Abstract
Tumor necrosis factor (TNF)-deficient mice were challenged with Leishmania donovani to characterize TNF in the response of visceral intracellular infection to antileishmanial chemotherapy. In wild-type controls (i) liver infection peaked at week 2 and resolved, (ii) discrete liver granulomas developed at weeks 2 to 4 and involuted, and (iii) leishmanicidal responses to antimony (Sb), amphotericin B (AmB), and miltefosine were intact. In TNF knockout (KO) mice (i) initial liver infection was unrestrained, plateaued, and then declined somewhat by week 6, (ii) an absent early granulomatous reaction abruptly accelerated with striking tissue inflammation, widespread hepatic necrosis, and 100% mortality by week 10, and (iii) while the initial response to AmB and miltefosine was intact, killing induced by Sb therapy was reduced by >50%. Although initial AmB treatment during weeks 2 to 3 killed 98% of liver parasites, 75% of AmB-treated KO mice subsequently relapsed and died by week 12; however, additional maintenance AmB preserved long-term survival. These results for a model of visceral infection indicate that endogenous TNF is required early on to control intracellular L. donovani, support granuloma development, and mediate optimal initial effects of Sb and prevent relapse after ordinarily curative AmB treatment. A compensatory, TNF-independent antileishmanial mechanism developed in TNF KO mice; however, its effect was uncontrolled fatal inflammation. Chemotherapeutic elimination of the parasite stimulus reversed the hyperinflammatory response and preserved survival.
The pleiotropic cytokine tumor necrosis factor (TNF) appears to be a prominent component of a diverse spectrum of both beneficial and deleterious inflammatory responses (2, 34). Among the beneficial effects of endogenous TNF is its complex role in inducing macrophage activation and enhancing host antimicrobial defense, particularly against intracellular pathogens (2, 34). Such a role, initially demonstrated by the capacity of treatment with anti-TNF antibodies to exacerbate infection, has recently been confirmed in a number of models using TNF- and TNF receptor-deficient (knockout [KO]) mice (1, 4, 6, 7, 12, 21, 23, 26, 35, 36).
In a previous report, we illustrated the critical role of endogenous TNF in the multicytokine-mediated host defense response which controls experimental visceral leishmaniasis, a disseminated protozoal infection in which macrophages of the liver, spleen, and bone marrow are targeted (31, 33; reviewed in reference 13). Challenging normal BALB/c mice with Leishmania donovani induced TNF in infected liver and spleen, and increasing tissue TNF levels reflected both initial control over parasite replication and subsequent near resolution of visceral infection by week 8 (31, 33). Repeated injections of anti-TNF antiserum abolished acquired resistance, permitting intracellular amastigotes to replicate freely within visceral macrophages. At the same time, 8 weeks of anti-TNF treatment did not appear to interfere with the orderly assembly of inflammatory mononuclear cells into well-circumscribed granulomas at infected tissue foci (13) and did not cause death in treated animals despite high parasite burdens (33).
This report extends the analysis of endogenous TNF's role in this model of visceral infection by asking whether this cytokine also acts with or regulates the in vivo response to antileishmanial chemotherapy (pentavalent antimony [Sb] or amphotericin B [AmB]). We posed this particular question for two reasons. First, gamma interferon (IFN-γ), another pivotal endogenous antileishmanial cytokine (13, 31) is required for the in vivo expression of Sb's leishmanicidal action (16) and is closely intertwined with TNF in inflammatory events including macrophage activation and the generation of toxic intermediates for L. donovani killing (2, 11, 24, 25, 28, 30, 34). Second, both Sb and AmB (as well as miltefosine, a new antileishmanial agent [15, 29] stimulate mononuclear phagocytes to secrete TNF (10, 32, 37), raising the possibility that induced TNF may act along with or enhance the local drug effect. To answer this question about endogenous TNF, we turned to well-characterized TNF KO mice (8, 12) for an in vivo test environment strictly free of the cytokine and characterized the host reaction and the behavior of visceral L. donovani in the absence of TNF and then the response to treatment.
MATERIALS AND METHODS
Mice and visceral infection.
Randomly selected male and female TNF KO mice (−/−) and their wild-type (WT) littermates (+/+), generated on a C57BL/6 × 129/Sv background (12), were used in these experiments. Groups of three to five mice were injected via the tail vein with 1.5 × 107 hamster spleen-derived L. donovani amastigotes (one Sudan strain) (33). Visceral infection was monitored microscopically using Giemsa-stained liver imprints, and liver parasite burdens were measured by counting in a blinded fashion the amastigotes per 500 cell nuclei and multiplying this number by the liver weight in milligrams (Leishman-Donovan units [LDUs]) (13). The histologic reaction in the liver was assessed using formalin-fixed tissue sections stained with hematoxylin and eosin. Granuloma formation at infected foci was scored as none, developing, or mature (13, 33).
Treatment.
Two weeks after infection (day 0), liver parasite burdens were determined and mice then received no treatment, a single intraperitoneal injection of Sb, three alternate-day intraperitoneal injections of AmB, or five consecutive once-daily doses of oral miltefosine by gavage as in previous studies (15, 16). Optimal doses of each drug were administered: Sb (sodium stibogluconate, Pentostam; Wellcome Foundation Ltd., London, United Kingdom), 500 mg/kg of body weight on day 0; AmB (Gensia Laboratories Ltd., Irvine, Calif.), 5 mg/kg on days 0, +2, and +4; and miltefosine (ASTA Medica AG, Frankfurt, Germany), 25 mg/kg on days 0 to +4 (15, 16). On day +7 (1 week after treatment was started), mice were sacrificed and liver parasite burdens were measured. Day +7 LDUs were compared to day 0 LDUs to determine percent parasite killing (15, 16). Differences between mean values were analyzed by a two-tailed Student t test.
RESULTS
Initial response to L. donovani infection.
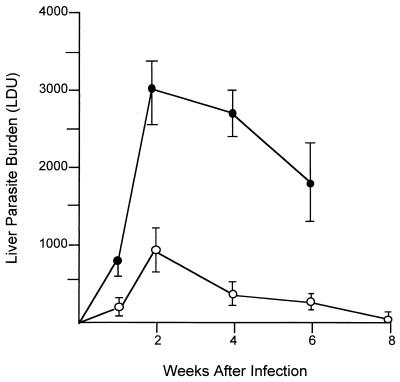
The kinetics of visceral infection in TNF KO mice promptly diverged from those in WT mice; 2 weeks after infection, liver parasite burdens were threefold higher in TNF-deficient animals (Fig. 1). In addition, while mononuclear cell influx into the liver and early granuloma assembly at parasitized tissue foci were evident in WT mice at the 2-week time point, there was little or no inflammatory response in livers of KO animals (Fig. 2a and b). The few granulomas that were present in KO mice were also poorly developed (not shown). We concluded from these results that endogenous TNF not only is required to control early visceral parasite replication but also helps to direct initial mononuclear cell (T cells and blood monocytes [13]) recruitment and influx into infected tissue.
FIG. 1.
Course of L. donovani infection in livers of TNF KO (solid circles) and WT control mice (open circles). Results are from four experiments and indicate means ± standard errors of the means for 9 to 14 mice per group at weeks 2, 4, and 6 and for 3 mice per group at weeks 1 and 8. Observations of TNF KO mice ended at week 6 since 50% had died by week 8 (see Fig. 3). Differences in mean LDUs for KO versus WT mice were significant (P < 0.05) at weeks 2 and 4 but not at week 1 or week 6 (P < 0.05).
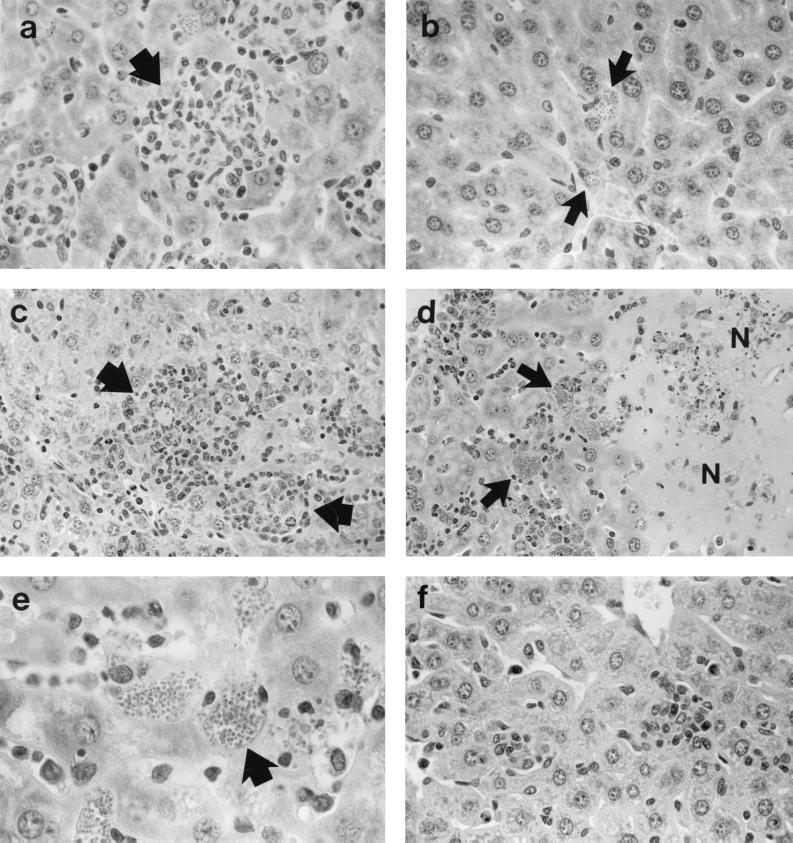
FIG. 2.
Liver histologic response to L. donovani 2 to 4 weeks after infection in WT controls (a) and TNF KO mice (b to f). (a and b) Week 2. WT mice (a) show normal developing granulomas (arrow) at sites of parasitized Kupffer cells; in contrast, in KO mice (b), there is no cellular response at parasitized foci (arrows). (c) Week 3. Rapid granuloma development in KO mice. (d and e) Week 4. KO mice show destructive inflammation with few recognizable granulomas, widespread areas of necrosis (N), and abundant cellular debris (d). Necrotic areas in panel d are ringed by partially preserved tissue containing large masses of intracellular amastigotes (arrows), also shown in panel e. (f) Week 3. Near absence of inflammatory changes in KO mice treated with AmB during the previous week. Magnifications, ×315 (a, b, and f), ×200 (c and d), and ×500 (e).
Responses at weeks 3 to 4 after infection.
After week 2, liver parasite burdens in KO mice plateaued but remained high (Fig. 1). Between weeks 2 and 3, infected KO mice also developed a histologic response in the liver that appeared nearly normal, with early granulomas (Fig. 2c), which were appropriate to this stage of infection (13) and which were similar to those present at week 2 in WT animals. However, by week 4 in KO mice, the inflammatory response in the liver abruptly became intense, widespread, and clearly exaggerated at the majority of parasitized foci. Areas anticipated to house developing or mostly mature granulomas at this stage of infection (week 4 [13]) were largely replaced with necrotic material and cell debris (Fig. 2d); adjacent areas contained heavily infected cells with uncharacteristically large masses of amastigotes (Fig. 2e). None of these inflammatory or heavily parasitized lesions developed in WT controls.
Fatal outcome in KO mice after week 4.
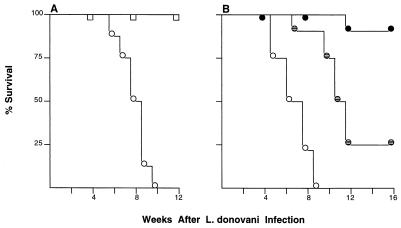
Liver parasite burdens in KO mice showed some decline after week 4; however, these animals nevertheless appeared ill and began to die starting at week 6. As illustrated in Fig. 3A, 100% of TNF KO mice were dead by the end of week 10; in contrast, no WT mice died and all showed barely detectable liver burdens at week 8 (Fig. 1).
FIG. 3.
Survival after L. donovani infection in untreated (A) and AmB-treated mice (B). (A) Results are from two experiments in which a total of 18 WT controls (squares) and 20 TNF KO mice (circles) were infected but were not given antileishmanial treatment. (B) Results are from a single experiment in which infected TNF KO mice were (i) left untreated (open circles; n = 12 mice), (ii) given three AmB injections during weeks 2 to 3 only (hatched circles; n = 8), or (iii) treated similarly with AmB during weeks 2 to 3 and then given once-weekly AmB during weeks 4 to 7 (filled circles; n = 8).
Initial response to antileishmanial treatment.
We tested the short-term response to antileishmanial therapy in KO mice that appeared healthy by administering treatment starting 2 weeks after infection (day 0), before the onset of the hyperinflammatory response. Efficacy was measured on day +7, 7 days after treatment was initiated. KO and WT mice responded equally well to AmB and miltefosine, with leishmanicidal activity induced in both mouse types by both drugs (Table 1); however, killing by Sb was appreciably impaired in TNF KO mice (32% killing versus 83% killing in WT controls).
TABLE 1.
Response to antileishmanial treatmenta
| Mouse type | Treatment | Liver parasite burden (LDU) at day:
|
% Killingb | |
|---|---|---|---|---|
| 0 | +7 | |||
| WT controls | None | 825 ± 113 | 1,137 ± 264 | 0 |
| Sb | 825 ± 113 | 140 ± 87c | 83 | |
| AmB | 825 ± 113 | 78 ± 25c | 91 | |
| MILT | 825 ± 113 | 148 ± 71c | 82 | |
| TNF KO | None | 1,332 ± 358 | 1,690 ± 365 | 0 |
| Sb | 1,332 ± 358 | 906 ± 257 | 32 | |
| AmB | 1,332 ± 358 | 26 ± 10c | 98 | |
| MILT | 1,332 ± 358 | 342 ± 99c | 74 | |
Two weeks after L. donovani challenge (day 0), LDUs were determined and mice received no treatment, a single dose of Sb (500 mg/kg), three injections of AmB (5 mg/kg) on alternate days, or five once-daily oral doses of miltefosine (MILT) (25 mg/kg). One week after treatment was started (day +7), all mice were sacrificed. Results are from two to four experiments and represent means ± standard errors of the means for LDUs from 7 to 14 mice per group at each time point.
% Killing = (day 0 LDU − day +7 LDU)/day 0 LDU × 100.
Significantly lower (P < 0.05) than day 0 value.
In addition to reducing liver parasite burdens, treatment also blunted the emerging inflammatory response, presumably by decreasing the overall magnitude of (miltefosine, Sb) or nearly eliminating (AmB) the triggering stimulus, replicating intracellular parasites. In contrast to what was seen in untreated KO mice, there was little or no disordered inflammatory reaction at week 3 (day +7) in livers of AmB-treated animals (Fig. 2f); inflammatory changes in the livers of KO mice given either miltefosine or Sb were also reduced (not shown).
Durability of the response to treatment and effect on survival.
To determine if the preceding initial response to chemotherapy was maintained in the absence of endogenous TNF and, at the same time, to test if antileishmanial therapy could prevent the subsequent death of KO mice due to inflammation, we used AmB because of its superior activity (98% killing; Table 1). Starting 2 weeks after infection, KO animals were randomly separated into three groups and received (i) no treatment (group A), (ii) three alternate-day 5-mg/kg injections of AmB during weeks 2 to 3 with no further treatment (as in Table 1) (group B), or (iii) the same three injections of AmB during weeks 2 to 3 followed by four maintenance injections (5 mg/kg) given once weekly at the beginning of weeks 4 to 7 (group C). Maintenance injections in group C were discontinued after week 7 at the time when 50% of untreated mice had died (Fig. 3B).
As shown in Fig. 3B, all untreated KO mice died by week 9, at which time 88 to 100% of AmB-treated mice in groups B and C were alive. However, group B animals (treated with AmB only during weeks 2 to 3) appeared ill after week 8 and only 25% eventually survived. In contrast, 88% of group C KO mice, which had received additional once-weekly maintenance Amb therapy to week 7, remained healthy at week 16. These mice were then sacrificed to examine their livers. Few amastigotes were present in 16-week liver imprints (42 ± 10 LDU; n = 8 mice examined), and the histologic appearance of this tissue was essentially unremarkable, with little evidence of either infection or inflammation (not shown).
DISCUSSION
These results for cytokine-deficient animals suggest several conclusions about the role of endogenous TNF in visceral antileishmanial resistance and its specific role in modulating the host response to antileishmanial chemotherapy.
First, in the absence of endogenous TNF, intracellular replication of L. donovani proceeded rapidly, reaching high levels 2 weeks after challenge. At this time, the early granulomatous tissue reaction, characteristic of this model (13), was largely absent at the majority of infected liver foci in KO mice. Both observations confirm the initial requirement for TNF in these two visceral responses. Second, the response in KO mice spontaneously shifted such that, by weeks 3 to 4, unrestrained liver infection had plateaued, albeit at a high level. This response was accompanied by the delayed but intact emergence of normal early granuloma assembly. Yet, third, the apparently developing capacity in KO mice to control L. donovani (Fig. 1) and generate granulomas at infected foci, obviously mediated by a TNF-independent compensatory mechanism, was abruptly obscured by a florid, destructive inflammatory response. This response, well recognized in other microbial models for TNF-deficient mice (1, 4, 6, 8, 12, 21, 35), induced diffuse hepatic necrosis in L. donovani-infected mice. However, even though eventually uniformly fatal, this response was not uncontrollable once triggered. Thus, as judged by the partial action of short-term chemotherapeutic intervention with AmB and the curative effect when intervention is extended, inhibition or removal of the inciting microbial stimulus can slow or terminate the progressive inflammatory response.
We did not formally determine the cause of death in TNF-deficient mice, nor did we examine responses in other parasitized organs such as the spleen. However, the level of hepatic infection was decreasing at week 6 (Fig. 1) as mice started to die, suggesting a role for factors other than progressive infection. Given the nature and extent of the histologic lesions in the liver, we suspect that death most likely resulted from inflammation-induced tissue destruction. Injecting these same TNF KO mice with a nonviable stimulus, killed Corynebacterium parvum, for example, produces a similarly intense, necrotic inflammatory tissue reaction in liver and spleen with near-100% mortality within approximately the same time period (12).
Paradoxically, the Th1 cell-dependent, interleukin-12 (IL-12)- and IFN-γ-mediated mechanism which successfully induces macrophage activation and acquired resistance to L. donovani (13, 14, 31) may well be responsible for this dysregulated, lethal inflammation (8). The generation of cytokine-induced inducible nitric oxide synthase-derived reactive nitrogen intermediates may also be involved (19, 21, 23). Alternatively, since TNF can induce IL-10 (3), deficient IL-10-related downregulation may play some role as well. Prior observations have well highlighted this unanticipated but critical role for endogenous TNF in regulating and limiting the extent and duration of in vivo inflammatory reactions (1, 4, 6, 8, 12, 21, 35). Evaluation of TNF receptor KO mice with Leishmania major footpad infection has also shown a persistent local inflammatory response with failure of lesion healing (21, 35), which may reflect deficient apoptosis of infiltrating T cells (9). In this model, initial control over intracellular infection is also impaired; however, parasite clearance eventually develops (21, 35).
We believe that the presence and effects of this (or a similar) TNF-independent hypercompensatory mechanism in KO mice likely explains the differences we observed in our prior study of anti-TNF antiserum-treated animals in which there was no control over infection, no exaggerated inflammatory response, and no deaths (33). In antiserum-treated normal BALB/c mice, we suspect that either (i) sufficient endogenous TNF was still present to prevent aberrant inflammation (but not enough to control visceral infection) or (ii) there was no intrinsic compensatory mechanism poised to overshoot, thus preserving survival in the previous study (33).
Fourth, despite clearly heightened visceral infection at week 2 and the absence of the host defense effects of endogenous TNF, the short-term antileishmanial efficacy of AmB and miltefosine was maintained. Thus, the reported capacity of these two agents to induce macrophage secretion of TNF in vitro (32, 37) does not appear central to their antileishmanial action in vivo.
Sb also stimulates macrophages to produce TNF (10), and the response to this agent, conventional therapy for human visceral leishmaniasis, was clearly diminshed in KO mice. Such a finding, indicating that Sb and TNF interact in vivo, is consistent with Sb's specific requirement for endogenous host immune mechanisms for expression of its leishmanicidal activity in vivo (16, 20). (Neither AmB nor miltefosine has this requirement, and both appear to initially act directly in vivo without immunologic cofactors [15–17]. Our ongoing analysis indicates that host regulation of the response to Sb is strictly T cell dependent and requires two Th1 cell cytokines (IFN-γ and IL-12) intertwined with the expression in tissue of at least one adhesion molecule, ICAM-1 (16, 20; unpublished data). (ICAM-1 itself is also upregulated by IFN-γ and/or TNF [5, 27].) Although clearly present, the defect in Sb responsiveness seen here in TNF KO mice is not as complete as it is in parasitized IFN-γ- or IL-12-deficient mice. In these KO animals, similar Sb treatment induces 0% L. donovani killing (16; unpublished data).
In addition to demonstrating that the hyperinflammatory response triggered in TNF KO mice was not a runaway process but rather one which can be successfully terminated, the treatment studies using AmB also yielded a final conclusion: that compensatory, TNF-independent antileishmanial mechanisms were not sufficient to prevent reemergence of residual posttreatment infection. Thus, while the three-injection AmB regimen during weeks 2 to 3 initially eradicated 98% of liver amastigotes in KO mice (Table 1), residual infection nevertheless recurred, as judged by the eventual death of 75% of these animals not further treated. Therefore, endogenous TNF also appears to be a critical determinant of the relapse-free state in the treated host with L. donovani infection. Providing AmB-treated KO mice with additional short-term maintenance therapy enhanced survival. Thus, continued pharmacologic inhibition of residual parasite replication (or perhaps driving the parasite number even lower) appears to permit TNF-independent mechanisms, likely mediated by T cells (18, 22), time to develop sufficiently to preserve long-term survival.
ACKNOWLEDGMENT
This study was supported by NIH grant AI 16963.
REFERENCES
- 1.Bean A G D, Roach D R, Briscoe H, France M P, Korner H, Sedgwick J D, Britton W J. Structural deficiencies in granuloma formation in TNF gene-targeted mice underlie the heightened susceptibility to aerosol Mycobacterium tuberculosis infection, which is not compensated for by lymphotoxin. J Immunol. 1999;162:3504–3511. [PubMed] [Google Scholar]
- 2.Beutler B, Grau G E. Tumor necrosis factor in the pathogenesis of infectious diseases. Crit Care Med. 1993;21:S423–S435. [PubMed] [Google Scholar]
- 3.Daftarian P M, Kumar A, Kryworuchko M, Diaz-Mitoma F. IL-10 production is enhanced in human T cells by IL-12 and IL-6 and in monocytes by tumor necrosis factor-α. J Immunol. 1996;157:12–20. [PubMed] [Google Scholar]
- 4.Deckert-Schluter M, Bluethmann H, Rang A, Hof H, Schluter D. Crucial role of TNF receptor type 1 (p55), but not of TNF receptor type 2 (p75) in murine toxoplasmosis. J Immunol. 1998;160:3427–3436. [PubMed] [Google Scholar]
- 5.Dustin M L, Rothlein R, Bhan A K, Dinarello C A, Springer T A. Induction by IL-1 and interferon-γ: tissue distribution, biochemistry, and function of a natural adherence molecule (ICAM-1) J Immunol. 1986;137:245–254. [PubMed] [Google Scholar]
- 6.Ehlers S, Benini L, Kutsch S, Endres R, Rietschel E T, Pfeffer K. Fatal granuloma necrosis without exacerbated mycobacterial growth in tumor necrosis factor receptor p55 gene-deficient mice intravenously infected with Mycobacterium avium. Infect Immun. 1999;67:3571–3579. doi: 10.1128/iai.67.7.3571-3579.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Flynn J L, Goldstein M M, Chan J, Triebold K J, Pfeffer K, Lowenstein C J, Schreber R, Mak T W, Bloom B R. Tumor necrosis factor-α is required in the protective immune response against Mycobacterium tuberculosis in mice. Immunity. 1995;2:561–572. doi: 10.1016/1074-7613(95)90001-2. [DOI] [PubMed] [Google Scholar]
- 8.Hodge-Dufour J, Marino M W, Horton M R, Jungbluth A, Burdick M D, Streiter R M, Noble P W, Hunter C A, Pure E. Inhibition of interferon gamma induced interleukin 12 production: a potential mechanism for the anti-inflammatory activities of tumor necrosis factor. Proc Natl Acad Sci USA. 1998;95:13806–13811. doi: 10.1073/pnas.95.23.13806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kanaly S T, Nashleanas M, Hondowicz B, Scott P. TNF receptor p55 is required for elimination of inflammatory cells following control of intracellular pathogens. J Immunol. 1999;163:3883–3889. [PubMed] [Google Scholar]
- 10.Kaye P M, Gorak P, Murphy M, Ross S. Strategies for immune intervention in visceral leishmaniasis. Ann Trop Med Parasitol. 1995;89(Suppl. 1):75–81. doi: 10.1080/00034983.1995.11813016. [DOI] [PubMed] [Google Scholar]
- 11.Liew F Y, Li Y, Millott S. Tumor necrosis factor-α synergizes with IFN-γ in mediating killing of Leishmania major through the induction of nitric oxide. J Immunol. 1990;145:4306–4310. [PubMed] [Google Scholar]
- 12.Marino M W, Dunn A, Grail D, Inglese M, Noguchi Y, Richards E, Jungbluth A, Wada H, Moore M, Williamson B, Basu S, Old L J. Characterization of tumor necrosis factor-deficient mice. Proc Natl Acad Sci USA. 1997;94:8093–8098. doi: 10.1073/pnas.94.15.8093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Murray H W. Granulomatous inflammation: host antimicrobial defense in the tissues in visceral leishmaniasis. In: Gallin J I, Snyderman R, Fearon D T, Haynes B F, Nathan C F, editors. Inflammation: basic principles and clinical correlates. 3rd ed. Philadelphia, Pa: Lippincott-Raven; 1999. pp. 977–993. [Google Scholar]
- 14.Murray H W. Endogenous interleukin-12 regulates acquired resistance in experimental visceral leishmaniasis. J Infect Dis. 1997;175:1477–1479. doi: 10.1086/516482. [DOI] [PubMed] [Google Scholar]
- 15.Murray H W, Delph-Etienne S. Visceral leishmanicidal activity of hexadecylphosphocholine (miltefosine) in mice deficient in T cells and activated macrophage microbicidal mechanisms. J Infect Dis. 2000;181:795–799. doi: 10.1086/315268. [DOI] [PubMed] [Google Scholar]
- 16.Murray H W, Delph-Etienne S. Roles of endogenous gamma interferon and macrophage microbicidal mechanisms in host response to chemotherapy in experimental visceral leishmaniasis. Infect Immun. 2000;68:288–293. doi: 10.1128/iai.68.1.288-293.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Murray H W, Hariprashad J, Fichtl R E. Treatment of experimental visceral leishmaniasis in a T-cell-deficient host: response to amphotericin B and pentamidine. Antimicrob Agents Chemother. 1993;37:1504–1505. doi: 10.1128/aac.37.7.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Murray H W, Hariprashad J, Fichtl R E. Models of relapse of experimental visceral leishmaniasis. J Infect Dis. 1996;173:1041–1043. doi: 10.1093/infdis/173.4.1041. [DOI] [PubMed] [Google Scholar]
- 19.Murray H W, Nathan C F. Macrophage microbicidal mechanisms in vivo: reactive nitrogen versus oxygen intermediates in the killing of intracellular visceral Leishmania donovani. J Exp Med. 1999;189:741–746. doi: 10.1084/jem.189.4.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Murray H W, Oca M J, Granger A M, Schreiber R D. Successful response to chemotherapy in experimental visceral leishmaniasis: requirement for T cells and effect of lymphokines. J Clin Investig. 1989;83:1254–1259. doi: 10.1172/JCI114009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nashleanas M, Kanaly S, Scott P. Control of Leishmania major infection in mice lacking TNF receptors. J Immunol. 1998;160:5506–5513. [PubMed] [Google Scholar]
- 22.Nashleanas M, Scott P. Activated T cells induce macrophages to produce NO and control Leishmania major in the absence of tumor necrosis factor receptor p55. Infect Immun. 2000;68:1428–1434. doi: 10.1128/iai.68.3.1428-1434.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rayhane N, Lortholary O, Fitting C, Callebert J, Huerre M, Dromer F, Caraillon J-M. Enhanced sensitivity of tumor necrosis factor/lymphotoxin-α-deficient mice to Cryptococcus neoformans infection despite increased levels of nitrite/nitrate, interferon-γ, and interleukin-12. J Infect Dis. 1999;180:1637–1647. doi: 10.1086/315061. [DOI] [PubMed] [Google Scholar]
- 24.Reiner N E, Ng W, Wilson C B, McMaster W B, Burchett S K. Modification of in vitro monocyte cytokine responses to Leishmania donovani: interferon-γ prevents parasite-induced inhibition of interleukin-1 production and primes monocytes to respond to Leishmania by producing both tumor necrosis factor-α and interleukin-1. J Clin Investig. 1990;85:1914–1924. doi: 10.1172/JCI114654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roach T I A, Kiderlen A F, Blackwell J M. Role of inorganic nitrogen oxides and tumor necrosis factor alpha in killing Leishmania donovani amastigotes in gamma interferon-lipopolysaccharide-activated macrophages from Lshs and Lshr congenic mouse strains. Infect Immun. 1991;59:3935–3944. doi: 10.1128/iai.59.11.3935-3944.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rothe J, Lesslauer W, Loetscher H, Lang Y, Koebel P, Koentgen F, Althage A, Zinkernagel R, Steinmetz M, Bluethmann H. Mice lacking the tumour necrosis factor receptor 1 are resistant to TNF-mediated toxicity but highly susceptible to infection by Listeria monocytogenes. Nature. 1993;364:798–802. doi: 10.1038/364798a0. [DOI] [PubMed] [Google Scholar]
- 27.Semnani R T, Nutman T B, Hochman P, Shaw S, van Seventer G A. Costimulation by purified intracellular adhesion molecule 1 and lymphocyte function-associated antigen 3 induces distinct proliferation, cytokine and cell surface antigen profiles in human “naive” and “memory” CD4+ cells. J Exp Med. 1994;180:2125–2135. doi: 10.1084/jem.180.6.2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stenger S, Solbach W, Rollinghoff M, Bogdan C. Cytokine interactions in experimental cutaneous leishmaniasis. II. Endogenous tumor necrosis factor-α production by macrophages is induced by synergistic action of interferon (IFN)-γ and interleukin (IL) 4 and accounts for the antiparasitic effect mediated by IFN-γ and IL 4. Eur J Immunol. 1991;21:1669–1675. doi: 10.1002/eji.1830210713. [DOI] [PubMed] [Google Scholar]
- 29.Sundar S, Rosenkaimer F, Makharia M K, Goyal A K, Mandal A K, Voss A, Hilgard P, Murray H W. Trial of oral miltefosine for visceral leishmaniasis. Lancet. 1998;352:1821–1823. doi: 10.1016/S0140-6736(98)04367-0. [DOI] [PubMed] [Google Scholar]
- 30.Talmadge J E, Tribble H R, Pennington R W, Phillips H, Wiltrout R H. Immunomodulatory and immunotherapeutic properties of recombinant interferon-γ and recombinant tumor necrosis factor in mice. Cancer Res. 1987;47:2563–2570. [PubMed] [Google Scholar]
- 31.Taylor A, Murray H W. Intracellular antimicrobial activity in the absence of interferon-γ: effect of interleukin-12 in experimental visceral leishmaniasis in interferon-γ gene-disrupted mice. J Exp Med. 1997;185:1231–1239. doi: 10.1084/jem.185.7.1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tokuda Y, Tsuji M, Yamazaki M, Kimura S, Abe S, Yamaguchi H. Augmentation of murine tumor necrosis factor production by amphotericin B in vitro and in vivo. Antimicrob Agents Chemother. 1993;37:2228–2230. doi: 10.1128/aac.37.10.2228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tumang M C T, Keogh C, Moldawer L L, Helfgott D C, Teitlbaum R, Hariprashad J, Murray H W. Role and effect of TNF-α in experimental visceral leishmaniasis. J Immunol. 1994;153:768–775. [PubMed] [Google Scholar]
- 34.Vassalli P. The pathophysiology of tumor necrosis factors. Annu Rev Immunol. 1992;10:411–452. doi: 10.1146/annurev.iy.10.040192.002211. [DOI] [PubMed] [Google Scholar]
- 35.Vieira L Q, Goldschmidt M, Nashleanas M, Pfeffer K, Mak T, Scott P. Mice lacking the TNF receptor p55 fail to resolve lesions caused by infection with Leishmania major, but control parasite replication. J Immunol. 1996;157:827–835. [PubMed] [Google Scholar]
- 36.Yap G S, Scharton-Kersten T, Charest H, Sher A. Decreased resistance of TNF receptor p55- and p75-deficient mice to chronic toxoplasmosis despite normal activation of inducible nitric oxide synthase in vivo. J Immunol. 1998;160:1340–1345. [PubMed] [Google Scholar]
- 37.Zeisig R, Rudolf M, Eue I, Arndt D. Influence of hexadecylphosphocholine on the release of tumor necrosis factor and nitroxide from peritoneal macrophages in vitro. J Cancer Res Clin Oncol. 1995;121:69–75. doi: 10.1007/BF01202215. [DOI] [PMC free article] [PubMed] [Google Scholar]