Background:
Previous studies have reported controversial results on levels of inflammatory cytokines in patients with arsenic exposure. This study aims to evaluate the associations between arsenic exposure and inflammatory cytokines and C-reaction protein (CRP).
Methods:
We searched the databases including PubMed, Embase, Web of Science, and China national knowledge infrastructure (CNKI) for studies reporting levels of cytokines and CRP in patients with arsenic exposure compared to the controls. The retrieval time was from January 2000 to September 2022.
Results:
13 observational studies involving 1665 arsenic exposed and 1091 unexposed individuals were included. Among these studies, 6 from China, 4 from India, 2 from Bangladesh and 1 from Turkey. Our result showed that interleukin (IL)-6, IL-8, and IL-12 levels were significantly higher in arsenic-exposed individuals compared to the control group, IL-2 level was significantly lower, and Tumor necrosis factor-α, Interferon-γ, CRP, and IL-10 levels were not changed. After sensitivity analyses, tumor necrosis factor-α and Interferon-γ levels were significantly higher in arsenic-exposed individuals compared to the control group. High heterogeneity was detected in most studies.
Conclusion:
Many cytokines (such as IL-6, IL-8, and IL-12) have altered in individuals with arsenic exposure, this indicates arsenic exposure could trigger the cell-mediated inflammatory response. Regular examining immune function (such as inflammatory cytokines) in individuals with the risk of arsenic exposure is important to human health.
Keywords: arsenic, C-reactive protein (CRP), cytokines, immunotoxicity, inflammation
1. Introduction
Arsenic is a naturally existing toxic metal-like element which considered a common environmental pollutant.[1] Worldwide, arsenic exposure is a major public health challenge that affected more than 200 million individuals through drinking water which was considered the main source of arsenic exposure.[2,3] It is believed that chronic arsenic exposure could have serious harmful effects on human health, such as skin lesions, intestinal maladies, vascular diseases, neurological diseases, immunotoxicity, and various cancer.[4–7] However, the pathogenesis of arsenic exposure was still not clear enough.[8] Recently, researchers pay more attention to immunotoxicity and inflammation associated with chronic arsenic exposure.[9] Inflammation is a response considered to be caused by tissue injury or infection. In animal models, low chronic arsenic exposure could initiate inflammation and high exposure was believed to accentuate inflammation and trigger immunostimulatory.[10–12] Moreover, arsenic exposure could damage the functions of immune cells and alter the expression of inflammatory cytokines, such as tumor necrosis factor-α (TNF-α), interferon-γ (IFN-γ), interleukin-1β (interleukin [IL]-1β), interleukin-2 (IL-2), interleukin-6 (IL-6), interleukin-10 (IL-10), interleukin-12 (IL-12).[12–14] C-reactive protein (CRP) is a protein considered an acute-phase reactant and the production of CRP is part of the nonspecific response to inflammation.[15] Many studies have reported altered levels of inflammatory cytokines in individuals with arsenic exposure, the results of studies existed different, and some findings were even inconsistent with the results of animal experiments.[16–19]
Currently, the discussion of the association between arsenic exposure with inflammation in humans remains controversial. Therefore, we performed a systematic review and meta-analysis to evaluate the levels of inflammatory cytokines such as TNF-α and IL-2 among individuals with arsenic exposure compared to the controls. This study aims to investigate the association between arsenic exposure with inflammation according to published literature. Our results may provide more elucidation on the immunological effects of cytokines of chronic arsenic exposure.
2. Method
This study was designed and performed according to the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA).[20] The protocol of this meta-analysis was registered at PROSPERO (registration number: CRD42022359688 (https://www.crd.york.ac.uk/PROSPERO/)).
2.1. Search strategy
Two researchers (Z.Y.Z and R.Z.P) independently conducted a literature review of the search for this study. We searched the databases including PubMed, Embase, Web of Science, and China national knowledge infrastructure (CNKI). The retrieval time was from January 2000 to September 2022. The language was limited to English and Chinese. The following was the search strategy: (arsenic OR Arsenicum OR Arsenium OR arsenic exposure) AND (Inflammation OR inflammatory OR cytokines OR chemokine OR IL-1β OR IL-2 OR IL-4 OR IL-5 OR IL-6 OR IL-8 OR IL-10 OR IL-12 OR “Tumor Necrosis Factor-alpha” OR TNF-α OR CRP OR “C-reactive protein” OR IFN-γ OR “Interferons” OR interferon gamma). The literature retrieval process of PubMed database was shown in Supplementary material 1, Supplemental Digital Content, http://links.lww.com/MD/I163.
2.2. Inclusion and exclusion criteria
Inclusion criteria: The study participants must be patients with a history of arsenic exposure (e.g., occupational arsenic exposure, Arsenic-endemic study areas, etc.), the participants in the control group were healthy controls without a history of arsenic exposure; Study reported arsenic levels in the drinking water or in the participants (e.g., hair, nail, or plasma); The outcomes of the study included at least 1 of serum level of cytokines (IL-1β, IL-2, IL-4, IL-5, IL-6, IL-8, IL-10, IL-12, TNF-α, TGF-β, IFN-γ, and CRP)
Exclusion criteria: Case reports, literature reviews, editorials, animal studies, or republished studies; Single arm study; Raw data cannot be extracted or the full text was not available; study did not report any outcome; Participants < 18 years old, having diseases that affect immune function and recent infections.
2.3. Data extraction
Two researchers (Z.Y.Z and J.Y.L) independently extracted the raw data from included studies. The following data were extracted in each study: Title, journal of publication, the 1st author, years of publication, country, details of arsenic exposure (source, duration, and arsenic levels), patient characteristics, and level of cytokines (Mean ± standard deviation).
2.4. Assessment of the risk of bias
Two researchers (Z.Y.Z and R.Z.P) independently assessed the risk of bias in each included study. The Newcastle-Ottawa quality assessment scale (NOS) was used for assessing the risk of bias in case-control studies in meta-analysis.[21] NOS has 3 domains: selection of study, comparability, and evaluation of outcomes, with a total score of 9 points. Scores ≥ 7 are considered a high-quality study.
2.5. Statistical analysis
Meta-analyses were performed by using RevMan5.3 software according to the Cochrane Manual for Systematic Evaluation of Interventions. Meta-analysis would be performed when more than 2 studies (≥2) have reported levels of a kind of cytokine. Mean difference and 95% confidence intervals (CI) were used to assess the pooled effect size when the concentration units of included studies are the same. Otherwise, standardized mean difference (SMD) and 95% CI were used. For studies with multiple arsenic exposure groups according to different levels of exposure, subgroup data were combined for meta-analysis.[22] The Cochran’s Q test combined I2 statistics were used to evaluate the statistical heterogeneity in this meta-analysis. The fixed-effects model (Mantel-Haenszel method) was selected when no significant heterogeneity was detected (I2 < 50% or P-value for heterogeneity > .1). The random-effects model (Der Simonian-Laird method) was selected when significant heterogeneity was detected (I2 ≥ 50% or P value for heterogeneity ≤ .1). Subgroup analyses are performed when significant heterogeneity was found. If subgroup analyses could not find the source of heterogeneity, sensitivity analyses would be performed to find difference between studies. For meta-analysis with the number of included studies > 5, the publication bias was assessing both through Funnel plots and Egger’s tests. Egger’s test was performing using Stata15 Software. A P value of > .05 was considered statistically significant.
3. Results
3.1. Literature search
The literature search yielded 4927 studies. After duplication removal, a total of 1943 studies were removed. Following screening the titles and abstracts, 2753 studies were excluded. 131 articles met our eligibility criteria and a full-text evaluation was performed in these studies. Finally, a total of 13 studies[16–19,23–31] were included in this meta-analysis. Among included studies, 4 were in Chinese,[24–27] and 9 were in English.[16–19,23,28–31] Besides, all studies were conducted in developing countries, 6 from China,[24–27,30,31] 4 from India,[16,17,23,28] 2 from Bangladesh[19,29] and 1 from Turkey.[18] Most exposure sources of included studies were from environment exposure[16,17,19,23–29,31] except 2 studies[18,30] from occupational exposure. The flow diagram of study selection and exclusion was shown in Figure 1.
Figure 1.
The flow diagram of study selection and exclusion.
3.2. Characteristics of included studies
13 studies with 1665 arsenic exposed and 1091 unexposed individuals were included in the final analysis and 8 cytokines were assessed for association with arsenic exposure. The main characteristics of included studies were presented in Table 1. The risk of bias in included studies was assessed by NOS. All included studies were assessed as high-quality studies for scoring ≥ 7. NOS scoring details were presented in Table 2.
Table 1.
The main characteristics of included studies.
| Study | Country | Exposure sources | Exposure variables | Age, yr (exposed/unexposed) | Sample size (exposed/unexposed) | Outcomes | Detection method | |
|---|---|---|---|---|---|---|---|---|
| Biswas 2008[16] | India | Environment exposure | Drinking water, urine, nail, hair | 41.05 | 46.05 | 38 (20/18) | ①②④⑦ | CBA |
| Ji 2011[23] | China | Environment exposure | Drinking water | 49.92 ± 9.06 | 53.33 ± 8.28 | 90 (58/32) | ③ | Nephelometry |
| Das 2012[27] | India | Environment exposure | Drinking water, urine | 40.13 ± 13.36 | 40.03 ± 13.01 | 65 (32/33) | ⑤⑥ | ELISA |
| Karim 2013[28] | Bangladesh | Environment exposure | Drinking water, nail, hair | 37.55 ± 11.71 | 35.04 ± 10.93 | 313 (207/106) | ③ | ELISA |
| Ning 2014[25] | China | Environment exposure | Not mentioned | 55.7 ± 6.2 | 53.8 ± 4.9 | 58 (28/30) | ① | ELISA |
| Dutta 2015[29] | India | Environment exposure | Drinking water | 38 ± 6 | 39 ± 4 | 273 (142/131) | ①⑤⑥⑦⑧ | ELISA |
| Wang 2016[26] | China | Environment exposure | Drinking water, urine | 28.92 ± 3.86 | 28.28 ± 5.05 | 168 (120/48) | ④ | ELISA |
| Prasad 2017[17] | India | Environment exposure | Drinking water | 38 ± 7 | 39 ± 5 | 233 (123/110) | ①③⑤⑥⑧ | ELISA |
| Gao 2018[30] | China | Occupational exposure | Urine | 26.43 ± 5.76 | 25.76 ± 6.57 | 394 (114/280) | ③ | Not mentioned |
| Xu 2020[31] | China | Environment exposure | Urine | 49.07 ± 10.04 | 50.52 ± 9.97 | 246 (201/45) | ①④⑤ | ELISA |
| Fang 2019[24] | China | Environment exposure | Urine | 50.69 ± 6.14 | 45.76 ± 7.88 | 190 (149/41) | ④ | ELISA |
| Tutkun 2019[18] | Turkey | Occupational exposure | Serum | Not mentioned (>18) | 135 (62/73) | ①③⑤⑦ | ELISA | |
| Rahman 2021[19] | Bangladesh | Environment exposure | Drinking water | 37.5 ± 11.3 | 35.9 ± 11.2 | 553 (409/144) | ①② | ELISA |
CBA = cytometric bead array, ELISA = enzyme linked immunosorbent assay.
①: TNF-α ②: IFN-γ ③: CRP ④: IL-2 ⑤: IL-6 ⑥: IL-8 ⑦: IL-10 ⑧: IL-12.
Table 2.
Risk of bias assessment of included studies.
| Study | Selection | Comparability | Outcome | Score | |||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | ||
| Biswas 2008[16] | * | * | * | * | ** | * | * | * | 9 |
| Ji 2011[23] | * | * | * | * | ** | * | * | * | 9 |
| Das 2012[27] | * | * | * | * | ** | * | * | * | 9 |
| Karim 2013[28] | * | * | * | * | ** | * | * | 8 | |
| Ning 2014[25] | * | * | * | * | * | * | * | 7 | |
| Dutta 2015[29] | * | * | * | * | ** | * | * | 8 | |
| Wang 2016[26] | * | * | * | * | * | * | * | 7 | |
| Prasad 2017[17] | * | * | * | * | * | * | * | * | 8 |
| Gao 2018[30] | * | * | * | * | ** | * | * | * | 9 |
| Xu 2020[31] | * | * | * | * | ** | * | * | * | 9 |
| Fang 2019[24] | * | * | * | * | ** | * | * | 8 | |
| Tutkun 2019[18] | * | * | * | * | * | * | * | 7 | |
| Rahman 2021[19] | * | * | * | * | ** | * | * | * | 9 |
(1) Sufficient definition of the cases (2) Representativeness of the cases (3) Selection of the controls (4) Definition of the controls (5) Comparability of cases and controls based on the design or analysis (6) Assessment of exposure (7) Same method of ascertainment for cases and controls (8) non-response rate.
3.3. TNF-α
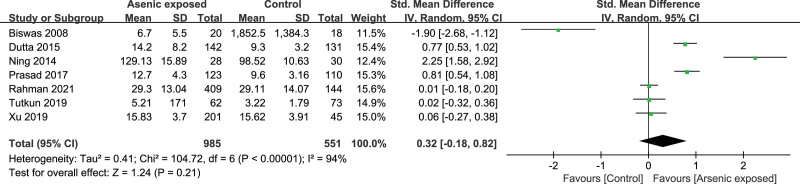
Seven studies[16–19,23,26,31] reported the TNF-α level in the As-exposed (n = 985) and control (n = 551) groups. Significant heterogeneity was detected between studies (I2 = 94%, heterogeneity P < .00001), and the random-effects model was used. Our study showed that the TNF-α level was not significantly changed in As-exposed patients compared to the controls (SMD = 0.32, 95%CI (-0.18, 0.82), P = .21) (Fig. 2).
Figure 2.
Forest plot for TNF-α. TNF-α = tumor necrosis factor-α.
3.4. IFN-γ
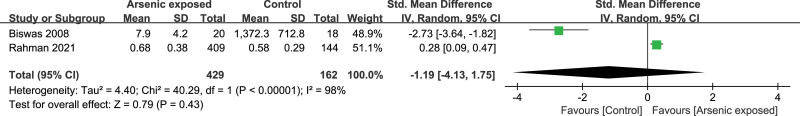
Two studies[16,19] reported the IFN-γ level in the As-exposed (n = 429) and control (n = 161) groups. Significant heterogeneity was detected between studies (I2 = 98%, heterogeneity P < .00001), and the random-effects model was used. Our study showed that the IFN-γ level was not significantly changed in As-exposed patients compared to the controls (SMD = -1.19, 95%CI (-4.13, 1.75), P = .43) (Fig. 3).
Figure 3.
Forest plot for IFN-γ. IFN-γ = interferon-γ.
3.5. CRP
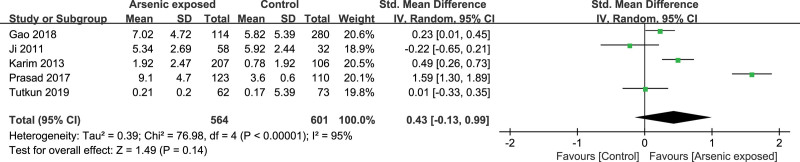
Five studies[17,18,24,29,30] reported the CRP level in the As-exposed (n = 564) and control (n = 601) groups. Significant heterogeneity was detected between studies (I2 = 95%, heterogeneity P < .00001), and the random-effects model was used. Our study showed that the CRP level was not significantly changed in As-exposed patients compared to the controls (SMD = 0.43, 95%CI (-0.13, 0.99), P = .14) (Fig. 4).
Figure 4.
Forest plot for CRP. CRP = C-reactive protein.
3.6. IL-2
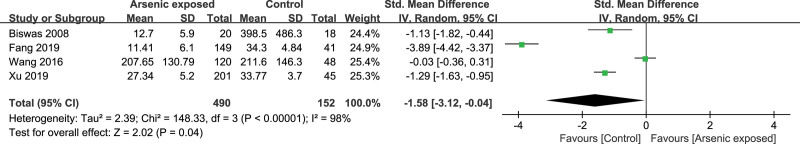
Four studies[16,25,27,31] reported the IL-2 level in the As-exposed (n = 490) and control (n = 152) groups. Significant heterogeneity was detected between studies (I2 = 98%, heterogeneity P < .00001), and the random-effects model was used. Our study showed that the IL-2 level was significantly decreased in As-exposed patients compared to the controls (SMD = -1.58, 95%CI (-3.12, -0.04), P = .04) (Fig. 5).
Figure 5.
Forest plot for IL-2. IL = interleukin.
3.7. IL-6
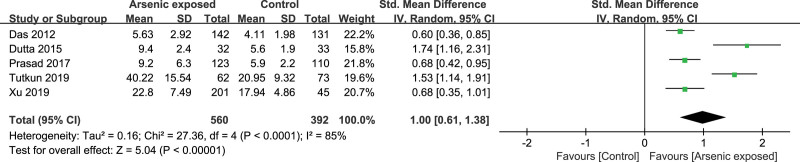
Five studies[17,18,23,28,31] reported the IL-6 level in the As-exposed (n = 560) and control (n = 392) groups. Significant heterogeneity was detected between studies (I2 = 85%, heterogeneity P < .00001), and the random-effects model was used. Our study showed that the IL-6 level was significantly increased in As-exposed patients compared to the controls (SMD = 1.00, 95%CI (0.61, 1.38), P < .00001) (Fig. 6).
Figure 6.
Forest plot for IL-6. IL = interleukin.
3.8. IL-8
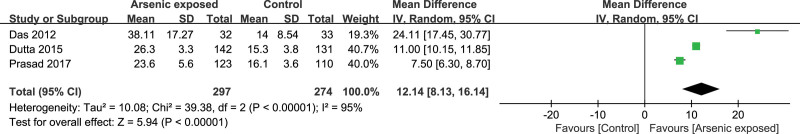
Three studies[17,23,28] reported the IL-8 level in the As-exposed (n = 297) and control (n = 274) groups. Significant heterogeneity was detected between studies (I2 = 95%, heterogeneity P < .00001), and the random-effects model was used. Our study showed that the IL-8 level was significantly increased in As-exposed patients compared to the controls (SMD = 12.14, 95%CI (8.13, 16.14), P < .00001) (Fig. 7).
Figure 7.
Forest plot for IL-8. IL = interleukin.
3.9. IL-10
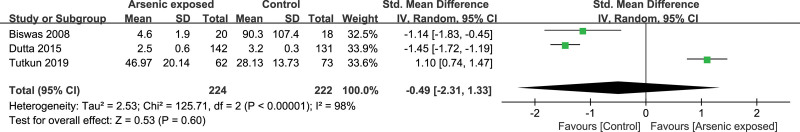
Three studies[16,18,23] reported the IL-10 level in the As-exposed (n = 224) and control (n = 222) groups. Significant heterogeneity was detected between studies (I2 = 96%, heterogeneity P < .00001), and the random-effects model was used. Our study showed that the IL-10 level was not significantly changed in As-exposed patients compared to the controls (SMD = -2.34, 95%CI (22.26, 17.58), P = .82) (Fig. 8).
Figure 8.
Forest plot for IL-10. IL = interleukin.
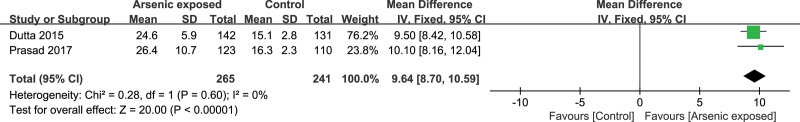
3.10. IL-12
Two studies[17,23] reported the IL-12 level in the As-exposed (n = 265) and control (n = 241) groups. No significant heterogeneity was detected between studies (I2 = 0%, heterogeneity P = .60), and the fixed-effects model was used. Our study showed that the IL-12 level was significantly increased in As-exposed patients compared to the controls (SMD = 9.64, 95%CI (8.70, 10.59), P < .00001) (Figure 9.).
Figure 9.
Forest plot for IL-12. IL = interleukin.
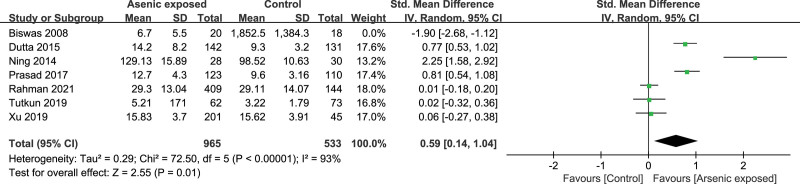
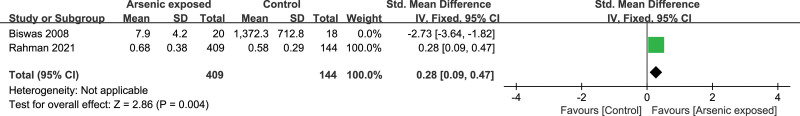
3.11. Subgroup analyses and Sensitivity analyses
Subgroup analyses could not explain the heterogeneity in this meta-analysis. Sensitivity analyses showed significantly higher levels of TNF-α (SMD = 0.59, 95%CI (0.14, 1.04), P = .01) (Fig. 10) and IFN-γ (SMD = 0.28, 95%CI (0.09, 0.47), P = .004) (Fig. 11) in Arsenic exposed group compared to the control group when Biswas’s study[16] was excluded.
Figure 10.
Forest plot of sensitivity for TNF-α. TNF-α = tumor necrosis factor-α.
Figure 11.
Forest plot of sensitivity for IFN-γ. IFN-γ = interferon-γ.
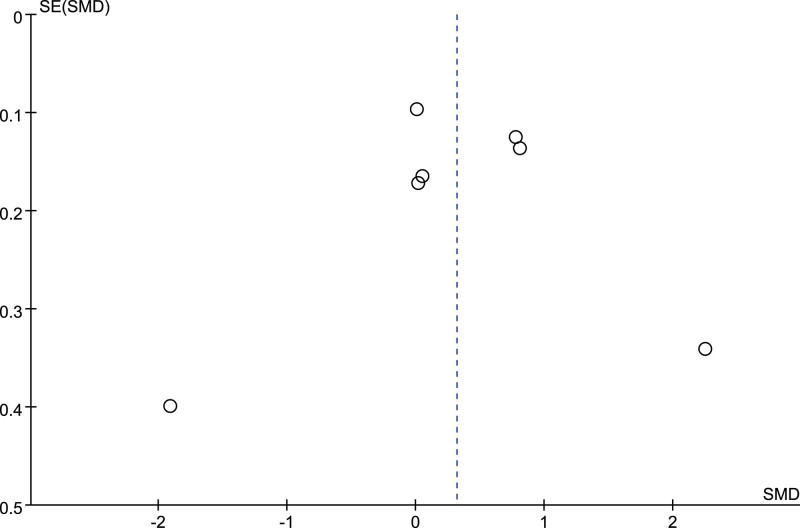
3.12. Publication bias
Only the number of included studies of meta-analysis of TNF-α were more than 5. The Funnel plot (Fig. 12) and Egger’s test (P = .994) both suggested no publication bias was found in this meta-analysis.
Figure 12.
The funnel plot.
4. Discussion
To the best of our knowledge, this is the 1st meta-analysis to evaluate the effect of arsenic exposure on human immunity by conducting a systemic review and meta-analysis. Studies reporting inflammatory cytokines levels in arsenic-exposed and unexposed individuals were identified by literature search. Our results showed that IL-6, IL-8, and IL-12 levels were significantly higher in arsenic-exposed individuals compared to the control group, IL-2 level was significantly lower, and TNF-α, IFN-γ, CRP, and IL-10 levels were not changed. When Biswas’s study was excluded, TNF-α and IFN-γ levels were significantly higher in arsenic-exposed individuals compared to the control group. Biswas’s study showed that all the 6 cytokines significantly decreased in the arsenic-exposed individuals compared to the control group, which was different from other included studies. This may be attributed to the severity of the arsenic exposure group because all individuals with arsenic exposure suffered skin lesions. Prolonged and high-dose arsenic exposure may trigger immunosuppression in humans. Due to the limited number of included studies, the dose-response analysis could not be performed for finding the association between the severity of arsenic exposure and levels of cytokines in humans.
Cytokines can mediate inflammatory responses. The interaction between proinflammatory cytokines (IL-2, IL-6, IL-8, IL-12, TNF-α, IFN-γ, and et. al.) and anti-inflammatory (IL-4, IL-10, and et. al.) cytokine regulates the immune response in human.[32] Current findings included the tendency of elevated IL-6, IL-8, IL-12, TNF-α, and IFN-γ, while a reduction in IL-2 levels in individuals with arsenic exposure as compared to the control group, which were in line with the results of animal experiments basically.[12,13,33,34] Arsenic-induced pro-inflammatory response may lead to various diseases, such as autoimmune disorders, allergic diseases, and cardiovascular events.[19,28,29] Moreover, Polymorphisms of inflammatory genes could increase the risk of disorders of body function and diseases in arsenic-exposure populations.[35–38] These results indicated that the expression of cytokines may be a potential and useful biomarker of arsenic exposure and arsenic-induced damage to tissues and organs.
Several limitations existed in this present study. First, high heterogeneity between studies was detected and cannot be explained by subgroup analysis or sensitivity analysis. Second, the number of included studies was relatively small and the types of cytokines were different, resulting in a small sample size for meta-analysis of each cytokine. Third, this present study failed to compare the differential effects of low versus high arsenic exposure on levels of cytokines in humans.
5. Conclusions
In summary, arsenic exposure is a public health problem that affected hundreds of millions of people. Exposure to arsenic demonstrated altered inflammatory cytokines, which indicated that arsenic exposure could trigger inflammatory responses. The immunotoxicity of arsenic exposure may be related to cell-mediated responses. This present study has the limitations of a small sample size and large heterogeneity. Large sample sizes and high-quality case-control studies on the association between inflammatory cytokines and arsenic exposure are still necessary in the future. Important tissues and organ damage due to arsenic exposure may be mediated by inflammatory responses. Thus, regular examining immune function (such as inflammatory cytokines) in individuals with the risk of arsenic exposure is important to human body health.
Author contributions
Conceptualization: Baofei Sun.
Data curation: Zheyu Zhang, Ruozheng Pi, Jieya Luo.
Formal analysis: Zheyu Zhang, Ruozheng Pi.
Methodology: Zheyu Zhang, Ji Liu.
Software: Zheyu Zhang, Jieya Luo.
Supervision: Baofei Sun, Aihua Zhang.
Writing – original draft: Zheyu Zhang, Ruozheng Pi.
Writing – review & editing: Zheyu Zhang, Baofei Sun.
Supplementary Material
Abbreviations:
- CI =
- confidence interval
- CRP =
- C-reactive protein
- IFN-γ =
- interferon-γ
- IL =
- interleukin
- NOS =
- Newcastle-Ottawa quality assessment scale
- SMD =
- standardized mean difference
- TNF-α =
- tumor necrosis factor-α
ZZ, and RP contributed equally to this work.
Supplemental Digital Content is available for this article.
The authors have no conflicts of interest to disclose.
This work was supported by the National Natural Science Foundation of China (grant number: 81760573).
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
How to cite this article: Zhang Z, Pi R, Luo J, Liu J, Zhang A, Sun B. Association between arsenic exposure and inflammatory cytokines and C-reaction protein: A systematic review and meta-analysis. Medicine 2022;101:50(e32352).
Contributor Information
Zheyu Zhang, Email: aihuagy@yahoo.com.cn.
Ruozheng Pi, Email: 327746461@qq.com.
Jieya Luo, Email: jieyaluo2000@163.com.
Ji Liu, Email: 424374103@qq.com.
Aihua Zhang, Email: aihuagy@yahoo.com.cn.
References
- [1].Nordstrom DK. Public health. Worldwide occurrences of arsenic in ground water. Science. 2002;296:2143–5. [DOI] [PubMed] [Google Scholar]
- [2].Naujokas MF, Anderson B, Ahsan H, et al. The broad scope of health effects from chronic arsenic exposure: update on a worldwide public health problem. Environ Health Perspect. 2013;121:295–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Carlin DJ, Naujokas MF, Bradham KD, et al. Arsenic and environmental health: state of the science and future research opportunities. Environ Health Perspect. 2016;124:890–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Palma-Lara I, Martínez-Castillo M, Quintana-Pérez JC, et al. Arsenic exposure: a public health problem leading to several cancers. Regul Toxicol Pharmacol. 2020;110:104539. [DOI] [PubMed] [Google Scholar]
- [5].Monteiro De OE, Caixeta ES, Santos VSV, et al. Arsenic exposure from groundwater: environmental contamination, human health effects, and sustainable solutions. J Toxicol Environ Health B Crit Rev. 2021;24:119–35. [DOI] [PubMed] [Google Scholar]
- [6].Rahaman MS, Rahman MM, Mise N, et al. Environmental arsenic exposure and its contribution to human diseases, toxicity mechanism and management. Environ Pollut. 2021;289:117940. [DOI] [PubMed] [Google Scholar]
- [7].Dangleben NL, Skibola CF, Smith MT. Arsenic immunotoxicity: a review. Environ Health. 2013;12:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Minatel BC, Sage AP, Anderson C, et al. Environmental arsenic exposure: from genetic susceptibility to pathogenesis. Environ Int. 2018;112:183–97. [DOI] [PubMed] [Google Scholar]
- [9].Ferrario D, Gribaldo L, Hartung T. Arsenic exposure and immunotoxicity: a review including the possible influence of age and sex. Curr Environ Health Rep. 2016;3:1–12. [DOI] [PubMed] [Google Scholar]
- [10].Zhao M, Sun Y, Jiang Y. Anti-VEGF therapy is not a magic bullet for diabetic retinopathy. Eye (Lond). 2020;34:609–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Suntararuks S, Worasuttayangkurn L, Akanimanee J, et al. Sodium arsenite exposure impairs B cell proliferation and enhances vascular inflammation in Plasmodium berghei mouse model. Environ Toxicol Pharmacol. 2019;66:7–13. [DOI] [PubMed] [Google Scholar]
- [12].Yan N, Xu G, Zhang C, et al. Chronic arsenic exposure induces the time-dependent modulation of inflammation and immunosuppression in spleen. Cell Biosci. 2020;10:91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Duan X, Gao S, Li J, et al. Acute arsenic exposure induces inflammatory responses and CD4(+) T cell subpopulations differentiation in spleen and thymus with the involvement of MAPK, NF-kB, and Nrf2. Mol Immunol. 2017;81:160–72. [DOI] [PubMed] [Google Scholar]
- [14].Ahmed G, Thakur AK, Pushpanjali, et al. Modulation of the immune response and infection pattern to Leishmania donovani in visceral leishmaniasis due to arsenic exposure: an in vitro study. PLoS One. 2019;14:e0210737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Pathak A, Agrawal A. Evolution of C-reactive protein. Front Immunol. 2019;10:943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Biswas R, Ghosh P, Banerjee N, et al. Analysis of T-cell proliferation and cytokine secretion in the individuals exposed to arsenic. Hum Exp Toxicol. 2008;27:381–6. [DOI] [PubMed] [Google Scholar]
- [17].Prasad P, Sinha D. Low-level arsenic causes chronic inflammation and suppresses expression of phagocytic receptors. Environ Sci Pollut Res Int. 2017;24:11708–21. [DOI] [PubMed] [Google Scholar]
- [18].Tutkun L, Gunduzoz M, Turksoy VA, et al. Arsenic-induced inflammation in workers. Mol Biol Rep. 2019;46:2371–8. [DOI] [PubMed] [Google Scholar]
- [19].Rahman A, Islam MS, Tony SR, et al. T helper 2-driven immune dysfunction in chronic arsenic-exposed individuals and its link to the features of allergic asthma. Toxicol Appl Pharmacol. 2021;420:115532. [DOI] [PubMed] [Google Scholar]
- [20].Page MJ, Moher D, Bossuyt PM, et al. PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. Bmj. 2021;372:n160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Wells GA, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. 2019. Available at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. [access date June 2022].
- [22].Higgins J, Thomas J, Chandler J, et al. Cochrane handbook for systematic reviews of interventions version 6.3 (updated February 2022). 2022. Cochrane. Available at: www.training.cochrane.org/handbook. Published [accessed 2022]. [Google Scholar]
- [23].Dutta K, Prasad P, Sinha D. Chronic low level arsenic exposure evokes inflammatory responses and DNA damage. Int J Hyg Environ Health. 2015;218:564–74. [DOI] [PubMed] [Google Scholar]
- [24].Ji C, Fu C, Xiang Q, et al. Biomarkers of early vascular endothelial injury with low-arsenic exposure in drinking water. Chin J Endemiol. 2011;30:493–7. [Google Scholar]
- [25].Fang X, Xia S, Zhu K, et al. Differential expression and significance of Foxp3, TGF-β1 and IL-2 in peripheral blood of patients with coal-burning arsenic poisoning. Chin J Endemiol. 2019;28:91–5. [Google Scholar]
- [26].Ning Y, Wang X. Changes of TNF and T lymphocyte subsets in patients with drinking water arsenic poisoning. Chin J Ctrl Endem Dis. 2014;29:202. [Google Scholar]
- [27].Wang R, Wang Q. Effect of high arsenic drinking water on the levels of T lymphocyte subsets, immunoglobulin and interleukin 2 in pregnant women. Chin J Ctrl Endem Dis. 2016;31:680–1. [Google Scholar]
- [28].Das N, Paul S, Chatterjee D, et al. Arsenic exposure through drinking water increases the risk of liver and cardiovascular diseases in the population of West Bengal, India. BMC Public Health. 2012;12:639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Karim MR, Rahman M, Islam K, et al. Increases in oxidized low-density lipoprotein and other inflammatory and adhesion molecules with a concomitant decrease in high-density lipoprotein in the individuals exposed to arsenic in Bangladesh. Toxicol Sci. 2013;135:17–25. [DOI] [PubMed] [Google Scholar]
- [30].Gao Y, Zhao Z, Yang L, et al. Arsenic exposure assists ccm3 genetic polymorphism in elevating blood pressure. Oncotarget. 2018;9:4915–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Xu Y, Zou Z, Liu Y, et al. miR-191 is involved in renal dysfunction in arsenic-exposed populations by regulating inflammatory response caused by arsenic from burning arsenic-contaminated coal. Hum Exp Toxicol. 2020;39:37–46. [DOI] [PubMed] [Google Scholar]
- [32].Kany S, Vollrath JT, Relja B. Cytokines in inflammatory disease. Int J Mol Sci . 2019;20:6008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Liu S, Sun Q, Wang F, et al. Arsenic induced overexpression of inflammatory cytokines based on the human urothelial cell model in vitro and urinary secretion of individuals chronically exposed to arsenic. Chem Res Toxicol. 2014;27:1934–42. [DOI] [PubMed] [Google Scholar]
- [34].Zhao L, Yang S, Guo Y, et al. Chronic arsenic exposure in drinking water interferes with the balances of T lymphocyte subpopulations as well as stimulates the functions of dendritic cells in vivo. Int Immunopharmacol. 2019;71:115–31. [DOI] [PubMed] [Google Scholar]
- [35].Wu CC, Huang YK, Chung CJ, et al. Polymorphism of inflammatory genes and arsenic methylation capacity are associated with urothelial carcinoma. Toxicol Appl Pharmacol. 2013;272:30–6. [DOI] [PubMed] [Google Scholar]
- [36].Hsieh YC, Hsieh FI, Lien LM, et al. Risk of carotid atherosclerosis associated with genetic polymorphisms of apolipoprotein E and inflammatory genes among arsenic exposed residents in Taiwan. Toxicol Appl Pharmacol. 2008;227:1–7. [DOI] [PubMed] [Google Scholar]
- [37].Chen TH, Huang JJ, Kung WS, et al. The Association of Serum TNF-α Levels and Blood Multi-Elements Modified by TNF-α Gene Polymorphisms in Metal Industrial Workers. Int J Environ Res Public Health. 2019;16:4097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Escobar-García DM, Del Razo LM, Sanchez-Peña LC, et al. Association of glutathione S-transferase Ω 1-1 polymorphisms (A140D and E208K) with the expression of interleukin-8 (IL-8), transforming growth factor beta (TGF-β), and apoptotic protease-activating factor 1 (Apaf-1) in humans chronically exposed to arsenic in drinking water. Arch Toxicol. 2012;86:857–68. [DOI] [PubMed] [Google Scholar]