Abstract
It has long been appreciated that polymorphonuclear leukocytes (PMN) kill Cryptococcus neoformans, at least in part via generation of fungicidal oxidants. The aim of this study was to examine the contribution of nonoxidative mechanisms to the inhibition and killing of C. neoformans. Treatment of human PMN with inhibitors and scavengers of respiratory burst oxidants only partially reversed anticryptococcal activity, suggesting that both oxidative and nonoxidative mechanisms were operative. To define the mediators of nonoxidative anticryptococcal activity, PMN were fractionated into cytoplasmic, primary (azurophil) granule, and secondary (specific) granule fractions. Incubation of C. neoformans with these fractions for 18 h resulted in percents inhibition of growth of 67.4 ± 3.4, 84.6 ± 4.4, and 29.2 ± 10.5 (mean ± standard error, n = 3), respectively. Anticryptococcal activity of the cytoplasmic fraction was abrogated by zinc and depletion of calprotectin. Antifungal activity of the primary granules was significantly reduced by pronase treatment, boiling, high ionic strength, and magnesium but not calcium. Fractionation of the primary granules by reverse phase high-pressure liquid chromatography on a C4 column over an acetonitrile gradient revealed multiple peaks with anticryptococcal activity. Of these, peaks 1 and 6 had substantial fungistatic and fungicidal activity. Peak 1 was identified by acid-urea polyacrylamide gel electrophoresis (PAGE) and mass spectroscopy as human neutrophil proteins (defensins) 1 to 3. Analysis of peak 6 by sodium dodecyl sulfate-PAGE revealed multiple bands. Thus, human PMN have nonoxidative anticryptococcal activity residing principally in their cytoplasmic and primary granule fractions. Calprotectin mediates the cytoplasmic activity, whereas multiple proteins, including defensins, are responsible for activity of the primary granules.
Cryptococcus neoformans is an encapsulated fungus that is a significant cause of morbidity and mortality in patients with impaired cell-mediated immunity, especially those with AIDS (9, 11). Polymorphonuclear leukocytes (PMN) are thought to contribute to innate defenses against cryptococcosis, particularly early in the course of infection before an acquired immune response has had time to develop. Thus, in animal models, a PMN influx into tissues is seen soon after cryptococcal challenge and is associated with rapid, albeit partial, clearance of the organism (21, 47). In murine models of cryptococcosis, boosting PMN defenses by administration of granulocyte colony-stimulating factor resulted in reduced brain tissue burden and prolonged survival of mice treated with fluconazole (27). PMN are frequently found in pathology specimens taken from humans with cryptococcosis, although in only a minority of cases do PMN predominate (3, 30). In vitro, human PMN kill C. neoformans, provided opsonins in the form of complement or antibody are present to facilitate recognition of the fungi (29, 33, 37, 42).
PMN can exert antimicrobial activity by two general mechanisms, oxidative and nonoxidative. The former is characterized by the respiratory burst, a process whereby molecular oxygen is consumed and reduced to superoxide anion (2, 49). A series of reactions then results in the generation of reactive oxygen intermediaries with potent antimicrobial activity. Several studies have demonstrated that oxidative mechanisms contribute to PMN anticryptococcal activity (10, 15, 60). In vitro oxidative inhibition and killing of C. neoformans was reported by several investigators (15, 42, 43, 60). Moreover, in cell-free systems, C. neoformans is susceptible to killing by oxidants known to be generated by PMN, including hydrogen peroxide (10, 15). However, concentrations of oxidants required to kill fungi are generally considerably higher than those required to kill bacteria.
A multitude of effector molecules with nonoxidative antimicrobial activity have been found in PMN. Density gradient separation of disrupted PMN reveals three major fractions with antimicrobial activity: primary (azurophil) granules, secondary (specific) granules, and cytoplasm (14). Following phagocytosis, granules fuse with the phagosome and release their contents directly onto the microbe. There are about 1,500 primary granules contained in a mature PMN (31). Antimicrobial substances known to reside in primary granules include defensins, elastase, cathepsin G, collagenase, proteinase 3 or p29b or AGP7, bacterial permeability factor, and azurocidin/CAP37 (4, 22, 31, 46, 64). Secondary granule proteins include lysozyme and lactoferrin (31). The major cytoplasmic antimicrobial protein appears to be the zinc-binding protein calprotectin (44).
A putative role for PMN nonoxidative anticryptococcal activity can be found in studies in which purified components of PMN, including defensins (1, 22, 32, 61), calprotectin (59), lysozyme (21), and lactoferrin (63), inhibited or killed C. neoformans. However, in those studies, it was often unclear whether the concentrations and conditions tested were physiological. Moreover, by using purified components, synergistic activity, if present, would be missed. The aim of the present study was to define the contribution of PMN nonoxidative mechanisms to inhibition and killing of C. neoformans by directly testing the three major PMN fractions for anticryptococcal activity at concentrations likely to be physiologically relevant and then determining the individual components of the fractions responsible for the activity.
MATERIALS AND METHODS
Materials.
Reagents used were purchased from Sigma Chemical Co. (St. Louis, Mo.) unless otherwise stated. RPMI 1640 was purchased from Bio-Whittaker (Walkersville, Md.) and supplemented with penicillin, streptomycin, and l-glutamine. Reagents used for reverse-phase high-pressure liquid chromatography (RP-HPLC) were purchased from Fisher Scientific (Fair Lawn, N.J.). Pronase attached to agarose beads (immobilized pronase) was purchased from Pierce Chemical Co. (Rockford, Ill.). Pooled human serum (PHS) was obtained by combining sera from at least 10 healthy donors under conditions which preserved complement activity and stored in aliquots at −70°C until use (50).
C. neoformans.
The encapsulated serotype A C. neoformans strain 145 (ATCC 62070) was used for all studies. This strain has been shown previously to be susceptible to killing by human PMN (43, 50). Fungi were harvested after 72 h growth on Sabouraud dextrose agar plates at 30°C, washed twice in phosphate-buffered saline, counted on a hemocytometer, and resuspended at the desired concentration.
Isolation of PMN and fractionation of subcellular components.
Human PMN were isolated from heparinized peripheral blood by sequential dextran sedimentation, Ficoll-Hypaque centrifugation, and hypotonic lysis (40, 50). Granule and cytoplasmic fractions were obtained by nitrogen cavitation and Percoll gradient sedimentation as described elsewhere (5, 14). Briefly, PMN (5 × 108 to 13 × 108) isolated from 250 ml of blood were suspended in ice-cold relaxation buffer [125 mM KCl, 5 mM NaCl, 3.5 mM MgCl2, 10 mM piperazine-N,N′-bis(2-ethanesulfonic acid) (PIPES), 1 mM Na2ATP, 10 μM leupeptin, 5 μM pepstatin A (pH 7.4)] and pressurized in a nitrogen bomb (Parr Instrument Company, Moline, Ill.) at 425 lb/in2 for 30 min with constant stirring. Nuclei and cell debris were removed by centrifugation at 500 × g for 10 min at 4°C. The supernatant was layered over a discontinuous Percoll gradient of 1.02, 1.05, 1.10, and 1.12 g/ml and centrifuged at 100,000 × g for 30 min. The cytoplasmic fraction is found on top of the density gradient at ρ = 1.02, the secondary granule fraction was located at the bottom of gradient at ρ = 1.05, the mix fraction was located at the bottom of gradient at ρ = 1.10, and the primary granule fraction was located in the middle of gradient at ρ = 1.12. The fractions were recovered from the Percoll by ultracentrifugation at 180,000 × g for 3 h. The layer that sedimented above the packed Percoll was aspirated and resuspended in relaxation buffer, and the granules were disrupted by freeze-thawing five times. Where indicated, fractions were desalted and concentrated using a centrifuge filter with a molecular weight cutoff of 4,000 as instructed by the manufacturer (Nalge Nunc International, Rochester, N.Y.). All fractions were stored at −70°C until use.
Assays for lactoferrin and elastase.
To assess purity of the granule preparations, fractions were assayed for the primary and secondary granule markers elastase and lactoferrin, respectively. Elastase activity was measured by spectrofluorimetry using O-methyl-Suc-Ala-Ala-Ala-methylcoumarin as the substrate (24, 54). Lactoferrin was measured by sandwich enzyme-linked immunosorbent assay (23). Total protein concentration was determined by the bicinchoninic acid method (Pierce), with bovine serum albumin in relaxation buffer as the standard (14).
Inhibition and killing of C. neoformans.
Inhibition and killing of C. neoformans were measured as in our previous studies (34, 50). Briefly, PMN or purified PMN fractions were incubated for 2 h (killing assay) or 18 h (growth inhibition assay) with C. neoformans in a 96-well flat-bottom microtiter plate (Costar, Cambridge, Mass.). For wells containing intact PMN, the cells were lysed by the addition of a final concentration of 0.1% Triton X-100, and contents of wells were transferred to test tubes containing 1 ml of distilled water, diluted, and spread on Sabouraud agar plates. These conditions completely release phagocytosed C. neoformans without affecting fungal viability (50). Wells containing PMN fractions were treated identically except that Triton X-100 was omitted. Following 48 to 72 h of incubation at 30°C, C. neoformans colonies were counted and the number of CFU per well was calculated. For each assay, sets of wells containing C. neoformans, medium, and PHS (but no effector cells) were incubated at 37°C to determine control fungal growth. Results are expressed as percent anticryptococcal activity according to the formula [1 − (CFU experimental/CFU control)] × 100. We have found that replication of C. neoformans, which is harvested at the stationary phase of growth, does not occur following incubation at 37°C for 2 h (34, 50). Thus, percent anticryptococcal activity is equivalent to percent killing when the incubation period is 2 h.
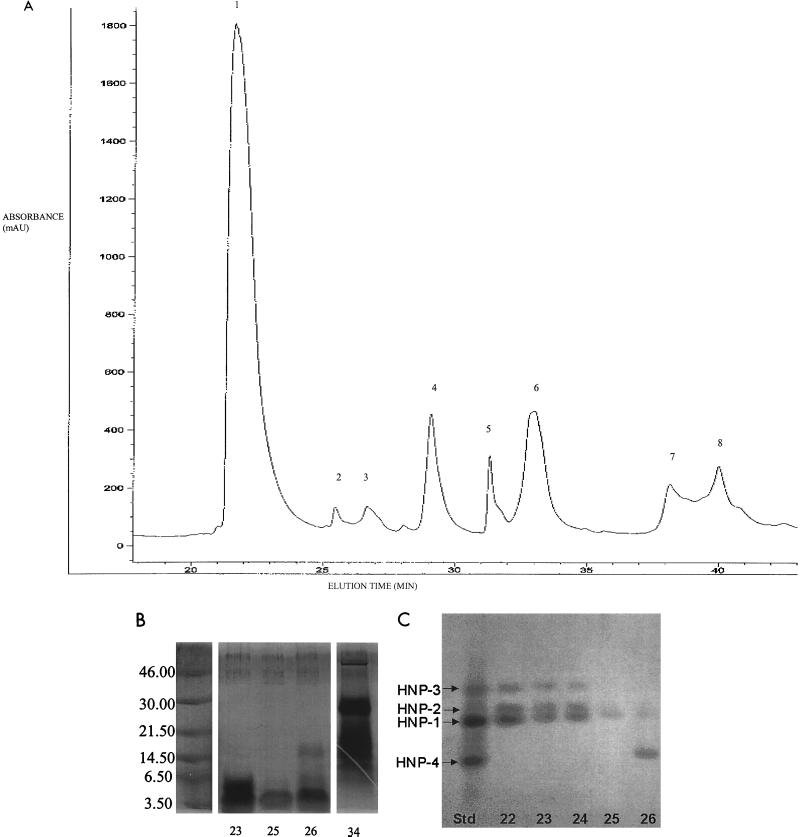
For the studies described in Table 2, the anticryptococcal activity of RP-HPLC fractions was determined visually by assessing growth under an inverted microscope. Fractions were diluted in 10 mM phosphate buffer (pH 5.5) containing 2% RPMI 1640 and incubated with 2.5 × 103 C. neoformans cells for 18 h in 384 flat-bottom well plates containing a final volume of 25 μl. Growth in the wells was scored from 0 to +++, with 0 indicating no fungistasis (growth equivalent to that seen in the absence of fractions), 1+ indicating a modest degree of fungistasis (0 to 50%), 2+ indicating moderate (>50%) fungistasis, and 3+ indicating complete fungistasis (no growth). Wells were read with the observer blinded as to experimental group.
TABLE 2.
Bioassay of HPLC fractionsa
| Db | Fungistasis
|
|||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 20 | 21 | 22 | 23 | 24 | 25 | 26 | 27 | 28 | 29 | 30 | 31 | 32 | 33 | 34 | 35 | 36 | 37 | 38 | 39 | 40 | 41 | 42 | 43 | 44 | 45 | |
| 1/2.5 | 0 | ++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ | 0 | 0 | 0 | +++ | ++ | ++ | ++ | + | + | 0 |
| 1/25 | 0 | 0 | +++ | +++ | +++ | + | + | ++ | 0 | 0 | + | 0 | + | 0 | ++ | + | 0 | 0 | 0 | + | + | + | + | + | 0 | 0 |
| 1/250 | 0 | 0 | 0 | ++ | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
C. neoformans was incubated for 18 h with reconstituted RP-HPLC fractions 20 to 45, obtained as demonstrated in Fig. 7. Fungistasis was scored on a scale of 0 (no fungistasis) to +++ (complete fungistasis) as described in Materials and Methods.
D, dilution of the reconstituted RP-HPLC fraction.
Calprotectin studies with Mac 387 antibody.
To study the role of calprotectin in mediating anticryptococcal activity, the cytoplasmic fraction was depleted of calprotectin by the procedure of Sohnle et al. (56). Briefly, monoclonal antibody to calprotectin, Mac 387 (Dako, Carpinteria, Calif.), and the irrelevant isotype-matched control antibody 36.65, specific for the hapten azophenylarsonate (51), were desalted (HiTrap desalting column; Pharmacia, Uppsala, Sweden), and then each antibody was incubated with cytoplasmic fractions at a concentration of 1 μg of antibody to 2 μg of cytosol protein for 30 min at 37°C. The preparation then was centrifuged at 10,000 × g for 1 h at 4°C, and the supernatants were tested for anticryptococcal activity as described above.
Separation of the primary granule fraction by RP-HPLC.
PMN primary granules obtained from 250 ml of blood were acid extracted by freeze-thawing five times in the presence of an equal volume of 100 mM glycine (pH 2.0) (64). The sample was vigorously agitated for 40 min and centrifuged at 1,400 × g to remove visible debris. The extracted proteins then were separated by RP-HPLC (Hewlett-Packard [Burlington, Mass.] 1090 series II) using a C4 column (214TP54; Vydac, Heperia, Calif.) equilibrated in solvent A (aqueous 0.1% trifluoroacetic acid [TFA]). Elution was with a gradient to 20% solvent B (0.09% TFA in acetonitrile) over 10 min, then to 80% solvent B for an additional 60 min, and finally to 100% solvent B over the last 10 min. The flow rate was 1 ml/min (64). Fractions (1 ml) were dried using a speed vacuum pump, reconstituted in 100 μl of 10 mM phosphate buffer (pH 5.5), and tested for anticryptococcal activity as described above. The most active fractions were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) in 8 to 16% polyacrylamide gels (Bio-Rad Laboratories, Hercules, Calif.).
Identification of human neutrophil defensins.
Fractions were analyzed for the presence of human neutrophil defensins on a 12.5% acid-urea polyacrylamide gel loaded with 1.2 to 2 μg of peptide. A standard containing the defensins human neutrophil peptides 1 to 4 (HNP-1 to -4) was run in parallel. Bands were visualized by staining with formalin-Coomassie blue (53). The presence of defensins was further studied using mass spectroscopy performed by matrix-assisted laser desorption ionization–time of flight (MALDI/TOF) on a PE Biosystems Voyager RP mass spectrometer in a linear mode. Samples (1 to 10 pmol) were dissolved in water-acetonitrile (1:1) containing 0.1% TFA. Masses obtained were for protonated monoisotopic species and in all cases were within 1% of the calculated masses for HNP-1, -2, -3, or -4 (62).
Statistics.
The two-tailed Student t test or paired t test was used to compare experimental and control groups, while the Mann-Whitney rank sum test was used when tests for normality failed.
RESULTS
Effect of inhibitors of respiratory burst oxidants on neutrophil anticryptococcal activity.
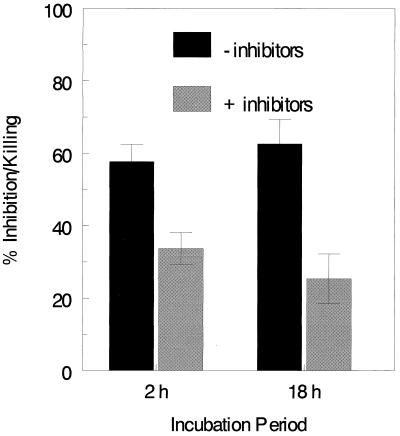
Initial experiments sought to examine the relative contributions of oxidative and nonoxidative mechanisms to neutrophil anticryptococcal activity. PMN were incubated for 2 and 18 h with C. neoformans in the presence and absence of superoxide dismutase, catalase, and mannitol. This cocktail inhibits the generation of reactive oxygen intermediaries as well as scavenges oxygen metabolites. At both time points, PMN had significant anticryptococcal activity (Fig. 1). Moreover, the presence of the cocktail of inhibitors significantly reduced, but did not abolish, the anticryptococcal activity of the PMN. These data suggest that both oxidative and nonoxidative mechanisms of growth inhibition or killing of C. neoformans are operative in PMN.
FIG. 1.
Effects of inhibitors of the respiratory burst oxidants on PMN anticryptococcal activity. PMN (105) were incubated for 2 and 18 h with C. neoformans (104) in RPMI 1640 containing 10% PHS in the absence (−inhibitors) or presence (+inhibitors) of 80 μg of bovine liver catalase per ml, 8 μg of bovine erythrocyte superoxide dismutase per ml, and 100 mM mannitol. Data represent means ± SE of five experiments performed in triplicate. Using the paired t test, there were significant differences (P < 0.0001) in percent anticryptococcal activity at both 2 and 18 h when the absence of inhibitors was compared to the presence of inhibitors. Furthermore, there were significant differences (P < 0.0001) in C. neoformans CFU between wells lacking PMN (not shown) and those containing PMN. These differences were observed regardless of whether inhibitors were present and at both 2- and 18-h time points. Using the paired t test, there were significant differences (P < 0.0001) in growth inhibition at both 2 and 18 h when the absence of inhibitors was compared to the presence of inhibitors. Furthermore, there were significant differences (P < 0.0001) between the control wells (not shown) and those containing PMN in the presence and absence of inhibitors at both 2 and 18 h. These differences were observed regardless of whether inhibitors were present and at both 2- and 18-h time points.
Growth inhibition or killing of C. neoformans by PMN fractions.
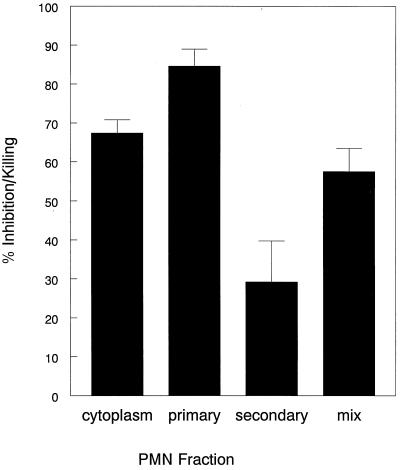
Having suggestive evidence that PMN utilize nonoxidative anticryptococcal mechanisms, we next sought to determine the mediators responsible for this activity. PMN were subjected to nitrogen cavitation and then separated on a discontinuous Percoll gradient. Four fractions were obtained: cytoplasm, secondary, primary, and mix (an impure fraction that has a mixture of granules from the primary and secondary fractions). Purity of the fractions was determined by assaying for the secondary and azurophil markers lactoferrin and elastase, respectively (Table 1). Of the PMN fractions, the primary granule fraction had the greatest anticryptococcal activity, followed in order by the cytoplasmic and secondary granule fractions (Fig. 2).
TABLE 1.
Protein, lactoferrin, and elastase content of PMN fractionsa
| PMN fraction | Concn (mean ± SEb)
|
||
|---|---|---|---|
| Protein (μg/ml) | Lactoferrin (ng/mg of total protein) | Elastase (μg/mg of total protein) | |
| Cytoplasm | 3,136.5 ± 3.6 | 1.42 ± 0.1 | 0.03 ± 0.0 |
| Mixc | 432.1 ± 1.5 | 15.99 ± 0.0 | 1.63 ± 0.2 |
| Secondary granule | 587.8 ± 2.9 | 166.55 ± 1.3 | 7.69 ± 3.4 |
| Primary granule | 661.3 ± 1.8 | 24.91 ± 29.2 | 98.08 ± 2.1 |
PMN were isolated, disrupted by nitrogen cavitation, and separated by discontinuous Percoll gradient fractionation. Fractions were then analyzed for protein content, the secondary granule marker lactoferrin, and the primary granule marker elastase as described in Materials and Methods.
For three nitrogen cavitations from three healthy donors.
Impure fraction containing granules from both primary and secondary fractions.
FIG. 2.
Anticryptococcal activity of PMN fractions. PMN fractions in 100 μl of relaxation buffer were incubated with 104 C. neoformans suspended in 20 μl of RPMI 1640 for 18 h. Anticryptococcal activity was measured as described in Materials and Methods. Data represent means ± SE of four experiments each performed in triplicate. Each group showed a statistical significance compared to the 37°C control group (P < 0.001) except for the secondary granule group. The statistical test used was the paired t test.
Role of calprotectin in mediating the anticryptococcal activity of the cytoplasmic fraction.
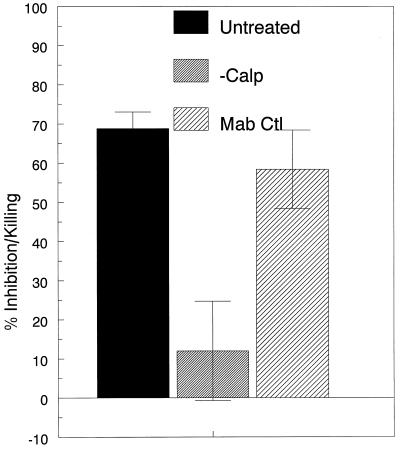
The next set of studies sought to determine the mediator(s) responsible for the anticryptococcal activity of the cytoplasmic fraction. Several reports (12, 38, 44, 52) have linked the zinc-binding protein calprotectin with antimicrobial properties contained in the PMN cytoplasm. In three independent experiments, addition of 10 μM ZnCl significantly reversed the inhibitory activity of the cytoplasmic fraction (40.7% ± 6.9% and −76.7% ± 34.3% [mean ± standard error {SE}]) anticryptococcal activity over 18 h in the absence and presence of ZnCl, respectively; P < 0.0001, using the Mann-Whitney rank sum test). To further study the role of calprotectin in mediating the anticryptococcal activity, the PMN cytoplasmic fraction was depleted of calprotectin by immunoprecipitation with the monoclonal antibody to calprotectin, Mac 387 (Fig. 3). Anticryptococcal activity was nearly completely abolished with calprotectin-depleted cytoplasm. In contrast, there was no significant effect when the cytoplasmic fraction was treated with a control antibody in lieu of Mac 387. Taken together, these data suggest that calprotectin is responsible for the anticryptococcal activity in the cytoplasmic fraction and that inhibition is mediated by zinc deprivation.
FIG. 3.
Role of calprotectin in mediating the anticryptococcal activity of the cytoplasmic fraction. The PMN cytoplasmic fraction was left untreated or depleted of calprotectin by immunoprecipitation with the monoclonal antibody Mac 387 (−Calp). Control fractions were treated with the irrelevant isotype-matched control antibody 36.65 (Mab Ctl). The samples then were incubated for 18 h with C. neoformans, and anticryptococcal activity was measured as described in Materials and Methods. Data represent means ± SE of three experiments. P = 0.005 and 0.0152 comparing −Calp with Untreated and Mab Ctl, respectively, using the Student t test.
Requirements for the anticryptococcal activity of the azurophil fraction.
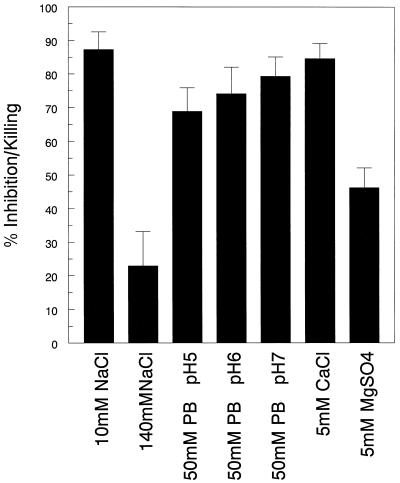
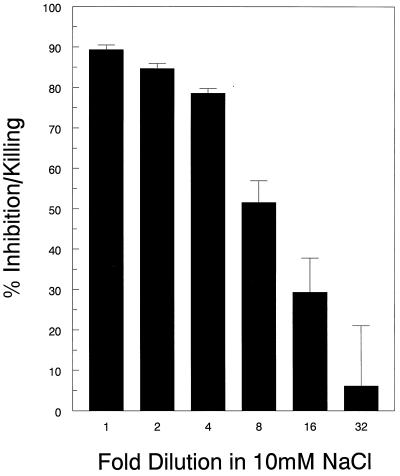
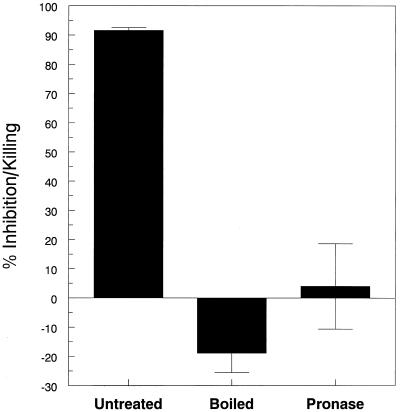
Next, we focused on defining the conditions under which anticryptococcal activity of the primary granule fraction occurred. The anticryptococcal activity of this fraction was significantly reduced at high ionic strength and in the presence of 5 mM magnesium but was unaffected by 5 mM calcium and pH over a range from 5 to 7 (Fig. 4). Significant anticryptococcal activity was observed even when the primary granule fraction was diluted up to eightfold (Fig. 5). To determine if peptides or proteins contributed to the anticryptococcal activity, the primary fraction was subjected to boiling and treated with pronase. Both treatments completely abrogated anticryptococcal activity (Fig. 6), suggesting that the antifungal effects are mediated by peptides and/or proteins.
FIG. 4.
Effects of ionic strength, pH, and divalent cations on anticryptococcal activity of the primary granule fraction. The primary granule fraction was desalted and brought to its original volume in 10 mM NaCl, 140 mM NaCl, 10 mM phosphate buffer (PB) at pH 5, 6, or 7, 10 mM calcium chloride, and 10 mM magnesium sulfate, as indicated on the abscissa. The samples then were incubated for 18 h with C. neoformans in the presence of 2% RPMI 1640, and anticryptococcal activity was measured as described in Materials and Methods. Data represent means ± SE of three experiments each performed in triplicate. P < 0.0001 comparing 10 mM NaCl and 140 mM NaCl; P < 0.0001 comparing 10 mM NaCl and 10 mM MgSO4, using the paired t test.
FIG. 5.
Dose-response curve of the primary granule fraction. The primary granule fraction was left undiluted or successively diluted twofold, as indicated, in 10 mM NaCl. Samples were then incubated for 18 h with C. neoformans in 10 mM NaCl containing 2% RPMI 1640. Anticryptococcal activity was measured as described in Materials and Methods. Data represent means ± SE of three experiments each performed in triplicate. Mean protein concentration in the undiluted fraction was 661.26 μg/ml. P < 0.0001 comparing anticryptococcal activity at all dilutions up to eightfold to controls incubated with medium containing no primary granules, using the paired t test.
FIG. 6.
Effects of boiling and pronase treatment on the anticryptococcal activity of the primary granule fraction. The primary fraction was left untreated, boiled for 10 min, or treated with immobilized pronase for 2 h at 37°C. The samples then were incubated for 18 h with C. neoformans, and anticryptococcal activity was measured as described in Materials and Methods. Data represent means ± SE of three experiments each performed in triplicate. There was statistical significance when the untreated group was compared to the boiled and pronase-treated group by using the Mann-Whitney rank sum test (P < 0.001).
HPLC and identity of major fractions.
In the final set of experiments, we sought to determine the component(s) of the primary granule fraction with anticryptococcal activity. Following acid extraction, primary granule proteins were separated by RP-HPLC using a C4 column and eluted over an acetonitrile gradient. Eight major peaks were observed (Fig. 7A). Fractions of 1 ml were collected and tested for fungistatic capacity. There were varying degrees of fungistasis at regions that corresponded to the peaks, but the fractions representing the first and sixth peaks had substantial fungistatic activity (Table 2). Moreover, in a 2-h killing assay, 95% ± 0.5% and 61% ± 6.3% of the fungal inoculum was killed by peaks 1 (fractions 22 to 24) and 6 (fractions 33 to 34), respectively (mean ± SE of two experiments performed in duplicate). Killing was inhibited by all major peaks by high ionic strength and in the presence of magnesium but not calcium (data not shown). Fractions from the remaining peaks had less fungicidal activity than peaks 1 and 6. SDS-PAGE analysis of the fractions comprising peak 1 and 2 revealed a broad band at approximately 3 kDa (Fig. 7B). This, and the elution profile on RP-HPLC (64), suggested that peak 1 is comprised of the HNPs (members of the defensin family). Mass spectroscopy (data not shown) and acid-urea PAGE (Fig. 7C) confirmed this and specifically demonstrated the presence of HNP-1 to -3. Peak 2, which was contained in fraction 25, was predominately HNP-1 and -2, while peak 3 (fraction 26) was HNP-1 and -4. SDS-PAGE analysis of fraction 34 from peak 6 revealed a single broad band at 30 kDa and multiple bands at 14.3 to 21.5 kDa (Fig. 7B). Further fractionation of peak 6 by RP-HPLC on a C18 column with elution over an acetonitrile gradient resulted in a single peak, which on SDS-PAGE had an apparent mass of 30 kDa. This peak lacked anticryptococcal activity (data not shown).
FIG. 7.
Fractionation and analysis of the primary granule proteins. (A) Primary granule proteins were fractionated by RP-HPLC on a C4 column equilibrated in solvent A (0.1% aqueous TFA) and eluted with a gradient to 20% solvent B (0.9% TFA in acetonitrile over 5 min, then 70% over 65 min, followed by 100% over 85 min and 0% over 90 min). Fractions of 1 ml were collected and assayed for anticryptococcal activity as shown in Table 2. The chromatogram is representative of two. Peaks were not observed in the parts of the chromatogram not shown. (B) SDS-PAGE of RP-HPLC fractions. The silver-stained SDS-polyacrylamide (8 to 16%) gel shows selected RP-HPLC fractions that demonstrated anticryptococcal activity. Sizes are indicated in kilodaltons. (C) Acid-urea PAGE of HPLC fractions. The formalin-Coomassie blue-stained acid-urea polyacrylamide (12.5%) gel shows selected RP-HPLC fractions. The standard shown in the left lane (Std) is a mix of HNP-1 to -4. All unknowns were run at 2 μg per lane.
DISCUSSION
The data presented herein demonstrate a role for nonoxidative anticryptococcal activity and define many of the major mediators responsible for activity. Incubation of PMN with a potent cocktail of inhibitors and scavengers of respiratory burst products resulted in approximately 50% retention of PMN anticryptococcal activity at both the 2- and 18-h time points. These data are consistent with results obtained with PMN from chronic granulomatous disease (CGD) patients (15). CGD PMN, which are incapable of generating a respiratory burst, did not kill C. neoformans as well as control PMN but still effected significant killing. In contrast, using the same concentrations of inhibitors as utilized in our study, Chaturvedi et al. found that superoxide dismutase completely inhibited PMN killing of C. neoformans strain H99 (10). However, another C. neoformans strain, characterized by relatively low mannitol production, was only partially inhibited by superoxide dismutase, suggesting that there may be strain-related differences in susceptibility to PMN nonoxidative killing. Additionally, the PMN respiratory burst is short-lived and usually over well within 2 h (8). Thus, it is likely that for fungi that resist initial killing, the PMN must rely nearly exclusively on nonoxidative antimicrobial mechanisms.
The importance of PMN nonoxidative mechanisms has been demonstrated for other fungi as well. Stein et al. (58), using anucleate, granule-poor cytoplasts (U-CYT) derived from human PMN, demonstrated that PMN granule components were necessary for killing of Candida albicans hyphae (58). The hyphae could not be killed by large numbers of U-CYT, even in the presence of exogenous myeloperoxidase, unless purified sublethal concentrations of PMN granule extracts were added. This suggested that both oxidative and nonoxidative mechanisms could have been working in synergy to effect complete killing of the organism. In a study on PMN-mediated activity against Histoplasma capsulatum (45), there was no difference in fungistatic activity between CGD patients and healthy controls, suggesting that nonoxidative mechanisms predominated.
All three PMN fractions tested had anticryptococcal activity, with the primary granule fraction having the greatest activity, followed by the cytoplasmic and secondary granule fractions. Importantly, fractions were not concentrated prior to testing. Thus, the concentrations of the fractions used in the assays should approximate those found in the PMN. The primary granule fraction also had the greatest activity against H. capsulatum, with fungistasis demonstrable for up to 7 days (45).
Our data provide strong support for the role of calprotectin in mediating the anticryptococcal activity of the cytoplasmic fraction. Calprotectin is a heterodimeric, zinc-binding protein that accounts for up to 40% of the protein content of the cytoplasm (16, 19, 26, 52). Activity of the cytoplasmic fraction was lost following immunodepletion of calprotectin and by the addition of 10 μM zinc. The latter effect suggests that the mechanism of action of calprotectin against C. neoformans is nutritional deprivation of zinc (38, 44, 55). In support of our data, purified calprotectin has previously been shown to have anticryptococcal activity (59). Similar effects have been noted for calprotectin activity against Candida albicans (44, 56).
The relevance of the anticryptococcal activity of the cytoplasmic fraction is unclear. When phagocytosed, C. neoformans is thought to reside in a phagolysosome (35), and thus intracellular contact with cytoplasmic contents is likely to be minimal. Interestingly though, in murine macrophages, C. neoformans has been shown to disrupt the phagosomal membrane (20). If the same process occurs with human PMN, then the cytoplasm could have extensive contact with phagocytosed C. neoformans. Moreover, following lysis of PMN, calprotectin is released to the immediate environment (48). In rare cases of cryptococcosis where abscess formation occurs (3), the possibility that calprotectin may inhibit growth of extracellular C. neoformans cannot be dismissed. A similar role for calprotectin has been postulated in abscesses due to Candida species (48).
The primary granule fraction retained significant potency against C. neoformans even when diluted up to eightfold. Moreover, activity of the primary fraction granule was retained over a pH range of 5 to 7. The pH of the cryptococcal phagolysosome is approximately 5, although it rises to 7 in the presence of chloroquine (35). Thus, the primary granules had potent activity over a biologically relevant pH range. Newman et al. (45) also showed that over a range of pH 4 to 8 there was no difference in anti-H. capsulatum activity of the primary granule fraction.
There was a significant reduction in anticryptococcal activity of primary granules when ionic strength was increased and with the addition of magnesium but not calcium. The primary granule components defensins and azurocidin are inhibited by increasing ionic strength (7, 39). The antimicrobial activity of azurocidin is also inhibited by calcium, an effect not found in our primary granule preparations. The effect of divalent cations on the antimicrobial activity of the defensins varies depending on the target organism (39).
Fractionation of the primary granule contents by RP-HPLC on a C4 column revealed eight major distinct peaks following elution with an acetonitrile gradient. This chromatogram profile was similar to that obtained by Wilde and coworkers (64). Peak 1 was the largest and also had the greatest anticryptococcal activity. Using SDS-PAGE, acid-urea PAGE, and MALDI-TOF mass spectroscopy, peaks 1 and 2 were demonstrated to be members of the defensin family, HNP-1 to -4. Defensins are cationic proteins that are widely distributed in nature, including mammals, birds, amphibia, invertebrates, and plants (39). They are prominent components of human PMN, comprising 30 to 50% of the primary granule protein (32), but are also expressed by other cell types, including pulmonary macrophages, intestinal epithelial cells, and cells in the skin, urogenital tract, and kidneys (28, 36).
Members of the defensin family appear to have particularly potent antifungal activity. Using purified defensins, others have demonstrated the anticryptococcal activity of human, rhesus macaque, and rabbit defensins (1, 22, 61, 62). Moreover, activity of defensins against other fungi, including Aspergillus fumigatus, Rhizopus oryzae, Candida albicans, and H. capsulatum has been demonstrated (13, 17, 36). In our studies, the defensin-containing fraction maintained fungistasis even at a 200-fold dilution. Moreover, activity was seen at both 2 and 18 h. As C. neoformans does not replicate over 2 h under the conditions of the assay (34), these data demonstrate the capacity of defensins to kill C. neoformans.
Substantial anticryptococcal activity, including killing, was also associated with peak 6. SDS-PAGE of peak 6 revealed several bands including a predominant band at approximately 30 kDa. Attempts to purify the active component(s) in peak 6 were unsuccessful, as activity was completely lost upon further purification by RP-HPLC on a C18 column. Loss of activity could be due to a requirement for multiple granule proteins acting in synergy, although the possibility that an antifungal protein was lost in the purification step cannot be totally eliminated. Primary granule proteins with known antimicrobial activity that have molecular masses of approximately 30 kDa include cathepsin G, azurocidin, CAP37, and proteinase 3 (18). Several of the other peaks also had anticryptococcal activity, albeit weaker than that associated with peaks 1 and 6. Moreover, weak activity was also found in the secondary granules, although this was not statistically significant. This activity was not further characterized, although others have associated anticryptococcal activity with the secondary granule components lactoferrin (63) and lysozyme (6). A small amount of elastase activity was detected in the secondary granule fraction, thus raising the possibility that some of the antifungal activity associated with this fraction was actually due to contamination from primary granules.
Taken together, the data demonstrate the existence of a broad range of PMN granule proteins with nonoxidative anticryptococcal activity. The clinical implications of our findings are speculative. There is great interest in developing therapies that boost natural host defenses against opportunistic fungi (41). Nowadays, the vast majority of patients with cryptococcosis has severely impaired T-cell function due to AIDS or immunosuppressive medications (25). For those patients, it may be unrealistic to expect to acutely boost T-cell function. In contrast, augmenting PMN host defenses against C. neoformans could be a more realistic approach, as PMN function is relatively preserved in most patients with cryptococcosis (15, 57, 60). Our data demonstrating that both oxidative and nonoxidative antimicrobial mechanisms are important in PMN defenses against cryptococcosis suggest that approaches aimed at boosting PMN anticryptococcal activity should be directed at both mechanisms.
ACKNOWLEDGMENTS
This work was supported by NIH grants DK31056, HL07501, AI-37532, AI-25780, and AI-22931 and by Large Scale Biology, Inc. S.M.L. is the recipient of a Burroughs Wellcome Fund Scholar Award in Pathogenic Mycology.
REFERENCES
- 1.Alcouloumre M S, Ghannoum M A, Ibrahim A S, Selsted M E, Edwards J E., Jr Fungicidal properties of defensin NP-1 and activity against Cryptococcus neoformans in vitro. Antimicrob Agents Chemother. 1993;37:2628–2632. doi: 10.1128/aac.37.12.2628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Babior B M, Curnutte J T, McMurrich B J. The particulate superoxide-forming system from human neutrophils. Properties of the system and further evidence supporting its participation in the respiratory burst. J Clin Investig. 1976;58:989–996. doi: 10.1172/JCI108553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baker R D, Haugen R K. Tissue changes and tissue diagnosis in cryptococcosis. A study of 26 cases. Am J Clin Pathol. 1955;25:14–24. doi: 10.1093/ajcp/25.1.14. [DOI] [PubMed] [Google Scholar]
- 4.Blondin J, Janoff A. The role of lysosomal elastase in the digestion of Escherichia coli proteins by human polymorphonuclear leukocytes: experiments with living leukocytes. J Clin Investig. 1976;58:971–979. doi: 10.1172/JCI108551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Borregaard N, Heiple J M, Simons E R, Clark R A. Subcellular localization of the b-cytochrome component of the human neutrophil microbicidal oxidase: translocation during activation. J Cell Biol. 1983;97:52–61. doi: 10.1083/jcb.97.1.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Borregaard N, Lollike K, Kjeldsen L, Sengelov H, Bastholm L, Nielsen M H, Bainton D F. Human neutrophil granules and secretory vesicles. Eur J Haematol. 1993;51:187–198. doi: 10.1111/j.1600-0609.1993.tb00629.x. [DOI] [PubMed] [Google Scholar]
- 7.Campanelli D, Detmers P A, Nathan C F, Gabay J E. Azurocidin and a homologous serine protease from neutrophils. Differential antimicrobial and proteolytic properties. J Clin Investig. 1990;85:904–915. doi: 10.1172/JCI114518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carey P D, Jenkins J K, Byrne K, Schneider A M, Walsh C J, Fowler A A, III, Sugerman H J. Neutrophil short-lived oxidant production: enhancement following onset of sepsis-induced lung injury. Circ Shock. 1992;36:256–264. [PubMed] [Google Scholar]
- 9.Casadevall A, Perfect J R. Cryptococcus neoformans. Washington, D.C.: ASM Press; 1998. [Google Scholar]
- 10.Chaturvedi V, Wong B, Newman S L. Oxidative killing of Cryptococcus neoformans by human neutrophils. Evidence that fungal mannitol protects by scavenging reactive oxygen intermediates. J Immunol. 1996;156:3836–3840. [PubMed] [Google Scholar]
- 11.Chuck S L, Sande M A. Infections with Cryptococcus neoformans in the acquired immunodeficiency syndrome. N Engl J Med. 1989;321:794–799. doi: 10.1056/NEJM198909213211205. [DOI] [PubMed] [Google Scholar]
- 12.Clohessy P A, Golden B E. Calprotectin-mediated zinc chelation as a biostatic mechanism in host defence. Scand J Immunol. 1995;42:551–556. doi: 10.1111/j.1365-3083.1995.tb03695.x. [DOI] [PubMed] [Google Scholar]
- 13.Couto M A, Liu L, Lehrer R I, Ganz T. Inhibition of intracellular Histoplasma capsulatum replication by murine macrophages that produce human defensin. Infect Immun. 1994;62:2375–2378. doi: 10.1128/iai.62.6.2375-2378.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Del Buono B J, Luscinskas F W, Simons E R. Preparation and characterization of plasma membrane vesicles from human polymorphonuclear leukocytes. J Cell Physiol. 1989;141:636–644. doi: 10.1002/jcp.1041410323. [DOI] [PubMed] [Google Scholar]
- 15.Diamond R D, Root R, Bennett J E. Factors influencing killing of Cryptococcus neoformans by human leukocytes in vitro. J Infect Dis. 1972;125:367–375. doi: 10.1093/infdis/125.4.367. [DOI] [PubMed] [Google Scholar]
- 16.Edgeworth J, Gorman M, Bennett R, Freemont P, Hogg N. Identification of p8,14 as a highly abundant heterodimeric calcium binding protein complex of myeloid cells. J Biol Chem. 1991;266:7706–7713. [PubMed] [Google Scholar]
- 17.Eisenhauer P B, Harwig S S, Szklarek D, Ganz T, Selsted M E, Lehrer R I. Purification and antimicrobial properties of three defensins from rat neutrophils. Infect Immun. 1989;57:2021–2027. doi: 10.1128/iai.57.7.2021-2027.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Elsbach P, Weiss J. Oxygen-independent antimicrobial systems of phagocytes. In: Gallin J I, Goldstein I M, Snyderman R, editors. Inflammation: basic principles and clinical correlates. 2nd ed. New York, N.Y: Raven Press, Ltd.; 1992. pp. 603–636. [Google Scholar]
- 19.Fagerhol M K, Dale I, Anderson T. Release and quantitation of a leucocyte derived protein (L1) Scand J Haematol. 1980;24:393–440. [Google Scholar]
- 20.Feldmesser M, Rivera J, Kress Y, Kozel T R, Casadevall A. Antibody interactions with the capsule of Cryptococcus neoformans. Infect Immun. 2000;68:3642–3650. doi: 10.1128/iai.68.6.3642-3650.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gadebusch H H, Johnson A G. Natural host resistance to infection with Cryptococcus neoformans. IV. The effect of some cationic proteins on the experimental disease. J Infect Dis. 1966;116:551–565. doi: 10.1093/infdis/116.5.551. [DOI] [PubMed] [Google Scholar]
- 22.Ganz T, Selsted M E, Szklarek D, Harwig S S, Daher K, Bainton D F, Lehrer R I. Defensins. Natural peptide antibiotics of human neutrophils. J Clin Investig. 1985;76:1427–1435. doi: 10.1172/JCI112120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gewirtz A T, Seetoo K F, Simons E R. Neutrophil degranulation and phospholipase D activation are enhanced if the Na+/H+ antiport is blocked. J Leukoc Biol. 1998;64:98–103. doi: 10.1002/jlb.64.1.98. [DOI] [PubMed] [Google Scholar]
- 24.Gewirtz A T, Simons E R. Phospholipase D mediates Fc gamma receptor activation of neutrophils and provides specificity between high-valency immune complexes and fMLP signaling pathways. J Leukoc Biol. 1997;61:522–528. doi: 10.1002/jlb.61.4.522. [DOI] [PubMed] [Google Scholar]
- 25.Gold J W. Clinical spectrum of infections in patients with HTLV-III-associated diseases. Cancer Res. 1985;45:4652s–4654s. [PubMed] [Google Scholar]
- 26.Gottsch J D, Eisinger S W, Liu S H, Scott A L. Calgranulin C has filariacidal and filariastatic activity. Infect Immun. 1999;67:6631–6636. doi: 10.1128/iai.67.12.6631-6636.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Graybill J R, Bocanegra R, Lambros C, Luther M F. Granulocyte colony stimulating factor therapy of experimental cryptococcal meningitis. J Med Vet Mycol. 1997;35:243–247. doi: 10.1080/02681219780001221. [DOI] [PubMed] [Google Scholar]
- 28.Hoffmann J A, Kafatos F C, Janeway C A, Ezekowitz R A. Phylogenetic perspectives in innate immunity. Science. 1999;284:1313–1318. doi: 10.1126/science.284.5418.1313. [DOI] [PubMed] [Google Scholar]
- 29.Kozel T R, Tabuni A, Young B J, Levitz S M. Influence of opsonization conditions on C3 deposition and phagocyte binding of large- and small-capsule Cryptococcus neoformans cells. Infect Immun. 1996;64:2336–2338. doi: 10.1128/iai.64.6.2336-2338.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee S C, Dickson D W, Casadevall A. Pathology of cryptococcal meningoencephalitis: analysis of 27 patients with pathogenetic implications. Hum Pathol. 1996;27:839–847. doi: 10.1016/s0046-8177(96)90459-1. [DOI] [PubMed] [Google Scholar]
- 31.Lehrer R I, Ganz T. Antimicrobial polypeptides of human neutrophils. Blood. 1990;76:2169–2181. [PubMed] [Google Scholar]
- 32.Lehrer R I, Ganz T, Selsted M E. Defensins: endogenous antibiotic peptides of animal cells. Cell. 1991;64:229–230. doi: 10.1016/0092-8674(91)90632-9. [DOI] [PubMed] [Google Scholar]
- 33.Levitz S M. Overview of host defenses in fungal infections. Clin Infect Dis. 1992;14(Suppl. 1):S37–S42. doi: 10.1093/clinids/14.supplement_1.s37. [DOI] [PubMed] [Google Scholar]
- 34.Levitz S M, Harrison T S, Tabuni A, Liu X. Chloroquine induces human mononuclear phagocytes to inhibit and kill Cryptococcus neoformans by a mechanism independent of iron deprivation. J Clin Investig. 1997;100:1640–1646. doi: 10.1172/JCI119688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Levitz S M, Nong S H, Seetoo K F, Harrison T S, Speizer R A, Simons E R. Cryptococcus neoformans resides in an acidic phagolysosome of human macrophages. Infect Immun. 1999;67:885–890. doi: 10.1128/iai.67.2.885-890.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Levitz S M, Selsted M E, Ganz T, Lehrer R I, Diamond R D. In vitro killing of spores and hyphae of Aspergillus fumigatus and Rhizopus oryzae by rabbit neutrophil cationic peptides and bronchoalveolar macrophages. J Infect Dis. 1986;154:483–489. doi: 10.1093/infdis/154.3.483. [DOI] [PubMed] [Google Scholar]
- 37.Levitz S M, Tabuni A. Binding of C. neoformans by cultured human macrophages: requirements for multiple complement receptors and actin. J Clin Investig. 1991;87:528–535. doi: 10.1172/JCI115027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Loomans H J, Hahn B L, Li Q Q, Phadnis S H, Sohnle P G. Histidine-based zinc-binding sequences and the antimicrobial activity of calprotectin. J Infect Dis. 1998;177:812–814. doi: 10.1086/517816. [DOI] [PubMed] [Google Scholar]
- 39.Martin E, Ganz T, Lehrer R I. Defensins and other endogenous peptide antibiotics of vertebrates. J Leukoc Biol. 1995;58:128–136. doi: 10.1002/jlb.58.2.128. [DOI] [PubMed] [Google Scholar]
- 40.Metcalf J A, Gallin J I, Nauseef W M, Root R K. Material and cell preparation for assays. New York, N.Y: Raven Press; 1986. pp. 1–23. [Google Scholar]
- 41.Meunier F. Targeting fungi: a challenge. Am J Med. 1995;99:60S–67S. doi: 10.1016/s0002-9343(99)80291-5. [DOI] [PubMed] [Google Scholar]
- 42.Miller G P G, Kohl S. Antibody-dependent leukocyte killing of Cryptococcus neoformans. J Immunol. 1983;131:1455–1459. [PubMed] [Google Scholar]
- 43.Miller M F, Mitchell T G. Killing of Cryptococcus neoformans strains by human neutrophils and monocytes. Infect Immun. 1991;59:24–28. doi: 10.1128/iai.59.1.24-28.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Murthy A R, Lehrer R I, Harwig S S, Miyasaki K T. In vitro candidastatic properties of the human neutrophil calprotectin complex. J Immunol. 1993;151:6291–6301. [PubMed] [Google Scholar]
- 45.Newman S L, Gootee L, Gabay J E. Human neutrophil-mediated fungistasis against Histoplasma capsulatum. Localization of fungistatic activity to the azurophil granules. J Clin Investig. 1993;92:624–631. doi: 10.1172/JCI116630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Oderberg H, Olsson I. Mechanisms for the microbicidal activity of cationic proteins of human granulocytes. Infect Immun. 1976;14:1269–1275. doi: 10.1128/iai.14.6.1269-1275.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Perfect J R, Lang S D, Durack D T. Chronic cryptococcal meningitis: a new experimental model in rabbits. Am J Pathol. 1980;101:177–194. [PMC free article] [PubMed] [Google Scholar]
- 48.Radke L L, Hahn B L, Wagner D K, Sohnle P G. Effect of abscess fluid supernatants on the kinetics of Candida albicans growth. Clin Immunol Immunopathol. 1994;73:344–349. doi: 10.1006/clin.1994.1208. [DOI] [PubMed] [Google Scholar]
- 49.Robinson J M, Badwey J A. Production of active oxygen species by phagocytic leukocytes. In: Zwilling B S, Einsenstein T K, editors. Macrophage-pathogen Interactions. New York, N.Y: Marcel Dekker, Inc.; 1994. pp. 159–160. [PubMed] [Google Scholar]
- 50.Roseff S A, Levitz S M. Effect of endothelial cells on phagocyte-mediated anticryptococcal activity. Infect Immun. 1993;61:3818–3824. doi: 10.1128/iai.61.9.3818-3824.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rothstein T L, Margolies M N, Gefter M L, Marshak-Rothstein A. Fine specificity of idiotope suppression in the A/J anti-azophenylarsonate response. J Exp Med. 1983;157:795–800. doi: 10.1084/jem.157.2.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Santhanagopalan V, Hahn B L, Sohnle P G. Resistance of zinc-supplemented Candida albicans cells to the growth inhibitory effect of calprotectin. J Infect Dis. 1995;171:1289–1294. doi: 10.1093/infdis/171.5.1289. [DOI] [PubMed] [Google Scholar]
- 53.Selstead M E. Investigational approaches for studying the structures and biological functions of myeloid antimicrobial peptides. Vol. 15. 1993. Coordinating ed., J. K. Setlow. Plenum Press, New York, N.Y. [DOI] [PubMed] [Google Scholar]
- 54.Sklar L A, Oades Z G, Finney D A. Neutrophil degranulation detected by right angle light scattering: spectroscopic methods suitable for simultaneous analyses of degranulation or shape change, elastase release, and cell aggregation. J Immunol. 1984;133:1483–1487. [PubMed] [Google Scholar]
- 55.Sohnle P G, Collins-Lech C, Wiessner J H. Antimicrobial activity of an abundant calcium-binding protein in the cytoplasm of human neutrophils. J Infect Dis. 1991;163:187–192. doi: 10.1093/infdis/163.1.187. [DOI] [PubMed] [Google Scholar]
- 56.Sohnle P G, Collins-Lech C, Wiessner J H. The zinc-reversible antimicrobial activity of neutrophil lysates and abscess fluid supernatants. J Infect Dis. 1991;164:137–142. doi: 10.1093/infdis/164.1.137. [DOI] [PubMed] [Google Scholar]
- 57.Spitznagel J K, Shafer W M. Neutrophil killing of bacteria by oxygen-independent mechanisms: a historical summary. Rev Infect Dis. 1985;7:398–403. doi: 10.1093/clinids/7.3.398. [DOI] [PubMed] [Google Scholar]
- 58.Stein D K, Malawista S E, Van Blaricom G, Wysong D, Diamond R D. Cytoplasts generate oxidants but require added neutrophil granule constituents for fungicidal activity against Candida albicans hyphae. J Infect Dis. 1995;172:511–520. doi: 10.1093/infdis/172.2.511. [DOI] [PubMed] [Google Scholar]
- 59.Steinbakk M, Naess-Andresen C F, Lingaas E, Dale I, Brandtzaeg P, Fagerhol M K. Antimicrobial actions of calcium binding leucocyte L1 protein, calprotectin. Lancet. 1990;336:763–765. doi: 10.1016/0140-6736(90)93237-j. [DOI] [PubMed] [Google Scholar]
- 60.Tacker J R, Farhi F, Bulmer G S. Intracellular fate of Cryptococcus neoformans. Infect Immun. 1972;6:162–167. doi: 10.1128/iai.6.2.162-167.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tang Y Q, Yuan J, Miller C J, Selsted M E. Isolation, characterization, cDNA cloning, and antimicrobial properties of two distinct subfamilies of alpha-defensins from rhesus macaque leukocytes. Infect Immun. 1999;67:6139–6144. doi: 10.1128/iai.67.11.6139-6144.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tang Y Q, Yuan J, Osapay G, Osapay K, Tran D, Miller C J, Ouellette A J, Selsted M E. A cyclic antimicrobial peptide produced in primate leukocytes by the ligation of two truncated alpha-defensins. Science. 1999;286:498–502. doi: 10.1126/science.286.5439.498. [DOI] [PubMed] [Google Scholar]
- 63.Vorland L H, Ulvatne H, Andersen J, Haukland H H, Rekdal O, Svendsen J S, Gutteberg T J. Antibacterial effects of lactoferricin B. Scand J Infect Dis. 1999;31:179–184. doi: 10.1080/003655499750006245. [DOI] [PubMed] [Google Scholar]
- 64.Wilde C G, Snable J L, Griffith J E, Scott R W. Characterization of two azurophil granule proteases with active-site homology to neutrophil elastase. J Biol Chem. 1990;265:2038–2041. [PubMed] [Google Scholar]