Abstract
Background
The severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) has an affinity for the angiotensin-converting enzyme 2 (ACE2) receptors, which are present abundantly on the diaphragm. This study aims to describe temporal changes in diaphragmatic thickness and excursion using ultrasonography in subjects with acute COVID-19.
Methods
This prospective observational study included adults hospitalized with COVID-19 in the past 48 hours. The diaphragm thickness at end-expiration (DTE), diaphragm thickening fraction (DTF), and diaphragm excursion during tidal breathing (DE) and maximal inspiration (DEmax) were measured using ultrasonography daily for 5 days. The changes in DTE, DTF, DE, and Demax from day 1 to day 5 were assessed.
Results
This study included 64 adults (62.5% male) with a mean (SD) age of 50.2 (17.5) years. A majority (91%) of the participants had mild or moderate illness. The median (IQR) DTE, DTF (%), DE and Demax on day 1 were 2.2 (1.9, 3.0) mm, 21.5% (14.2, 31.0), 19.2 (16.5, 24.0) mm, and 26.7 (22.0, 30.2) mm, respectively. On day 5, there was a significant reduction in the DTE (p=0.002) with a median (IQR) percentage change of -15.7% (-21.0, 0.0). The DTF significantly increased on day 5 with a median (IQR) percentage change of 25.0% (-19.2, 98.4), p=0.03. There was no significant change in DE and Demax from day 1 to day 5, with a median (IQR) percentage change of 3.6% (-5.2, 15) and 0% (-6.7, 5.9), respectively.
Conclusions
Non-intubated patients with COVID-19 exhibited a temporal decline in diaphragm thickness with increase in thickening fraction over 5 days of hospital admission. Further research is warranted to assess the impact of COVID-19 pneumonia on diaphragmatic function.
Keywords: Ultrasonography, Diaphragm, Diaphragm atrophy, Diaphragm function, Respiratory muscle, SARS-CoV-2
1. Introduction
Coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), has resulted in an unprecedented pandemic affecting millions of people worldwide [1]. Although it is predominantly a pulmonary disease, extrapulmonary involvement by SARS-CoV-2 is common and has important clinical implications [2,3]. The angiotensin-converting enzyme 2 (ACE2) receptors play a pivotal role in the entry of SARS-CoV-2 in various cells and subsequent injury [4]. The ACE2 receptors are expressed in multiple organs – lung, heart, kidney, intestine, and muscles [4,5]. The diaphragm, which is the main muscle of respiration, also expresses ACE2 receptors and may possibly be infiltrated by SARS-CoV-2 [5].
Among non-COVID patients with acute respiratory failure, poor contractile strength and atrophy of the diaphragmatic muscle are frequent findings [6]. The optimally functioning diaphragm generates adequate alveolar ventilation, whereas a poorly functioning diaphragm is associated with worsening of respiratory failure, difficult weaning, prolonged ventilation, prolonged hospital stay, and mortality [6], [7], [8]. Till date, there are no prospective studies that have assessed diaphragmatic function among subjects with COVID-19.
Most of the tools available for the assessment of either the thickness or contractility of the diaphragm, such as measurement of maximal inspiratory pressure (MIP), fluoroscopy, dual-energy X-ray absorptiometry (DEXA) scan, computed tomography (CT) scan, or magnetic resonance imaging (MRI) are not practically useful in acutely ill patients [6]. Similarly, placement of an esophageal balloon and measurement of transdiaphragmatic pressure (Pdi) by phrenic nerve stimulation is invasive, cumbersome and impractical in the care of COVID-19 patients. Ultrasonography (USG) is an excellent bedside, non-invasive, quick and easy-to-perform technique that has been used for the assessment of the thickness as well as contractility of the diaphragm among non-COVID critically ill patients [6,9,10]. Critical care ultrasonography has become standard of care, and diaphragm ultrasound can potentially be incorporated into the same routine. Importantly, the diaphragm characteristics assessed by USG were also shown to correlate with outcomes [9]. This study aimed to report the trends of changes in the diaphragm thickness and movement assessed by USG among subjects hospitalized with COVID-19. We hypothesized that patients who require hospitalization for COVID-19 pneumonia would demonstrate abnormalities of diaphragmatic thickness and excursion.
2. Methods
2.1. Study design, settings and participants
This prospective, observational study was conducted at a tertiary care teaching hospital in July 2021. All consecutive adults (aged 18 years or older) hospitalized with COVID-19 (confirmed by rapid antigen test, cartridge-based nucleic acid amplification test, or real-time RT-PCR for SARS-CoV-2) within the previous 48 hrs were eligible for inclusion. Subjects with a pre-existing neuromuscular disease (e.g., myasthenia gravis, Guillain-Barré syndrome, or phrenic nerve palsy), need for invasive mechanical ventilation at admission, surgical dressing over the right lower rib cage, or those who declined consent to participate were excluded. The disease severity was categorized according to the COVID-19 management guidelines issued by the Indian Ministry of Health & Family Welfare. Accordingly, severe disease was defined by shock, hypoxemia (SpO2 < 90% on room air) or tachypnea (respiratory rate > 30 breaths per minute); moderate disease was defined as presence of dyspnea, SpO2 between 90 and 94%, and respiratory rate between 24 and 30 breaths per minute. All other patients were considered to have mild disease [11].
The study protocol was approved by the institute ethics committee. Informed consent was taken from each patient prior to enrolment in the study.
2.2. Study outcomes
The primary outcome of our study was to assess the temporal change in the diaphragm thickness and excursion using ultrasonography over 5 days of admission for COVID-19 pneumonia. The secondary outcome was to determine the factors at baseline associated with the decline of diaphragmatic thickness and excursion during hospital stay. We also aimed to study the association of the trend of diaphragmatic thickness and excursion with the in-hospital outcomes.
2.3. Ultrasonography schedule and techniques
All measurements were performed by a trained fellow (AR) using a portable USG machine (FUJIFILM Sonosite, Inc. Bothell, WA, USA) daily for 5 days following enrolment, or till discharge/death, whichever was earlier.
The diaphragm thickness (DT) was measured using a 7-10 MHz linear array probe set to B-mode in the zone of apposition of the diaphragm on the right side in the supine position as described previously [9,12]. The linear probe was positioned at the 8th or 9th intercostal space with vertical orientation in the mid-axillary line and adjusted until an optimum image of the hemidiaphragm is visualized. The diaphragm was identified as a three-layered structure superficial to the liver, consisting of a relatively non-echogenic diaphragm muscle bounded on either side by echogenic pleura and peritoneum (figure in supplementary file). The thickness of the diaphragm was measured at end-expiration (DTE) and end-inspiration (DTI) during tidal breathing. The diaphragm thickening fraction (DTF) was calculated using the equation, DTF = (DTI-DTE)/DTE*100.
The excursion of the diaphragm (DE) was assessed using a curvilinear probe (3.5 – 5.0 MHz) [13]. The probe was set to the B-mode and positioned below the right costal margin along the mid-clavicular line, directed medially, cranially, and dorsally so that the ultrasound beam was perpendicular to the posterior third of the right hemidiaphragm. The scanning was performed along the long axis of the intercostal spaces with the liver serving as an acoustic window. The excursion of the diaphragm was measured using the M-mode. The excursion was recorded during tidal breathing (DE) and maximal inspiration, i.e., from residual volume to total lung capacity (DEmax). During deep breathing the probe position and angle were adjusted so that the descending lung does not obscure the image. The DE and DEmax were measured by placing the first caliper at the foot of the inspiratory slope of the diaphragm line and the second caliper at the apex of the slope (figure in supplementary file). The average of three measurements was recorded for all the above-described diaphragm parameters.
Besides daily USG, the WHO 8-point ordinal scale of COVID-19 severity [14], the need for escalation of respiratory support (supplementary oxygen, HFNC/NIV or endotracheal intubation), transfer to ICU, discharge or death, and functional status at the time of hospital discharge using the post-COVID functional status (PCFS) scale [15] were also recorded. Those who were switched to a higher level of respiratory support (from supplemental oxygen to HFNC or NIV, or from HFNC or NIV to intubation), or shifted from ward to ICU were classified to have deterioration. Based on the functional status at the time of hospital discharge, participants were classified as having poor and good functional status.
2.4. Statistical analysis
Categorical variables were presented as number and percentage. Continuous variables were reported as mean ± standard deviation (SD) or median (IQR) as appropriate. The changes in diaphragm thickness and excursion from day 1 to day 5 of enrolment were calculated and depicted as percentage changes. The Friedman test was used to assess the daily trend of change of the diaphragm characteristics from day 1 to day 5. The Wilcoxon signed-rank test was used to analyze the percentage changes in diaphragm thickness and excursion between day 1 and day 5. The temporal change in diaphragmatic characteristics between day 1 and day 5 was compared between subgroups based on various outcomes using Wilcoxon rank sum test. To determine the factors at baseline associated with changes in diaphragm thickness or excursion, the participants were categorized in two groups based on median values of clinically important parameters such as such age, gender, acute physiology and chronic health evaluation (APACHE) score, sequential organ failure assessment (SOFA) score, C-Reactive protein, ferritin, lactate dehydrogenase (LDH), and IL6 levels, and were compared using the Wilcoxon rank sum test. STATA version 16 (College Station, TX, USA) software was used to analyze the data. A p-value of less than 0.05 was considered significant. Given the lack of similar study in COVID, the sample size was based on pragmatic approach and no calculation was done for the same.
3. Results
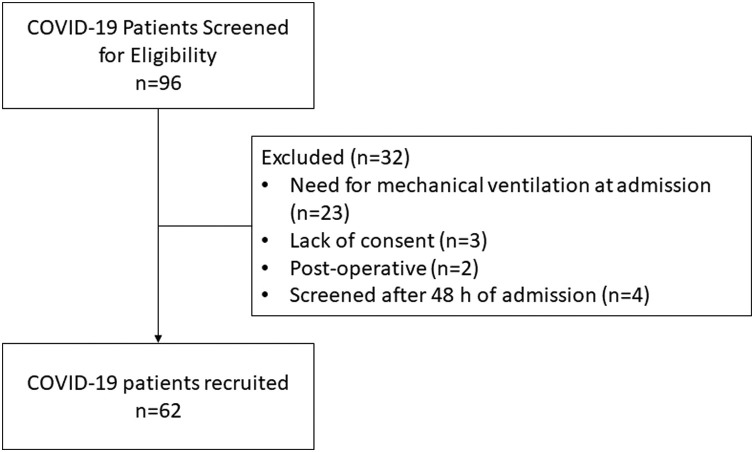
During the study period, 96 consecutive subjects with COVID-19 were screened for eligibility. A total of 32 subjects were excluded, most commonly due to the need for invasive mechanical ventilation at the time of admission (n=23). Finally, 64 subjects were included in the study (Fig. 1 ).
Fig. 1.
Study flow.
The mean (SD) age of the study participants was 50.2 (17.5) years, and 40 (62.5%) were men. The median (IQR) duration between the onset of the symptoms and hospitalization was 7.0 (5.0, 12.3) days. The majority of the participants (90.6%) had a mild or moderate illness. The median levels of inflammatory markers were as follows: C–Reactive Protein (CRP) - 7.8 mg/L (normal: 1.0–5.0 mg/L); lactate dehydrogenase (LDH) - 351 U/L (normal: 135–214 U/L); ferritin - 669 ng/mL (normal: 15–150 ng/mL); and interleukin-6 (IL-6) - 11.65 pg/mL (normal: 5–15 pg/mL). Thirty-two subjects had one or more co-morbidities (coronary artery diseases, diabetes, hypertension, acute myeloid leukaemia, carcinoma breast and oesophagus, hypothyroidism). The demographic characteristics of the study participants are summarized in Table 1 .
Table 1.
Demographic profile of the study participants.
| Parameters (n=64) | Results |
|---|---|
| Age, mean (SD), years | 50.2 (17.5) |
| Duration of symptoms, median (IQR), days | 7.0 (5.0, 12.3) |
| APACHE-II score, median (IQR) | 3.5 (1.8, 8.0) |
| SOFA score, median (IQR) | 2 (0, 3) |
| Leukocyte count, median (IQR), 109 per L | 8.9 (6.5, 13.0) |
| Neutrophil count, median (IQR), 109 per L | 6.5 (4.1, 10.8) |
| Lymphocyte count, mean (IQR), 109 per L | 1.0 (0.7, 1.6) |
| Neutrophil:Leukocyte ratio (NLR), median (IQR) | 5.9 (3.1, 10.6) |
| Platelet Count, median (IQR), × 109 per L | 240 (180, 350) |
| Thrombocytopenia, n(%) | 9 (14) |
| Blood urea, median (IQR), mg/dL | 33 (21.8, 51.5) |
| Creatinine, median (IQR), mg/dL | 0.8 (0.5, 1.0) |
| Albumin, median (IQR), g/dL | 3.7 (3.4, 4.0) |
| CRP, median (IQR), mg/L | 7.8 (3.1, 13.1) |
| Ferritin, median (IQR), ng/L | 669 (325,1319) |
| Lactate dehydrogenase, median (IQR), U/L | 351 (279, 509) |
| Interleukin 6, median (IQR), ng/mL | 11.65 (5.1, 26.5) |
| Modes of oxygen support, n (%) | |
| Room air | 38 (59.4) |
| Supplemental O2 by mask/nasal cannula | 20 (31.2) |
| NIV / HFNC | 6 (9.4) |
| Use of Corticosteroids, n (%) | 29 (45.3) |
| Duration Of Oxygen, median (IQR) days; n=23 | 4 (2.5, 6.5) |
| Duration of HFNC, median (IQR) days; n=9 | 4 (2, 10) |
| Duration of NIV, median (IQR) days; n=9 | 2 (1, 3) |
| Duration of MV, median (IQR) days; n=8 | 3 (2, 8) |
| HDU Stay, median (IQR) days; n=13 | 6 (3, 10) |
| ICU Stay, median (IQR) days; n=11 | 9 (6, 15.5) |
| Hospital Stay, median (IQR) days | 10 (5, 13) |
APACHE – Acute physiology and chronic health evaluation, SOFA – Sequential organ failure assessment, CRP – C-reactive protein, NIV – non-invasive ventilation, HFNC – high flow nasal cannula, MV – mechanical ventilation, HDU – high dependency unit, ICU – intensive care unit
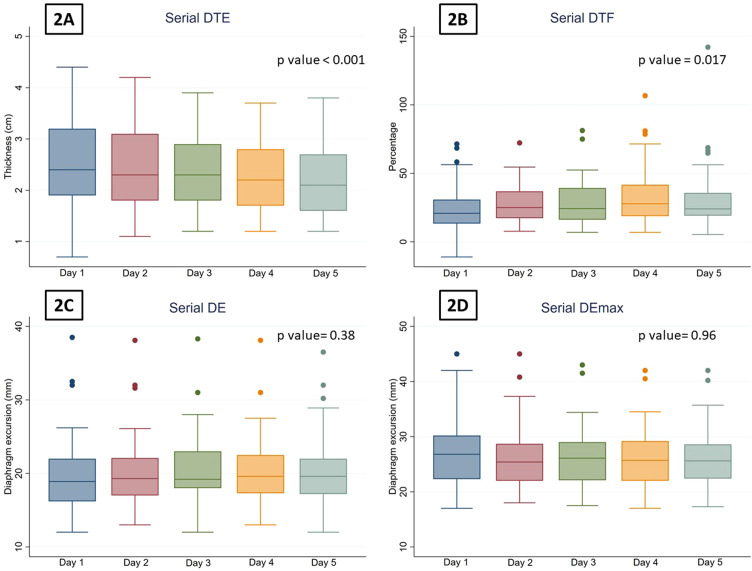
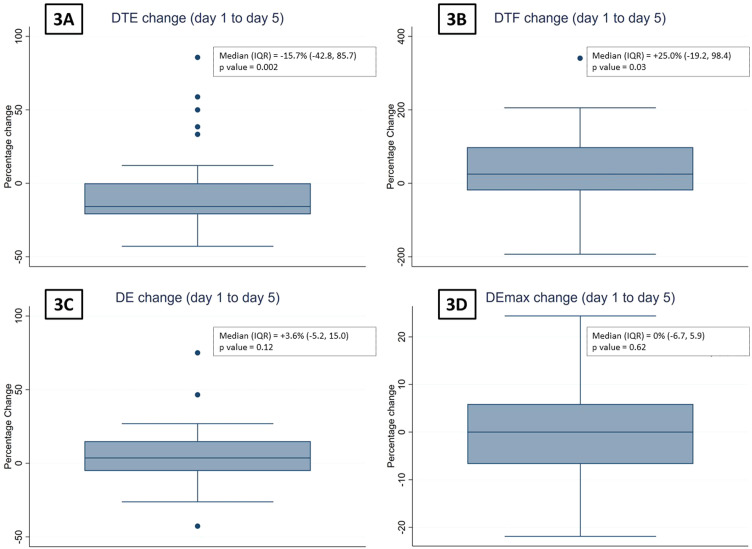
The number of subjects who underwent serial ultrasonography of the diaphragm on days 1, 2, 3, 4 and 5 were 64, 64, 57, 47, and 37, respectively. The trends of the DTE and DTF on consecutive days is depicted in Fig. 2, Fig. 3 . The DTE showed a decline from a median (IQR) of 2.2 (1.9, 3.0) mm on day 1 (baseline) to 2.1 (1.6, 2.7) mm on day 5. From day 1 to day 5, the median (IQR) percentage change in DTE was -15.7% (-42.8, 85.7), p = 0.002. The median (IQR) DTF on day 1 and day 5 were 20.8% (13.3, 30.8) and 24.0% (19.0, 35.7), respectively. The median (IQR) percentage change in DTF between day 1 and day 5 was 25.0% (-19.2, 98.4), p = 0.03.
Fig. 2.
Percentage reductions in the (2A) diaphragm thickness at end-expiration (DTE), (2B) diaphragm thickening fraction (DTF), (2C) the diaphragm excursion during tidal breathing (DE) and (2D) the diaphragm excursion during deep breathing (DEmax) on days 1 through 5 of enrolment.
Fig. 3.
The box plots depicting the percentage reductions in (3A) the diaphragm thickness at end-expiration (DTE), (3B) diaphragm thickening fraction (DTF), (3C) the diaphragm excursion during tidal breathing (DE), and (3D) the diaphragm excursion during deep breathing (DEmax) on day 5 compared with day 1 of enrolment.
There was no significant change in DE or Demax from day 1 to day 5, with a median (range) percentage change of 3.6% (-5.2, 15.0) and 0% (-6.7, 5.9), respectively (Figs. 2 and 3).
The temporal changes in the DTE, DTF DE, and Demax were compared between various subgroups based on the median values of age (<51 vs ≥51) years, duration of symptoms (<7 vs ≥7) days, CRP (<7.8 vs ≥7.8) mg/L, LDH (<351 vs ≥351) U/L, ferritin (<669.0 vs ≥669.0) ng/L, IL6 (<11.65 vs ≥11.65) pg/L, APACHE (<3.5 vs ≥3.5), and gender. No statistically significant differences were found (Table 2 ).
Table 2.
Comparison of median percentage change in diaphragm thickness and excursion between day 1 and day 5 in various subgroups based on baseline characteristics*
| Subgroup | Median (IQR) change (%) in diaphragm characters |
|||
|---|---|---|---|---|
| DTE | DTF | DE | DEmax | |
| Age (years) | ||||
| <51 (n = 23) | -12.2 (-21.0, 0) | 50 (-18.4, 123.5) | 4.5 (0, 15.8) | 1.4 (-7.0, 8.1) |
| ≥51 (n = 14) | -16.2 (-24.0, -5.0) | 12.8 (-36.5, 88.2) | 2.7 (-8.8, 9.8) | 3.6 (-5.9, 4.6) |
| p value | p = 0.51 | p = 0.36 | p= 0.39 | p = 0.60 |
| Sex | ||||
| Male (n = 26) | -14.0 (-27.3, 0) | 44.4 (-3.6, 123.5) | 4.6 (-7.7, 18.3) | -1.7 (-7.3, 5.6) |
| Female (n = 11) | -15.8 (-21.0, 2.7) | -18.4 (-44.4, 78.1) | 1.1 (-5.2, 8.4) | 3.1 (-4.3, 8.7) |
| p value | p = 0.98 | p = 0.09 | p = 0.60 | p = 0.23 |
| Symptom duration (days) | ||||
| <7 (n = 16) | -17.2 (-27.6, -10.5) | 31.0 (-1.6, 81.6) | 0.5 (-6.4, 11.9) | 0.2 (-6.4, 5.8) |
| ≥7 (n = 21) | -11.1 (-19.4, 2.7) | 0 (-27.8, 140) | 4.8 (-4.5, 15.8) | -3.4 (-7.0, 8.1) |
| p value | p = 0.13 | p = 0.84 | p = 0.42 | p = 0.88 |
| APACHE | ||||
| <3.5 (n = 19) | -12.5 (-28.6, -10) | 51.9 (-3.6, 123.5) | 3.3 (-6.9, 18.3) | 0 (-7.9, 4.0) |
| ≥3.5 (n = 18) | -15.8 (-19.0, 3.7) | -1.6 (-27.8, 78.1) | 6.4 (-5.2, 15) | -1.2 (-5.3, 8.7) |
| p value | p=0.29 | p=0.14 | p=0.81 | p=0.41 |
| CRP (mg/L) | ||||
| <7.8 (n = 16) | -14.1 (-23.4, -7.5) | 43.6 (-9.2, 84.9) | 0 (-11.0, 11.3) | -1.7 (-8.8, 8.4) |
| ≥7.8 (n = 21) | -15.8 (-21.0, 2.7) | 0.5 (-28.6, 104.9) | 6.1 (1, 15) | 0 (-5.3 vs 4) |
| p value | p=0.84 | p = 0.74 | p=0.16 | p=0.57 |
| IL-6 (ng/mL) | ||||
| <11.65 (n = 16) | -12.3 (-24.2, -4.3) | 50.9 (-1.8, 107.6) | -2.2 (-11.7, 2.8) | -3.2 (-9.8, 4.8) |
| ≥11.65 (n = 20) | -16.2 (-23.7, 1.4) | 12.8 (-36.1, 101.7) | 7.9 (3.4, 15.6) | 0.8 (-5.8, 8.4) |
| p value | p=0.71 | p=0.36 | p=0.015 | p = 0.22 |
| Ferritin(ng/mL) | ||||
| <669.0 (n = 17) | -12.5 (-21.0, -10) | 0 (-28.6, 78.1) | 1.0 (-6.9, 6.7) | -4.3 (-7.9, 3.1) |
| ≥669.0 (n = 20) | -16.7 (-23.3, 1.8) | 44.45 (-3.6, 119.1) | 8.7 (-2.2, 19.1) | 2.0 (-5.5, 7.0) |
| p value | p=0.76 | p=0.16 | p=0.10 | p=0.12 |
| LDH (U/L) | ||||
| <351 (n = 13) | -18.2 (-28, -10.5) | 85.2 (-19.2, 104.9) | 8.4 (1.1, 18.3) | 3.6 (-3.7, 8.1) |
| ≥351 (n = 24) | -12.4 (-20.0, 0) | 2.6 (-20.2, 84.0) | 1.6 (-7.3, 9.3) | -3.8 (-6.8, 4.1) |
| p value | p=0.37 | p=0.46 | p=0.13 | p=0.12 |
DTE – diaphragm thickness at end-expiration, DTF – diaphragm thickening fraction, DE – diaphragm excursion during tidal breathing, DEmax – diaphragm excursion during maximal inspiration, APACHE – acute physiology and chronic health evaluation, CRP – C-reactive protein, IL6 – interleukin 6, LDH – lactate dehydrogenase
A total of 37 out of 64 enrolled patients underwent ultrasonography of the diaphragm on day 5.
During the study period, 13 (20.3%) subjects experienced worsening of the respiratory failure requiring escalation of respiratory support; 8 (12.5%) subjects required prolonged hospital stay (>14 days); and 17 (26.6%) subjects were discharged with poor functional status (PCFS scale of grade III and IV), respectively. The changes in DTE, DTF, DE and Demax were comparable between those with and without deterioration, those who required hospital stay >14 days and ≤14 days, and those who were discharged with good and poor functional status (Table 3 ).
Table 3.
Comparison of median percentage change in diaphragm thickness and excursion between day 1 and day 5 in various subgroups based on various in-hospital outcomes*
| Subgroup | Median (IQR) change (%) in diaphragm characters |
|||
|---|---|---|---|---|
| DTE | DTF | DE | DEmax | |
| In-hospital course | ||||
| Deterioration (n=6) | -8.2 (-19.0, 38.5) | -20.2 (-27.8, 20.3) | 3.4 (-11.5, 15.8) | -1.6 (-10, 8.7) |
| No deterioration (n=31) | -15.8 (-28, 0) | 50 (-7.5, 104.9) | 3.6 (-5.2, 15) | 0 (-6.7, 5.9) |
| p value | p = 0.32 | p = 0.16 | p = 0.87 | p = 0.71 |
| Hospital stays | ||||
| ≥14 days (n=6) | -7 (-12.5, 0.0) | -20.2 (-28.6, 133.3) | -5.7 (-11.5, 2.1) | -5.0 (-10, -3.4) |
| <14 days (n=31) | -16.7 (-28, 0.0) | 37.1 (-7.5, 98.4) | 4.8 (-3.6, 15.8) | 1.1 (-6.7, 8.1) |
| p value | p= 0.12 | p= 0.44 | p = 0.052 | p = 0.08 |
| Functional status at discharge | ||||
| Poor (n = 5) | -10.5 (-38.2, -10) | -7.5 (-27.8, 25) | 3.3 (-5.2, 12.5) | 0 (-4.3, 0.3) |
| Good (n = 28) | -15.8 (-20.2, 0) | 50.9 (-11.4, 111.0) | 4.0 (-4.0, 15.2) | -0.4 (-6.8, 7) |
| p value | p = 0.74 | p = 0.28 | p = 0.88 | p = 0.96 |
| Outcome | ||||
| Death (n = 4) | -15.8 (-23.2, 18.8) | 1.0 (-42.8, 76.8) | -0.1 (-13.2, 19.1) | -4.4 (-12.6, 5.7) |
| Discharge (n = 33) | -15.8 (-21.0, 0) | 37.1 (-19.2, 98.4) | 3.6 (-4.5, 15) | 0 (-6.2, 5.9) |
| p value | p = 0.96 | p = 0.52 | p = 0.73 | p = 0.43 |
DTE – diaphragm thickness at end-expiration, DTF – diaphragm thickening fraction, DE – diaphragm excursion during tidal breathing, DEmax – diaphragm excursion during maximal inspiration
A total of 37 out of 64 enrolled patients underwent ultrasonography of the diaphragm on day 5.
4. Discussion
This single-center, prospective study showed that among hospitalized subjects with COVID-19, the diaphragm exhibited a decline in the thickness at end-expiration over a period of 5 days. Simultaneously, there was a significant increase in the diaphragm thickening fraction. However, we found no change in the diaphragm excursion, either during tidal or deep breathing during this period.
Among patients with COVID-19, diaphragmatic dysfunction can occur due to a myriad of mechanisms such as critical illness neuromyopathy, ventilator-induced diaphragm dysfunction, iatrogenic phrenic nerve injury (during line placement), post-infectious inflammatory neuropathy, or direct involvement by SARS-CoV-2; these mechanisms may act either alone or in combination [5,6]. Until recently, the recognition of diaphragmatic dysfunction in COVID-19 was not getting enough attention. In a seminal study, involving autopsy specimens of diaphragm from 34 critically ill subjects (COVID, 26 and non-COVID, 8), Shi and colleagues [5] demonstrated that epimysial and perimysial fibrosis was two times higher among patients with COVID-19 as compared to the non-COVID patients. The authors also demonstrated the presence of ACE2 receptors on the myofiber membrane, infiltration of the diaphragmatic muscle by SARS-CoV-2, and an altered expression of the genes involved in the activation of fibrosis [5]. Formenti et al. assessed the diaphragm, the parasternal muscles, and the rectus femoris muscle among subjects with COVID-related ARDS using USG. Authors reported that the muscle quality (assessed by echogenicity) was significantly poorer among survivors as compared to non-survivors [16]. Lower diaphragm thickness among non-survivors was also reported by Corradi and colleagues [17]. The same group also found that a lower DTF is associated with failure of continuous positive airway pressure leading to invasive mechanical ventilation [18]. On the contrary, an observational study enrolling 57 patients requiring mechanical ventilation for COVID-19 did not find DTF to be a predictive factor for weaning failure [19]. To summarize, these findings highlight that SARS-CoV-2 has an affinity for the diaphragm and underscores the importance of assessment of the diaphragm among subjects with COVID-19. Our study has further substantiated this belief by demonstrating a decline in the diaphragm thickness measured by bedside USG during the hospital stay for COVID-19. It should be noted that all the aforementioned studies [5,[16], [17], [18]] were conducted among critically ill subjects who either had ARDS or succumbed to severe COVID-19. In contrast, our study included COVID-19 subjects who were relatively less severely ill (median APACHE score of 3.5 and median SOFA score of 2). Despite enrolling a milder cohort, we found a reduction in the DTE of 15.7%. This raises the question whether diaphragmatic involvement is a distinctive feature of COVID-19 itself. There are studies that have reported decline in DTE and DTI on day 5 among non-COVID subjects [9,18,20]. In a previous study [9], non-COVID critically ill subjects showed a lesser decline in the DTE (6.6% vs 15.7%) in comparison to the COVID-19 subjects in our study. On the contrary, other authors have reported a decline in diaphragm thickness of approximately 12% and 28% at the comparable time-points in non-COVID subjects [20,21]. Interestingly, we found an increase in the DTF between day 1 and day 5 despite the reduction in diaphragm thickness. The DTF is believed to correlate with the diaphragm function and respiratory effort [22]. While there is a dearth of studies examining serial DTF in COVID-19, we propose that the change may have been driven by onset of recovery of diaphragmatic contraction over the course of the predominantly mild illness in our study. Further prospective studies are needed to confirm this hypothesis.
Diaphragm excursion is a less accurate reflection of its strength and function compared to DTF [22]. However, the DE has been found to predict weaning failure among subjects on mechanical ventilation [23,24]. In the emergency department, the DE on USG can help triage subjects with respiratory failure for the need of ventilatory support [25]. Many patients with COVID-19 are hospitalized with various degrees of respiratory failure and triaging of these patients may be crucial for the optimum utilization of limited hospital resources. However, we did not find any correlation between the diaphragmatic parameters and worsening of respiratory failure needing a higher level of respiratory support. Older age, male gender, and increased levels of CRP, IL6, LDH, and ferritin are associated with severe COVID-19 and worse outcomes [26,27]. However, our results failed to find any association of either age, gender, inflammatory markers (CRP, IL6, and LDH) or severity of disease with diaphragmatic dysfunction. Among non-COVID subjects with respiratory failure, diaphragmatic dysfunction is associated with poor outcomes [6]. This study found no association of either change in diaphragm thickness or diaphragm excursion with worsening of oxygenation, prolonged hospital stay, and functional status at discharge. The finding of lack of association may be consequential to the small sample size. Nonetheless, the study indicates that COVID-19 affects the diaphragm, irrespective of the severity of the disease.
Although most studies of diaphragm ultrasonography in critically ill patients have been conducted among intubated patients to predict weaning success, a few studies have enrolled non-intubated patients. Such studies have been performed among both non-COVID and COVID-19 patients prior to non-invasive ventilation to predict deterioration of respiratory failure [18,28].
This is the first study that describes the temporal changes in diaphragmatic thickness and excursion during acute COVID-19. The major strengths of our study are consecutive recruitment and assessment of diaphragm on a daily basis. The small sample size, single-center recruitment, and absence of histopathological examination are the major limitations. Also, this study cannot determine the mechanism of diaphragmatic involvement. In the absence of a control group of non-COVID patients, we also cannot specifically attribute the diaphragmatic changes to the SARS-CoV-2 virus rather than to the acute illness itself. Lastly, we are unable to comment on the effect of diaphragmatic dysfunction on symptoms or disability following discharge from the hospital.
5. Conclusions
The present study suggests that the diaphragm is commonly affected among hospitalized COVID-19 subjects with declining thickness at end-expiration during the hospital stay. However, the impact of the same on clinical outcomes is still unclear. Larger, well-designed, multicenter studies are warranted among COVID-19 patients for further substantiating the decline in the diaphragm thickness and its clinical implications.
Author contributions
VH, SM, KM, and AM conceived the work. VH, AR and TMS participated in data acquisition. VH, AR, TMS and MAK performed data analysis. VH, AR and TMS wrote the first draft. All authors critically reviewed and revised the manuscript. All authors approved the final version of the manuscript for submission.
Sources of financial support
None.
Declaration of Competing Interests
The authors declare that they have no competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.resmer.2022.100960.
Appendix. Supplementary materials
References
- 1.WHO Coronavirus (COVID-19) dashboard n.d. https://covid19.who.int (accessed August 19, 2022).
- 2.Gupta A, Madhavan MV, Sehgal K, Nair N, Mahajan S, Sehrawat TS, et al. Extrapulmonary manifestations of COVID-19. Nat Med. 2020;26:1017–1032. doi: 10.1038/s41591-020-0968-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hussain A, Yadav S, Hadda V, Suri TM, Tiwari P, Mittal S, et al. Covid-19: a comprehensive review of a formidable foe and the road ahead. Expert Rev Respir Med. 2020;14:869–879. doi: 10.1080/17476348.2020.1782198. [DOI] [PubMed] [Google Scholar]
- 4.Beyerstedt S, Casaro EB, Rangel ÉB. COVID-19: angiotensin-converting enzyme 2 (ACE2) expression and tissue susceptibility to SARS-CoV-2 infection. Eur J Clin Microbiol Infect Dis. 2021;40:905–919. doi: 10.1007/s10096-020-04138-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shi Z, de Vries HJ, Vlaar APJ, van der Hoeven J, Boon RA, Heunks LMA, et al. Diaphragm pathology in critically ill patients with COVID-19 and postmortem findings from 3 medical centers. JAMA Intern Med. 2021;181:122–124. doi: 10.1001/jamainternmed.2020.6278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Supinski GS, Morris PE, Dhar S, Callahan LA. Diaphragm dysfunction in critical illness. Chest. 2018;153:1040–1051. doi: 10.1016/j.chest.2017.08.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Supinski GS, Callahan LA. Diaphragm weakness in mechanically ventilated critically ill patients. Crit Care. 2013;17:R120. doi: 10.1186/cc12792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jung B, Moury PH, Mahul M, de Jong A, Galia F, Prades A, et al. Diaphragmatic dysfunction in patients with ICU-acquired weakness and its impact on extubation failure. Intensive Care Med. 2016;42:853–861. doi: 10.1007/s00134-015-4125-2. [DOI] [PubMed] [Google Scholar]
- 9.Hadda V, Kumar R, Tiwari P, Mittal S, Kalaivani M, Madan K, et al. Decline in diaphragm thickness and clinical outcomes among patients with sepsis. Heart Lung. 2021;50:284–291. doi: 10.1016/j.hrtlng.2020.12.014. [DOI] [PubMed] [Google Scholar]
- 10.Haaksma ME, Smit JM, Boussuges A, Demoule A, Dres M, Ferrari G, et al. EXpert consensus On Diaphragm UltraSonography in the critically ill (EXODUS): a Delphi consensus statement on the measurement of diaphragm ultrasound-derived parameters in a critical care setting. Crit Care. 2022;26:99. doi: 10.1186/s13054-022-03975-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clinical management protocol for COVID-19 (For adults). Government of India, Ministry of Health and Family Welfare (version 6) n.d. 2022
- 12.Dhungana A, Khilnani G, Hadda V, Guleria R. Reproducibility of diaphragm thickness measurements by ultrasonography in patients on mechanical ventilation. World J Crit Care Med. 2017;6:185–189. doi: 10.5492/wjccm.v6.i4.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boussuges A, Gole Y, Blanc P. Diaphragmatic motion studied by m-mode ultrasonography: methods, reproducibility, and normal values. Chest. 2009;135:391–400. doi: 10.1378/chest.08-1541. [DOI] [PubMed] [Google Scholar]
- 14.WHO R&D Blueprint novel Coronavirus. COVID-19 therapeutic trial synopsis n.d. 2022
- 15.Klok FA, Boon GJAM, Barco S, Endres M, Geelhoed JJM, Knauss S, et al. The post-COVID-19 functional status scale: a tool to measure functional status over time after COVID-19. Eur Respir J. 2020;56 doi: 10.1183/13993003.01494-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Formenti P, Coppola S, Umbrello M, Froio S, Caccioppola A, De Giorgis V, et al. Time course of the bioelectrical impedance vector analysis and muscular ultrasound in critically ill patients. J Crit Care. 2022;68:89–95. doi: 10.1016/j.jcrc.2021.11.014. [DOI] [PubMed] [Google Scholar]
- 17.Corradi F, Isirdi A, Malacarne P, Santori G, Barbieri G, Romei C, et al. Low diaphragm muscle mass predicts adverse outcome in patients hospitalized for COVID-19 pneumonia: an exploratory pilot study. Minerva Anestesiol. 2021;87:432–438. doi: 10.23736/S0375-9393.21.15129-6. [DOI] [PubMed] [Google Scholar]
- 18.Corradi F, Vetrugno L, Orso D, Bove T, Schreiber A, Boero E, et al. Diaphragmatic thickening fraction as a potential predictor of response to continuous positive airway pressure ventilation in Covid-19 pneumonia: a single-center pilot study. Respir Physiol Neurobiol. 2021;284 doi: 10.1016/j.resp.2020.103585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vetrugno L, Orso D, Corradi F, Zani G, Spadaro S, Meroi F, et al. Diaphragm ultrasound evaluation during weaning from mechanical ventilation in COVID-19 patients: a pragmatic, cross-section, multicenter study. Respir Res. 2022;23:210. doi: 10.1186/s12931-022-02138-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thabet DB, Makhlouf HA, Hasan AA, Mekkawy AI, Ghanem MK. Serial ultrasonographic monitoring of diaphragmatic and mid-upper arm muscle thickness in mechanically ventilated respiratory patients: a single-center observational study. Clin Respir J. 2021;15:11–18. doi: 10.1111/crj.13263. [DOI] [PubMed] [Google Scholar]
- 21.Schepens T, Verbrugghe W, Dams K, Corthouts B, Parizel PM, Jorens PG. The course of diaphragm atrophy in ventilated patients assessed with ultrasound: a longitudinal cohort study. Crit Care. 2015;19:422. doi: 10.1186/s13054-015-1141-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Umbrello M, Formenti P, Longhi D, Galimberti A, Piva I, Pezzi A, et al. Diaphragm ultrasound as indicator of respiratory effort in critically ill patients undergoing assisted mechanical ventilation: a pilot clinical study. Crit Care. 2015;19:161. doi: 10.1186/s13054-015-0894-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zambon M, Greco M, Bocchino S, Cabrini L, Beccaria PF, Zangrillo A. Assessment of diaphragmatic dysfunction in the critically ill patient with ultrasound: a systematic review. Intensive Care Med. 2017;43:29–38. doi: 10.1007/s00134-016-4524-z. [DOI] [PubMed] [Google Scholar]
- 24.Jiang J-R, Tsai T-H, Jerng J-S, Yu C-J, Wu H-D, Yang P-C. Ultrasonographic evaluation of liver/spleen movements and extubation outcome. Chest. 2004;126:179–185. doi: 10.1378/chest.126.1.179. [DOI] [PubMed] [Google Scholar]
- 25.Bobbia X, Clément A, Claret PG, Bastide S, Alonso S, Wagner P, et al. Diaphragmatic excursion measurement in emergency patients with acute dyspnea: toward a new diagnostic tool? Am J Emerg Med. 2016;34:1653–1657. doi: 10.1016/j.ajem.2016.05.055. [DOI] [PubMed] [Google Scholar]
- 26.Guan W-J, Ni Z-Y, Hu Y, Liang W-H, Ou C-Q, He J-X, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020 doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Berlin DA, Gulick RM, Martinez FJ. Severe Covid-19. N Engl J Med. 2020;383:2451–2460. doi: 10.1056/NEJMcp2009575. [DOI] [PubMed] [Google Scholar]
- 28.Cammarota G, Sguazzotti I, Zanoni M, Messina A, Colombo D, Vignazia GL, et al. Diaphragmatic ultrasound assessment in subjects with acute hypercapnic respiratory failure admitted to the emergency department. Respir Care. 2019;64:1469–1477. doi: 10.4187/respcare.06803. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.