Abstract
Patients with chronic lymphocyticleukemia (CLL) typically have innate/adaptive immune system dysregulation, thus the protective effect of coronavirus disease 2019 (COVID-19) vaccination remains uncertain. This prospective review evaluates vaccination response in these patients, including seropositivity rates by CLL treatment status, type of treatment received, and timing of vaccination. Antibody persistence, predictors of poor vaccine response, and severity of COVID-19 infection in vaccinated patients were also analyzed. Practical advice on the clinical management of patients with CLL is provided. Articles reporting COVID-19 vaccination in patients with CLL, published January 1, 2021–May 1, 2022, were included. Patients with CLL displayed the lowest vaccination responses among hematologic malignancies; however, seropositivity increased with each vaccination. One of the most commonly reported independent risk factors for poor vaccine response was active CLL treatment; others included hypogammaglobulinemia and age >65–70 years. Patients who were treatment-naive, off therapy, in remission, or who had a prior COVID-19 infection displayed the greatest responses. Further data are needed on breakthrough infection rates and a heterologous booster approach in patients with hematologic malignancies. Although vaccine response was poor for patients on active therapy regardless of treatment type, CLL management in the context of COVID-19 should aim to avoid delays in antileukemic treatment, especially with the advent of numerous strategies to mitigate risk of severe COVID-19 such as pre-exposure prophylaxis, and highly effective antivirals and monoclonal antibody therapy upon confirmed infection. Patients with CLL should remain vigilant in retaining standard prevention measures such as masks, social distancing, and hand hygiene.
INTRODUCTION
Since the beginning of the coronavirus disease 2019 (COVID-19) pandemic, there have been over 500 million cases and over 6 million deaths reported worldwide.1 Patients with hematologic malignancies and COVID-19 infection have significantly higher rates of hospitalizations (57.1% versus 37.8%), intensive care unit (ICU) stays (29.8% versus 11.2%), and a 3.88-fold higher mortality rate (13.1% versus 3.7%) than respective patients with solid tumor malignancies.2 A meta-analysis consisting of 3377 adult patients with hematologic malignancies conducted earlier in the pandemic showed a 34% risk of death in a predominantly hospitalized population, and a subgroup analysis of patients with chronic lymphocytic leukemia (CLL) estimated a pooled mortality risk of 31%.3 Additionally, in a multicenter international cohort study comprising 198 patients with CLL and COVID-19, hospital admission occurred in 90% of patients, among whom, the case fatality rate was 37%.4 In a retrospective, international, multicenter study by the European Research Initiative on CLL (ERIC) and Campus CLL including 190 patients with CLL and confirmed COVID-19, 79% presented with severe COVID-19 (requiring hospitalization and oxygen or ICU admission), with a reported CFR of 36.4%.5 In an update to this analysis through March 2021, with an expanded population of 941 patients (887 with CLL) the CFR had decreased (27.3% overall, 38.4% for patients with severe COVID-19).6 Similar results were seen in a recent retrospective analysis of 188 patients with CLL and COVID-19, which reported a CFR of 27%.7 As COVID-19 infection management improved over time, one retrospective analysis in 374 patients with CLL and COVID-19 evaluated CFR stratified by date; they observed a higher percentage of hospital admissions (85% versus 55%) and required ICU admissions (32% versus 15%), and a higher CFR (35% versus 11%) in the early cohort (February–April 2020) compared with the later cohort (May 2020–Feb 2021), respectively, indicating a trend toward lower mortality rates over time for patients with CLL and COVID-19.8
Factors affecting COVID-19–related risk of death in patients with CLL have been investigated. In the recently published retrospective analysis mentioned above on outcomes in 188 patients with CLL and COVID-19, the type of CLL-directed therapy did not have any impact on COVID-19–related risk of death.7 Advanced age, poor performance status, low platelets, and elevated lactate dehydrogenase levelswere associated with increased risk of death due to COVID-19. Similar results have been seen in other large retrospective analyses where treatment type did not appear to impact COVID-19 outcomes.9,10 However, most patients in these studies had not been vaccinated.
A key feature of CLL is innate and adaptive immune system dysregulation, which may worsen over the course of the disease. This includes increased mobilization, inhibition, and impairment of T cells, and inhibition and decreased numbers of antibody-producing cells, among other immune deficiencies. Thus, the protective effects of vaccination against a variety of pathogens are variable in patients with CLL.11 Given the added concern regarding the CLL treatment impact on immunity and response to vaccination,12–16 the pandemic may influence the decision of whether to treat CLL, and when. Optimal timing of vaccination in relation to treatment has also been unclear, with heterogeneity in recommendations across national and international guidelines. The current consensus supported by the American Society of Hematology (ASH), the European Hematology Association, ERIC, and the National Comprehensive Cancer Network® (NCCN®) is that, while all patients should be assessed on a case-by-case basis, and exceptions apply, generally patients should be vaccinated as soon as possible.17–19
Since the COVID-19 vaccination program initiation, numerous studies have been conducted to evaluate antibody response to COVID-19 vaccination in CLL, including patient populations receiving treatment, and a few studies have evaluated T-cell–mediated response. While many prior reviews have included a broader population of hematologic illnesses,20–22 we aim to focus on prospective data in patients with CLL specifically to determine the overall humoral and cellular response, and the impact of CLL treatment and COVID-19 infection on immunity. This article also aims to provide practical thoughts on managing these specific groups of patients in clinical practice.
METHODS
Data search strategy
An electronic search of the PubMed database was performed to gather articles reporting on COVID-19 vaccination in patients with CLL, published between January 1, 2021, and May 1, 2022. Search strings covering the terms CLL, COVID19, vaccine, and vaccination were used (Suppl. Table S1). Abstracts presented at the ASH 2021 Annual Meeting and Exposition were also searched using the terms COVID-19 and CLL. To be eligible for inclusion, studies needed to be conducted in humans, and published in English from January 2021 to May 2022. Prospective clinical trials, observational studies, meta-analyses, systematic reviews, and registry studies were included. Finally, studies were required to include serology results in ≥20 patients with CLL; this enabled the inclusion of a sizeable number of studies while still providing an adequate number of patients for analysis of subgroups within the data.
Data screening and extraction
Key outcomes included seropositivity rates post-COVID-19 vaccination, defined in most studies as the presence of antibodies against the SARS-CoV-2 spike receptor-binding domain at or above the cutoff level for positivity, for patients with CLL versus healthy controls or patients with other hematologic malignancies. Studies that only included neutralizing antibodies as an endpoint were excluded.22–24 Rates based on treatment status were considered, as well as type of treatment, and timing of vaccination. Antibody persistence, predictors of poor vaccine response, and severity of COVID-19 breakthrough infection in vaccinated patients were analyzed.
Data summary
A descriptive summary of the findings is presented. For the purpose of this review, a full initial vaccine series is defined as two doses of an mRNA vaccine, or one dose of adenovirus vector vaccine. Unless otherwise stated, vaccine response refers to antibody response. It should be noted that currently there is no standard definition of vaccine response – as such, definition of response varied across studies.
RESULTS
Search and study selection
In total, 27 studies were included in the review (Figure 1 and Suppl. Table S2).
Figure 1.
Included studies. aStudy not conducted in humans; not published in English between January 21 and May 2022; not one of the following study types: prospective clinical trial, observational study, meta-analysis, systematic review, or registry study; not including serology results in ≥20 patients with CLL. CLL = chronic lymphocytic leukemia.
How does vaccine response in CLL compare with other hematologic illnesses?
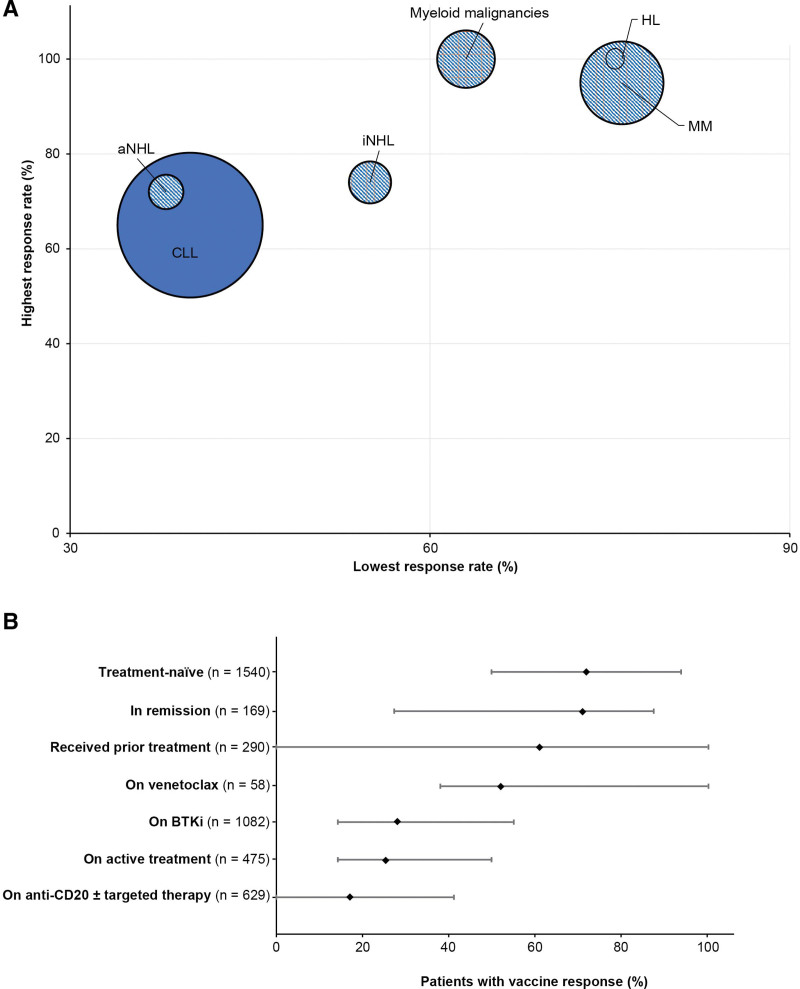
The data from several studies, including 3 large meta-analyses, provided vaccine response results for multiple hematologic malignancies (Table 1 and Figure 2A). Across all studies, with 3 exceptions,25,28,30 the lowest seropositivity rate was seen in the CLL population. The humoral response in patients with CLL after a full initial vaccine series ranged from 40% to 67% across all studies, compared with a range of 44%–100% for other hematologic malignancies. Eight studies also included healthy controls with vaccine responses between 97% and 100%.20,21,27,33,34,36,40
Table 1.
Seropositivity Rates Post Initial Vaccine Series Reported for Patients With CLL or Other Hematologic Malignancies
| Author | Patients With CLL | Other Hematologic Malignancies | ||
|---|---|---|---|---|
| N | Response (%) | Disease Type, n | Response (%) | |
| Giuliano et al (2022)25 | 23 | 65.2 | HL, 10 | 100 |
| AML, 30 | 100 | |||
| MDS, MPN, or MDS/MPN, 49 | 85.7 | |||
| B-cell NHL, 55 | 58.2 | |||
| MM, 88 Other, <20 patients eacha |
93.2 | |||
| Teh et al (2021)21 | 1446 | 51 | AML/MDS, 126 | 93 |
| MPN/CML, 281 | 87–88 | |||
| Myeloma, 1218 | 76–80 | |||
| Lymphoma, 1296 | 52–55 | |||
| Gagelmann et al (2021)20 | 1753 | 50 | HL, 133 | 91 |
| MPN, 365 | 83 | |||
| Aggressive NHL, 386 | 58 | |||
| Indolent NHL, 494 | 61 | |||
| Myeloma, 1564 | 76 | |||
| Gong et al (2022)26 | 1709 | 44 | PCD, 2066 | 72 |
| Lymphoid malignancies, 1904 | 52 | |||
| MPN, 464 | 81 | |||
| Acute leukemias, 147 | 83 | |||
| MDS, 79 | 63 | |||
| Šušol et al (2022)24 | 53 | 39.6 | HL, 21 | 76.2 |
| aNHL, 32 | 37.5 | |||
| iNHL, 62 | 54.8 | |||
| MM, 119 | 87.4 | |||
| AL/MDS, 33 | 84.8 | |||
| cMPN, 54 | 92.6 | |||
| Shen et al (2021)27 | 160 | 55 | MBL, 21 | 90.5 |
| Greenberger et al (2021)28 | 650 | 64.2 | MCL, 27 | 44.4 |
| Smoldering MM, 29 | 100 | |||
| AML, 34 | 91.2 | |||
| CML, 34 | 97.1 | |||
| MZL, 34 | 61.8 | |||
| MDS/MPN, 35 | 97.1 | |||
| NHL NOS, 48 | 79.2 | |||
| DLBCL, 52 | 78.8 | |||
| HL, 65 | 98.5 | |||
| WM, 97 | 74.2 | |||
| FL, 98 | 77.6 | |||
| MM, 184 | 95.1 | |||
| Other, <20 patients eacha | ||||
| Herzog Tzarfati et al (2021)29 | 34 | 47 | CML, 22 | 91 |
| Indolent NHL, 40 | 60 | |||
| Aggressive NHL, 51 | 71 | |||
| MM, 53 | 76 | |||
| MPN, 68 | 84 | |||
| Dong et al (2021)30 | 23 | 65.2 | B-cell NHL, 55 Other, <20 patients eacha | 58.2 |
| Molica et al (2022)31 | 2082 | 52 | ||
| Bagacean et al (2022)32 | 506 | 52 | ||
| Molica et al (2022)33 | 70 | 58.5 | ||
| Bergman et al (2021)34 | 79 | 63.3 | ||
| Sun et al (2021)35 | 58 | 60.3 | ||
| Parry et al (2022)36 | 500 | 67 | ||
| Benjamini et al (2022)37 | 373 | 43 | ||
| Roeker et al (2021)38 | 44 | 52 | ||
| Tadmor et al (2021)39 | 84 | 58.3 | ||
| Herishanu et al (2021)40 | 167 | 39.5 | ||
| Ujjani et al (2022)23 | 37 | 41 | ||
| Haydu et al (2022)41 | 36 | 55.6 | ||
aResponse rates only reported for hematologic malignancies where the reference cites results for .20 patients.
ALL = acute lymphoblastic leukemia; AML = acute myeloid leukemia; CLL = chronic lymphocyticleukemia; CML = chronic myeloid leukemia; CMPN = chronic myeloproliferative neoplasm; DLBCL = diffuse large B-cell lymphoma; FL = follicular lymphoma; HL = Hodgkin lymphoma; MBL = monoclonal B-lymphocytosis; MDS = myelodysplastic syndromes; MM = multiple myeloma; MPN = myeloproliferative neoplasm; n = number of patients; NHL = non-Hodgkin lymphoma; NK = natural killer.
Figure 2.
Vaccine antibody responses by disease area (A)a and treatment status and type (B)b. aThe data for other hematologic malignancies represent only data present in those references that were included for the CLL analysis, and are not a comprehensive evaluation of response rates in those malignancies. The visual shows the range of vaccine responses for each disease area, with the lowest response rate within the range shown on the x-axis and the highest response on the y-axis. The size of the bubbles represent the number of patients within that disease area across all studies; bDiamond represents median; bar represents range. Median (range) % responders by treatment status or treatment type: treatment-naive, 72 (50–94); active treatment, 25.3 (14–50); prior treatment, 61 (0–100); remission, 71 (27.2–87.5); venetoclax, 52 (38–100); BTKi, 28 (14.3–55); anti-CD20, 17 (0–41). AML/MDS = acute myeloid leukemia/myelodysplastic syndromes; BTKi = Bruton tyrosine kinase inhibitor; CLL = chronic lymphocytic leukemia; CR = complete remission; HL = Hodgkin lymphoma; MM = multiple myeloma; MPN = myeloproliferative neoplasm; NHL = non-Hodgkin lymphoma; TN = treatment-naive; ven = venetoclax.
As vaccination programs have accelerated in several countries, many patients with CLL have received a third dose of vaccine. In 6 studies, where patients were offered a third dose, 0%–80% (median 44.7%) achieved a serological response. The broad range of responses seen after the third dose may be due to the differences in response to the initial vaccine series. In the 2 studies, with the lowest responses to the third dose, for example, the patients were seronegative after the initial vaccine series,24,42 whereas in the study with the highest response rate, 72% were seronegative after the initial vaccine series.43 One study reported a 55% conversion rate in initially seronegative patients; however, patient numbers were low.41 Six studies included data on response after a single dose of vaccine in addition to response following the second dose; continued vaccination led to a higher percentage of patients reaching seropositivity (Table 2). Limited data are available on the fourth vaccine dose during the period of interest (January 1, 2021–May 1, 2022).
Table 2.
Seroconversion Rates in Patients With CLL for First, Second, and Third Dose of Vaccines
| Author | Response (n Evaluable) | |||
|---|---|---|---|---|
| N | First Dose Response | Second Dose Response | Third Dose Response | |
| Bagacean et al (2022)32 | 530 | 27% (158) | 52% (506) | 35% (95)a |
| Dong et al (2021)30 | 23 | 21.7% (23) | 65.2% (23) | NA |
| Shen et al (2021)27 | 160 | 20.8% (125) | 55.0% (160) | NA |
| Herishanu et al (2022)42 | 172 | NA | NA | 23.8% (172)a |
| Greenberger et al (2021)43 | 25 | NA | NA | 80% (25)b |
| Del Poeta et al (2021)44 | 46 | NA | NA | 54.3% (46)c |
| Haydu et al (2022)41 | 36 | NA | NA | 55% (11)a |
| Giuliano et al (2022)25 | 23 | 21.7% (23) | 65.2% (23) | NA |
| Šušol et al (2022)24 | 53 | NA | NA | 0% (15)a |
| Teh et al (2021)21,d | 1446 | 18% (111) | 51% (1446) | NA |
aSeronegative after initial vaccine series.
b72% seronegative after initial vaccination.
cInitial vaccination response not reported.
dMeta-analysis, rate after first dose is from 1 study with 111 patients, rate after second dose is pooled across included studies of patients with CLL.
CLL = chronic lymphocytic leukemia; n = number of patients; NA = not available.
How does treatment status impact vaccination response?
The majority of studies (17/27) included subgroup analyses on response based on treatment status. The most commonly reported independent factor for poor response was being on active treatment, with responses ranging from 14% to 50% (median 25.3%) after the initial vaccine series, compared with 50% to 94% (median 72%) in those who were treatment-naive (Table 3). For patients who were off therapy but who had received prior treatment, response rates of 42%–100% (median 61%) were reported,27,32,34,35,39,40,42 with 2 outliers of 23% (n = 26)38 and 0% (n = 5).41 The heterogeneous results seen across studies is reflective of the many variables that can impact vaccine response in patients with CLL, including factors such as age and comorbidities, time since last treatment administration (in particular for anti-CD20 therapy), and study design elements such as the assay used and timing of response evaluation following vaccination. Patients in remission had response rates similar to those who were treatment-naive (67.5%–87.5%; median 71%),27,33,36,40 with 1 outlier of 27.2% (n = 22).39 A similar pattern was observed in patients who were offered a third dose, with higher response in treatment-naive patients compared with those on active treatment.42,44 In 1 study, patients with prior COVID-19 infection had the highest response rate (100%) with just 1 vaccination.32 Another study reported antibody titers in patients with CLL who had a prior COVID-19 infection that were comparable with titers seen in healthy controls.36
Table 3.
Seropositivity Rates According to Treatment Status
| Author | CLL N | Response (n Evaluable) | |||
|---|---|---|---|---|---|
| Treatment-naive | Active Treatment | Prior Treatment | In Remission | ||
| Second dose | |||||
| Sun et al (2021)35 | 58 | 71% (21) | 50% (34) | 100% (3) | NA |
| Dong et al (2021)30 | 23 | 81.3% (16) | 28.6% (7) | NA | NA |
| Shen et al (2021)27 | 160 | 63.4% (82) | 21.9% (32) | NA | 67.5% (40)a |
| Molica et al (2021)33 | 70 | 87% (23) | 41.7% (36) | NA | 87.5% (8)a |
| Parry et al (2022)36 | 500 | 78% (284) | 43% (200) [on/prior] | 73% (11)b | 71% (75) |
| Bagacean et al (2022)32 | 506 | 72% (210) | 22% (166) | 60% (130) | NA |
| Benjamini et al (2022)37 | 373 | 61% (158) | 14% (113)c | 42% (55)d | NA |
| Herishanu et al (2021)40 | 167 | 55.2% (58) | 16% (75) | 64.7% (34) | 79.2% (24) |
| Roeker et al (2021)38 | 44 | 94% (18) | NA | 23% (26) | NA |
| Tadmor et al (2021)39 | 84 | 76.2% (16) | 28.6% (6) | 61.9% (26) | 27.2% (22) |
| Bergman et al (2021)34 | 79 | 84.6% (26) | NA | NAe | NA |
| Ujjani et al (2022)23 | 37 | 50% (14) | NA | NA | NA |
| Haydu et al (2022)41 | 36 | 72% (25) | 33% (6) | 0% (5) | NA |
| Molica et al (2022)31 | 2082 | 73% (533) | NA | NA | NA |
| Haggenburg et al (2022)45 | 94 | 70% (56) | NA | NA | NA |
| Third dose (booster results) | |||||
| Herishanu et al (2022)42 | 172 | 40% (40) | 12% (100) | 40.6% (32) | 41.7% (24) |
| Del Poeta et al (2021)44 | 46 | 88.2% (17)f | 34.5% (29) | NA | NA |
aComplete or partial remission.
bPrior targeted therapy.
cTargeted therapy.
dPrior targeted therapy.
ePrior ibrutinib: 9 (55.6%); prior anti-CD20 (median 13 mo): 18 (88.9%). fIncludes prior treatment.
CLL = chronic lymphocytic leukemia; n = number of patients; NA = not available.
How do specific therapies impact vaccination response?
Targeted therapies (inhibitors of Bruton tyrosine kinase [BTKi] and B-cell lymphoma-2 [BCL-2i]), as well as anti-CD20 antibodies, are widely used in CLL. The effect of anti-CLL–targeted therapies on humoral responses is unclear, and may be due to off-target effects on other kinases affecting CD4 T-cell function in the case of BTKi’s or reduction in absolute numbers of B cells and T cells, as with BCL2i’s11; however, monoclonal anti-CD20 therapy (most commonly with rituximab or obinutuzumab), used in CLL treatment, depletes CD20+ B-cell function, which is vital in forming a serologic response. Seventeen out of 27 studies included subgroup analyses on seroconversion rates for specific therapies (Table 4). Vaccine response for venetoclax monotherapy ranged from 38% to 100% (median 52%).32,35–37,40 Response rates in patients on BTKi monotherapy ranged from 14.3% to 55% (median 28%), with 11 of the 14 studies reporting response rates less than 36% after 2 doses of vaccine, and in those on anti-CD20s from 0% to 41% (median 17%) (Figure 2B). For those on any venetoclax-based regimen, including anti-CD20 or BTKi combinations, response was reduced (0%–39%); it should be noted that the studies included in this range did not provide a breakdown of the venetoclax-based regimens (ie, mono or combo and specific combination partner).28,31,36,37,39,40 The NCCN recommendations do not distinguish between therapies, stating that patients on maintenance therapies for CLL, including anti-CD20 antibodies and BTKi’s, may have an attenuated response to vaccination.18 Currently, it is unclear if the rates of vaccine response correlate to better outcomes in patients with CLL.
Table 4.
Seroconversion Rates Post Initial Vaccination According to Type of Targeted Therapy Received
| Author | CLL N | Response (n Evaluable) | ||||
|---|---|---|---|---|---|---|
| Targeted Treatment | Venetoclax Mono | BCL2i ± Combo | BTKi | Anti-CD20 Response ± Targeted | ||
| Second dose | ||||||
| Sun et al (2021)35 | 58 | NA | 100% (1) | NA | 55% (29) | 20% (5) |
| Molica et al (2021)33 | 70 | 43% (35) | NA | NA | NA | 10% (10) |
| Parry et al (2022)36 | 500 | 24% (114) | 38% (16) | 0% (5)a | 34% (93)b | NA |
| Bagacean et al (2022)32 | 506 | NA | 52% (23) | 0% (6)a | 22% (104) | 0% (19) |
| Bergman et al (2021)34 | 79 | NA | NA | NA | 26.9% (26) | NA |
| Molica et al (2022)31 | 2082 | NA | NA | 32% (94) | 29% (325) | 41% (254) |
| Benjamini et al (2022)37 | 373 | 14% (113) | 62% (13) | 6% (34) | 18% (79) | 14% (49) |
| Roeker et al (2021)38 | 44 | NA | NA | NA | 21% (14) | 10% (21) |
| Tadmor et al (2021)39 | 84 | NA | NA | 16.7% (6) | 36.4% (4) | NA |
| Herishanu et al (2021)40 | 167 | NA | 40% (5) | 13.6% (22) | 16% (50) | NA |
| Greenberger et al (2021)28 | 650 | NA | NA | 39.3% (28) | 47.5% (282) | 36.9% (263) |
| Shen et al (2022)27 | 160 | 16% (25) | NAc | NA | 14.3% (21) | NA |
| Ujjani et al (2022)23 | 37 | NA | NA | NA | 33% (15) | 38% (8)d |
| Haydu et al (2022)41 | 36 | NA | NA | NA | 33% (6) | NA |
| Haggenburg (2022)45 | 94 | NA | NA | NA | 27% (34) | NA |
| Third dosee | ||||||
| Herishanu et al (2022)42 | 172 | NA | 60% (5) | NA | 15.3% (59) | 7.7% (39) |
| Del Poeta et al (2021)44 | 46 | NA | 25% (8) | NA | 38.1% (21) | NA |
aPatients receiving venetoclax + a BTKi.
bBTKi monotherapy.
cFour patients (response 25%) were on venetoclax, but had completed anti-CD20 therapy within the last 12 mo.
dVenetoclax + anti-CD20 therapy ongoing/within 1 y.
eHerishanu: seronegative after initial vaccination; Del Poeta: any patient (may include prior treatment).
BCL2i = B-cell lymphoma-2 inhibitor; BTKi = Bruton tyrosine kinase inhibitor; CLL = chronic lymphocytic leukemia; Combo = combination therapy; Mono = monotherapy; n = number of patients; NA = not available.
How does timing of vaccination post-anti-CD20 anti-CD20 treatment impact vaccine response?
As mentioned previously, patients who had had prior therapy, but were no longer on active treatment had a greater response than those on active treatment.32,35–41 However, the optimal time for vaccine administration post-anti-CD20 treatment has yet to be determined. Table 5 summarizes prospective studies that analyzed vaccine response in patients on anti-CD20 therapy, based on the timing of vaccination. Patients who were given their initial vaccine series at least 12 months following end of anti-CD20 therapy had a greater vaccine response (35%–56.2%) compared with a range of 0%–24% for those who were vaccinated less than 12 months after anti-CD20 therapy.27,31,36–40 One study reported a response rate of 20% (n = 10) when the vaccine was given as early as within 6 months after treatment with an anti-CD20, so some patients do respond earlier than 12 months post-treatment.24 A similar pattern was seen in the 1 study that measured response based on timing of a third vaccine in relation to anti-CD20 therapy (22.7% of patients not treated with an anti-CD20 in the 12 months before vaccination versus 3.6% of those who were).37 Studies did not report correlations between vaccine timing post-targeted therapy specifically and vaccine response.
Table 5.
Seroconversion Rates According to Timing of Vaccination Post-anti-CD20 Therapy
| Author | CLL, N | Response (n Evaluable) | |
|---|---|---|---|
| CD20 <12 mo | CD20 ≥12 mo | ||
| Second dose | |||
| Shen et al (2022)27 | 160 | 22.2% (9) | NA |
| Parry et al (2022)36 | 500 | 24% (17) | 56% (136) |
| Herishanu et al (2021)40 | 167 | 0% (22) | 45.5% (55) |
| Molica et al (2022)31 | 2082 | 4% (94) | NA |
| Benjamini et al (2022)37 | 373 | 5% (39) | 35% (92) |
| Roeker et al (2021)38 | 44 | 14.3% (14) | NA |
| Tadmor et al (2021)39 | 84 | NA | 56.2% (18) |
| Third dose (booster) | |||
| Herishanu et al 202242 | 172 | 3.6% (28) | 22.7% (66) |
CLL = chronic lymphocytic leukemia; n = number of patients; NA = not available.
What factors were shown to be predictors of poor vaccine response?
The most commonly reported independent risk factors for poor vaccine response were low immunoglobulin (Ig)M, IgG, or IgM and/or IgA27,30,32,34,36,39,42,44; anti-CD20 therapy (active or within the last 12 months)23,24,28–30,32,36,40,42,43; BTKi therapy28,29,34–36,40,42,45; active therapy in general30,32,33,39,42,44; age >65 years29,32,38,42; CLL-directed therapy of any kind in the last 12 months27,32,38; and BCL-2i regimens29,42 (+ anti-CD2035,40). Other factors identified in more than one study included the number of prior therapies/lines of therapy29,42; Cumulative Illness Rating Scale score >646 or ≥637; vaccine type (BNT162b2)28,32; low B-lymphocyte count23,30; and gender (male36,44 and female28,39).
How long did patients maintain seropositivity?
Five studies provided results on antibody persistence in patients with CLL. In 4 of these studies, 64%–90% of patients remained seropositive over a 3–6-month timeframe following the initial vaccine series.36,39,47 In the other study, 100% (n = 14) of patients remained seropositive at 6 months; however, 7 of the patients had also received a third dose.23 Although patients with CLL may start out with a lower response rate, in 1 study, it was noted that the decay in antibody response over time is similar between patients with CLL and healthy controls aged ≥70 years.39
In terms of whether anti-CD20 therapy post-vaccination impacts antibody persistence, none of the studies captured in the literature search addressed immune response when treatment is initiated postvaccination. However, in a recent study (published January, 2022) evaluating vaccine response in 126 patients with lymphoma, 15 patients received a full initial vaccine series before beginning anti-CD20 therapy.48 Ten of these patients generated an antibody response, and rates of persistence in this group were comparable with age-matched healthy controls. This study demonstrates little to no impact on vaccine response when patients are vaccinated before anti-CD20 therapy.
What was the severity of illness for patients who developed an infection postvaccination (breakthrough infection)?
There were only 3 studies that reported breakthrough COVID-19 infection in patients with CLL during the study period. In 1 study, 3 of 400 patients developed a COVID-19 infection following vaccination, one in between the first and second dose of vaccine and 2 after the initial vaccine series was complete (14 and 24 days post-vaccination) – all 3 recovered uneventfully.37 In another study, 1 patient (out of 37) who did not seroconvert upon initial vaccination, and was not assessed after a subsequent booster, developed a COVID-19 infection. The patient received casirivimab/imdevimab and recovered without significant complications.23 In the third study,42 4 patients, who had received a third dose of an mRNA vaccine after being seronegative with the first 2 doses, developed a COVID-19 infection, 2 with severe disease, and 2 with mild disease. One patient died due to their infection, while the others recovered. Three of the patients were seronegative after the third dose and 1 was seropositive with a low antibody titer. Although patients who were seronegative seemed to have more severe disease in this analysis, given the small number of patients with COVID-19 infection across all studies, further research is needed to characterize outcomes of breakthrough infections postvaccination.
In a retrospective cohort study with 984 fully vaccinated patients with CLL, identified between December 2020 and October 2021, there was a 15.2% risk of breakthrough infection (confidence interval: 13–17.6) versus 4.5% in 508,457 fully vaccinated patients without cancer. In the same study, hospitalization risk and mortality were shown to be significantly higher in patients with versus those without breakthrough infections across all hematologic malignancies (hazard ratios: 34.49 and 10.25, respectively).49
How did the type of vaccine impact seropositivity, is there an advantage to mixing vaccines?
Ten studies reported on the use of different vaccine types (eg, mRNA1273 or BNT162b2 or Ad26.COV2.S), though not all specifically in patients with CLL: 4 studies showed no difference in serology and/or titers between the vaccines used27,35,36,43; 6 studies reported a difference in vaccine response depending on the vaccine used, of which 3 identified vaccine type as a predictor of response,28,32,41 and 3 did not analyze the difference observed.22,23,38 Overall, for the studies reporting response rates by vaccine type, mRNA1273 had the highest response rates, followed by BNT162b2 (both mRNA-based vaccines), and finally the adenovirus-based vaccine, Ad26.COV2.S. One study reported that this difference was only seen in hematologic patients and not in healthy controls.28 Similarly, in a retrospective analysis of 239 hematologic patients, seroconversion rates were significantly higher for patients who received mRNA1273 (57%) versus BNT162b2 (36%; P = 0.006).50 This difference has also been seen in immune compromised patients in general.51
Additional vaccinations (third and fourth doses) currently approved under emergency use authorization (EUA) are restricted to homologous mRNA vaccines.52 There have been studies reporting results on heterologous, or mixing of, booster vaccinations. In one phase 1/2 study of 458 individuals, a homologous booster increased antibody titers 4.2–20-fold, whereas heterologous boosters increased titers 6.2–76-fold (pre-print).53 Other studies have shown similar increases in either systemic reactogenicity or immunogenicity with a heterologous booster approach45,54,55; however, these studies are in healthy adults.56,57 Only 1 study evaluated heterologous boosters in patients with hematologic malignancies.58 Seventeen percent of 18 patients with CLL mounted a response to the Ad26.COV2.S vaccine after being seronegative on the initial full series of BNT162b2 vaccine, yet there was no comparison with a homologous booster. Further data are needed on the use of heterologous vaccines to boost vaccine response in hematologic patients.
Expert opinion on management of patients with CLL and COVID-19: clinical scenarios
The following perspectives on the clinical management of patients with CLL and COVID-19 infection is based on expert opinion and should not be perceived as clinical practice recommendations. Clinical judgement is required to ensure that treatment decisions are appropriate for individual patients. It is important to note that not all opinions here are based on published data; practices are discussed that may go beyond the scope of this review.
Should I vaccinate all of my patients?
Patients who are moderately or severely immunocompromised, such as patients with CLL, should be vaccinated and offered subsequent boosters regardless of treatment status or type of B-cell directed therapy. We agree with the current recommendation of the Center for Disease Control (CDC) for a fifth dose for patients aged 12 and older who are moderately or severely immunocompromised. It is advisable to follow the relevant treatment guidelines for patients receiving hematopoietic stem cell transplantation or chimeric antigen receptor T-cell therapy.
Should I stop or delay treatment to vaccinate?
There is no evidence to support interruption of CLL treatment for vaccination. Where possible, it is preferable to vaccinate before initiating therapy; however, therapy should not be delayed in order to complete the vaccine series.
Should I measure B lymphocytes or other biomarkers for response before vaccination?
B-cell levels and/or antibody levels are not predictive of the quality and quantity of immune protection nor the capacity to develop a response to vaccination. Accordingly, Haggenburg et al45 states the following: “effective B-cell depleting therapy precludes generation of antibody responses, although B-cell numbers do not need to be normalized to generate sufficient antibody concentrations.” Currently, there are no validated biomarkers for predicting vaccine response, and decisions regarding vaccination should not be based on such variables.
Should I use antibody titers to assess immunity after COVID-19 vaccination?
Similar to the above, antibody testing is not currently recommended to assess immunity after COVID-19 vaccination (outside of a research study) – this is in line with the NCCN, and Food and Drug Administration (FDA) Safety Communication, and guidance from the CDC, and other international agencies.18,59–61
Should I avoid anti-CD20 therapy?
Vaccine response is poor for patients on any active treatment, not only anti-CD20 therapy. Patients who need treatment should not be limited to certain classes of medications based upon potential vaccine response. The best available therapy should be used for the patient, especially during an endemic state. We now have several strategies available to mitigate risk of severe COVID-19 infection, even among those patients who may have a poor vaccine response, such as pre-exposure prophylaxis with anti-SARS-CoV-2 monoclonal antibodies (tixagevimab/cilgavimab), highly effective antiviral therapies (nirmaltrelvir/ritonavir), and postexposure prophylaxis with monoclonal antibody therapy (bebtelovimab). All patients with CLL on active treatment, including with anti-CD20 therapy, should be offered prophylactic anti-SARS-CoV-2 monoclonal antibody injection to lower the risk of severe infection.
Should I stop treatment if a patient develops a COVID-19 infection?
In cases of severe COVID-19, where there is a need for hospitalization and oxygen, it should be verified whether treatment can be postponed until the patient is cleared of COVID-19 infection (as confirmed by a negative swab). In most cases of mild infection, therapy can continued.62–64 The decision to stop treatment can be based on factors such as length of therapy, response, absence of signs of progression, and minimal expected impact of treatment break.
To what extent do T cells contribute to immunity against COVID-19?
Current evidence is unclear and contradictory: most data suggest that a T-cell response may be impaired when antibodies are not produced, while some studies have indicated otherwise (Table 6). The current level of evidence is not adequate to propose any clinical guidance. In addition, routine assessment of a T-cell response is challenging without any commercially available assays, and therefore difficult to implement in regular clinical practice, and is not recommended.
Table 6.
T-cell Response to COVID-19 Vaccination in Patients With CLL
| Author | CLL N | T-cell Response (n Evaluable) | ||
|---|---|---|---|---|
| Seropositive | Seronegative | Correlation | ||
| Sun et al (2021)35 | 58 | 52.4% (21)a | 42.9% (21)a | P = 0.4b |
| Itchaki et al (2021)46 | 68 | 32% (68) | 68% (68) | P = 0.048b |
| Haydu et al (2022)41 | 36 | NA | 78% (9) | NA |
| Shen et al (2022)27 | 160 | 81% (25)c | 19% (6)d | NA |
aData missing for 1 patient.
bP value for correlation of T-cell response with antibody response.
cPatients with a normal response, albeit of variable intensity.
dPatients with a weak or negative response.
CLL = chronic lymphocytic leukemia; COVID-19 = coronavirus disease 2019; n = number of patients; NA = not available.
Could T-cell response compensate for lack of seroconversion and provide sufficient protection against COVID-19 infections?
Most studies show a correlation between IgG and T-cell responses; in the absence of an antibody response, a cellular response is less likely to occur. While efforts have been made to assess T-cell response, the data are insufficient and inconsistent. Factors such as the evolving state of the pandemic, and differing vaccine statuses/policies and treatment availability across locations have confounded efforts to fully understand this topic.
Should I use a COVID-19 monoclonal antibody for pre-exposure prophylaxis in all my patients with CLL, and is there an optimal time to utilize them?
Tixagevimab/cilgavimab is a combination of long-acting monoclonal antibodies that is indicated for pre-exposure prophylaxis under FDA EUA for immunocompromised individuals who may not mount an adequate response to vaccination. Tixagevimab/cilgavimab is currently recommended in all patients with CLL, particularly those who are on active treatment. However, as with vaccines, it will be important to follow changes over time in circulating variants that may evade protection, thereby limiting the efficacy of tixagevimab/cilgavimab. Active monitoring of symptoms remains critical to start effective antiviral therapy after exposure. Tixagevimab/cilgavimab is indicated for those individuals without active COVID-19 infection or recent exposure and, as of June 2022, the FDA EUA Fact Sheet indicates that repeat doses can be given every 6 months.65 It is not a substitute for vaccination but may provide an additional layer of protection against symptomatic infection. Special attention should be given to drug–drug interactions that can occur with available SARS-CoV-2 antiviral drugs (Table 7), for which a precautionary hold of the antileukemic treatment may be suggested while also considering the short duration of the antiviral therapy (5 days).
Table 7.
Regimens/Agents Used in the Management of COVID-19 That Interact With Therapies for CLL
| COVID-19 Treatment | Metabolism/Elimination | CLL Treatment With Potential Drug Interaction | CLL Treatment Considerations |
|---|---|---|---|
| Tocilizumab66 | Inhibition of IL-6 may lead to increased metabolism of drugs that are CYP450 substrates | Acalabrutinib, duvelisib, ibrutinib, idelalisib, venetoclax, zanubrutinib | Caution should be exercised when coadministering tocilizumab with CYP3A4 substrate drugs where decrease in effectiveness is undesirable. The effect of tocilizumab on CYP450 enzyme activity may persist for several weeks after stopping therapy |
| Remdesivir67 | In vitro, remdesivir is a substrate for drug metabolizing enzyme CYP3A4 and P-gp transporters | Acalabrutinib, duvelisib, ibrutinib, idelalisib, venetoclax, zanubrutinib | Because remdesivir is an inhibitor of CYP3A4, coadministration of remdesivir and CLL drugs may increase levels and toxicities of CLL drugs. It is not clear if remdesivir is a strong or moderate CYP3A4 inhibitor. Either interrupt dosing or reduce dose of CLL drug while remdesivir is being used; refer to USPIs of CLL drugs for dose modification recommendations |
| Nirmatrelvir plus ritonavir68 | A strong inhibitor of CYP3A; components are CYP3A substrates | Ibrutinib, venetoclax, acalabrutinib, idelalisib, zanubrutinib | Because nirmatrelvir plus ritonavir is a strong inhibitor of CYP3A, coadministration of nirmatrelvir plus ritonavir and CLL drugs (ibrutinib, venetoclax, acabrutinib, duvelisib, idelalisib, zanubrutinib) may increase levels and toxicities of CLL drugs. Avoid coadministration or interrupt dosing of CLL drug while nirmatrelvir plus ritonavir is being used |
CLL = chronic lymphocytic leukemia; COVID-19 = coronavirus disease 2019; IL = interleukin; IV = intravenous; mAb = monoclonal antibody; MoA = mechanism of action; OATP1B1 = organic anion transporting polypeptides 1B1; P-gp = P-glycoprotein; USPI = United States Prescribing Information.
Do you recommend your patients who are vaccinated to continue COVID-19 prevention measures?
Despite vaccination, patients with CLL are still at risk of COVID-19 infection and therefore should continue to use standard infection prevention measures such as masks, social distancing, and hand hygiene.
DISCUSSION
The pandemic has been a challenge to navigate for patients living with CLL and their healthcare providers. Since the start of the vaccination program, numerous studies have been conducted to look at serologic response and, less frequently, T-cell-mediated response to COVID-19 vaccination in the CLL population, the data from which can now be analyzed to provide guidance around treatment and vaccination.
Despite having the lowest vaccination response relative to other malignancies in the majority of studies included, seropositivity rates in patients with CLL are shown to increase with each vaccination, and in some cases even when a response had not been mounted prior.32,41,42 This may be due to the timing of the third dose post-treatment; however, it indicates that additional vaccinations are warranted regardless of initial response. The CDC recommends an updated COVID-19 vaccine booster to help restore protection that has decreased since the last vaccine administration and that may provide improved protection against newer variants. Updated boosters, also known as bivalent boosters, target the most recent Omicron subvariants, in addition to the original SARS-CoV-2.60
One of the most common independent predictors of poor vaccine response was being on active CLL therapy, with response rates as low as 14% across all studies.37 Regarding the impact of specific treatments on vaccine response, the findings of this review indicate that vaccine response is poor regardless of the treatment given, with responses ranging from 14.3% to 55% (median 28%) for BTKi monotherapy27,35 (11 of 14 studies with response rate <36%) and 0% to 41% (median 17%) for anti-CD20 therapies given with or without venetoclax.31,32,35 The wide range of responses seen may be reflective of the varied time points at which serology was evaluated postvaccination. Greater responses were seen in patients who were vaccinated more than 12 months post-anti-CD20 treatment.36,37,40,42 Nevertheless, serological responses have been reported in patients being vaccinated within 12 months of treatment; therefore, patients should still be offered vaccination before the 12-month mark.8,25,27,36 In addition, vaccination response was shown to wane over time (eg, 33% loss of vaccine response at 4 months36), which may explain the lower responses observed through continuous BTKi therapy.33,39,42 According to emerging data, treatment given post-vaccination, if feasible, does not appear to impact the persistence of vaccine response nor does the rate of decay differ between patients on treatment and healthy controls.
There is a question as to whether cellular response is still present in the absence of antibody response and whether serology to evaluate humoral response is the best measure of viral protection. Cellular response was seen in the absence of antibody response, with 2 studies showing no association with antibody response35,41; however, 1 study did show a correlation,46 therefore the data are conflicting. A small retrospective cohort study of patients treated with B-cell-targeting therapies (of whom 47% had CLL) compared with healthy participants also found no trends between antibody and cellular responses, and the probability of developing a cell-mediated immune response negatively correlated with disease burden, mainly in patients with CLL.69 A more recent cohort study of patients with CLL found that cellular responses after the second and third vaccine doses were comparable with those in healthy participants, but also noted that cellular response was markedly higher in patients who had received a heterologous vaccine series.70 Therefore, cellular responses to vaccination may play a role in preventing severe disease but may vary between vaccine types/series.
There are also limited-to-no data on breakthrough infection and survival outcomes in patients who achieve a cellular versus antibody response. Further research is needed in this area. Real-world evidence from the National COVID Cohort Collaborative showed that patients with solid tumors and hematologic malignancies had significantly higher risk for breakthrough infections and severe outcomes compared with non-cancer patients. Compared with solid tumors, hematologic malignancies were associated with an increased risk for breakthrough infections, with the highest observed for lymphoid leukemia. Unexpectedly, however, patients with lymphoid leukemia were at decreased risk of severe COVID outcomes versus those with solid tumors.71
In addition to being on active CLL therapy, there were other common independent factors identified for poor vaccine response, most notably hypogammaglobulinemia and age >65–70 years.32 These may be additional factors to consider if there is a need to prioritize recipients of COVID-19 prophylaxis, alongside standard infection prevention measures. In contrast, patients who were treatment-naive, currently off therapy, in remission, or who had a prior COVID-19 infection had the greatest responses, with the latter being similar to that of healthy controls.35,69 As many patients are able to achieve remission with B-cell directed therapies, and with the recent availability of COVID-19 monoclonal antibodies for pre-exposure prophylaxis that could be used for patients at risk for poor vaccine response, antileukemic treatment should not be delayed in patients with CLL.
Limitations
Different vaccines were used in the studies eg, BNT162b2 (Pfizer), mRNA-1273 (Moderna), Ad26.COV2.S (J&J), and vaccine type was shown to be a predictor of vaccine response in 3 studies.28,32,41 Although in most studies, the time between the 2 doses of vaccination was 3 weeks, in 1 study in particular, the majority of patients were on an extended vaccine series with 10–12 weeks between doses, which the authors implied may enhance seropositivity.36 The studies evaluated serology at various time points post-vaccination, ranging from 14 to 139 days, which may impact results, as antibody responses may wane over time.33,36,39,42 Many different testing methods/assays were utilized in the studies, with various thresholds for adequate response depending on the type of test used. There were also varying time points from last treatment to final vaccination dose, which may impact response.
SUMMARY OF FINDINGS
All patients with CLL should be offered vaccination, regardless of treatment type or status. There is no evidence for CLL treatment to be interrupted for vaccination; however, when possible, it is preferable to vaccinate prior to CLL treatment initiation.
Currently, there are no validated biomarkers for predicting vaccine response, and antibody testing is not recommended to assess immunity after COVID-19 vaccination (outside of a research study).
All patients on active therapy are at risk of having a poor response to vaccination regardless of therapy type; the best available CLL therapy should be given to the patient, with anti-SARS-CoV-2 monoclonal antibody pre-exposure prophylaxis administered to reduce the risk of severe COVID-19 infection.
Pre-exposure prophylaxis with SARS-CoV-2 monoclonal antibody injection (tixagevimab/cilgavimab) is recommended in all patients with CLL, particularly those who are on active treatment. It is not a substitute for vaccination or standard infection prevention measures (mask wearing, social distancing, hand washing).
Targeted therapies (eg, BTKi) are not routinely interrupted when a patient presents with mild COVID-19 symptoms. In cases of severe COVID-19, where there is a need for hospitalization and oxygen, it should be evaluated whether treatment can be postponed until after the patient’s infection has resolved.
AUTHOR CONTRIBUTIONS
All authors were involved in interpreting the data and writing the manuscript. All authors approved the final version for submission.
DISCLOSURES
MS reports consulting and advisory boards, and is a part of steering committees, or data safety monitoring committees for AbbVie, Genentech, Inc., AstraZeneca, Sound Biologics, Pharmacyclics, BeiGene, Bristol Myers Squibb, Morphosys/Incyte, TG Therapeutics, Innate Pharma, Kite Pharma, Adaptive Biotechnologies, Epizyme, Eli Lilly, Adaptimmune, Mustang Bio, Regeneron, Merck, Fate Therapeutics, MEI Pharma, and Atara Biotherapeutics; reports research funding from Mustang Bio, Celgene, Bristol Myers Squibb, Pharmacyclics, Gilead, Genentech, Inc., AbbVie, TG Therapeutics, BeiGene, AstraZeneca, Sunesis, Atara Biotherapeutics, Genmab, and Morphosys/Incyte. KE, HJH, JMLB are employed by Genentech, Inc./F. Hoffmann-La Roche Ltd, and have equity in F. Hoffmann-La Roche Ltd. PG reports consulting, board of directors, advisory committee and has received honoraria from AbbVie, AstraZeneca, BeiGene, Bristol Myers Squibb, Lilly/Loxo, Janssen, MSD, F. Hoffmann-La Roche Ltd; reports research funding from AbbVie, AstraZeneca, Janssen; and is an editor for HemaSphere. ARM served as a consultant advisory board/steering committee for AbbVie, AstraZeneca, BeiGene, Bristol Myers Squibb, Celgene, Genentech, Laboratorios Pfizer Ltda., LOXO Oncology, Octopharma, Pharmacyclics LLC, NURIX, GENMAB, TG Therapeutics; is a Data Safety Monitoring Board participant for Celgene, TG Therapeutics; a member of a Medical Advisory Board for CLL Society; a CME speaker for Curio; a consultant for DAVA, Medscape; and an ad hoc scientific advisor for the Lymphoma Research Foundation. CL has no conflicts of interest to disclose.
SOURCES OF FUNDING
Genentech Inc. provided financial support for this comprehensive literature review, for the third-party medical writing and editorial assistance that, under the direction of the authors, was provided by Sinéad Holland, PhD, of Ashfield MedComms, an Inizio company, and was funded by Genentech Inc.
Supplementary Material
Footnotes
MS and CL are co-first authors. PG and ARM are co-last and corresponding authors.
Supplemental digital content is available for this article.
REFERENCES
- 1.World Health Organization. WHO Coronavirus (COVID-19) Dashboard. Available at: https://covid19.who.int/. Accessed March 23, 2022.
- 2.Yun NK, Chebrolu P, Yarnold PR, et al. Four-fold increased mortality from sars-Cov-2 infection in patients with hematologic versus non-hematologic malignancies treated at the largest tertiary COVID-19 center in Chicago/Rush University Medical Center (March 1, 2020-December 31,2020). Blood. 2021;138(suppl 1):1947–1947. [Google Scholar]
- 3.Vijenthira A, Gong IY, Fox TA, et al. Outcomes of patients with hematologic malignancies and COVID-19: a systematic review and meta-analysis of 3377 patients. Blood. 2020;136:2881–2892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mato AR, Roeker LE, Lamanna N, et al. Outcomes of COVID-19 in patients with CLL: a multicenter international experience. Blood. 2020;136:1134–1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scarfò L, Chatzikonstantinou T, Rigolin GM, et al. COVID-19 severity and mortality in patients with chronic lymphocytic leukemia: a joint study by ERIC, the European Research Initiative on CLL, and CLL Campus. Leukemia. 2020;34:2354–2363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chatzikonstantinou T, Kapetanakis A, Scarfò L, et al. COVID-19 severity and mortality in patients with CLL: an update of the international ERIC and Campus CLL study. Leukemia. 2021;35:3444–3454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Puła B, Pruszczyk K, Pietrusza E, et al. Outcome of SARS-CoV-2-infected polish patients with Chronic Lymphocytic Leukemia. Cancers. 2022;14:558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roeker LE, Eyre TA, Thompson MC, et al. COVID-19 in patients with CLL: improved survival outcomes and update on management strategies. Blood. 2021;138:1768–1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kochneva ОL, Kislova M, Zhelnova EI, et al. COVID-19 in patients with chronic lymphocytic leukemia: a Moscow observational study. Leuk Lymphoma. 2022;63:1607–1616. [DOI] [PubMed] [Google Scholar]
- 10.Aumann S, Tsubary U, Israel S, et al. COVID-19 among patients with hematological malignancies: experience from a tertiary center showing lower than expected mortality and establishing the safety of in-hospital patient care during the pandemic. Blood. 2021;138(suppl 1):4088–4088. [Google Scholar]
- 11.Langerbeins P, Eichhorst B. Immune dysfunction in patients with chronic lymphocytic leukemia and challenges during COVID-19 pandemic. Acta Haematol. 2021;144:508–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hartkamp A, Mulder AH, Rijkers GT, et al. Antibody responses to pneumococcal and haemophilus vaccinations in patients with B-cell chronic lymphocytic leukaemia. Vaccine. 2001;19:1671–1677. [DOI] [PubMed] [Google Scholar]
- 13.Sinisalo M, Aittoniemi J, Oivanen P, et al. Response to vaccination against different types of antigens in patients with chronic lymphocytic leukaemia. Br J Haematol. 2001;114:107–110. [DOI] [PubMed] [Google Scholar]
- 14.Forconi F, Moss P. Perturbation of the normal immune system in patients with CLL. Blood. 2015;126:573–581. [DOI] [PubMed] [Google Scholar]
- 15.van der Velden AM, Mulder AH, Hartkamp A, et al. Influenza virus vaccination and booster in B-cell chronic lymphocytic leukaemia patients. Eur J Intern Med. 2001;12:420–424. [DOI] [PubMed] [Google Scholar]
- 16.Shadman M, Ujjani C. Vaccinations in CLL: implications for COVID-19. Blood. 2021;137:144–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.CDC. Interim guidelines for COVID-19 antibody testing. 2022. Available at: https://www.cdc.gov/coronavirus/2019-ncov/lab/resources/antibody-tests-guidelines.html. Accessed May 27, 2022.
- 18.NCCN: Cancer and COVID-19 Vaccination. Recommendations of the National Comprehensive Cancer Network® (NCCN®) Advisory Committee on COVID-19 Vaccination and Pre-exposure Prophylaxis*. 2022. Available at: https://www.nccn.org/docs/default-source/covid-19/2021_covid-19_vaccination_guidance_v5-0.pdf?sfvrsn=b483da2b_114. Accessed April 28, 2022.
- 19.Buske C, Dreyling M, Alvarez-Larrán A, et al. Managing hematological cancer patients during the COVID-19 pandemic: an ESMO-EHA interdisciplinary expert consensus. ESMO Open. 2022;7:100403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gagelmann N, Passamonti F, Wolschke C, et al. Antibody response after vaccination against SARS-CoV-2 in adults with haematological malignancies: a systematic review and meta-analysis. Haematologica. 2021;107:1840–1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Teh JSK, Coussement J, Neoh ZCF, et al. Immunogenicity of COVID-19 vaccines in patients with hematological malignancy: a systematic review and meta-analysis. Blood Adv. 2021;6:2014–2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gong I, Vijenthira A, Betschel S, et al. COVID-19 vaccine response in patients with hematologic malignancy: a systematic review and meta-analysis. Blood. 2021;138(suppl 1):4113–4113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ujjani C, Shadman M, Lynch RC, et al. The impact of B-cell-directed therapy on SARS-CoV-2 vaccine efficacy in chronic lymphocytic leukaemia. Br J Haematol. 2022;197:306–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Šušol O, Hájková B, Zelená H, et al. Third dose of COVID-19 vaccine restores immune response in patients with haematological malignancies after loss of protective antibody titres. Br J Haematol. 2022;197:302–305. [DOI] [PubMed] [Google Scholar]
- 25.Giuliano AR, Lancet JE, Pilon-Thomas S, et al. Evaluation of antibody response to SARS-CoV-2 mRNA-1273 vaccination in patients with cancer in Florida. JAMA Oncol. 2022;8:748–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gong IY, Vijenthira A, Betschel SD, et al. COVID-19 vaccine response in patients with hematologic malignancy: a systematic review and meta-analysis. Am J Hematol. 2022;97:E132–E135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shen Y, Freeman JA, Holland J, et al. COVID-19 vaccine failure in chronic lymphocytic leukaemia and monoclonal B-lymphocytosis; humoural and cellular immunity. Br J Haematol. 2021;197:41–51. [DOI] [PubMed] [Google Scholar]
- 28.Greenberger LM, Saltzman LA, Senefeld JW, et al. Antibody response to SARS-CoV-2 vaccines in patients with hematologic malignancies. Cancer Cell. 2021;39:1031–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Herzog Tzarfati K, Gutwein O, Apel A, et al. BNT162b2 COVID-19 vaccine is significantly less effective in patients with hematologic malignancies. Am J Hematol. 2021;96:1195–1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dong N, Jain AG, Tan ES, et al. Immunogenicity of SARS-CoV-2 mRNA 1273 vaccine in patients with lymphoid malignancies. Blood. 2021;138:2504–2504. [Google Scholar]
- 31.Molica S, Giannarelli D, Montserrat E. mRNA COVID-19 vaccines in patients with chronic lymphocytic leukemia: a systematic review and meta-analysis. Eur J Haematol. 2022;108:264–267. [DOI] [PubMed] [Google Scholar]
- 32.Bagacean C, Letestu R, Al-Nawakil C, et al. Humoral response to mRNA anti–COVID-19 vaccines BNT162b2 and mRNA-1273 in patients with chronic lymphocytic leukemia. Blood Adv. 2022;6:207–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Molica S, Giannarelli D, Lentini M, et al. Efficacy of the BNT162b2 mRNA COVID-19 vaccine in patients with chronic lymphocytic leukemia: a serologic and cellular study. Chemotherapy. 2021;67:91–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bergman P, Blennow O, Hansson L, et al. Safety and efficacy of the mRNA BNT162b2 vaccine against SARS-CoV-2 in five groups of immunocompromised patients and healthy controls in a prospective open-label clinical trial. EBioMedicine. 2021;74:103705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sun C, Gaglione EM, Vaughn LT, et al. Humoral and cellular immunogenicity of COVID-19 vaccinations in patients with CLL. Blood. 2021;138(suppl 1):3724–3724. [Google Scholar]
- 36.Parry H, McIlroy G, Bruton R, et al. Impaired neutralisation of SARS-CoV-2 delta variant in vaccinated patients with B cell chronic lymphocytic leukaemia. J Hematol Oncol. 2022;15:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Benjamini O, Rokach L, Itchaki G, et al. Safety and efficacy of the BNT162b mRNA COVID-19 vaccine in patients with chronic lymphocytic leukemia. Haematologica. 2022;107:625–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Roeker LE, Knorr DA, Thompson MC, et al. COVID-19 vaccine efficacy in patients with chronic lymphocytic leukemia. Leukemia. 2021;35:2703–2705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tadmor T, Benjamini O, Braester A, et al. Antibody persistence 100 days following the second dose of BNT162b mRNA Covid19 vaccine in patients with chronic lymphocytic leukemia. Leukemia. 2021;35:2727–2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Herishanu Y, Avivi I, Aharon A, et al. Efficacy of the BNT162b2 mRNA COVID-19 vaccine in patients with chronic lymphocytic leukemia. Blood. 2021;137:3165–3173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Haydu JE, Maron JS, Redd RA, et al. Humoral and cellular immunogenicity of SARS-CoV-2 vaccines in chronic lymphocytic leukemia: a prospective cohort study. Blood Adv. 2022;6:1671–1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Herishanu Y, Rahav G, Levi S, et al. Efficacy of a third BNT162b2 mRNA COVID-19 vaccine dose in patients with CLL who failed standard 2-dose vaccination. Blood. 2022;139:678–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Greenberger LM, Saltzman LA, Senefeld JW, et al. Anti-spike antibody response to SARS-CoV-2 booster vaccination in patients with B cell-derived hematologic malignancies. Cancer Cell. 2021;39:1297–1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Del Poeta G, Bomben R, Polesel J, et al. COVID-19 vaccination: evaluation of risk for protection failure in chronic lymphocytic leukemia patients. Hematol Oncol. 2021;39:712–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Haggenburg S, Lissenberg-Witte BI, van Binnendijk RS, et al. Quantitative analysis of mRNA-1273 COVID-19 vaccination response in immunocompromised adult hematology patients. Blood Adv. 2022;6:1537–1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Itchaki G, Rokach L, Benjamini O, et al. Cellular immune responses to BNT162b2 mRNA COVID-19 vaccine in patients with chronic lymphocytic leukemia. Blood. 2021;138:638–638. [Google Scholar]
- 47.Herishanu Y, Avivi I, Levi S, et al. Six-month antibody persistence after BNT162b2 mRNA COVID-19 vaccination in patients with chronic lymphocytic leukemia. Blood Adv. 2022;6:148–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shree T, Shankar V, Lohmeyer JJK, et al. CD20-targeted therapy ablates De Novo antibody response to vaccination but spares preestablished immunity. Blood Cancer Discov. 2022;3:95–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang L, Kaelber DC, Xu R, et al. COVID-19 breakthrough infections, hospitalizations and mortality in fully vaccinated patients with hematologic malignancies: a clarion call for maintaining mitigation and ramping-up research. Blood Rev. 2022;54:100931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ollila TA, Masel R, Reagan JL, et al. Factors associated with seroconversion after COVID-19 vaccination in patients with hematologic malignancies. Blood. 2021;138:3511–3511. [Google Scholar]
- 51.Bajema KL, Dahl RM, Evener SL, et al. Comparative effectiveness and antibody responses to moderna and Pfizer-BioNTech COVID-19 vaccines among hospitalized veterans - five veterans affairs medical centers, United States, February 1-September 30, 2021. MMWR Morb Mortal Wkly Rep. 2021;70:1700–1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.FDA. Coronavirus (COVID-19) Update: FDA Authorizes Second Booster Dose of Two COVID-19 Vaccines for Older and Immunocompromised Individuals. 2022. Available at: https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-second-booster-dose-two-covid-19-vaccines-older-and. Accessed May 27, 2022.
- 53.Atmar RL, Lyke KE, Deming ME, et al. Heterologous SARS-CoV-2 booster vaccinations - preliminary report. medRxiv. 2021:2021.2010.2010.21264827. [Google Scholar]
- 54.Groß R, Zanoni M, Seidel A, et al. Heterologous ChAdOx1 nCoV-19 and BNT162b2 prime-boost vaccination elicits potent neutralizing antibody responses and T cell reactivity against prevalent SARS-CoV-2 variants. EBioMedicine. 2022;75:103761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stuart ASV, Shaw RH, Liu X, et al. Immunogenicity, safety, and reactogenicity of heterologous COVID-19 primary vaccination incorporating mRNA, viral-vector, and protein-adjuvant vaccines in the UK (Com-COV2): a single-blind, randomised, phase 2, non-inferiority trial. Lancet (London, England). 2022;399:36–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liu X, Shaw RH, Stuart ASV, et al. Safety and immunogenicity of heterologous versus homologous prime-boost schedules with an adenoviral vectored and mRNA COVID-19 vaccine (Com-COV): a single-blind, randomised, non-inferiority trial. Lancet (London, England). 2021;398:856–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Costa Clemens SA, Weckx L, Clemens R, et al. Heterologous versus homologous COVID-19 booster vaccination in previous recipients of two doses of CoronaVac COVID-19 vaccine in Brazil (RHH-001): a phase 4, non-inferiority, single blind, randomised study. Lancet (London, England). 2022;399:521–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Reimann P, Ulmer H, Mutschlechner B, et al. Efficacy and safety of heterologous booster vaccination with Ad26.COV2.S after BNT162b2 mRNA COVID-19 vaccine in haemato-oncological patients with no antibody response. Br J Haematol. 2022;196:577–584. [DOI] [PubMed] [Google Scholar]
- 59.FDA. Antibody testing is not currently recommended to assess immunity after COVID-19 vaccination: FDA safety communication. 2022. Available at: https://www.fda.gov/medical-devices/safety-communications/antibody-testing-not-currently-recommended-assess-immunity-after-covid-19-vaccination-fda-safety#:~:text=Safety%20Communications-,Antibody%20Testing%20Is%20Not%20Currently%20Recommended%20to%20Assess%20Immunity%20After,19%20Vaccination%3A%20FDA%20Safety%20Communication&text=For%20the%20most%2Dup%2Dto,Information%20for%20Patients%20and%20Consumers. Accessed May 27, 2022.
- 60.CDC. COVID-19 vaccines for people who are moderately or severely immunocompromised. 2022. Available at: https://www.cdc.gov/coronavirus/2019-ncov/vaccines/recommendations/immuno.html?s_cid=10483:immunocompromised%20and%20covid%20vaccine:sem.ga:p:RG:GM:gen:PTN:FY21. Accessed May 27, 2022.
- 61.European Centre for Disease Prevention and Control. Considerations for the use of antibody tests for SARS-CoV-2 - first update. 2022. Available at: https://www.ecdc.europa.eu/en/publications-data/use-antibody-tests-sars-cov-2. Accessed May 27, 2022.
- 62.Food and Drug Administration US. CALQUENCE® (acalabrutinib) highlights of prescribing information. 2022. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/210259s009lbl.pdf. Accessed September 2, 2022.
- 63.Food and Drug Administration US. VENCLEXTA® (venetoclax) highlights of prescribing information. 2022. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/208573s027lbl.pdf. Accessed September 2, 2022.
- 64.Food and Drug Administration US. IMBRUVICA® (ibrutinib) highlights of prescribing information. 2022. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/205552s035,215063s011lbl.pdf. Accessed September 2, 2022.
- 65.Food and Drug Administration US. EVUSHELD® (tixagevimab co-packaged with cilgavimab) highlights of emergency use authorization. 2022. Available at: https://www.fda.gov/media/154701/download. Accessed September 15, 2022.
- 66.Genentech Inc. ACTEMRA® (tocilizumab) highlights of emergency use authorization. 2021. Available at: https://www.gene.com/download/pdf/actemra_eua_hcp_fact_sheet.pdf. Accessed September 8, 2022.
- 67.Gilead Sciences Inc. VEKLURY® (remdesivir) highlights of prescribing information. 2022. Available at: https://www.gilead.com/-/media/files/pdfs/medicines/covid-19/veklury/veklury_pi.pdf. Accessed September 8, 2022.
- 68.Food and Drug Administration US. PAXLOVID® (nirmatrelvir tablets; ritonavir tablets) highlights of emergency use authorization. 2021. Available at: https://www.fda.gov/media/155050/download. Accessed September 8, 2022.
- 69.Bacova B, Kohutova Z, Zubata I, et al. Cellular and humoral immune response to SARS-CoV-2 mRNA vaccines in patients treated with either Ibrutinib or Rituximab. Clin Exp Med. 2022:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Parry H, Bruton R, Roberts T, et al. COVID-19 vaccines elicit strong cellular immunity and robust clinical protection in CLL. Cancer Cell. 2022;40:584–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Song Q, Bates B, Shao YR, et al. Risk and outcome of breakthrough COVID-19 infections in vaccinated patients with cancer: real-world evidence from the National COVID Cohort Collaborative. J Clin Oncol. 2022;40:1414–1427. [DOI] [PMC free article] [PubMed] [Google Scholar]