More than 750 000 Americans are hospitalized with sepsis annually, and one in five patients may not survive [1]. Emergency medical services (EMS) provide essential care for more than half of hospitalized sepsis patients, yet the diagnosis of sepsis may be delayed or missed. However, the prehospital interval is a key opportunity for early sepsis recognition, initiation of treatment and emergency department (ED) notification [2]. Prior prehospital studies developed sepsis recognition models based on subjective data and vital signs with limited success [3,4].
Plasma biomarkers provide key information about the presence of acute infection and organ dysfunction in other acute care settings, yet are understudied during prehospital care [5]. One important sepsis biomarker is the high mobility group box protein 1 (HMGB1), a ubiquitously expressed nuclear tolerance protein of the damage-associated molecular pattern family. HMGB1 is passively and actively released into circulation as part of an early inflammatory response, where it activates innate immune cells. By binding to cell surface receptors, HMGB1 activates endothelial cells, further increasing the production of pro-inflammatory cytokines and chemokines [6]. Higher concentrations of HMGB1 are associated with mortality in sepsis, and modulation of HMGB1-mediated responses has been shown to reduce mortality in animal models [7,8].
This study prospectively determined the association between prehospital circulating HMGB1 and sepsis among nontrauma, nonarrest patients transported to a hospital. An individually matched case–control cohort of subjects was drawn from the Pittsburgh Prehospital LINking Evaluation (PIPeLINE) study (NIH GM104022) that enrolled adult (age ≥18 years) nontrauma, nonarrest prehospital patients at risk for sepsis who were transported by the City of Pittsburgh Bureau of EMS between August 2013 and February 2014 (N = 345) [9,10]. We randomly selected 20 cases using Sepsis-3 criteria [4] defined as suspected infection and acute organ dysfunction using a sequential organ failure assessment score greater than 2 within 24 h of hospital admission. Cases were individually matched with 20 nearest neighbor controls not meeting Sepsis-3 criteria on (1) sex, (2) age, (3) race and (4) Elixhauser comorbidity index. This project was approved by the Human Research Protection Office of the University of Pittsburgh (STUDY19070378).
EMS clinical data were gathered for each patient encounter from a computerized medical record database (emsCharts, Inc; Warrendale, Pennsylvania, USA), and linked to electronic health record data at University of Pittsburgh Medical Center hospitals (Cerner Powerchart; Cerner, Kansas City, Missouri, USA). Prehospital vital signs were used to calculate a validated clinical risk score [11]. From the inpatient record, length of stay, rates of ICU admission and in-hospital mortality were determined.
Lactate was measured at the point of care (Lactate Pro, FACT, Canada) and prehospital blood samples were collected by EMS at the time of prehospital peripheral IV catheter placement. Samples were processed by the research team upon ED arrival, as described elsewhere [9]. Plasma samples were frozen at −80°C until assay. They were batch analyzed after a single freeze-thaw cycle for interleukin-6 (IL-6), interleukin-10 (IL-10) and tumor necrosis factor [10]. Plasma HMGB1 was quantified using an express enzyme-linked immunosorbent assay (Tecan; Cat. No. 30164033). Blood processing and analysis were completed by the Clinical Research, Investigation, and Systems Modeling of Acute Illness Biospecimen Core.
Demographic and clinical characteristics of patients with and without Sepsis-3 were described. Comparisons between groups were completed with Wilcoxon signed-rank tests for continuous data and Fisher’s exact tests for categorical data. We considered outlier values as two SDs above the mean, log-transformed HMGB1 values, and reported results as significant at a two-sided P-value of 0.05. R statistical software version 4.1.2 (R Core Team, Vienna, Austria) was employed for statistical analysis (https://github.com/stuthi-iyer/HMGB1).
Baseline demographics were similar between prehospital patients with and without sepsis [mean (SD) age: 65 (16) vs. 60 (14) years; male sex: 11 (58%) vs. 10 (53%); white race: 15 (79%) vs. 14 (74%); and mean (SD) Elixhauser comorbidity index: 0.5 (0.6) vs. 0.4 (0.6)]. Prehospital vital signs across groups were also similar (Table 1), as was the validated prehospital critical illness risk score [mean (SD) 0.2 (0.4) vs. 0.2 (0.4), P = 0.65].
Table 1.
Cohort characteristics
| Feature | Sepsis | No Sepsis |
|---|---|---|
| N (%) | 19 (50) | 19 (50) |
| Age (years): mean (SD) | 65 (16) | 60 (14) |
| Sex (male): no. (%) | 11 (58) | 10 (53) |
| Elixhauser: mean (SD)a | 0.5 (0.6) | 0.4 (0.6) |
| Race: no. (%)b | ||
| White | 15 (79) | 14 (74) |
| Black | 4 (21) | 4 (21) |
| Other | 0 (0) | 1 (5) |
| Prehospital assessmentc | ||
| Critical illness risk score: mean (SD)d | 0.2 (0.4) | 0.2 (0.4) |
| Respiratory rate (breaths per min): mean (SD) | 20 (7) | 19 (4) |
| SBP (mm Hg): median (IQR) | 128 (80–172) | 152 (100–244) |
| Pulse oximetry (%): median (IQR) | 98 (88–100) | 98 (93–100) |
| Heart rate (beats per min): mean (SD) | 90 (18) | 100 (23) |
| Prehospital biomarkerse | ||
| HMGB1 (ng/mL): median (IQR) | 6.5 (0.6–14.6) | 2.7 (1.2–12.9) |
| Serum lactate (mmol/L): median (IQR) | 1.6 (0–7.2) | 2.0 (0–6.5) |
| IL-6 (pg/mL): median (IQR) | 63 (5–2300) | 12 (3–420) |
| IL-10 (pg/mL): median (IQR) | 5 (2–410) | 3 (1–1000) |
| TNF (pg/mL): median (IQR) | 7 (5–6500) | 7 (5–61) |
| Hospital outcomes | ||
| Hospital length of stay (days): median (IQR) | 5 (2–19) | 1.5 (1–5) |
| Admitted to ICU: no. (%) | 5 (21) | 0 (0) |
| In-hospital mortality: no (%) | 2 (11) | 0 (0) |
HMGB1, high mobility group box-1; IL, interleukin; IQR, interquartile range; TNF, tumor necrosis factor.
Elixhauser is a method of categorizing comorbidities of patients based on the International Classification of Diseases (ICD) diagnosis codes found in administrative data, ranging from 0 to 31.
Other race corresponds to Chinese, Filipino, Hawaiian, American Indian/Alaskan, Asian, Hawaiian/Other Pacific Islander, Middle Eastern, Native American, Not specified or Pacific Islander.
Vital signs are first reported by EMS responders.
Critical illness risk score is a validated prehospital risk score based on EMS vital signs and ranges from 0–8.
Prehospital biomarkers measured at time of IV catheter placement.
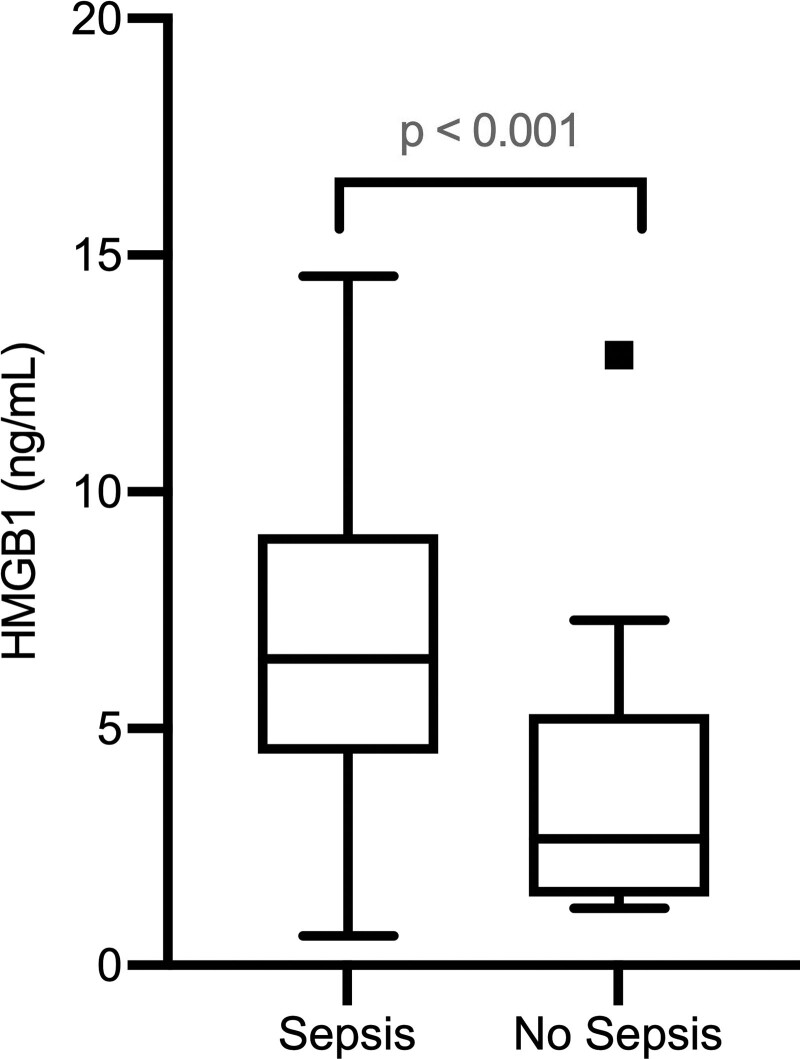
Patients with Sepsis-3 within 24 h of presentation had significantly higher prehospital HMGB1 [median and interquartile range (IQR): 6.5 (0.6–14.6) vs. 2.7 (1.2–12.9) ng/mL; P < 0.001] compared to nonseptic controls (Fig. 1). There were no significant differences in prehospital serum lactate [median (IQR): 1.6 (0–7.2) vs. 2.0 (0–6.5) mmol/L; P = 0.53], but patients with Sepsis-3 had a longer length of hospital stay [median (IQR) 5.0 (2–19) vs. 1.5 (1–5) days; P < 0.001] and greater rates of ICU admissions [5 (21%) vs. 0 (0%); P = 0.11].
Fig. 1.
Box plots of HMGB1 concentration measured in prehospital setting at time of IV catheter placement, with N = 19 per group (N = 38 overall).
In this individually matched case–control study of the prospective PIPeLINE cohort, median prehospital HMGB1 was significantly higher among patients with Sepsis-3 on arrival compared with those without sepsis.
These data suggest that the measurement of HMGB1 may contribute to the early recognition of sepsis. Prior prehospital work has focused on markers of organ dysfunction and inflammatory response, such as cytokines or serum lactate. This study is an extension by measuring HMGB1, a damage-associated molecular pattern known to activate innate immune cells either passively or actively, and to activate endothelial cells, thereby producing pro-inflammatory cytokines and chemokines by binding to cell surface receptors [6]. Preclinical work reveals that HMGB1 is elevated within 12–24 h of cecal ligation and puncture [12]. Human studies show that HMGB1 is elevated within 24 h of hospital presentation and remains elevated for 144 h [7,13]. These results suggest that HMGB1 is an important regulator of an early inflammatory response to an insult, including trauma and sepsis. These findings parallel other prehospital biomarkers, such as point-of-care lactate [9,10].
This study has limitations. First, HMGB1 can be elevated in conditions other than sepsis, including cancer [14], traumatic brain injury, neuroinflammation, epilepsy and cognitive dysfunction [15]. We did not assess the presence of these conditions in this study. Second, sepsis is a heterogeneous syndrome that may encompass many underlying patterns of disease. We conducted clinical adjudication of Sepsis-3 among cases to reduce misclassification, but cases may not be representative of all sepsis subtypes. Third, the small sample implies that these findings should be considered exploratory. Fourth, the PIPeLINE cohort did not incorporate biomarkers into decision making or clinical care, and the feasibility of point-of-care prehospital measurement is beyond the scope of this study.
In conclusion, prehospital HMGB1 was associated with Sepsis-3 on arrival and may contribute to early sepsis recognition.
Acknowledgements
None of the authors received any payments or influence from a third-party source for the work presented. This work did not receive external funding. Dr. Seymour is supported in part by grants from the NIH (R35GM119519, K23GM104022).
Conflicts of interest
There are no conflicts of interest.
References
- 1.Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med 2001; 29:1303–1310. [DOI] [PubMed] [Google Scholar]
- 2.Seymour CW, Kahn JM, Martin-Gill C, Callaway CW, Yealy DM, Scales D, Angus DC. Delays from first medical contact to antibiotic administration for sepsis. Crit Care Med 2017; 45:759–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Polito CC, Isakov A, Yancey AH, 2nd, Wilson DK, Anderson BA, Bloom I, et al. Prehospital recognition of severe sepsis: development and validation of a novel EMS screening tool. Am J Emerg Med 2015; 33:1119–1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Seymour CW, Liu VX, Iwashyna TJ, Brunkhorst FM, Rea TD, Scherag A, et al. Assessment of clinical criteria for sepsis: for the third international consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016; 315:762–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tobias AZ, Guyette FX, Seymour CW, Suffoletto BP, Martin-Gill C, Quintero J, et al. Pre-resuscitation lactate and hospital mortality in prehospital patients. Prehosp Emerg Care 2014; 18:321–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Denning NL, Aziz M, Gurien SD, Wang P. DAMPs and NETs in sepsis. Front Immunol 2019; 10:2536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Angus DC, Yang L, Kong L, Kellum JA, Delude RL, Tracey KJ, Weissfeld L; GenIMS Investigators. Circulating high-mobility group box 1 (HMGB1) concentrations are elevated in both uncomplicated pneumonia and pneumonia with severe sepsis. Crit Care Med 2007; 35:1061–1067. [DOI] [PubMed] [Google Scholar]
- 8.Lee W, Ku SK, Bae JS. Zingerone reduces HMGB1-mediated septic responses and improves survival in septic mice. Toxicol Appl Pharmacol 2017; 329:202–211. [DOI] [PubMed] [Google Scholar]
- 9.Brant EB, Martin-Gill C, Callaway CW, Angus DC, Seymour CW. Prehospital identification of community sepsis using biomarkers of host response. Intensive Care Med 2020; 46:823–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brant EB, Kennedy J, Martin-Gill C, Jackson V, Peck Palmer OM, Callaway CW, et al. Association between intravenous fluid bolus and biomarker trajectory during prehospital care. Prehosp Emerg Care 2020; 24:196–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Seymour CW, Kahn JM, Cooke CR, Watkins TR, Heckbert SR, Rea TD. Prediction of critical illness during out-of-hospital emergency care. JAMA 2010; 304:747–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li J, Kokkola R, Tabibzadeh S, Yang R, Ochani M, Qiang X, et al. Structural basis for the proinflammatory cytokine activity of high mobility group box 1. Mol Med 2003; 9:37–45. [PMC free article] [PubMed] [Google Scholar]
- 13.Sundén-Cullberg J, Norrby-Teglund A, Rouhiainen A, Rauvala H, Herman G, Tracey KJ, et al. Persistent elevation of high mobility group box-1 protein (HMGB1) in patients with severe sepsis and septic shock. Crit Care Med 2005; 33:564–573. [DOI] [PubMed] [Google Scholar]
- 14.He J, Zhang P, Li Q, Zhou D, Liu P. Expression of high mobility group box 1 protein predicts a poorer prognosis for patients with osteosarcoma. Oncol Lett 2016; 11:293–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Paudel YN, Shaikh MF, Chakraborti A, Kumari Y, Aledo-Serrano Á, Aleksovska K, et al. HMGB1: a common biomarker and potential target for TBI, neuroinflammation, epilepsy, and cognitive dysfunction. Front Neurosci 2018; 12:628. [DOI] [PMC free article] [PubMed] [Google Scholar]