Abstract
Background:
World Health Organization recommends that influenza vaccines should benefit as much of the population as possible, especially where resources are limited. Corona virus disease 2019 (COVID-19) has become one of the greatest threats to health systems worldwide. The present study aimed to extend the evidence of the association between influenza vaccination and COVID-19 to promote the former.
Methods:
In this systematic review, four electronic databases, including the Cochrane Library, PubMed, Embase, and Web of Science, were searched for related studies published up to May 2022. All odds ratios (ORs) with 95% confidence intervals (CIs) were pooled by meta-analysis.
Results:
A total of 36 studies, encompassing 55,996,841 subjects, were included in this study. The meta-analysis for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection provided an OR of 0.80 (95% CI: 0.73–0.87). The statistically significant estimates for clinical outcomes were 0.83 (95% CI: 0.72–0.96) for intensive care unit admission, 0.69 (95% CI: 0.57–0.84) for ventilator support, and 0.69 (95% CI: 0.52–0.93) for fatal infection, while no effect seen in hospitalization with an OR of 0.87 (95% CI: 0.68–1.10).
Conclusion:
Influenza vaccination helps limit SARS-CoV-2 infection and severe outcomes, but further studies are needed.
Registration:
PROSPERO, CRD42022333747.
Keywords: Influenza vaccine, COVID-19, Infection, Outcomes, Prevention
Introduction
Corona virus disease 2019 (COVID-19) is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pathogen, now in its third year of pandemic. Up to May 2022, the number of cases has exceeded 500 million people and countless deaths.[1] Its heavy burden has struck the health system unexpectedly without a chance to recuperate. If non-pharmaceutical interventions (NPIs) are relaxed, many seasonal and endemic respiratory diseases with the same route of transmission will return, in addition to SARS-CoV-2. Co-infection of influenza virus with SARS-CoV-2 worsens the illness and makes it more complex to treat.[2] Compared with SARS-CoV-2 alone, co-infection with influenza virus has a 4-fold increase in the use of invasive mechanical ventilation and led to 2.35 odds of death.[3] Therefore, an excessive rate of co-infections would be disastrous.
Influenza viruses and SARS-CoV-2 are mechanistically similar in that they use surface glycoproteins to bind cell membrane receptors of the respiratory system. Since the outbreak, both NPIs and SARS-CoV-2 have disrupted influenza circulation individually and in combination.[4,5] Some anti-influenza drugs are used clinically to treat COVID-19 patients.[6,7] These findings suggest a common intervention may work. Intuitively, some questions about the influenza vaccine as a potential COVID-19 intervention have arisen. Higher influenza vaccine coverage is related to a lower cumulative incidence, morbidity, or fatality, as observed at the population level.[8,9] The disadvantage of ecological studies lying in ecological fallacies, is often due to insufficiency of individual exposure, outcome, and confounding information. Influenza vaccination behavior is perceived differently between cultures.[7] For instance, Asians see it as a beneficial preventive method like wearing masks, while Westerners abstain due to misunderstanding.[10,11] However, performing clinical trials to determine the role of influenza vaccines in preventing SARS-CoV-2 infection and clinical outcomes is unreasonable.
Though contrasting results have been presented on the grounds of different study designs, participants, settings, or limited sample size, previous meta-analyses draw a unanimous beneficial conclusion on the association between influenza vaccination and the incidence of COVID-19.[12–14] Since additional studies have been reported, it is essential to update pooled results before the influenza rebound. Therefore, we added new findings at the individual level to determine if this benefit remains. Routine immunization, including seasonal influenza vaccination, backslid because of the COVID-19 pandemic in many countries and regions. However, the 2019/2020 influenza vaccination record that this review studied was less affected. Because measures leading to backslide were taken after WHO characterized COVID-19 as a pandemic in March, it was the time when the 2019/2020 influenza vaccination campaign closed to the end in most countries. Since the influenza vaccine is effective after 2 weeks, this was selected as the minimum start point for outcomes, and those until 12 months were used.[15,16] Using this vaccination time frame prevents any potential effect of the COVID-19 vaccines in late 2020. Thus, this systematic review and meta-analysis aim to determine if the 2019/2020 influenza vaccination help to reduce initial SARS-CoV-2 infection and clinical outcomes in the following 12 months. The pooled results were analyzed by special populations and levels of evidence. The findings from this study provide immunological insights for future COVID-19 vaccines co-administered with the influenza vaccine.
Methods
Search strategy
This systematic review was performed in Embase, PubMed, Web of Science, and Cochrane Library, according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.[17] The search strategy used a combination of MeSH, Emtree, and text words, with the following topics: (influenza vaccin∗ OR flu vaccin∗) AND (‘COVID-19’ OR ‘SARS-CoV-2’) and related terms. The search strategy is elaborated in Appendix 1 in [Supplementary Materials]. We also manually searched references that the selected articles cited with the same inclusion criteria to ensure that relevant studies were not missed. The study protocol was registered in the International Prospective Register for Systematic Reviews (https://www.crd.york.ac.uk/prospero/, registration number: CRD42022333747).
Inclusion and exclusion criteria
Two authors independently screened the search results in two rounds based on both title and abstract, and then full text, while any discrepancies were resolved by the senior reviewer. Published articles that were included met the following criteria:
(1) The exposure factor was the administration of the influenza vaccine. (2) The articles compared the effects of influenza vaccination history on individuals with COVID-19 outcomes of interest, including infection, hospitalization, intensive care unit (ICU) admission, ventilator use, or death. (3) Influenza vaccination occurred during the 2019 to 2020 seasonal campaign and was within one year of COVID-19 outcomes. (4) Participants were limited to those who had never had a SARS-CoV-2 infection, articles studied on reinfection were beyond this study scope. (5) Studies reported the odds ratios (ORs) or risk ratios (RRs) and 95% confidence intervals (CIs). (6) Studies were published between January 2020 and April 2022. No sample size, status, or language restrictions were applied. (7) Reviews, case reports, case series, editorials, conference papers, and animal experiments were excluded, and articles using ecological study designs were also excluded.
When results from a study were reported more than once, the results were compared for consistency, and only the latest results were included.
Study selection and data extraction
The primary outcome was the association between influenza vaccination and SARS-CoV-2 infection. The secondary outcomes were the association between influenza vaccination and SARS-CoV-2 infection severity. The extracted data included:[1] (1) published information (first author, year of publication, and study design);[2] (2) the characteristics of the study (study settings, geographic origin, and sample size);[3] (3) exposure information (the time of influenza vaccination, influenza vaccination coverage, and ascertainment of influenza vaccination);[4] (4) SARS-CoV-2 infection and COVID-19 outcomes (identification of COVID-19, time period of COVID-19 outcomes, and crude or adjusted estimates with 95% CIs of COVID-19 outcomes and its adjusted variables). If a study reported both crude and adjusted estimates for a same variable, adjusted ones were adopted in the pooled analysis.
Statistical analysis and quality assessment
The statistical analyses of all data were performed using Microsoft Excel (version 2019, Redmond, WA, USA), RStudio (version 1.4.1, RStudio Inc., Boston, MA, USA), and R (version 4.1.3, R Foundation for Statistical Computing, Vienna, Austria). The extracted data were used to calculate pooled ORs with 95% CI to demonstrate the association between influenza vaccination and the risk of COVID-19 incidence, hospitalization, ICU admission, and death. RR was applied if the incidence of the outcome in the unvaccinated group was <10% and the RR is between 0.5 and 2.5 in those studies without OR estimates.[18] Subgroup analyses were performed on studies grouped by study designs, study population, and type of influenza vaccines to determine possible sources of heterogeneity. Statistical significance was established when the P value was <0.05. Heterogeneity was assessed using the Q test (significant heterogeneity is indicated by P < 0.05) and the I2 test (significant heterogeneity is indicated by I2 > 50%). A random-effects model was used when significant heterogeneity (P < 0.05, I2 ≥ 50%) was observed. Otherwise, a fixed-effect model was applied. Forest plots were used to present the data. Funnel plot and Egger's test, which calculates the funnel plot asymmetry by a linear regression of the intervention effect estimates on their standard errors, was used to assess publication bias. Egger's test could not only compensate for the small-study effect of funnel plots, but also test an assumption that the potentially unpublished studies have negative or positive treatment effects by P value. Sensitivity analyses were performed by omitting each study one by one to determine any included studies with notable impact and examine the robustness of the overall effect. The α value was set at 0.05.
The Newcastle–Ottawa Scale (NOS) was used to assess the quality and bias of cohort and case-control studies, the bias was rated as follows: high level for 0 to 3, moderate level for 4 to 6, low level for 7 to 9.[19] For cross-sectional study designs, the Agency for Healthcare Research and Quality (AHRQ) was used where a score above seven was regarded as low risk of bias, 4 to 7 and 0 to 3 were regarded as moderate risk or high risk level, respectively.[20] Any score disagreement was resolved by consensus, and a final agreed-upon rating was assigned to each study.
Results
Literature selection and study characteristics
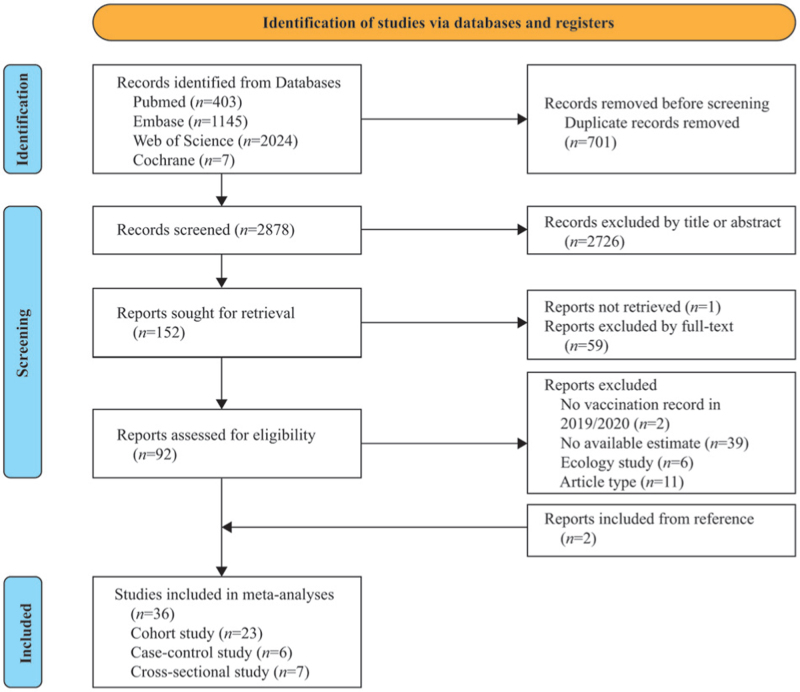
A total of 3579 records were retrieved after systematically searching the four databases. After duplicates were removed, 2878 record titles and abstracts were screened. Of the 92 full-text articles identified, 58 were excluded because they did not meet the inclusion criteria, and two others were obtained from the references. The remaining 36 articles reported on 55,996,841 subjects outcomes were included in this analysis.[21–56] None of the participants had received COVID-19 vaccination during the study period. A flowchart of the study selection process [Figure 1] was prepared according to the PRISMA guidelines. The characteristics and outcomes of the included studies are listed in [Table 1 and Appendix 2 in Supplementary Materials]. Most included studies were cohort designs (n = 23), including five prospective and 18 retrospective. Among the rest, six were case-control studies, and seven were cross-sectional designed.
Figure 1.
PRISMA flow diagram of the study search and selection process.
Table 1.
Main characteristics of 36 included studies in the meta-analysis.
| Studies | Country | Sample size | Study design | Follow-up or investigated timespan | COVID-19 diagnostic approach | Vaccination identification | IVC (%) | Participants | Risk of bias |
| Yang et al[21] | US | 2005 | Retrospective cohort | 2020.3–2020.8 | Laboratory confirmed | Records | 10.7 | COVID-19 patients (>18 years) | Low |
| Wilcox et al[22] | UK | 6921 | Retrospective cohort | 2020.1.1–2020.7.31 | PCR | Records | 37.8 | Confirmed and suspected cases | Low |
| Umasabor-Bubu et al[23] | US | 588 | Retrospective cohort | 2020.3.12–2020.6.30 | PCR | Self-reported | 35.0 | COVID-19 hospitalized patients | Low |
| Taghioff et al[24] | US, UK, Italy, Germany, Israel, and Singapore | 37,377 | Retrospective cohort | Before 2021.1 | Laboratory confirmed | Records | 1.3 | SARS-CoV-2 positive patients | Low |
| Sánchez-García et al[55] | Mexico | 16,879 | Retrospective cohort | 2020.3.17–2020.8.16 | PCR | Records | 17.2 | COVID-19 positive patients (>18 years) | Low |
| Pedote et al[54] | Italy | 3872 | Retrospective cohort | 2020.2–2020.5 | PCR | Records | 28.7 | COVID-19 patients | Low |
| Pastorino et al[53] | Italy | 741 | Prospective cohort | 2020.3.1–2020.6.1 | PCR | Records | 32.4 | COVID-19 patients visited emergency room | Low |
| Paganoti et al[52] | Brazil | 1664 | Retrospective cohort | 2020.2.16–2021.5.1 | PCR or antigen | Records | 24.7 | Pregnant and postpartum women of a childbearing age (10–55 years old) with severe SARS-CoV-2 | Moderate |
| Massoudi and Mohit[51] | Iran | 261 | Case-control | 2020.3.10–2020.4.10 | PCR or pulmonologist-confirmed | Records | 34.5 | HCWs | Low |
| Kristensen et al[50] | Denmark | 35,168 | Prospective cohort | 2020.4.15–2020.5.7, 2020.6.2–2020.6.10, 2020.9.30–2020.10.7 | Serological test | Self-reported | 29.7 | HCWs | Low |
| Kowalska et al[49] | Poland | 5479 | Cross-sectional | 2020.8–2020.10 | Serological test | Self-reported | 16.5 | Residents | Low |
| Kline et al[48] | US | 149 | Retrospective cohort | 2020.3.20–2020.5.10 | Not mention | Records | 65.8 | COVID-19 positive patients | Low |
| Kissling et al[47] | European | 1701 | Test-negative case-control | 2020.3.1–2020.8.31 | PCR | Records or self-reported | 25.3 | Patients with acute respiration infection | Moderate |
| Huang et al[45] | US | 55,667,977 | Cross-sectional | 2020.1.15–2020.6.30 | Not mention | Records | 23.4 | Older adults (≥65 years) | Low |
| Green et al[44] | Israel | 22,563 | Cross-sectional | 2020.2.1–2020.4.30 | PCR | Records | 10.2 | Health maintenance organization members | Low |
| Greco et al[42] | Italy | 952 | Retrospective cohort | 2020.3.15–2020.6.13 | PCR | Records | 40.0 | COVID-19 patients (≥18 years) | Low |
| Fink et al[43] | Brazil | 39,745 | Retrospective cohort | 2020.1.1–2020.6.23 | Clinical COVID-19 diagnosis | Self-reported | 31.2 | Hospitalized COVID-19 cases | Low |
| El-Qutob et al[37] | Spain | 255 | Retrospective cohort | 2020.2.26–2020.5.20 | PCR | Records | 42.0 | Hospitalized COVID-19 cases (≥18 years) | Low |
| Diallo et al[41] | French | 819 | Retrospective cohort | 2020.3.10–2020.3.31 | PCR and/or clinically/radiologically diagnosis | Self-reported | 45.8 | People with diabetes hospitalized for COVID-19 | Moderate |
| Conlon et al[40] | US | 27,201 | Retrospective cohort | 2020.2.27–2020.7.15 | Laboratory confirmed | Records | 47.8 | Hospitalized patients | Low |
| Arce-Salinas et al[39] | Mexico | 560 | Age-matched case-control | From 2020.4 | PCR | Records | 33.4 | Hospitalized COVID-19 cases (≥18 years) | Low |
| Xiang et al[38] | UK | 30,835 | Prospective cohort | 2020.1.31–11.3 | Laboratory confirmed | Records | NA | Record in UK Biobank (40–69 years) | Low |
| Candelli et al[73] | Rome | 602 | Retrospective cohort | 2020.3.1–2020.6.30 | PCR | Not mentioned | 24.9 | COVID-19 patients admission in emergency department | Moderate |
| Scozzari et al[35] | Italy | 8761 | Prospective cohort | 2020.5.4–2020.11.20 | Serological test | Self-reported | 18.0 | Workers at the University Hospital and University | Low |
| Pawlowski et al[74] | US | 25,582 | Retrospective cohort | 2020.2.15–2020.7.14 | PCR | Records | NA | Suspected SARS-CoV-2 infected cases | Low |
| Ilic et al[33] | Serbia | 107 | Retrospective cohort | 2020.3.20–2020.4.22 | PCR | Self-reported | 70.1 | COVID-19 confirmed HCWs | Moderate |
| Fernández-Prada et al[32] | Spain | 188 | Test-negative case-control | 2020.2.28–2020.5.8 | PCR | Not mentioned | 43.1 | Patients in a regional hospital | Low |
| Debisarun et al[75] | Netherlands | 6856 | Cross-sectional | 2020.3–2020.6 | PCR | Records | 53.3 | HCWs | Low |
| Noale et al[30] | Italy | 6680 | Cross-sectional | 2020.4–2020.6 | Not mention | Self-reported | 21.0 | Adults (≥18 years) | Low |
| Ragni et al[29] | Italy | 17,608 | Test-negative retrospective cohort | 2020.2.15–2020.5.22 | PCR | Records | 30.8 | Residents underwent nasal and oropharyngeal swab test | Low |
| Martínez-Baz et al[28] | Spain | 8935 | Prospective cohort | 2020.3.1–2020.5.31 | PCR or serological test | Records | 34.1 | Tested HCWs | Low |
| Olivar-López et al[26] | Mexico | 510 | Cross-sectional | 2020.3–2020.6 | PCR | Not mentioned | 23.3 | Patients under 18 years of age who came for a consultation with a clinical picture compatible with SARS-CoV-2 and who underwent RT-PCR testing | Low |
| King et al[46] | US | 1736 | Test-negative case control | 2020.6.1–2020.9.30 | PCR | Records | 65.1 | Patients with an acute illness | Low |
| Ortiz-Prado et al[25] | Ecuador | 9468 | Retrospective cohort | 2020.2.27–2020.4.18 | PCR | Self-reported | 0.9 | COVID-19 positive patients | Moderate |
| Belingheri et al[27] | Italy | 3520 | Cross-sectional | Before 2020.5 | PCR | Records | 23.2 | HCWs and medical residents | Low |
| Tayar[56] | Qatar | 2576 | Test-negative case-control | 2020.9.17–2020.12.31 | PCR | Records | 28.0 | HCWs | Low |
HCWs: Healthcare workers; IVC: Influenza vaccine coverage; NA: Not available; PCR: Polymerase chain reaction; SARS-CoV-2: Severe acute respiratory syndrome coronavirus 2.
This study extracted crude and adjusted ORs or RRs from the original data for synthesis. Seventeen of the 20 studies on the association between influenza vaccination and SARS-CoV-2 infection provided adjusted estimates. For COVID-19 clinical outcomes, the association with hospitalization, intensive care, ventilator, and death were examined by 9, 10, 8, or 15 articles, respectively. Only one study discussed the death without adjusted OR. Although the adjusted confounding factors were not consistent, studies generally controlled sex, age, and comorbidities (adjusted factors shown in Appendix 2 in Supplementary Materials). Considering the risk of bias, 79% (23/29) were graded with low risk based on the NOS grading system, and the cross-sectional studies (n = 7) were evaluated as having a low risk of bias on the AHRQ scale. The quality evaluation is presented in Table 1 and Appendix 3 in Supplementary Materials.
Pooled results of the association between influenza vaccine and susceptibility of SARS-CoV-2
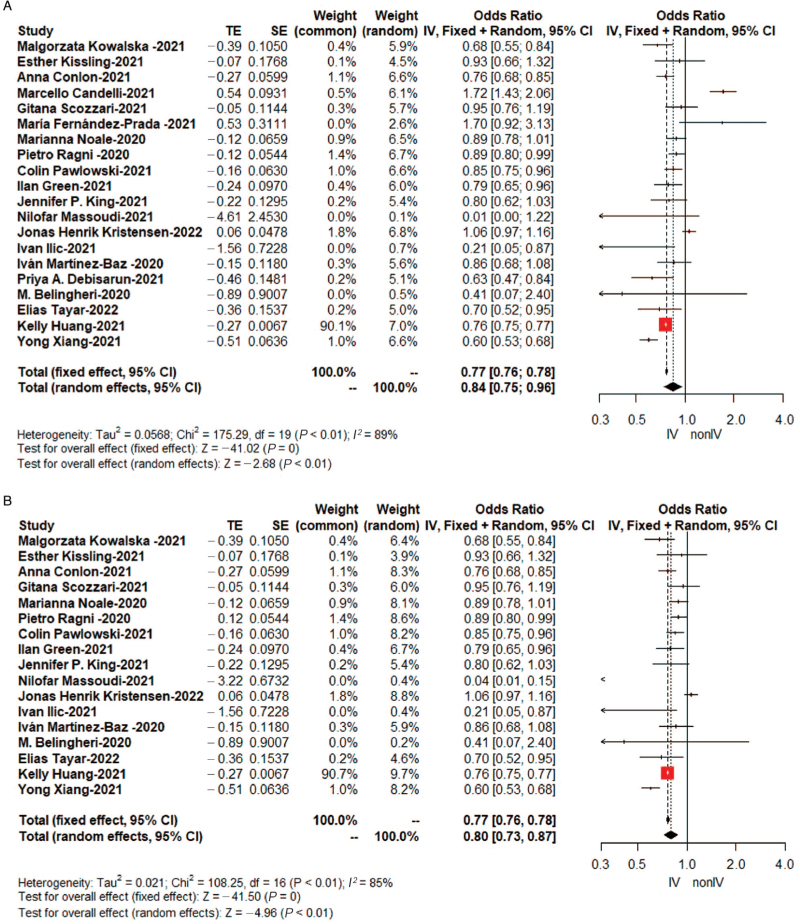
As shown in Table 2, the pooled OR of SARS-CoV-2 infection, synthesized by a random-effects model due to the high heterogeneity (I2 = 89%), was 0.84 (95%CI: 0.75–0.96, Figure 2A) in a total of 55,867,805 individuals. Omitting crude ORs, the pooled adjusted OR was 0.80 (95%CI: 0.73–0.87, Figure 2B) in consideration of potential confounding, which was encompassed by ten statistically significant original studies and seven studies without significant results.
Table 2.
Associated risk and publication bias between influenza vaccination and the risk of SARS-CoV-2 infection.
| Egger's test | |||||||
| Studies | Number of studies | Cumulative sample size | OR (95% CI) | I2 (%) | Effect model | t | P value |
| Infection | 20 | 55,867,805 | 0.84 (0.75–0.96)∗ | 89 | Random | 0.99 | 0.34 |
| Adjusted estimate | 17 | 55,860,337 | 0.80 (0.73–0.87)∗ | 85 | Random | 0.41 | 0.68 |
| Population | |||||||
| General | 9 | 55,780,161 | 0.79 (0.71–0.87)∗ | 75 | Random | −0.10 | 0.92 |
| HCWs | 8 | 59,570 | 0.74 (0.59–0.93)∗ | 85 | Random | −2.66 | 0.04 |
| Elders | 6 | 55,718,816 | 0.76 (0.75–0.77)∗ | 0 | Fix | 0.94 | 0.40 |
| Study design | |||||||
| Cohort study | 8 | 147,844 | 0.83 (0.72–0.95)∗ | 88 | Random | −0.81 | 0.45 |
| Case-control study | 3 | 6013 | 0.80 (0.67–0.94)∗ | 0 | Fix | 0.44 | 0.74 |
| Cross-sectional study | 6 | 55,713,075 | 0.76 (0.75–0.77)∗ | 45 | Fix | −0.13 | 0.91 |
| Type of influenza vaccine | |||||||
| Quadrivalent | 8 | 56,433 | 0.74 (0.67–0.81)∗ | 71 | Random | −3.39 | 0.02 |
| Trivalent | 2 | 4429 | 1.00 (0.77–1.29) | 71 | Random | NA | |
| Inactivated | 5 | 35,101 | 0.77 (0.66–0.89)∗ | 59 | Random | −2.42 | 0.09 |
| Diagnostic approach | |||||||
| Polymerase chain reaction test | 13 | 85,704 | 0.87 (0.72–1.06) | 84 | Random | −0.88 | 0.40 |
| Serological test | 5 | 55,510 | 0.90 (0.75–1.08) | 74 | Random | −1.05 | 0.37 |
| Others/not mention | 5† | 55,732,954 | 0.48 (0.19–1.21) | 90 | Random | −0.96 | 0.41 |
P < 0.05.
Including two original studies did not mention this information, one for pulmonologist-confirmed and two for laboratory confirmed but not specified it. CI: Confidence interval; COVID-19: Corona virus disease 2019; NA: Not available due to limited number of studies; OR: Odds ratio; SARS-CoV-2: Severe acute respiratory syndrome coronavirus 2; HCWs: Healthcare workers.
Figure 2.
Forest plots for the association between influenza vaccination and SARS-CoV-2 infection: (A) OR by random-effects model (B) Only adjusted OR by random-effects model. IV: Vaccinated against influenza group; nonIV: Unvaccinated against influenza group; SARS-CoV-2: Severe Acute Respiratory Syndrome Coronavirus 2; SE: Standard error of treatment estimate; TE: Treatment effect; OR: Odds ratio.
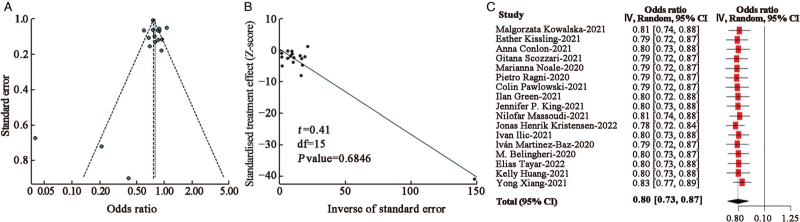
No significant publication bias was noted with Egger's test (t = 0.82, Egger's test P value = 0.43, Figure 3A and 3B). For the sake of hierarchy of evidence, pooled results presented a negative association between influenza vaccine and incidence of SARS-CoV-2 positivity of 0.83 (95% CI: 0.72–0.95) in cohort studies (n = 8), 0.80 (95% CI: 0.67–0.94) in case-control studies (n = 3), and 0.76 (95% CI: 0.75–0.77) in cross-sectional studies (n = 6) [Table 2].
Figure 3.
Publication bias and sensitivity analysis of the estimated result of COVID-19 infection including adjust estimates. (A) Funnel plot. (B) Linear regression test of funnel plot asymmetry: Egger's bias plot of random-effects model. (C) Adjusted OR of sensitivity analysis applied random-effect model by omitting studies one by one. COVID-19: Corona virus disease 2019; OR: Odds ratio.
Pooled results of the association between influenza vaccine and COVID-19 clinical outcomes
Table 3 shows that COVID-19 patients who have received the influenza vaccine in the past year were less likely to develop severe disease. Specifically, compared with unvaccinated patients, the vaccinated individuals had reduced rates of ICU admission (OR = 0.83, 95% CI: 0.72–0.96, I2 = 61%), ventilator support (OR = 0.69, 95% CI: 0.57–0.84, I2 = 69%), and death (OR = 0.69, 95% CI: 0.52–0.93, I2 = 87%). However, a preventive role in hospitalization was not observed (OR = 0.87, 95% CI: 0.68–1.10, I2 = 79%). Similar qualitative results were produced on the basis of cohort studies by the random-effects model.
Table 3.
Associated between influenza vaccination and the risk of COVID-19 clinical outcomes by random-effects model.
| Hospitalization | ICU admission | Ventilator support | Death | |||||||||||||
| Studies | n | Sample size | OR (95% CI) | I2 (%) | n | Sample size | OR (95% CI) | I2 (%) | n | Sample size | OR (95% CI) | I2 (%) | n | Sample size | OR (95% CI) | I2 (%) |
| All | 9 | 57,424 | 0.87 (0.68–1.10) | 79 | 10 | 83,838 | 0.83 (0.72–0.96)∗ | 61 | 8 | 55,672,128 | 0.69 (0.57–0.84)∗ | 69 | 15 | 139,342† | 0.69 (0.52–0.93)∗ | 87 |
| Population | ||||||||||||||||
| General | 8 | 54,045 | 0.86 (0.66–1.12) | 82 | 9 | 82,299 | 0.91 (0.87–0.96)∗ | 39‡ | 5 | 2585 | 0.82 (0.76–0.88)∗ | 43‡ | 14 | 137,678† | 0.74 (0.55–1.00) | 86 |
| Elders | 3 | 1365† | 1.00 (0.82–1.22) | 0‡ | 4 | 1365† | 1.06 (0.71–1.56) | 37‡ | 2 | 1093 | 0.96 (0.42–2.17) | 64 | 3 | 406† | 1.35 (0.91–1.99) | 0‡ |
| Pregnant women | 0 | NA | NA | NA | 0 | NA | NA | NA | 0 | NA | NA | NA | 2 | 1724 | 0.35 (0.25–0.48)∗ | 0‡ |
| Study design | ||||||||||||||||
| Cohort study | 9 | 57,424 | 0.87 (0.68–1.10) | 79 | 10 | 83,838 | 0.83 (0.72–0.96)∗ | 61 | 6 | 3538 | 0.72 (0.54–0.96)∗ | 66 | 14 | 138,782 | 0.70 (0.51–0.97)∗ | 88 |
| Cross-sectional study | 0 | NA | 0 | NA | 2 | 55,667,988 | 0.72 (0.68–0.76)∗ | 49‡ | 0 | NA | ||||||
P < 0.05.
The actual sample size was larger, since the sample size in some of the original studies was not available for the subgroup analysis.
The pooled OR was calculated by a fixed-effects model. CI: Confidence interval; COVID-19: Corona virus disease 2019; ICU: Intensive care unit; n: Number of studies; NA: Not available; OR: Odds ratio.
Subgroup analyses
In Tables 2 and 3, subgroups were divided by healthcare workers (HCWs), pregnant women, and those over 65 years of age within the available data since all of these groups indicate getting a yearly influenza vaccine. Eight studies reported the SARS-CoV-2 infection odds between those with or without influenza vaccination for a total of 59,570 HCWs (OR = 0.74, 95% CI: 0.59–0.93, I2 = 85%). For infections in older populations, six reports gave an OR as the primary outcome or subgroup outcome. Both subgroups received a negative association with statistical significance, with little heterogeneity in the older populations (OR = 0.76, 95%CI: 0.75–0.77, I2 = 0%, n = 6). Few articles reported severe outcomes in HCWs, possibly for timely treatment advantages. The pooled ORs were not significant in the association among older populations in the COVID-19 clinical outcomes. Despite the small number of studies, influenza vaccination was associated with a reduced risk of mortality in 1724 COVID-19 pregnant patients, and the pooled OR was 0.35 (95% CI: 0.25–0.48, I2 = 0%, n = 2). The type of influenza vaccines could not be overlooked. Quadrivalent vaccines (OR = 0.74, 95% CI: 0.67–0.81, I2 = 71%, n = 8) and inactivated vaccines were well-behaved when preventing from SARS-CoV-2 infection (OR = 0.77, 95% CI: 0.66–0.89, I2 = 59%, n = 5). The diagnostic approach was another important factor in cases confirmed. A negative relationship was not observed in the PCR test group (OR = 0.87, 95% CI: 0.72–1.06) or the serological test group (OR = 0.90, 95% CI: 0.75–1.08).
Publication bias and sensitivity analyses
We determined the potential publication bias in the statistically significant results with Egger's test and funnel plot analysis. No significant publication bias was found in the results of an Egger's test except for the subgroup analyses of HCWs infection; details are shown in [Table 2 and Figure 3A]. To examine the strength of the pooled results, we performed a sensitivity analysis by omitting one study at a time. The pooled results [Figure 3B] were not significantly affected by any of the individual studies, suggesting the high stability and reliability of our results.
Discussion
This systematic review and meta-analysis summarized the ORs of SARS-CoV-2 incidence and related clinical outcomes between individuals with or without flu vaccination. A total of 36 articles (14 only on infection, 16 on clinical outcomes, and 6 on both) met our PICO (Patient, Intervention, Comparison, and Outcome) question. The pooled results showed that being vaccinated for influenza in the 2019 to 2020 season reduced the risk of COVID-19 infection by 20% (OR = 0.80, 95% CI: 0.74–0.87) within 1 year. Moreover, influenza vaccination was negatively associated with worse outcomes, mainly based on the retrospective cohort studies. For COVID-19 patients, influenza vaccination reduced ICU admission by 17%, 31% in ventilator support, and 31% in death the following year, but no statistical significant association was found with the risk of hospitalization. The primary finding was verified in several ecological perspectives, where the significance level of severity outcomes was inconsist with meta-analysis studies.[57–59] The reason why clinical outcomes were inconsistent among studies is speculated to be limited by the number of published studies and heterogeneous the original research settings. Therefore, our work confirms and extends these results.
Compared with the pooled OR of infection (OR = 0.84), statistically significant pooled ORs of severe outcomes (OR of 0.83, 0.69, 0.69 for ICU admission, ventilator support, and death, respectively) indicated good association in preventing complex treatments and fatal infection rather than infection. This brought up the distinction between specific and non-specific immune efficacy. Although influenza vaccines provide specific protection against influenza viruses, the non-specific immune phenomenon may help against SARS-CoV-2 which is similar to the non-specific effects described by the reduction in all-cause mortality attributed to measles and Bacille Calmette-Guerin (BCG) vaccines administration.[60] Besides, this result showed a marginally greater protection effect in clinical outcomes compared to preventing infection. Since live attenuated influenza vaccination before SARS-CoV-2 infection reduced SARS-CoV-2 replication and shedding in the upper respiratory tract in the ferret model, it was speculated that the non-specific effects of influenza vaccination played a role in the progression against SARS-CoV-2 viruses.[61] The infectivity, pathogenicity, and virulence of SARS-CoV-2 is another possible interpretation. The various characteristics of different strains led to the change of the infection spectrum. Strong infectivity and less virulence would magnify this protection of infection outcomes. Uncorrelated results could be affected by the constrained capacity of detection, which was concentrated on patients visiting hospitals in the early days of the pandemic. Infected patients with comorbidities were more prone to hospitalization. Studies performed were dependent on electronic hospital records for retrospective studies and a history of complications may have led to more hospital visits.
The results of our study reduce the chance that the negative associations were found by chance or coincidence. The results merit broad attention from epidemiologists to immunologists. From the perspective of an individual, the protection afforded by the vaccine should not be ignored, especially for disease transmission. Those who are vaccinated often pay more attention to their health for multiple reasons, and they take extra care to get vaccinated and are more likely to follow the NPIs to prevent infection. High influenza vaccination coverage normally affects vulnerable populations because of their immunodeficiency, for example, cardiovascular patients and health workers who are at an increased risk of exposure to the influenza virus.[62]
Influenza vaccine protection against SARS-CoV-2 infection has been hypothesized to affect innate or adaptive immunity, but it is still unknown. Debisarun et al[31] clarified the epidemiological research with immunological evidence. Quadrivalent inactivated influenza vaccination modulates the responses to SARS-CoV-2 antigen invasion. Additionally, previous influenza history or active infection status may increase susceptibility to SARS-CoV-2.[63] Vaccination records show that the 2019/2020 season, as well as previous years immunization affected preventing SARS-CoV-2 infection.[38] It is recommended that health departments facilitate routine influenza vaccination by taking advantage of established forms of COVID-19 vaccination.
The hypothesizes of the heterologous effects of vaccines, which are not the first to help train the immune system to improve the responsiveness of cells to heterologous viral stimuli. Cross-reactivity has been observed between influenza virus hemagglutinin and SARS-CoV-2 anti-spike neutralizing antibody titers, even though its effect remains controversial.[64] Mei-Mei et al[65] noted the similarity between the influenza virus and the SARS-CoV-2 infection process by reviewing the structure, cell membrane binding, and the action processes after cell invasion. This theoretical evidence supports that shared infection or immunologic mechanisms offered by influenza vaccines may limit COVID-19 unintentionally. Unfortunately, the analogous infection mechanism is an open door to viruses. It is the time gap that troubles the identification of co-infection, which was caused by the limited coincidence time of incubation and viral shedding of influenza virus and SARS-CoV-2 virus.[2] Since most symptoms caused by influenza cannot be distinguished from COVID-19, being unaware of co-infection may result in missed medical treatments, where severe outcomes could be averted. In this way, influenza vaccination reduced the adverse events of COVID-19 confirmed patients who were coinfected with influenza. Slight protection degree of pooled results was observed under the circumstance of flattened flu seasons attributed to the impact of NPIs instituted for COVID-19. As more western countries have initiated the removal of NPIs, influenza vaccination could limit virus activity rebound.[66] Therefore, controlling influenza, COVID-19, and co-infection could be eased with influenza vaccination.
Subgroup analysis was performed to speed the uptake of influenza vaccines currently. The reverse relationship between influenza vaccination and confirmed COVID-19 in elders was obtained with little heterogeneity (OR = 0.76, n = 6, I2 = 0) but not observed in clinical outcomes of hospitalization, ICU admission, and death. Ineffectiveness of preventing severe COVID-19 was contributed to modest vaccine effectiveness, which was generally seen with influenza in people older than 65 years.[67,68] Although older populations fail to have an adequate protective response to traditional vaccines due to senescent immune function,[69] annual influenza vaccination is still a cost-effective way to provide protection for them against severe influenza-associated disease and death. Also, by changing the concentration and composition of the adjuvant in the vaccine, it is preferable to prescribe high-dose inactivated influenza vaccines for the elderly to achieve antibody titers comparable to those in the young.[70] Inspired by this, pharmaceutical enterprises may develop exclusive COVID-19 vaccines for older populations in the future. The efficacy of COVID-19 vaccines with the aid of the geriatric influenza vaccine needs to be clarified.
Subgroup analysis pooled the estimates based on study design and diagnostic approach, which has been ignored in previous studies.[12–14] It is crucial for top priority to be given to the quality and comparability of the data. The results did not change in either subgroup. Lower evidence hierarchy amplified the protective effect of the influenza vaccine, but the degree was not determined. Real-world studies are needed to clarify influenza vaccine effectiveness toward SARS-CoV-2 variants. Also, qualitative results highlight the influenza vaccination in the context of COVID-19 and help to dispel vaccine hesitancy. Given sufficient thought to the greater hazard of either influenza or COVID-19 infection, WHO indicated influenza vaccines and more recently inactivated ones should be administered with global COVID-19 vaccines.[71,72]
There are several advantages to this study. First, the use of meta-analysis dilutes the peculiar characteristic of a single population and increases the statistical power of observational studies. The final results pooled the estimates after controlling for important confounding factors. Second, strict inclusion criteria of timespan improve the power of our results because cross-protection from the two forms of immunization may have introduced confounders and disturbed the results to some extent. Also, this is the first study focused on the protective effect in several high-risk groups. Apart from population-based vaccination, tendentious interventions could limit COVID-19 spread. It is pragmatic for vaccination practice to occur when health resources are unbalanced and the disease burden is high.
We acknowledge several limitations in this meta-analysis. First, the highest hierarchy of evidence stemmed from cohort studies, a majority of which were conducted retrospectively. Therefore, the advantage of influenza vaccines is merely considered an assumption, and the conclusions drawn need more data. Second, part of the subgroup analyses on the outcomes was restricted by the number of original studies or sample sizes, such as the estimate of ventilator support in older populations, the estimated risk of death in pregnant women, and the estimate of influenza vaccine types. The pooled results of HCWs failed to pass Egger's test which indicated that result interpretation needed to be cautious. Since the immune mechanisms among HCWs were less likely various depend on their profession, the main results were still credible and robust. Moreover, though the influenza vaccination status and COVID-19 records were mainly based on various medical records, confounders may be introduced in retrospective studies, especially minority collected by self-reported. Meanwhile, the difference in sensitivity and specificity of the COVID-19 test was also inevitable among studies. However, sensitivity analysis was conducted to avoid the confounders’ impact from original studies. Besides, specific population and age groups as well as SARS-CoV-2 variants and vaccines co-administration are needed to further elaborate. More information helps to advance vaccine co-administration and develop a combined one.
In conclusion, heterogeneity from original studies was inevitable yet, the thrilling association made the influenza vaccines promising for reducing COVID-19 infection risks and serious cases. Grantly, the mechanism was unclear as yet, but the massive susceptible population of COVID-19 and clear advantage toward influenza vaccines stress that influenza vaccination has been highly recommended. This correlation gives a new reason to obtain an influenza vaccine, especially for those at high risk, to address the low adoption. Integrating the influenza vaccine with other primary health services should increase efficacy. Further understanding of the differences and interactions of these two viruses and their vaccines should help people get better protection. Pooled results give way to new questions about people who received COVID-19 vaccines, and the change of influenza vaccine strains in each season would impact the protective effects. Further studies are needed with the changing landscape of the pandemic. These results suggest a possible association between COVID-19 and other respiratory diseases may be targeted with a broad vaccine.
Supplementary Material
Footnotes
How to cite this article: Jiang B, Huang Q, Jia M, Xue X, Wang Q, Yang W, Feng L. Association between influenza vaccination and SARS-CoV-2 infection and its outcomes: systematic review and meta-analysis. Chin Med J 2022;135:2282–2293. doi: 10.1097/CM9.0000000000002427
Supplemental digital content is available for this article.
References
- 1. COVID-19 Dashboard. The Center for Systems Science and Engineering at Johns Hopkins University; 2020. Available from: ht∗∗tps://www.arcgis.com/apps/dashboards/bda7594740fd40299423467b48e9ecf6. [Accessed on May 26, 2022]. [Google Scholar]
- 2.Dao TL, Hoang VT, Colson P, Million M, Gautret P. Co-infection of SARS-CoV-2 and influenza viruses: a systematic review and meta-analysis. J Clin Virol Plus 2021; 1:100036.doi: 10.1016/j.jcvp.2021.100036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Swets MC, Russell CD, Harrison EM, Docherty AB, Lone N, Girvan M, et al. SARS-CoV-2 co-infection with influenza viruses, respiratory syncytial virus, or adenoviruses. Lancet 2022; 399:1463–1464. doi: 10.1016/S0140-6736(22)00383-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Han S, Zhang T, Lyu Y, Lai S, Dai P, Zheng J, et al. Influenza's plummeting during the COVID-19 pandemic: the roles of mask-wearing, mobility change, and SARS-CoV-2 interference. Engineering (Beijing) 2022; doi: 10.1016/j.eng.2021.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Feng L, Zhang T, Wang Q, Xie Y, Peng Z, Zheng J, et al. Impact of COVID-19 outbreaks and interventions on influenza in China and the United States. Nat Commun 2021; 12:3249.doi: 10.1038/s41467-021-23440-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang M, Cao R, Zhang L, Yang X, Liu J, Xu M, et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res 2020; 30:269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lu H. Drug treatment options for the 2019-new coronavirus (2019-nCoV). Biosci Trends 2020; 14:69–71. doi: 10.5582/bst.2020.01020. [DOI] [PubMed] [Google Scholar]
- 8.Cocco P, Meloni F, Coratza A, Schirru D, Campagna M, De Matteis S. Vaccination against seasonal influenza and socio-economic and environmental factors as determinants of the geographic variation of COVID-19 incidence and mortality in the Italian elderly. Prev Med 2021; 143:106351.doi: 10.1016/j.ypmed.2020.106351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Root-Bernstein R. Pneumococcal and influenza vaccination rates and pneumococcal invasive disease rates set geographical and ethnic population susceptibility to serious COVID-19 cases and deaths. Vaccines 2021; 9:474.doi: 10.3390/vaccines9050474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen YC, Ko P, Lee WC, Lien WC. Ecological fallacy in COVID-19 epidemiological inference: influenza vaccination rate as an example. J Formos Med Assoc 2021; 120:1655–1656. doi: 10.1016/j.jfma.2021.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tse SC, Wyatt LC, Trinh-Shevrin C, Kwon SC. Racial/ethnic differences in influenza and pneumococcal vaccination rates among older adults in New York City and Los Angeles and Orange Counties. Prev Chronic Dis 2018; 15:E159.doi: 10.5888/pcd15.180101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Su W, Wang H, Sun C, Li N, Guo X, Song Q, et al. The association between previous influenza vaccination and COVID-19 infection risk and severity: a systematic review and meta-analysis. Am J Prev Med 2022; 63:121–130. doi: 10.1016/j.amepre.2022.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang R, Liu M, Liu J. The association between influenza vaccination and COVID-19 and its outcomes: a systematic review and meta-analysis of observational studies. Vaccines 2021; 9:529.doi: 10.3390/vaccines9050529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zeynali Bujani M, Behnampour M, Rahimi N, Safari T, Khazaei Feizabad A, Hossein Sarbazi A, et al. The effect of influenza vaccination on COVID-19 morbidity, severity and mortality: systematic review and meta-analysis. Malays J Med Sci 2021; 28:20–31. doi: 10.21315/mjms2021.28.6.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grohskopf LA, Alyanak E, Ferdinands JM, Broder KR, Blanton LH, Talbot HK, et al. Prevention and control of seasonal influenza with vaccines: recommendations of the advisory committee on immunization practices, United States, 2021-22 influenza season. MMWR Recomm Rep 2021; 70:1–28. doi: 10.15585/mmwr.rr7005a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Key Facts About Seasonal Flu Vaccine. US CDC; 2022. Available from: ht∗∗tps://www.cdc.gov/flu/prevent/keyfacts.htm. [Accessed May 27, 2022]. [Google Scholar]
- 17.Moher D, Liberati A, Tetzlaff J, Altman DG. PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 2009; 339:b2535.doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang J, Yu KF. What's the relative risk? A method of correcting the odds ratio in cohort studies of common outcomes. JAMA 1998; 280:1690–1691. doi: 10.1001/jama.280.19.1690. [DOI] [PubMed] [Google Scholar]
- 19.Lo CK, Mertz D, Loeb M. Newcastle-Ottawa scale: comparing reviewers’ to authors’ assessments. BMC Med Res Methodol 2014; 14:45.doi: 10.1186/1471-2288-14-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smith SR. Preface to the AHRQ supplement. J Gen Intern Med 2014; 29:S712–S713. doi: 10.1007/s11606-014-2922-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang MJ, Rooks BJ, Le TT, Santiago IO, 3rd, Diamond J, Dorsey NL, et al. Influenza vaccination and hospitalizations among COVID-19 infected adults. J Am Board Fam Med 2021; 34: (Suppl): S179–S182. doi: 10.3122/JABFM.2021.S1.200528. [DOI] [PubMed] [Google Scholar]
- 22.Wilcox CR, Islam N, Dambha-Miller H. Association between influenza vaccination and hospitalisation or all-cause mortality in people with COVID-19: a retrospective cohort study. BMJ Open Respir Res 2021; 8:e000857.doi: 10.1136/bmjresp-2020-000857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Umasabor-Bubu OQ, Bubu OM, Mbah AK, Nakeshbandi M, Taylor TN. Association between influenza vaccination and severe COVID-19 outcomes at a designated COVID-only hospital in Brooklyn. Am J Infect Control 2021; 49:1327–1330. doi: 10.1016/j.ajic.2021.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Taghioff SM, Slavin BR, Holton T, Singh D. Examining the potential benefits of the influenza vaccine against SARS-CoV-2: a retrospective cohort analysis of 74,754 patients. PLoS One 2021; 16:e0255541.doi: 10.1371/journal.pone.0255541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ortiz-Prado E, Simbaña-Rivera K, Barreno LG, Diaz AM, Barreto A, Moyano C, et al. Epidemiological, socio-demographic and clinical features of the early phase of the COVID-19 epidemic in Ecuador. PLoS Negl Trop Dis 2021; 15:e0008958.doi: 10.1371/journal.pntd.0008958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Olivar-López V, Leyva-Barrera A, López-Martínez B, Parra-Ortega I, Márquez-González H. Clinical risk profile associated with SARS-CoV-2 infection and complications in the emergency area of a pediatric COVID-19 center. Bol Med Hosp Infant Mex 2020; 77:221–227. doi: 10.24875/BMHIM.20000198. [DOI] [PubMed] [Google Scholar]
- 27.Belingheri M, Paladino ME, Latocca R, De Vito G, Riva MA. Association between seasonal flu vaccination and COVID-19 among healthcare workers. Occup Med 2020; 70:665–671. doi: 10.1093/occmed/kqaa197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Martínez-Baz I, Trobajo-Sanmartin C, Arregui I, Navascues A, Adelantado M, Indurain J, et al. Influenza vaccination and risk of SARS-CoV-2 infection in a cohort of health workers. Vaccines 2020; 8:611.doi: 10.3390/vaccines8040611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ragni P, Marino M, Formisano D, Bisaccia E, Scaltriti S, Bedeschi E, et al. Association between exposure to influenza vaccination and COVID-19 diagnosis and outcomes. Vaccines 2020; 8:675.doi: 10.3390/vaccines8040675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Noale M, Trevisan C, Maggi S, Antonelli Incalzi R, Pedone C, Di Bari M, et al. The association between influenza and pneumococcal vaccinations and SARS-Cov-2 infection: data from the EPICOVID19 web-based survey. Vaccines 2020; 8:471.doi: 10.3390/vaccines8030471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Debisarun PA, Gossling KL, Bulut O, Kilic G, Zoodsma M, Liu Z, et al. Induction of trained immunity by influenza vaccination - impact on COVID-19. PLoS Pathog 2021; 17:e1009928.doi: 10.1371/journal.ppat.1009928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fernández-Prada M, García-González P, García-Morán A, Ruiz-Álvarez I, Ramas-Diez C, Calvo-Rodríguez C. Personal and vaccination history as factors associated with SARS-CoV-2 infection. Med Clin 2021; 157:226–233. doi: 10.1016/j.medcli.2021.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ilic I, Zdravkovic M, Timcic S, Stojanovic DU, Bojic M, Loncar G. Pneumonia in healthcare workers during a COVID-19 outbreak at a cardiovascular hospitals. Int J Infect Dis 2021; 103:188–193. doi: 10.1016/j.ijid.2020.11.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Petrie JG, Bazzi LA, McDermott AB, Follmann D, Esposito D, Hatcher C, et al. Coronavirus occurrence in the household influenza vaccine evaluation (HIVE) cohort of Michigan households: reinfection frequency and serologic responses to seasonal and severe acute respiratory syndrome coronaviruses. J Infect Dis 2021; 224:49–59. doi: 10.1093/infdis/jiab161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Scozzari G, Costa C, Migliore E, Coggiola M, Ciccone G, Savio L, et al. Prevalence, persistence, and factors associated with sars-cov-2 IgG seropositivity in a large cohort of healthcare workers in a tertiary care university hospital in northern Italy. Viruses 2021; 13:1064.doi: 10.3390/v13061064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zelka FZ, Kocaturk RR, Ozcan OO, Karahan M. Can nutritional supports beneficial in other viral diseases be favorable for COVID-19? K J Fam Med 2022; 43:3–15. doi: 10.4082/kjfm.20.0134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.El-Qutob D, Nieto M, Alvarez-Arroyo L, Carrera-Hueso FJ. Is there any effect of flu vaccine on the SARS-CoV-2 infected patients? Vacunas 2022; 23:71–76. doi: 10.1016/j.vacun.2021.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xiang Y, Wong KC, So HC. Exploring drugs and vaccines associated with altered risks and severity of COVID-19: a UK Biobank cohort study of all ATC level-4 drug categories reveals repositioning opportunities. Pharmaceutics 2021; 13:1514.doi: 10.3390/pharmaceutics13091514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Arce-Salinas CA, Esquivel-Torruco YN, Bejarano-Juvera AA, Bustamante-Flores AK, Aguilar-Martínez N, Azcorra-López JG, et al. Association between influenza vaccination and mortality due to COVID-19. Vacunas 2022; 23:113–118. doi: 10.1016/j.vacun.2021.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Conlon A, Ashur C, Washer L, Eagle KA, Hofmann Bowman MA. Impact of the influenza vaccine on COVID-19 infection rates and severity. Am J Infect Control 2021; 49:694–700. doi: 10.1016/j.ajic.2021.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Diallo A, Pichelin M, Wargny M, Gourdy P, Bonnet JB, Hadjadj S, et al. Influenza vaccination and prognosis for COVID-19 in hospitalized patients with diabetes: results from the CORONADO study. Diabetes Obes Metab 2022; 24:343–347. doi: 10.1111/dom.14577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Greco S, Bella A, Bonsi B, Fabbri N, Califano A, Morrone S, et al. SARS-CoV-2 infection and H1N1 vaccination: does a relationship between the two factors really exist? A retrospective analysis of a territorial cohort in Ferrara, Italy. Eur Rev Med Pharmacol Sci 2021; 25:2795–2801. doi: 10.26355/eurrev_202103_25441. [DOI] [PubMed] [Google Scholar]
- 43.Fink G, Orlova-Fink N, Schindler T, Grisi S, Ferrer APS, Daubenberger C, et al. Inactivated trivalent influenza vaccination is associated with lower mortality among patients with COVID-19 in Brazil. BMJ Evid Based Med 2021; 26:192–193. doi: 10.1136/bmjebm-2020-111549. [DOI] [PubMed] [Google Scholar]
- 44.Green I, Ashkenazi S, Merzon E, Vinker S, Golan-Cohen A. The association of previous influenza vaccination and coronavirus disease-2019. Hum Vaccin Immunother 2021; 17:2169–2175. doi: 10.1080/21645515.2020.1852010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Huang K, Lin SW, Sheng WH, Wang CC. Influenza vaccination and the risk of COVID-19 infection and severe illness in older adults in the United States. Sci Rep 2021; 11:11025.doi: 10.1038/s41598-021-90068-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.King JP, McLean HQ, Belongia EA. Risk of symptomatic severe acute respiratory syndrome coronavirus 2 infection not associated with influenza vaccination in the 2019-2020 season. Influenza Other Respir Viruses 2021; 15:697–700. doi: 10.1111/irv.12880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kissling E, Hooiveld M, Brytting M, Vilcu AM, de Lange M, Martínez-Baz I, et al. Absence of association between 2019-20 influenza vaccination and COVID-19: results of the European I-MOVE-COVID-19 primary care project, March-August 2020. Influenza Other Respir Viruses 2021; 15:429–438. doi: 10.1111/irv.12839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kline A, Trinh LN, Hussein MH, Elshazli RM, Toraih EA, Duchesne J, et al. Annual flu shot: does it help patients with COVID-19? Int J Clin Pract 2021; 75:e14901.doi: 10.1111/ijcp.14901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kowalska M, Niewiadomska E, Barański K, Kaleta-Pilarska A, Brożek G, Zejda JE. Association between influenza vaccination and positive sars-coV-2 igG and igM tests in the general population of Katowice region, Poland. Vaccines 2021; 9:415.doi: 10.3390/vaccines9050415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kristensen JH, Hasselbalch R, Pries-Heje M, Nielsen PB, Dehlbaek Knudsen A, Fogh K, et al. Effect of influenza vaccination on risk of COVID-19 - a prospective cohort study of 46,000 health care workers. J Infect Dis 2022; 226:6–10. doi: 10.1093/infdis/jiac001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Massoudi N, Mohit B. A case-control study of the 2019 influenza vaccine and incidence of COVID-19 among healthcare workers. J Clin Immunol 2021; 41:324–334. doi: 10.1007/s10875-020-00925-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Paganoti CF, Rodrigues AS, Francisco RPV, Costa RAD. The influenza vaccine may protect pregnant and postpartum women against severe COVID-19. Vaccines 2022; 10:206.doi: 10.3390/vaccines10020206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pastorino R, Villani L, La Milia DI, Ieraci R, Chini F, Volpe E, et al. Influenza and pneumococcal vaccinations are not associated to COVID-19 outcomes among patients admitted to a university hospital. Vaccine 2021; 39:3493–3497. doi: 10.1016/j.vaccine.2021.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pedote PD, Termite S, Gigliobianco A, Lopalco PL, Bianchi FP. Influenza vaccination and health outcomes in COVID-19 patients: a retrospective cohort study. Vaccines 2021; 9:358.doi: 10.3390/vaccines9040358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sánchez-García C, Salinas-Aguirre JE, Rodríguez-Muñoz L, Rodríguez-Sánchez R, Díaz-Castaño A, Bernal-Gómez R. History of influenza immunization in COVID-19 patients: impact on mortality. Gac Med Mex 2021; 157:102–106. doi: 10.24875/gmm.M21000527. [DOI] [PubMed] [Google Scholar]
- 56.Tayar E. Effectiveness of influenza vaccination against SARS-CoV-2 infection among healthcare workers in Qatar. medRxiv 2022; doi: 10.1101/2022.05.09.22274802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wehenkel C. Positive association between COVID-19 deaths and influenza vaccination rates in elderly people worldwide. PeerJ 2020; 8:e10112.doi: 10.7717/peerj.10112. [Google Scholar]
- 58.Amato M, Werba JP, Frigerio B, Coggi D, Sansaro D, Ravani A, et al. Relationship between influenza vaccination coverage rate and COVID-19 outbreak: an Italian ecological study. Vaccines 2020; 8:535.doi: 10.3390/vaccines8030535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zanettini C, Omar M, Dinalankara W, Imada EL, Colantuoni E, Parmigiani G, et al. Influenza vaccination and COVID-19 mortality in the USA: an ecological study. Vaccines 2021; 9:427.doi: 10.3390/vaccines9050427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pollard AJ, Bijker EM. A guide to vaccinology: from basic principles to new developments. Nat Rev Immunol 2021; 21:83–100. doi: 10.1038/s41577-020-00479-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ryan KA, Schewe KE, Crowe J, Fotheringham SA, Hall Y, Humphreys R, et al. Sequential delivery of live attenuated influenza vaccine and severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in the ferret model can reduce SARS-CoV-2 shedding and does not result in enhanced lung pathology. J Infect Dis 2022; 225:404–412. doi: 10.1093/infdis/jiab594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Casado I, Dominguez A, Toledo D, Chamorro J, Astray J, Egurrola M, et al. Repeated influenza vaccination for preventing severe and fatal influenza infection in older adults: a multicentre case-control study. CMAJ 2018; 190:E3–E12. doi: 10.1503/cmaj.170910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Domnich A, Orsi A, Trombetta CS, Guarona G, Panatto D, Icardi G. COVID-19 and seasonal influenza vaccination: cross-protection, co-administration, combination vaccines, and hesitancy. Pharmaceuticals 2022; 15:322.doi: 10.3390/ph15030322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Netea MG, Dominguez-Andres J, Barreiro LB, Chavakis T, Divangahi M, Fuchs E, et al. Defining trained immunity and its role in health and disease. Nat Rev Immunol 2020; 20:375–388. doi: 10.1038/s41577-020-0285-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mei-Mei B, Kai Y, Bing Y. Influenza virus and coronavirus: cellular binding and internalization. Acta Phys Sin 2020; 69:208701–1208701. doi: 10.7498/aps.69.20201161. [Google Scholar]
- 66.Han S, Zhang T, Lyu Y, Lai S, Dai P, Zheng J, et al. The incoming influenza season - China, UK, US, 2021-2022. China CDC Wkly 2021; 3:1039–1045. doi: 10.46234/ccdcw2021.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Demicheli V, Jefferson T, Di Pietrantonj C, Ferroni E, Thorning S, Thomas RE, et al. Vaccines for preventing influenza in the elderly. Cochrane Database Syst Rev 2018; 2:CD004876.doi: 10.1002/14651858.CD004876.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Belongia EA, McLean HQ. Influenza vaccine effectiveness: defining the H3N2 problem. Clin Infect Dis 2019; 69:1817–1823. doi: 10.1093/cid/ciz411. [DOI] [PubMed] [Google Scholar]
- 69.Witkowski JM, Fulop T, Bryl E. Immunosenescence and COVID-19. Mech Ageing Dev 2022; 204:111672.doi: 10.1016/j.mad.2022.111672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.ECDC. Systematic review of the efficacy, effectiveness and safety of newer and enhanced seasonal influenza vaccines for the prevention of laboratory confirmed influenza in individuals aged 18 years and over. Stockholm: ECDC; 2020. [Google Scholar]
- 71. World Health Organization. Vaccines Against Influenza: WHO Position Paper - May 2022. Wkly Epidemiol Rec 2022; 97:185-208. Available from: ht∗∗tps://apps.who.int/iris/bitstream/handle/10665/354264/WER9719-eng-fre.pdf?sequence=1&isAllowed=y. [Accessed on 15 July, 2022]. [Google Scholar]
- 72. Coadministration of Seasonal Inactivated Influenza and COVID-19 Vaccines. World Health Organization; 2021. Available from: ht∗∗tps://www.who.int/publications/i/item/WHO-2019-nCoV-vaccines-SAGE_recommendation-coadministration-influenza-vaccines. [Accessed May 28, 2022]. [Google Scholar]
- 73.Candelli M, Pignataro G, Torelli E, Gullì A, Nista EC, Petrucci M, et al. Effect of influenza vaccine on COVID-19 mortality: a retrospective study. Intern Emerg Med 2021; 16:1849–1855. doi:10.1007/s11739-021-02702-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pawlowski C, Puranik A, Bandi H, Venkatakrishnan AJ, Agarwal V, Kennedy R, et al. Exploratory analysis of immunization records highlights decreased SARS-CoV-2 rates in individuals with recent non-COVID-19 vaccinations. Sci Rep 2021; 11:4741.doi:10.1038/s41598-021-83641-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Debisarun PA, Gössling KL, Bulut O, Kilic G, Zoodsma M, Liu Z, et al. Induction of trained immunity by influenza vaccination - impact on COVID-19. PLoS Pathog 2021; 17:e1009928.doi: 10.1371/journal.ppat.1009928. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.