Background and purpose:
Endoscopic mucosal resection (EMR) is frequently used for the removal of colorectal neoplasms. However, the use of prophylactic antibiotics in patients undergoing EMR is debatable. The aim of this randomized controlled trial was to assess whether antimicrobial prophylaxis is crucial in the perioperative period of EMR, especially for 10- to 20-mm lesions in this setting.
Methods:
Two hundred and sixty-four patients were randomized equally into 2 groups, the antibiotic (cefixime) group and the control group. The occurrence of adverse events was examined at 1 to 3 days after EMR. Plasma levels of inflammatory markers were analyzed at pre-operation, 1 day post-operation and 3 days post-operation. Blood samples collected at 1 day post-operation were used for culture.
Results:
A total of 264 and 268 polyps were removed by EMR in the antibiotic group and the control group, respectively. There were 5 cases of fever, with 2 in the antibiotic group and 3 in the control group. In the antibiotic group, 12 patients had abdominal pain and 10 suffered bleeding, whereas in the control group, abdominal pain and bleeding were observed in 10 and 11 patients, respectively. There were no significant differences in the proportion of patients with fever or the incidences of postoperative complications between the groups. No significant differences between the groups were reported in plasma levels of white blood cell count, erythrocyte sedimentation rate, C-reactive protein or procalcitonin at pre-operation or post-operation. No patients provided positive blood cultures.
Conclusions:
The use the prophylactic antibiotics for EMR procedures in the perioperative period is no longer required when the lesions are 10 to 20 mm in size.
Keywords: adverse events, antibiotic, endoscopic mucosal resection
1. Introduction
Colorectal cancer (CRC) is the third and second most common cancer reported in males and females, respectively. It is also a leading cause of cancer-related deaths across the globe.[1] Since CRC may develop from adenoma, the primary strategy for the prevention of CRC is screening colonoscopies and polypectomies.
Endoscopic mucosal resection (EMR) is a simple, safe and effective procedure and one of the main modalities of polypectomies that finds wide usage in the treatment of superficial colorectal lesions. Presently, the major concerns associated with EMR are high rates of recurrence (3%–39%), bleeding (0.4%–16%), and perforation (0%–5%).[2–6] There has been little research on the occurrence and management of postoperative infection in patients undergoing EMR. The use of prophylactic antibiotics in the perioperative period of EMR remains controversial,[7,8] especially for the size 10 mm or more.
The objective of this study was to prospectively assess the impact of prophylactic antibiotics in the perioperative period on the occurrence of clinical adverse events and plasma levels of inflammatory markers in patients undergoing EMR for 10- to 20-mm colorectal polyps. We hypothesized that the use of prophylactic antibiotics is not necessary in the perioperative period of EMR for lesions 10 to 20 mm in size.
2. Patients and Methods
2.1. Study design and inclusion criteria
This was a prospective, randomized, controlled single-blind study. In the single-blind design, the doctors and nurses knew whether the patients were assigned to the antibiotic group or the control group, but the patients did not to which group they were assigned. The study was approved by the ethics committee of the 900th Hospital of PLA (Fujian, China) and was registered as Chinese Clinical trial registry ID: ChiCTR-IOR-15007613.
The inclusion criteria were as follows: patients who were aged between 18 and 80 years; patients who had been diagnosed with 10- to 20-mm colorectal polyps by colonoscopy at the outpatient clinic or other hospitals; patients who did not suffer from other major diseases, such as cardiopulmonary diseases or hepatic and renal failure; patients who had not recently taken any drugs that might have an impact on the experiment, for example, those not taking antibiotics a week ago; and patients who had normal clotting time and bleeding time. Meanwhile, if polyps were smaller than 10mm in the enrolled patients, we used cold snare polypectomy or biopsy forceps to remove diminutive polyps. The exclusion criteria of the study were coagulation dysfunction, taking anticoagulants, submucosal invasion lesions, allergy to cefixime and organ-related diseases such as myocardial and cerebral infarction.
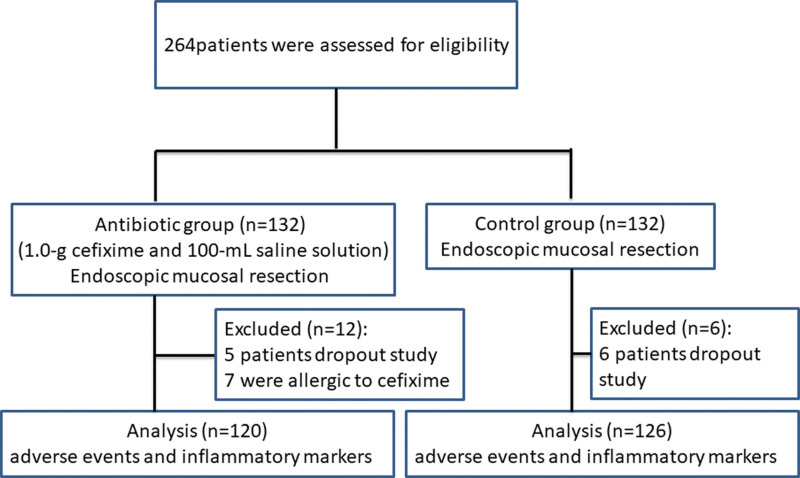
Previous study has reported that the incidences of fever in patients of the antibiotic and non-antibiotic groups were 0.9% and 8.4%, respectively,[8] following endoscopic polypectomy for colorectal polyps. Assuming a significance level of 5%, a statistical power of 80% and a grouping ratio of 1:1, the sample size was 120 participants per group. The final sample size was estimated to be 132 participants per group based on a potential drop-out rate of 10%. A total of 264 patients who met the inclusion criteria were referred for endoscopic resection and admitted to the hospital for EMR from January 2016 to November 2019. Among them, 5 patients dropout study and 7 patients were allergic to cefixime in antibiotic group, 6 patients dropout in the control group. All the remaining patients underwent EMR (Fig. 1).
Figure 1.
Flowchart of the prospective randomized trial.
2.2. Treatment allocation and intervention
The digital random method was employed for allocating patients to treatment using Microsoft Excel 2010 (Microsoft, Redmond, WA). After the random digitals were generated, all patients enrolled in the study were randomized into 2 groups, the antibiotic group (n = 132) and the control group (n = 132). The random table was preordered by a researcher who was not involved in the study.
In the antibiotic group, patients were administered 1.0-g cefixime and 100-mL saline solution intravenously 30 minutes before EMR, followed by another dose 6 hours after surgery. Conversely, patients in the control group were not given any antibiotic. The preoperative preparation of EMR included measurement of prothrombin time and international normalized ratio, in addition to electrocardiography. pretreatment of the colon comprised ingestion of 3-L polyethylene glycol solution to have clean bowel preparation.
2.3. EMR procedure
All EMR procedures were executed by experienced endoscopists using an endoscope (CF-HQ260; Olympus Co., Tokyo, Japan), with an endoclip (HX-610-135 EZ CLIP; Olympus Co.), polypectomy snare (Captivator M00562301, Captivator M00562321; Boston Scientific Co, Natick, MA) and an injection needle (NM-4U; Olympus Co.). The local injection solution consisted of 1:10,000 adrenaline and 0.5% methylene blue solution. Other device included an incisional generator (VIO200S; Elektromedizin Gmbh, Tubingen, Germany).
2.4. Data collection
Demographic and clinical data of the patients were collected from their medical records. The characteristics of polyps were also recorded, including the size, number, location and shape, in operation. While polyps were endoscopically classified using the Paris classification,[9] colorectal adenomas were histologic classified according to the Vienna classification.[10]
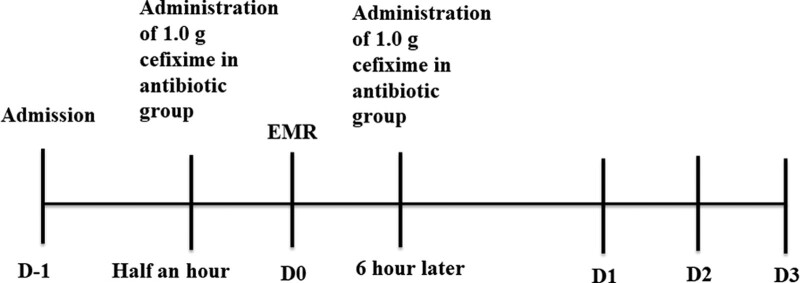
The primary outcome measure was the proportion of patients with fever after EMR. Secondary outcome measures included the incidence of postoperative complications and plasma levels of inflammatory markers. Clinical adverse events including fever (axillary temperature > 37.2°C), abdominal pain, bleeding, postpolypectomy coagulation syndrome (PPCS) and perforation, were documented. The body temperature of patients was recorded at 6 am and 8 pm during their hospital stay. In addition, blood samples were collected from all patients for assessing white blood cell count (WBCC), erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), and procalcitonin (PCT) at pre-operation, 1 day post-operation and 3 days post-operation. Blood samples collected at 1 day post-operation were also used for bacterial culture under aerobic and anaerobic conditions (Fig. 2).
Figure 2.
A schematic diagram of the study protocol. Blood samples of inflammatory markers, such as the white blood cell count, ESR, CRP, procalcitonin, were tested on D-1, D1, and D3. In addition, blood cultures were collected on D1. In the antibiotic group, cefixime was intravenously administered 30 min before EMR, followed by one more dose 6 h later. The body temperature was recorded at 6 am and 8 pm, and adverse events were recorded on D1, D2, and D3. CRP = C-reactive protein, EMR = endoscopic mucosal resection, ESR = erythrocyte sedimentation rate.
2.5. Statistical analysis
Outcome data were assessed by using SPSS 19.0 (IBM Corp., Armonk, NY). Continuous variables (patient characteristics and inflammatory markers) are expressed as the mean ± SD or median (interquartile range), whereas categorical variables (polyp characteristics and adverse events) are expressed as the count (percentage). All data were first checked for the normality of the distribution by Shapiro–Wilk test. The Student’s t test was used to compare normally distributed continuous variables (e.g., WBCC and ESR) between the 2 groups, and Mann–Whitney U test was applied to non-normally distributed continuous variables (e.g., CRP and PCT). The chi-square test was applied for the comparison of categorical variables between the 2 groups, except that the proportion of patients with fever was compared by the Fisher exact test. The statistical significance was set at P < .05.
3. Results
Our study comprised 246 patients who underwent EMR of 10- to 20-mm colorectal polyps. The antibiotic group comprised 63 males and 57 females, with a mean age of 52.74 ± 8.89 years. The control group comprised 68 males and 58 females, with a mean age of 51.11 ± 9.52 years. No significant differences were detected between the groups in terms of patient age and gender (Table 1). During the course of the treatment, none of the patients had perforation or major bleeding and sudden changes in the clinical conditions that required surgical intervention. Thus, all randomized patients successfully completed the study and they were included in per-protocol analyses.
Table 1.
Demographic and clinical data of patients and characteristics of polyps in the 2 randomized groups (PP analysis).
| Antibiotic group | Control group | P value | |
|---|---|---|---|
| Age (yr) | 52.74 ± 8.89 | 51.11 ± 9.52 | .169 |
| Gender (M:F) | 63:57 | 68:58 | .818 |
| Polyp size (15–20 mm) | 44 (36.7%) | 50 (39.7%) | .627 |
| Location of polyps | .972 | ||
| Cecum-ascending | 48 (18%) | 47 (18%) | |
| Transverse | 55 (21%) | 56 (21%) | |
| Descending | 56 (21%) | 52 (20%) | |
| Sigmoid | 55 (21%) | 57 (21%) | |
| Rectum | 50 (19%) | 56 (20%) | |
| Macroscopic type of polyps | .888 | ||
| Pedunculated | 67 (25%) | 66 (25%) | |
| Semipedunculated | 67 (25%) | 73 (27%) | |
| Sessile | 130 (50%) | 129 (48%) | |
| Histological type of polyps | .871 | ||
| Tubular | 94 (36%) | 91 (35%) | |
| Tubulovillous | 65 (25%) | 65 (24%) | |
| Villous | 62 (23%) | 61 (23%) | |
| Serrated | 43 (16%) | 51 (18%) | |
| Polyps with dysplasia or carcinogenesis | .917 | ||
| Polyps with no dysplasia or carcinogenesis | 163 (62%) | 159 (59%) | |
| Polyp-LG | 76 (29%) | 80 (30%) | |
| Polyp-HG | 19 (7%) | 21 (8%) | |
| CA | 6 (2%) | 8 (3%) |
CA = adenocarcinoma, HG = high-grade dysplasia, LG = low-grade dysplasia, PP = per-protocol.
A total of 264 polyps in the antibiotic group and 268 polyps in the control group were removed by EMR. In the antibiotic group, polyps were detected in the cecum–ascending colon (48 lesions; 18%), transverse colon (55 lesions; 21%), descending colon (56 lesions; 21%), sigmoid colon (55 lesions; 21%) and rectum (50 lesions; 19%). Regarding the macroscopic classification, pedunculated, semipedunculated and sessile polyps accounted for 67 (25%), 67 (25%) and 130 (50%) polyps, respectively. In the control group, the lesion sites where polyps were detected were the cecum–ascending colon (47 lesions; 18%), transverse colon (56 lesions; 21%), descending colon (52 lesions; 20%), sigmoid colon (57 lesions; 21%) and rectum (56 lesions; 20%). Pedunculated, semipedunculated and sessile polyps accounted for 66 (25%), 73 (27%) and 129 (48%) polyps in the control group, respectively. No significant differences were observed in the proportion of polyps with size 15 to 20 mm and the location of lesions between the 2 groups. The histopathological examination of resected polyps revealed no significant difference between the groups in the proportions of tubular adenomas, tubulovillous adenomas, villous adenomas, serrated adenomas, hyperplastic polyps and polyps with or without dysplasia or carcinogenesis (Table 1).
The clinical outcomes of EMR in the 2 randomized groups are presented in Table 2. Following the EMR procedure, 5 patients had an axillary temperature of >37.2°C, but none of them reached 38.5°C. Two cases (1.7%) of fever were found in the antibiotic group and 3 (2.4%) were in the control group. No significant difference was found in the proportion of patients with fever between the groups. None of the fever cases were treated with antipyretics, and their body temperature returned to normal after 10 to 12 hours. Blood cultures were negative in these 5 cases.
Table 2.
Clinical outcomes of endoscopic mucosal resection in the 2 randomized groups (PP analysis).
| Antibiotic group | Control group | P value | |
|---|---|---|---|
| Adverse events | |||
| Fever | 2 (1.7%) | 3 (2.4%) | 1 |
| Abdominal pain | 12 (10.0%) | 10 (7.9%) | .571 |
| Bleeding* | 10 (8.3%) | 11 (8.7%) | .911 |
| White blood cell count | |||
| Before surgery | 6.01 ± 1.62 | 6.02 ± 1.77 | .975 |
| 1 d after surgery | 6.18 ± 1.39 | 6.38 ± 1.41 | .266 |
| 3 d after surgery | 5.77 ± 1.67 | 6.06 ± 1.99 | .212 |
| Erythrocyte sedimentation rate | |||
| Before surgery | 12.08 ± 7.01 | 12.89 ± 6.68 | .317 |
| 1 d after surgery | 11.48 ± 10.18 | 11.10 ± 8.18 | .741 |
| 3 d after surgery | 10.06 ± 8.15 | 10.92 ± 8.46 | .417 |
| C-reactive protein | |||
| Before surgery | 0.36 (0.36–0.36) | 0.36 (0.36–0.36) | .372 |
| 1 d after surgery | 0.36 (0.36–0.36) | 0.36 (0.36–0.36) | .732 |
| 3 d after surgery | 0.36 (0.36–0.36) | 0.36 (0.36–0.36) | .357 |
| Procalcitonin | |||
| Before surgery | 0.025 (0.025–0.025) | 0.025 (0.025–0.025) | 1.000 |
| 1 d after surgery | 0.025 (0.025–0.100) | 0.025 (0.025–0.100) | .469 |
| 3 d after surgery | 0.025 (0.025–0.025) | 0.025 (0.025–0.025) | .324 |
Delayed bleeding was defined as bleeding symptoms or hemoglobin loss (≥ 2 g/dL) within 30 days after endoscopic mucosal resection, PP = per-protocol.
Abdominal pain was observed in 22 patients, with 12 (10.0%) in antibiotic group and 10 (7.9%) in the control group. However, the pain was mild and could be relieved by a spasmolytic. In addition, bleeding was reported in 21 patients (10 in antibiotic group, 8.3%; and 11 in the control group, 8.7%) after EMR. The bleeding was controlled with clips, and no subsequent bleeding was reported. No cases of PPCS or perforation were observed in any groups. In comparison to the control group, no significant differences were observed in WBCC, ESR, CRP, and PCT levels at pre-operation, 1 day post-operation and 3 days post-operation in the antibiotic group. No patients had a positive blood culture.
4. Discussion
Nowadays, the development of endoscopic technique has caused a progressive shift in the management strategy for colonic polyps from surgery to endoscopic removal techniques.[11] Owing to its minimally invasive procedure, minimal trauma, few complications and reliable clinical efficacy, EMR is widely used these days.[12–14] However, a recent upsurge in the EMR frequency is likely to escalate the incidence of complications, which, in fact, are inherent to this procedure.[15] The primary complications associated with EMR are bleeding and perforation, which can be treated by endoscopy and rarely need surgical treatment. However, very few people are concerned with the occurrence and management of infection caused by EMR. Reportedly, the rates of bacteremia related to colonoscopy range from 0% to 25% (mean, 4.4%). Since bacteremia is usually short-lived and does not cause clinical manifestations,[16] the American Society Gastrointestinal guidelines do not specify whether prophylactic antibiotics should be used for patients undergoing EMR for colonic polyps.
The PPCS is a rare complication of colonoscopic polypectomy (rate: 0.003%–0.1%[17]), yet it could result in abdominal pain, fever, peritoneal tenderness, guarding, leucocytosis and abnormal levels of inflammatory markers. These events could be managed by antibiotics without surgical intervention.[18] The risk factors associated with PPCS are hypertension, large lesion size (≥10 mm) and non-polypoid configuration of lesions.[17] In a prospective and randomized study, Lee et al[19] demonstrated that the prophylactic use of antibiotics in colorectal endoscopic submucosal dissection (ESD) was associated with the reduced risk of post-ESD electrocoagulation syndrome, decreased CRP levels and decreased abdominal pain. Although reasons for post-ESD electrocoagulation syndrome are unidentified, it seems to be related to submucosal injection during the ESD procedure. A catheter-related infection may directly inoculate bacteria into the blood stream during submucosal injection,[20,21] and mucosal defect is exposed after polypectomy.[20–22] Therefore, in practice, hospitals frequently use antibiotics to prevent intestinal infection during the EMR and ESD procedures, especially when the lesions are >10 mm in size.
Here, we examined whether the use of prophylactic antibiotics is necessary during the perioperative period of EMR for 10 to 20 mm polyps through a randomized controlled trial. For surgery, the second-generation cephalosporins, such as cefixime, are the first choice of prophylactic antibiotics.[23] In our study, the first dose of cefixime (1.0 g) was intravenously administered 30 min before the initiation of the EMR procedure, followed by an additional injection 6 hours after EMR. In total, we found 5 cases of fever in our patients, with 2 in the antibiotic group and 3 in the control group. Their axillary temperature did not reach 38.5°C and without antipyretic therapy, the temperature returned to normal 10 to 12 hours later. As the blood cultures were negative in all cases, the fever might be related to common cold or stress. Reportedly, the frequency of post-ESD fever was over 40% in a cohort of 199 patients with colorectal lesions,[24] which was much higher than rates of post-EMR fever in our patients (mean, 1.9%). This difference could be attributed to significant mucosal wounds because of ESD that is influenced by exposure to some enterobacteria rather than exposure of EMR.
Furthermore, we reported 22 patients with mild abdominal pain that could be relieved by a spasmolytic in the 2 groups. The assumed cause for this complication was the excessive insufflation air during colonoscopy. Bleeding, one of the complications of EMR, is categorized by intraprocedural bleeding and post-procedural bleeding.[25] Its incidence varies widely from 4% to 38%.[26] In our study, we observed 21 cases of delayed bleeding in the 2 groups after the EMR procedure. The bleeding was controlled with clips, and we observed no subsequent bleeding. Moreover, we did not report any occurrence of PPCS, and no patient presented with signs of perforation in either group. These adverse events need to be explored by future studies with a larger number of participants.
In comparison to the control group, we observed no significant difference in the antibiotic group with regard to plasma levels of inflammatory markers (WBCC, ESR, CRP, and PCT) at pre-operation or post-operation. In addition, no patient displayed positive blood culture, suggesting no impact of the administration of prophylactic antibiotics on endotoxemia of the patients undergoing EMR. Recently, a study discouraged the use of antibiotics after EMR of colon polyps to prevent infection,[27] but it did not specify the size of polyps. In 2015, Zhang et al[8] reported the efficacy of prophylactic antibiotics in patients with colorectal lesions undergoing polypectomy and found that the administered antibiotics could reduce the incidence of clinical adverse events such as abdominal pain, diarrhea, hematochezia and fever. However, the study examined many small lesions (<10 mm in size) and included ESD cases.
The study has a few limitations. First, this is a single-center study. Even though the results will serve as guidelines for the current clinical practice, a multi-center, randomized controlled trial is warranted to confirm our findings. Second, sample size of the study is moderate, but the number of patients could be thought as scarce to explore infrequent adverse events such as fever, PPCS and perforation. Further studies with larger sample size are needed for verification purpose. Third, other risk factors are not considered in the randomized controlled trial. For example, one must take into account the ratio of coagulating and cutting current when using high-frequency current in EMR, as a high ratio may increase the proportion of patients with PPCS after EMR.[28]
In conclusion, this study highlights that the prophylactic use of antibiotics does not affect the occurrence of adverse events (such as fever, abdominal pain and bleeding) or plasma levels of inflammatory markers (such as WBCC, ESR, CRP, and PCT) in colorectal EMR of 10 to 20 mm polyps. Therefore, we do not recommend the conventional application of antibiotics in the perioperative period of EMR for healthy people without comorbidities with 10 to 20 mm colorectal polyps.
Author contributions
Conceptualization: Linfu Zheng, Liping Jiang.
Data curation: Longke Xie, Linxin Zhou, Jianxiao Huang, Meiyan Liu.
Formal analysis: Liping Jiang.
Funding acquisition: Linfu Zheng, Longke Xie, Wen Wang.
Methodology: Dazhou Li, Longping Chen, Chuanshen Jiang.
Project administration: Wen Wang.
Software: Linfu Zheng, Liping Jiang.
Supervision: Wen Wang.
Writing – original draft: Linfu Zheng, Liping Jiang.
Writing – review & editing: Wen Wang.
Abbreviations:
- CRC =
- colorectal cancer
- CRP =
- C-reactive protein
- EMR =
- endoscopic mucosal resection
- ESD =
- endoscopic submucosal dissection
- ESR =
- erythrocyte sedimentation rate
- PCT =
- procalcitonin
- PP =
- Per-protocol
- PPCS =
- postpolypectomy coagulation syndrome
- WBCC =
- white blood cell count
LZ and LJ contributed equally to this work and should be regarded as co-first authors.
This project was supported in part by the Fujian Medical University Starting Fund (2016QH130), the project of the 900th Hospital of PLA (2020L32), and the Fujian Science and Technology Innovation Joint Fund Project (2018Y9116).
The authors have no conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request. All data generated or analyzed during this study are included in this published article [and its supplementary information files].
How to cite this article: Zheng L, Jiang L, Li D, Chen L, Jiang C, Xie L, Zhou L, Huang J, Liu M, Wang W. Antimicrobial prophylaxis in patients undergoing endoscopic mucosal resection for 10- to 20-mm colorectal polyps: A randomized prospective study. Medicine 2022;101:50(e31440).
Contributor Information
Linfu Zheng, Email: 2010zlf@163.com.
Liping Jiang, Email: jiangcs1982@163.com.
Dazhou Li, Email: LDZ7302999@sina.com.
Longping Chen, Email: 346403058@qq.com.
Chuanshen Jiang, Email: jiangcs1982@163.com.
Longke Xie, Email: 1075072560@qq.com.
Linxin Zhou, Email: 971152478@qq.com.
Jianxiao Huang, Email: 380631141@qq.com.
Meiyan Liu, Email: 15205052482@163.com.
References
- [1].Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115–32. [DOI] [PubMed] [Google Scholar]
- [2].Santos CE, Malaman D, Pereira-Lima JC. Endoscopic mucosal resection in colorectal lesion: a safe and effective procedure even in lesions larger than 2 cm and in carcinomas. Arq Gastroenterol. 2011;48:242–7. [DOI] [PubMed] [Google Scholar]
- [3].Repici A, Pellicano R, Strangio G, Danese S, Fagoonee S, Malesci A. Endoscopic mucosal resection for early colorectal neoplasia: pathologic basis, procedures, and outcomes. Dis Colon Rectum. 2009;52:1502–15. [DOI] [PubMed] [Google Scholar]
- [4].Hurlstone DP, Sanders DS, Cross SS, et al. Colonoscopic resection of lateral spreading tumours: a prospective analysis of endoscopic mucosal resection. Gut. 2004;53:1334–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Higaki S, Hashimoto S, Harada K, et al. Long-term follow-up of large flat colorectal tumors resected endoscopically. Endoscopy. 2003;35:845–9. [DOI] [PubMed] [Google Scholar]
- [6].Seo GJ, Sohn DK, Han KS, et al. Recurrence after endoscopic piecemeal mucosal resection for large sessile colorectal polyps. World J Gastroenterol. 2010;16:2806–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].La Regina D, Mongelli F, Fasoli A, et al. Clinical adverse events after endoscopic resection for colorectal lesions: a meta-analysis on the antibiotic prophylaxis. Dig Dis. 2020;38:15–22. [DOI] [PubMed] [Google Scholar]
- [8].Zhang QS, Han B, Xu JH, Gao P, Shen YC. Antimicrobial prophylaxis in patients with colorectal lesions undergoing endoscopic resection. World J Gastroenterol. 2015;21:4715–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].The Paris endoscopic classification of superficial neoplastic lesions: esophagus, stomach, and colon: November 30 to December 1, 2002. Gastrointest Endosc. 2003;58(6 Suppl):S3–43. [DOI] [PubMed] [Google Scholar]
- [10].Schlemper RJ, Riddell RH, Kato Y, et al. The Vienna classification of gastrointestinal epithelial neoplasia. Gut. 2000;47:251–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Ahlenstiel G, Hourigan LF, Brown G, et al. Actual endoscopic versus predicted surgical mortality for treatment of advanced mucosal neoplasia of the colon. Gastrointest Endosc. 2014;80:668–76. [DOI] [PubMed] [Google Scholar]
- [12].Kim HH, Kim SE, Cho EJ. What can be the criteria of outpatient-based endoscopic resection for colon polyp. World J Gastrointest Endosc. 2014;6:493–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Othman MO, Wallace MB. Endoscopic mucosal resection (EMR) and endoscopic submucosal dissection (ESD) in 2011, a Western perspective. Clin Res Hepatol Gastroenterol. 2011;35:288–94. [DOI] [PubMed] [Google Scholar]
- [14].Wada M, Yamamoto H. [From EMR to ESD]. Gan To Kagaku Ryoho. 2007;34:1163–7. [PubMed] [Google Scholar]
- [15].Kim HW. What is different between postpolypectomy fever and postpolypectomy coagulation syndrome. Clin Endosc. 2014;47:205–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Nelson DB. Infectious disease complications of GI endoscopy: part I, endogenous infections. Gastrointest Endosc. 2003;57:546–56. [DOI] [PubMed] [Google Scholar]
- [17].Cha JM, Lim KS, Lee SH, et al. Clinical outcomes and risk factors of post-polypectomy coagulation syndrome: a multicenter, retrospective, case-control study. Endoscopy. 2013;45:202–7. [DOI] [PubMed] [Google Scholar]
- [18].Fisher DA, Maple JT, Ben-Menachem T, et al. ASGE Standards of Practice Committee. Complications of colonoscopy. Gastrointest Endosc. 2011;74:745–52. [DOI] [PubMed] [Google Scholar]
- [19].Lee SP, Sung IK, Kim JH, et al. A randomized controlled trial of prophylactic antibiotics in the prevention of electrocoagulation syndrome after colorectal endoscopic submucosal dissection. Gastrointest Endosc. 2017;86:349–57.e2. [DOI] [PubMed] [Google Scholar]
- [20].Min BH, Chang DK, Kim DU, et al. Low frequency of bacteremia after an endoscopic resection for large colorectal tumors in spite of extensive submucosal exposure. Gastrointest Endosc. 2008;68:105–10. [DOI] [PubMed] [Google Scholar]
- [21].Itaba S, Iboshi Y, Nakamura K, et al. Low-frequency of bacteremia after endoscopic submucosal dissection of the stomach. Dig Endosc. 2011;23:69–72. [DOI] [PubMed] [Google Scholar]
- [22].Fujihara S, Mori H, Kobara H, et al. The efficacy and safety of prophylactic closure for a large mucosal defect after colorectal endoscopic submucosal dissection. Oncol Rep. 2013;30:85–90. [DOI] [PubMed] [Google Scholar]
- [23].Edwards FH, Engelman RM, Houck P, Shahian DM, Bridges CR; Society of Thoracic Surgeons. The Society of Thoracic Surgeons Practice Guideline Series: Antibiotic Prophylaxis in Cardiac Surgery, Part I: Duration. Ann Thorac Surg. 2006;81:397–404. [DOI] [PubMed] [Google Scholar]
- [24].Izumi K, Osada T, Sakamoto N, et al. Frequent occurrence of fever in patients who have undergone endoscopic submucosal dissection for colorectal tumor, but bacteremia is not a significant cause. Surg Endosc. 2014;28:2899–904. [DOI] [PubMed] [Google Scholar]
- [25].Ferlitsch M, Moss A, Hassan C, et al. Colorectal polypectomy and endoscopic mucosal resection (EMR): European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline. Endoscopy. 2017;49:270–97. [DOI] [PubMed] [Google Scholar]
- [26].Kim SH, Moon JS, Youn YH, Lee KM, Lee SJ. Management of the complications of endoscopic submucosal dissection. World J Gastroenterol. 2011;17:3575–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Shi Z, Qiu H, Liu H, Yu H. Should antibiotics be administered after endoscopic mucosalresection in patients with colon polyps. Turk J Med Sci. 2016;46:1486–90. [DOI] [PubMed] [Google Scholar]
- [28].Waye JD. Endoscopic mucosal resection of colon polyps. Gastrointest Endosc Clin N Am. 2001;11:537–48, vii. [PubMed] [Google Scholar]