodes, and spleen (Figure S7C through S7E) but not in nondraining lymph nodes (Figure S7F). c-Met+ T cells displayed signs of recent activation in the heart tissue and draining lymph nodes, but less in the spleen. In addition, we detected a significant increase of c-Met+ CD4+ CD25high FoxP3+ Treg cells in heart, draining lymph nodes, and spleen, but not in nondraining lymph nodes. Treatment with PHA-665752 blunted the expansion of c-Met+ T cells in the heart, draining lymph nodes, and spleen, as well as reducing the proportion of Tregs.
As a control, we also investigated the presence of circulating c-Met+ T cell subsets in prediabetic and diabetic NOD (nonobese diabetic) mice, a model of autoimmune type I diabetes, but we were unable to detect an expansion of this T cell subset in this model of autoimmunity, suggesting that c-Met+ T cells selectively associate with heart autoimmunity (Figure S7G and S7H).
The specificity of c-Met+ T cells was determined by assaying proliferation of carboxyfluorescein succinimidyl ester–labeled splenocytes from diseased and control mice exposed to cardiac myosin after 5 days of culture.
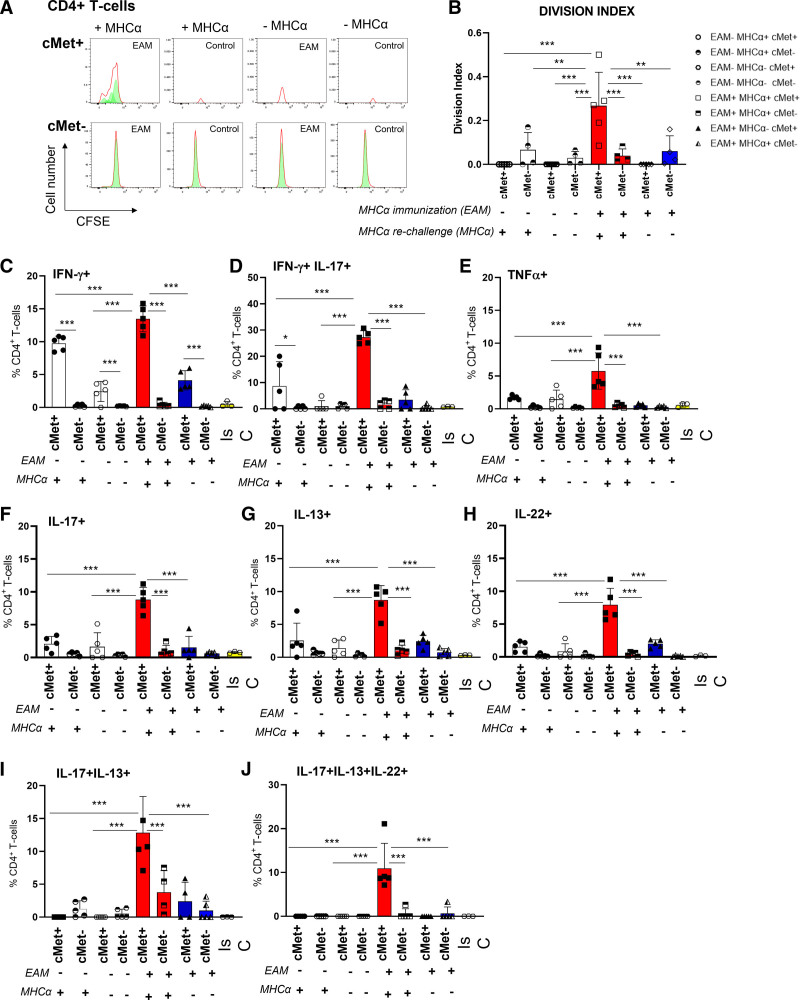
As shown in Figure 6A and 6B, proliferation to autoantigen was detected only in the c-Met+ T cell population of immunized mice, confirming their autoreactive nature. As expected, no proliferative responses against the MHCα peptide were detected in either c-Met+ or c-Met− CD8+ T cells (Figure S9A). Unlike in human AM, c-Met+ CD4+ T cells also produced IFN-γ (Figure 6C), with a proportion of these T cells also coproducing IL-17 (Figure 6D) and tumor necrosis factor–α (Figure 6E). IFN-γ was also produced by c-Met+ T cells from mice that received adjuvant alone (Figure 6C), possibly because of nonspecific systemic immune activation by the adjuvant. Similar to what was observed in human PBMCs from patients with AM or iDCM, IL-17–producing and IL-13–producing CD4+ T cells were significantly increased in the c-Met+ T cell populations (Figure 6F and 6G). IL-13 was used as surrogate for Th2 responses because IL-4 could not be measured by intracellular antibody staining. These cytokines were not produced by c-Met− T cells. IL-22–producing T cells were also enriched in the c-Met+ T cell population (Figure 6H). When multiple cytokine producers were analyzed, as in human AM, IL-17+ IL-13+ double-positive and IL-17+ IL-13+ IL-22+ triple-positive c-Met+ T cells were significantly increased in the c-Met+ T cell population (Figure 6I and 6J) in the heart-draining lymph nodes, but not the spleen of immunized mice (Figure S9, depicting cytokine production by c-Met+ and c-Met− T cells in the spleen).
Figure 6.
Specificity and functional characteristics of c-Met+ T cells in experimental autoimmune myocarditis. BALB/cAnN male mice were immunized with murine MHCα (myosin heavy chain) peptide (RSLKLMATLFSTYASADR). Fourteen days after the second immunization, mice were killed and CD4+ T cells from heart-draining lymph nodes restimulated in vitro with autologous splenocytes, stimulatory anti-CD28 antibody, and MHCα peptide. Controls included mice treated with adjuvant alone. The white bars correspond to the negative controls, where mice were not immunized or rechallenged with MHCα peptide (ie, experimental autoimmune myocarditis [
EAM], MHCα−). Red and blue bars correspond to immunized mice (ie, EAM+) with and without MHCα peptide rechallenge (red bars, MHCα+; blue bars, MHCα−), respectively. A and B, Before stimulation, some T cells were labeled with carboxyfluorescein succinimidyl ester. Cells were harvested and analyzed 5 days later for carboxyfluorescein succinimidyl ester dilution and c-Met+ expression. Representative histograms are shown in A. In B, the division index measured in samples from 5 animals is shown (n=2). C through J, Production of the indicated cytokines by c-Met+ and c-Met− T cells was measured 6 hours after restimulation with MHCα peptide by intracellular staining. In the experimental design, 2 independent variables were applied on the same level (ie, EAM+/−, and MHCα+/−) in nonindependent samples. Downstream of EAM immunization and MHCα peptide rechallenge, c-Met+ and c-Met− populations were discriminated through flow cytometry evaluation. A mixed-effects analysis of variance test was used for statistical analysis to account for the fact that some samples are paired and correlated.
P values are highlighted as follows: ***P<0.001, **P<0.01, *P<0.05. Data are represented as median ± interquartile range. IL indicates interleukin.
Discussion
On the basis of our previous description of cardiac-tropic c-Met+ memory T cells,15 we investigated the possibility that bloodborne c-Met–expressing memory T cells might mark and define adaptive autoimmune inflammation of the heart. c-Met expression by highly cytotoxic CD8+41 but not CD4+ T cells has been reported in encephalitogenic T cells42 and has been transduced in T cells to target them to the liver,43 in line with our previous observations.15
c-Met+ memory T cells display features that implicate them in the pathogenesis of cardiac autoimmunity. c-Met+ T cells are detected in large numbers in inflammatory infiltrates of AM and iDCM hearts and respond to the autoantigen cardiac myosin.
c-Met+ T cells display certain unique functional characteristics, including patterns of cytokine secretion dominated by IL-4, IL-17, and IL-22. Production of IL-17A has been previously associated with AM, in which it is upregulated in response to cardiac myosin stimulation.14 Although limited data describe a role for IL-4 in human myocarditis, work in mice has shown the importance of IL-4 in driving AM.20 Relevant to this study, in a model of autoimmune myocarditis, the c-Met ligand hepatocyte growth factor was shown to enhance Th2 responses,44,45 providing a mechanistic link for IL-4 production by c-Met+ T cells.
IL-22 plays a central role in wound healing and antimicrobial immunity in the skin.46 However, under pathologic conditions, IL-22 can play a proinflammatory role in psoriasis47 and murine viral myocarditis,48 particularly when associated with IL-17 production.47 Furthermore, a recombinant IL-22–immunoglobulin molecule has been shown to ameliorate EAM in mice.48 Similar to IL-4, the role of IL-22 in cardiac inflammation has not been described in humans, but IL-22 production in EAM was also reduced by c-Met inhibition, suggesting a common induction pathway.
A unique feature of c-Met+ T helper cell (Th) subsets is the ability to simultaneously produce IL-4/IL-13, IL-17, and IL-22 in both AM and EAM. Precursors of Th17 cells can differentiate into Th17/Th1 cells in response to IL-12, whereas an IL-4–rich microenvironment can induce memory Th17/Th2 cells.50,51 Similar observations have been made in the Th22 subset.52,53 The protective or pathogenic role of these subsets in human health and disease is unclear.54 A similar IL-4+ IL-17+ IL-22+ Th cell subset has been reported to play a protective role in pregnancy outcomes,55 with transcripts of relevant transcription factors, including GATA3, RORc (RAR-related orphan receptor C), and AHR54 detected in the sites infiltrated by this subset. Further studies are needed to establish the role of c-Met signals in defining cytokine production by T cells, particularly because our data in mice suggest that only IL-13 and IL-22, but not IL-17, production is promoted by this pathway.
We also observed an increased production of IFN-γ by c-Met− T cells in iDCM. The role of this cytokine in inflammatory cardiomyopathy remains contentious. A number of murine studies suggest that IFN-γ plays a protective role in myocarditis because of its role in maintaining the suppressive function of Treg cells.56–58 Other works in mouse models of persistent adenoviral myocardial infection and EAM have suggested that IFN-γ is proinflammatory, although it is not clear whether the IFN-γ was produced by T cells in these studies because it also can be produced by natural killer cells.59–61 Genetic testing of children with DCM has shown higher frequencies of an IFN-γ genotype (TT) that is associated with higher IFN-γ expression compared with HCs.62 However, another study involving 16 patients with undifferentiated but severe DCM (average left ventricular ejection fraction=22) has shown that, after in vitro stimulation, there was a reduction in the proportion of CD4+ T cells producing IFN-γ compared with HCs, suggesting that IFN-γ–producing CD4+ T cells do not make a substantial contribution to severe DCM.63 Increased IFN-γ production might be part of the attempt of the immune system to control inflammation. Our finding that c-Met+ GARP+ Tregs are increased in AM but not in iDCM suggests a failure to develop a protective response in the latter, as has been reported previously.64 Increased IFN-γ+ T cells in these patients might therefore reflect a compensatory mechanism to increase Treg suppressive and anti-inflammatory activity.
Our observations also indicate that differences in circulating c-Met+ memory T cell subpopulations may reflect distinct pathogenic mechanisms of inflammatory cardiomyopathies. Whereas similar proportions of c-Met+ CD4+ memory T cells are detectable in both iDCM and fHMD, c-Met+ CD8+ memory T cells are nearly undetectable in patients with fHMD. CD8+ T cell responses are essential to viral containment and the presence of CD8+ T cells has been described as a diagnostic feature for AM by endomyocardial biopsy immunohistochemistry analysis,65–67 consistent with commonly described viral triggers of this condition. Other work has shown that CD4+ T cells are the predominant T cell infiltrate in endomyocardial biopsy from inherited arrhythmogenic cardiomyopathies.68 Relevant to this observation, the role of CD8+ T cells in autoimmune myocarditis has not been clarified. Similar to CD4+ T cells, they are presumed to be induced to express c-Met during priming. In human AM, they could be remnants of an antiviral immune response. In EAM, although we find CD8+ c-Met+ T cells, we cannot detect proliferation of these T cells in response to cardiac myosin (as expected, because the immunogen is major histocompatibility complex class II restricted). Whereas epitope spreading during autoimmunity might account for this effect, the precise role of CD8+ c-Met+ T cells in autoimmune heart muscle inflammation remains to be clarified.
In addition, we show that c-Met+ stem memory T cells are detectable in patients with AM or iDCM, but not in patients with fHMD. Stem memory T cells play a role in the maintenance of T cell–mediated autoimmune diseases, in which they provide a reservoir of self-reactive T cells that sustain chronic adaptive inflammation,39,40 suggesting persistence of autoimmunity in AM and in iDCM. Their absence in patients with fHMD indicates that in these conditions, autoimmunity might arise occasionally or intermittently, possibly in response to transient triggers, such as after exercise or during systemic inflammatory responses after otherwise unrelated disorders.
We provide evidence that preventing the development of c-Met+ T cells by selective pharmacologic inhibition of c-Met is effective in significantly blunting disease severity in EAM, in which the functional phenotype of c-Met+ cells is remarkably similar to that of their human counterpart. Furthermore, their presence in the peripheral blood can predict EAM development, suggesting a causative role for this T cell subset in cardiac autoimmunity.
Study Limitations
A limitation of these experiments is that we cannot exclude an effect of the c-Met inhibitor on other immune cells, such as antigen-presenting cells. An alternative approach would use inducible, T cell–selective c-Met–deficient mice, but these are not available on the BALB/cAnN genetic background.
Single-cell transcriptomic analysis of both AM and DCM c-Met+ T cells would greatly help the full characterization of c-Met+ T cells in cardiac autoimmunity.
An additional limitation is that AM diagnoses were made on the basis of clinical presentations and cardiac imaging (angiography and cardiac magnetic resonance imaging) and were not confirmed by biopsy. However, endomyocardial biopsy is not recommended in the diagnostic workup for most patients with AM.69 Although human cohorts were small, sample sizes exceeded those indicated by power analyses.
Conclusions
In a novel study with a limited number of patients, the potential translational implications of this work are evident, namely the diagnostic and prognostic potential of monitoring c-Met+ T cells in the venous blood, and provide a new target pathway for therapeutic intervention. Multicenter studies will be required for the translation of these findings in the diagnosis, prognosis, and treatment of inflammatory cardiomyopathies.
Article Information
Acknowledgments
The authors thank the patients and healthy blood donors. This work forms part of the research areas contributing to the translational research portfolio of the Cardiovascular Biomedical Research Unit at Barts, which is supported and funded by the National Institute for Health Research.
Sources of Funding
This work was supported by the British Heart Foundation (grant FS/16/18/31973 to Drs E. Stephenson‚ Marelli-Berg, and Mohiddin; grant FS/18/82/34024 to Drs Protonotarios and Elliott; grant PG/18/27/33616 to Dr Asimaki; grant CH/15/2/32064 and British Heart Foundation Accelerator Award AA/18/5/34222 to Dr Marelli-Berg), the Barts’ Charity (grant MRC0230 to Drs E. Stephenson‚ Marelli-Berg, and Mohiddin), and donations from the Thompson, Hughes, and Parsons Foundations. Dr Rocha-Vieira was the recipient of a Brazilian National Council for Scientific and Technological Development Fellowship (CNPq- 20813/2017-5).
Disclosures
None.
Supplemental Material
Expanded Methods
Tables S1–S5
Figures S1–S9
Supplementary Material
Nonstandard Abbreviations and Acronyms
- AHR
- aryl hydrocarbon receptor
- AM
- acute myocarditis
- c-Met
- c-mesenchymal epithelial transition factor
- DCM
- dilated cardiomyopathy
- EAM
- experimental autoimmune myocarditis
- fHMD
- familial heart muscle disease
- GARP
- glycoprotein A repetitions predominant
- GATA3
- GATA family of conserved zinc-finger transcription factors 3
- HC
- healthy control
- HGF
- hepatocyte growth factor
- iDCM
- idiopathic dilated cardiomyopathy
- IFN
- interferon
- IHF
- ischemic heart failure
- IL
- interleukin
- MHC
- myosin heavy chain
- NOD
- nonobese diabetic
- PBMC
- peripheral blood mononuclear cell
- RORc
- RAR-related orphan receptor C
- RORγt
- RAR-related orphan receptor gamma isoform t
- STEMI
- ST-segment–elevation myocardial infarction
- TEMRA
- effector memory RA+ T cell
- Th
- T-helper cell subset
- Treg
- regulatory T cell
- TT
- tetanus toxoid
S. Fanti, E. Stephenson, S.A. Mohiddin, and F.M. Marelli-Berg contributed equally.
Circulation is available at www.ahajournals.org/journal/circ
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/CIRCULATIONAHA.121.055610.
For Sources of Funding and Disclosures, see page 1943.
Contributor Information
Silvia Fanti, Email: s.fanti@qmul.ac.uk.
Edward Stephenson, Email: e.stephenson4@nhs.net.
Etel Rocha-Vieira, Email: etelvieira@terra.com.br.
Alexandros Protonotarios, Email: alexandros.protonotarios.10@ucl.ac.uk.
Stavroula Kanoni, Email: s.kanoni@qmul.ac.uk.
M. Paula Longhi, Email: m.longhi@qmul.ac.uk.
Vishal S. Vyas, Email: v.s.vyas@qmul.ac.uk.
Carlene Dyer, Email: c.dyer@qmul.ac.uk.
Elena Pontarini, Email: e.pontarini@qmul.ac.uk.
Angeliki Asimaki, Email: aasimaki@sgul.ac.uk.
Carlos Bueno-Beti, Email: cbuenobe@sgul.ac.uk.
Monica De Gaspari, Email: monica.deg1@gmail.com.
Stefania Rizzo, Email: s.rizzo@unipd.it.
Cristina Basso, Email: cristina.basso@unipd.it.
Michele Bombardieri, Email: m.bombardieri@qmul.ac.uk.
David Coe, Email: d.coe@bristol.ac.uk.
Guosu Wang, Email: g.wang@qmul.ac.uk.
Daniel Harding, Email: d.harding@qmul.ac.uk.
Iain Gallagher, Email: i.j.gallagher@stir.ac.uk.
Egle Solito, Email: e.solito@qmul.ac.uk.
Perry Elliott, Email: perry.elliott@ucl.ac.uk.
Stephane Heymans, Email: s.heymans@maastrichtuniversity.nl.
Maurits Sikking, Email: m.sikking@maastrichtuniversity.nl.
Konstantinos Savvatis, Email: k.savvatis@nhs.net.
Saidi A. Mohiddin, Email: saidi.mohiddin@nhs.net.
Federica M. Marelli-Berg, Email: f.marelli-berg@qmul.ac.uk.
References
- 1.Stephenson E, Savvatis K, Mohiddin SA, Marelli-Berg FM. T cell immunity in myocardial inflammation: pathogenic role and therapeutic manipulation. Br J Pharmacol. 2017;174:3914–3925. doi: 10.1111/bph.13613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Caforio AL, Pankuweit S, Arbustini E, Basso C, Gimeno-Blanes J, Felix SB, Fu M, Helio T, Heymans S, Jahns R, et al. ; European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: a position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J. 2013;34:2636–2648, 2648a–2648d. doi: 10.1093/eurheartj/eht210 [DOI] [PubMed] [Google Scholar]
- 3.Rose NR. Learning from myocarditis: mimicry, chaos and black holes. F1000Prime Rep. 2014;6:25. doi: 10.12703/P6-25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gallagher S, Jones DA, Anand V, Mohiddin S. Diagnosis and management of patients with acute cardiac symptoms, troponin elevation and culprit-free angiograms. Heart. 2012;98:974–981. doi: 10.1136/heartjnl-2011-301121 [DOI] [PubMed] [Google Scholar]
- 5.Heidecker B, Ruedi G, Baltensperger N, Gresser E, Kottwitz J, Berg J, Manka R, Landmesser U, Luscher TF, Patriki D. Systematic use of cardiac magnetic resonance imaging in MINOCA led to a five-fold increase in the detection rate of myocarditis: a retrospective study. Swiss Med Wkly. 2019;149:w20098. doi: 10.4414/smw.2019.20098 [DOI] [PubMed] [Google Scholar]
- 6.Ali-Ahmed F, Dalgaard F, Al-Khatib SM. Sudden cardiac death in patients with myocarditis: evaluation, risk stratification, and management. Am Heart J. 2019;220:29–40. doi: 10.1016/j.ahj.2019.08.007 [DOI] [PubMed] [Google Scholar]
- 7.Rodrigues P, Joshi A, Williams H, Westwood M, Petersen SE, Zemrak F, Schilling RJ, Kirkby C, Wragg A, Manisty C, et al. Diagnosis and prognosis in sudden cardiac arrest survivors without coronary artery disease: utility of a clinical approach using cardiac magnetic resonance imaging. Circ Cardiovasc Imaging. 2017;10:e006709. doi: 10.1161/CIRCIMAGING.117.006709 [DOI] [PubMed] [Google Scholar]
- 8.Looi JL, Edwards C, Armstrong GP, Scott A, Patel H, Hart H, Christiansen JP. Characteristics and prognostic importance of myocardial fibrosis in patients with dilated cardiomyopathy assessed by contrast-enhanced cardiac magnetic resonance imaging. Clin Med Insights Cardiol. 2010;4:129–134. doi: 10.4137/CMC.S5900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kubo N, Morimoto S, Hiramitsu S, Uemura A, Kimura K, Shimizu K, Hishida H. Feasibility of diagnosing chronic myocarditis by endomyocardial biopsy. Heart Vessels. 1997;12:167–170. doi: 10.1007/BF02767044 [DOI] [PubMed] [Google Scholar]
- 10.Kuhl U. Antiviral treatment of myocarditis and acute dilated cardiomyopathy. Heart Fail Clin. 2005;1:467–474. doi: 10.1016/j.hfc.2005.06.014 [DOI] [PubMed] [Google Scholar]
- 11.Kuhl U, Noutsias M, Seeberg B, Schultheiss HP. Immunohistological evidence for a chronic intramyocardial inflammatory process in dilated cardiomyopathy. Heart. 1996;75:295–300. doi: 10.1136/hrt.75.3.295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kuhl U, Pauschinger M, Noutsias M, Seeberg B, Bock T, Lassner D, Poller W, Kandolf R, Schultheiss HP. High prevalence of viral genomes and multiple viral infections in the myocardium of adults with “idiopathic” left ventricular dysfunction. Circulation. 2005;111:887–893. doi: 10.1161/01.CIR.0000155616.07901.35 [DOI] [PubMed] [Google Scholar]
- 13.Gil-Cruz C, Perez-Shibayama C, De Martin A, Ronchi F, van der Borght K, Niederer R, Onder L, Lutge M, Novkovic M, Nindl V, et al. Microbiota-derived peptide mimics drive lethal inflammatory cardiomyopathy. Science. 2019;366:881–886. doi: 10.1126/science.aav3487 [DOI] [PubMed] [Google Scholar]
- 14.Myers JM, Cooper LT, Kem DC, Stavrakis S, Kosanke SD, Shevach EM, Fairweather D, Stoner JA, Cox CJ, Cunningham MW. Cardiac myosin-Th17 responses promote heart failure in human myocarditis. JCI Insight. 2016;1. doi: 10.1172/jci.insight.85851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Komarowska I, Coe D, Wang G, Haas R, Mauro C, Kishore M, Cooper D, Nadkarni S, Fu H, Steinbruchel DA, et al. Hepatocyte growth factor receptor c-Met instructs T cell cardiotropism and promotes T cell migration to the heart via autocrine chemokine release. Immunity. 2015;42:1087–1099. doi: 10.1016/j.immuni.2015.05.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kortekaas KA, van der Baan A, Aarts LP, Palmen M, Cobbaert CM, Verhagen JC, Engbers FH, Klautz RJ, Lindeman JH. Cardiospecific sevoflurane treatment quenches inflammation but does not attenuate myocardial cell damage markers: a proof-of-concept study in patients undergoing mitral valve repair. Br J Anaesth. 2014;112:1005–1014. doi: 10.1093/bja/aet588 [DOI] [PubMed] [Google Scholar]
- 17.Bobbert P, Weikert U, Schmidt-Lucke C, Skurk C, Meyer A, Steffens D, Schultheiss HP, Rauch U. Platelet activation and thrombus formation relates to the presence of myocardial inflammation in patients with cardiomyopathy. J Cardiol. 2014;63:379–384. doi: 10.1016/j.jjcc.2013.09.014 [DOI] [PubMed] [Google Scholar]
- 18.Barber LD, Whitelegg A, Madrigal JA, Banner NR, Rose ML. Detection of vimentin-specific autoreactive CD8+ T cells in cardiac transplant patients. Transplantation. 2004;77:1604–1609. doi: 10.1097/01.tp.0000129068.03900.25 [DOI] [PubMed] [Google Scholar]
- 19.Weinzierl AO, Rudolf D, Maurer D, Wernet D, Rammensee HG, Stevanovic S, Klingel K. Identification of HLA-A*01- and HLA-A*02-restricted CD8+ T cell epitopes shared among group B enteroviruses. J Gen Virol. 2008;89:2090–2097. doi: 10.1099/vir.0.2008/000711-0 [DOI] [PubMed] [Google Scholar]
- 20.Afanasyeva M, Wang Y, Kaya Z, Park S, Zilliox MJ, Schofield BH, Hill SL, Rose NR. Experimental autoimmune myocarditis in A/J mice is an interleukin-4-dependent disease with a Th2 phenotype. Am J Pathol. 2001;159:193–203. doi: 10.1016/S0002-9440(10)61685-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maisch B, Pankuweit S. Inflammatory dilated cardiomyopathy: etiology and clinical management. Herz. 2020;45:221–229. doi: 10.1007/s00059-020-04900-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kumar A, Cannon CP. Acute coronary syndromes: diagnosis and management, part I. Mayo Clin Proc. 2009;84:917–938. doi: 10.1016/S0025-6196(11)60509-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Verstappen GM, Kroese FGM, Bootsma H. T cells in primary Sjögren’s syndrome: targets for early intervention. Rheumatology (Oxford). 2019;60:3088–3098. doi: 10.1093/rheumatology/kez004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fu H, Ward EJ, Marelli-Berg FM. Mechanisms of T cell organotropism. Cell Mol Life Sci. 2016;73:3009–3033. doi: 10.1007/s00018-016-2211-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tian Y, Babor M, Lane J, Schulten V, Patil VS, Seumois G, Rosales SL, Fu Z, Picarda G, Burel J, et al. Unique phenotypes and clonal expansions of human CD4 effector memory T cells re-expressing CD45RA. Nat Commun. 2017;8:1473. doi: 10.1038/s41467-017-01728-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sun L, Jin H, Li H. GARP: a surface molecule of regulatory T cells that is involved in the regulatory function and TGF-beta releasing. Oncotarget. 2016;7:42826–42836. doi: 10.18632/oncotarget.8753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhu J, Yamane H, Paul WE. Differentiation of effector CD4 T cell populations (*). Annu Rev Immunol. 2010;28:445–489. doi: 10.1146/annurev-immunol-030409-101212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Massberg D, Hatt H. Human olfactory receptors: novel cellular functions outside of the nose. Physiol Rev. 2018;98:1739–1763. doi: 10.1152/physrev.00013.2017 [DOI] [PubMed] [Google Scholar]
- 29.Wang TW, Zhang H, Gyetko MR, Parent JM. Hepatocyte growth factor acts as a mitogen and chemoattractant for postnatal subventricular zone-olfactory bulb neurogenesis. Mol Cell Neurosci. 2011;48:38–50. doi: 10.1016/j.mcn.2011.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rao M, Wang X, Guo G, Wang L, Chen S, Yin P, Chen K, Chen L, Zhang Z, Chen X, et al. Resolving the intertwining of inflammation and fibrosis in human heart failure at single-cell level. Basic Res Cardiol. 2021;116:55. doi: 10.1007/s00395-021-00897-1 [DOI] [PubMed] [Google Scholar]
- 31.Wang Z, Liao Y, Dong J, Li S, Wang J, Fu ML. Clinical significance and pathogenic role of anti-cardiac myosin autoantibody in dilated cardiomyopathy. Chin Med J (Engl). 2003;116:499–502. [PubMed] [Google Scholar]
- 32.Bondue A, Arbustini E, Bianco A, Ciccarelli M, Dawson D, De Rosa M, Hamdani N, Hilfiker-Kleiner D, Meder B, Leite-Moreira AF, et al. Complex roads from genotype to phenotype in dilated cardiomyopathy: scientific update from the Working Group of Myocardial Function of the European Society of Cardiology. Cardiovasc Res. 2018;114:1287–1303. doi: 10.1093/cvr/cvy122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mestroni L, Rocco C, Gregori D, Sinagra G, Di Lenarda A, Miocic S, Vatta M, Pinamonti B, Muntoni F, Caforio AL, et al. Familial dilated cardiomyopathy: evidence for genetic and phenotypic heterogeneity: Heart Muscle Disease Study Group. J Am Coll Cardiol. 1999;34:181–190. doi: 10.1016/s0735-1097(99)00172-2 [DOI] [PubMed] [Google Scholar]
- 34.Heymans S, Eriksson U, Lehtonen J, Cooper LT, Jr. The quest for new approaches in myocarditis and inflammatory cardiomyopathy. J Am Coll Cardiol. 2016;68:2348–2364. doi: 10.1016/j.jacc.2016.09.937 [DOI] [PubMed] [Google Scholar]
- 35.Smith ED, Lakdawala NK, Papoutsidakis N, Aubert G, Mazzanti A, McCanta AC, Agarwal PP, Arscott P, Dellefave-Castillo LM, Vorovich EE, et al. Desmoplakin cardiomyopathy, a fibrotic and inflammatory form of cardiomyopathy distinct from typical dilated or arrhythmogenic right ventricular cardiomyopathy. Circulation. 2020;141:1872–1884. doi: 10.1161/CIRCULATIONAHA.119.044934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Piriou N, Marteau L, Kyndt F, Serfaty JM, Toquet C, Le Gloan L, Warin-Fresse K, Guijarro D, Le Tourneau T, Conan E, et al. Familial screening in case of acute myocarditis reveals inherited arrhythmogenic left ventricular cardiomyopathies. ESC Heart Fail. 2020;7:1520–1533. doi: 10.1002/ehf2.12686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Limas CJ, Limas C. Beta-adrenoceptor antibodies and genetics in dilated cardiomyopathy: an overview and review. Eur Heart J. 1991;12 Suppl D:175–177. doi: 10.1093/eurheartj/12.suppl_d.175 [DOI] [PubMed] [Google Scholar]
- 38.Caforio ALP, Vinci A, Iliceto S. Anti-heart autoantibodies in familial dilated cardiomyopathy. Autoimmunity. 2008;41:462–469. doi: 10.1080/08916930802031546 [DOI] [PubMed] [Google Scholar]
- 39.Vignali D, Cantarelli E, Bordignon C, Canu A, Citro A, Annoni A, Piemonti L, Monti P. Detection and characterization of CD8(+) autoreactive memory stem T cells in patients with type 1 diabetes. Diabetes. 2018;67:936–945. doi: 10.2337/db17-1390 [DOI] [PubMed] [Google Scholar]
- 40.Hosokawa K, Muranski P, Feng X, Townsley DM, Liu B, Knickelbein J, Keyvanfar K, Dumitriu B, Ito S, Kajigaya S, et al. Memory stem T cells in autoimmune disease: high frequency of circulating CD8+ memory stem cells in acquired aplastic anemia. J Immunol. 2016;196:1568–1578. doi: 10.4049/jimmunol.1501739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Benkhoucha M, Molnarfi N, Kaya G, Belnoue E, Bjarnadottir K, Dietrich PY, Walker PR, Martinvalet D, Derouazi M, Lalive PH. Identification of a novel population of highly cytotoxic c-Met-expressing CD8(+) T lymphocytes. EMBO Rep. 2017;18:1545–1558. doi: 10.15252/embr.201744075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Benkhoucha M, Senoner I, Lalive PH. c-Met is expressed by highly autoreactive encephalitogenic CD8+ cells. J Neuroinflammation. 2020;17:68. doi: 10.1186/s12974-019-1676-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jiang W, Li T, Guo J, Wang J, Jia L, Shi X, Yang T, Jiao R, Wei X, Feng Z, et al. Bispecific c-Met/PD-L1 CAR-T cells have enhanced therapeutic effects on hepatocellular carcinoma. Front Oncol. 2021;11:546586. doi: 10.3389/fonc.2021.546586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Diny NL, Baldeviano GC, Talor MV, Barin JG, Ong S, Bedja D, Hays AG, Gilotra NA, Coppens I, Rose NR, et al. Eosinophil-derived IL-4 drives progression of myocarditis to inflammatory dilated cardiomyopathy. J Exp Med. 2017;214:943–957. doi: 10.1084/jem.20161702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Okunishi K, Dohi M, Fujio K, Nakagome K, Tabata Y, Okasora T, Seki M, Shibuya M, Imamura M, Harada H, et al. Hepatocyte growth factor significantly suppresses collagen-induced arthritis in mice. J Immunol. 2007;179:5504–5513. doi: 10.4049/jimmunol.179.8.5504 [DOI] [PubMed] [Google Scholar]
- 46.Bollyky PL, Wilson SB. CD1d-restricted T cell subsets and dendritic cell function in autoimmunity. Immunol Cell Biol. 2004;82:307–314. doi: 10.1111/j.0818-9641.2004.01253.x [DOI] [PubMed] [Google Scholar]
- 47.Kim JH, Hu Y, Yongqing T, Kim J, Hughes VA, Le Nours J, Marquez EA, Purcell AW, Wan Q, Sugita M, et al. CD1a on Langerhans cells controls inflammatory skin disease. Nat Immunol. 2016;17:1159–1166. doi: 10.1038/ni.3523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Guo Y, Wu W, Cen Z, Li X, Kong Q, Zhou QI. IL-22-producing Th22 cells play a protective role in CVB3-induced chronic myocarditis and dilated cardiomyopathy by inhibiting myocardial fibrosis. Virol J. 2014;11:230. doi: 10.1186/s12985-014-0230-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Deleted in proof.
- 50.Lee YK, Turner H, Maynard CL, Oliver JR, Chen D, Elson CO, Weaver CT. Late developmental plasticity in the T helper 17 lineage. Immunity. 2009;30:92–107. doi: 10.1016/j.immuni.2008.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cosmi L, Maggi L, Santarlasci V, Capone M, Cardilicchia E, Frosali F, Querci V, Angeli R, Matucci A, Fambrini M, et al. Identification of a novel subset of human circulating memory CD4(+) T cells that produce both IL-17A and IL-4. J Allergy Clin Immunol. 2010;125:222–230. doi: 10.1016/j.jaci.2009.10.012 [DOI] [PubMed] [Google Scholar]
- 52.Plank MW, Kaiko GE, Maltby SA-O, Weaver J, Tay HL, Shen W, Wilson MS, Durum SK, Foster PS. Th22 cells form a distinct Th lineage from Th17 cells in vitro with unique transcriptional properties and Tbet-dependent Th1 plasticity. J Immunol. 2017;198:2182–2190. doi: 10.4049/jimmunol.1601480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Czarnowicki T, Gonzalez J, Shemer A, Malajian D, Xu H, Zheng X, Khattri S, Gilleaudeau P, Sullivan-Whalen M, Suárez-Fariñas M, et al. Severe atopic dermatitis is characterized by selective expansion of circulating TH2/TC2 and TH22/TC22, but not TH17/TC17, cells within the skin-homing T cell population. J Allergy Clin Immunol. 2015;136:104–115. doi: 10.1016/j.jaci.2015.01.020 [DOI] [PubMed] [Google Scholar]
- 54.Fujita H. The role of IL-22 and Th22 cells in human skin diseases. J Dermatol Sci. 2013;72:3–8. doi: 10.1016/j.jdermsci.2013.04.028 [DOI] [PubMed] [Google Scholar]
- 55.Logiodice F, Lombardelli L, Kullolli O, Haller H, Maggi E, Rukavina D, Piccinni MA-O, Aguerre-Girr M, Casart Y, Berrebi A, et al. Decidual interleukin-22-producing CD4+ T cells (Th17/Th0/IL-22+ and Th17/Th2/IL-22+, Th2/IL-22+, Th0/IL-22+), which also produce IL-4, are involved in the success of pregnancy. Int J Mol Sci. 2019;20:428. doi: 10.3390/ijms20020428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fairweather D, Frisancho-Kiss S, Yusung SA, Barrett MA, Davis SE, Gatewood SJ, Njoku DB, Rose NR. Interferon-gamma protects against chronic viral myocarditis by reducing mast cell degranulation, fibrosis, and the profibrotic cytokines transforming growth factor-beta 1, interleukin-1 beta, and interleukin-4 in the heart. Am J Pathol. 2004;165:1883–1894. doi: 10.1016/s0002-9440(10)63241-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wood KJ, Sawitzki B. Interferon gamma: a crucial role in the function of induced regulatory T cells in vivo. Trends Immunol. 2006;27:183–187. doi: 10.1016/j.it.2006.02.008 [DOI] [PubMed] [Google Scholar]
- 58.Fu H, Kishore M, Gittens B, Wang G, Coe D, Komarowska I, Infante E, Ridley AJ, Cooper D, Perretti M, et al. Self-recognition of the endothelium enables regulatory T cell trafficking and defines the kinetics of immune regulation. Nat Commun. 2014;5:3436. doi: 10.1038/ncomms4436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Perez Leiros C, Goren N, Sterin-Borda L, Borda ES. Myocardial dysfunction in an experimental model of autoimmune myocarditis: role of IFN-gamma. Neuroimmunomodulation. 1997;4:91–97. doi: 10.1159/000097326 [DOI] [PubMed] [Google Scholar]
- 60.Afanasyeva M, Georgakopoulos D, Belardi DF, Bedja D, Fairweather D, Wang Y, Kaya Z, Gabrielson KL, Rodriguez ER, Caturegli P, et al. Impaired up-regulation of CD25 on CD4+ T cells in IFN-gamma knockout mice is associated with progression of myocarditis to heart failure. Proc Natl Acad Sci USA. 2005;102:180–185. doi: 10.1073/pnas.0408241102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Murray PD, McGavern DB, Pease LR, Rodriguez M. Cellular sources and targets of IFN-gamma-mediated protection against viral demyelination and neurological deficits. Eur J Immunol. 2002;32:606–615. doi: 10.1002/1521-4141(200203)32:3<606::AID-IMMU606>3.0.CO;2-D [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Balci SO, Col-Araz N, Baspinar O, Sever T, Balat A, Pehlivan S. Cytokine gene polymorphisms in childhood dilated cardiomyopathy: interferon-gamma, tumor necrosis factor-alpha and transforming growth factor-beta 1 genes are associated with the disease in Turkish patients. Iran J Pediatr. 2013;23:603–604. [PMC free article] [PubMed] [Google Scholar]
- 63.Lindberg E, Andersson B, Hornquist EH, Magnusson Y. Impaired activation of IFN-gamma+CD4+ T cells in peripheral blood of patients with dilated cardiomyopathy. Cell Immunol. 2010;263:224–229. doi: 10.1016/j.cellimm.2010.04.003 [DOI] [PubMed] [Google Scholar]
- 64.Wei Y, Yu K, Wei H, Su X, Zhu R, Shi H, Sun H, Luo Q, Xu W, Xiao J, et al. CD4(+) CD25(+) GARP(+) regulatory T cells display a compromised suppressive function in patients with dilated cardiomyopathy. Immunology. 2017;151:291–303. doi: 10.1111/imm.12728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Luzuriaga K, Koup RA, Pikora CA, Brettler DB, Sullivan JL. Deficient human immunodeficiency virus type 1-specific cytotoxic T cell responses in vertically infected children. J Pediatr. 1991;119:230–236. doi: 10.1016/s0022-3476(05)80732-2 [DOI] [PubMed] [Google Scholar]
- 66.Pikora CA, Sullivan JL, Panicali D, Luzuriaga K. Early HIV-1 envelope-specific cytotoxic T lymphocyte responses in vertically infected infants. J Exp Med. 1997;185:1153–1161. doi: 10.1084/jem.185.7.1153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Noutsias M, Fechner H, de Jonge H, Wang X, Dekkers D, Houtsmuller AB, Pauschinger M, Bergelson J, Warraich R, Yacoub M, et al. Human coxsackie-adenovirus receptor is colocalized with integrins alpha(v)beta(3) and alpha(v)beta(5) on the cardiomyocyte sarcolemma and upregulated in dilated cardiomyopathy: implications for cardiotropic viral infections. Circulation. 2001;104:275–280. doi: 10.1161/01.cir.104.3.275 [DOI] [PubMed] [Google Scholar]
- 68.Burke AP, Farb A, Tashko G, Virmani R. Arrhythmogenic right ventricular cardiomyopathy and fatty replacement of the right ventricular myocardium: are they different diseases? Circulation. 1998;97:1571–1580. doi: 10.1161/01.cir.97.16.1571 [DOI] [PubMed] [Google Scholar]
- 69.Cooper LT, Baughman KL, Feldman AM, Frustaci A, Jessup M, Kuhl U, Levine GN, Narula J, Starling RC, Towbin J, et al. The role of endomyocardial biopsy in the management of cardiovascular disease: a scientific statement from the American Heart Association, the American College of Cardiology, and the European Society of Cardiology Endorsed by the Heart Failure Society of America and the Heart Failure Association of the European Society of Cardiology. Eur Heart J. 2007;28:3076–3093. doi: 10.1093/eurheartj/ehm456 [DOI] [PubMed] [Google Scholar]
- 70.Friedrich MG, Sechtem U, Schulz-Menger J, Holmvang G, Alakija P, Cooper LT, White JA, Abdel-Aty H, Gutberlet M, Prasad S, et al. ; International Consensus Group on Cardiovascular Magnetic Resonance in M. Cardiovascular magnetic resonance in myocarditis: a JACC white paper. J Am Coll Cardiol. 2009;53:1475–1487. doi: 10.1016/j.jacc.2009.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ferreira VM, Schulz-Menger J, Holmvang G, Kramer CM, Carbone I, Sechtem U, Kindermann I, Gutberlet M, Cooper LT, Liu P, et al. Cardiovascular magnetic resonance in nonischemic myocardial inflammation: expert recommendations. J Am Coll Cardiol. 2018;72:3158–3176. doi: 10.1016/j.jacc.2018.09.072 [DOI] [PubMed] [Google Scholar]
- 72.Kwong RY, Schussheim AE, Rekhraj S, Aletras AH, Geller N, Davis J, Christian TF, Balaban RS, Arai AE. Detecting acute coronary syndrome in the emergency department with cardiac magnetic resonance imaging. Circulation. 2003;107:531–537. doi: 10.1161/01.cir.0000047527.11221.29 [DOI] [PubMed] [Google Scholar]
- 73.Larose E, Rodes-Cabau J, Pibarot P, Rinfret S, Proulx G, Nguyen CM, Dery JP, Gleeton O, Roy L, Noel B, et al. Predicting late myocardial recovery and outcomes in the early hours of ST-segment elevation myocardial infarction traditional measures compared with microvascular obstruction, salvaged myocardium, and necrosis characteristics by cardiovascular magnetic resonance. J Am Coll Cardiol. 2010;55:2459–2469. doi: 10.1016/j.jacc.2010.02.033 [DOI] [PubMed] [Google Scholar]
- 74.Cury RC, Shash K, Nagurney JT, Rosito G, Shapiro MD, Nomura CH, Abbara S, Bamberg F, Ferencik M, Schmidt EJ, et al. Cardiac magnetic resonance with t2-weighted imaging improves detection of patients with acute coronary syndrome in the emergency department. Circulation. 2008;118:837–844. doi: 10.1161/CIRCULATIONAHA.107.740597 [DOI] [PubMed] [Google Scholar]
- 75.Petersen SE, Aung N, Sanghvi MM, Zemrak F, Fung K, Paiva JM, Francis JM, Khanji MY, Lukaschuk E, Lee AM, et al. Reference ranges for cardiac structure and function using cardiovascular magnetic resonance (CMR) in Caucasians from the UK Biobank population cohort. J Cardiovasc Magn Reson. 2017;19:18. doi: 10.1186/s12968-017-0327-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-delta delta c(t)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.