Supplemental Digital Content is available in the text.
Background.
Social inequalities in health lead to an increased risk of chronic kidney disease and less access to renal transplantation. The objective of this study was to assess the association between social deprivation estimated by the fifth quintile of the European Deprivation Index (EDI) and preemptive kidney transplantation (PKT) and to explore the potential mediators of this association.
Methods.
This retrospective observational multicenter study included 8701 patients who received their first renal transplant in France between 2010 and 2014. Mediation analyses were performed to assess the direct and indirect effects of the EDI on PKT.
Results.
Among the 8701 transplant recipients, 32.4% belonged to the most deprived quintile of the EDI (quintile 5) and 16% received a PKT (performed either with a deceased- or living-donor). There was a significant association between quintile 5 of the EDI and PKT (total effect: odds ratio [OR]: 0.64 [95% confidence interval (CI): 0.55-0.73]). Living-donor kidney transplantation was the main mediator of this association (natural indirect effect: OR: 0.92 [0.89–0.95]). To a lesser extent, positive cytomegalovirus and hepatitis C serologies and blood group B were also mediators (respective natural indirect effects: OR: 0.98 [95% CI: 0.95-1.00], OR: 0.99 [95% CI: 0.99-1.00], and OR: 0.99 [95% CI: 0.98-1.00], P < 0.05).
Conclusions.
Our study suggests that social deprivation is associated with a decreased proportion of PKT. This association might be mitigated by promoting living-donor transplantation.
It is widely accepted that, compared with dialysis, renal transplantation is associated with a lower risk of mortality and a greater quality of life.1 Large-scale studies have demonstrated that patients transplanted before beginning dialysis had a lower risk of death than subjects who were transplanted while on dialysis.2,3 Furthermore, it has been shown that the time spent on dialysis before transplantation affects patient survival.3 The beneficial effect of preemptive transplantation on renal allograft survival is more controversial.4 Nevertheless, longer transplant survival was found when transplantation was performed from a living donor5-7 and when living-donor transplantation was performed preemptively rather than after the initiation of dialysis.7 Recent recommendations encourage preemptive living-donor kidney transplantation (LDKT).8
On the other hand, it has been demonstrated that there are social inequalities in access to preemptive renal transplantation.9-11 In France, it has been suggested that social deprivation could influence access to preemptive registration.12 There is a lack of data regarding the underlying mechanism by which social deprivation affects preemptive renal transplantation. Decreased access to living-donor transplantation of the most deprived population may partially explain this social inequity.9,10,13
The European Deprivation Index (EDI) is a composite index that measures social deprivation. It contains variables common, on the one hand, to the European Union Statistics on Income and Living survey that studied objective poverty and the concept of individual perception of needs called “subjective poverty” in 26 European countries14 and, on the other hand, to the census-based data of the National Institute for Statistics and Economic Studies. The 10 individual items that constitute the EDI are: foreign nationality, low level of education, unemployment, unskilled worker, no access to a car, no access to central or electric heating, single-parent family, household ≥ 6 people, overcrowded housing, and nonowner. As these variables are applied to the smallest French geographical area (Ilôts Regroupés pour l’Information Statistique [IRIS]), the EDI constitutes an ecological index of social deprivation. It allows comparisons between European countries where it has been validated.14,15 Two studies from our team demonstrated that the EDI was associated with the outcomes of renal transplantation and with the proportion of living-donor transplantation.16,17
To our knowledge, there are no data about the effects of social disparities in health, estimated by a European transnational indicator, and preemptive transplantation. Moreover, although 3 studies have evaluated the role of social disparities on access to transplantation using mediation analyses,18-20 none have examined the mediators that could explain the association between social deprivation and preemptive renal transplantation.
The objective of our study was to assess the association between social deprivation, estimated by the most deprived quintile of the EDI, and preemptive renal transplantation. The objective was also to estimate the direct and indirect effects of social deprivation on the outcome.21-23
MATERIALS AND METHODS
Study Population and Data Sources
This was a retrospective study, using the CRISTAL database of the French Biomedicine Agency (Agence de la Biomédecine, Saint-Denis, France) where the data of transplanted patients of 32 French transplantation centers were registered. The patients older than 18 y receiving a first deceased- or living-donor kidney ransplantation between January 1, 2010, and December 31, 2014, and for whom a precise home address in France was provided at registration for the waiting list (required for EDI calculation) were included in the study. Patients who had already been transplanted (renal, cardiac, or liver transplant) or who had received a multiorgan transplantation were excluded. Of the 9205 transplanted patients from the original dataset, home address was missing for 504 of them. There was no statistical difference regarding the patients’ characteristics between individuals excluded because of missing address and the other patients. Ultimately, 8701 patients were included.
Study Variables
Event of Interest
The event of interest was preemptive renal transplantation, with either a deceased- or living-kidney donor, among the study population. Preemptive kidney transplantation (PKT) was defined as transplantation with no previous history of dialysis.
Social Deprivation
The explanatory variable was the EDI. The EDI is a validated European ecological index, for which there is a French version,14 calculated for the smallest geographical scale available in France (IRIS) corresponding to about 2000 inhabitants. Each patient’s home address corresponds to an IRIS for which the EDI has been measured and has been interpreted as a proxy of the individual social deprivation. The induced ecological bias of this proxy is considered to be limited because the accuracy of socioeconomic measures decreases with the size of the geographical unit used.
The EDI was categorized into 5 quintiles to describe the general population (quintile 5 being the most deprived), as recommended by Pornet et al14 after studying its distribution. Subsequently, as was necessary for the mediation analyses, the EDI was dichotomized and used as a binary variable (quintile 5 versus other quintiles).
Of note, ethnicity could not be studied in our work since the collection of this variable is not authorized by the French law.
Patient Characteristics
Patient information provided at registration for the renal transplant waiting list is collected in the CRISTAL database of the Agence de la Biomédecine. The following variables were extracted: age, sex, body mass index (BMI), underlying nephropathy, diabetes, cardiovascular disease (coronaropathy, myocardial infarction, chronic heart failure, angiopathy, and stroke), tobacco use, hypertension, hepatitis C virus (HCV) serology, cytomegalovirus (CMV) serology, Epstein-Barr virus serology, positive HLA antibodies, blood group, preemptive registration, and preemptive transplantation.
Potential Confounders and Mediators
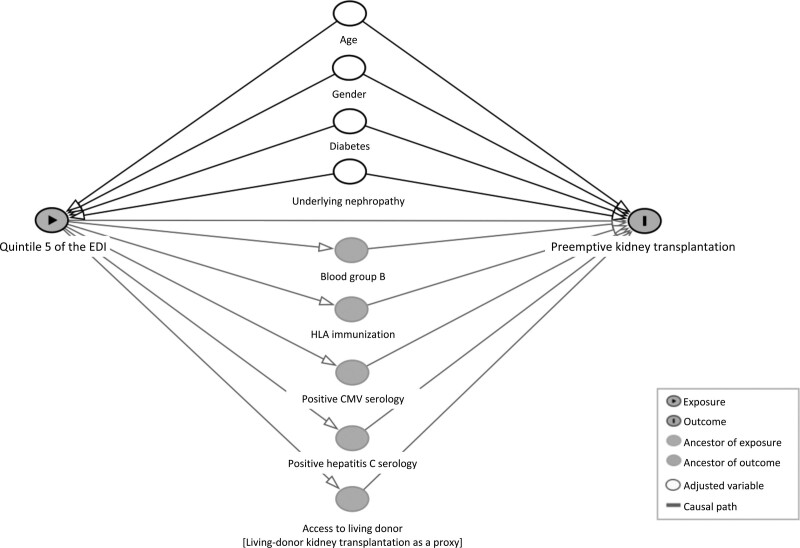
Our assumptions for potential confounders and mediators were defined based on the literature data (SDC, http://links.lww.com/TXD/A357) and their hypothetical relations with quintile 5 of the EDI and PKT are graphically represented in a directed acyclic graph (DAG) (Figure 1).24
FIGURE 1.
Directed acyclic graph describing the causal assumptions in the relationship between social deprivation, estimated by quintile 5 of the European Deprivation Index, and preemptive kidney transplantation (potential confounders and potential mediators). CMV, cytomegalovirus; EDI, European Deprivation Index.
This study was conducted in the French regulation context regarding living donor transplantation. Patients awaiting renal transplantation, whatever the modality, must be registered on the waiting list for deceased-donor transplantation. That means that even patients due for living donor are registered on the waiting list pending the end of the transplantation work up of their living donor. Consequently, of the patients who have a living donor, there are individuals that accept a proposal for a deceased-donor transplant before the completion of the living-donor transplantation. “Registration with a living donor,” which is on the pathway to preemptive transplantation, could have been considered as a marker of the access to LDKT, unfortunately this information is not collected in the registry. We hypothesized that the most deprived patients had a lower likelihood of preemptive transplantation that could be partly explained by a lower access to living donor. As the variable “registration with a living donor” was not available in the database, “LDKT” was used as a proxy and considered as a mediator of the effect of social deprivation on the rate of preemptive transplantation.
Statistical Analyses
For the univariate analysis, characteristics of the complete cohort were described according to the 5 quintiles of the EDI. Categorical data were described by frequencies and percentages.
Using a logistic regression model, 2 bivariate analyses were performed to estimate the association of each covariate with quintile 5 of the EDI and with PKT. Regression splines were used to explore the functional form of the continuous variables “age” and “BMI.” As there was no linear relationship between these covariates and the logit event of interest, they were transformed into categorical variables.
Mediation analyses explore the underlying mechanism by which one exposure variable influences an outcome variable through a mediator variable. The relationship between the exposure variable, the outcome variable, potential confounders, and potential mediators are graphically represented by rows in a DAG (Figure 1). In traditional regression modeling, when controlling for the mediator, the association between the exposure and outcome is reduced.
Based on the DAG, mediation analyses using a counterfactual approach were performed to assess the extent to which the effect of social deprivation on PKT was direct or mediated by other variables.21-23 Confidence intervals (CIs) of the estimates were obtained using a nonparametric bootstrap procedure.
As there were 5 potential mediators, a mediation analysis with 1 mediator at a time was first performed. Subsequently, a sequential mediation analysis was conducted (model 1: single mediator “living-donor transplantation,” model 2: model 1 + “positive CMV serology,” model 3: model 2 + “HLA immunization,” model 4: model 3 + “positive HCV serology,” model 5: model 4 + “blood group B”). Mediators were entered based on the strength of their association with the outcome in the bivariate analysis.
All mediation analyses were adjusted for age, gender, diabetes, and underlying nephropathy. The “total effect” of the exposure on the outcome was decomposed into a “natural direct effect” (the effect of the exposure on the outcome) and into a “natural indirect effect” (the effect of the exposure on the outcome through a mediator). There were no exposure–mediator or mediator–mediator interactions.
This study using data from the CRISTAL database was conducted with the approval of the French National Ethics Committee (Commission Nationale de l’Informatique et des Libertés).
Statistical analyses were performed using R3.3.3 (R Foundation for Statistical Computing, Vienna, Austria), using medflex and dagitty packages.
RESULTS
Patient Characteristics
Among the 8701 transplant recipients, 2818 patients (32.4 %) were in quintile 5 of the EDI and 1397 (16%) received a PKT. Compared with the patients of the other quintiles, subjects of quintile 5 were younger (older than 60 y: 32% versus 41% in quintile 1, 36% in quintile 2, and 39% in quintiles 3 and 4), were more frequently female (39% in quintile 5 versus 34% in quintiles 1 and 2, 35% in quintile 3, and 36% in quintile 4), and had a higher BMI. Patients in quintile 5 were more frequently diabetic, positive for HCV serology and blood group B (15% in quintile 5 versus 11% in quintiles 1 to 3 and 10% in quintile 4). The rate of HLA immunization was greater in the quintile 5 group than in the other groups (29% in quintile 5 versus 25% in quintiles 1 and 2 and 26% in quintiles 3 and 4). The proportion of PKT decreased from quintile 1 to quintile 5 (20% in quintile 1, 19% in quintile 2, and 17% in quintiles 3 and 4 versus 12% in quintile 5) as did the proportion of living-donor transplants (16% in quintiles 1 and 2, 14% in quintile 3, and 15% in quintile 4 versus 12% in quintile 5). Patient characteristics are presented in Table 1 and Table S1 (SDC, http://links.lww.com/TXD/A357).
TABLE 1.
Patient characteristics (complete cohort) according to deprivation quintiles of the European Deprivation Index (quintile 5 is the most deprived)
| All(N=8701) | Quintile 1 (N=1272) | Quintile 2 (N=1391) | Quintile 3 (N=1524) | Quintile 4 (N=1696) | Quintile 5 (N=2818) | |
|---|---|---|---|---|---|---|
| Patient characteristics | ||||||
| Age (y) | ||||||
| 18–30 | 685 (8%) | 88 (7%) | 98 (7%) | 110 (7%) | 143 (8%) | 246 (9%) |
| 30–60 | 4839 (56%) | 659 (52%) | 788 (57%) | 821 (54%) | 899 (53%) | 1672 (59%) |
| >60 | 3177 (37%) | 525 (41%) | 505 (36%) | 593 (39%) | 654 (39%) | 900 (32%) |
| Gender (female) | 3150 (36%) | 434 (34%) | 470 (34%) | 534 (35%) | 608 (36%) | 1104 (39%) |
| BMI (kg/m2) | ||||||
| <20 | 247 (3%) | 36 (3%) | 35 (3%) | 46 (3%) | 49 (3%) | 81 (3%) |
| 20–25 | 4203 (48%) | 638 (50%) | 675 (49%) | 719 (47%) | 854 (50%) | 1317 (47%) |
| >25 | 4251 (49%) | 598 (47%) | 681 (49%) | 759 (50%) | 793 (47%) | 1420 (50%) |
| Underlying nephropathy | ||||||
| Diabetic | 895 (10%) | 100 (8%) | 120 (9%) | 138 (9%) | 169 (10%) | 368 (13%) |
| Glomerulonephritis | 1979 (23%) | 309 (24%) | 352 (25%) | 368 (24%) | 401 (24%) | 549 (19%) |
| Interstitial nephritis | 789 (9%) | 102 (8%) | 128 (9%) | 144 (9%) | 152 (9%) | 263 (9%) |
| PKD (ref) | 1650 (19%) | 289 (23%) | 324 (23%) | 320 (21%) | 334 (20%) | 383 (14%) |
| Systemic disease | 302 (3%) | 43 (3%) | 48 (3%) | 60 (4%) | 59 (3%) | 92 (3%) |
| Uropathy | 204 (2%) | 29 (2%) | 37 (3%) | 36 (2%) | 47 (3%) | 55 (2%) |
| Vascular | 866 (10%) | 133 (10%) | 116 (8%) | 132 (9%) | 149 (9%) | 336 (12%) |
| Miscellaneous | 477 (5%) | 73 (6%) | 64 (5%) | 80 (5%) | 87 (5%) | 173 (6%) |
| Unknown | 1539 (18%) | 194 (15%) | 202 (15%) | 146 (16%) | 298 (18%) | 599 (21%) |
| Diabetes | 1466 (17%) | 190 (15%) | 228 (16%) | 228 (15%) | 266 (16%) | 554 (20%) |
| Cardiovascular disease | 2041 (23%) | 301 (24%) | 328 (24%) | 368 (24%) | 379 (22%) | 665 (24%) |
| Tobacco (smoker) | 4497 (52%) | 611 (48%) | 672 (48%) | 727 (48%) | 843 (50%) | 1351 (48%) |
| Hypertension | 5948 (68%) | 882 (69%) | 952 (68%) | 1036 (68%) | 1131 (67%) | 1947 (69%) |
| Positive hepatitis C serology | 222 (3%) | 26 (2%) | 35 (3%) | 18 (1%) | 41 (2%) | 102 (4%) |
| Positive CMV serology | 5147 (59%) | 602 (47%) | 702 (50%) | 818 (54%) | 983 (58%) | 2042 (72%) |
| Positive EBV serology | 8329 (96%) | 1213 (95%) | 1325 (95%) | 1452 (95%) | 1626 (96%) | 2713 (96%) |
| HLA antibody class I and/or II > 0% | 2309 (27%) | 317 (25%) | 347 (25%) | 395 (26%) | 438 (26%) | 812 (29%) |
| Blood group | ||||||
| B | 1043 (12%) | 134 (11%) | 149 (11%) | 172 (11%) | 162 (10%) | 426 (15%) |
| Others | 7658 (88%) | 1138 (89%) | 1242 (89%) | 1352 (89%) | 1534 (90%) | 2392 (85%) |
| Transplantation characteristics | ||||||
| Preemptive registration | 3026 (35%) | 532 (42%) | 547 (39%) | 573 (38%) | 584 (34%) | 790 (28%) |
| Preemptive transplantation | 1397 (16%) | 252 (20%) | 268 (19%) | 264 (17%) | 283 (17%) | 330 (12%) |
| Donor source | ||||||
| Living donor | 1225 (14%) | 209 (16%) | 220 (16%) | 219 (14%) | 251 (15%) | 326 (12%) |
| Of which PKT | 478 (39%) | 94 (45%) | 96 (44%) | 89 (41%) | 92 (37%) | 107 (33%) |
| Deceased donor | 7476 (86%) | 1063 (84%) | 1171 (84%) | 1305 (86%) | 1445 (85%) | 2492 (88%) |
| Of which PKT | 919 (12%) | 158 (15%) | 172 (15%) | 175 (13%) | 191 (13%) | 223 (9%) |
BMI, body mass index; CMV, cytomegalovirus; EBV, Epstein-Barr virus; PKD, polycystic kidney disease; PKT, preemptive kidney transplantation.
Bivariate Analyses
Patient characteristics of age older than 60 y, female gender, diabetes, positive HCV serology, positive CMV serology, HLA immunization, blood group B, and the underlying nephropathy were associated with quintile 5 of the EDI (Table 2). Patients in quintile 5 were less likely to be transplanted from a living donor and before dialysis initiation (odds ratio [OR]: 0.73 [95% CI: 0.63-0.83], OR: 0.60 [95% CI: 0.52-0.68]).
TABLE 2.
Bivariate analysis (logistic regression)
| Patient characteristics (N = 2818) | OR [95% CI] |
|---|---|
| Age (y) | |
| 18–30 | Ref |
| 30–60 | 0.94 [0.80-1.11] |
| >60 | 0.71 [0.59-0.84] |
| Gender (female) | 1.21 [1.10-1.33] |
| BMI (kg/m2) | |
| <20 | 1.07 [0.81-1.40] |
| 20–25 | Ref |
| >25 | 1.10 [1.00-1.20] |
| Underlying nephropathy | |
| Diabetic | 2.31 [1.94-2.75] |
| Glomerulonephritis | 1.27 [1.09-1.48] |
| Interstitial nephritis | 1.65 [1.37-1.99] |
| PKD | Ref |
| Systemic disease | 1.45 [1.10-1.89] |
| Uropathy | 1.22 [0.87-1.69] |
| Vascular | 2.10 [1.75-2.51] |
| Miscellaneous | 1.88 [1.51-2.34] |
| Unknown | 2.11 [1.81-2.46] |
| Diabetes | 1.33 [1.19-1.50] |
| Cardiovascular disease | 1.01 [0.91-1.12] |
| Tobacco (smoker) | 0.98 [0.89-1.07] |
| Hypertension | 1.05 [0.95-1.16] |
| Positive hepatitis C serology | 1.80 [1.38-2.36] |
| Positive CMV serology | 2.35 [2.14-2.60] |
| HLA antibody class I and/or II > 0% | 1.19 [1.07-1.31] |
| Blood group | |
| B | 1.52 [1.33-1.73] |
| Others | Ref |
| Donor source (living donor) | 0.73 [0.63-0.83] |
| Preemptive kidney transplantation | 0.60 [0.52-0.68] |
Factors associated with quintile 5 of the European Deprivation Index.
BMI, body mass index; CI, confidence interval; CMV, cytomegalovirus; OR, odds ratio; PKD, polycystic kidney disease.
Factors associated with preemptive transplantation were age older than 30 y, female gender, diabetes, cardiovascular disease, tobacco use, positive HCV serology, positive CMV serology, HLA immunization, blood group B, and underlying nephropathy (Table 3). Quintile 5 of the EDI and living-donor transplantation were associated with preemptive transplantation (OR: 0.60 [95% CI: 0.52-0.68]; OR: 4.57 [95% CI: 3.99-5.22]).
TABLE 3.
Bivariate analysis (logistic regression)
| Patient characteristics (N = 1397) | OR [95% CI] |
|---|---|
| Age (y) | |
| 18–30 | Ref |
| 30–60 | 0.71 [0.59-0.87] |
| >60 | 0.67 [0.55-0.83] |
| Gender (female) | 1.13 [1.01-1.27] |
| BMI (kg/m2) | |
| <20 | 1.03 [0.73-1.44] |
| 20–25 | Ref |
| >25 | 0.93 [0.83-1.04] |
| Underlying nephropathy | |
| Diabetic | 0.40 [0.31-0.51] |
| Glomerulonephritis | 0.69 [0.58-0.81] |
| Interstitial nephritis | 0.95 [0.77-1.17] |
| PKD | Ref |
| Systemic disease | 0.30 [0.19-0.46] |
| Uropathy | 1.27 [0.90-1.76] |
| Vascular | 0.46 [0.36-0.58] |
| Miscellaneous | 0.68 [0.51-0.88] |
| Unknown | 0.51 [0.42-0.62] |
| Diabetes | 0.56 [0.46-0.66] |
| Cardiovascular disease | 0.52 [0.44-0.61] |
| Tobacco (smoker) | 0.79 [0.71-0.89] |
| Hypertension | 0.89 [0.79-1.00] |
| Positive hepatitis C serology | 0.43 [0.25-0.68] |
| Positive CMV serology | 0.75 [0.67-0.84] |
| HLA antibody class I and/or II > 0% | 0.76 [0.66-0.87] |
| Blood group | |
| B | 0.75 [0.61-0.90] |
| Others | Ref |
| Donor source (living donor) | 4.57 [3.99-5.22] |
| EDI (quintile 5) | 0.60 [0.52-0.68] |
Factors associated with preemptive kidney transplantation.
BMI, body mass index; CI, confidence interval; CMV, cytomegalovirus; OR, odds ratio; PKD, polycystic kidney disease.
Tables S2 and S3 (SDC. http://links.lww.com/TXD/A357), respectively, represent the comparison of factors between each quintile of the EDI and factors associated with preemptive registration.
Mediation Analyses
After adjustment for potential confounders, the mediation analysis showed a significant total effect (OR: 0.64 [95% CI: 0.55-0.73]) and direct effect of quintile 5 of the EDI on preemptive transplantation. In the mediation analyses performed separately for each mediator, quintile 5 had a significant indirect effect on preemptive transplantation through living-kidney donor (OR: 0.92 [95% CI: 0.89-0.95]). To a lesser extent, positive CMV serology (OR: 0.98 [95% CI: 0.95-1.00], P < 0.05), positive HCV serology (OR: 0.99 [95% CI: 0.99-1.00], P < 0.05), and blood group B (OR: 0.99 [95% CI: 0.98-1.00], P < 0.05) were also mediators of the effect of social deprivation on PKT. Quintile 5 of the EDI had no indirect effect on preemptive transplantation through the HLA immunization (OR: 1.00 [95% CI: 0.99-1.00]). The results of the mediation analysis examining one mediator at a time are displayed in Table 4. When the mediators were entered sequentially in the mediation analyses, the indirect effect of quintile 5 of the EDI through the mediators remained within the same range (OR: 0.92 [95% CI: 0.89-0.95] for model 1, OR: 0.90 [95% CI: 0.87-0.94] for model 2, OR: 0.90 [95% CI: 0.87-0.94] for model 3, OR: 0.90 [95% CI: 0.86-0.93] for model 4, and OR: 0.89 [95% CI: 0.85-0.92] for model 5). The results of the sequential mediation analysis are provided in Table 5.
TABLE 4.
Mediation analyses: effects of social deprivation on preemptive kidney transplantation (one mediator at a time)
| Living-donor transplantation | Positive CMV serology | HLA immunization | Positive hepatitis C serology | Blood group B | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI | |
| Natural direct effect | 0.69 | [0.61-0.78]** | 0.65 | [0.57-0.75]** | 0.64 | [0.56-0.73]** | 0.64 | [0.55-0.73]** | 0.64 | [0.56-0.73]** |
| Natural indirect effect | 0.92 | [0.89-0.95]** | 0.98 | [0.95-1.00]* | 1.00 | [0.99-1.00] | 0.99 | [0.99-1.00]* | 0.99 | [0.98-1.00]* |
| Total effect | 0.64 | [0.56-0.73]** | 0.63 | [0.56-0.73]** | 0.63 | [0.55-0.73]** | 0.63 | [0.55-0.73]** | 0.63 | [0.55-0.73]** |
The analyses were adjusted for confounders: age, sex, diabetes, and underlying nephropathy.
*P < 0.05.
**P < 0.001.
CI, confidence interval; CMV, cytomegalovirus; OR, odds ratio.
TABLE 5.
Sequential mediation analyses: effects of social deprivation on preemptive kidney transplantation
| Model 1 | Model 2 | Model 3 | Model 4 | Model 5 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI | |
| Natural direct effect | 0.69 | [0.61-0.78] | 0.70 | [0.62-0.80] | 0.70 | [0.62-0.80] | 0.71 | [0.62-0.81] | 0.72 | [0.63-0.82] |
| Natural indirect effect | 0.92 | [0.89-0.95] | 0.90 | [0.87-0.94] | 0.90 | [0.87-0.94] | 0.90 | [0.86-0.93] | 0.89 | [0.85-0.92] |
| Total effect | 0.64 | [0.56-0.73] | 0.64 | [0.56-0.73] | 0.64 | [0.55-0.73] | 0.64 | [0.55-0.73] | 0.64 | [0.55-0.73] |
The analyses were adjusted for confounders: age, sex, diabetes, and underlying nephropathy.
Model 1: mediation analysis with a single mediator—living-donor transplantation.
Model 2: mediation analysis with 2 mediators—living-donor transplantation and positive CMV serology.
Model 3: mediation analysis with 3 mediators—living-donor transplantation, positive CMV serology, and HLA immunization.
Model 4: mediation analysis with 4 mediators—living-donor transplantation, positive CMV serology, HLA immunization, and positive hepatitis C serology.
Model 5: complete mediation analysis with the 5 mediators described in the DAG—living-donor transplantation, positive CMV serology, HLA immunization, positive hepatitis C serology, and blood group B.
CI, confidence interval; CMV, cytomegalovirus; DAG, directed acyclic graph; OR, odds ratio.
Additional Analyses
Additional mediation analyses focusing on preemptive registration as the outcome are presented in Tables S4 and S5 (SDC, http://links.lww.com/TXD/A357).
The results of the multivariable analysis stratified by donor type are presented in Table S6 (SDC, http://links.lww.com/TXD/A357). In this analysis, quintile 5 of the EDI was associated with preemptive registration and preemptive transplantation in the deceased-donor kidney transplanted group but not in the living-donor kidney transplanted group. This could be explained by the fact that patients belonging to quintile 5 who received a living-donor transplant may have particular characteristics not measured in our study or may have a different treatment modality. However, the interaction term between EDI (exposure) and donor type (mediator) had no effect on PKT (outcome), which allowed us to conduct the mediation analysis reflecting the natural effect models.
DISCUSSION
Our study showed that in France, social deprivation estimated by the EDI was highly prevalent among transplanted patients and that it was associated with a lower proportion of preemptive renal transplantation directly but also indirectly through living-donor transplantation, which appeared to be the main mediator. Our findings were consistent with the results of previous studies, which highlighted social disparities in access to preemptive transplantation9-12 and confirmed that the EDI can be used in the nephrology field of social deprivation.12,16-18,25,26 The factors that may explain this association could depend on the healthcare system, the recipient, and the donor, as well as the physician.
Disparities in access to primary care, necessary for referral to a nephrologist, may partially explain the lower proportion of preemptive transplantation in the most deprived patients compared with the other transplanted patients. It has been shown that individuals with a greater income received more prescription medication and more often visited medical specialists and underwent advanced imaging procedures.27 In one study from the United States, routine healthcare visits were a mediator between socioeconomic status and chronic kidney disease (CKD).28 In France, inequities in access to primary care have been recently described in the general population.29
A faster decline in renal function in the most deprived population could also affect the timing of the nephrology referral. It has been demonstrated that low socioeconomic status was associated with a higher risk of chronic diseases30 and with risk factors for CKD progression.28 Lower socioeconomic status was associated with albuminuria, estimated glomerular filtration rate decline, and end-stage renal disease in previous reports.31,32 In Europe, low education levels were associated with CKD progression.33 In a report from the United Kingdom, socially deprived populations had a higher risk of severe CKD at presentation to a renal service.34 Moreover, a French study showed that the most deprived patients had a higher risk of requiring emergency dialysis than the other patients,26 whereas the emergency start was associated with a lower chance of being waitlisted in a recent study.35 Lower socioeconomic and educational status resulted in delayed nephrology referral,36 even though late referral is known to reduced transplantation access.37
An association among health literacy, socioeconomic status, and CKD has been demonstrated.38 Thus, the lack of understanding of what CKD is and what it involves is influenced by socioeconomic status and could also affect access to healthcare and referral to nephrology.
Moreover, there is a potential association between social deprivation and geographic isolation, which would suggest that distance to the transplantation center might delay registration on the waiting list; however, previous studies among dialysis patients have reported controversial results.39,40 To the best of our knowledge, there are no data about the effect of the distance from patient home to transplantation center and preemptive registration. Socioeconomic status could impact the whole process of kidney transplantation from the potential recipient to the potential donor because of difficulty in navigating the health system. Navigator programs may help the most deprived subjects move through the complex pathway that leads to transplantation.41
The cost associated with the transplantation workup may as well decrease access to preemptive registration on the waiting list. Disparities between private centers or for-profit ownership of dialysis facilities were described regarding access to the exams required for registration.42 In a recent US study, disparities in patients’ access to PKT were associated with the type of health insurance.11 However, in Sweden, despite a universal healthcare coverage, low income was also associated with lower registration on the waiting list for renal transplantation.43
The sequential mediation analysis we conducted has identified LDKT as being the main and above all modifiable mediator between social deprivation and PKT. To our knowledge, our study is the first to focus on the mediators involved in the causal pathway between social deprivation and PKT. Indeed, in a pediatric French cohort, Driollet et al18 studied the mediators between the EDI and graft failure. In the United Kingdom, Bailey et al19 studied the indirect effect of education and income on LDKT. And last, in a US study by Murphy et al,20 socioeconomic status was a mediator between ethnicity and access to registration and renal transplantation.
To our knowledge, there is no mediation analysis that has evaluated LDKT as an outcome. Large-scale studies conducted in Australia, the United Kingdom, and the United States showed that socially deprived patients had lower access to LDKT.9-11 Since socially deprived relatives are exposed to the same risk factors as deprived recipients,28 the prevalence of chronic diseases among potential donors could reduce the possibility of organ donation in the most disadvantaged populations. Data about living donors in the United States showed that living donors were more frequently employed than the general population.44 Gill et al13 reported that the rate of LDKT was greater in the highest income population and that the least deprived living donors had greater access to medical specialists who performed the transplantation workup. It can also be speculated that difference in health literacy related to socioeconomic status, cultural, and religious factors may influence the willingness toward living organ donation.45-47 To decrease ethnic disparities in access to living-donor transplantation, web programs for education on LDKT have been developed.48
Finally, the socioeconomic status of patients may influence physicians’ perception and practice. In support of this, a qualitative study conducted in Australia and New Zealand highlighted that nephrologists tended to consider that the most socially disadvantaged patients were less likely to receive a living-donor kidney.49
In our mediation analyses, positive CMV serology, known to be associated with an increased risk of cardiovascular disease50; hepatitis C, which is more prevalent in disadvantaged populations51; and blood group B had an indirect effect on preemptive transplantation, but the magnitude of the effect was marginal compared with living-donor.
Our study has limitations. Residual confounders not collected in the database may affect the association between the EDI and preemptive transplantation. As an example, ethnicity has not been studied because the collection of this data is not authorized in France. The EDI potentially contains mediators of the relationship between social deprivation and PKT, such as foreign nationality, no access to a car, low level of education, unemployment, single-parent family, or household ≥6 people.14 Last, the association between the EDI and preemptive transplantation was estimated among transplanted patients, whereas a study of patients registered for renal transplantation would have provided a more accurate estimation.
Our work demonstrated that social deprivation estimated by the EDI was associated with PKT. Our study emphasized that the effect of social deprivation on preemptive transplantation was mainly mediated by living-donor transplantation. This result is interesting since, unlike the other mediators highlighted in our study, living-donor transplantation is a modifiable factor. Further studies are needed to explore the role of social deprivation on LDKT. To increase preemptive renal transplantation in the most deprived populations, measures should be implemented to promote living-donor transplantation, targeting both practitioners and patients. Physicians should be able to promote health literacy and to provide information about living-donor transplantation tailored to the patients’ health literacy.52 Widespread use of web or mobile programs for shared decision aid in the treatment of CKD should be encouraged48,53 to help inform socially deprived patients.
Supplementary Material
Footnotes
E.C., T.L., and V.C. participated in research design, in the performance of the research, in data analysis, and in the writing of the paper. L.L., A.B., and G.L. participated in research design and in the performance of the research.
The authors declare no conflicts of interest.
The study was funded by a grant from the “Agence de la Biomédecine.”
Supplemental digital content (SDC) is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML text of this article on the journal’s Web site (www.transplantationdirect.com).
REFERENCES
- 1.Tonelli M, Wiebe N, Knoll G, et al. Systematic review: kidney transplantation compared with dialysis in clinically relevant outcomes. Am J Transplant. 2011;11:2093–2109. [DOI] [PubMed] [Google Scholar]
- 2.Asderakis A, Augustine T, Dyer P, et al. Pre-emptive kidney transplantation: the attractive alternative. Nephrol Dial Transplant. 1998;13:1799–1803. [DOI] [PubMed] [Google Scholar]
- 3.Haller MC, Kammer M, Oberbauer R. Dialysis vintage and outcomes in renal transplantation. Nephrol Dial Transplant. 2019;34:555–560. [DOI] [PubMed] [Google Scholar]
- 4.Kessler M, Ladriere M, Giral M, et al. Does pre-emptive kidney transplantation with a deceased donor improve outcomes? Results from a French transplant network. Transpl Int. 2011;24:266–275. [DOI] [PubMed] [Google Scholar]
- 5.Molnar MZ, Streja E, Kovesdy CP, et al. Age and the associations of living donor and expanded criteria donor kidneys with kidney transplant outcomes. Am J Kidney Dis. 2012;59:841–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cecka JM. The UNOS scientific renal transplant registry. Clin Transpl. 1999;1–21. [PubMed] [Google Scholar]
- 7.Mange KC, Joffe MM, Feldman HI. Effect of the use or nonuse of long-term dialysis on the subsequent survival of renal transplants from living donors. N Engl J Med. 2001;344:726–731. [DOI] [PubMed] [Google Scholar]
- 8.Chadban SJ, Ahn C, Axelrod DA, et al. Summary of the Kidney Disease: Improving Global Outcomes (KDIGO) Clinical Practice Guideline on the Evaluation and Management of Candidates for Kidney Transplantation. Transplantation. 2020;104:708–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grace BS, Clayton PA, Cass A, et al. Transplantation rates for living- but not deceased-donor kidneys vary with socioeconomic status in Australia. Kidney Int. 2013;83:138–145. [DOI] [PubMed] [Google Scholar]
- 10.Wu DA, Robb ML, Watson CJE, et al. Barriers to living donor kidney transplantation in the United Kingdom: a national observational study. Nephrol Dial Transplant. 2017;32:890–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.King KL, Husain SA, Jin Z, et al. Trends in disparities in preemptive kidney transplantation in the United States. Clin J Am Soc Nephrol. 2019;14:1500–1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Riffaut N, Lobbedez T, Hazzan M, et al. Access to preemptive registration on the waiting list for renal transplantation: a hierarchical modeling approach. Transpl Int. 2015;28:1066–1073. [DOI] [PubMed] [Google Scholar]
- 13.Gill J, Dong J, Rose C, et al. The effect of race and income on living kidney donation in the United States. J Am Soc Nephrol. 2013;24:1872–1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pornet C, Delpierre C, Dejardin O, et al. Construction of an adaptable European transnational ecological deprivation index: the French version. J Epidemiol Community Health. 2012;66:982–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guillaume E, Pornet C, Dejardin O, et al. Development of a cross-cultural deprivation index in five European countries. J Epidemiol Community Health. 2016;70:493–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Châtelet V, Bayat-Makoei S, Vigneau C, et al. Renal transplantation outcome and social deprivation in the French healthcare system: a cohort study using the European Deprivation Index. Transpl Int. 2018;31:1089–1098. [DOI] [PubMed] [Google Scholar]
- 17.Châtelet V, Gatault P, Hazzan M, et al. Transplant center characteristics associated with living-donor kidney transplantation: a cohort study with a hierarchical modeling approach. Transpl Int. 2019;32:865–875. [DOI] [PubMed] [Google Scholar]
- 18.Driollet B, Bayer F, Chatelet V, et al. Social deprivation is associated with poor kidney transplantation outcome in children. Kidney Int. 2019;96:769–776. [DOI] [PubMed] [Google Scholar]
- 19.Bailey PK, Caskey FJ, MacNeill S, et al. Mediators of socioeconomic inequity in living-donor kidney transplantation: results from a UK multicenter case-control study. Transplant Direct. 2020;6:e540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Murphy KA, Jackson JW, Purnell TS, et al. Association of socioeconomic status and comorbidities with racial disparities during kidney transplant evaluation. Clin J Am Soc Nephrol. 2020;15:843–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vansteelandt S. Understanding counterfactual-based mediation analysis approaches and their differences. Epidemiology. 2012;23:889–891. [DOI] [PubMed] [Google Scholar]
- 22.VanderWeele TJ, Vansteelandt S. Mediation analysis with multiple mediators. Epidemiol Methods. 2014;2:95–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tennant PWG, Murray EJ, Arnold KF, et al. Use of directed acyclic graphs (DAGs) to identify confounders in applied health research: review and recommendations. Int J Epidemiol. 2021;50:620–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Suttorp MM, Siegerink B, Jager KJ, et al. Graphical presentation of confounding in directed acyclic graphs. Nephrol Dial Transplant. 2015;30:1418–1423. [DOI] [PubMed] [Google Scholar]
- 25.Châtelet V, Lobbedez T, Harambat J, et al. Précarité et greffe rénale: pourquoi et comment estimer son effet sur la santé des populations? Néphrol Thér. 2018;14:81–84. [DOI] [PubMed] [Google Scholar]
- 26.Beaumier M, Béchade C, Dejardin O, et al. Is self-care dialysis associated with social deprivation in a universal health care system? A cohort study with data from the Renal Epidemiology and Information Network Registry. Nephrol Dial Transplant. 2020;35:861–869. [DOI] [PubMed] [Google Scholar]
- 27.Filc D, Davidovich N, Novack L, et al. Is socioeconomic status associated with utilization of health care services in a single-payer universal health care system? Int J Equity Health. 2014;13:115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vart P, Gansevoort RT, Crews DC, et al. Mediators of the association between low socioeconomic status and chronic kidney disease in the United States. Am J Epidemiol. 2015;181:385–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Safon MO. Soins de Santé Primaires: Les Pratiques Professionnelles en France. Centre de documentation de l’IRDES; 2020. [Google Scholar]
- 30.Pathirana TI, Jackson CA. Socioeconomic status and multimorbidity: a systematic review and meta-analysis. Aust N Z J Public Health. 2018;42:186–194. [DOI] [PubMed] [Google Scholar]
- 31.Vart P, Gansevoort RT, Joosten MM, et al. Socioeconomic disparities in chronic kidney disease: a systematic review and meta-analysis. Am J Prev Med. 2015;48:580–592. [DOI] [PubMed] [Google Scholar]
- 32.Ward MM. Socioeconomic status and the incidence of ESRD. Am J Kidney Dis. 2008;51:563–572. [DOI] [PubMed] [Google Scholar]
- 33.Zeng X, Liu J, Tao S, et al. Associations between socioeconomic status and chronic kidney disease: a meta-analysis. J Epidemiol Community Health. 2018;72:270–279. [DOI] [PubMed] [Google Scholar]
- 34.Bello AK, Peters J, Rigby J, et al. Socioeconomic status and chronic kidney disease at presentation to a renal service in the United Kingdom. Clin J Am Soc Nephrol. 2008;3:1316–1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pladys A, Morival C, Couchoud C, et al. ; REIN registry. Outcome-dependent geographic and individual variations in the access to renal transplantation in incident dialysed patients: a French nationwide cohort study. Transpl Int. 2019;32:369–386. [DOI] [PubMed] [Google Scholar]
- 36.Navaneethan SD, Aloudat S, Singh S. A systematic review of patient and health system characteristics associated with late referral in chronic kidney disease. BMC Nephrol. 2008;9:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cass A, Cunningham J, Snelling P, et al. Late referral to a nephrologist reduces access to renal transplantation. Am J Kidney Dis. 2003;42:1043–1049. [DOI] [PubMed] [Google Scholar]
- 38.Oniscu GC, Schalkwijk AAH, Johnson RJ, et al. Equity of access to renal transplant waiting list and renal transplantation in Scotland: cohort study. BMJ. 2003;327:1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McPherson LJ, Barry V, Yackley J, et al. ; Southeastern Kidney Transplant Coalition. Distance to kidney transplant center and access to early steps in the kidney transplantation process in the Southeastern United States. Clin J Am Soc Nephrol. 2020;15:539–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fraser SD, Roderick PJ, Casey M, et al. Prevalence and associations of limited health literacy in chronic kidney disease: a systematic review. Nephrol Dial Transplant. 2013;28:129–137. [DOI] [PubMed] [Google Scholar]
- 41.Sullivan C, Leon JB, Sayre SS, et al. Impact of navigators on completion of steps in the kidney transplant process: a randomized, controlled trial. Clin J Am Soc Nephrol. 2012;7:1639–1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Garg PP, Frick KD, Diener-West M, et al. Effect of the ownership of dialysis facilities on patients’ survival and referral for transplantation. N Engl J Med. 1999;341:1653–1660. [DOI] [PubMed] [Google Scholar]
- 43.Zhang Y, Gerdtham UG, Rydell H, et al. Socioeconomic inequalities in the kidney transplantation process: a registry-based study in Sweden. Transplant Direct. 2018;4:e346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rodrigue JR, Paek M, Whiting J, et al. Trajectories of perceived benefits in living kidney donors: association with donor characteristics and recipient outcomes. Transplantation. 2014;97:762–768. [DOI] [PubMed] [Google Scholar]
- 45.Taylor DM, Bradley JA, Bradley C, et al. ; ATTOM investigators. Limited health literacy is associated with reduced access to kidney transplantation. Kidney Int. 2019;95:1244–1252. [DOI] [PubMed] [Google Scholar]
- 46.Navaneethan SD, Singh S. A systematic review of barriers in access to renal transplantation among African Americans in the United States. Clin Transplant. 2006;20:769–775. [DOI] [PubMed] [Google Scholar]
- 47.Dageforde LA, Petersen AW, Feurer ID, et al. Health literacy of living kidney donors and kidney transplant recipients. Transplantation. 2014;98:88–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Patzer RE, McPherson L, Redmond N, et al. A culturally sensitive web-based intervention to improve living donor kidney transplant among African Americans. Kidney Int Rep. 2019;4:1285–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hanson CS, Chadban SJ, Chapman JR, et al. Nephrologists’ perspectives on recipient eligibility and access to living kidney donor transplantation. Transplantation. 2016;100:943–953. [DOI] [PubMed] [Google Scholar]
- 50.Wang H, Peng G, Bai J, et al. Cytomegalovirus infection and relative risk of cardiovascular disease (ischemic heart disease, stroke, and cardiovascular death): a meta-analysis of prospective studies up to 2016. J Am Heart Assoc. 2017;6:e005025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Parmar P, Corsi DJ, Cooper C. Distribution of hepatitis C risk factors and HCV treatment outcomes among central canadian aboriginal. Can J Gastroenterol Hepatol. 2016;2016:8987976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Voigt-Barbarowicz M, Brütt AL. The agreement between patients’ and healthcare professionals’ assessment of patients’ health literacy-a systematic review. Int J Environ Res Public Health. 2020;17:2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Patzer RE, Basu M, Larsen CP, et al. iChoose kidney: a clinical decision aid for kidney transplantation versus dialysis treatment. Transplantation. 2016;100:630–639. [DOI] [PMC free article] [PubMed] [Google Scholar]