Abstract
Because most pathogens initially challenge the body at epithelial surfaces, it is important to dissect the mechanisms that underlie T-cell responses to infected epithelial cells in vivo. The coccidian parasites of the genus Eimeria are protozoan gut pathogens that elicit a potent, protective immune response in a wide range of host species. CD4+ αβ T cells and gamma interferon (IFN-γ) are centrally implicated in the primary immunoprotective response. To define any additional requirements for the primary response and to develop a comparison between the primary and the secondary response, we have studied Eimeria infections of a broad range of genetically altered mice. We find that a full-strength primary response depends on β2-microglobulin (class I major histocompatibility complex [MHC] and class II MHC and on IFN-γ and interleukin-6 (IL-6) but not on TAP1, perforin, IL-4, Fas ligand, or inducible nitric oxide synthetase. Indeed, MHC class II-deficient and IFN-γ-deficient mice are as susceptible to primary infection as mice deficient in all αβ T cells. Strikingly, the requirements for a highly effective αβ-T-cell-driven memory response are less stringent, requiring neither IFN-γ nor IL-6 nor class I MHC. The class II MHC dependence was also reduced, with adoptively transferable immunity developing in MHC class II−/− mice. Besides the improved depiction of an immune response to a natural gut pathogen, the finding that effective memory can be elicited in the absence of primary effector responses appears to create latitude in the design of vaccine strategies.
A primary goal of immunological research is to understand how protective primary and secondary immune responses against natural infectious pathogens are mounted. This understanding will aid in the management of infectious disease and the design of vaccines that elicit effective secondary responses. To this end, recent research has developed operational paradigms for immune responses to different pathogens. For example, the linkage of disease resistance to the effective operation of either T-helper-cell type 1 (Th1) or T-helper-cell type 2 (Th2) responses during challenge with pathogens such as Leishmania major, Listeria monocytogenes, Toxoplasma gondii, and various nematodes (1,4,8,24).
Commonly, pathogens make primary contact with the host via infection of an external body surface, particularly the intestinal epithelium. Thus, the immune responses active at body surfaces are an essential component of the host-pathogen relationship. Consistent with this, the intestine displays a complex gut-associated lymphoid tissue (GALT), with lymphocytes present in mesenteric lymph nodes, specialized follicles such as Peyer's patches, the lamina propria, and the epithelium itself. At the same time, the gut immune system must remain tolerant to innocuous foreign antigens such as those ingested as food. Thus, effective understanding of GALT has the potential both to aid oral vaccination programs for infectious diseases and to guide oral tolerance strategies to ameliorate autoimmunity (55).
Nonetheless, there are few instances in which the primary and secondary immune responses to natural and widespread infectious pathogens of the gut epithelium have been defined in detail. This is in part because many studies have focused on pathogens delivered experimentally via subcutaneous, intravenous, and/or intraperitoneal injection. Such studies cannot assess whether immune responses to infected epithelia adhere strictly to general rules established for systemic responses or whether there are site-specific immune effector mechanisms. Significant questions include the degree to which Th1 and Th2 dichotomies and nonconventional antigen presentation mechanisms might be involved in intestinal responses and the differences in the respective requirements for mounting primary and secondary responses. By the direct study of a natural, widespread infection of the gut epithelium, this paper provides answers to these questions.
The epithelium-tropic protozoan Eimeria vermiformis is an example of an organism causing a natural intestinal infection to which vertebrates mount a highly effective immune response. Eimeria spp. are apicomplexans closely related to human pathogens Cryptosporidium spp., T. gondii, and Sarcocystis spp., all of which reside in or enter the body via the intestinal epithelium (10). Additionally, eimerian parasites have an inherent biological significance: there are estimated to be over 10,000 species that together target most vertebrates prior to and throughout breeding age. Hence, Eimeria may have provided a significant evolutionary pressure for the development of effective intestinal immune responses. Currently, significant economic cost is caused by infection of livestock with different Eimeria spp., leading to a clinical condition known as intestinal coccidiosis (47).
Infection by E. vermiformis is largely limited to the intestinal epithelium and can be initiated via the natural route by intubation of the esophagus. The resistance or susceptibility of an animal to primary infection can be reliably quantified by enumeration of parasites released into the feces and by the length of the “patent period” over which this occurs (36, 39). Previous studies have clearly established that αβ-T-cell immunity is central to the effective attenuation of primary infection (reviewed in reference 35; 54). Moreover, the immune response to E. vermiformis is highly effective, and within a 2- to 3-week period all conventional inbred mouse strains develop essentially complete immunity to reinfection by an inoculum 1,000 times greater than the primary challenge (39; A. L. Smith and A. C. Hayday, unpublished observations). We previously showed that αβ T cells are likewise necessary for the memory response and that the resistance to neither primary nor secondary infection is affected by the congenital absence of γδ cells (33). These results are consistent with the findings that αβ-T-cell-deficient mice are defective in specific T-cell-mediated immunity to the majority of (possibly all) pathogens so far employed (14, 22, 26, 52).
Although the host response attenuates primary infection, Eimeria spp. are intrinsically self-limiting. Hence, within 3 to 4 weeks even highly immunocompromised hosts become completely cleared of the pathogen (33, 36). Experimentally, this is a useful property, because although it limits the quantitative difference in susceptibility between fully immunocompetent and fully immunocompromised mice (e.g., SCID mice), the complete clearance of the primary infection allows unequivocal evaluation of the secondary response in variably immunocompromised animals. This contrasts with other popular models of infection, where attenuated pathogens or uncertain drug treatment strategies are required to eliminate the primary inoculum from immunodeficient animals. This property was exploited in this study to allow an improved understanding of respective mechanisms of the αβ-T-cell-mediated primary and secondary responses. To date, antibody depletion and adoptive-transfer experiments have indicated that CD4+ T cells and gamma interferon (IFN-γ) are involved in the development of the primary response, suggesting that it may conform to the Th1 paradigm (37, 38, 40, 41). Nonetheless, a detailed genetic analysis of the primary response had yet to be undertaken. Likewise, the requirements for the secondary response over and above an essential role for αβ T cells had heretofore remained opaque.
MATERIALS AND METHODS
Animals.
I-A−/−, T-cell receptor-β−/− (TCRβ−/−, and TCR (β × δ) mice were crossed onto a C57BL/6 background (10 generations) and bred and maintained at Yale University in specific-pathogen-free isolators in an accredited facility. C57BL/6, F2(129 × C57BL/6), β2-microglobulin−/− (β2m−/−) (both C57BL/6 and mixed backgrounds), TAP1−/− (mixed background), IFN-γ−/− (C57BL/6 background), interleukin-4−/−(IL-4−/−) (C57BL/6 background), IL-6−/− (mixed background bred back an unknown number of times to C57BL/6), perforin−/− (C57BL/6 background), and some TCRβ−/− (C57BL/6 background) mice were originally purchased from Jackson Laboratory (Bar Harbor, Maine). Mice were given an invariant diet (Hamster chow 3500) and water ad libitum. Animals were 6 to 10 weeks of age for primary infection, and secondary infections were initiated 4 to 6 weeks after primary infection.
Parasites and oocyst enumeration.
E. vermiformis (kind gift from K. S. Todd, University of Illinois) was maintained by passage in vivo, with oocysts purified and sporulated as described previously (39). Sporulation was scored microscopically, and mice were given 102 or 103 sporulated oocysts in 100 μl of water by oral gavage. At the beginning of patency (7 days postinfection), mice were individually caged and maintained over autoclaved sand, preventing reingestion of fecal material. All fetal material was collected at 24-h periods until no oocysts could be detected. Total oocysts in each collected fecal sample were counted after salt flotation in McMaster chambers, and the susceptibility for each infection was described by the total oocyst counts over the patent period. It was not appropriate to correct oocyst yields to the weight in grams of fecal material, because fecal consistency inevitably changed across the course of the infection. Because the Eimeria stocks were prepared by passage in vivo, the potency inevitably varied among inocula. For this reason, all experiments were strictly internally controlled.
Preparation of lymphocytes.
Spleens and mesenteric lymph nodes were removed from freshly euthanized mice using a sterile technique, and lymphocyte suspensions were prepared by gently disrupting diced organs through a steel mesh into sterile phosphate-buffered saline (PBS; pH 7.2)–2% fetal calf serum and maintained on ice. Erythrocytes were removed from spleen preparations by “flash lysis” according to a standard technique (56). Small intestine intraepithelial lymphocytes were prepared as described previously (17). Lymphocytes were washed, resuspended, and counted microscopically; viability always exceeded 90% according to trypan blue exclusion.
Adoptive transfer.
For adoptive transfer, cells were resuspended in PBS, pH 7.2, and injected intraperitoneally into recipient mice. Controls received PBS, pH 7.2, with no cells. Recipient mice were challenged with E. vermiformis 24 h after adoptive transfer, as described by Rose et al. (38, 42).
RESULTS
αβ T cells are essential for immunity to primary and secondary infection by E. vermiformis.
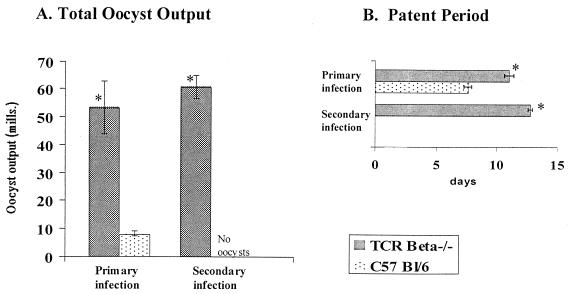
Data shown in Fig. 1 demonstrate that TCRβ−/− mice are highly susceptible to primary infection and remain highly susceptible to subsequent infection whereas intact mice are completely immune to reinfection. This supports an earlier report in which TCRα−/− mice were used (33) and in which γδ-cell-deficient mice were shown to have no overt susceptibility to either primary or secondary infection. Collectively, these data provide the basis for examining the antigen presentation and effector requirements for αβ T cells that are relevant to the anticoccidial response.
FIG. 1.
TCRβ−/−mice are highly susceptible to primary infection with E. vermiformis and do not develop immune memory to reinfection. C57BL/6 and C57BL/6 TCRβ−/− mice were infected with 1,000 sporulated oocysts of E. vermiformis, and secondary infection was initiated 30 days after primary infection. Results of oocyst counts and patent periods are means ± standard errors from groups of 6 to 10 individually housed animals. ∗, significant difference between experimental groups infected at the same time (P < 0.05).
Antigen presentation pathways involved in the primary response to Eimeria.
Previous studies have shown that mice depleted of CD4+ cells by administration of L3T4 antibody in vivo are highly sensitive to primary infection by E. vermiformis (37, 38). Likewise, protection conferred on naive mice by adoptive transfer of mesenteric lymph node T cells from infected mice was abrogated by depletion of CD4+ T cells from the inoculum (38). Conversely, anti-CD8 antibodies had no effect (38). To investigate the pathway(s) of antigen presentation to which active CD4+ cells are restricted, a series of infections was carried out in mice deficient in major histocompatibility complex (MHC) functions (Table 1). C57BL/6 I-A−/− mice are deficient in class II MHC because the targeted disruption of I-Aβ is set against a mutation in I-E; there are very few CD4+ cells in these mice (7, 19). β2m−/− mice are severely deficient in the expression of most conventional class I MHC molecules and nonconventional class Ib MHC molecules; there are very few CD8+ cells in these mice (5, 59). Likewise, TAP1−/− mice harbor reduced numbers of CD8+ MHC class I-restricted cells, because they cannot stabilize conventional, peptide-dependent MHC class I molecules (53). However, there are greater numbers of class I and class Ib MHC-reactive cells in TAP1−/− mice than in β2m−/− mice because there is scope in TAP1−/− mice for the stabilization of some conventional class I molecules by TAP-independent leader peptides and for the expression of MHC class Ib molecules, such as CD1 and TL, that are β2m dependent and TAP1 independent (6).
TABLE 1.
Characteristics of E. vermiformis infectionsa in mice with defects in antigen presentation
| Expt no. | Strain of mouseb | No. of mice | Primary infection
|
Secondary infection
|
||
|---|---|---|---|---|---|---|
| Oocyst output (106) | Patent period (days) | Oocyst output (106) | Patent period (days) | |||
| 1 | I-Aβ−/− | 5 | 94.5 ± 10.3* | 13.8 ± 0.4* | 5.4 ± 1.9* | 9.0 ± 2.1* |
| C57BL/6 | 4 | 9.7 ± 3.2† | 8.0 ± 0.0† | 0.0 ± 0.0† | 0.0 ± 0.0† | |
| I-Aβ−/− primary controlc | 6 | ndd | nd | 93.4 ± 11.1‡ | 12.3 ± 0.2‡ | |
| 2 | TCRβ−/− | 5 | 43.3 ± 4.5* | 13.7 ± 0.2* | nd | |
| I-Aβ−/− | 5 | 50.6 ± 5.0* | 11.8 ± 0.4† | nd | ||
| C57BL/6 | 7 | 6.5 ± 1.2† | 9.9 ± 0.0‡ | nd | ||
| 3 | β2m−/− | 5 | 32.3 ± 1.5* | 9.6 ± 1.0* | 0.0 ± 0.0* | 0.0 ± 0.0* |
| C57BL/6 | 5 | 10.5 ± 1.7† | 8.4 ± 1.1* | 0.0 ± 0.0* | 0.0 ± 0.0* | |
| β2m−/− primary control | 4 | nd | nd | 48.5 ± 13.3† | 8.5 ± 0.4† | |
| 4 | β2m−/− | 4 | 48.5 ± 13.3* | 8.5 ± 0.4* | 0.0 ± 0.0* | 0.0 ± 0.0* |
| β2m+/− | 6 | 15.2 ± 2.3† | 9.0 ± 0.0* | 0.0 ± 0.0* | 0.0 ± 0.0* | |
| C57BL/6 | 6 | 12.2 ± 2.8† | 8.5 ± 0.4* | 0.0 ± 0.0* | 0.0 ± 0.0* | |
| C57BL/6 primary control | 4 | nd | nd | 9.7 ± 3.2† | 8.0 ± 0.0† | |
| 5 | TAP1−/− | 7 | 8.4 ± 1.9* | 8.0 ± 0.3* | 0.0 ± 0.0* | 0.0 ± 0.0* |
| β2m−/− | 6 | 56.0 ± 6.1† | 10.4 ± 0.4† | 0.0 ± 0.0* | 0.0 ± 0.0* | |
| TAP+/+ β2m+/+ | 6 | 5.7 ± 1.1* | 7.0 ± 0.3* | 0.0 ± 0.0* | 0.0 ± 0.0* | |
All infections were initiated with 103 sporulated oocysts, with secondary infections initiated 4 to 6 weeks after the primary infection. Oocyst outputs and patent periods are means ± standard errors. Different symbols indicate significant differences in oocyst output or patent period (P < 0.05).
Mouse strain designations: −/−, homozygous targeted gene disruption; +/+, wild type.
Primary infection controls were naive mice infected in parallel with the secondary infections shown.
nd, not done.
Data derived from five independent experiments are shown in Table 1. Because Eimeria stocks are prepared by passage in vivo, their potency inevitably varies among inocula and with storage over time (compare data for C57BL/6 mice in different experiments in Table 1). Although this makes it difficult to use the same inocula to infect a wide range of different hosts, all experiments are tightly internally controlled (e.g., relative to C57BL/6 mice), and all comparisons are made within rather than across experimental groups. Some clear conclusions can be drawn. First, experiments 1 and 2 demonstrate that I-A−/− mice are ∼10-fold more susceptible than congenic C57BL/6 mice and have susceptibility similar to that of TCRβ−/− mice (experiment 2, Table 1). Compared to a normal yield of ∼10 million oocysts shed over a patent period of ∼8 days, I-A−/− mice yield ∼100 million parasites over ∼14 days. (The results of secondary infection, also shown in Table 1, will be considered below.)
Experiment 5 demonstrates that F2(129 × C57BL/6)TAP1−/− and F2(129 × C57BL/6)TAP1+/+ mice are essentially indistinguishable from one another in oocyst yield and in patent period. This insensitivity to TAP1 deficiency is consistent with previous data that depletion of CD8+ cells does not compromise the immune response (37). However, TAP1−/− mice are more resistant than F2(129 × C57BL/6)β2m−/− mice. Experiments 3 and 4 confirm the increased susceptibility to primary infection of inbred C57BL/6 β2m−/− mice compared to that of congenic C57BL/6 β2m+/− or β2m+/+ mice. The sensitivity to β2m deficiency but not to TAP1 deficiency suggests a role for cells regulated either by a nonconventional MHC class I pathway of antigen presentation (e.g., TAP-independent loading of leader peptides) or by TAP-independent, β2m-dependent MHC class I-related molecules, e.g., TL and CD1. Given that CD8 depletion, albeit by antibody treatment rather than null mutation, was previously shown to have no effect on the primary response (37), it is possible that the cells in question are either CD4+ CD8− or CD4− CD8− (double-negative) cells (see Discussion). Likewise, because the primary response is insensitive to γδ-cell deficiency (33), the β2m dependence is not likely to be a manifestation of γδ-cell involvement.
Effector molecules involved in the primary response to Eimeria.
The effector mechanism utilized by the MHC class II-restricted response was analyzed in mice deficient in a range of molecules. These included mice deficient in cytokines (Table 2) IFN-γ (since it is the defining Th1 cytokine [27]), IL-4 (since it is a defining Th2 cytokine [27]), IL-6 (since IL-6 has been reported to have effects on priming Th2 responses [32] and on Th1 and neutrophil responses [12, 34, 43]) and mice deficient in cytotoxic activities (Table 3) perforin (since Th1-type CD4+ T cells, as well as CD8+ T cells and NK cells, have been reported to utilize this effector molecule [20]) and Fas ligand (FasL), which is required for the susceptibility of cells to Fas-mediated cytolysis. Other mice examined were deficient in the receptor for IFN-γ or inducible nitric oxide synthase (iNOS), which is reported to be an IFN-γ-inducible effector against pathogens that adopt intracellular niches such as the phagolysosome utilized by Eimeria.
TABLE 2.
Characteristics of E. vermifomis infectionsa in mice with defects in cytokine production
| Expt no. | Strain of mouseb | No. of mice | Primary infection
|
Secondary infection
|
||
|---|---|---|---|---|---|---|
| Oocyst output (106) | Patent period (days) | Oocyst output (106) | Patent period (days) | |||
| 1 | IFN-γ−/− | 5 | 98.3 ± 14.8* | 11.4 ± 0.7* | 0.0 ± 0.0* | 0.0 ± 0.0* |
| C57 BL/6 | 5 | 16.4 ± 4.0† | 8.2 ± 0.4† | 0.0 ± 0.0* | 0.0 ± 0.0* | |
| IFN-γ−/− primary controlc | 5 | ndd | nd | 47.5 ± 4.6† | 11.4 ± 0.4† | |
| C57 BL/6 primary control | 5 | nd | nd | 10.8 ± 1.5‡ | 8.6 ± 0.2‡ | |
| 2 | IL-4−/− | 7 | 13.2 ± 1.6* | 8.0 ± 0.3* | 0.0 ± 0.0* | 0.0 ± 0.0* |
| C57BL/6 | 8 | 12.5 ± 2.2* | 7.9 ± 0.4* | 0.0 ± 0.0* | 0.0 ± 0.0* | |
| IL-4−/− primary control | 6 | nd | nd | 9.4 ± 1.6† | 8.2 ± 0.5† | |
| C57 BL/6 primary control | 8 | nd | nd | 8.2 ± 0.9† | 7.6 ± 0.3† | |
| 3 | IL-6−/− | 6 | 21.4 ± 1.5* | 7.3 ± 0.3* | 0.0 ± 0.0* | 0.0 ± 0.0* |
| IL-6+/+ | 6 | 8.8 ± 1.9† | 7.0 ± 0.0* | 0.0 ± 0.0* | 0.0 ± 0.0* | |
| C57BL/6 | 6 | 10.2 ± 0.8† | 7.2 ± 0.2* | 0.0 ± 0.0* | 0.0 ± 0.0* | |
| IL-6−/− primary control | 6 | nd | nd | 16.9 ± 3.8† | 7.7 ± 0.2† | |
| IL-6+/+ primary control | 6 | nd | nd | 5.1 ± 1.2‡ | 7.0 ± 0.4‡ | |
| C57 BL/6 primary control | 6 | nd | nd | 7.6 ± 1.4‡ | 6.8 ± 0.2‡ | |
All infections were initiated with 103 sporulated oocysts with secondary infections initiated 4 to 6 weeks after the primary infection. Oocyst outputs and patent periods are means, ± standard errors. Different symbols indicate significant differences in oocyst output or patent period (P < 0.05).
Mouse strain designations: −/−, homozygous targeted gene disruption; +/+, wild type.
Primary infection controls were naive mice infected in parallel with the secondary infections shown.
nd, not done.
TABLE 3.
Characteristics of E. vermifomis infectionsa in mice with defects in cytolytic function
| Expt no. | Strain of mouseb | No. of mice | Primary infection
|
Secondary infection
|
||
|---|---|---|---|---|---|---|
| Oocyst output (106) | Patent period (days) | Oocyst output (106) | Patent period (days) | |||
| 1 | Perforin−/− | 6 | 10.2 ± 1.2* | 9.3 ± 0.2* | 0.0 ± 0.0* | 0.0 ± 0.0* |
| C57BL/6 | 8 | 10.5 ± 1.3* | 9.2 ± 0.2* | 0.0 ± 0.0* | 0.0 ± 0.0* | |
| C57BL/6 primary controlc | 6 | ndd | nd | 11.7 ± 1.2† | 9.0 ± 0.4† | |
| 2 | Perforin−/− | 6 | 9.6 ± 1.0* | 9.3 ± 0.3* | 0.0 ± 0.0* | 0.0 ± 0.0* |
| gld/gld | 6 | 13.1 ± 1.8* | 8.3 ± 0.4* | 0.0 ± 0.0* | 0.0 ± 0.0* | |
| C57BL/6 | 6 | 11.7 ± 1.2* | 9.0 ± 0.4* | 0.0 ± 0.0* | 0.0 ± 0.0* | |
| C57BL/6 primary control | 6 | nd | nd | 8.6 ± 1.4† | 8.8 ± 0.2* | |
All infections were initiated with 103 sporulated oocysts with secondary infections initiated 4 to 6 weeks after the primary infection. Oocyst outputs and patent periods are means ± standard errors. Different symbols indicate significant differences in oocyst output or patent period (P < 0.05).
Mouse strain designations: −/−, homozygous targeted gene disruption; gld/gld, FasL deficient.
Primary infection controls were naive mice infected in parallel with the secondary infections shown.
nd, not done.
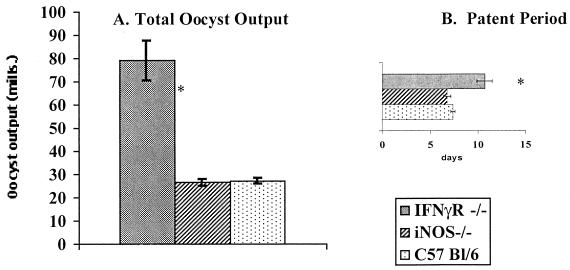
IFN-γ−/− mice were consistently up to 10-fold more susceptible to infection than congenic C57BL/6 mice and, like I-A−/− mice, shed for a longer patent period (Table 2, experiment 1). Similar results were obtained with IFN-γ receptor−/− (IFN-γR−/−) mice (Fig. 2). By contrast, the primary response was insensitive to IL-4 deficiency (Table 2, experiment 2), whereas IL-6 deficiency produced reproducible increases, albeit small, in oocyst output but no increase in patent period (Table 2, experiment 3). Although the difference was small, the internal controls were sufficiently rigorous that statistical significance could be easily assigned (P < 0.05). Conversely, primary responses were insensitive to mutations in perforin, FasL, and iNOS (Table 3 and Fig. 2). Integration of the data in Tables 1 to 3 with previously published work supports the hypothesis that MHC class II-restricted, IFN-γ-producing CD4+ cells are important in the anti-Eimeria response but it adds a role for cells responsive to β2m-associated molecules and defines a contribution of IL-6. The insensitivity to either perforin or FasL deficiency supports the findings that neither CD8+ cytolytic T cells nor NK cells are required for immunity against this parasite (49). Moreover, the data in Fig. 2 demonstrate that although the primary response depends on IFN-γ and its receptor, this dependency does not reflect any requirement for iNOS.
FIG. 2.
IFNγR−/− mice but not iNOS−/− mice are more highly susceptible than C57BL/6 mice to primary infection with E. vermiformis. Mice were infected with 1,000 sporulated oocysts of E. vermiformis. Results of oocyst counts and patent periods are means ± standard errors from groups of 6 to 10 individually housed animals. ∗, significant difference between experimental groups infected at the same time (P < 0.05).
Requirements for the secondary response to Eimeria.
Many of the mice studied in Tables 1 to 3 were subsequently rechallenged, so that the requirements for the secondary response could be compared to the requirements for the primary response. In each case, a simultaneous primary infection of syngeneic mice was undertaken to control for the reproductive index of the inoculum used. Not surprisingly, complete immunity was evident in TAP1−/− (Table 1), IL-4−/− (Table 2), perforin−/−, FasL mutant (Table 3), and iNOS−/− mice (data not shown) in which strong primary responses developed. Likewise, the slightly reduced primary response of IL-6−/− mice was followed by a robust secondary response (Table 2). However, at variance with the primary infection data was the complete immunity to rechallenge of β2m−/− mice and IFN-γ−/− mice (Tables 1 and 2). Likewise, I-A−/− mice, which are an order of magnitude more susceptible to primary infection, showed an almost 20-fold increase in resistance to rechallenge (Table 1). It cannot be argued that the secondary response is insensitive to any mutation, since αβ-T-cell deficient mice are highly susceptible to primary and secondary infections, with no decrease in oocyst output at secondary infection compared with that at primary infection (Fig. 1).
Adoptive transfer of the secondary response to Eimeria from mutant mice.
The development of immunity to E. vermiformis can be demonstrated by adoptive transfer of mesenteric lymph node cells (MLNC) to irradiated naive recipients (38, 42) or T-cell-deficient recipients (A. L. Smith and A. C. Hayday, unpublished data) (see below). Although complete protection is rarely achieved, naive recipients of MLNC from mice 8 to 12 days after primary infection are invariably more resistant to infection than mice inoculated with cells from naive mice or mice mock inoculated with PBS (38; A. L. Smith and A. C. Hayday, unpublished observations). Therefore, this well-established approach was used to test whether the relative resistance to secondary infection in I-A−/− mice reflects the development of true, transferable, cell-mediated immunity.
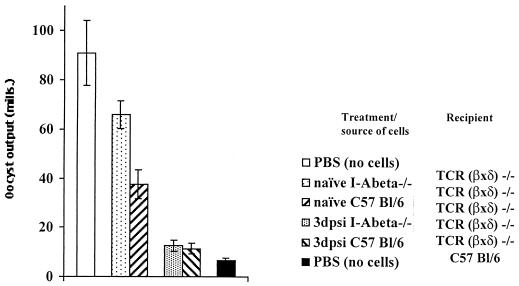
T-cell-deficient TCR(β × δ)−/− mice were used as recipients, as they are highly susceptible to infection (A. L. Smith and A. C. Hayday, submitted for publication) and do not require irradiation prior to transfer, a particular concern with intestinal epithelium-tropic pathogens since the epithelium itself is sensitive to irradiation. The data collected into groups in Fig. 3 are derived from a single large experiment in which all mice were infected and analyzed in parallel. The data are fully representative of other experiments in which smaller component groups of the experiment were examined (data not shown).
FIG. 3.
MHC class II−/− mice develop transferable immunity to E. vermiformis that functions in MHC class II+ recipients. The recipient mice listed were infected with 1,000 sporulated oocysts of E. vermiformis 24 h after receiving inocula listed (3dpsi, cells harvested on the third day following secondary infection). Oocyst counts are means ± standard errors from groups of 6 to 10 individually housed animals.
The mice of group 1 showed that TCR(β × δ)−/− mice mock inoculated with PBS were over 1 order of magnitude more susceptible than congenic C57BL/6 mice mock inoculated with PBS in the same experiment (Fig. 3). In fact, TCR(β × δ)−/− mice, unlike any other strains, would occasionally die because of overwhelming infection (the uniquely high susceptibility of these mice is the focus of a separate study [A. L. Smith and A. C. Hayday, submitted]). Similarly, TCR(β × δ)−/− mice that received cells from naive I-A−/− mice were also approximately 1 order of magnitude more susceptible than C57BL/6 controls (Fig. 3) and were not significantly different in their susceptibility from unmanipulated TCR(β × δ)−/− mice. By contrast, adoptive transfer of T cells from naive but immunocompetent C57BL/6 mice measurably reduced the susceptiblity of TCR(β × δ)−/− mice (note that resistance in adoptive-transfer recipients is never as great as in infected, unmanipulated donors [Fig. 3] [42]). These differences in the capacities of cells from naive I-A−/− mice and naive C57BL/6 mice to confer protection parallel the high susceptibility of naive I-A−/− mice to primary infection (Table 1).
Nonetheless, these differences largely disappear when donor cells are derived from previously-infected I-A−/− or C57BL/6 mice (Table 4). Relative to cells from naive C57BL/6 mice, cells taken either from mice at the peak of primary infection or from mice 3 days after secondary infection both show increased capacity to confer protection (Fig. 3; A. L. Smith and A. C. Hayday, unpublished). Strikingly, a comparable capacity was shown by cells harvested from I-A−/− mice (Table 4). This indicates that the dramatically improved resistance of I-A−/− mice to secondary, as opposed to primary, challenge is manifest in transferable immunity. Importantly, the effectors which develop in the infected I-A−/− mice function in an I-A-intact mouse, indicating that they are not specific to atypical antigen presentation pathways that may be up-regulated in MHC class II−/− mice.
TABLE 4.
Immunoprotection of TCR (β × δ)−/− mice with cells taken from I-Aβ−/− mice after secondary infectiona
| Group | Recipient strainb | Donor strain or inoculumc | No. of mice | Oocyst output | Patent period |
|---|---|---|---|---|---|
| 1 | TCR (β × δ)−/− | PBS | 8 | 90.9 ± 13.1* | 13.4 ± 0.3* |
| 2 | TCR (β × δ)−/− | Naive I-A−/− | 5 | 66.0 ± 5.6* | 12.3 ± 0.3† |
| 3 | TCR (β × δ)−/− | Naive C57BL/6 | 5 | 37.6 ± 5.9† | 11.6 ± 0.6† |
| 4 | TCR (β × δ)−/− | 3 dpsi I-A−/− | 7 | 12.6 ± 2.3‡ | 9.9 ± 0.5‡ |
| 5 | TCR (β × δ)−/− | 3 dpsi C57Bl/6 | 7 | 11.3 ± 2.1‡ | 13.8 ± 0.3* |
| 6 | I-A−/− | PBS | 5 | 50.6 ± 5.0*† | 11.8 ± 0.4† |
| 7 | C57BL/6 | PBS | 7 | 6.5 ± 1.2§ | 9.9 ± 0.0‡ |
All infections were initiated with 103 sporulated oocysts 48 h after transfer of a mixture of 108 spleen cells and MLNC. Oocyst outputs and patent periods are means ± standard errors. Different symbols indicate significant differences in oocyst output or patent period (P < 0.05).
−/−, homozygous targeted gene disruption.
dpsi, days after secondary infection.
DISCUSSION
The results in this paper are from a large number of internally controlled experiments aimed at clarifying how an animal mounts an immune response to a natural infection of its gut epithelium. The infection system under study, coccidiosis, is a protozoan-induced disease that afflicts most vertebrates before and throughout breeding age and as such may have provided an evolutionary pressure on intestinal immune responses. In addition to being economically important in its own right, Eimeria is closely related to several human pathogens that either reside in or enter the body via the intestinal epithelium, e.g., Cryptosporidium parvum and T. gondii.
The data presented support the previously published hypothesis that the immune response to primary infection is dominated by MHC class II-restricted CD4+, IFN-γ-producing αβ T cells; for example, IFN-γ−/− and I-A−/− mice are as susceptible as TCRβ−/− mice. In any studies of knockout mice, important physiological mediators can fail to score as essential because of functional redundancy. This may be the case for class II MHC and IFN-γ in the secondary response. Nonetheless, neither of these components can be substituted for in the primary response. Thus, one can conclude that the primary infection is most likely characterized by a dominant Th1-type response, while the secondary response can be both primed and executed in the absence of class II MHC or IFN-γ.
Evidence that cells other than MHC class II-restricted T cells are activated during the primary response is provided in this study by data that the primary response is also sensitive to β2m deficiency and influenced, albeit weakly, by IL-6 deficiency (Table 1). This seems to parallel other instances in which more than one class of antigen-presenting molecule is important for the establishment of the full T-cell response (57). Future studies will determine whether the cells sensitive to β2m deficiency recognize antigens presented by classical class Ia MHC, but in a TAP-independent fashion, or TAP1-independent, class I-related molecules, of which there are a growing number of candidates, e.g., CD1, TL, RAE, H60, and any putative murine homolog of MICA/B that is expressed on activated human enterocytes (9, 16, 29, 45, 48, 50).
There is precedent for the involvement of T cells reactive to such antigens in the response to protozoal infection. Thus, murine T-cell types including TCRαβ CD4+ NK1.1 cells have been shown to be CD1 restricted (23), and antibody synthesis supported by such cells was reported following infection by Plasmodium, another apicomplexan (44). One of the characteristics of such cells is an oligoclonal TCR repertoire, most utilizing Vα14Jα281 and a limited set of Vβs. Intriguingly, one such set of cells expressing Vβ3 has been documented exclusively in the intestine (25). Although CD1-restricted, CD4+ NK1.1+ cells are notable for their capacity to produce IL-4 within 30 min of anti-CD3 treatment (58), they can also produce IL-2 and IFN-γ (3, 13). Hence the sensitivity of the antieimerian response to β2m deficiency but not to IL-4 deficiency does not preclude a role for TCRαβ CD4+ NK1.1 cells. Interestingly, β2m deficiency has been reported to reduce the number of IFN-γ-producing intestinal intraepithelial lymphocytes in response to intestinal L. monocytogenes infection (15).
The rapidity and effectiveness of the secondary response to Eimeria are both extremely high and represent hoped-for standards for oral vaccines for a plethora of infectious diseases. Moreover, antieimerian immunity is long-lived, in contrast to the commonly cited transient status of memory for mucosal pathogens (reviewed in reference 2). Nonetheless, it had previously been inferred from antibody depletion studies that the requirements for the secondary response might be less than those for the primary response. For example, both anti-CD4 and anti-IFN-γ antibodies administered in vivo inhibited the primary but not the secondary response (38, 40, 41). Heretofore, the interpretation of such studies had to be qualified by the uncertain capacity of administered antibodies to penetrate the GALT, where memory cells might reside. Such qualifications are offset by the current experiments that show that a memory response, either 100% complete or 95 to 99% complete, clearly develops in IFN-γ−/− and MHC class II−/− mice, respectively, even though these mice cannot develop primary responses of any magnitude. These data are quite different from those on the effects of αβ-T-cell deficiency that obliterates both the primary and secondary responses equally (Fig. 1) (33). Moreover, we show that the increased resistance of I-A−/− mice is reflected in genuine transferable immunity (Table 4). Such memory is not provided by γδ T cells or B cells (A. L. Smith and A. C. Hayday, submitted and unpublished studies), and it will be interesting to determine the nature of the cells that mediate such immunity.
The complete dependence of the memory response on αβ T cells (Fig. 1) means that in the β2m−/− mice and IA−/− mice the memory response is most likely driven by different T-cell populations: those selected on β2m-associated MHC in IA−/− mice or those selected on class II MHC in β2m−/− mice. One might hypothesize that a variety of different memory cells can be primed during the initial encounter with an antigen. Possibly these cells compete with each other for long-term survival and reactivation. This would be consistent with the demonstration that B cells compete with each other for a limited number of available niches during recirculation (11). It would also seem consistent with the concept that Th1 and Th2 dichotomies reflect population effects rather than the exclusive and precise commitment of every cell clone to one particular phenotype (21). Hence, in the absence of one population (e.g., IFN-γ-producing, MHC class II-resticted CD4+ αβ T cells) other antigen-experienced cell types may develop more successfully and subsequently act to provide memory. The factors that prevent similar functional redundancy in the primary response need to be clarified, but, until proven otherwise, it may simply be the time constraint in making a response de novo to a rapidly proliferating pathogen. This could likewise explain the capacity to “see” fully effective recall responses in mice, such as the IFN-γ−/− strain, in which there was no measurable primary response.
The capacity to elicit pathogen-limiting memory responses in the absence of all molecules except for TCRαβ may be germane to vaccine programs that would be designed to recapitulate the efficacy of the anti-Eimeria response. Different adjuvants and vaccine delivery regimens are known to promote some effector functions better than others. The findings presented here suggest, perhaps surprisingly, that there may be room for some latitude in vaccine design. So long as appropriate, neutralizing epitopes can be presented and recognized, effective immunoprotective T cells may develop in multiple compartments.
ACKNOWLEDGMENTS
The work was supported by NIH grants AI 27855 and AI 38932 to A.H., by the Wellcome Trust (A.H.), and by the BBSRC (A.L.S). Laboratory facilities were supported by the Dunhill Medical Trust.
We thank Bob Tigelaar, Scott Roberts, Jasmine Sia, Craig Findly, Elizabeth Ramsburg, and other colleagues for their valuable input during this study.
REFERENCES
- 1.Abbas A K, Murphy K M, Sher A. Functional diversity of helper T lymphocytes. Nature (London) 1996;383:787–793. doi: 10.1038/383787a0. [DOI] [PubMed] [Google Scholar]
- 2.Ahmed R, Gray D. Immunological memory and protective immunity: understanding their relation. Science. 1996;272:54–60. doi: 10.1126/science.272.5258.54. [DOI] [PubMed] [Google Scholar]
- 3.Arase H, Arase N, Saito T. Interferon γ production by natural killer (NK) cells and NK1.1+ T cells upon NKRP1 crosslinking. J Exp Med. 1996;183:2391–2396. doi: 10.1084/jem.183.5.2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beaman M H, Wong S Y, Remington J S. Cytokines, toxoplasma and intracellular parasitism. Immunol Rev. 1992;127:97–117. doi: 10.1111/j.1600-065x.1992.tb01410.x. [DOI] [PubMed] [Google Scholar]
- 5.Bix M, Raulet D. Inefficient positive selection of T cells directed by haematopoietic cells. Nature (London) 1992;359:330–333. doi: 10.1038/359330a0. [DOI] [PubMed] [Google Scholar]
- 6.Cheroute H, Holcombe H R, Tangri S, Castano A R, Teitell M, Miller J E W, Cardell S, Benoist C, Mathis D, Huse W D, Peterson P A, Kronenburg M. Antigen presenting function of the TL antigen and mouse CD1 molecules. Immunol Rev. 1995;147:31–51. doi: 10.1111/j.1600-065x.1995.tb00086.x. [DOI] [PubMed] [Google Scholar]
- 7.Cosgrove D, Gray D, Dierich A, Kaufman J, Lemeur M, Benoist C, Mathis D. Mice lacking MHC class II molecules. Cell. 1991;66:1051–1066. doi: 10.1016/0092-8674(91)90448-8. [DOI] [PubMed] [Google Scholar]
- 8.Cox F E G, Liew F Y. T cell subsets and cytokines in parasitic infections. Immunol Today. 1992;13:445–448. doi: 10.1016/0167-5699(92)90072-F. [DOI] [PubMed] [Google Scholar]
- 9.Crowley M P, Reich Z, Mavaddat N, Altman J D, Chien Y-H. The recognition of the non-classical major histocompatibility complex (MHC) class I molecule, T10, by the γδ T cell, G8. J Exp Med. 1997;185:1223–1230. doi: 10.1084/jem.185.7.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Current W L, Upton S J, Long P L. Taxonomy and life cycles. In: Long P L, editor. Coccidiosis of man and domestic animals. Boca Raton, Fla: CRC Press; 1990. pp. 1–16. [Google Scholar]
- 11.Cyster J, Hartley S, Goodnow C. Competition for follicular niches excludes self-reactive cells from the recirculating B-cell repertoire. Nature. 1994;371:389–395. doi: 10.1038/371389a0. [DOI] [PubMed] [Google Scholar]
- 12.Dalrymple S A, Lucian L A, Slattery R, McNiel T, Aud D M, Fuchino S, Lee F, Murray R. Interleukin-6-deficient mice are highly susceptible to Listeria monocytogenes infection: correlation with inefficient neutrophilia. Infect Immun. 1995;63:2262–2268. doi: 10.1128/iai.63.6.2262-2268.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Denkers E Y, Scharton-Kirsten T, Barbieri S, Caspar P, Sher A. A role for CD4+NK1.1+ T lymphocytes as major histocompatibility complex class II-independent helper cells in the generation of CD8+ effector function against intracellular infection. J Exp Med. 1996;184:131–139. doi: 10.1084/jem.184.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eichelberger M, McMickle A, Blackman M, Mombaerts P, Tonegawa S, Doherty P C. Functional analysis of the TCR alpha− beta+ cells that accumulate in the pneumonic lung of influenza virus-infected TCR-alpha−/−mice. J Immunol. 1995;154:1569–1576. [PubMed] [Google Scholar]
- 15.Emoto M, Neuhaus O, Emoto Y, Kaufmann S H E. Influence of β2-microglobulin expression on gamma interferon secretion and target cell lysis by intraepithelial lymphocytes during intestinal Listeria monocytogenes infection. Infect Immun. 1996;64:569–575. doi: 10.1128/iai.64.2.569-575.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Exley M, Garcia J, Balk S P, Porcelli S. Requirements for CD1d recognition by human invariant Vα24+CD4-CD8-T cells. J Exp Med. 1997;186:109–120. doi: 10.1084/jem.186.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Findly R C, Roberts S J, Hayday A C. Dynamic response of murine gut intraepithelial T cells after infection by the coccidian parasite Eimeria. Eur J Immunol. 1993;23:2557–2564. doi: 10.1002/eji.1830231027. [DOI] [PubMed] [Google Scholar]
- 18.Fu Y-X, Roark C E, Kelly K, Drevets D, Campbell P, O'Brien R, Born W. Immune protection and control of inflammatory tissue necrosis by gamma delta T cells. J Immunol. 1994;150:550–555. [PubMed] [Google Scholar]
- 19.Grusby M J, Johnson R S, Papaioannou V E, Glimcher L H. Depletion of CD4(+) T cells in major histocompatibility complex II-deficient mice. Science. 1991;253:1417–1420. doi: 10.1126/science.1910207. [DOI] [PubMed] [Google Scholar]
- 20.Hahn S, Gehri R, Erb P. Mechanism and biological significance of CD4-mediated cytotoxicity. Immunol Rev. 1995;146:56–79. doi: 10.1111/j.1600-065x.1995.tb00684.x. [DOI] [PubMed] [Google Scholar]
- 21.Kelso A. Th1 and Th2 subsets: paradigms lost? Immunol Today. 1995;16:374–379. doi: 10.1016/0167-5699(95)80004-2. [DOI] [PubMed] [Google Scholar]
- 22.Ladel C H, Blum C, Dreher K, Reifenburg K, Kaufmann S H E. Protective role of γδ T cells and αβ T cells in tuberculosis. Eur J Immunol. 1995;25:2877–2881. doi: 10.1002/eji.1830251025. [DOI] [PubMed] [Google Scholar]
- 23.Lantz O, Bendelac A. An invariant T cell receptor alpha chain is used by a unique subset of major histocompatibility complex class I-specific CD4+ and CD4-8- T cells in mice and humans. J Exp Med. 1994;180:1097–1106. doi: 10.1084/jem.180.3.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Locksley R M, Louis J A. Immunology of leishmaniasis. Curr Opin Immunol. 1992;4:413–418. doi: 10.1016/s0952-7915(06)80032-4. [DOI] [PubMed] [Google Scholar]
- 25.Masuda K, Makino Y, Cui J, Ito T, Tokuhisa T, Takahama Y, Koseki H, Tsuchida K, Koike T, Moriya H, Amano M, Taniguchi M. Phenotypes and invariant alpha beta TCR expression of peripheral V alpha 14+ NK T cells. J Immunol. 1997;158:2076–2082. [PubMed] [Google Scholar]
- 26.Mombaerts P, Arnoldi J, Russ F, Tonogawa S, Kaufmann S H E. Different roles of αβ and γδ T cells in immunity against an intracellular bacterial pathogen. Nature (London) 1993;365:53–56. doi: 10.1038/365053a0. [DOI] [PubMed] [Google Scholar]
- 27.Mosmann T R, Coffman R L. Heterogeneity of cytokine secretion patterns and function of helper T cells. Adv Immunol. 1989;46:111–147. doi: 10.1016/s0065-2776(08)60652-5. [DOI] [PubMed] [Google Scholar]
- 28.Mukasa A, Hiromatsu K, Matsuzaki G, O'Brien R, Born W, Nomoto K. Bacterial infection of the testis leading to autoagressive immunity triggers apparently opposed responses of alpha beta and gamma delta T cells. J Immunol. 1995;155:2047–2056. [PubMed] [Google Scholar]
- 29.Panga A, Barone A, Mayer L. Stimulation of lamina propria lymphocytes by intestinal epithelial cells: evidence for recognition of non-classical restriction elements. J Exp Med. 1994;179:943–950. doi: 10.1084/jem.179.3.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pao W, Wen L, Smith A L, Gulbranson-Judge A, Zheng B, Kelsoe G, Owen M J, MacLennan I, Hayday A C. γδ T cell help of B cells is induced by repeated parasitic infection, in the absence of other T cells. Curr Biol. 1996;6:1317–1325. doi: 10.1016/s0960-9822(02)70718-5. [DOI] [PubMed] [Google Scholar]
- 31.Peng S, Madaio M P, Hayday A C, Craft J. Propagation and regulation of autoimmunity by γδ T cells. J Immunol. 1996;157:5689–5698. [PubMed] [Google Scholar]
- 32.Rincon M, Anguita J, Nakamura T, Fikrig E, Flavell R A. Interleukin (IL)-6 directs the differentiation of IL-4-producing CD4+ T cells. J Exp Med. 1997;185:461–469. doi: 10.1084/jem.185.3.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roberts S J, Smith A L, West A B, Wen L, Findly R C, Owen M J, Hayday A C. T cell αβ+ and γδ+ deficient mice display abnormal but distinct phenotypes toward a natural, widespread infection of the intestinal epithelium. Proc Natl Acad Sci USA. 1996;93:11774–11779. doi: 10.1073/pnas.93.21.11774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Romani L, Mencacci A, Cenci E, Spaccapello R, Toniatti C, Puccetti P, Bistoni F, Poli V. Impaired neutrophil response and CD4+ T helper cell 1 development in interleukin-6 deficient mice infected with Candida albicans. J Exp Med. 1996;183:1345–13555. doi: 10.1084/jem.183.4.1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rose M E. Eimeria, isospora and cryptosporidium. In: Soulsby E J L, editor. Immunology, immunopathology and immunoprophylaxis of parasitic infections. Vol. 3. Boca Raton, Fla: CRC Press; 1987. pp. 275–312. [Google Scholar]
- 36.Rose M E, Hesketh P. Eimerian life cycles: the patency of Eimeria vermiformis, but not Eimeria pragensis is subject to host (Mus musculus) influence. J Parasitol. 1986;72:949–952. [PubMed] [Google Scholar]
- 37.Rose M E, Hesketh P, Wakelin D. Immune control of murine coccidiosis: CD4+ and CD8+ lymphocytes contribute differentially in resistance to primary and secondary infections. Parasitology. 1992;105:349–354. doi: 10.1017/s0031182000074515. [DOI] [PubMed] [Google Scholar]
- 38.Rose M E, Joysey H S, Hesketh P, Grencis R K, Wakelin D. Mediation of immunity to Eimeria vermiformis in mice by L3T4+ T cells. Infect Immun. 1988;56:1760–1765. doi: 10.1128/iai.56.7.1760-1765.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rose M E, Owen D G, Hesketh P. Susceptibility to coccidiosis: effect of strain of mouse on reproduction of Eimeria vermiformis. Parasitology. 1984;88:45–54. doi: 10.1017/s0031182000054330. [DOI] [PubMed] [Google Scholar]
- 40.Rose M E, Wakelin D, Hesketh P. Gamma interferon controls Eimeria vermiformis primary infection in BALB/c mice. Infect Immnun. 1989;57:1599–1603. doi: 10.1128/iai.57.5.1599-1603.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rose M E, Wakelin D, Hesketh P. Interferon-gamma-mediated effects upon immunity to coccidial infections in the mouse. Parasite Immunol. 1991;13:63–74. doi: 10.1111/j.1365-3024.1991.tb00263.x. [DOI] [PubMed] [Google Scholar]
- 42.Rose M E, Wakelin D, Joysey H S, Hesketh P. Immunity to coccidiosis: adoptive transfer in NIH mice challenged with Eimeria vermiformis. Parasite Immunol. 1988;10:59–69. doi: 10.1111/j.1365-3024.1988.tb00203.x. [DOI] [PubMed] [Google Scholar]
- 43.Ruzek M C, Miller A H, Opal S M, Pearce B D, Biron C A. Characterization of early cytokine responses and an interleukin (IL)-6-dependent pathway of endogenous glucocorticoid induction during murine cytomegalovirus infection. J Exp Med. 1997;185:1185–1192. doi: 10.1084/jem.185.7.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schofield L, McConville M J, Hansen D, Campbell A S, Fraser-Reid B, Grusby M J, Tachado S D. CD1d-restricted immunoglobulin G formation to GPI-anchored antigens mediated by NK T cells. Science. 1999;283:225–229. doi: 10.1126/science.283.5399.225. [DOI] [PubMed] [Google Scholar]
- 45.Shawar S M, Vyas J M, Rodgers J R, Rich R R. Antigen presentation by major histocompatibity complex class Ib molecules. Annu Rev Immunol. 1994;12:839–880. doi: 10.1146/annurev.iy.12.040194.004203. [DOI] [PubMed] [Google Scholar]
- 46.Shiohara T, Moriya N, Hayakawa J, Itohara S, Ishikawa H. Resistance to cutaneous graft-vs.-host disease is not induced in T cell receptor delta gene-mutant mice. J Exp Med. 1996;183:1483–1489. doi: 10.1084/jem.183.4.1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shirley M W. Research on avian coccidia: an update. Br Vet J. 1992;148:479–499. doi: 10.1016/0007-1935(92)90004-K. [DOI] [PubMed] [Google Scholar]
- 48.Sieling P A, Chatterjee D, Porcelli S A, Prigozy T I, Mazzaccaro R J, Soriano T, Bloom B R, Brenner M B, Kronenberg M, Brennan P J, Modlin R L. CD1-restricted T cell recognition of microbial lipoglycan antigens. Science. 1995;269:227–230. doi: 10.1126/science.7542404. [DOI] [PubMed] [Google Scholar]
- 49.Smith A L, Rose M E, Wakelin D. The role of natural killer cells in resistance to coccidiosis; investigations in a murine model. Clin Exp Immunol. 1994;97:273–279. doi: 10.1111/j.1365-2249.1994.tb06080.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sugita M, Jackman R M, van Donselaar E, Behar S M, Rogers R A, Peters P J, Brenner M B, Porcelli S A. Cytoplasmic tail-dependent localization of CD1b antigen presenting molecules to MIIC's. Science. 1996;273:349–352. doi: 10.1126/science.273.5273.349. [DOI] [PubMed] [Google Scholar]
- 51.Szczepnik M, Anderson L R, Ushio H, Ptak W, Owen M J, Hayday A C, Askenase P W. Gamma delta T cells from tolerized alpha beta T cell receptor (TCR)-deficient mice inhibit contact sensitivity-effector T cells in vivo, and their inteferon-gamma production in vitro. J Exp Med. 1996;184:2129–2139. doi: 10.1084/jem.184.6.2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tsuji M, Mombaerts P, Lefrancois L, Nussensweig R S, Zavala F, Tonegawa S. γδ T cells contribute to immunity against the liver stages of malaria in αβ T-cell-deficient mice. Proc Natl Acad Sci USA. 1994;91:345–349. doi: 10.1073/pnas.91.1.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Van-Kaer L, Ashton-Rickardt P G, Ploegh H L, Tonegawa S. TAP1 mutant mice are deficient in antigen presentation, surface class I molecules and CD4-8+ T cells. Cell. 1992;71:1205–1214. doi: 10.1016/s0092-8674(05)80068-6. [DOI] [PubMed] [Google Scholar]
- 54.Wakelin D, Rose M E. Immunity to coccidiosis. In: Long P L, editor. Coccidiosis of man and domestic animals. Boca Raton, Fla: CRC Press; 1990. pp. 281–306. [Google Scholar]
- 55.Weiner H L. Oral tolerance for the treatment of autoimmune diseases. Annu Rev Med. 1997;48:341–351. doi: 10.1146/annurev.med.48.1.341. [DOI] [PubMed] [Google Scholar]
- 56.Weir D M. Handbook of experimental immunology. 4th ed. II. Oxford, United Kingdom: Blackwell Scientific Publications; 1986. [Google Scholar]
- 57.Wicker L S, Leiter E H, Todd J A, Renjilian R J, Peterson E, Fischer P L, Zijlstra M, Jaenisch R, Peterson L B. Beta 2 microglobulin deficient NOD mice do not develop insulin dependent diabetes. Diabetes. 1994;43:500–504. doi: 10.2337/diab.43.3.500. [DOI] [PubMed] [Google Scholar]
- 58.Yoshimoto T, Paul W. CD4pos, NK1.1pos T cells promptly produce interleukin 4 in response to in vivo challenge with anti-CD3. J Exp Med. 1994;174:1285. doi: 10.1084/jem.179.4.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zijlstra M, Bix M, Simister N E, Loring J M, Raulet D H, Jaenisch R. β2-Microglobulin deficient mice lack CD4(−)CD8(+) cytolytic T cells. Nature (London) 1990;344:742–746. doi: 10.1038/344742a0. [DOI] [PubMed] [Google Scholar]