Abstract
The muscle spindle is an important sense organ for motor control and proprioception. Specialized intrafusal fibers are innervated by both stretch sensitive afferents and γ motor neurons that control the length of the spindle and tune the sensitivity of the muscle spindle afferents to both dynamic movement and static length. γ motor neurons share many similarities with other skeletal motor neurons, making it challenging to identify and specifically record or stimulate them. This short review will discuss recent advances in genetic and molecular biology techniques, electrophysiological recording, optical imaging, computer modelling, and stem cell culture techniques that have the potential to help answer important questions about fusimotor function in motor control and disease.
Introduction
The mammalian muscle spindle is a unique somatosensory mechanoreceptor in that it is innervated by both stretch sensitive sensory neurons and γ motor neurons that modulate its length to maintain stretch sensitivity. Muscle spindle afferents report muscle length and movement and provide the primary sensory input for proprioception, or the sense of body and limb position in space. Group Ia muscle spindles also comprise the sensory arm of the myotatic stretch reflex. By tuning both the dynamic and static sensitivity of muscle spindle afferents to stretch, γ motor neurons play an important role in motor control, locomotion, and balance [1,2]. The three types of skeletal motor neurons can be distinguished based on their muscle targets. The α motor neurons innervate the force-generating extrafusal fibers, the γ motor neurons the intrafusal fibers of the muscle spindle, and the β motor neurons both intrafusal and extrafusal fibers. γ motor neurons comprise roughly 30% of the motor pool, typically have smaller soma than α motor neurons, have simpler and less branched dendritic trees, and do not receive Group Ia monosynaptic input [3]. Functionally, γ motor neurons can fire at increased rates, are more excitable than α motor neurons, and have other electrophysiological differences that likely vary based on age and species [4–6]. The neuromuscular junction and the γ motor neuron endplate are functionally similar and share a common molecular basis for development [7]. Static γ motor neurons innervate the bag2 and/or chain intrafusal fibers and dynamic γ motor neurons innervate the bag1 fibers [8]. Co-activation of α and γ motor neurons is thought to maintain muscle spindle afferent sensitivity during planned movements and to allow the muscle spindle afferents to provide a sensory template of the expected movement [2,9]. In cats, there is strong evidence for independent control of dynamic and static γ motor neurons during locomotion and other behaviors [2], but in humans the role of independent fusimotor control seems to be more modest [10]. There are many unanswered questions about γ motor neuron function in motor control and disease progression, but technical challenges in identifying, recording, and manipulating them independently from other skeletal motor neurons mean they are relatively understudied. Here I will review recent advances in genetic and molecular biology techniques, electrophysiological recording, optical imaging, computer modelling, and stem cell culture techniques which provide additional avenues for the study of γ motor neuron function.
Identification of molecular markers for gamma motor neurons and transgenic tools
While adult γ motor neurons tend to have smaller soma than α motor neurons, using size to identify γ motor neurons is not definitive, especially during development [11] and disease when cell size may be altered [12]. Size is also not clearly differentiating in certain motor nuclei like the dorsolateral Trigeminal Motor Nucleus where there is a physiologically distinct group of α motor neurons of similar size to the γ motor neuron population [6,13]. In the past decade, a variety of molecular markers for γ motor neurons have been identified (recently reviewed in Ref. [14]), including high expression of the nuclear hormone receptor Err3 [11], the GDNF receptor Gfrα1 [15], the secreted signaling protein Wnt7a [16], the serotonin receptor 1d (5Ht1d) [5], and a low expression of neuronal nuclear protein (NeuN) and homeobox protein Hb9:: GFP transgene [11,15]. These markers have been identified using mouse genetic technologies including gene reporter mice that can be used to identify cells expressing a gene of interest or by using mouse models that lead to the reduction or absence of specifically γ motor neurons [11,15,16].
However, most of the markers are best used in combination and may have residual expression in other motor neuron subtypes. Recent advances in single cell profiling allow for high-throughput searches for identifying additional and more-specific molecular markers. Candidate markers for α and γ motor neurons have been identified in the early postnatal spinal cord using a novel and relatively low cost split-pool ligation-based transcriptome sequence method [17•]. These markers still need to be validated for specificity, as others have already identified a few of the candidate markers in other subtypes of motor neurons [18••,19]. Motor neurons comprise only a small percentage of cells in the spinal cord and their size makes them hard to dissociate into single cells, so using a sample of only the choline acetyl transferase (ChAT) positive pool of spinal cord nuclei is a more promising approach. Two groups have independently used mice with fluorescently tagged ChAT expressing neurons to increase the number of motor neuron nuclei they sequence and restrict their profiling to visceral and skeletal motor neurons and ChAT positive interneurons. Both groups found three main clusters of skeletal motor neurons, although there were differences in the subgroup markers they identified that will need to be studied further. Both groups hypothesize that these clusters may correspond to α, β, and γ motor neurons [18••,19], however they may alternatively correspond to α, static γ, and dynamic γ motor neurons if — as Banks has postulated — the β motor neurons represent α motor neurons that innervated intrafusal fibers because during development their axons encountered them by chance [8]. The analysis of ChAT-enriched motoneuron pools shows great promise not only for identifying molecular markers, but also for identifying functionally important genes for γ motor neuron function and development, and as a tool to compare transcription profiles following disease or development. Identifying a gene that is uniquely expressed in γ motor neurons is a prerequisite for uniquely targeting other transgenic tools like expression of optogenetic channels [20], chemogenetic tools [21], genetically encoded calcium- [22] or voltage indicators [23], or marker proteins (Figure 1).
Figure 1.
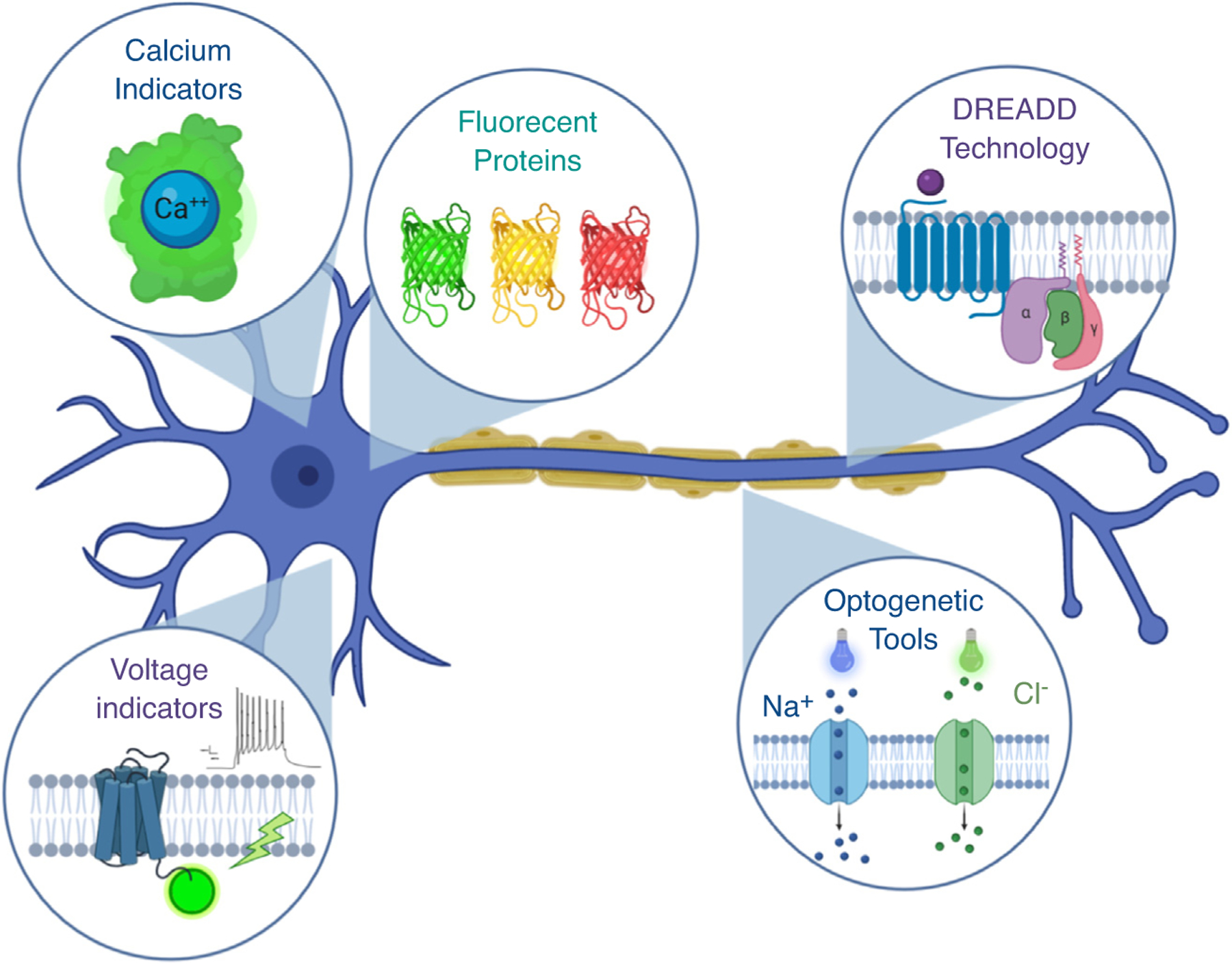
Promising transgenic tools for the study of γ motor neurons. Many technologies available in the mouse could allow for targeted recording, control, or identification of γ motor neurons, especially if a suitable γ motor neuron-specific driver was identified. Examples include fluorescent marker proteins like GFP, optogenetic channels like channelrhodopsin or halorhodopsin, Designer Receptors Exclusively Activated by Designer Drugs (DREADD) or other chemogenetic technologies, or genetically encoded calcium or voltage indicators. Figure created in BioRender.com.
Tools to study gamma motor neuron physiology
The majority of electrophysiological recordings of γ motor neurons have been done in the cat using laborious single fiber recordings [2]. The cat is an excellent model in that concurrent recordings of γ motor neurons and proprioceptive afferents can be accomplished during locomotor behaviors. A method for identifying and stimulating multiple dynamic and static γ motor neurons while recording the response of muscle spindle afferents has even been described [24]. However, the transgenic tools available in mice make it an attractive study species. Neonatal mouse spinal cord slice preparations have been used to record and compare membrane properties between skeletal motor neurons by using the presence of 5Ht1d-GFP fusion protein to identify γ motor neurons [5]. Motor neurons and spinal circuits are not completely developed at that point, though, so thin slice preparations for recording in adult mice are more suitable for experimental questions regarding mature motor neurons [25]. Muscle spindle afferent responses to passive stretch are relatively easy to record in a mouse muscle-nerve preparation and targeted expression of the light activated channel rhodopsin 2 would allow for γ motor neuron stimulation [26]. Electrophysiological recordings of motor neurons in intact mice is extremely challenging due to their small size, although some groups have successfully recorded α motor neurons from anesthetized [27] or decerebrate mice [28]. Electrophysiological recordings of γ motor neurons in mice should be theoretically possible, but technically very challenging. Imaging activity of motor neuron populations using genetically encoded indicators for calcium or voltage is a promising approach as is using genetically encoded fluorescent tags to study cellular dynamics or interactions. For instance, two-photon imaging of GFP-tagged microglia has been used in adult ex vivo spinal cord slices to study microglia interactions with α motor neurons following nerve injury [29]. Imaging in the deeper layers of the intact spinal cord is very difficult due to the light-scattering dorsal white matter, but two-photon laser scanning microscopy and a ventrolateral surgical approach allows for the acute imaging of motor neurons in the ventral horn in vivo [30••]. Further advances in three photon excited fluorescence imaging and chronic imaging chambers may allow for long-term imaging of the ventral horn[31].The ability to image populations of γ motor neurons in vivo could allow for a better understanding of fusimotor control during different types of movement as well as how disease states affect γ motor neurons.
Some important differences exist between fusimotor control in animal models and humans, including lower firing rates and smaller fusimotor-induced changes in firing in muscle spindle afferents, and less evidence for independent fusimotor control in humans [10]. There has been only one reported electrophysiological recording of γ motor neurons in humans [32] and fusimotor activity is normally extrapolated from changes in muscle spindle afferent firing rates. However, central reflex facilitation or fusimotor-independent peripheral changes in muscle spindle tension cannot be completely ruled out in these studies [33]. Muscle movement makes holding recordings difficult and limits the use of microneurography during many natural movements. Only superficial nerves can be recorded, limiting the muscles that can be studied [10], although recently a group has published methods to record from spindle afferents in the foot during standing [34•]. Coupling experimental recordings of muscle or spindle afferent activity with computer modeling is a promising approach to test hypotheses about fusimotor control [35•,36]. For instance, EMG recordings of physiological tremor at different muscle lengths were used to determine that shorter muscle lengths were accompanied by larger tremor amplitudes in human subjects. Using a closed-loop model of an afferented gastrocnemius muscle, increased static γ motor neuron drive was identified as the likely causal factor [36]. A similar computer model of the proprioceptive circuit was coupled with servo motor control of a cadaver finger to test hypotheses about altering γ motor neuron drive on the stretch reflex using realistic muscle and tendon forces [37,38]. Computer models hold great promise for testing hypotheses about the role of fusimotor control in normal movement and disease, but they would benefit from a greater understanding of the biophysical properties of γ motor neurons [39]. Future advances in recording techniques, experimental paradigms, and computer models can shed further light on human fusimotor control.
Cell culture tools to study gamma motor neurons
Advances in cell culture and stem cell technology have increased the utility of cell culture systems for studying motor neurons in vitro, which is a useful platform for studying the effect of disease mutation and development, or high throughput screening of drugs. Mature motor neurons are most useful for studying motor neuron behavior during age-related diseases [40] and methods for isolating spinal motor neurons from embryonic and adult mice as well as selecting for γ motor neurons have been developed [41,42]. However, yields from these techniques are relatively low and the cells recovered are likely to be the most resistant motor neurons and not those vulnerable to disease [40]. Using both rat and human stem cells, 2D co-culture systems have been created with intrafusal fibers and innervating muscle spindle afferents [43–45]. A human stem cell culture model with both bag and chain intrafusal fibers, innervating γ motor neurons, and functional neuromuscular junctions has also been developed [46]. These models show promise, however, the complex structure of the muscle spindle is not completely recapitulated and delivering reproducible stretches is difficult in vitro. Additionally, 2D monolayers can cause alterations in gene transcription and don’t model in vivo characteristics as well as 3D cultures, nor do they replicate the microenvironments seen by different cell types as well as compartmentalized microfluidic culture systems [47]. A 3D motor unit model in a compartmentalized microfluidic device has been developed using human induced pluripotent stem cells from a healthy control and an ALS patient and will be useful for screening therapeutic drug candidates [48••]. The development of a similar 3D culture system with intrafusal fibers, proprioceptive neurons, and γ motor neurons would provide a powerful tool to test questions about proprioceptive circuit development and potentially disease progression.
Conclusion
There are many unanswered questions about how the fusimotor system contributes to motor control and is affected by disease. For instance, the importance of independent fusimotor control in animal and human models during a variety of behaviors is still not well understood [2,10]. Why γ motor neurons are preferentially spared from degeneration in two neuromuscular disorders, amyotrophic lateral sclerosis (ALS) and spinal muscle atrophy (SMA), and how the surviving γ motor neurons may exacerbate disease progression is still unclear [12,49]. In contrast, γ but not α motor neurons are lost in a mouse model of Spinal Muscular Atrophy Lower Extremity Predominant (SMALED) [50•]. Exciting advances in genetics and molecular biology have led to better tools to identify-specific molecular markers for γ motor neurons that can be leveraged to target expression of other genetic technologies, including light-gated ion channels or genetically encoded calcium or voltage sensors. Coupled with advances in imaging technologies, these could allow for the control and/or recording of activity in populations of γ motor neurons, potentially even during normal behavior. There are important differences between human and animal model fusimotor control, so coupling computer modeling with electrophysiological recordings can overcome some of the limits to direct manipulation in human subjects. Stem cell technology allows for the development of more physiologically relevant 3D culture systems derived from patient cells which can be used to screen drug candidates and study disease progression. In short, the expanded toolbox for studying γ motor neurons should lead to exciting new discoveries about the fusimotor system.
Acknowledgements
The author would like to thank Stephan Kröger for his thoughtful suggestions which improved the article.
Funding sources
Research in the laboratory of the author is supported by the National Institutes of Health (SC3 GM127195).
Footnotes
Conflicts of interest statement
Nothing declared.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as
• of special interest
•• of outstanding interest
- 1.Matthews PBC: Where anatomy led, physiology followed: a survey of our developing understanding of the muscle spindle, what it does and how it works. J Anat 2015, 227:104–114 10.1111/joa.12345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ellaway PH, Taylor A, Durbaba R: Muscle spindle and fusimotor activity in locomotion. J Anat 2015, 227:157–166 10.1111/joa.12299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kanning KC, Kaplan A, Henderson CE: Motor neuron diversity in development and disease. Annu Rev Neurosci 2010, 33:409–440 10.1146/annurev.neuro.051508.135722. [DOI] [PubMed] [Google Scholar]
- 4.Ellaway PH, Murphy PR: A comparison of the recurrent inhibition of alpha- and gamma-motoneurones in the cat. J Physiol 1981, 315:43–58 10.1113/jphysiol.1981.sp013731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Enjin A, Leão KE, Mikulovic S, Le Merre P, Tourtellotte WG, Kullander K: Sensorimotor function is modulated by the serotonin receptor 1d, a novel marker for gamma motor neurons. Mol Cell Neurosci 2012, 49:322–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nishimura K, Ohta M, Saito M, Morita-Isogai Y, Sato H, Kuramoto E, Yin DX, Maeda Y, Kaneko T, Yamashiro T, Takada K et al. : Electrophysiological and morphological properties of α and γ motoneurons in the rat trigeminal motor nucleus. Front Cell Neurosci 2018, 12 10.3389/fncel.2018.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang Y, Lin S, Karakatsani A, Ruegg MA, Kroger S: Differential regulation of achr clustering in the polar and equatorial region of murine muscle spindles. Eur J Neurosci 2015, 41:69–78 10.1111/ejn.12768. [DOI] [PubMed] [Google Scholar]
- 8.Banks RW: The innervation of the muscle spindle: a personal history. J Anat 2015, 227:115–135 10.1111/joa.12297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Prochazka A: Sensory control of normal movement and of movement aided by neural prostheses. J Anat 2015, 227:167–177 10.1111/joa.12311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Macefield VG, Knellwolf TP: Functional properties of human muscle spindles. J Neurophysiol 2018, 120:452–467 10.1152/jn.00071.2018. [DOI] [PubMed] [Google Scholar]
- 11.Friese A, Kaltschmidt JA, Ladle DR, Sigrist M, Jessell TM, Arber S: Gamma and alpha motor neurons distinguished by expression of transcription factor err3. Proc Natl Acad Sci U S A 2009, 106:13588–13593 10.1073/pnas.0906809106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Powis RA, Gillingwater TH: Selective loss of alpha motor neurons with sparing of gamma motor neurons and spinal cord cholinergic neurons in a mouse model of spinal muscular atrophy. J Anat 2016, 228:443–451 10.1111/joa.12419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morita-Isogai Y, Sato H, Saito M, Kuramoto E, Yin DX, Kaneko T, Yamashiro T, Takada K, Oh SB, Toyoda H, Kang Y: A distinct functional distribution of alpha and gamma motoneurons in the rat trigeminal motor nucleus. Brain Struct Funct 2017, 222:3231–3239 10.1007/s00429-017-1400-8. [DOI] [PubMed] [Google Scholar]
- 14.Manuel M, Zytnicki D: Molecular and electrophysiological properties of mouse motoneuron and motor unit subtypes. Curr Opin Physiol 2019, 8:23–29 10.1016/j.cophys.2018.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shneider NA, Brown MN, Smith CA, Pickel J, Alvarez FJ: Gamma motor neurons express distinct genetic markers at birth and require muscle spindle-derived gdnf for postnatal survival. Neural Dev 2009, 4:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ashrafi S, Lalancette-Hebert M, Friese A, Sigrist M, Arber S, Shneider NA, Kaltschmidt JA: Wnt7a identifies embryonic gamma-motor neurons and reveals early postnatal dependence of gamma-motor neurons on a muscle spindle-derived signal. J Neurosci 2012, 32:8725–8731 10.1523/JNEUROSCI.1160-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17•.Rosenberg AB, Roco CM, Muscat RA, Kuchina A, Sample P, Yao Z, Graybuck LT, Peeler DJ, Mukherjee S, Chen W, Pun SH et al. : Single-cell profiling of the developing mouse brain and spinal cord with split-pool barcoding. Science 2018, 360:176–182 10.1126/science.aam8999. [DOI] [PMC free article] [PubMed] [Google Scholar]; Development of a relatively low cost method for single-cell profiling in the spinal cord. Identified a few candidate γ motor neuron-specific markers.
- 18••.Blum JA, Klemm S, Nakayama L, Kathiria A, Guttenplan KA, Hoang PT, Shadrach JL, Kaltschmidt JA, Greenleaf WJ, Gitler AD: Single-cell transcriptomic analysis of the adult mouse spinal cord. bioRxiv 2020. 10.1101/2020.03.16.992958. 2020.2003.2016.992958. [DOI] [PMC free article] [PubMed] [Google Scholar]; Single cell profiling of the spinal cord using an enrichment strategy to increase motor neuron yield and identify three main skeletal motor neuron gene expression clusters.
- 19.Alkaslasi MR, Piccus ZE, Silberberg H, Chen L, Zhang Y, Petros TJ, Le Pichon CE: Single nucleus rna-sequencing defines unexpected diversity of cholinergic neuron types in the adult mouse spinal cord. bioRxiv 2020. 10.1101/2020.07.16.193292. 2020.2007.2016.193292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim CK, Adhikari A, Deisseroth K: Integration of optogenetics with complementary methodologies in systems neuroscience. Nat Rev Neurosci 2017, 18:222–235 10.1038/nrn.2017.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roth BL: Dreadds for neuroscientists. Neuron 2016, 89:683–694 10.1016/j.neuron.2016.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang W, Yuste R: In vivo imaging of neural activity. Nat Methods 2017, 14:349–359 10.1038/nmeth.4230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Knöpfel T, Song C: Optical voltage imaging in neurons: moving from technology development to practical tool. Nat Rev Neurosci 2019, 20:719–727 10.1038/s41583-019-0231-4. [DOI] [PubMed] [Google Scholar]
- 24.Hulliger M, Banks RW: A method for the study of the effects of combining multiple pseudorandom fusimotor stimulation on the responses of muscle-spindle primary-ending afferents. J Neurosci Methods 2009, 178:103–115 10.1016/j.jneumeth.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 25.Mitra P, Brownstone RM: An in vitro spinal cord slice preparation for recording from lumbar motoneurons of the adult mouse. J Neurophysiol 2012, 107:728–741 10.1152/jn.00558.2011. [DOI] [PubMed] [Google Scholar]
- 26.Wilkinson KA, Kloefkorn HE, Hochman S: Characterization of muscle spindle afferents in the adult mouse using an in vitro muscle-nerve preparation. PLoS One 2012, 7:e39140 10.1371/journal.pone.0039140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Manuel M, Iglesias C, Donnet M, Leroy F, Heckman CJ, Zytnicki D: Fast kinetics, high-frequency oscillations, and subprimary firing range in adult mouse spinal motoneurons. J Neurosci 2009, 29:11246–11256 10.1523/JNEUROSCI.3260-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meehan CF, Mayr KA, Manuel M, Nakanishi ST, Whelan PJ: Decerebrate mouse model for studies of the spinal cord circuits. Nat Protoc 2017, 12:732–747 10.1038/nprot.2017.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rotterman TM, Alvarez FJ: Microglia dynamics and interactions with motoneurons axotomized after nerve injuries revealed by two-photon imaging. Sci Rep 2020, 10:8648 10.1038/s41598-020-65363-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30••.Cartarozzi LP, Rieder P, Bai X, Scheller A, d Oliveira ALR, Kirchhoff F: In vivo two-photon imaging of motoneurons and adjacent glia in the ventral spinal cord. J Neurosci Methods 2018, 299:8–15 10.1016/j.jneumeth.2018.01.005. [DOI] [PubMed] [Google Scholar]; Methods to image mouse spinal cord ventral horn in vivo.
- 31.Cheng YT, Lett KM, Schaffer CB: Surgical preparations, labeling strategies, and optical techniques for cell-resolved, in vivo imaging in the mouse spinal cord. Exp Neurol 2019, 318:192–204 10.1016/j.expneurol.2019.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ribot E, Roll JP, Vedel JP: Efferent discharges recorded from single skeletomotor and fusimotor fibres in man. J Physiol 1986, 375:251–268 10.1113/jphysiol.1986.sp016115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Horslen BC, Zaback M, Inglis JT, Blouin J-S, Carpenter MG: Increased human stretch reflex dynamic sensitivity with height-induced postural threat. J Physiol 2018, 596:5251–5265 10.1113/jp276459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34•.Knellwolf TP, Burton AR, Hammam E, Macefield VG: Firing properties of muscle spindles supplying the intrinsic foot muscles of humans in unloaded and freestanding conditions. J Neurophysiol 2019, 121:74–84 10.1152/jn.00539.2018. [DOI] [PubMed] [Google Scholar]; Microneurographic recording of human muscle spindle afferents during standing.
- 35•.Hardesty RL, Boots MT, Yakovenko S, Gritsenko V: Computational evidence for nonlinear feedforward modulation of fusimotor drive to antagonistic co-contracting muscles. Sci Rep 2020, 10:10625 10.1038/s41598-020-67403-w. [DOI] [PMC free article] [PubMed] [Google Scholar]; Study that used both human EMG recordings and computational modeling of the proprioceptive circuit to test a hypothesis about fusimotor function.
- 36.Jalaleddini K, Nagamori A, Laine CM, Golkar MA, Kearney RE, Valero-Cuevas FJ: Physiological tremor increases when skeletal muscle is shortened: Implications for fusimotor control. J Physiol 2017, 595:7331–7346 10.1113/JP274899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jalaleddini K, Minos Niu C, Chakravarthi Raja S, Joon Sohn W, Loeb GE, Sanger TD, Valero-Cuevas FJ: Neuromorphic meets neuromechanics, part ii: the role of fusimotor drive. J Neural Eng 2017, 14 10.1088/1741-2552/aa59bd025002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Niu CM, Jalaleddini K, Sohn WJ, Rocamora J, Sanger TD, Valero-Cuevas FJ: Neuromorphic meets neuromechanics, part i: the methodology and implementation. J Neural Eng 2017, 14 10.1088/1741-2552/aa593c025001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Elias LA, Matoso D Ed C, Watanabe RN, Kohn AF: Perspectives on the modeling of the neuromusculoskeletal system to investigate the influence of neurodegenerative diseases on sensorimotor control. Res Biomed Eng 2018, 34:176–186. [Google Scholar]
- 40.Bucchia M, Merwin SJ, Re DB, Kariya S: Limitations and challenges in modeling diseases involving spinal motor neuron degeneration in vitro. Front Cell Neurosci 2018, 12 10.3389/fncel.2018.00061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fujiki R, Lee JY, Jurgens JA, Whitman MC, Engle EC: Isolation and culture of oculomotor, trochlear, and spinal motor neurons from prenatal islmn:Gfp transgenic mice. J Visualized Exp: JoVE 2019, 153 10.3791/60440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Beaudet M-J, Yang Q, Cadau S, Blais M, Bellenfant S, Gros-Louis F, Berthod F: High yield extraction of pure spinal motor neurons, astrocytes and microglia from single embryo and adult mouse spinal cord. Sci Rep 2015, 5:16763 10.1038/srep16763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rumsey JW, Das M, Bhalkikar A, Stancescu M, Hickman JJ: Tissue engineering the mechanosensory circuit of the stretch reflex arc: sensory neuron innervation of intrafusal muscle fibers. Biomaterials 2010, 31:8218–8227 10.1016/j.biomaterials.2010.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Guo X, Colon A, Akanda N, Spradling S, Stancescu M, Martin C, Hickman JJ: Tissue engineering the mechanosensory circuit of the stretch reflex arc with human stem cells: sensory neuron innervation of intrafusal muscle fibers. Biomaterials 2017, 122:179–187 10.1016/j.biomaterials.2017.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Qiao Y, Cong M, Li J, Li H, Li Z: The effects of neuregulin-1β on intrafusal muscle fiber formation in neuromuscular coculture of dorsal root ganglion explants and skeletal muscle cells. Skeletal Muscle 2018, 8:29 10.1186/s13395-018-0175-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Colón A, Guo X, Akanda N, Cai Y, Hickman JJ: Functional analysis of human intrafusal fiber innervation by human γ-motoneurons. Sci Rep 2017, 7:17202 10.1038/s41598-017-17382-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Badiola-Mateos M, Hervera A, del Río JA, Samitier J: Challenges and future prospects on 3d in-vitro modeling of the neuromuscular circuit. Front Bioeng Biotechnol 2018, 6 10.3389/fbioe.2018.00194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48••.Osaki T, Uzel SGM, Kamm RD: Microphysiological 3d model of amyotrophic lateral sclerosis (als) from human ips-derived muscle cells and optogenetic motor neurons. Sci Adv 2018, 4: eaat5847 10.1126/sciadv.aat5847. [DOI] [PMC free article] [PubMed] [Google Scholar]; 3D motor unit cell culture system in a compartmentalized microfluidic device generated from ALS patient stem cells.
- 49.Lalancette-Hebert M, Sharma A, Lyashchenko AK, Shneider NA: Gamma motor neurons survive and exacerbate alpha motor neuron degeneration in als. Proc Natl Acad Sci U S A 2016, 113: E8316–E8325 10.1073/pnas.1605210113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50•.Rossor AM, Sleigh JN, Groves M, Muntoni F, Reilly MM, Hoogenraad CC, Schiavo G: Loss of bicd2 in muscle drives motor neuron loss in a developmental form of spinal muscular atrophy. Acta Neuropathol Commun 2020, 8:34 10.1186/s40478-020-00909-6. [DOI] [PMC free article] [PubMed] [Google Scholar]; Identification of a disease mutation leading to specific loss of muscle spindles and γ motor neurons.


