Abstract
Cell-mediated immunity has been reported to play an important role in defense against Encephalitozoon cuniculi infection. Previous studies from our laboratory have underlined the importance of cytotoxic CD8+ T lymphocytes (CTL) in survival of mice infected with E. cuniculi. In the present study, immune response against E. cuniculi infection in CD4+ T-cell-deficient mice was evaluated. Similar to resistant wild-type animals, CD4−/− mice were able to resolve E. cuniculi infection even at a very high challenge dose (5 × 107 spores/mouse). Tissues from infected CD4−/− mice did not exhibit higher parasite loads in comparison to the parental wild-type mice. Conversely, at day 21 postinfection, susceptible CD8−/− mice had 1014 times more parasites in the liver compared to control wild-type mice. Induction of the CD8+ T-cell response in CD4−/− mice against E. cuniculi infection was studied. Interestingly, a normal antigen-specific CD8+ T-cell response to E. cuniculi infection was observed in CD4−/− mice (precursor proliferation frequency, 1/2.5 × 104 versus 1/104 in wild-type controls). Lack of CD4+ T cells did not alter the magnitude of the antigen-specific CTL response (precursor CTL frequency; 1/1.4 × 104 in CD4−/− mice versus 1/3 × 104 in control mice). Adoptive transfer of immune CD8+ T cells from both CD4−/− and wild-type animals prevented the mortality in CD8−/− mice. E. cuniculi infection thus offers an example of an intracellular parasitic infection where CD8+ T-cell immunity can be induced in the absence of CD4+ T cells.
Microsporidia are obligate intracellular parasites that infect a wide range of hosts, including vertebrates and invertebrates (4). With the onset of the AIDS pandemic, more attention has been paid to several microsporidians, including Encephalitozoon, Enterocytozoon, Pleistophora, Nosema, and most recently Brachiola, which have been identified as causative agents of opportunistic infections in immunocompromised hosts (3, 10, 30, 41).
Most of what is known about the biology of microsporidia is based on the microsporidian Encephalitozoon cuniculi, which commonly infects rodents and has been found in humans as well (44). E. cuniculi, which was previously observed in laboratory animals, is considered a zoonotic organism. Immunologically competent hosts that are naturally infected with E. cuniculi usually express few clinical signs of disease (10). Several cases of human immunodeficiency virus (HIV)-infected patients with E. cuniculi infection have been reported in recent years (27, 42). These patients have presented a wide variety of symptoms, including renal failure, pneumonitis, sinusitis, keratopathy, granulomatous liver necrosis, and peritonitis (9, 17, 37, 44). In a recent report, autopsy findings in a patient with AIDS showed a disseminated E. cuniculi infection involving the brain (42).
Protective immunity against E. cuniculi infection is primarily dependent on the cellular immune response. Studies involving athymic or SCID mice have shown that these immunodeficient animals are highly susceptible to E. cuniculi infection (16). Adoptive transfer of sensitized syngeneic T cells protected athymic mice inoculated with E. cuniculi (32). In contrast, transfer of naïve T lymphocytes or hyperimmune antiserum failed to protect or prolong survival in these mice. Previous studies from our laboratory suggested that among the T-cell population, CD8+ T cells play a more important role in the protection against E. cuniculi infection (22). Gene knockout mice lacking CD8+ T cells were highly susceptible to E. cuniculi infection. However, mice lacking CD4+ T cells survived E. cuniculi infection and showed no signs of sickness. In the present study, the CD8+ T-cell immune response against E. cuniculi infection in the absence of CD4+ T cells was analyzed.
MATERIALS AND METHODS
Mice.
T. W. Mak (Amgen Institute, Toronto, Ontario, Canada) kindly provided a breeding pair of CD8−/− mice on the C57BL/6 background. Animals were bred under approved conditions at the Animal Research Facility at Dartmouth Medical School. CD4−/− mice on the same genetic background were obtained from Jackson Laboratory (Bar Harbor, Maine). CD40L−/− mice were obtained from Randy Noelle, Dartmouth Medical School. Age- and sex-matched C57BL/6 mice from Jackson Laboratory were used as wild-type controls.
Parasites and infection.
A rabbit isolate of E. cuniculi, kindly provided by Elizabeth Didier (Tulane Medical Research Center), was used throughout the study. The parasites were maintained by continuous passage in rabbit kidney (RK-13) cells, obtained from the American Type Culture Collection. The RK-13 cells were maintained in RPMI 1640 (Gibco BRL) containing 10% fetal calf serum (FCS; HyClone Laboratories). Organisms were collected from the culture medium and centrifuged at 325 × g for 10 min. After two washes with phosphate-buffered saline (PBS), the parasites were resuspended and injected intraperitoneally (i.p.; 107 spores/mouse).
Phenotypic analysis.
Following euthanasia, the spleens from infected CD4−/− and parental C57BL/6 animals were removed and homogenized in a petri dish. The contaminating red blood cells were lysed in red blood cell lysis buffer (Sigma Chemical Co., St. Louis, Mo.). Splenocytes were washed, suspended in 3% PBS–bovine serum albumin, and analyzed by fluorescence-activated cell sorter (FACS; Becton Dickinson) for CD8+ T-cell expression using a direct immunofluorescence assay. Cells (106/ml) were incubated with 1 μg of fluorescein isothiocyanate-labeled anti-CD8 (Pharmingen, San Diego, Calif.) in 3% PBS–bovine serum albumin. After 1 h of incubation at 4°C, the cells were washed several times in buffer, fixed in 1% methanol-free formaldehyde, and stored at 4°C for FACS analysis.
Lymphoproliferation assay.
The frequency of E. cuniculi-specific proliferative response of purified T cells was measured by precursor proliferation frequency (PPF) analysis. The splenocytes from 15-day-postinfection (p.i.) mice were separated into adherent and nonadherent populations by a previously described procedure (20). Briefly, 2.5 × 108 spleen cells were incubated in a glass petri dish (Fisherbrand, Pittsburgh, Pa.) at 37°C in a humidified atmosphere containing 5% CO2. After 2 h of incubation, the nonadherent population was collected and separated into an enriched cell population on a T-cell affinity column (Biotecx Laboratories, Houston, Tex.). Purity of the eluted cell population was determined by binding to fluorescein-labeled anti-mouse antibody Thy1.2 (Pharmingen) and subsequent FACS analysis. Limiting dilution assay (LDA) of purified T cells was performed by plating spleen cells in serial fivefold dilutions starting at 5 × 104 cells/well in round-bottom 96-well plates. For each dilution, there were 24 replicates; 105 irradiated syngeneic feeder cells (3,000 rads) and 5 × 103 irradiated spores (3,000 rads) were added to each well. When stimulated with irradiated spores, splenocytes from nonimmunized mice showed background proliferation. Twelve control wells were prepared as above by replacing spores with extract from host cell lysate antigen. The lysate was prepared from RK-13 cells, which were sonicated and centrifuged at 10,000 × g for 15 min. The concentration of protein was determined by the bicinchoninic acid assay; Pierce (Rockford, Ill.) 15 μg of soluble antigen per ml was added to each control well. After 5 days, 1 μCi of thymidine (Amersham, Arlington Heights, Ill.) was pulsed for 12 h to determine DNA synthesis. Wells were scored as positive if the counts per minute from the control wells were greater than 3 standard deviations (SD) above the mean counts from the control wells. The precursor frequencies were calculated by a standard method (31).
CTL assays.
A cytotoxic T-lymphocyte (CTL) assay was performed to determine the CTL response of spleen cells from infected animals at day 15 p.i. Cytolytic activity was quantitated by determining the precursor CTL (pCTL) frequency of the infected mice by LDA. Whole splenocytes from the infected animals were cultured by limiting dilution in 96-well round-bottom plates. Dilutions of cells ranging from 1,250 to 25,000 per well were grown in RPMI 1640 medium containing appropriate growth factors including interleukin-2 (IL-2; 15 U/ml; R&D Chemicals, Minneapolis, Minn.) irradiated (3,000 rads) spores (5 × 103/well). Irradiated splenocytes (3,000 rads), obtained from naïve syngeneic mice at the concentration of 105 cells/well, were used as feeder cells. Wells containing only irradiated parasites and feeder cells (without effector cells) served as controls. After 1 week, the cells were harvested and incubated with 51Cr-labeled parasite-infected and uninfected macrophages. Macrophages were collected and labeled as described elsewhere (22). Briefly, mouse peritoneal macrophages were obtained by lavage 2 days after i.p. inoculation with 1 ml of thioglycolate. The macrophages were washed three times in PBS and dispensed at a concentration of 5 × 104 cells/well in 96-well U-bottom tissue culture plates. After overnight incubation, the cells were infected with 2 × 105 spores of E. cuniculi per well for 48 h. The wells were washed extensively with PBS to clear extracellular parasites. Macrophages were labeled with 51Cr (0.5 μCi/well; New England Nuclear Research Products, Boston, Mass.) for 3 h at 37°C. Macrophages were washed five times in PBS and incubated with spleen cell cultures. The amount of radioisotope release was measured after 4 h of incubation. The wells were considered positive for lytic activity if total counts per minute released by effector cells was greater than 3 SD above the total for control wells (mean counts per minute released by target cells incubated with feeder cells and irradiated parasites alone). The pCTL frequency was calculated according to a standard formula (36).
Measurement of cytokines.
Intracellular cytokine staining was used to determine gamma interferon (IFN-γ), IL-4, and IL-10 production at the single-cell level as described previously (6). Spleen cells from day 15-day-p.i. mice were isolated and resuspended in RPMI 1640 containing 10% FCS. The cells were cultured at the concentration of 106 cells/well in a 96-well plate and stimulated with phorbol myristate acetate (PMA; 10 ng/ml; Sigma), ionomycin (500 ng/ml; Sigma), and monensin (GolgiStop; 2 μM; Pharmingen). Cultures were incubated for 4 h at 37°C in 5% CO2 in a humidified incubator. After incubation, cells were washed with PBS–1% FCS and stained with anti-CD8 or anti-CD4 conjugated with fluorescein (Pharmingen) for 30 min at 4°C. Intracellular staining was performed using a Cytofix/Cytoperm kit (Pharmingen) in accordance with the manufacturer's recommendations. Briefly, following cell surface staining, cells were washed and then treated with formaldehyde and saponin for fixation and permeabilization. Intracellular staining was then performed with anti-IFN-γ, anti-IL-4, anti-IL-10, or irrelevant isotype-matched control antibody conjugated with phycoerythrin (Pharmingen). Samples were resuspended in PBS containing 2% formaldehyde, acquired on a FACScan flow cytometer, and analyzed using Cellquest software (Becton Dickinson).
Quantitation of tissue parasite burden.
Tissues (liver and kidney) were recovered from mice 0, 7, 14, and 21 days after infection with E. cuniculi. The parasite load in the tissues was estimated by semiquantitative PCR. Briefly, DNA was extracted from the tissues by using a Qiamp tissue kit (Qiagen, Chatsworth, Calif.), and 3 μg of each sample was analyzed. The PCR was performed with a pair of primers that amplified a 549-bp fragment from a cloned small subunit rRNA (SSU-RNA) sequence from E. cuniculi (N. J. Pieniazek et al., GenBank accession no. L17072). The forward primer 5′-ATGAGAAGTGATGTGTGCG-3′ and the reverse primer 5′-TGCCATGCACTCACAGGCATC-3′ are specific for E. cuniculi and do not react with other microsporidia or published nonmicrosporidian sequences. A 510-bp competitive internal standard was generated by a method reported by Kiristis et al. (23) that employed a linker primer and the two primers listed above. The original 5′ primer was used in reamplification of the 549-bp product, but the original 3′ primer was replaced by a 30-bp internal linker (5′CACAGGCATCCCGCACACTCCACTCCTTGT-3′). In the 30-bp internal linker primer, 20 bp corresponded to a sequence 39 bp downstream from the original and 10 bp at the 5′ end that was identical to the first 10 bp at the 3′ end of the original 3′ primer, so that the linker primer contains sequences from the original primer. Since the 510-bp DNA fragment generated by this PCR contains the same primer template sequences at the 549-bp segment of the SSU-rRNA, it was amplified by the original two primers and used as internal standard for a competitive PCR. The 549-bp segment of the SSU-RNA and the 510-bp segment of the internal standard were amplified using the following conditions: 35 cycles of denaturation at 94°C for 45 s, annealing at 53°C for 1 min, and elongation at 72°C for 30 s. Amplification was performed with an Eppendorf master kit (Eppendorf Scientific Inc., Westbury, N.Y.) according to the manufacturer's directions, 0.2 mM each dGTP, dATP, dTTP, and dCTP, and 0.4 μM each E. cuniculi primer for each reaction. Various amounts of the internal standard were added to each reaction to determine the relative amount of SSU-RNA gene in each sample. Amplification products were analyzed after electrophoresis on a 1.5% agarose gel (Perkin-Elmer, Foster City, Calif.) and visualized with ethidium bromide. The number of parasites was determined by amplification of a known amount of parasite with a dilution of internal standard, using the PCR conditions described above.
Adoptive transfer of CD8+ T cells.
Parental C57BL/6 mice and CD4−/− mice were infected i.p. with 107 spores of E. cuniculi. At day 15 p.i., the mice were splenectomized, and spleen cells were isolated and collected. Splenic CD8+ T cells were isolated by magnetic separation using microbeads coated with anti-CD8 antibody (Miltenyi Biotec, Auburn Calif.) as recommended by the manufacturer. The purity of the separated cells was >95% as determined by FACS analysis. A total of 107 CD8+ T cells were adoptively transferred to naïve CD8−/− mice via intravenous tail vein inoculation. At 24 h after the adoptive transfer of immune cells, the CD8−/− mice were challenged with l07 spores of E. cuniculi.
Statistical analysis.
Statistical analysis of the data was performed by Student's t test (29).
RESULTS
CD4−/− mice survive high doses of E. cuniculi infection.
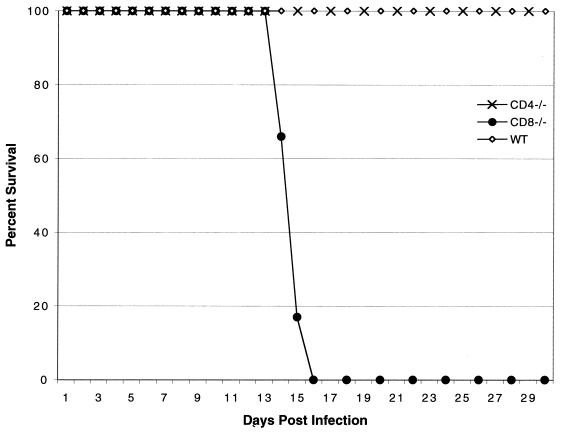
Previous studies from our laboratory reported that CD4+ T-cell-deficient mice, similar to parental wild-type controls, were able to survive an infective dose of 107 E. cuniculi spores (22), whereas CD8−/− mice infected with the same challenge dose succumbed to infection by day 21 p.i. When the challenge dose was increased fivefold, CD8−/− mice died sooner and all were dead by day 16 p.i. (Fig. 1). However, none of the CD4−/− or wild-type mice died or showed any sign of sickness (lethargy or development of ascites) even at a higher challenge dose. CD40/CD40L is important for the activation of CD4+ T cells (38). To further confirm the results obtained with the CD4−/− mice, we tested CD40L−/− mice. CD40L−/− mice on a C57BL/6 background were challenged i.p. with 5 × 107 spores of E. cuniculi. Similar to CD4−/− and parental wild-type animals, none of these mice succumbed to infection (data not shown).
FIG. 1.
Survival of gene knockout mice challenged with a high dose of E. cuniculi spores. Five- to six-week-old female CD4−/−, CD8−/−, and wild-type (WT) C57BL/6 mice were infected i.p. with 5 × 107 spores of E. cuniculi. Animals were monitored on a daily basis. The study was performed twice with similar results.
CD4−/− mice infected with E. cuniculi are able to clear the parasite burden in the tissues.
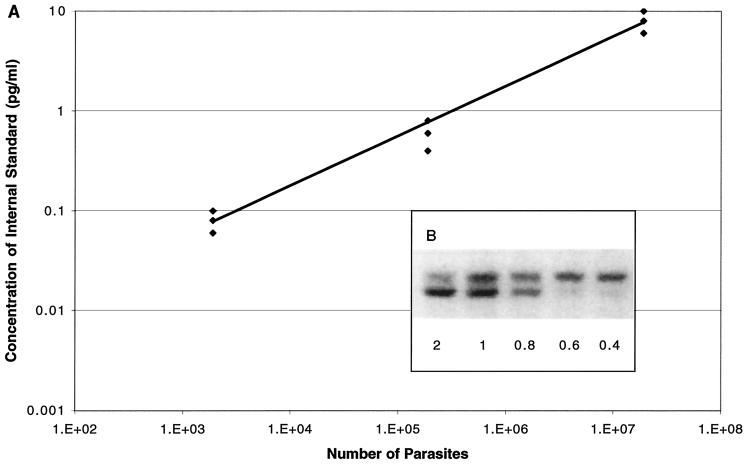
Next we determined if E. cuniculi-infected CD4−/− animals have a reduced ability to clear the parasites. Tissues from CD4−/−, CD8−/−, and parental wild-type mice were isolated and analyzed for parasite load by quantitative PCR performed at days 0, 7, 14, and 21 p.i. In the preliminary studies, PCRs were carried out to determine the amplification efficiency of the internal standard and the gene of interest as follows. DNA from a homogenate of a naïve mouse liver and a known amount of parasites, plus various dilutions of internal standard, were amplified. The range of highest and lowest values of the internal standard for a defined number of parasites was determined (Fig. 2A). The concentration of the internal standard in the sample containing a known number of parasites was determined by comparing the intensities of bands obtained for the genomic DNA and the internal control (Fig. 2B).
FIG. 2.
Standardization of quantitative PCR for detection of E. cuniculi parasites. (A) Correlation of number of parasites with amount of internal standard. A competitive PCR was performed as described in Materials and Methods with a known amount of parasites and different dilutions of the internal standard. The 50 ng of DNA used in the quantitative PCR was obtained by adding a known number of parasites to 75 mg of liver from a naive mouse before DNA extraction. The range of the internal standard indicates the highest and lowest possible values for each number of parasites tested. (B) Detection of E. cuniculi DNA by competitive PCR. A competitive PCR was done using various dilutions of internal standard added to samples with a constant amount of DNA. The result shows a decrease of the signal of the genomic DNA as the concentration of the internal standard increases. The point at which the intensities of the two bands are equal is considered the concentration of the internal standard used to determine the number of parasites in each sample.
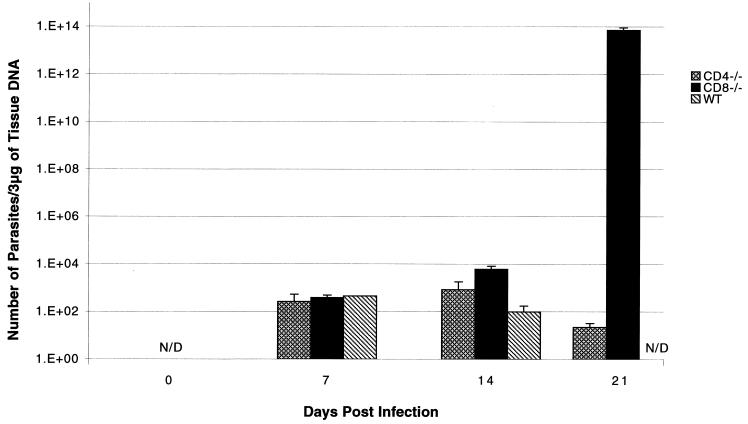
Analysis of the liver at day 7 p.i. showed no major difference in the level of parasite load between wild-type, CD4−/−, and CD8−/− mice (Fig. 3). By day 14 p.i., CD4−/− mice had a 10-fold greater number of parasites compared to parental wild-type animals (Fig. 3). However, the differences were much greater in CD8−/− mice, which at this time point exhibited almost a 100-fold increase in parasite number over the parental controls. The level of parasite DNA in the livers of CD4−/− mice was reduced >50% by day 21 p.i. Amounts of parasite DNA were below detectable levels in the wild-type mice at this time point. In contrast, CD8−/− mice infected with E. cuniculi during this period developed a substantial parasite load in the liver. The levels of parasite DNA in the CD8−/− animals at day 21 p.i. increased almost 1014 times in comparison to wild-type C57BL/6 mice.
FIG. 3.
Levels of parasite DNA in organs of E. cuniculi-infected knockout (CD4−/− and CD8−/−) and parental C57BL/6 mice. CD4−/−, CD8−/−, and wild-type (WT) C57BL/6 mice were infected i.p. with 107 spores of E. cuniculi. At days 0, 7, 14, and 21 p.i., the mice were sacrificed and the livers were isolated. The liver DNA was extracted as described in Materials and Methods; 3 μg of purified DNA was amplified by PCR using E. cuniculi-specific primers. Each time point comprised three mice, and data are expressed as mean ± SD. The experiment was performed twice with similar results.
Parasites were nondetectable in the kidneys of parental C57BL/6 mice at all time points tested. The low level of organisms (75 parasites) in the kidneys of CD4−/− mice at day 7 p.i. increased to 1.6 × 103 at day 14 p.i. (data not shown). No parasites were detectable in CD4−/− mice at day 21 p.i., suggesting the clearance of infection in these animals. In contrast, as in the liver, an abundance of E. cuniculi parasites was observed in CD8−/− mice; at day 14 p.i., almost a 5,000-fold increase in parasite levels in comparison to CD4−/− mice was observed in the kidneys of these animals. The number of parasites in CD8−/− mice persisted until day 21 p.i., when the animals were close to death (data not shown).
E. cuniculi infection induces a normal CD8+ T-cell response in CD4−/− mice.
To determine if a lack of CD4+ T cells can affect the CD8+ T-cell response in E. cuniculi-infected mice, phenotypic analysis of CD4−/− mice was performed. Spleen cells from knockout and parental wild-type mice were isolated at days 7, 14, and 21 after E. cuniculi infection and analyzed for expression of the CD8+ T-cell phenotype. An increase in the CD8+ T-cell population as a result of E. cuniculi infection was observed at day 7 p.i. in both CD4−/− and parental C57BL/6 mice (Table 1). No difference in the absolute numbers of CD8+ T cells between infected CD4+ T-cell-deficient and wild-type controls was noticed throughout the course of study (Table 1).
TABLE 1.
CD8+ T-cell response following E. cuniculi infectiona
| Day p.i. | Treatment | Splenic CD8+ expression (absolute no. of phenotypic cells [108])
|
|
|---|---|---|---|
| CD4−/− mice | WT mice | ||
| 7 | Uninfected | 0.13 ± 0.03 | 0.17 ± 0.02 |
| Infected | 0.36 ± 0.07 | 0.43 ± 0.09 | |
| 14 | Uninfected | 0.21 ± 0.04 | 0.22 ± 0.07 |
| Infected | 0.65 ± 0.09 | 0.75 ± 0.18 | |
| 21 | Uninfected | 0.16 ± 0.02 | 0.24 ± 0.06 |
| Infected | 0.49 ± 0.12 | 0.56 ± 0.14 | |
CD4−/− and parental wild-type (WT) C57BL/6 mice (n = 3/group) were infected i.p with 107 spores of E. cuniculi. Splenocytes were isolated and pooled at various time points p.i. The cells were phenotyped for the expression of CD8 receptor by direct immunofluorescence using FACS. The data represent mean ± SD of two similar experiments.
E. cuniculi-induced precursor proliferation responses in infected mice.
CD4+ T cells are an important source of IL-2, which is critical for the priming of CD8+ T-cell responses against a number of infectious diseases (15). Next, we determined if the absence of CD4+ T cells results in the generation of fewer antigen-specific CD8+ T cells in the infected animals. Quantitative assay of antigen-reactive T cells in E. cuniculi-infected mice was done by estimating the frequency of antigen-specific T cells in the infected animals. The experiment was performed at day 15 after E. cuniculi infection, a time point at which the rise in total T-cell populations in wild-type C57BL/6 mice is observed (22). Moreover, CD8−/− mice develop ascites and begin to look sick at this time. By LDA, it was determined that PPFs of both CD4−/− and CD8−/− mice are similar to that of parental wild-type C57BL/6 mice. The PPF of the T-cell population was 1/104 cells in the parental control group, compared to 1/2.5 × 104 in CD4−/− and 1/4.3 × 104 in CD8−/− mice (Fig. 4). Control splenocytes from uninfected mice showed background proliferation. The magnitude of the E. cuniculi-specific T-cell response generated in CD8−/− mice was similar to that in CD4−/− and parental wild-type animals, since these values are considered within the range of variability for this assay (11).
FIG. 4.
Antigen-induced proliferation of T cells from E. cuniculi-infected mice in an LDA. Five- to eight-week-old female CD4−/−, CD8−/−, and parental wild-type (WT) C57BL/6 mice were infected i.p. with 107 spores of E. cuniculi. At day 15 p.i., total T cells (>95% pure) from the pooled splenocytes (n = 3 mice/group) were isolated and cultured in the presence of E. cuniculi spores and irradiated feeder cells. After 1 week in culture, PPF of T cells was determined. Data are representative of one of two separate experiments.
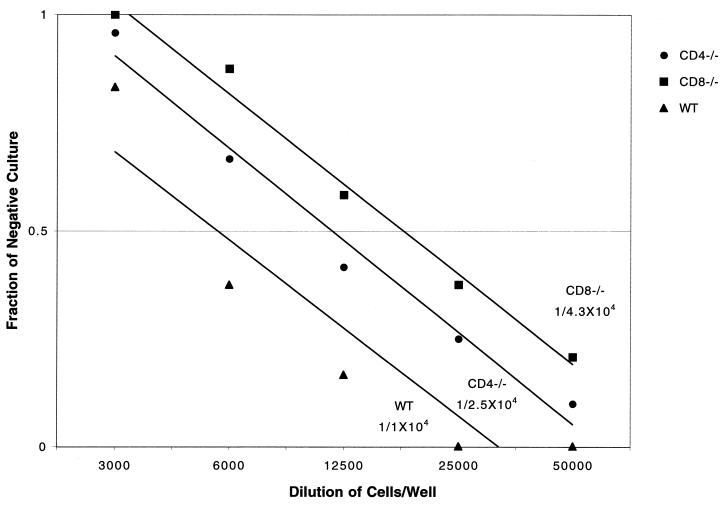
E. cuniculi-infected CD4−/− mice have normal pCTL frequency.
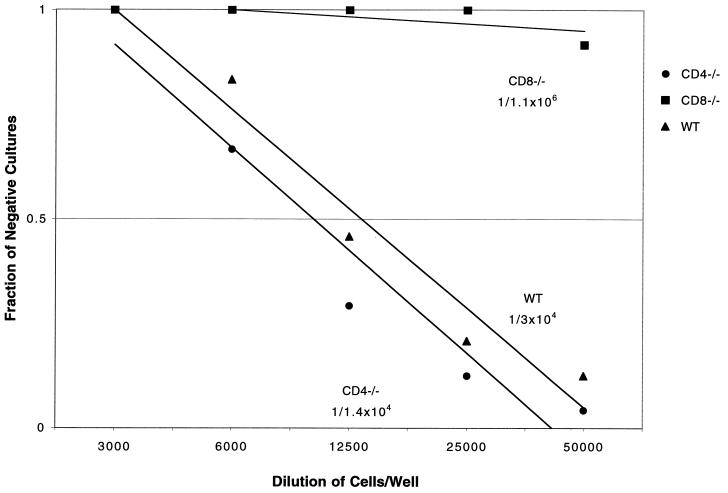
A previous report from our laboratory demonstrated that the CTL response in E. cuniculi-infected animals was important for host protection against the parasite (21). Based on these findings, we evaluated the frequency of antigen-specific CTL in the spleen cells of parental wild-type and knockout mice following E. cuniculi infection. The assay was carried out at day 15 p.i., when the CTL response in spleen cell cultures from C57BL/6 mice was maximal. By LDA, we determined that the pCTL frequency of CD4−/− mice was similar to that of wild-type controls. The pCTL frequency of CD4−/− mice was 1/1.4 × 104, compared to 1/3 × 104 in the parental control group (Fig. 5). In contrast, the frequency of antigen-specific CTL was reduced by almost 2 logs (1/1.1 × 106 cells) in CD8−/− mice. The CTL response was antigen specific, as splenocytes from infected mice failed to lyse uninfected targets (22) or macrophages infected with irrelevant antigen-like Toxoplasma gondii tachyzoites (data not shown). As in the proliferation assay, splenocytes from uninfected mice incubated with E. cuniculi-infected macrophages exhibited background cytotoxicity at all effector/target ratios (data not shown). Thus, it appears that the absence of CD4+ T cells does not result in the downregulation of the CTL response in E. cuniculi-infected mice.
FIG. 5.
In an LDA, E. cuniculi-infected mice generate pCTLs when stimulated in vitro with E. cuniculi spores. Five- to eight-week-old CD4−/−, CD8−/−, and wild-type (WT) C57BL/6 mice were infected with E. cuniculi as described in Materials and Methods. At day 17 p.i., splenocytes from each group of mice were isolated, pooled (three mice/group), and cultured by LDA in the presence of spores and irradiated feeder cells. After 1 week in culture, pCTL frequency of spleen cells was determined. Data shown are representative of one of the two separate experiments performed.
Cytokine responses in E. cuniculi-infected animals.
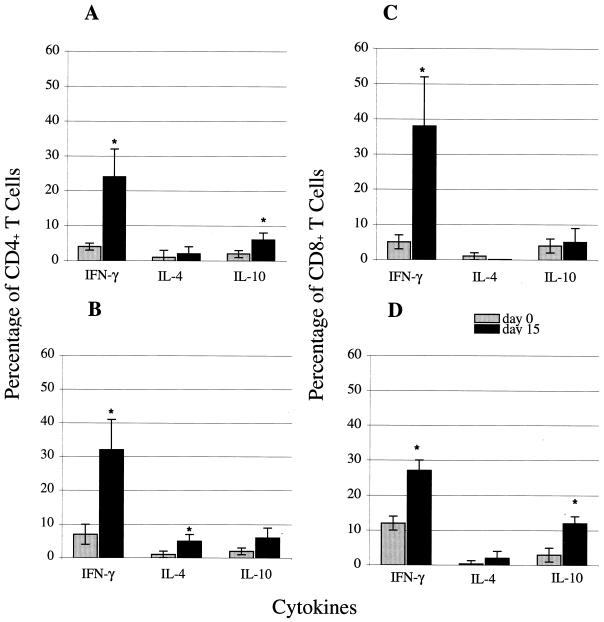
In addition to cytotoxic activity, IFN-γ production is an important feature of CD8+ CTLs (28). IFN-γ-secreting CD8+ T cells have been demonstrated to confer protective immunity against number of intracellular infections (12, 18, 25). IFN-γ has also been reported to play an important role in protection against E. cuniculi infection (21). To determine if the lack of CD4+ T cells can affect IFN-γ production in E. cuniculi-infected animals, mice deficient in the CD4 or CD8 gene were analyzed for cytokine message by quantitative PCR (19). No difference in the kinetic of IFN-γ message was detected between susceptible CD8−/− and resistant CD4−/− mice (data not shown). CD8+ and CD4+ T cells from infected CD4−/−, CD8−/−, and wild-type mice were further analyzed for cytokine production by intracellular staining at day 15 p.i. E. cuniculi infection caused a rise in IFN-γ-positive CD4+ T cells in both CD8−/− mice and parental controls (Fig. 6A and B). Similarly, an increase in IFN-γ-positive CD8+ T cells as a result of E. cuniculi infection was observed in both CD4−/− and wild-type mice (Fig. 6C and D). Although the percentage of CD8+ T cells positive for IFN-γ production was slightly lower in CD4−/− mice (27% ± 3%) than in parental wild-type controls (38% ± 10%), the differences were not statistically significant (Fig. 6C and D). Very minimal levels of IL-4-producing CD4+ or CD8+ T cells were detected in the infected parental wild-type mice (Fig. 6A and C). However, a small but significant increase in IL-4-producing CD4+ T cells was noticed in CD8−/− animals (Fig. 6B). An increase in IL-10-producing CD4+ T cells due to E. cuniculi infection was observed in wild-type C57BL/6 mice. Interestingly, no significant increase in IL-10-positive CD4+ T cells was noted in CD8−/− mice. No increase in IL-10-producing CD8+ T cells was observed in the infected wild-type animals (Fig. 6C). However, a rise in IL-10-producing CD4+ T cells was detected in CD8−/− mice (Fig. 6D). Overall, the lack of CD4+ T cells during E. cuniculi infection had no major effect on the cytokine profile of CD8+ T cells.
FIG. 6.
Detection of cytokine production by intracellular staining. Five- to six-week-old wild-type C57BL/6 and age-matched knockout mice were infected with 107 spores of E. cuniculi as described in Materials and Methods. At day 15 p.i., total splenocytes from CD4−/− (D), CD8−/− (B), and wild-type (A and C) infected mice were cultured in vitro with PMA, ionomycin, and monensin for 4 h. Cultured cells were then labeled for CD4+ (A and B) or CD8+ (C and D) T cells before intracellular staining for IFN-γ, IL-4, and IL-10. Values are the mean percentage of cells positive for IFN-γ, IL-4, or IL-10. Error bars represent the SD for four mice per group. Statistical significance was determined using the Student t test (∗, P < 0.05).
Adoptive transfer of immune CD8+ T cells from CD4−/− mice protects against E. cuniculi challenge.
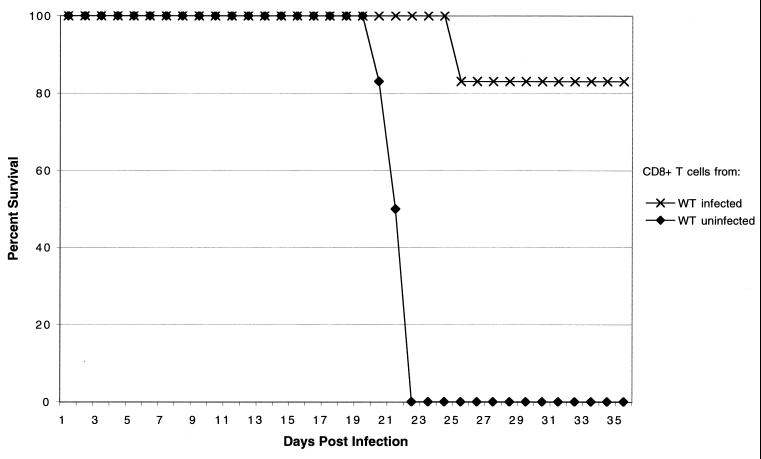
We evaluated the ability of immune CD8+ T cells from E. cuniculi-infected parental mice to protect hosts lacking the CD8 gene. CD8+ T cells were isolated from immunocompetent C57BL/6 mice at day 15 p.i. Purified CD8+ T cells (>95% pure) were adoptively transferred to naïve CD8−/− mice, and animals were challenged the following day with E. cuniculi spores as described above. Animals were observed daily for mortality or development of ascites. None of the animals treated with immune CD8+ T cells died or showed any signs of sickness throughout the experiment (Fig. 7), whereas mice treated with an equal number of nonimmune CD8+ T cells succumbed to infection as observed earlier.
FIG. 7.
Adoptive transfer of immune CD8+ T cells from wild-type (WT) E. cuniculi-infected mice protects naive CD8−/− mice against a lethal E. cuniculi challenge. CD8+ T cells from pooled splenocytes (n = 3/group) from E. cuniculi-infected parental C57BL/6 mice were isolated by magnetic separation at day 17 after challenge. A total of 107 CD8+ T cells (>95% pure) were injected intravenously into CD8−/− mice (n = 6 mice/group). Control animals received an equal amount of cells from uninfected mice. After 24 h, mice were challenged i.p. with 107 spores of E. cuniculi, and survival was monitored until the end of the experiment. The experiment was performed twice with similar results.
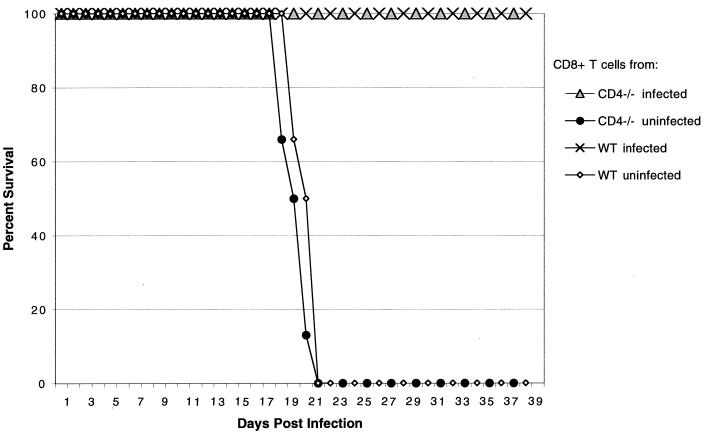
We then determined if CD8+ T cells from CD4−/− mice can protect CD8−/− mice against E. cuniculi challenge. CD8+ T cells from parental C57BL/6 and CD4−/− mice were isolated at day 15 p.i. Purified CD8+ T cells (>95% pure) were adoptively transferred to naïve CD8−/− mice, and animals were challenged the following day as described above. Animals were observed daily for mortality or development of ascites. None of the mice treated with immune CD8+ T cells from wild-type or CD4−/− mice died or showed signs of sickness for the duration of the experiment (Fig. 8), whereas control mice injected with nonimmune CD8+ T cells died of the infection.
FIG. 8.
Adoptive transfer of immune CD8+ T cells from CD4−/− E. cuniculi-infected mice protect naive CD8−/− mice against a lethal E. cuniculi challenge. CD8+ T cells from pooled splenocytes (n = 3 mice/group) from E. cuniculi-infected CD4−/− and parental wild-type (WT) C57BL/6 mice were isolated by magnetic separation at day 17 after challenge. A total of 107 CD8+ T cells (>95% pure) were injected intravenously to CD8−/− mice (n = 6 mice/group). Control animals received an equal amount of cells from uninfected mice. After 24 h, mice were challenged i.p. with 107 spores of E. cuniculi, and survival was monitored until the end of the experiment. The experiment was performed twice with similar results.
DISCUSSION
Microsporidia are being increasingly associated with patients infected with AIDS (9, 17). E. cuniculi, which was previously considered to cause a zoonotic infection, has been recently implicated in complications of HIV infection (42, 44). Moreover, E. cuniculi shares biological features with other microsporidians that are responsible for morbidity and mortality in individuals suffering from AIDS (7).
The role of T cells during natural E. cuniculi infection has been documented (16, 32). Previous studies from our laboratory have demonstrated that among the T-cell subtypes, CD8+ T cells play an essential role in protection against E. cuniculi infection (22). This interpretation was based on the findings that mice lacking CD8+ T cells, unlike parental wild-type animals, succumbed to E. cuniculi infection. Interestingly, E. cuniculi infection poses a problem for HIV-infected patients, who suffer a major defect in CD4+ T-cell immunity. However, in an experimental model, lack of CD4+ T cells did not affect the resistance of mice to infection. Thus, it is likely that similar to T. gondii, another opportunistic pathogen (14), E. cuniculi may be a problem during advanced stages of HIV infection when CD8+ T-cell immunity is also compromised (34).
Although CD4−/− mice survive E. cuniculi infection, the role of CD4+ T cells in the immune response against this parasite has not been elucidated. In this study, we demonstrate that the lack of CD4+ T cells has no effect on the CD8+ T-cell response in E. cuniculi-infected animals. PPF analysis indicated that the absence of CD4+ T cells had no significant effect on the generation of an antigen-specific CD8+ T-cell response in knockout mice. No difference in the proliferation of antigen-specific T cells was observed between the CD8−/− mice, which died, and CD4−/− or parental C57BL/6 strains, which survived. Thus, it seems that in the absence of CD8+ T cells, other immune cells in E. cuniculi-infected mice respond to antigenic stimulation. This could be due to the redundant mechanisms available in gene knockout animals, as found in other systems (24). It could also be the reason for the absence of major differences in cytokine production between wild-type, CD4−/−, and CD8−/− mice. However, even in the presence of normal cytokine production and optimal proliferation of immune cells, CD8−/− mice succumb to E. cuniculi infection. By pCTL analysis, CD8−/− mice showed almost a 100-fold loss of antigen-specific cytotoxicity. Thus, the loss of cytolytic ability seems to be the determining factor in the outcome of E. cuniculi infection. These findings confirm our previous observation that mice lacking the perforin gene, similar to CD8−/− mice, are unable to resolve E. cuniculi infection (22).
The important feature of our observations is that the absence of CD4+ T cells does not seem to have a profound effect on the efficacy of CD8+ T cells in controlling E. cuniculi infection. Mice lacking this cell type did not carry an overwhelming parasite burden. The role of CD4+ T cells in induction of the CD8+ T-cell response has been studied in other infectious disease models (14, 26). CD4+ T cells are an important source of early IL-2, which may be important for priming of the CD8+ T-cell response against intracellular infections (35). During lymphocytic choriomeningitis virus infection, mice lacking CD4+ T cells develop a significantly lower pCTL response compared to wild-type controls (26) and as a result are unable to clear the virus. In contrast, lack of CD4+ T cells does not affect mice infected with vaccinia virus (1). Coordinated interaction between CD4+ and CD8+ T cells is required to resolve infection with the intracellular bacterium Listeria monocytogenes (39). This also has been reported for T. gondii, where simultaneous depletion of CD4+ and CD8+ T cells results in the reactivation of chronic infection (14). As stated above, the lack of CD4+ T cells does not seem to compromise the CD8+ T-cell function of E. cuniculi-infected animals. By comparison, in recent studies of Plasmodium yoelii infection, CD8+ T-cell priming was dependent on IL-12 and NK cells (12). However, while in P. yoelii-infected mice CD8+ T-cell immunity was mediated by IFN-γ, protective immunity during E. cuniculi infection was primarily dependent on the cytolytic function of immune CD8+ T cells. As recent evidence suggests that microsporidia are closely related to fungi (43), the importance of CD8+ T cells in resolving systemic infection with other fungal pathogens has been reported (5, 8).
Previous studies from our laboratory have reported the importance of IFN-γ in protection against E. cuniculi infection (21). Mice lacking the IFN-γ gene survived longer than CD8−/− or perforin−/− animals but ultimately succumbed to E. cuniculi infection (22). In the present study, a significant percentage of CD4+ T cells in CD8−/− mice produced IFN-γ in response to E. cuniculi infection. Thus, it seems that although important, IFN-γ may not be the ultimate effector molecule during E. cuniculi infection. This possibility is further supported by our earlier observation that mice lacking the inducible nitric oxide synthase gene could withstand very high infective doses of E. cuniculi (21). The importance of IFN-γ during E. cuniculi infection may be due to its role in antigen presentation and augmentation of the CD8+ T-cell response as reported for other infectious disease models (13, 33, 40). The interactions between IFN-γ and CD8+ T cells during E. cuniculi infection need to be studied further.
Based on our current observations, we propose the following hypothesis. Natural infection with E. cuniculi induces a strong host immune reaction manifest by IFN-γ production. This probably leads to upregulation of major histocompatibility complex class I molecules on antigen-presenting cells, thereby enhancing the quality of antigen presentation. The role of IFN-γ in class I antigen regulation and processing has been previously described (2). All of this leads to the generation of an antigen-specific CD8+ CTL response, which is responsible for the elimination of parasites. One would assume that in the absence of IL-2-producing CD4+ T cells, robust CD8+ CTL immunity may not be generated. However, this does not seem to be the case with E. cuniculi infection. These observations raise two important questions. (i) What are the cell types responsible for priming the CD8+ T-cell immunity during E. cuniculi infection? Obviously in the absence of conventional CD4+ T cells, other cell types provide help to CD8+ T cells. The interesting point is that CD40-CD40L interactions may not be needed. (ii) Is long-term effective CD8+ T-cell immunity maintained in the absence of CD4+ T cells? Studies in this direction are in progress in our laboratory.
ACKNOWLEDGMENTS
We are thankful to Alice Givans, flow cytometry lab at Dartmouth Medical School, for help in FACS analysis. The assistance provided by Ken Ely and Martha Williams during preparation of the manuscript is acknowledged.
This work was supported by National Institutes of Health grant AI43693.
REFERENCES
- 1.Binder D, Kundig T M. Antiviral protection by CD8+ versus CD4+ T cells. CD8+ T cells correlating with cytotoxic activity in vitro are more efficient in antivaccinia virus protection than CD4-dependent IL. J Immunol. 1991;146:4301–4307. [PubMed] [Google Scholar]
- 2.Boehm U, Klamp T, Groot M, Howard J C. Cellular responses to interferon-gamma. Annu Rev Immunol. 1997;15:749–795. doi: 10.1146/annurev.immunol.15.1.749. [DOI] [PubMed] [Google Scholar]
- 3.Cali A, Takvorian P M, Lewin S, Rendel M, Sian C S, Wittner M, Tanowitz H B, Keohane E, Weiss L M. Brachiola vesicularum, n. g., n. sp., a new microsporidium associated with AIDS and myositis. J Eukaryot Microbiol. 1998;45:240–251. doi: 10.1111/j.1550-7408.1998.tb04532.x. [DOI] [PubMed] [Google Scholar]
- 4.Canning E, Lom J. The microsporidia of vertebrates. New York, N.Y: Academic Press; 1986. [Google Scholar]
- 5.Cano L E, Singer-Vermes L M, Costa T A, Mengel J O, Xidieh C F, Arruda C, Andre D C, Vaz C A, Burger E, Calich V L. Depletion of CD8+ T cells in vivo impairs host defense of mice resistant and susceptible to pulmonary paracoccidioidomycosis. Infect Immun. 2000;68:352–359. doi: 10.1128/iai.68.1.352-359.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carter L L, Swain S L. Single cell analyses of cytokine production. Curr Opin Immunol. 1997;9:177–182. doi: 10.1016/s0952-7915(97)80132-x. [DOI] [PubMed] [Google Scholar]
- 7.Coyle C M, Wittner M, Kotler D P, Noyer C, Orenstein J M, Tanowitz H B, Weiss L M. Prevalence microsporidiosis due to Enterocytozoon bieneusi and Encephalitozoon (Septata) intestinalis among patients with AIDS-related diarrhea: determination by polymerase chain reaction to the microsporidian small-subunit rRNA gene. Clin Infect Dis. 1996;23:1002–1006. doi: 10.1093/clinids/23.5.1002. [DOI] [PubMed] [Google Scholar]
- 8.Deepe G S., Jr Role of CD8+ T cells in host resistance to systemic infection with Histoplasma capsulatum in mice. J Immunol. 1994;152:3491–3500. [PubMed] [Google Scholar]
- 9.De Groote M A, Visvesvara G, Wilson M L, Pieniazek N J, Slemenda S B, daSilva A J, Leitch G J, Bryan R T, Reves R. Polymerase chain reaction and culture confirmation of disseminated Encephalitozoon cuniculi in a patient with AIDS: successful therapy with albendazole. J Infect Dis. 1995;171:1375–1378. doi: 10.1093/infdis/171.5.1375. [DOI] [PubMed] [Google Scholar]
- 10.Didier E S. Microsporidiosis. Clin Infect Dis. 1998;27:1–7. doi: 10.1086/514607. [DOI] [PubMed] [Google Scholar]
- 11.Doherty P C, Topham D J, Tripp R A. Establishment and persistence of virus-specific CD4+ and CD8+ T cell memory. Immunol Rev. 1996;150:23–44. doi: 10.1111/j.1600-065x.1996.tb00694.x. [DOI] [PubMed] [Google Scholar]
- 12.Doolan D L, Hoffman S L. IL-12 and NK cells are required for antigen-specific adaptive immunity against malaria initiated by CD8+ T cells in the Plasmodium yoelii model. J Immunol. 1999;163:884–892. [PubMed] [Google Scholar]
- 13.Ely K H, Kasper L H, Khan I A. Augmentation of the CD8+ T cell response by IFN-gamma in IL-12-deficient mice during Toxoplasma gondii infection. J Immunol. 1999;162:5449–5449. [PubMed] [Google Scholar]
- 14.Gazzinelli R, Xu Y, Hieny S, Cheever A, Sher A. Simultaneous depletion of CD4+ and CD8+ T lymphocytes is required to reactivate chronic infection with Toxoplasma gondii. J Immunol. 1992;149:175–180. [PubMed] [Google Scholar]
- 15.Ghezzi S, Vicenzi E, Soldini L, Tambussi G, Murone M, Lazzarin A, Poli G. Experiences in immune reconstitution. The rationale for interleukin-2 administration to HIV-infected individuals. J Biol Regul Homeost Agents. 1997;11:74–78. [PubMed] [Google Scholar]
- 16.Hermanek J, Koudela B, Kucerova Z, Ditrich O, Travnicek J. Prophylactic and therapeutic immune reconstitution of SCID mice infected with Encephalitozoon cuniculi. Folia Parasitol. 1993;40:287–291. [PubMed] [Google Scholar]
- 17.Hollister W S, Canning E U, Colbourn N I, Aarons E J. Encephalitozoon cuniculi isolated from the urine of an AIDS patient, which differs from canine and murine isolates. J Eukaryot Microbiol. 1995;42:367–372. doi: 10.1111/j.1550-7408.1995.tb01595.x. [DOI] [PubMed] [Google Scholar]
- 18.Khan I A, Ely K H, Kasper L H. Antigen-specific CD8+ T cell clone protects against acute Toxoplasma gondii infection in mice. J Immunol. 1994;152:1856–1860. [PubMed] [Google Scholar]
- 19.Khan I A, Matsuura T, Fonseka S, Kasper L H. Production of nitric oxide (NO) is not essential for protection against acute Toxoplasma gondii infection in IRF-1−/− mice. J Immunol. 1996;156:636–643. [PubMed] [Google Scholar]
- 20.Khan I A, Matsuura T, Kasper L H. IL-10 mediates immunosuppression following primary infection with Toxoplasma gondii in mice. Parasite Immunol. 1995;17:185–195. doi: 10.1111/j.1365-3024.1995.tb00888.x. [DOI] [PubMed] [Google Scholar]
- 21.Khan I A, Moretto M. Role of gamma interferon in cellular immune response against murine Encephalitozoon cuniculi infection. Infect Immun. 1999;67:1887–1893. doi: 10.1128/iai.67.4.1887-1893.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khan I A, Schwartzman J D, Kasper L H, Moretto M. CD8+ CTLs are essential for protective immunity against Encephalitozoon cuniculi infection. J Immunol. 1999;162:6086–6091. [PubMed] [Google Scholar]
- 23.Kiristis M, Mui E, Mcleod R. A new method to study the efficacy of vaccines and anti microbial therapy against toxoplasmosis. Int J Parasitol. 2000;30:149–155. doi: 10.1016/s0020-7519(00)00009-6. [DOI] [PubMed] [Google Scholar]
- 24.Locksley R M, Reiner S L, Hatam F, Littman D R, Killeen N. Helper T cells without CD4: control of leishmaniasis in CD4-deficient mice. Science. 1993;261:1448–1451. doi: 10.1126/science.8367726. [DOI] [PubMed] [Google Scholar]
- 25.Low H P, Santos M A, Wizel B, Tarleton R L. Amastigote surface proteins of Trypanosoma cruzi are targets for CD8+ CTL. J Immunol. 1998;160:1817–1823. [PubMed] [Google Scholar]
- 26.Matloubian M, Concepcion R J, Ahmed R. CD4+ T cells are required to sustain CD8+ cytotoxic T-cell responses during chronic viral infection. J Virol. 1994;68:8056–8063. doi: 10.1128/jvi.68.12.8056-8063.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mertens R B, Didier E S, Fishbein M C, Bertucci D C, Rogers L B, Orenstein J M. Encephalitozoon cuniculi microsporidiosis: infection of the brain, heart, kidneys, trachea, adrenal glands, and urinary bladder in a patient with AIDS. Mod Pathol. 1997;10:68–77. [PubMed] [Google Scholar]
- 28.Miyahira Y, Murata K, Rodriguez D, Rodriguez J R, Esteban M, Rodrigues M M, Zavala F. Quantification of antigen specific CD8+ T cells using an ELISPOT assay. J Immunol Methods. 1995;181:45–54. doi: 10.1016/0022-1759(94)00327-s. [DOI] [PubMed] [Google Scholar]
- 29.Neter J, Wasserman W, Kuter M H. Applied linear statistical models. 2nd ed. Homewood, Ill: Irwin; 1985. [Google Scholar]
- 30.Orenstein J M. Microsporidiosis in the acquired immunodeficiency syndrome. J Parasitol. 1991;77:843–864. [PubMed] [Google Scholar]
- 31.Posavad C M, Koelle D M, Shaughnessy M F, Corey L. Severe genital herpes infections in HIV-infected individuals with impaired herpes simplex virus-specific CD8+ cytotoxic T lymphocyte responses. Proc Natl Acad Sci USA. 1997;94:10289–10294. doi: 10.1073/pnas.94.19.10289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schmidt E C, Shadduck J A. Mechanisms of resistance to the intracellular protozoan Encephalitozoon cuniculi in mice. J Immunol. 1984;133:2712–2719. [PubMed] [Google Scholar]
- 33.Sharma D P, Ramsay A J, Maguire D J, Rolph M S, Ramshaw I A. Interleukin-4 mediates down regulation of antiviral cytokine expression and cytotoxic T-lymphocyte responses and exacerbates vaccinia virus infection in vivo. J Virol. 1996;70:7103–7107. doi: 10.1128/jvi.70.10.7103-7107.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shearer G M, Bernstein D C, Tung K S, Via C S, Redfield R, Salahuddin S Z, Gallo R C. A model for the selective loss of major histocompatibility complex self-restricted T cell immune responses during the development of acquired immune deficiency syndrome (AIDS) J Immunol. 1986;137:2514–2521. [PubMed] [Google Scholar]
- 35.Smith K A. Interleukin-2: inception, impact, and implications. Science. 1988;240:1169–1176. doi: 10.1126/science.3131876. [DOI] [PubMed] [Google Scholar]
- 36.Taswell C. Limiting dilution assays for the determination of immunocompetent cell frequencies. I. Data analysis. J Immunol. 1981;126:1614–1619. [PubMed] [Google Scholar]
- 37.Terada S, Reddy K R, Jeffers L J, Cali A, Schiff E R. Microsporidan hepatitis in the acquired immunodeficiency syndrome. Ann Intern Med. 1987;107:61–62. doi: 10.7326/0003-4819-107-1-61. [DOI] [PubMed] [Google Scholar]
- 38.Toes R E M, Schoenberger S P, van der Voort E I H, Offringa R, Melief C J M. CD40-CD40 ligand interactions and their role in cytotoxic T lymphocyte priming and anti-tumor immunity. Semin Immunol. 1998;10:443–448. doi: 10.1006/smim.1998.0147. [DOI] [PubMed] [Google Scholar]
- 39.Unanue E R. Studies in listeriosis show the strong symbiosis between the innate cellular system and the T-cell response. Immunol Rev. 1997;158:11–25. doi: 10.1111/j.1600-065x.1997.tb00988.x. [DOI] [PubMed] [Google Scholar]
- 40.Utermohlen O, Dangel A, Tarnok A, Lehmann-Grube F. Modulation by gamma interferon of antiviral cell-mediated immune responses in vivo. J Virol. 1996;70:1521–1526. doi: 10.1128/jvi.70.3.1521-1526.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Weber R, Bryan R T, Schwartz D A, Owen R L. Human microsporidial infections. Clin Microbiol Rev. 1994;7:426–461. doi: 10.1128/cmr.7.4.426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Weber R, Deplazes P, Flepp M, Mathis A, Baumann R, Sauer B, Kuster H, Luthy R. Cerebral microsporidiosis due to Encephalitozoon cuniculi in a patient with human immunodeficiency virus infection. New Engl J Med. 1997;336:474–478. doi: 10.1056/NEJM199702133360704. [DOI] [PubMed] [Google Scholar]
- 43.Weiss L M, Edlind T D, Vossbrinck C R, Hashimoto T. Microsporidian molecular phylogeny: the fungal connection. J Eukaryot Microbiol. 1999;46:17S–18S. [PubMed] [Google Scholar]
- 44.Zender H O, Arrigoni E, Eckert J, Kapanci Y. A case of Encephalitozoon cuniculi peritonitis in a patient with AIDS. Am J Clin Pathol. 1989;92:352–356. doi: 10.1093/ajcp/92.3.352. [DOI] [PubMed] [Google Scholar]