Abstract
Background:
Decreased scam awareness may be an early indicator of impending Alzheimer dementia and its precursor, mild cognitive impairment, but prior studies have not systematically examined the associations between scam awareness and adverse cognitive outcomes.
Objective:
To test the hypothesis that low scam awareness is associated with increased risk for incident Alzheimer dementia, mild cognitive impairment, and Alzheimer disease pathology in the brain.
Design:
Prospective cohort study of aging.
Setting:
Community-based study in the greater Chicago metropolitan area.
Participants:
935 older persons initially free of dementia.
Measurements:
Scam awareness was measured via questionnaire, incident Alzheimer dementia and mild cognitive impairment were documented in detailed annual cognitive and clinical evaluations, and Alzheimer disease neuropathology was quantified after death among a subset of persons who died (n = 264). Proportional hazards models examined associations between scam awareness and incident Alzheimer dementia and mild cognitive impairment. Regression models examined associations between scam awareness and Alzheimer disease pathology, particularly β-amyloid burden and tau tangle density.
Results:
During a mean of about 6 years (SD, 2.4) of observation, 151 persons (16.1%) developed Alzheimer dementia. Low scam awareness was associated with increased risk for Alzheimer dementia (hazard ratio [HR], 1.56 [95% CI, 1.21 to 2.01]; P < 0.001), such that each 1-unit increase in scam score (indicating lower awareness) was associated with about a 60% increase in dementia risk. Low scam awareness was also associated with increased risk for mild cognitive impairment (HR, 1.47 [CI, 1.20 to 1.81]; P < 0.001). These associations persisted even after adjustment for global cognitive function. Finally, low scam awareness was associated with a higher burden of Alzheimer pathology in the brain, particularly β-amyloid (estimated increase [±SE] in β-amyloid per 1-unit increase in scam score, 0.22 ± 0.10 unit; P = 0.029).
Limitation:
The measure of scam awareness used here is too weak for prediction at the individual level.
Conclusion:
Low scam awareness among older persons is a harbinger of adverse cognitive outcomes and is associated with Alzheimer disease pathology in the brain.
Primary Funding Source:
National Institute on Aging.
The persistent difficulty of determining who is at high risk for adverse cognitive outcomes in old age has led to intense interest in identifying behavioral changes that predict the onset of Alzheimer dementia and its precursor, mild cognitive impairment (MCI), earlier than standard neuropsychological measures currently do (1, 2). Early detection of persons at risk for MCI in particular might enable better targeting of potential disease-modifying therapies.
Despite awareness of the need to identify predictors of dementia and MCI, the aspects of behavior to target are unclear. Evidence suggests that impairment in complex behaviors (such as decision making, particularly financial) is associated with progression from MCI to Alzheimer dementia, but little is known about their association with the transition from normality to MCI (3–7). Scam awareness is a key component of decision making in old age. Fraudsters frequently target and victimize older persons, and an awareness of deceptive tactics and behaviors that increase susceptibility to scams or exploitation is essential for sound decision making (8–13). Emerging findings indicate that older persons, particularly those with MCI or other cognitive syndromes, are vulnerable to decreased scam awareness (8, 14). Further, even subtle changes in cognition related to age—those observed among “cognitively intact” persons—have a deleterious effect on scam awareness (15). Together, these findings suggest that decreased scam awareness may be an early manifestation of pathologic cognitive aging and a harbinger of adverse cognitive outcomes.
Here, we test the hypothesis that low scam awareness is associated with increased risk for incident MCI and Alzheimer dementia using data from more than 900 participants in a community-based study of aging (16). A subset of participants who died provided autopsy data to further explore whether low scam awareness in old age is a consequence of accumulating Alzheimer disease (AD) pathology in the brain.
Methods
Participants
Data came from an ongoing clinical pathologic study of aging (16), the Rush Memory and Aging Project. The study was approved by the Institutional Review Board of Rush University Medical Center. Enrollees have no known dementia, receive annual clinical evaluations, and agree to organ donation. Each participant provided written informed consent and a document of anatomical gift.
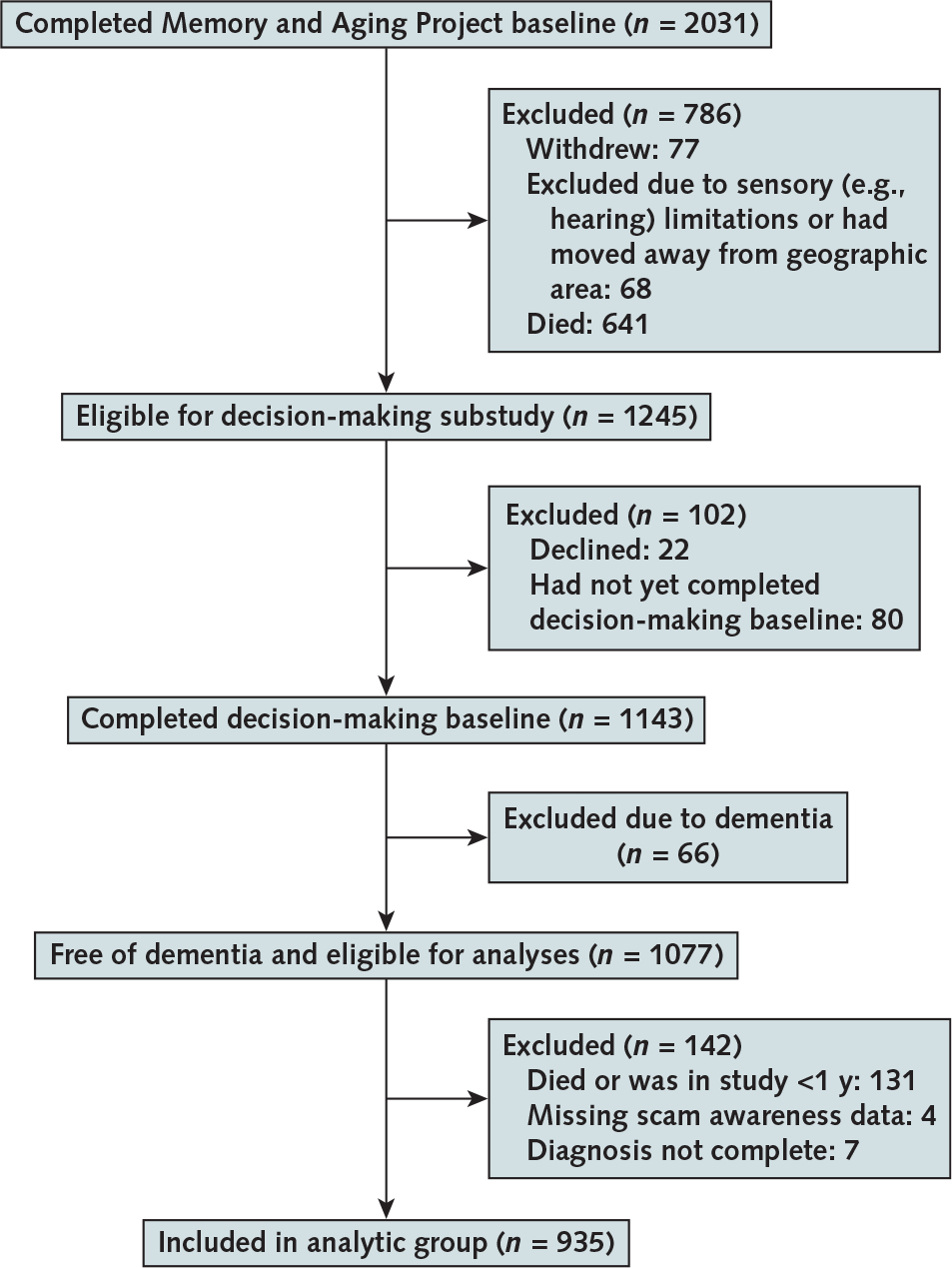
The Memory and Aging Project started in 1997, and assessment of scam awareness was introduced in 2010. At the time of these analyses, 2031 participants had enrolled and completed the baseline evaluation. Figure 1 details the assembly of the final analytic sample of 935 older persons free of dementia.
Figure 1. Flow chart showing the assembly of the analytic cohort.
Assessment of Scam Awareness
Scam awareness was assessed using a measure that addresses knowledge of tactics used to deceive older persons and willingness to engage in behaviors that may increase risk for falling prey to financial scams and other forms of exploitation (14, 15, 17–19). We focused on these behaviors because exploitation of elders is a growing public health problem (8–13, 20). Participants were asked to rate their agreement on a 7-point Likert scale (Appendix Table, available at Annals.org) with 5 statements that assess openness to sales pitches (items 1, 2, and 5), interest in potentially risky investments (item 3), and awareness of heightened vulnerability due to older age (item 4). Items in this measure have moderate internal consistency, with an intraclass correlation coefficient of 0.63. In prior work from this cohort, lower scam awareness was associated with older age, lower cognition, lower financial and health literacy, lower psychological well-being, and poorer decision making (14, 15, 17, 18). The average of ratings across the 5 items is the total score, and higher scores indicate lower scam awareness.
Assessment of Cognition
Cognitive function was assessed annually using 21 performance tests (15, 16, 21–23). Results from 19 tests were used to create a composite measure of global cognition. These included 7 measures of episodic memory (immediate and delayed recall of Logical Memory Story A and the East Boston Story, Word List Memory, Word List Recall, and Word List Recognition), 3 measures of semantic memory (Boston Naming Test, Verbal Fluency, and Word Reading), 3 measures of working memory (Digit Span Forward, Digit Span Backward, and Digit Ordering), 4 measures of perceptual speed (Symbol Digit Modalities Test, Number Comparison, and 2 indices from a modified Stroop Neuropsychological Screening Test), and 2 measures of visuospatial ability (Standard Progressive Matrices and Judgment of Line Orientation). Raw test scores were converted to Z scores using the baseline mean and SD of the cohort, and Z scores from all 19 tests were averaged to yield the composite measure of global cognitive function (16, 22, 23).
Clinical Diagnosis of Alzheimer Dementia and MCI
All participants had structured annual clinical evaluations that included the cognitive performance tests described in the previous paragraph, medical history interviews, and in-person neurologic examinations (16). Clinical classification of cognitive impairment and dementia followed a 3-step process. First, 11 cognitive tests were scored by computer, and an education-adjusted rating of impairment was provided for 5 cognitive domains (16, 21). Second, impairment ratings were reviewed by a neuropsychologist blinded to scam awareness and other information except cognitive data, education, sensorimotor function, and motivation. The neuropsychologist rendered a judgment on impairment. Third, an experienced clinician reviewed the cognitive data, neuropsychologist’s ratings, medical history, and neurologic examination results and rendered a decision regarding dementia and its likely cause. Clinical diagnosis of Alzheimer dementia was based on criteria from the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer’s Disease and Related Disorders Association; these criteria include a history of cognitive decline with impairment in memory and at least 1 other cognitive domain (16, 24). Mild cognitive impairment was defined as cognitive impairment that did not meet criteria for dementia (14, 16, 22).
Neuropathologic Indices of AD
Brain autopsy followed standard procedures (25, 26). β-Amyloid immunoreactive plaques were labeled with N-terminus–directed monoclonal antibodies (4G8 [Covance], 1:9000; 6F/3D [Dako North America], 1:50; or 10D5 [Elan Pharmaceuticals], 1:600). Neurofibrillary tangles were labeled with an antibody specific for phosphorylated tau (AT8 [Innogenetics], 1:1000). Computer-assisted image analysis quantified the areas occupied by β-amyloid and tau tangles, and regional measures were averaged to yield composite measures of β-amyloid burden and tangle density.
Statistical Analysis
Spearman correlations and t tests were used to describe the bivariate relationships between demographic variables and scam awareness at baseline. Cox proportional hazards models adjusted for age, sex, and education were used to examine the associations of scam awareness with incident Alzheimer dementia and MCI. Scores on the scam awareness measure served as the predictor, and time in years until the first incident event (Alzheimer dementia and MCI, separately) was the outcome. Thus, the increase in log hazard ratio (HR) is proportional with every 1-unit increase (1.4 SD) in scam awareness. Participants who had no event or died before an event were right-censored at the last evaluation. We assessed the proportional hazards assumption by using cumulative sums of Martingale residuals. We did sensitivity analyses that further examined the influence of baseline global cognitive function, extremes of age, and item-level associations. Mixed-effects models were used to examine the association of scam awareness with the starting level of and rate of decline in global cognitive function. Finally, among participants who had an autopsy (n = 264), linear regression models were used to examine the association between scam awareness and 2 molecularly specific markers of AD pathology.
Role of the Funding Source
This research was funded by the National Institute on Aging, which had no role in the design or conduct of the study, analysis of the data, or decision to submit the manuscript for publication.
Results
Descriptive Properties of the Sample
Table 1 provides descriptive data on the analytic cohort. The mean scam awareness score was 2.8 (SD, 0.7), with higher scores indicating lower scam awareness. In bivariate analyses, lower scam awareness was associated with older age (P < 0.001), lower education (P < 0.001), and lower global cognition (P < 0.001) at baseline.
Table 1.
Baseline Characteristics of the Analytic Group and Participants Who Did and Did Not Develop Alzheimer Dementia*
| Characteristic | Analytic Cohort (n = 935) | Unaffected Participants (n = 784) | Participants With Incident Alzheimer Dementia (n = 151) | P Value |
|---|---|---|---|---|
|
| ||||
| Age, y | <0.001 | |||
| Mean (SD) | 81.2 (7.5) | 80.4 (7.5) | 85.6 (5.8) | |
| Median (IQR) | 82.1 (76.3 to 86.6) | 81.2 (75.3 to 85.7) | 85.8 (82.1 to 89.8) | |
| Education, y | 0.111 | |||
| Mean (SD) | 15.4 (3.6) | 15.5 (3.0) | 15.0 (3.3) | |
| Median (IQR) | 16.0 (13.0 to 18.0) | 16.0 (13.0 to 18.0) | 15.0 (12.0 to 17.0) | |
| Women, % | 76.9 | 76.3 | 80.1 | 0.34 |
| Global cognition (Z score) | ||||
| Mean (SD) | 0.21 (0.52) | 0.31 (0.47) | −0.28 (0.47) | <0.001 |
| Median (IQR) | 0.24 (−0.14 to 0.59) | 0.33 (−0.001 to 0.64) | −0.28 (−0.56 to 0.02) | |
| Scam awareness † | ||||
| Mean (SD) | 2.8 (0.7) | 2.7 (0.7) | 3.1 (0.7) | <0.001 |
| Median (IQR) | 2.8 (2.2 to 3.2) | 2.8 (2.2 to 3.2) | 3.0 (2.6 to 3.6) | |
IQR = interquartile range.
Statistical significance is based on t tests or χ2 tests, as appropriate.
The average of the Likert ratings across all 5 items, with higher scores reflecting lower awareness.
Incidence rates for Alzheimer dementia and MCI were higher among participants with lower scam awareness (Table 2). Rates of incident Alzheimer dementia ranged from 11 cases per 1000 person-years for high scam awareness (10th percentile) to 42 cases per 1000 person-years for low awareness (90th percentile); those of MCI ranged from 38 cases per 1000 person-years (10th percentile) to 91 cases per 1000 person-years (90th percentile). Appendix Figure 1 (available at Annals.org) shows incident Alzheimer dementia and MCI by percentile of scam awareness score.
Table 2.
Incidence Rates of Alzheimer Dementia and MCI for Different Percentiles of Scam Awareness
| Scam Awareness* | Incidence Rate per 1000 Person-Years (95% CI) |
|---|---|
|
| |
| Alzheimer dementia | |
| 10th percentile | 11.02 (3.00–26.20) |
| 25th percentile | 33.43 (16.69–57.87) |
| 50th percentile | 28.02 (21.22–36.03) |
| 75th percentile | 40.11 (21.93–65.50) |
| 90th percentile | 42.17 (26.10–63.24) |
| MCI | |
| 10th percentile | 37.97 (19.62–64.31) |
| 25th percentile | 52.63 (28.02–87.44) |
| 50th percentile | 78.97 (65.25–94.34) |
| 75th percentile | 73.08 (44.00–111.77) |
| 90th percentile | 90.61 (60.21–129.05) |
MCI = mild cognitive impairment.
Higher scores indicate lower scam awareness, such that 10th percentile reflects high scam awareness and 90th percentile reflects low scam awareness.
Scam Awareness and Risk for Alzheimer Dementia
During a mean of 5.7 years (SD, 2.4) of observation (median, 6.0 years; range, 2 to 9 years), 151 persons developed incident Alzheimer dementia. These participants were older and had lower levels of education, lower global cognition, and lower scam awareness than those who did not develop dementia (Table 1). Scores on the measure of scam awareness were approximately normally distributed among those who did and did not develop Alzheimer dementia (Appendix Figure 2, available at Annals.org). In a proportional hazards model adjusted for age, sex, and education, a higher scam awareness score (indicating lower awareness) was associated with increased risk for Alzheimer dementia (HR, 1.56 [95% CI, 1.21 to 2.01]; P < 0.001) (Table 3), such that each additional unit (1.4 SD) was associated with about a 60% increase in risk for Alzheimer dementia. Figure 2 shows that a typical participant with a high score was about 2.2 times more likely to develop Alzheimer dementia than a participant with a low score. Figure 3 shows the association with Alzheimer dementia at additional percentiles of scam awareness. The log HR for Alzheimer dementia of a 1-unit increase was equivalent to approximately 4 additional years of age.
Table 3.
Associations of Scam Awareness With Incident Alzheimer Dementia and MCI*
| Model Term | Alzheimer Dementia (n = 935) |
MCI (n = 746) |
||
|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | |
|
| ||||
| Age, per year | 1.13 (1.09–1.16) | <0.001 | 1.10 (1.07–1.12) | <0.001 |
| Male sex | 0.79 (0.52–1.21) | 0.28 | 1.23 (0.90–1.68) | 0.20 |
| Education, peryear | 0.99 (0.94–1.06) | 0.94 | 1.01 (0.96–1.06) | 0.73 |
| Scam awareness, per unit† | 1.56 (1.21–2.01) | <0.001 | 1.47 (1.20–1.81) | <0.001 |
HR = hazard ratio; MCI = mild cognitive impairment.
Derived from proportional hazards models.
The average of the Likert ratings across all 5 items, with higher scores reflecting lower awareness.
Figure 2. Cumulative hazards of developing Alzheimer dementia or MCI for representative women with high versus low scam awareness scores, with 95% confidence bands.
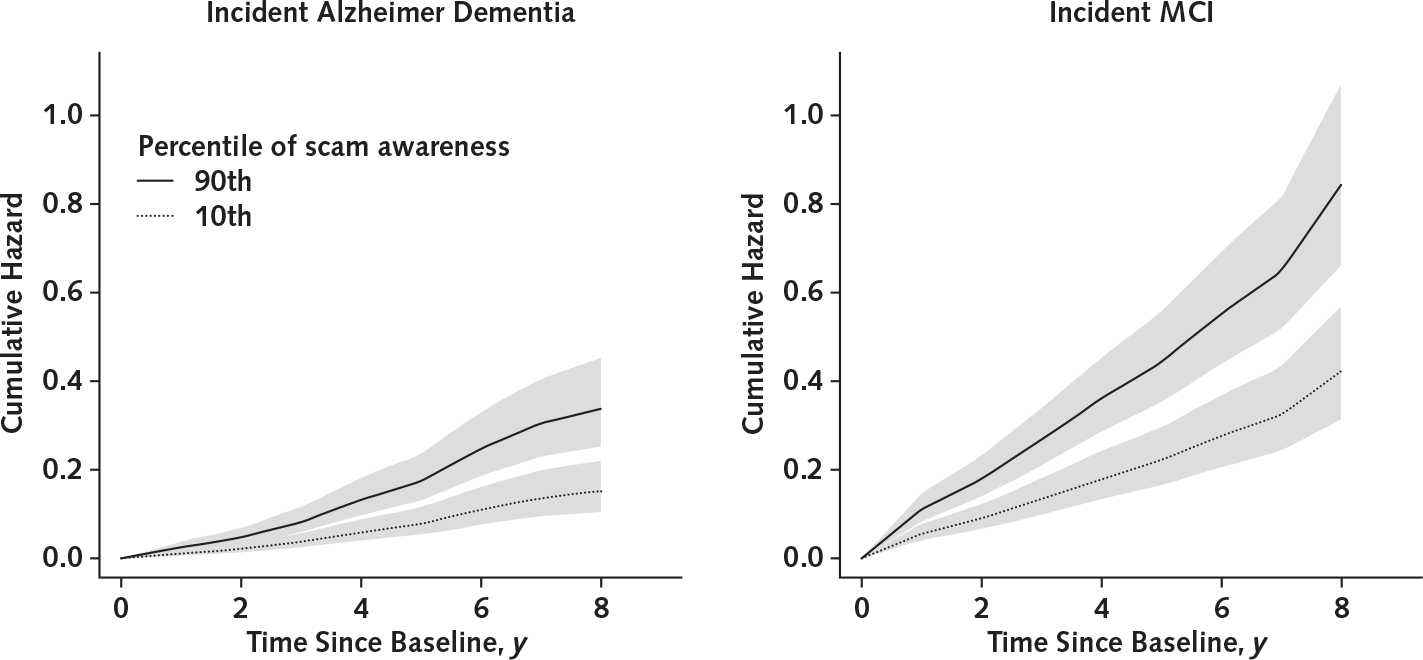
Representative women have mean age and mean years of education. High scores indicate low awareness (90th percentile), and low scores indicate high awareness (10th percentile). MCI = mild cognitive impairment.
Figure 3. Cumulative hazards of developing Alzheimer dementia or MCI for representative women, by percentile of scam awareness.
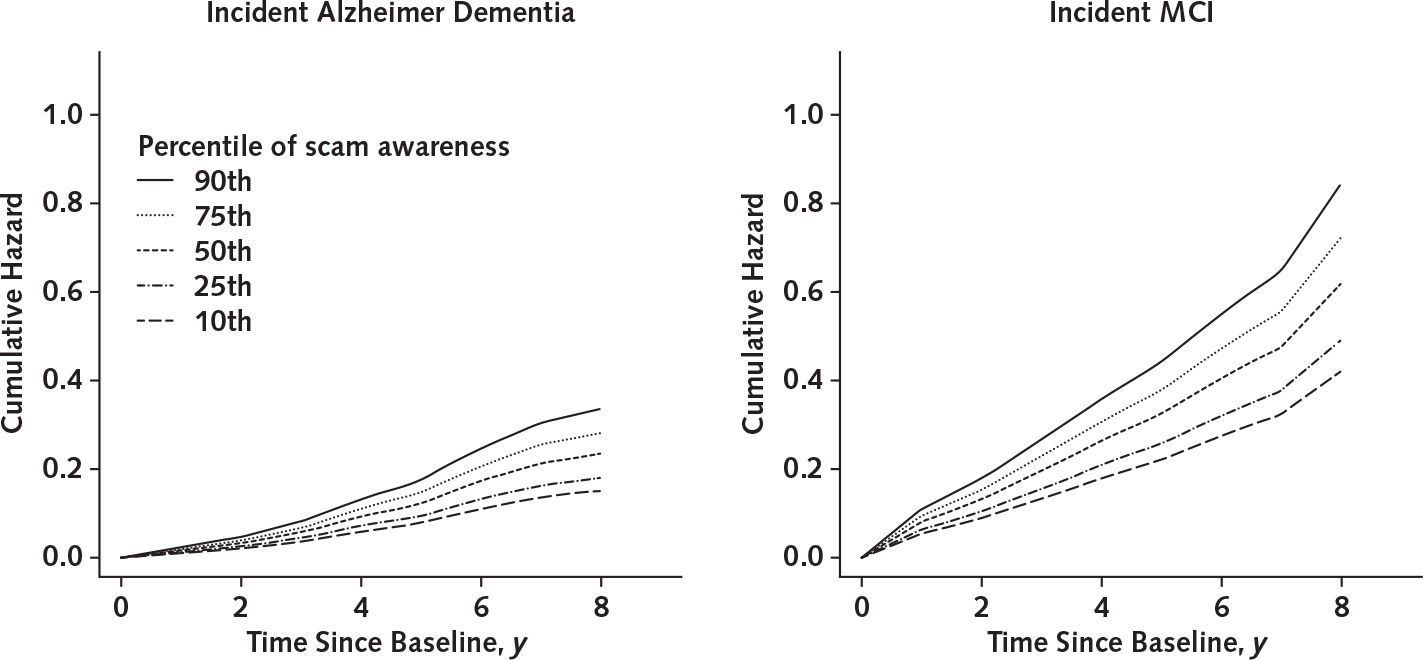
Representative women have mean age and mean years of education. MCI = mild cognitive impairment.
Scam Awareness and MCI
We next examined the association between scam awareness and risk for incident MCI. For these analyses, we excluded 189 participants who had MCI at baseline and repeated the primary proportional hazards model among the remaining 746 cognitively intact persons. Of these, the 255 (34.2%) who developed MCI were older and had lower cognition and lower scam awareness than those who did not. Scam awareness scores were approximately normally distributed among those who did and did not develop MCI (Appendix Figure 2).
In a proportional hazards model, a higher scam awareness score (indicating lower awareness) was associated with increased risk for MCI (HR, 1.47 [CI, 1.20 to 1.81]; P < 0.001) (Table 3), such that each additional unit was associated with about a 50% increase in risk for MCI. Figure 2 shows that a typical participant with a high score was about 2 times more likely to develop MCI than a participant with a low score (Figure 3).
Sensitivity Analysis
We did a series of sensitivity analyses. First, to determine whether the associations of scam awareness with Alzheimer dementia and MCI were independent of cognition, we repeated the primary proportional hazards models with an additional term to adjust for the starting level of global cognitive function. After this adjustment, associations persisted between scam awareness and Alzheimer dementia (HR, 1.37 [CI, 1.04 to 1.79]; P = 0.024) and MCI (HR, 1.29 [CI, 1.04 to 1.59]; P = 0.020). We also repeated the primary models after excluding persons in the top and bottom fifth percentiles of cognition, and the associations again persisted (Alzheimer dementia: HR, 1.48 [CI, 1.14 to 1.95]; P = 0.007; MCI: HR, 1.37 [CI, 1.10 to 1.71]; P = 0.005). These findings suggest that the observed associations were relatively independent of global cognition and were not driven by persons at the extremes of cognition.
Second, we repeated the primary proportional hazards models with an additional term for the interaction between scam awareness and baseline global cognition. The interaction terms were not significant (Alzheimer dementia: P for interaction = 0.72; MCI: P for interaction = 0.179).
Third, we constructed a mixed-effects model to simultaneously examine the associations between scam awareness and the starting level of and rate of change in global cognition. In this analysis, higher scores on the measure of scam awareness (indicating lower awareness) were associated with a lower level of global cognition (estimated decrease in mean baseline cognition per unit increase in scam awareness score [±SE], −0.09 ± 0.02; P < 0.001). With that association accounted for, higher scam awareness scores were also associated with a more rapid decline in global cognition (estimated additional rate of cognitive decline per unit increase in scam awareness score [±SE], 0.03 ± 0.01; P < 0.001). We then repeated this analysis among persons who were cognitively intact at baseline, and findings were essentially the same (decrease in mean baseline cognition [±SE], 0.08 ± 0.02 [P < 0.001]; additional rate of cognitive decline [±SE], 0.028 ± 0.01 [P < 0.001]).
Finally, because the associations of scam awareness with incident Alzheimer dementia and MCI may have been driven by persons at the extreme ends of age or responses to selected items, we repeated the primary proportional hazards models after excluding persons at the extremes of age and then examined each of the 5 scam awareness items separately. After the top and bottom fifth percentiles of age were excluded, associations persisted with Alzheimer dementia (HR, 1.68 [CI, 1.29 to 2.20]; P < 0.001) and MCI (HR, 1.41 [CI, 1.13 to 1.76]; P = 0.002). Further, responses to 3 of the 5 scam items were associated with incident Alzheimer dementia and MCI (Table 4).
Table 4.
Associations of individual Scam Items With Incident Alzheimer Dementia and MCI*
| Item† | Alzheimer Dementia (n = 935) |
MCI (n = 746) |
||
|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | |
|
| ||||
| 1 | 0.92 (0.83–1.02) | 0.80 | 0.99 (0.91–1.07) | 0.80 |
| 2 | 1.16 (1.03–1.30) | 0.015 | 1.17 (1.06–1.29) | 0.002 |
| 3 | 1.25 (1.09–1.43) | 0.001 | 1.28 (1.13–1.44) | <0.001 |
| 4 | 1.55 (1.31–1.83) | <0.001 | 1.14 (0.97–1.34) | 0.115 |
| 5 | 1.10 (0.99–1.22) | 0.057 | 1.09 (1.003–1.18) | 0.042 |
HR = hazard ratio; MCI = mild cognitive impairment.
Derived from proportional hazards models adjusted for age, sex, and education.
The Appendix Table describes the items.
Scam Awareness and AD Pathology
The associations of scam awareness with incident Alzheimer dementia and MCI suggest that deficits in scam awareness may be an early indicator of pathologic cognitive aging. We leveraged neuropathology data available in a subset of 264 deceased participants (mean age at death, 91.0 years [SD, 6.20]) and used linear regression models adjusted for age at death and education to estimate the relationship between scam awareness and 2 molecularly specific pathologic hallmarks of AD, β-amyloid load (square root percent area) and tau tangle density (square root per mm2). With each 1-unit increase in scam awareness score, β-amyloid (±SE) increased by 0.22 ± 0.10 unit (P = 0.029). The regression coefficient for tau tangles was similar but did not reach statistical significance (estimated increase [±SE], 0.20 ± 0.13; P = 0.113). β-Amyloid deposition is recognized as the first pathologic change in the continuum of AD and occurs before the accumulation of tangle pathology, and both β-amyloid and tau tangles follow region-specific patterns of accumulation (1). Thus, to examine whether scam awareness was related to the temporal sequence by which AD pathology accumulates, we did region-specific analyses separately for β-amyloid and tau tangles. Low scam awareness was related to increased β-amyloid throughout the brain, including in the neocortex (estimated increase [±SE], 0.25 ± 0.10; P = 0.010), entorhinal cortex (estimated increase [±SE], 0.28 ± 0.11; P = 0.014), and hippocampus (estimated increase [±SE], 0.22 ± 0.08; P = 0.010). It was associated with tangles in the entorhinal cortex, the earliest site of accumulation (estimated increase [±SE], 0.39 ± 0.16; P = 0.018). However, the associations between low scam awareness and tangles in the other regions did not reach statistical significance (estimated increase in hippocampus [±SE], 0.19 ± 0.20 [P = 0.36]; estimated increase in neocortex [±SE], 0.21 ± 0.12 [P = 0.090]).
Discussion
We found an association between low scam awareness and increased risk for incident Alzheimer dementia and MCI that was relatively independent of global cognitive function. Low scam awareness was also related to the pathologic hallmarks of AD, particularly β-amyloid deposition and tangles in their earliest site of accumulation. These findings provide compelling evidence that low scam awareness is an early harbinger of adverse cognitive outcomes and a manifestation of accumulating AD pathology in the brain.
The brains of older persons have accumulated a considerable burden of neuropathology by the time cognitive impairment is clinically manifest, which creates a challenge for both development and delivery of effective therapeutics (1, 2). There is a need to identify changes in behavior that occur before the onset of cognitive deficits detected by traditional measures. Emerging evidence suggests that dementia and MCI are associated with decrements in scam awareness and related aspects of decision making (4–8, 14, 15), but prior studies have not linked scam awareness with the subsequent development of adverse outcomes or examined the association between scam awareness and AD pathology.
The present findings suggest that low scam awareness is a harbinger of adverse cognitive outcomes among older persons without dementia and even those who are cognitively intact. That these associations were relatively independent of cognition suggests that reduced scam awareness occurs early in the course of age-related neurodegenerative disease. We conceptualize scam awareness as a component of decision making and a complex behavior that requires a variety of social cognitive abilities, including recognition that others may have intentions and desires that differ from one’s own, perception of the personality traits of others, and regulation of behavior even in highly pressurized situations (8, 18). The complexity of this behavior—particularly the need to integrate multiple abilities while managing a challenging social situation—may be critical to its sensitivity as an early harbinger of Alzheimer dementia and MCI.
The finding that scam awareness is related to β-amyloid (the first pathologic change in the AD continuum) and tangle pathology in its earliest site of accumulation provides novel neurobiological evidence that reduced scam awareness is an early manifestation of accumulating AD pathology (1). Of note, with few exceptions (for example, the main genetic risk factor for Alzheimer dementia, the apolipoprotein E4 allele), it is unusual for predictors of incident Alzheimer dementia and MCI to be associated with the pathologic hallmarks of AD (25, 27). Although other predictors of cognitive impairment, such as cognitive activity, purpose in life, and depressive symptoms, have been identified, most are not directly related to AD pathology (28–30). Further, because in vivo biomarkers for amyloid suggest that amyloid deposition predates overt cognitive impairment by many years and because amyloid is the major target of therapeutics under investigation, it is useful to identify changes in behavior that are associated with amyloid deposition (1, 27). The finding that decreased scam awareness is related to β-amyloid throughout the brain and tangles in the region in which they first accumulate provides strong support for the conclusion that decreased scam awareness in old age is in part a consequence of accumulating AD pathology and is an important early sign of adverse cognitive outcomes.
The measure used in this epidemiologic study is not suitable for prediction of cognitive impairment at an individual level. However, the findings suggest that new and appropriately validated measures of such complex behaviors as scam awareness may facilitate early identification of persons at risk for cognitive impairment, particularly when applied in conjunction with other behavioral or biological measures. That is, a single behavioral measure is unlikely to offer high sensitivity and specificity by itself, but such measures may increase the precision of a risk index. Efforts to develop and validate new measures of scam awareness and related behaviors may be of considerable clinical utility.
In addition to their relevance for Alzheimer dementia, we speculate that the present findings may have important implications for older persons’ vulnerability to financial fraud and other forms of exploitation (8, 10, 12, 13, 20). Older adults hold most of the nation’s household wealth, and recent estimates suggest that financial fraud alone results in losses exceeding $35 billion annually (31). Moreover, the public health costs of elder victimization far exceed the financial costs (32–36). Elder fraud is widely believed to be limited to persons with overt cognitive syndromes, but very recent data suggest that many persons who seem to be cognitively intact also fall prey to scams (8, 13, 37). Among our study participants, 76% reported that they answer the telephone whenever it rings even if they do not know who is calling, 24% reported that they listen to telemarketers, and 11% reported difficulty ending an unsolicited or unwanted communication with a telemarketer. We acknowledge that responses to the measure used here are not validated indicators of victimization, but such behaviors indicate a willingness to engage in behaviors associated with victimization (8–12, 19). Elder fraud is a major threat to the health and well-being of the aging population, and our findings suggest that all older persons—even those without MCI or dementia—could benefit from education on the tactics of fraudsters (20).
Data came from a large group of well-characterized, community-based, older persons followed prospectively; scam awareness was assessed using a measure previously shown to be associated with numerous aspects of health and well-being; the associations of scam awareness with Alzheimer dementia and MCI were relatively independent of global cognitive function; and autopsy data were unique. However, several limitations warrant mention, including the relatively short follow-up, largely white study population, and lack of validation of the scam awareness measure with actual victimization. Future studies are needed to establish the associations between such measures and victimization and to develop and validate measures of complex behaviors, such as scam awareness, for use in clinical settings.
Financial Support:
By grants R01AG33678, R01AG34374, and R01AG17917 from the National Institute on Aging (NIA) and by the Illinois Department of Public Health.
Appendix Table.
Items Used to Assess Scam Awareness and Descriptive Data on the Percentage of Respondents Who Responded Incorrectly to Each Item*
| Item | Respondents Who Answered Incorrectly (n = 935), % |
|---|---|
|
| |
| 1. I answer the telephone whenever it rings, even if I do not know who is calling. | 76 |
| 2. I have difficulty ending a telephone call, even if the caller is a telemarketer, someone I do not know, or someone I did not wish to call me. | 11 |
| 3. If something sounds too good to be true, it usually is. | 5 |
| 4. Persons older than 65 y are often targeted by con artists. | 5 |
| 5. When telemarketers call me, I usually listen to what they have to say. | 24 |
The total score used in analyses was the average of ratings of agreement or disagreement across all 5 statements; items 1, 2, and 5 were reverse-coded such that higher scores indicate lower awareness. However, responses to each item can be rated as correct or incorrect depending on a participant’s agreement or disagreement with each item. For example, for item 4, only responses indicating agreement are correct, because the fact that persons aged ≥65 y are frequently targeted by con artists is well established. For descriptive purposes, we scored responses to each item as correct or incorrect and calculated the percentage of persons who answered each item incorrectly. Of note, the percentages of persons who answered each item incorrectly were similar when calculated separately for those with mild cognitive impairment and those who were cognitively intact at baseline.
Appendix Figure 1. Kaplan–Meier curves for incident Alzheimer dementia (top) and MCI (bottom).
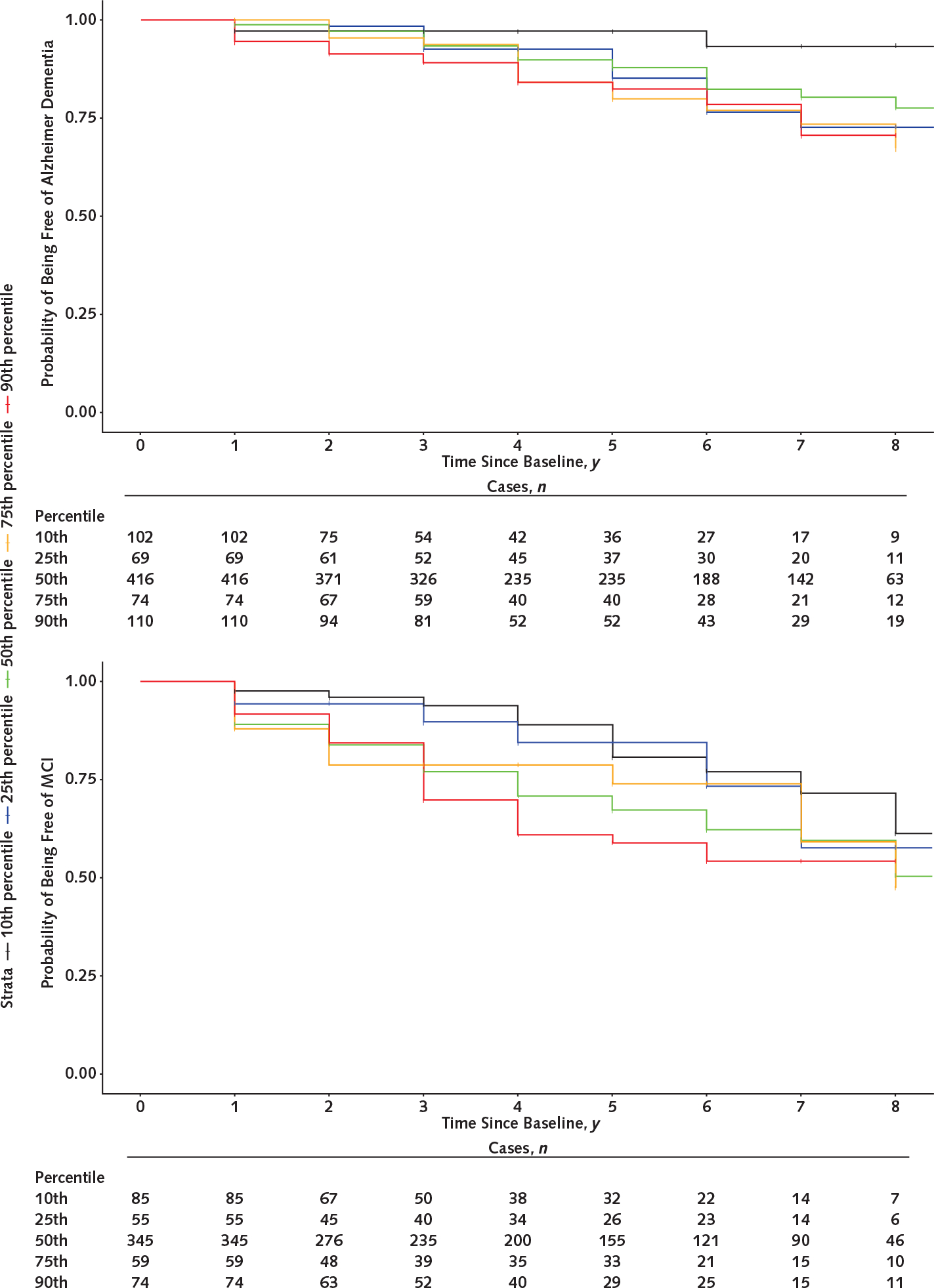
MCI = mild cognitive impairment.
Appendix Figure 2. Histograms showing the distribution of scores on the scam awareness measure among persons who did not and those who did develop Alzheimer dementia and MCI.
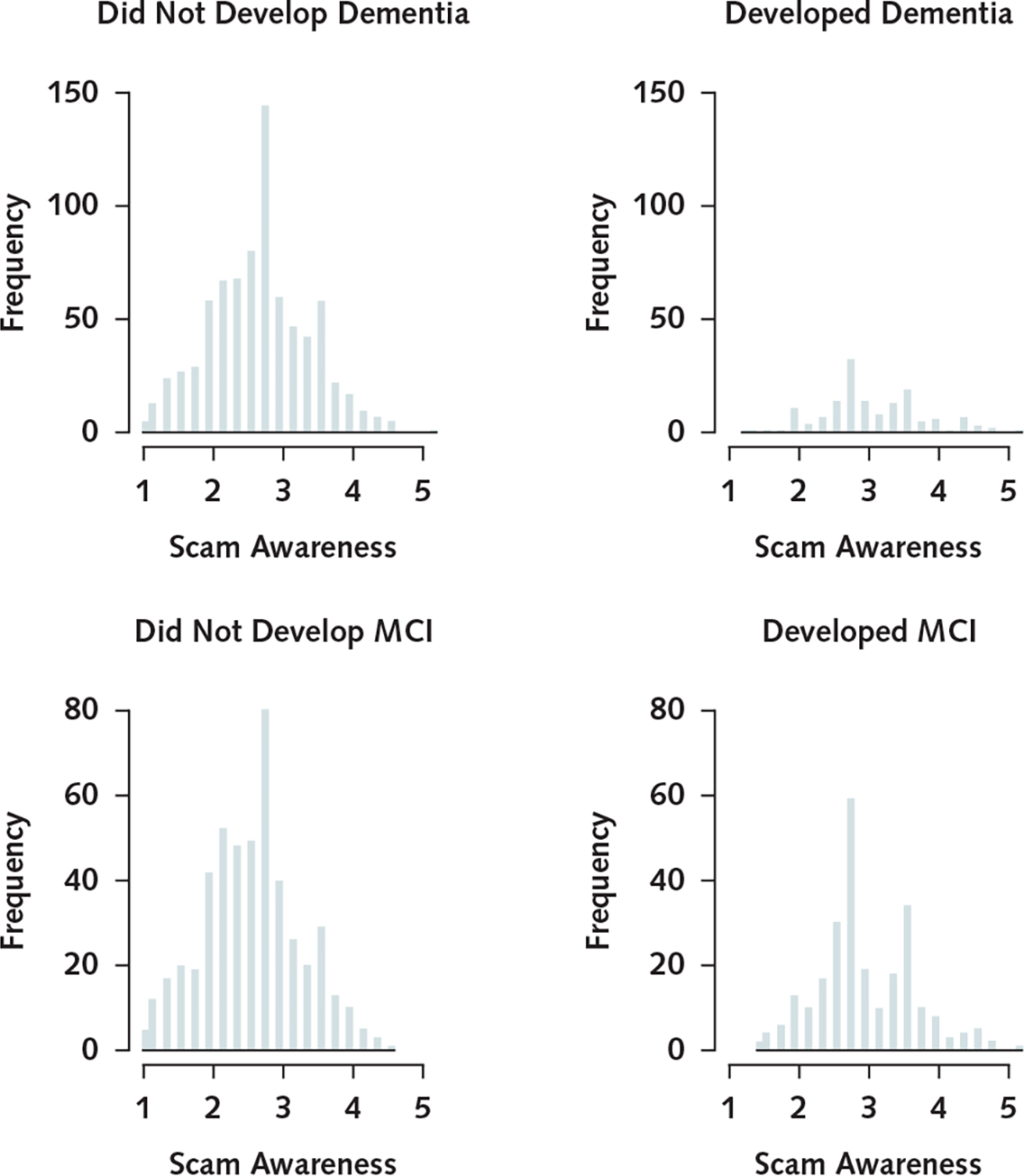
MCI = mild cognitive impairment.
Footnotes
Disclosures: Dr. Boyle reports grants from the NIA of the National Institutes of Health (NIH) and from the Illinois Department of Public Health during the conduct of the study. Dr. Yu reports grants from NIA during the conduct of the study. Dr. Schneider reports grants from NIA/NIH and from the Illinois Department of Public Health during the conduct of the study and personal fees from Grifols, Eli Lilly, Avid Radiopharmaceuticals, the National Hockey League, and the National Football League outside the submitted work. Dr. Wilson reports grants from NIA/NIH and from the Illinois Department of Public Health during the conduct of the study. Dr. Bennett reports grants from NIA/NIH and the state of Illinois during the conduct of the study. Authors not named here have disclosed no conflicts of interest. Disclosures can also be viewed at www.acponline.org/authors/icmje/ConflictOfInterestForms.do?msNum=M18-2711.
Disclaimer: Drs. Boyle and Yu had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Reproducible Research Statement: Study protocol: Available at www.radc.rush.edu. Statistical code and data set: Available by request at www.radc.rush.edu.
Current author addresses and author contributions are available at Annals.org.
References
- 1.Jack CR Jr, Bennett DA, Blennow K, Carrillo MC, Dunn B, Haeberlein SB, et al. ; Contributors. NIA-AA research framework: toward a biological definition of Alzheimer’s disease. Alzheimers Dement. 2018;14:535–62. doi: 10.1016/j.jalz.2018.02.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sperling RA, Aisen PS, Beckett LA, Bennett DA, Craft S, Fagan AM, et al. Toward defining the preclinical stages of Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:280–92. doi: 10.1016/j.jalz.2011.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Okonkwo OC, Griffith HR, Copeland JN, Belue K, Lanza S, Zamrini EY, et al. Medical decision-making capacity in mild cognitive impairment: a 3-year longitudinal study. Neurology. 2008;71:1474–80. doi: 10.1212/01.wnl.0000334301.32358.48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Triebel KL, Martin R, Griffith HR, Marceaux J, Okonkwo OC, Harrell L, et al. Declining financial capacity in mild cognitive impairment: a 1-year longitudinal study. Neurology. 2009;73:928–34. doi: 10.1212/WNL.0b013e3181b87971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Han SD, Boyle PA, James BD, Yu L, Bennett DA. Mild cognitive impairment is associated with poorer decision-making in community-based older persons. J Am Geriatr Soc. 2015;63:676–83. doi: 10.1111/jgs.13346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gerstenecker A, Triebel KL, Martin R, Snyder S, Marson DC. Both financial and cognitive decline predict clinical progression in MCI. Alzheimer Dis Assoc Disord. 2016;30:27–34. doi: 10.1097/WAD.0000000000000120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martin RC, Gerstenecker A, Triebel KL, Falola M, McPherson T, Cutter G, et al. Declining financial capacity in mild cognitive impairment: a six-year longitudinal study. Arch Clin Neuropsychol. 2019;34:152–61. doi: 10.1093/arclin/acy030 [DOI] [PubMed] [Google Scholar]
- 8.Spreng RN, Karlawish J, Marson DC. Cognitive, social, and neural determinants of diminished decision-making and financial exploitation risk in aging and dementia: a review and new model. J Elder Abuse Negl. 2016;28:320–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.American Association of Retired Persons. Telemarketing fraud and older Americans: an AARP study. Washington, DC: AARP; 1996. [Google Scholar]
- 10.FINRA Investor Education Foundation. Senior fraud risk survey. Washington, DC: FINRA Foundation; 2007. Accessed at www.saveandinvest.org/sites/default/files/FINRA-Foundation-Senior-Fraud-Risk-Survey-August-2007.pdf on 1 November 2018. [Google Scholar]
- 11.FINRA Investor Education Foundation. Financial Fraud and Fraud Susceptibility in the United States. Washington, DC: FINRA Foundation; 2013. Accessed at www.finrafoundation.org/sites/default/files/Financial-Fraud-And-Fraud-Susceptibility-In-The-United-States_0_0_0.pdf on 1 November 2018. [Google Scholar]
- 12.National Research Council (US) Panel to Review Risk and Prevalence of Elder Abuse and Neglect; Bonnie RJ, Wallace RB, eds. Elder Mistreatment: Abuse, Neglect and Exploitation in an Aging America. Washington, DC: National Academies Pr; 2003. [PubMed] [Google Scholar]
- 13.Burnes D, Henderson CR Jr, Sheppard C, Zhao R, Pillemer K, Lachs MS. Prevalence of financial fraud and scams among older adults in the United States: a systematic review and meta-analysis. Am J Public Health. 2017;107:e13–21. doi: 10.2105/AJPH.2017.303821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Han SD, Boyle PA, James BD, Yu L, Bennett DA. Mild cognitive impairment and susceptibility to scams in old age. J Alzheimers Dis. 2016;49:845–51. doi: 10.3233/JAD-150442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boyle PA, Yu L, Wilson RS, Gamble K, Buchman AS, Bennett DA. Poor decision making is a consequence of cognitive decline among older persons without Alzheimer’s disease or mild cognitive impairment. PLoS One. 2012;7:e43647. doi: 10.1371/journal.pone.0043647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bennett DA, Buchman AS, Boyle PA, Barnes LL, Wilson RS, Schneider JA. Religious Orders Study and Rush Memory and Aging Project. J Alzheimers Dis. 2018;64:S161–89. doi: 10.3233/JAD-179939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Duke Han S, Boyle PA, Yu L, Arfanakis K, James BD, Fleischman DA, et al. Grey matter correlates of susceptibility to scams in community-dwelling older adults. Brain Imaging Behav. 2016;10:524–32. doi: 10.1007/s11682-015-9422-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.James BD, Boyle PA, Bennett DA. Correlates of susceptibility to scams in older adults without dementia. J Elder Abuse Negl. 2014;26:107–22. doi: 10.1080/08946566.2013.821809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Financial Industry Regulatory Authority. Risk Meter. 2019. Accessed at https://tools.finra.org/risk_meter on 11 March 2019.
- 20.Centers for Disease Control and Prevention. Elder abuse prevention. Updated 5 June 2017. Accessed at www.cdc.gov/features/elderabuse/index.html on 1 June 2018.
- 21.Bennett DA, Schneider JA, Aggarwal NT, Arvanitakis Z, Shah RC, Kelly JF, et al. Decision rules guiding the clinical diagnosis of Alzheimer’s disease in two community-based cohort studies compared to standard practice in a clinic-based cohort study. Neuroepidemiology. 2006;27:169–76. [DOI] [PubMed] [Google Scholar]
- 22.Bennett DA, Wilson RS, Schneider JA, Evans DA, Beckett LA, Aggarwal NT, et al. Natural history of mild cognitive impairment in older persons. Neurology. 2002;59:198–205. [DOI] [PubMed] [Google Scholar]
- 23.Wilson RS, Beckett LA, Barnes LL, Schneider JA, Bach J, Evans DA, et al. Individual differences in rates of change in cognitive abilities of older persons. Psychol Aging. 2002;17:179–93. [PubMed] [Google Scholar]
- 24.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34:939–44. [DOI] [PubMed] [Google Scholar]
- 25.Farfel JM, Yu L, De Jager PL, Schneider JA, Bennett DA. Association of APOE with tau-tangle pathology with and without β-amyloid. Neurobiol Aging. 2016;37:19–25. doi: 10.1016/j.neurobiolaging.2015.09.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boyle PA, Yang J, Yu L, Leurgans SE, Capuano AW, Schneider JA, et al. Varied effects of age-related neuropathologies on the trajectory of late life cognitive decline. Brain. 2017;140:804–12. doi: 10.1093/brain/aww341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Villemagne VL, Pike KE, Chételat G, Ellis KA, Mulligan RS, Bourgeat P, et al. Longitudinal assessment of A and cognition in aging and Alzheimer disease. Ann Neurol. 2011;69:181–92. doi: 10.1002/ana.22248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wilson RS, Boyle PA, Yu L, Barnes LL, Schneider JA, Bennett DA. Life-span cognitive activity, neuropathologic burden, and cognitive aging. Neurology. 2013;81:314–21. doi: 10.1212/WNL.0b013e31829c5e8a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Boyle PA, Buchman AS, Wilson RS, Yu L, Schneider JA, Bennett DA. Effect of purpose in life on the relation between Alzheimer disease pathologic changes on cognitive function in advanced age. Arch Gen Psychiatry. 2012;69:499–505. doi: 10.1001/archgenpsychiatry.2011.1487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wilson RS, Boyle PA, Capuano AW, Shah RC, Hoganson GM, Nag S, et al. Late-life depression is not associated with dementia-related pathology. Neuropsychology. 2016;30:135–42. doi: 10.1037/neu0000223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.True Link Financial. Elder financial abuse. In: Pooled Special Needs Trust Best Practices. San Francisco, CA: True Link Financial; 2015. [Google Scholar]
- 32.Acierno R, Hernandez MA, Amstadter AB, Resnick HS, Steve K, Muzzy W, et al. Prevalence and correlates of emotional, physical, sexual, and financial abuse and potential neglect in the United States: the National Elder Mistreatment Study. Am J Public Health. 2010;100:292–7. doi: 10.2105/AJPH.2009.163089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dong X Medical implications of elder abuse and neglect. Clin Geriatr Med. 2005;21:293–313. [DOI] [PubMed] [Google Scholar]
- 34.Burnett J, Jackson SL, Sinha AK, Aschenbrenner AR, Murphy KP, Xia R, et al. Five-year all-cause mortality rates across five categories of substantiated elder abuse occurring in the community. J Elder Abuse Negl. 2016;28:59–75. doi: 10.1080/08946566.2016.1142920 [DOI] [PubMed] [Google Scholar]
- 35.Dong X, Simon M, Mendes de Leon C, Fulmer T, Beck T, Hebert L, et al. Elder self-neglect and abuse and mortality risk in a community-dwelling population. JAMA. 2009;302:517–26. doi: 10.1001/jama.2009.1109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lachs MS, Williams CS, O’Brien S, Pillemer KA, Charlson ME. The mortality of elder mistreatment. JAMA. 1998;280:428–32. [DOI] [PubMed] [Google Scholar]
- 37.Cooper C, Selwood A, Blanchard M, Walker Z, Blizard R, Livingston G. Abuse of people with dementia by family carers: representative cross sectional survey. BMJ. 2009;338:b155. doi: 10.1136/bmj.b155 [DOI] [PMC free article] [PubMed] [Google Scholar]


