Abstract
The gastric inflammatory and immune response in Helicobacter pylori infection may be due to the effect of different H. pylori products on innate immune mechanisms. The aim of this study was to determine whether bacterial components could modulate cytokine production in vitro and thus contribute to Th1 polarization of the gastric immune response observed in vivo. The effect of H. pylori recombinant urease, bacterial lysate, intact bacteria, and bacterial DNA on proliferation and cytokine production by peripheral blood mononuclear cells (PBMCs) from H. pylori-negative donors was examined as a model for innate cytokine responses. Each of the different H. pylori preparations induced gamma interferon (IFN-γ) and interleukin-12p40 (IL-12p40), but not IL-2 or IL-5, production, and all but H. pylori DNA stimulated release of IL-10. Addition of anti-IL-12 antibody to cultures partially inhibited IFN-γ production. In addition, each bacterial product inhibited mitogen-stimulated IL-2 production by PBMCs and Jurkat T cells. The inhibitory effect of bacterial products on IL-2 production correlated with inhibition of mitogen-stimulated lymphocyte proliferation, although urease inhibited IL-2 production without inhibiting proliferation, suggesting that inhibition of IL-2 production alone is not sufficient to inhibit lymphocyte proliferation. The results of these studies demonstrate that Th1 polarization of the gastric immune response may be due in part to the direct effects of multiple different H. pylori components that enhance IFN-γ and IL-12 production while inhibiting both IL-2 production and cell proliferation that may be necessary for Th2 responses.
Helicobacter pylori is one of the most common infections of humans, causing variable degrees of chronic gastritis in all infected individuals, which sometimes leads to peptic ulcer, gastric atrophy, gastric adenocarcinoma, or mucosa-associated lymphoid tissue lymphoma (6, 7, 14). An unexplained paradox of H. pylori infection is that while the immune and inflammatory response that accompanies natural infection rarely leads to spontaneous resolution of infection, prophylactic and therapeutic immunization with H. pylori products in animal models has been demonstrated to have efficacy in preventing or reducing colonization and inflammation (6, 12, 36, 37, 41). Following natural infection, the gastric mucosa, which normally contains few lymphocytes and inflammatory cells, is infiltrated with large numbers of neutrophils and lymphoid cells, which are highly polarized towards a Th1 cytokine response, such as gamma interferon (IFN-γ) and interleukin-12 (IL-12) (2, 11, 20, 36, 37). It has been suggested that the Th1 polarization contributes to ongoing tissue injury and inhibition of a possibly beneficial Th2 cytokine response, such as IL-5 and IL-10 (1, 36). Some of the inflammatory and immune events associated with H. pylori production seem to be due to innate responses of the epithelium that are not dependent on cognate immunity, such as marked upregulation of NF-κB (10), IL-8 production (10), iNOS (15, 38, 48), COX-2 (15), and inflammatory cytokines (8, 10, 38). Previous studies have suggested that H. pylori products may have direct, non-antigen-specific effects on production of regulatory lymphokines, such as IL-2 and IFN-γ (1, 16, 41, 46), and may modulate lymphocyte proliferation (4, 5, 9, 22, 23, 28–30, 39, 41, 43). Therefore, the aim of this investigation was to further examine the possibility that the Th1 regulatory cytokine polarization of the gastric immune response is largely dependent on innate, rather than antigen-specific recognition of H. pylori products. To study this question, a reductionist model system was used, namely cultures of peripheral blood mononuclear cells (PBMCs) containing a mixture of myeloid and lymphoid cells obtained from H. pylori-negative volunteers. A secondary rationale for these experiments was to determine whether there are differences in the potential for several different H. pylori vaccine candidates to elicit innate immune responses that could be important in vaccine efficacy.
MATERIALS AND METHODS
PBMCs and cell culture.
Blood was obtained from five healthy H. pylori-noninfected volunteers. H. pylori status was determined serologically (Hp Enzyme Immunoassay; Enteric Products, Inc., Westbury, N.Y.) according to the manufacturer's directions. There were no borderline values. PBMCs were isolated with a Histopaque-1077 (Sigma Diagnostics, St. Louis, Mo.) gradient. Mononuclear cells were separated, washed with phosphate-buffered saline (PBS), recentrifuged, and resuspended in RPMI 1640 medium (1.5 × 106 to 2 × 106 cells/ml of medium; Gibco BRL, Life Technologies, Inc., Grand Island, N.Y.) supplemented with 10% (vol/vol) fetal bovine serum (heat-inactivated, 54°C for 45 min, Gibco BRL) and gentamicin at 0.1 mg/ml (Sigma Chemical Co., St. Louis, Mo.). Cell number was calculated with a hemocytometer after staining cells with trypan blue solution (0.4%; Sigma Chemical Co.; diluted 1:1 [vol/vol]), excluding nonviable cells. PBMCs were cultured in round-bottom 96-well plates (200 μl of cell suspension/well; total cell number, 3 × 105 to 4 × 105 cells/well) in the presence or absence of bacterial products (see below) and/or mitogens (phytohemagglutinin [PHA] [5 μg/ml] plus phorbol myristate acetate [PMA] [2.5 ng/ml]; Sigma Chemical Co.) for 24 h. Jurkat T cells (see below) were cultured under the same conditions. To confirm cell viability, the intracellular cytosolic enzyme lactate dehydrogenase concentration in the supernatant of the cell culture was determined (Cytotoxicity Detection Kit; Boehringer Mannheim, Indianapolis, Ind.).
T-cell line.
Jurkat cells, a CD4+ leukemia T-cell line (clone E6-1), were obtained from the American Type Culture Collection (Rockville, Md.). Cells were maintained in culture medium (see above) in 10-ml culture flasks at 37°C in a 5% CO2 humified atmosphere. Forty-eight hours after passing of cells into new culture flasks, cells were washed and resuspended with fresh RPMI medium 1640 for in vitro experiments. Cell suspensions (200-μl total volume; 1.5 × 106 to 2 × 106 cells/ml) were used as described for PBMCs (see above).
H. pylori products.
Briefly, H. pylori (UMAB 41 strain [19], cagA positive) was cultured on blood agar (brucella agar; Becton Dickinson, Cockeysville, Md.) or with the Difco Laboratories (Detroit, Mich.) campylobacter agar kit, supplemented with defibrinated sheep blood (10%; Waltz Farm, Smithburg, Md.) and amphotericin B (2 μg/ml; Biofluids, Inc., Rockville, Md.) under microaerophilic conditions (BBL CampyPak; Becton Dickinson Microbiology Systems). After passing H. pylori cultures three or four times and 5 days of the final culture, bacteria were harvested by scraping colonies from the agar surface and transfering them into sterile ice-cold PBS. Bacteria were washed twice and resuspended in sterile PBS. The concentration of bacteria was estimated by using the formula an absorbance of 0.1 = 108 bacteria/ml. A portion of the intact bacteria was frozen at −80°C prior to use. These bacterial cells were not viable when recultured under the conditions described above. A portion of the bacterial solution was disrupted with a French press (American Instruments Co., Silver Spring, Md.) to produce bacterial lysates. Protein content was determined with the bicinchoninic acid protein assay (Pierce, Rockford, Ill.). Stock solutions were diluted to the appropriate concentrations (3.125 to 200 μg of protein/ml), aliquoted, and frozen at −80°C until use. Fifty micrograms of protein/ml of solution is defined as equivalent to 2.28 × 108 bacteria (48). Recombinant enzymatically inactive urease, containing both UreA and UreB, was kindly provided by Oravax, Inc. (Cambridge, Mass.). In some studies, urease, H. pylori lysate, and intact bacteria were either boiled for 30 min or treated with proteinase K (final concentration, 10 μg/ml; Gibco BRL, Life Technologies, Gaithersburg, Md.) for 1 h at 37°C prior to use in cell culture experiments. Genomic DNA was isolated by a previously described method (34). Briefly, H. pylori bacteria were harvested, washed, suspended in solubilization buffer supplemented with proteinase K (final concentration, 100 μg/ml), and incubated at 55°C for 5 h. DNA was extracted with phenol-chloroform-isoamyl alcohol (25/24/1 [vol/vol/vol]; Boehringer Mannheim, Co.), precipitated with 95% ethanol and sodium acetate (final concentration, 3 M [pH 5.2]; Sigma Chemical Co.), washed with ethanol (70%), dissolved in diethyl pyrocarbonate water (Sigma Chemical Co.), and finally incubated with RNase (final concentration, 50 μg/ml; Boehringer Mannheim Co.) at 37°C for 1 h. The extraction was repeated once without the RNase incubation step to achieve higher purity of protein-free genomic DNA. Samples were stored at −80°C until use.
Antibodies.
In some experiments, monoclonal mouse anti-human IFN-γ (final concentration, 10 μg/ml) and polyvalent goat anti-human IL-12p40 (final concentration, 5 μg/ml) antibodies (both obtained from R & D Systems, Minneapolis, Minn.) and their isotype-matched controls were added to cultures.
Cytokine assays.
Twenty-four hours after initiation of cell culture, 150 μl of supernatants from duplicate or triplicate samples was pooled and frozen at −80°C until cytokine content was determined by using commercial enzyme-linked immunosorbent assay (ELISA) kits for IL-2 (INCstar, Stillwater, Minn.), IFN-γ, IL-5, IL-10, and IL-12p40 (R & D Systems) according to the instructions of the manufacturer. Samples were thawed only once for analysis of each cytokine. The IL-2 assay is a solid-phase enzyme amplified-sensitivity immunoassay performed on a microtiter plate. The IFN-γ, IL-5, IL-10, and IL-12p40 assays (R & D Systems) employ the quantitative sandwich enzyme immunoassay technique. Standard curves were constructed according to the manufacturer's instructions. The minimum detectable cytokine concentrations are estimated to be 0.1 U/ml for IL-2, <3 pg/ml for IFN-γ and IL-5, 5 pg/ml for IL-12, and 1.5 pg/ml for IL-10, respectively. Results are expressed as means ± standard errors.
Proliferation assay.
Tritium incorporation was used as an estimate for cell growth and DNA synthesis. After 24 h of cell culture under different conditions, 1 μCi of [methyl-3H]thymidine (Amersham Co., Arlington Heights, Ill.) was added to each well of cell cultures for 12 h. Incubation was then stopped, and incorporated radioactivity was measured in cpm with a 1205 betaplate liquid scintillation counter (Wallac, Inc., Gaithersburg, Md.). The results are expressed as means ± standard errors.
Statistics.
Analyses were performed with Statview 4.5 and superANOVA software for the Macintosh (SAS Institute, Inc., Cary, N.C.). When single comparisons were made, the Student t test was used, applying paired or unpaired analysis as appropriate. The normal distribution was tested prior to use of the paired t test. When multiple comparisons between groups were performed, one-way analysis of variance was used, followed by the Student-Newman-Keuls multiple comparisons procedure. Correlation analysis was performed with the Z test. Differences of P < 0.05 were considered significant.
RESULTS
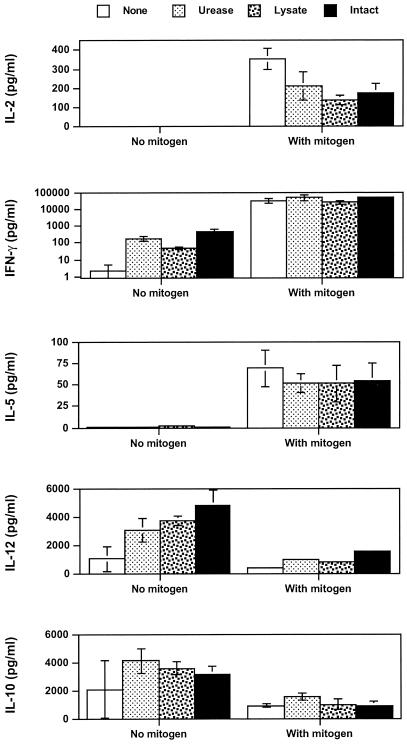
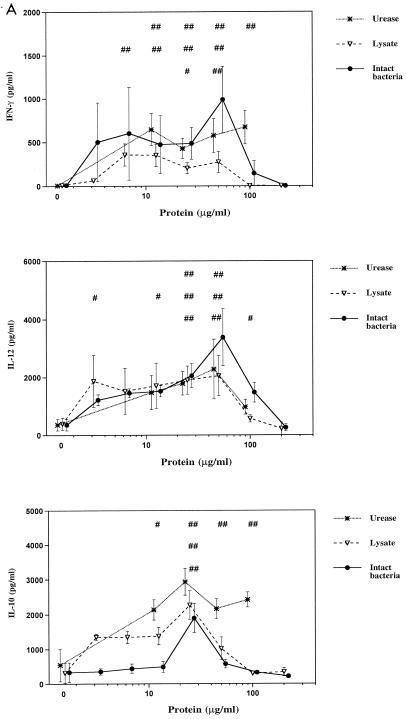
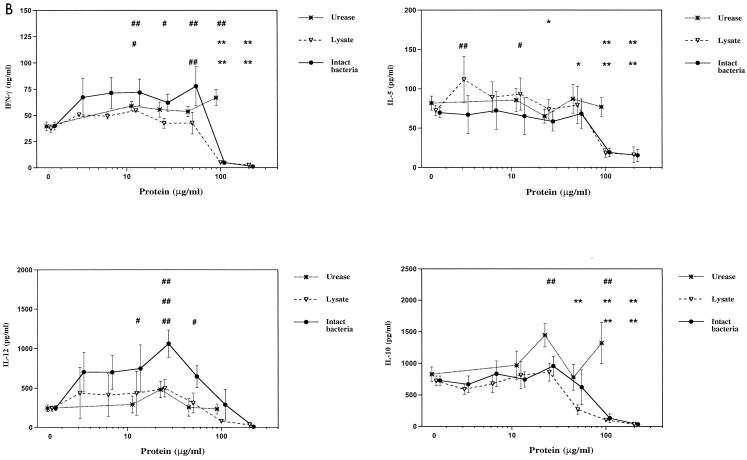
In initial experiments to examine the effects of different H. pylori preparations on cytokine production by naïve PBMCs, PBMCs were either cultured with H. pylori products alone or with the addition of a potent stimulus, PHA plus PMA. The choice of cytokines included examples of cytokines that play important roles in modulation of immune responses, including IL-2, IFN-γ, IL-5, IL-10, and IL-12. As shown in Fig. 1, H. pylori urease, whole-cell lysate, and intact, nonviable bacteria all had a similar ability to induce production of IFN-γ, IL-10, and IL-12 by naïve PBMCs at high concentrations of H. pylori products (50 μg/ml). Very low or undetectable levels of IL-2 and IL-5 were found in the same cultures. In mitogen-stimulated cultures, H. pylori preparations had no significant effect on production of mitogen-stimulated IFN-γ, IL-5, IL-10, and IL-12. However, mitogen-stimulated IL-2 production was lower in cultures containing H. pylori products (Fig. 1). Increased production of IFN-γ, IL-10, and IL-12, but not IL-2 or IL-5, was stimulated over a wide dose range by H. pylori products (Fig. 2A). There was no significant effect of H. pylori products on mitogen-stimulated production of IFN-γ, IL-5, IL-10, and IL-12 except at very high doses of bacterial products (Fig. 2B).
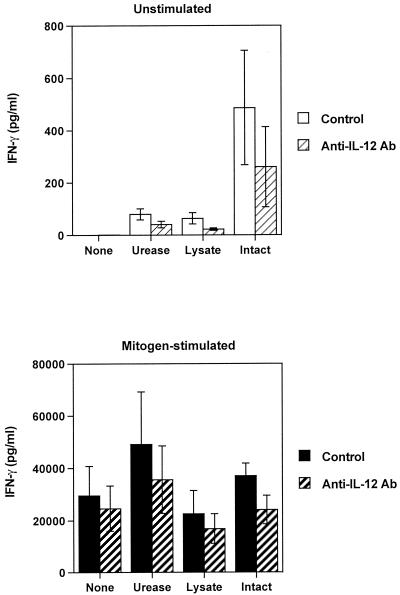
FIG. 1.
Effect of H. pylori products on cytokine production by resting and activated PBMCs from H. pylori-negative donors (n = 3). H. pylori products were urease (final concentration, 50 μg/ml), H. pylori French press lysate (50 μg of protein/ml), and intact bacteria (2.28 × 108 bacteria/ml) in vitro for 24 h with or without PHA plus PMA. Cytokine concentrations in culture supernatants were analyzed in duplicate by ELISA. Values are means ± standard errors.
FIG. 2.
Effects of H. pylori factors on cytokine production by PBMCs. Cultures were performed as in Fig. 1 with various concentrations of H. pylori urease (12.5, 25, 50, and 100 μg/ml), French press lysate, and intact bacteria (equivalent to protein concentrations of 3.125, 6.25, 12.5, 25, 50, 100, and 200 μg of protein/ml each) in the absence (A) and presence (B) of PHA plus PMA. Values are means ± standard errors of 4 to 12 experiments. A one- or two-tailed Dunnett's test was used as appropriate. # and ##, significant increase (#, P < 0.05; ##, P < 0.01). ∗ and ∗∗, significant decrease (∗, P < 0.05; ∗∗, P < 0.01). The top row of symbols refers to urease values, the middle row refers to lysate, and the bottom row refers to intact bacteria.
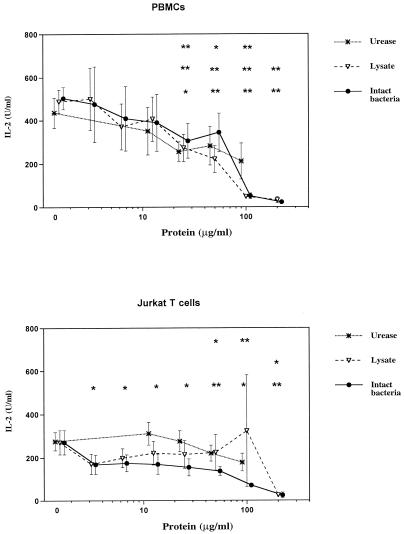
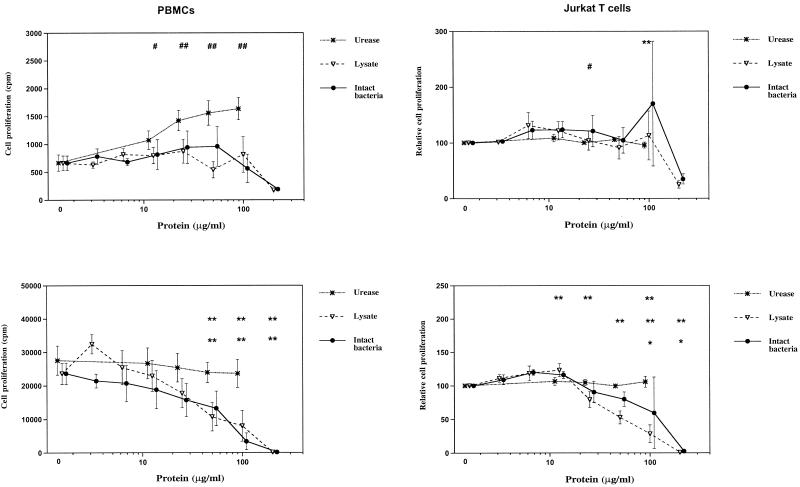
All three H. pylori products demonstrated dose-dependent inhibition of mitogen-stimulated IL-2 production by PBMCs (Fig. 3). To determine whether the inhibitory effect of H. pylori on IL-2 was due to interaction of different cell types in the PBMC preparations or whether H. pylori could have a direct effect on T cells, we carried out similar experiments with mitogen-stimulated Jurkat T cells and found similar dose-dependent inhibition of IL-2 production by this T-cell line (Fig. 3).
FIG. 3.
Effects of H. pylori products on IL-2 production by PBMCs and Jurkat T cells. PBMCs and Jurkat T cells were incubated with various concentrations of different H. pylori products with PHA plus PMA for 24 h as in Fig. 2. In the absence of PHA plus PMA, IL-2 secretion by resting cells was below the detection limit (0.1 U/ml). Values and number of experiments are as in Fig. 2. # and ##, significant increase (#, P < 0.05; ##, P < 0.01). ∗ and ∗∗, significant decrease (∗, P < 0.05; ∗∗, P < 0.01). The top row of symbols refers to urease values, the middle row refers to lysate, and the bottom row refers to intact bacteria.
Because of the significant upregulation of IFN-γ and IL-12 production by H. pylori-stimulated PBMCs, we sought to determine whether the IFN-γ production was dependent on IL-12. Addition of blocking doses of anti-IL-12p40 antibody to cultures of either resting or mitogen-stimulated PBMCs resulted in partial inhibition of IFN-γ production (Fig. 4), suggesting that the effect of H. pylori products on IFN-γ production was partially a direct effect, rather than an indirect effect mediated through induction of IL-12. Addition of blocking anti-IFN-γ antibodies had no significant effect on secretion of IL-12p40 by PBMCs stimulated with different H. pylori products (not shown).
FIG. 4.
Effect of IL-12p40-blocking antibody on H. pylori-induced IFN-γ secretion. Resting and activated PBMCs were cultured in vitro for 24 h in the presence or absence of different H. pylori products and anti-IL-12p40 or isotype-matched control antibody (Ab). Mean values ± standard errors of three experiments (same three donors as Fig. 1) performed in duplicates are shown.
We also examined the effects of H. pylori products on proliferation of PBMCs. H. pylori products stimulated minimal proliferation of resting PBMCs, and H. pylori lysate and intact bacteria, but not urease, inhibited mitogen-stimulated proliferation by PBMCs in a dose-dependent fashion (Fig. 5). There was a significant correlation between the ability of H. pylori bacteria and lysate to inhibit IL-2 production and the ability to inhibit proliferation of PBMCs (bacteria, r = 0.668; P = 0.015; lysate, r = 0.614, P = 0.03). Similarly, H. pylori products had little effect on the spontaneous proliferation of the Jurkat T-cell line; however, higher doses of H. pylori lysate and intact bacteria demonstrated inhibition of Jurkat T-cell proliferation when cultured with mitogens (Fig. 5).
FIG. 5.
Effect of H. pylori products on cell proliferation. (Top panels) PBMCs (left) or Jurkat T cells (right) cultured with H. pylori products alone. (Bottom panels) PBMCs (left) or Jurkat T cells (right) cultured with H. pylori products and PHA plus PMA. Cell cultures were performed as described in the legend to Fig. 2. After an incubation period of 24 h, tritiated thymidine incorporation was determined after an additional 12 h. Values are mean cpm ± standard errors. Due to variation in baseline proliferation of Jurkat T cells, values were normalized to 100% for these cells. # and ##, significant increase (#, P < 0.05; ##, P < 0.01). ∗ and ∗∗, significant decrease (∗, P < 0.05; ∗∗, P < 0.01). The top row of symbols refers to urease values, the middle row refers to lysate, and the bottom row refers to intact bacteria.
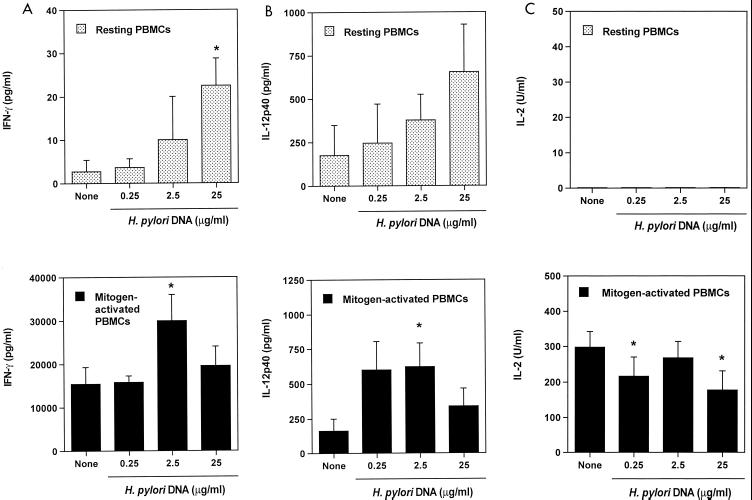
We next carried out experiments to determine whether the modulatory factors present in H. pylori preparations were protein or nonprotein factors. The inhibitory effect of H. pylori lysate (100 μg/ml) on mitogen-stimulated IL-2 production by PBMCs was found to be diminished from 98% to 40% by boiling and from 98% to 56% by protease treatment (not shown). Since bacterial CpG islands have been shown to be immunostimulatory in other systems (see below), we cultured resting or mitogen-stimulated PBMCs with H. pylori genomic DNA. Bacterial DNA enhanced secretion of IFN-γ and IL-12 by resting and mitogen-stimulated PBMCs (Fig. 6A and B) and inhibited expression of IL-2 (Fig. 6C), but had no significant effects on secretion of IL-5 or IL-10 (not shown).
FIG. 6.
Effect of H. pylori DNA on cytokine secretion by PBMCs. PBMCs were cultured with H. pylori DNA alone or with PHA plus PMA (mitogen-activated PBMCs). IFN-γ (A), IL-12p40 (B), and IL-2 (C) were assayed in duplicate by ELISA. Values are means ± standard errors of three experiments (same three donors as in Fig. 1). Dunnett's test was used for statistical analysis. ∗, P < 0.05 versus none.
DISCUSSION
Colonization of the gastric mucosa by H. pylori in most individuals is associated with a chronic inflammatory and immune response that on the one hand probably accounts for the diseases caused by H. pylori, but on the other hand does not clear the infection. The lifelong adaptation of H. pylori, which is noninvasive or minimally invasive (17, 18, 32, 38) with the host inflammatory and immune response (6, 7, 14, 38) is distinctly different from invasive enteric pathogens, such as Salmonella and Shigella species, that are usually associated with self-limited infection and generation of protective immunity (42, 47). Interestingly, both invasive enteric pathogens and H. pylori generate innate immune responses characterized by intense inflammation and immunity (6, 7, 14, 38, 42, 47) dominated by Th1 cytokine (such as IFN-γ and IL-12) production (2, 11, 20, 36, 37, 42). This raises the question as to whether the persistence of H. pylori is primarily due to inaccessibility of the intraluminal bacteria to mucosal immune effector mechanisms or is due to other qualitative or quantitative differences in the inflammatory and immune response that favor persistent bacterial colonization. Although enterocytes likely contribute critical signals in the response, such as IL-8 production (10), the innate and adaptive immune response to H. pylori most likely includes interaction of bacteria with intramucosal cells, including cells of macrophage and lymphoid lineage due to translocation of bacterial products across the epithelium (17, 18, 32). Therefore, the primary focus of this investigation was to evaluate the potential for different H. pylori products to elicit innate immune responses, and, in particular, cytokine responses that could play an important role in modulating adaptive immunity.
The initial experiments comparing three distinctly different H. pylori preparations—intact bacteria, a crude whole-bacterial-cell lysate, and recombinant enzymatically inactive urease—demonstrated dose-dependent induction of IL-10, IL-12p40, and IFN-γ secretion by PBMCs, but no significant induction of IL-2 or IL-5. These observations are confirmatory of the observations of previous studies which have demonstrated induction of IFN-γ, IL-10, and IL-12 in naïve PBMC cultures with several different types of bacterial preparations (2, 13, 16). It is interesting that there were no major differences in the cytokine-inducing activities of the three different preparations used, suggesting broad recognition of H. pylori products by innate mechanisms. Since IL-12 is thought to be a critical regulatory cytokine for IFN-γ production (2, 13, 16), we examined the effect of blocking anti-IL-12 antibodies and observed only modest effects on IFN-γ production. This suggests that the stimulatory effect of H. pylori products on their target cells, which are not defined, is largely independent of IL-12. The direct activation of IFN-γ and IL-12 production by H. pylori products suggests that this is a possible mechanism for the observed in vivo predominance of Th1 cytokine (IFN-γ)-expressing cells in the gastric mucosa of H. pylori-infected patients (1, 16, 41, 46) and the in vitro increased production of IL-10 and IL-12 in gastric biopsies from infected patients (2). Furthermore, based on studies of IFN-γ (−/−) mice, the presence of this cytokine is critical not only for inflammation, but also for downregulation of bacterial colonization (41). The biological effects of increased IL-10 production in H. pylori gastritis are unknown, but based on the observation of more severe gastritis in H. felis-infected IL-10(−/−) mice compared to controls (3), IL-10 may contribute to a protective response.
We simultaneously carried out experiments to evaluate the potential for the same three H. pylori preparations to modulate cytokine responses by adding them to mitogen-stimulated cultures. These experiments revealed inhibition of high-output mitogen-stimulated IL-5, IL-10, IL-12, and IFN-γ production only at very high doses of bacteria or bacterial protein. In contrast, dose-dependent inhibition of mitogen-stimulated IL-2 production was observed at lower concentrations of bacterial preparations. The effect of H. pylori products on inhibition of IL-2 production may be a direct effect on T cells, rather than an indirect effect mediated through other cell types, since we also observed direct inhibition of IL-2 production by a Jurkat T-cell line. We have previously shown that other bacterial products, isolated from enteropathogenic Escherichia coli, directly inhibit cytokine production by T cells (24, 25, 33). It is presently unknown whether H. pylori gastritis is associated with inhibition of IL-2 production in vivo or whether IL-2 plays an important role in disease pathogenesis, as might be determined by study of IL-2(−/−) mice.
If H. pylori products limit expression of IL-2 in vivo, then there are a number of possible implications of this finding. First, IL-2 is one critical cytokine necessary for expansion of immune responses, and thus, inhibition of IL-2 could limit the magnitude of the overall immune response. Second, cell proliferation is required for activation of IL-4 gene expression from naïve lymphocytes (39), and thus inhibition of IL-2 could limit expansion of a Th2 cytokine response. Our results clearly demonstrate that H. pylori products not only inhibit IL-2 production, but also inhibit mitogen-stimulated proliferation of PBMCs and Jurkat T cells. However, the inhibitory effect on cell proliferation was only observed for intact H. pylori bacteria or lysate, but not for urease, while inhibition of IL-2 production was observed with all three products. This observation suggests that inhibition of IL-2 by H. pylori products is not sufficient for inhibition of proliferation.
The natures of the factors present in intact bacteria and bacterial lysates that both induce and inhibit cytokine production are unknown. In preliminary experiments, we found that the factor or factors that inhibit IL-2 production are heat and protease sensitive, suggesting that bacterial proteins mediate the inhibitory activity. This observation is consistent with the observations of Knipp et al. (29) showing that a protein preparation of H. pylori inhibits proliferation. In addition, it has previously been shown that recombinant CagA protein inhibits proliferation of PBMCs (40). Our observation that recombinant urease also inhibits IL-2 production suggests that multiple different H. pylori proteins may have immunomodulatory activity.
In addition to bacterial proteins, there has been recent interest in the ability of bacterial DNA motifs to elicit innate immune responses in murine cells, including increases in NK activity, B- and T-cell activation, and production of multiple cytokines, including IL-6, IL-12, and IFN-γ, but not IL-5 or IL-10 (21, 26, 27, 31, 35, 44, 45, 49). Surprisingly, previous studies have demonstrated that the effects of bacterial DNA on innate immune cell activation may be greater than that of bacterial lipopolysaccharide (27). Our data demonstrate that H. pylori DNA also has the potential to activate IFN-γ and IL-12 production by human PBMCs. The concentrations of H. pylori DNA required to elicit increased cytokine responses were high and much greater than the estimated DNA concentrations in the bacterial lysates and intact bacterial preparations used in this study. Nonetheless, it remains possible that bacterial DNA released by cell death might achieve a sufficiently high concentration in proximity of immune cells to result in activation in vivo.
In summary, the results of these studies demonstrate that different products of H. pylori, including recombinant urease, crude protein preparations, intact bacteria, and bacterial DNA, all have the capacity to modulate innate immunity. In particular, the marked upregulation of IFN-γ and IL-12 production and inhibition of IL-2 production and proliferation by activated cells may contribute to Th1 polarization of the immune response observed in vivo. The capacity of a variety of different H. pylori products to elicit innate immune responses may significantly influence their potential to elicit protective immunity when used as vaccines.
ACKNOWLEDGMENTS
This work was supported by grants from the Deutsche Forschungsgemeinschaft Me 1400/1-1 (F.M.), the National Institutes of Health (DK02469 [K.T.W.], CA67497 [K.T.W.], DK53620 [K.T.W. and S.P.J.], N01-AI-65299 [S.P.J.]), and the Office of Medical Research, Department of Veterans Affairs (K.T.W.).
The authors gratefully acknowledge the technical assistance of Carol Malstrom, Jan-Michael Klapproth, and Oravax, Inc. (Cambridge, Mass.) for kindly providing recombinant urease.
REFERENCES
- 1.Bamford K B, Fan X, Crowe S E, Leary J F, Gourley W K, Luthra G K, Brooks E G, Graham D Y, Reyes V E, Ernst P B. Lymphocytes in the human gastric mucosa during Helicobacter pylori have a T helper cell 1 phenotype. Gastroenterology. 1998;114:482–492. doi: 10.1016/s0016-5085(98)70531-1. [DOI] [PubMed] [Google Scholar]
- 2.Bauditz J, Ortner M, Bierbaum M, Niedobitek G, Lochs H, Schreiber S. Production of IL-12 in gastritis relates to infection with Helicobacter pylori. Clin Exp Immunol. 1999;117:316–323. doi: 10.1046/j.1365-2249.1999.00968.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berg D J, Lynch N A, Lynch R G, Lauricella D M. Rapid development of severe hyperplastic gastritis with gastric epithelial dedifferentiation in Helicobacter felis-infected IL-10(−/−) mice. Am J Pathol. 1998;152:1377–1386. [PMC free article] [PubMed] [Google Scholar]
- 4.Birkholz S, Knipp U, Opferkuch W. Stimulatory effects of Helicobacter pylori on human peripheral blood mononuclear cells of H. pylori infected patients and healthy blood donors. Zentbl Bakteriol. 1993;280:166–176. doi: 10.1016/s0934-8840(11)80953-9. [DOI] [PubMed] [Google Scholar]
- 5.Birkholz S, Knipp U, Nietzki C, Adamek R J, Opferkuch W. Immunological activity of lipopolysaccharide of Helicobacter pylori on human peripheral mononuclear blood cells in comparison to lipopolysaccharides of other intestinal bacteria. FEMS Immunol Med Microbiol. 1993;6:317–324. doi: 10.1111/j.1574-695X.1993.tb00344.x. [DOI] [PubMed] [Google Scholar]
- 6.Blanchard T G, Czinn S J, Nedrud J G. Host response and vaccine development to Helicobacter pylori infection. Curr Top Microbiol Immunol. 1999;241:181–213. doi: 10.1007/978-3-642-60013-5_10. [DOI] [PubMed] [Google Scholar]
- 7.Blaser M J. Helicobacter pylori and gastric diseases. BMJ. 1998;316:1507–1510. doi: 10.1136/bmj.316.7143.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bodger K, Wyatt J I, Heatley R V. Gastric mucosal secretion of interleukin-10: relations to histopathology, Helicobacter pylori status, and tumour necrosis factor-alpha secretion. Gut. 1997;40:739–744. doi: 10.1136/gut.40.6.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chmiela M, Lelwala-Guruge J A, Wadstrom T, Rudnicka W. The stimulation and inhibition of T cell proliferation by Helicobacter pylori components. J Physiol Pharmacol. 1996;47:195–202. [PubMed] [Google Scholar]
- 10.Crabtree J E. Gastric mucosal inflammatory responses to Helicobacter pylori. Aliment Pharmacol Ther. 1996;10(Suppl):29–37. doi: 10.1046/j.1365-2036.1996.22164003.x. [DOI] [PubMed] [Google Scholar]
- 11.D'Elios M M, Manghetti M, De Carli M, Costa F, Baldari C T, Burroni D, Telford J L, Romagnani S, Del Prete G. T helper 1 effector cells specific for Helicobacter pylori in the gastric antrum of patients with peptic ulcer disease. J Immunol. 1997;158:962–967. [PubMed] [Google Scholar]
- 12.Dubois A, Lee C K, Fiala N, Kleanthous H, Mehlaman P T, Monath T. Immunization against natural Helicobacter pylori infection in nonhuman primates. Infect Immun. 1998;66:4340–4346. doi: 10.1128/iai.66.9.4340-4346.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Duchmann R, Scherer H, Neurath M, Knolle P, Meyer zum Buschenfelde K H. Normal interleukin-12 production in individuals with antibodies to Helicobacter pylori. APMIS. 1997;105:824–830. doi: 10.1111/j.1699-0463.1997.tb05090.x. [DOI] [PubMed] [Google Scholar]
- 14.Dunn B E, Cohen H, Blaser M J. Helicobacter pylori. Clin Microbiol Rev. 1997;10:720–741. doi: 10.1128/cmr.10.4.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fu S, Ramanujam K S, Wong A, Fantry G T, Drachenberg C B, James S P, Meltzer S J, Wilson K T. Increased expression and cellular localization of inducible nitric oxide synthase and cyclooxygenase 2 in Helicobacter pylori gastritis. Gastroenterology. 1999;116:1319–1329. doi: 10.1016/s0016-5085(99)70496-8. [DOI] [PubMed] [Google Scholar]
- 16.Haeberle H A, Kubin M, Bamford K B, Garofalo R, Graham D Y, El-Zaatari F, Karttunen R, Crowe S E, Reyes V E, Ernst P B. Differential stimulation of interleukin-12 (IL-12) and IL-10 by live and killed Helicobacter pylori in vitro and association of IL-12 production with gamma interferon-producing T cells in the human gastric mucosa. Infect Immun. 1997;65:4229–4235. doi: 10.1128/iai.65.10.4229-4235.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harris P R, Mobley H L, Perez-Perez G I, Blaser M J, Smith P D. Helicobacter pylori urease is a potent stimulus of mononuclear phagocyte activation and inflammatory cytokine production. Gastroenterology. 1996;111:419–425. doi: 10.1053/gast.1996.v111.pm8690207. [DOI] [PubMed] [Google Scholar]
- 18.Harris P R, Ernst P B, Kawabata S, Kiyono H, Graham M F, Smith P D. Recombinant Helicobacter pylori urease activates primary mucosal macrophages. J Infect Dis. 1998;178:1516–1520. doi: 10.1086/314426. [DOI] [PubMed] [Google Scholar]
- 19.Hu L-T, Foxall P A, Russell R, Mobley H L T. Purification of recombinant Helicobacter pylori urease apoenzyme encoded by ureA and ureB. Infect Immun. 1992;60:2657–2666. doi: 10.1128/iai.60.7.2657-2666.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Itoh T, Wakatsuki Y, Yoshida M, Usui T, Matsunaga Y, Kaneko S, Chiba T, Kita T. The vast majority of gastric T cells are polarized to produce T helper 1 type cytokines upon antigenic stimulation despite the absence of Helicobacter pylori infection. J Gastroenterol. 1999;34:560–570. doi: 10.1007/s005350050373. [DOI] [PubMed] [Google Scholar]
- 21.Jakob T, Walker P S, Krieg A M, Udey M C, Vogel J C. Activation of cutaneous dendritic cells by CpG-containing oligodeoxynucleotides: a role for dendritic cells in the augmentation of Th1 responses by immunostimulatory DNA. J Immunol. 1998;161:3042–3049. [PubMed] [Google Scholar]
- 22.Karttunen R. Blood lymphocyte proliferation, cytokine secretion and appearance of T cells with activation surface markers in cultures with Helicobacter pylori. Comparison of the responses of subjects with and without antibodies to H. pylori. Clin Exp Immunol. 1991;83:396–400. doi: 10.1111/j.1365-2249.1991.tb05650.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Karttunen R, Andersson G, Poikonen K, Kosunen T U, Karttunen T, Juutinen K, Niemela S. Helicobacter pylori induces lymphocyte activation in peripheral blood cultures. Clin Exp Immunol. 1990;82:485–488. doi: 10.1111/j.1365-2249.1990.tb05476.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Klapproth J M, Donnenberg M S, Abraham J M, James S P. Products of enteropathogenic E. coli inhibit lymphokine production by gastrointestinal lymphocytes. Am J Physiol. 1996;271:G841–G848. doi: 10.1152/ajpgi.1996.271.5.G841. [DOI] [PubMed] [Google Scholar]
- 25.Klapproth J-M, Donnenberg M S, Abraham J M, Mobley H L T, James S P. Products of enteropathogenic Escherichia coli inhibit lymphocyte activation and lymphokine production. Infect Immun. 1995;63:2248–2254. doi: 10.1128/iai.63.6.2248-2254.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kline J N, Waldschmidt T J, Businga T R, Lemish J E, Weinstock J V, Thorne P S, Krieg A M. Modulation of airway inflammation by CpG oligodeoxynucleotides in a murine model of asthma. J Immunol. 1998;160:2555–2559. [PubMed] [Google Scholar]
- 27.Klinman D M, Yi A K, Beaucage S L, Conover J, Krieg A M. CpG motifs present in bacteria DNA rapidly induce lymphocytes to secrete interleukin 6, interleukin 12, and interferon gamma. Proc Natl Acad Sci USA. 1996;93:2879–2883. doi: 10.1073/pnas.93.7.2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Knipp U, Birkholz S, Kaup W, Opferkuch W. Immune suppressive effects of Helicobacter pylori on human peripheral blood mononuclear cells. Med Microbiol Immunol. 1993;182:63–76. doi: 10.1007/BF00189374. [DOI] [PubMed] [Google Scholar]
- 29.Knipp U, Birkholz S, Kaup W, Opferkuch W. Partial characterization of a cell proliferation-inhibiting protein produced by Helicobacter pylori. Infect Immun. 1996;64:3491–3496. doi: 10.1128/iai.64.9.3491-3496.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Knipp U, Birkholz S, Kaup W, Mahnke K, Opferkuch W. Suppression of human mononuclear cell response by Helicobacter pylori: effects on isolated monocytes and lymphocytes. FEMS Immunol Med Microbiol. 1994;8:157–166. doi: 10.1111/j.1574-695X.1994.tb00438.x. [DOI] [PubMed] [Google Scholar]
- 31.Krieg A M, Yi A K, Matson S, Waldschmidt T J, Bishop G A, Teasdale R, Koretzky G A, Klinman D M. CpG motifs in bacterial DNA trigger direct B-cell activation. Nature. 1995;374:546–549. doi: 10.1038/374546a0. [DOI] [PubMed] [Google Scholar]
- 32.Mai U E H, Perez-Perez G I, Wahl L M, Wahl S M, Blaser M J, Smith P D. Soluble surface proteins from Helicobacter pylori activate monocytes/macrophages by lipopolysaccharide-independent mechanism. J Clin Investig. 1991;87:894–900. doi: 10.1172/JCI115095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Malstrom C, James S. Inhibition of murine splenic and mucosal lymphocyte function by enteric bacterial products. Infect Immun. 1998;66:3120–3127. doi: 10.1128/iai.66.7.3120-3127.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McAllister C F, Stephens D S. Analysis in Neisseria meningitidis and other Neisseria species of genes homologous to the FKBP immunophilin family. Mol Microbiol. 1999;10:13–23. doi: 10.1111/j.1365-2958.1993.tb00899.x. [DOI] [PubMed] [Google Scholar]
- 35.McCluskie M J, Davis H L. CpG DNA is a potent enhancer of systemic and mucosal immune responses against hepatitis B surface antigen with intranasal administration to mice. J Immunol. 1998;161:4463–4466. [PubMed] [Google Scholar]
- 36.Mohammadi M, Nedrud J, Redline R, Lycke N, Czinn S J. Murine CD4 T-cell response to Helicobacter infection: TH1 cells enhance gastritis and TH2 cells reduce bacterial load. Gastroenterology. 1997;113:1848–1857. doi: 10.1016/s0016-5085(97)70004-0. [DOI] [PubMed] [Google Scholar]
- 37.Mohammadi M, Czinn S J, Redline R, Nedrud J. Helicobacter-specific cell-mediated immune responses display a predominant Th1 phenotype and promote a delayed-type hypersensitivity response in the stomachs of mice. J Immunol. 1996;156:4729–4738. [PubMed] [Google Scholar]
- 38.Pérez-Pérez G I, Shepherd V L, Morrow J D, Blaser M J. Activation of human THP-1 cells and rat bone marrow-derived macrophages by Helicobacter pylori lipopolysaccharide. Infect Immun. 1995;63:1183–1187. doi: 10.1128/iai.63.4.1183-1187.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Richter A, Lohning M, Radbruch A. Instruction for cytokine expression in T helper lymphocytes in relation to proliferation and cell cycle progression. J Exp Med. 1999;190:1439–1450. doi: 10.1084/jem.190.10.1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rudnicka W, Covacci A, Wadstrom T, Chmiela M. A recombinant fragment of Helicobacter pylori CagA affects proliferation of human cells. J Physiol Pharmacol. 1998;49:111–119. [PubMed] [Google Scholar]
- 41.Sawai N, Kita M, Kodama T, Tanahashi T, Yamaoka Y, Tagawa Y-I, Iwakura Y, Imanishi J. Role of gamma interferon in Helicobacter pylori-induced gastric inflammatory responses in a mouse model. Infect Immun. 1999;67:279–285. doi: 10.1128/iai.67.1.279-285.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schlaak J F, Nieder P, Meyer zum Buschenfelde K H, Fleischer B. Human T helper cells reactive with somatic bacterial antigens belong to the Th1 subset. Med Microbiol Immunol. 1994;183:169–175. doi: 10.1007/BF00196051. [DOI] [PubMed] [Google Scholar]
- 43.Sharma S A, Miller G G, Perez-Perez G I, Gupta R S, Blaser M J. Humoral and cellular immune recognition of Helicobacter pylori proteins are not concordant. Clin Exp Immunol. 1994;97:126–132. doi: 10.1111/j.1365-2249.1994.tb06590.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sun S, Zhang X, Tough D F, Sprent J. Type I interferon-mediated stimulation of T cells by CpG DNA. J Exp Med. 1998;188:2335–2342. doi: 10.1084/jem.188.12.2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sur S, Wild J S, Choudhury B K, Sur N, Alam R, Klinman D M. Long term prevention of allergic lung inflammation in a mouse model of asthma by CpG oligodeoxynucleotides. J Immunol. 1999;162:6284–6293. [PubMed] [Google Scholar]
- 46.Tarkkanen J, Kosunen T U, Saksela E. Contact of lymphocytes with Helicobacter pylori augments natural killer cell activity and induces production of gamma interferon. Infect Immun. 1993;61:3012–3016. doi: 10.1128/iai.61.7.3012-3016.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.VanCott J L, Chatfield S N, Roberts M, Hone D M, Hohmann E L, Pascual D W, Yamamoto M, Kiyono H, McGhee J R. Regulation of host immune responses by modification of Salmonella virulence genes. Nat Med. 1998;4:1247–1252. doi: 10.1038/3227. [DOI] [PubMed] [Google Scholar]
- 48.Wilson K T, Ramanujam K S, Mobley H L T, Musselman R F, James S P, Meltzer S J. Helicobacter pylori stimulates inducible nitric oxide synthase expression and activity in a murine macrophage cell line. Gastroenterology. 1996;111:1524–1533. doi: 10.1016/s0016-5085(96)70014-8. [DOI] [PubMed] [Google Scholar]
- 49.Yi A K, Krieg A M. Rapid induction of mitogen-activated protein kinases by immune stimulatory CpG DNA. J Immunol. 1998;161:4493–4497. [PubMed] [Google Scholar]