Abstract
Outer membrane protein E (OMP E) is a 50-kDa protein of Moraxella catarrhalis which possesses several characteristics indicating that the protein will be an effective vaccine antigen. To study the antigenic structure of OMP E, eight monoclonal antibodies were developed and characterized. Three of the antibodies recognized epitopes which are present on the bacterial surface. Fusion peptides corresponding to overlapping regions of OMP E were constructed, and immunoblot assays were performed to localize the areas of the molecule bound by the monoclonal antibodies. These studies identified a surface-exposed epitope in the region of amino acids 80 through 180. To further study the protein, two mutants which lack OMP E were constructed. In bactericidal assays, the mutants were more readily killed by normal human serum compared to the isogenic parent strains. These results indicate that OMP E is involved in the expression of serum resistance of M. catarrhalis.
Moraxella catarrhalis is an important human respiratory tract pathogen (6, 8, 9, 21, 22). It is the third most common cause of otitis media, accounting for 15 to 20% of all episodes based on cultures of middle ear fluid obtained by tympanocentesis (13, 29). M. catarrhalis also causes lower respiratory tract infections, often called exacerbations, in adults with chronic obstructive pulmonary disease (COPD) (23, 25). It is difficult to state precisely the etiology of exacerbations in individual patients; however, one study estimated that approximately 30% are caused by M. catarrhalis (33). Nosocomial outbreaks of respiratory tract infections caused by M. catarrhalis have been recognized since the mid-1980s (18, 20, 26–28). Many of these outbreaks of infections have occurred in respiratory units where the presence of a susceptible population with underlying lung disease contributed to the clusters.
In view of the importance of M. catarrhalis as a human pathogen, there is interest in developing a vaccine to prevent these infections. Two populations would benefit most from such a vaccine. Infants would be immunized in an effort to prevent otitis media, with particular emphasis on preventing recurrent otitis media in otitis-prone children. The second population that would benefit from such a vaccine is adults with COPD.
Outer membrane protein E (OMP E) is a 50-kDa heat-modifiable outer membrane protein (OMP) which has characteristics that indicate that it may be an effective vaccine antigen (2, 3). The protein is abundantly expressed on the bacterial surface as demonstrated by immunofluorescence assays and flow cytometry with monoclonal antibodies (MAbs) (3). OMP E is highly conserved among strains of M. catarrhalis (2, 3). These two features of OMP E suggest that inducing an immune response to the protein may result in protection from infection.
The present study was undertaken to further characterize the antigenic structure of OMP E. MAbs were developed and characterized. The regions of the OMP E molecule bound by the MAbs were identified, and two mutants which are defective in expression of OMP E were constructed and characterized.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
M. catarrhalis strain 25240 is from the American Type Culture Collection. Strain 7169 was recovered by tympanocentesis from the middle ear fluid of a child with otitis media and was kindly provided by Howard Faden (Children's Hospital, Buffalo, N.Y.). M. catarrhalis strains were grown on brain heart infusion (BHI) plates or in BHI broth, unless otherwise noted.
SDS-PAGE and immunoblot assay.
Whole-bacterial-cell lysates, purified outer membrane preparations, purified recombinant OMP E, and fusion peptides were subjected to sodium dodecyl sulfate–12% polyacrylamide gel electrophoresis (SDS-PAGE) and immunoblot assay as previously described (3) but with the following modifications. Immunoblots were blocked with 3% nonfat dry milk for 1 h at room temperature. After 3 washes in buffer A (0.01 M Tris–0.15 M NaCl [pH 7.4]), immunoblots were incubated with tissue culture supernatants of MAbs overnight at room temperature. After three washes, blots were incubated with goat anti-mouse immunoglobulin G (IgG)-immunoglobulin M conjugated to peroxidase (Boehringer Mannheim, Indianapolis, Ind.) for 1 h at room temperature. The blots were then washed, and bands were visualized with H2O2 and HRP color developer (Bio-Rad Laboratories, Richmond, Calif.).
Purification of recombinant OMP E.
Recombinant OMP E was expressed in plasmid pESA, which was derived from pRSET (Invitrogen, San Diego, Calif.) and expresses the full-length mature OMP E protein with six histidines on the amino terminus (3). The protein was purified by elution from Talon resin (Clontech, Palo Alto, Calif.) as described previously (3).
Cloning of ompe fragments into pGEX4T.
Peptides corresponding to selected regions of the gene which encodes OMP E were expressed as fusion proteins by using the pGEX4T plasmid expression system (24, 31). Oligonucleotides were designed with BamHI and EcoRI sites so that DNA fragments amplified by PCR were cloned directionally into pGEX4T which had been cut with BamHI and EcoRI. PCR products corresponding to specific regions of ompe were ligated into pGEX4T with T4 DNA ligase (Ligation Express; Clontech). Plasmids were electroporated into Escherichia coli HB101 and plated. Clones selected for further study were confirmed by sequencing the entire insert.
Purification of GST fusion proteins.
Fusion proteins with glutathione-S-transferase (GST) were purified by elution from glutathione-Sepharose essentially as previously described (10).
Development of MAbs.
The eight MAbs were developed from two different fusions. MAbs 1B3 and 9G10d were described previously (3). The remaining six MAbs were developed in BALB/c mice using the following schedule of immunization: days 0 and 14, 50 μg of purified recombinant OMP E emulsified with incomplete Freund's adjuvant administered subcutaneously; day 28, ∼108 CFU of M. catarrhalis 25240 suspended in phosphate-buffered saline administered intraperitoneally. On day 31, the mice were euthanized, and the spleens were removed and perfused to collect splenocytes.
To perform the fusion, splenocytes were fused with SP2/0-Ag-14 plasmacytoma cells by a modification of the procedure of Kennett (12) and our own previously described methods (30). After clones producing antibodies were identified, the hybridomas were cloned by limiting dilution, and the isotypes were determined by using the mouse MonoAB ID kit (Zymed, San Francisco, Calif.). MAbs were purified by elution from protein G.
Flow cytometry.
Flow cytometry was performed by using a Becton Dickinson FACScan to determine whether antibodies recognized epitopes which were present on the intact bacterial cell as previously described (3).
Construction of mutants.
A PstI site was engineered into ompe in pESA with one-step overlap extension PCR as follows (15, 32). Primers were designed to amplify the 5′ 423 bp of ompe with a BamHI site at the 5′ end and a PstI site at the 3′ end (Table 1). A second set of primers was designed to amplify the remaining 3′ end of ompe with a PstI site at the 5′ end and an EcoRI site at the 3′ end. A one-step overlap extension PCR that used the two PCR products above as template and primers corresponding to the 5′ and 3′ ends of ompe was performed with VentI DNA polymerase. The resulting 1.38-kb PCR product was ligated back into pESA to yield a plasmid with ompe that had a PstI site. The aphA-3 nonpolar kanamycin cassette from pUC18K (19) was amplified by PCR using primers with PstI sites (Table 1) and ligated into the PstI site in ompe in the above described plasmid. The resultant kanamycin-resistant plasmid pESA::kan was prepared for electroporation.
TABLE 1.
Oligonucleotide primers used in construction of mutants
| Primer | Strand | Sequencea |
|---|---|---|
| ESA1-5′Bam | Gene | GCGCGCGGATCCGCAGGCCTGGATCGCTC |
| ESA425-3′Eco | Opposite | ATATATGAATTCCAAGGCATAGCCATCATTAC |
| 3′Pst-ESA | Opposite | GGCTTGTGCCTGCAGTGAATTGGTTTC |
| 5′Pst-ESA | Gene | ACCAATTCACTGCAGGCACAAGCCAAAC |
| 5′Pst-kan | Gene | ATATATCTGCAGTGACTAACTAGGAGGAATAAATG |
| 3′Pst-kan | Opposite | GCGCGCCTGCAGTCATTATTCCCTCCAGGTACT |
Restriction enzymes sites are underlined.
M. catarrhalis strains 25240 and 7169 were subjected to electroporation by using the method of Helminen et al. (11) and Luke et al. (17). The strains were electroporated with 1 μg of whole pESA::kan, linearized pESA::kan cut with ScaI, and the purified insert in separate electroporation experiments. Bacteria were plated on BHI plates containing 20 μg of kanamycin per ml. Colonies were picked and characterized as described in Results.
Southern blot assays.
DNA was transferred from agarose gels by vacuum transfer (Hoefer Trans-Vac TE 80) to Hybond-N+ membrane (Amersham). The blots were probed with oligonucleotides labeled by random priming using the Phototope Kit (New England Biolabs). Blots were developed using the Phototope Star detection kit (New England Biolabs).
Pulsed-field gel electrophoresis.
Strains were subjected to pulsed-field gel electrophoresis as described previously (14). Genomic DNA was cut individually with both SpeI and NheI.
Bactericidal assays.
Bactericidal assays were performed as described by Chen et al. (7). The complement source was normal human serum which was adsorbed with protein G to remove IgG. A pool of serum from healthy adults was heat inactivated by heating in a 56°C water bath for 30 min. Bacteria were grown on Mueller-Hinton plates for bactericidal assays.
RESULTS
Characterization of MAbs.
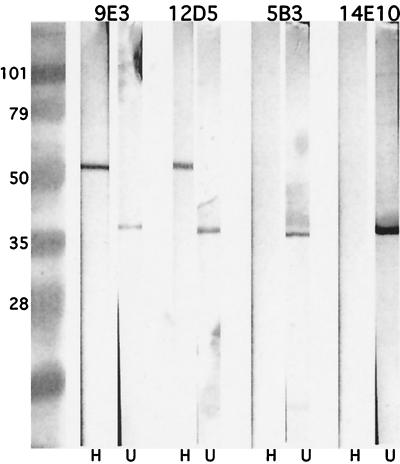
Eight MAbs to OMP E were developed (Table 2). Two have been reported previously (3). To assess the antigenic specificity of the MAbs, they were assayed in an immunoblot assay with whole-cell lysates, outer-membrane preparations, and purified, recombinant OMP E. All eight recognized OMP E. Figure 1 shows that MAbs 5B3 and 14E10 preferentially recognize the non-heat-modified form of OMP E (∼35 kDa), whereas the other six MAbs recognized both the heat-modified (∼50-kDa) and non-heat-modified forms of OMP E (some data not shown).
TABLE 2.
Characteristics of MAbs to OMP E of M. catarrhalis
| MAb | Isotype and subclass | Surface exposurea |
|---|---|---|
| 1B3b | IgG1 | Yes |
| 9G10db | IgG1 | Yes |
| 5B4 | IgG1 | Yes |
| 4C11 | IgG1 | No |
| 12D5 | IgG1 | No |
| 9E3 | IgM | No |
| 5B3 | IgG2a | No |
| 14E10 | IgG2a | No |
Surface exposure determined by flow cytometry.
Previously described (3).
FIG. 1.
Immunoblot assay. All lanes contain a whole bacterial cell lysate of M. catarrhalis 25240. Lanes H, lysate incubated at 100°C in sample buffer containing β-mercaptoethanol; lanes U, lysate incubated at room temperature in sample buffer in the absence of β-mercaptoethanol. MAbs are noted at the top and molecular mass markers are noted in kilodaltons on the left.
Flow cytometry was performed to determine whether the MAbs recognized epitopes which were present on the surface of the intact bacterium. MAbs 1B3, 9G10d, and 5B4 were reactive in flow cytometry, indicating that they recognize surface epitopes. The remaining 5 MAbs were nonreactive in flow cytometry, indicating that they recognized epitopes which are buried in the outer membrane and not available on the bacterial surface (Table 2). The three MAbs which recognized surface-exposed epitopes (all subclass IgG1) had no bactericidal activity when tested in bactericidal assays.
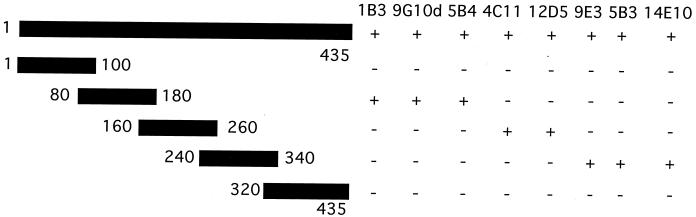
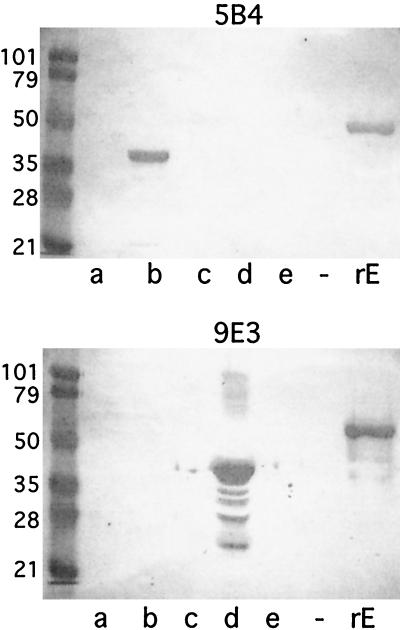
To begin to elucidate the regions of the OMP E molecule which are recognized by the MAbs, overlapping fusion peptides corresponding to regions of OMP E were constructed as depicted in Fig. 2. The GST fusion peptides were purified and subjected to immunoblot assay. Figure 3 shows that MAb 5B4 recognized an epitope in amino acids 80 through 180 of OMP E and MAb 9E3 recognized an epitope in amino acids 240 through 340. Figure 2 summarizes the results of immunoblot assays of the overlapping fusion peptides with all eight MAbs. The observation that MAbs 1B3, 9G10d, and 5B4 were reactive in flow cytometry (Table 2) along with the results of immunoblot assays with the fusion peptides which span the OMP E molecule (Fig. 2) allowed us to conclude that a surface-exposed epitope is present in the region of amino acids 80 through 180.
FIG. 2.
Schematic diagram depicting five fusion protein constructs of OMP E. Numbers correspond to amino acids in the mature protein in each construct. The reactivity of eight MAbs to OMP E with each fusion protein in immunoblot assays is noted on the right.
FIG. 3.
Immunoblot assays with MAbs 5B4 and 9E3. Lanes a through e contain GST fusion proteins corresponding to amino acids (aa) of OMP E as follows: a, aa 1 through 100; b, 80 through 180; c, 160 through 260; d, 240 through 340; e, 320 through 435. Lane −, empty; lane rE, recombinant OMP E. Molecular mass standards are noted in kilodaltons on the left. Lane d in the lower panel contains some proteolytic degradation of the fusion protein.
Construction and characterization of mutants.
Electroporation of M. catarrhalis 25240 with whole pESA::kan, which contains ompe with a kanamycin cassette inserted in the central portion of the gene, yielded 30 to 40 colonies, whereas electroporation of 25240 with a linearized plasmid and an isolated insert yielded no colonies. By contrast, strain 7169 yielded colonies following electroporation with all three forms of DNA including whole plasmid, linearized plasmid, and isolated insert. Two clones were picked and characterized further. Strain 25240E− was from an electroporation with whole pESA::kan, and 7169E− was from an electroporation with the isolated insert of pESA::kan.
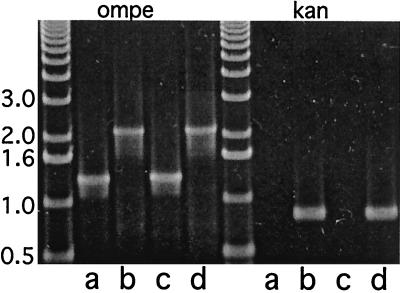
To confirm the insertion of the kanamycin cassette into ompe, genomic DNA from the mutants and the isogenic parent strains was subjected to PCR analysis. PCR amplification of ompe was performed with primers corresponding to the 5′ and 3′ ends of the gene. Figure 4 shows that the parent strains yielded amplicons of 1,299 bp, which corresponds to the predicted size of ompe plus restriction sites in the primers. The mutant strains yielded amplicons of 2,170 bp, which corresponds to the sum of ompe and the kanamycin cassette. PCR amplification with primers corresponding to the kanamycin cassette resulted in PCR products of 871 bp, the predicted size of the kanamycin cassette plus restriction sites in the primers and in the mutants and no PCR product in the parent strains (Fig. 4).
FIG. 4.
Agarose gel stained with ethidium bromide. PCRs were performed using primers to amplify ompe and the kanamycin cassette (Kan) as noted at the top of the gel. Templates for PCRs were genomic DNA from M. catarrhalis strains as follows: lanes a, 7169; b, 7169E−; c, 25240; d, 25240E−. Molecular size markers are noted in kilobases at the left.
To further confirm the insertion of the kanamycin cassette into ompe, genomic DNA was isolated from the mutant and parent strains, cut with NdeI, and subjected to agarose gel electrophoresis and Southern blot assay. Probing the blot with an oligonucleotide corresponding to ompe yielded a band of ∼5 kb in the parent strains and a band of ∼5.8 kb in the mutants (data not shown). Probing separately with an oligonucleotide corresponding to the kanamycin cassette revealed a band of ∼5.8 kb in the mutants and no band in the parent strains. These results indicated that the kanamycin cassette is inserted into ompe.
The nucleotide sequences of the entire PCR-amplified ompe with kanamycin cassettes from the mutants were determined. The sequence of ompe from strain 7169 was identical to ompe from strain 25240 (GenBank accession number L31788). In addition, the downstream sequences were in frame with the kanamycin cassette, confirming that these mutations were nonpolar.
To further confirm that the mutants are isogenic, strains 25240E−, 7169E−, and their respective parent strains 25240 and 7169 were subjected to pulsed-field gel electrophoresis. Digestion of DNA individually with SpeI and NheI produced banding patterns which were identical in corresponding mutant and parent strains (data not shown).
Expression of OMP E in mutant and parent strains.
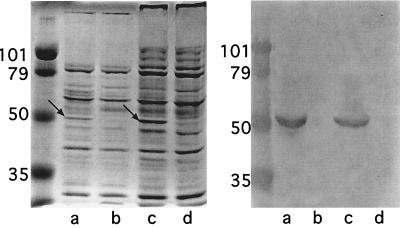
To assess the expression of OMP E in the mutant and parent strains, whole-cell lysates and outer membrane preparations were subjected to SDS-PAGE and immunoblot assays. Figure 5 shows that the OMP E band in outer-membrane preparations was absent in the mutants, but the banding patterns were otherwise identical with those of the respective parent strains. Immunoblot assays with MAbs 1B3 (Fig. 5) and 9E3 (data not shown) confirmed the absence of expression of OMP E in the mutants. MAb 1B3 recognized an epitope corresponding to a region upstream of the kanamycin cassette while MAb 9E3 recognized an epitope corresponding to a region downstream of the kanamycin cassette.
FIG. 5.
Left panel: Coomassie blue-stained sodium dodecyl sulfate gel of outer membranes. Lanes: a, 7169; b, 7169E−; c, 25240; d, 25240E−. Arrows denote OMP E. Right panel: immunoblot assay with MAb 1B3. Lanes a through d as per left panel. Molecular mass markers are noted in kilodaltons.
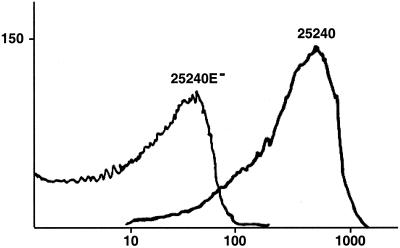
The mutant strains and their respective parent strains were subjected to flow cytometry with MAb 1B3, which recognizes an epitope on the surface of the intact bacterial cell (3). Figure 6 shows that MAb 1B3 recognized an abundantly expressed epitope on the bacterial surface of the parent strain 25240. The mutant strain 25240E− did not fluoresce in flow cytometry, confirming the absence of expression of this epitope on the bacterial surface. Strains 7169 and 7169E− gave identical results in that the parent strain was strongly positive while the mutant was negative in flow cytometry.
FIG. 6.
Results of flow cytometry with MAb 1B3 and strains 25240 and 25240E− as noted. x axis, fluorescence in arbitrary units; y axis, number of cells.
Growth characteristics of mutants.
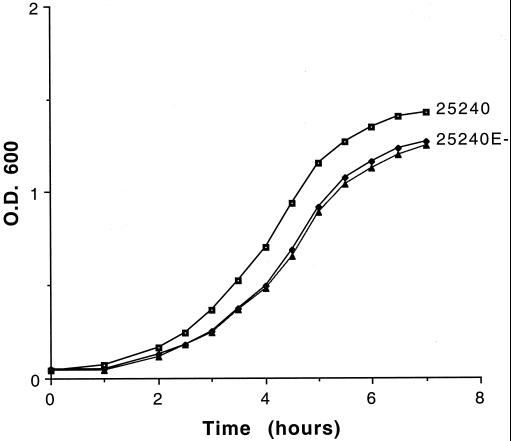
Mutants and parent strains had identical microscopic appearances when subjected to Gram stain. The rate of growth of the OMP E mutants in BHI broth was monitored in parallel with the respective parent strains. Figure 7 shows that the mutant 25240E− grew slightly more slowly in BHI broth compared to the parent strain. When growth was continued overnight, the optical density of the stationary-phase culture of the mutant remained lower than that of the parent strain. The growth rate of the mutant 7169E− was lower than that of its parent strain, but the difference was less pronounced than that of 25240E− and 25240.
FIG. 7.
Growth curves in BHI broth. x axis, time in hours; y axis, optical density (O.D.) at a wavelength of 600 nm. The two curves marked “25240E-” represent the mutant grown individually in BHI with kanamycin (▴) and BHI broth alone (⧫).
The growth rate of the mutant 25240E− was identical in the presence and absence of kanamycin (Fig. 7). Colony counts were performed by plating aliquots of broth cultures of the mutants grown in the absence of kanamycin on both BHI plus kanamycin plates and BHI plates. The colony counts were identical, indicating that individual bacterial cells of the mutant retained expression of kanamycin resistance while growing in broth in the absence of kanamycin.
Serum resistance.
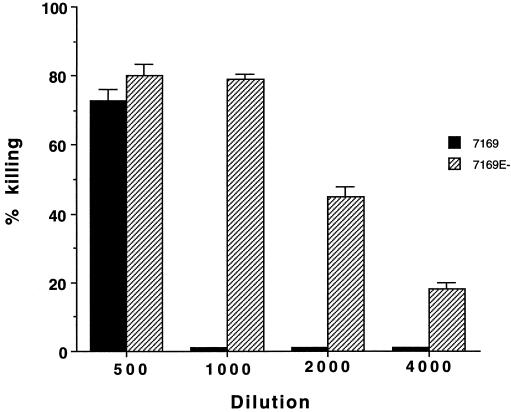
To assess the sensitivity of the mutants to complement-mediated killing by human serum, the mutants and their respective parent strains were tested in bactericidal assays with various dilutions of pooled normal human serum. Figure 8 shows that mutant 7169E− was more readily killed by normal human serum at essentially all dilutions tested compared with its isogenic parent strain 7169. The assay was repeated three times, and the results of all three were similar in showing that 7169E− was more sensitive to killing by normal human serum. M. catarrhalis 25240E− was more serum sensitive than its parent strain 25240; however, the difference was less striking.
FIG. 8.
Results of bactericidal assays. x axis, dilution of normal human serum; y axis, percent killing at 60 min. Error bars represent results of duplicate plates from colony counts.
DISCUSSION
In this report, to begin to define the antigenic structure of OMP E, MAbs were developed, and the regions on OMP E to which the antibodies bound were identified using immunoassays with fusion peptides constructed to correspond to regions of the protein. Several different epitopes were represented by the eight MAbs (Fig. 2). Three of the antibodies (1B3, 9G10d, and 5B4) bound to a surface-exposed epitope which is located between amino acids 80 and 180. MAbs 5B3 and 14E10 bound to epitopes which were denatured by reduction because they recognized OMP E exclusively in its nonreduced form.
In previous work, proteinase K treatment of intact M. catarrhalis cells resulted in the cleavage of the amino-terminal 184 amino acids of OMP E and loss of reactivity by MAbs 1B3 and 9G10d (3). These data led to the conclusion that the epitope recognized by MAbs 1B3 and 9G10d was located in the 184 amino acids at the amino terminus of OMP E. However, this conclusion was based on indirect evidence, i.e., loss of reactivity with the MAbs. Construction of fusion peptides and analysis of these constructs with the MAbs in the present study confirm this finding with direct evidence and extend the observation by further localizing this surface-exposed epitope. The identification of a surface epitope on OMP E will facilitate more detailed studies of the human antibody response to OMP E. For example, it will be important to determine what proportions of antibodies in human samples are directed at surface epitopes compared to epitopes buried in the outer membrane, since antibodies which mediate protection from infection are likely to be directed to surface-exposed epitopes.
Mutants deficient in the expression of OMP E were constructed and characterized. Several approaches were used to ensure that these mutants were isogenic. A nonpolar kanamycin cassette was used, and sequence analysis confirmed that the cassette is in frame with downstream sequences. Pulsed-field gel electrophoresis, PCR analysis, Southern blot analysis, and SDS-PAGE of outer membrane preparations all support the conclusion that the mutants differ only in expression of OMP E. These mutants will be important tools in a variety of experiments, such as controls in immunoassays; the availability of the mutants also will help elucidate the function of OMP E for the bacterium.
Mutants defective in expression of eight OMPs have been constructed in M. catarrhalis thus far. Six of these are involved in acquisition of iron, including CopB, TbpA, TbpB, LbpA, LbpB, and Orf3 (5, 11, 16, 17). In addition, mutants lacking expression of the high-molecular-weight proteins UspA1 and UspA2 have been constructed (1). OMP E mutants showed a slightly reduced rate of growth in broth (Fig. 7), whereas the previously reported mutants appeared to grow at rates similar to their respective parent strains in broth which includes sources of iron, such as BHI. OMP E shares borderline homology (49.1% similarity, 25.6% identity) with E. coli FadL, which is involved in uptake of long-chain fatty acids (4). The observation that a mutation in OMP E results in a reduced rate of growth is compatible with OMP E being involved in uptake of nutrients. However, a definitive conclusion regarding the function of OMP E awaits further experiments.
The mutants defective in expression of OMP E were more susceptible to killing by normal human serum compared to their isogenic parent strains (Fig. 8). A similar observation has been made with mutants of CopB and UspA2, suggesting that expression of several surface molecules affects susceptibility to killing of M. catarrhalis by serum (1, 11).
OMP E, a potential vaccine antigen, is abundantly expressed on the bacterial surface as indicated by results of immunofluorescence assays and flow cytometry with MAbs (3). It would be important to know whether the expression of OMP E is regulated and the extent to which the protein is expressed in vivo. Sera from adults with COPD and from some normal adults contain antibodies to OMP E, suggesting that the protein is expressed in vivo (3). Analysis of PCR restriction fragment length polymorphisms among clinical isolates and studies of strain specificity with polyclonal and monoclonal antibodies indicate that OMP E is conserved among strains of M. catarrhalis (2, 3). These characteristics of abundant expression on the bacterial surface and conservation among strains are highly desirable for a potential vaccine antigen. Future work should focus on determining whether immune responses to OMP E are protective against infection.
The present study contributes to further elucidating the antigenic structure of OMP E and provides new data indicating that OMP E is involved in serum resistance and suggesting that OMP E is involved in growth of the bacterium. The mutants constructed will contribute to future work to characterize the function of OMP E and further evaluate OMP E as a vaccine antigen for preventing infections caused by M. catarrhalis.
ACKNOWLEDGMENTS
This work was supported by grant AI28304 from the National Institute of Allergy and Infectious Diseases and the Department of Veterans Affairs.
Our thanks to Nicole Luke and Anthony Campagnari for advice in construction of the mutants.
REFERENCES
- 1.Aebi C, LaFontaine E R, Cope L D, Latimer J L, Lumbley S L, McCracken G H, Jr, Hansen E J. Phenotypic effect of isogenic uspA1 and uspA2 mutations on Moraxella catarrhalis 035E. Infect Immun. 1998;66:3113–3119. doi: 10.1128/iai.66.7.3113-3119.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bhushan R, Craigie R, Murphy T F. Molecular cloning and characterization of outer membrane protein E of Moraxella (Branhamella) catarrhalis. J Bacteriol. 1994;176:6636–6643. doi: 10.1128/jb.176.21.6636-6643.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bhushan R, Kirkham C, Sethi S, Murphy T F. Antigenic characterization and analysis of the human immune response to outer membrane protein E of Branhamella catarrhalis. Infect Immun. 1997;65:2668–2675. doi: 10.1128/iai.65.7.2668-2675.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Black P N. Primary sequence of the Escherichia coli fadL gene encoding an outer membrane protein required for long-chain fatty acid transport. J Bacteriol. 1991;173:435–442. doi: 10.1128/jb.173.2.435-442.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bonnah R A, Wong H, Loosmore S M, Schryvers A B. Characterization of Moraxella (Branhamella) catarrhalis lbpB, lpbA, and lactoferrin receptor orf3 isogenic mutants. Infect Immun. 1999;67:1517–1520. doi: 10.1128/iai.67.3.1517-1520.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Catlin B W. Branhamella catarrhalis: an organism gaining respect as a pathogen. Clin Microbiol Rev. 1990;3:293–320. doi: 10.1128/cmr.3.4.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen D, McMichael J C, van der Meid K R, Hahn D, Mininni T, Cowell J, Eldridge J. Evaluation of purified UspA from Moraxella catarrhalis as a vaccine in a murine model after active immunization. Infect Immun. 1996;64:1900–1905. doi: 10.1128/iai.64.6.1900-1905.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Christensen J J. Moraxella (Branhamella) catarrhalis: clinical, microbiological and immunological features in lower respiratory tract infections. APMIS. 1999;107(Suppl.):1–36. [PubMed] [Google Scholar]
- 9.Enright M C, McKenzie H. Moraxella (Branhamella) catarrhalis—clinical and molecular aspects of a rediscovered pathogen. J Med Microbiol. 1997;46:360–371. doi: 10.1099/00222615-46-5-360. [DOI] [PubMed] [Google Scholar]
- 10.Haase E M, Yi K, Morse G D, Murphy T F. Mapping of bactericidal epitopes on the P2 porin protein of nontypeable Haemophilus influenzae. Infect Immun. 1994;62:3712–3722. doi: 10.1128/iai.62.9.3712-3722.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Helminen M E, Maciver I, Paris M, Latimer J L, Lumbley S L, Cope L D, McCracken G H, Jr, Hansen E J. A mutation affecting expression of a major outer membrane protein of Moraxella catarrhalis alters serum resistance and survival in vivo. J Infect Dis. 1993;168:1194–1201. doi: 10.1093/infdis/168.5.1194. [DOI] [PubMed] [Google Scholar]
- 12.Kennett R H. Cell fusion. Methods Enzymol. 1979;58:345–359. doi: 10.1016/s0076-6879(79)58149-x. [DOI] [PubMed] [Google Scholar]
- 13.Klein J O. Otitis media. Clin Infect Dis. 1994;19:823–833. doi: 10.1093/clinids/19.5.823. [DOI] [PubMed] [Google Scholar]
- 14.Klingman K L, Pye A, Murphy T F, Hill S L. Dynamics of respiratory tract colonization by Moraxella (Branhamella) catarrhalis in bronchiectasis. Am J Respir Crit Care Med. 1995;152:1072–1078. doi: 10.1164/ajrccm.152.3.7663786. [DOI] [PubMed] [Google Scholar]
- 15.Ling M M, Robinson B H. Approaches to DNA mutagenesis: an overview. Anal Biochem. 1997;254:157–178. doi: 10.1006/abio.1997.2428. [DOI] [PubMed] [Google Scholar]
- 16.Luke N R, Campagnari A A. Construction and characterization of Moraxella catarrhalis mutants defective in expression of transferrin receptors. Infect Immun. 1999;67:5815–5819. doi: 10.1128/iai.67.11.5815-5819.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Luke N R, Russo T A, Luther N, Campagnari A A. The use of an isogenic mutant, constructed in Moraxella catarrhalis, to identify a protective epitope of OMP B1 defined by monoclonal antibody 11C6. Infect Immun. 1999;67:681–687. doi: 10.1128/iai.67.2.681-687.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McKenzie H, Morgan M G, Jordens J Z, Enright M C, Bain M. Characterisation of hospital isolates of Moraxella (Branhamella) catarrhalis by SDS-PAGE of whole-cell proteins, immunoblotting and restriction-endonuclease analysis. J Med Microbiol. 1992;37:70–76. doi: 10.1099/00222615-37-1-70. [DOI] [PubMed] [Google Scholar]
- 19.Menard R, Sansonetti P J, Parsot C. Nonpolar mutagenesis of the ipa genes defines IpaB, IpaC, and IpaD as effectors of Shigella flexneri entry into epithelial cells. J Bacteriol. 1993;175:5899–5906. doi: 10.1128/jb.175.18.5899-5906.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morgan M G, McKenzie H, Enright M C, Bain M, Emmanuel F X S. Use of molecular methods to characterize Moraxella catarrhalis strains in a suspected outbreak of nosocomial infection. Eur J Clin Microbiol Infect Dis. 1992;11:305–312. doi: 10.1007/BF01962069. [DOI] [PubMed] [Google Scholar]
- 21.Murphy T F. Branhamella catarrhalis: epidemiology, surface antigenic structure, and immune response. Microbiol Rev. 1996;60:267–279. doi: 10.1128/mr.60.2.267-279.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Murphy T F. Branhamella catarrhalis: epidemiological and clinical aspects of a human respiratory tract pathogen. Thorax. 1998;53:124–128. doi: 10.1136/thx.53.2.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Murphy T F. The role of bacterial infection in the course and pathogenesis of COPD. Respir Care Matters. 1998;2:14–15. [Google Scholar]
- 24.Murphy T F, Kirkham C, Denardin E, Sethi S. Analysis of antigenic structure and human immune response to outer membrane protein CD of Moraxella catarrhalis. Infect Immun. 1999;67:4578–4585. doi: 10.1128/iai.67.9.4578-4585.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Murphy T F, Sethi S. Bacterial infection in chronic obstructive pulmonary disease. Am Rev Respir Dis. 1992;146:1067–1083. doi: 10.1164/ajrccm/146.4.1067. [DOI] [PubMed] [Google Scholar]
- 26.Patterson T F, Patterson J E, Masecar B L, Barden G E, Hierholzer W J, Jr, Zervos M J. A nosocomial outbreak of Branhamella catarrhalis confirmed by restriction endonuclease analysis. J Infect Dis. 1988;157:996–1001. doi: 10.1093/infdis/157.5.996. [DOI] [PubMed] [Google Scholar]
- 27.Richards S J, Greening A P, Enright M C, Morgan M G, McKenzie H. Outbreak of Moraxella catarrhalis in a respiratory unit. Thorax. 1993;48:91–92. doi: 10.1136/thx.48.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Robert M C, Pang Y, Spencer R C, Winstanley T G, Brown B A, Wallace R J., Jr Tetracycline resistance in Moraxella (Branhamella) catarrhalis: demonstration of two clonal outbreaks by using pulsed-field gel electrophoresis. Antimicrob Agents Chemother. 1991;35:2453–2455. doi: 10.1128/aac.35.11.2453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ruuskanen O, Heikkinen T. Otitis media: etiology and diagnosis. Pediatr Infect Dis J. 1994;13:S23–S26. [PubMed] [Google Scholar]
- 30.Sarwar J, Campagnari A A, Kirkham C, Murphy T F. Characterization of an antigenically conserved heat-modifiable major outer membrane protein of Branhamella catarrhalis. Infect Immun. 1992;60:804–809. doi: 10.1128/iai.60.3.804-809.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Smith D B, Johnson K S. Single-step purification of polypeptides expressed in Escherichia coli as fusions with glutathione S-transferase. Gene. 1988;67:31–40. doi: 10.1016/0378-1119(88)90005-4. [DOI] [PubMed] [Google Scholar]
- 32.Urban A, Neukirchen S, Jaeger K E. A rapid and efficient method for site-directed mutagenesis using one-step overlap extension PCR. Nucleic Acids Res. 1997;25:2227–2228. doi: 10.1093/nar/25.11.2227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Verghese A, Roberson D, Kalbfleisch J H, Sarubbi F. Randomized comparative study of cefixime versus cephalexin in acute bacterial exacerbations of chronic bronchitis. Antimicrob Agents Chemother. 1990;34:1041–1044. doi: 10.1128/aac.34.6.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]