Abstract
For a long time, the morbidity and mortality rates of hepatocellular carcinoma (HCC) have remained high. Since the concept of ferroptosis was introduced in 2012, researchers’ perspectives have shifted toward finding novel ferroptosis-related treatment strategies, especially for tumors that are resistant to apoptosis. In recent years, there have been an increasing number of studies on ferroptosis, and these studies have found that ferroptosis has great potential and promise for cancer treatment. Ferroptosis is a kind of regulated cell death (RCD); unlike apoptosis, ferroptosis is an iron-dependent type of RCD driven by lipid peroxidation. The whole process of ferroptosis mainly revolves around three pathways (system xc−/ glutathione peroxidase 4 [GPX4]), lipid peroxidation, and iron metabolism), which are also regulated by various metabolic factors. This review will attempt to analyze the relationship between the system xc−/GPX4 pathway, lipid peroxidation, iron metabolism, and ferroptosis from three aspects (triggering, execution, and regulation), and the regulatory factors for ferroptosis will be summarized. In this review, we will also illustrate the relationship between ferroptosis and tumors as well as its application in tumors from the perspective of HCC. Finally, we will summarize the current limitations and needs and provide perspectives related to the focus of development in the future.
Keywords: Ferroptosis, Hepatocellular carcinoma, Molecular targets
Introduction
Primary liver cancer is the sixth most commonly diagnosed cancer and ranks third in terms of mortality rate; hepatocellular carcinoma (HCC) accounts for 75% of primary liver cancers.[1,2] In the long-term fight against cancer, we are committed to exploring strategies for the specific elimination of cancer cells while retaining normal cells. To date, most clinical anticancer drugs cause cancer cell apoptosis. Apoptosis is a type of programmed cell death, which is a constitutive suicide process cells undergo that can be regulated by various internal or external signals.[3] However, apoptosis resistance is a general feature of cancer that forces us to look for new opportunities to exploit regulated cell death (RCD), which is a broader concept.[4] RCD refers to cell death that is regulated by molecules or signals but can develop without going through a specific procedure.[5] Ferroptosis is an iron-dependent type of RCD driven by lipid peroxidation. Ferroptosis is distinct from apoptosis, necrosis, and autophagy in terms of morphological changes, biochemical characteristics and genetic standards.[6] With the increase in research on ferroptosis, there is an increasing evidence that ferroptosis may play a physiological role in the occurrence and development of tumors, which is particularly important, especially in the era of drug resistance. In this paper, we first introduced the factors mediating the triggering, execution and regulation of ferroptosis at the molecular level and then introduced the relationship of ferroptosis with HCC, including its application in HCC. Finally, we discussed the prospects regarding the development of ferroptosis.
Molecular Mechanism of Ferroptosis
The concept of ferroptosis was described by Dixon et al[7] in 2012. However, Yang and Stockwell[8] described the phenomenon of ferroptotic cell death for the first time in a high-throughout screening study to identify compounds that can be selectively lethal to cells carrying ras sarcoma (RAS) mutant subtypes as early as 2008. Dixon et al[7] found that erastin can induce a regulated but nonapoptotic form of cell death, which is accomplished by phospholipid peroxidation, and this process depends on the reactive oxygen species (ROS), polyunsaturated fatty acids (PUFAs), and iron metabolic products. Glutathione peroxidase 4 (GPX4) and system xc− are the two most important pathways and are involved in nearly the entire mechanism of triggering, executing, and regulating ferroptosis; thus, they have a direct impact on this process. Iron and lipid peroxidation also play vital roles in ferroptosis. The regulatory factors of ferroptosis, such as ferroptosis suppressor protein 1 (FSP1) and nuclear factor erythroid 2-related factor 2 (NRF2), were also briefly summarized in this article.
System xc− and GPX4
System xc− is an amino acid antiporter that belongs to the heteromeric amino acid transporter (HAT) family, whose function is to mediate the exchange of extracellular L-cystine and intracellular L-glutamate across the cellular plasma membrane.[9] It is a heterodimer formed by the 12-pass transmembrane transporter protein solute carrier family 7 member 11 (SLC7A11) and the single-pass transmembrane regulatory protein solute carrier family 3 member 2 (SLC3A2) with a disulfide bond as a link.[10] It is necessary to discuss GPX4 before introducing the role of system xc−. GPX4 is a restricted selenoprotein that is important for the survival of mammalian cells and can catalyze the reduction of lipid peroxides (LPOs) in a complex cell membrane environment.[11,12] It can restrain the activity of lipoxygenase (LOX) and resist lipid peroxidation by converting phospholipid hydroperoxide (PLOOH) to the corresponding alcohol phospholipid hydroxide.[13] A number of studies have shown that the inhibition of GPX4 can increase lipid peroxidation and eventually lead to ferroptosis, which also illustrates the source of LPOs in normal cells.[14–16] Research has also shown that GPX4 is the central regulatory factor of ferroptosis and one of the most fatal signals of ferroptosis. Notably, to play a corresponding role in the physiological state, glutathione (GSH) acts as the basic auxiliary factor for GPX4, and cysteine is a precursor of GSH synthesis.[12,17] Simultaneously, system xc− can regulate the level of intracellular cysteine directly, thus controlling the occurrence of ferroptosis by affecting the activity of GPX4. In addition, research has shown that SLC7A11 is regulated by multiple genes at the same time; for example, NRF2 can promote its expression and activation,[18] while some tumor suppressor genes, such as p53, breast cancer susceptibility gene 1 associated protein 1 (BAP1), and beclin 1 (BECN1), play a negative regulatory role.[19–21] System xc− and GPX4 expression can be restrained by erastin and RSL3, respectively.[14,22]
Lipid peroxidation
Previous studies have revealed that the association between cellular metabolism and ferroptosis is particularly complex.[23–26] It has been confirmed that ferroptosis is driven by lipid peroxidation.[7] Lipid peroxidation occurs via a sequential process (initiation, propagation, and termination), and these three stages are closely linked.[27,28] However, when GPX4 cannot eliminate redundant LPOs for some reasons, such as the inhibition of RSL3, LPOs will be converted to an alkoxyl radical (LO·) under the premise of the action of ferrous iron, and then LO· reacts with PUFAs, ultimately entering into another lipid radical chain reaction.[27] Consequently, the lipid peroxidation process cannot enter the final termination phase, which promotes the accumulation of LPOs, such as malondialdehyde (MDA), eventually leading to toxicity or the initiation of cell death cascades.[27,29] LOXs are nonheme, iron-containing enzymatic protein effectors, whose primary substrates are PUFAs. They play an important role in lipid peroxidation in ferroptosis, and LOX knockout can resist ferroptosis mediated by erastin.[30,31] In addition to the reported long-chain fatty acid–coenzyme A (CoA) ligase 4 (ACSL4)/lysophospholipid–acyltransferase 3 (LPCAT3)/15-LOX pathway involved in the production of LPO during ferroptosis,[15,32] a recent study found that the p53/SLC7A11/12-LOX pathway was also related to the production of LPO.[29] Although we understand the metabolic process of lipid peroxidation in ferroptosis, it is not clear how to induce cytotoxicity via lipid peroxidation or which mechanism regulates cell death in this context. Lipid peroxidation in the course of ferroptosis is a multicascade process that is organized by the balance between the oxidation system and the antioxidant system, lipid scavenging mechanisms, autophagy, and membrane repair mechanisms.[33] In addition, since GPX4 also plays a crucial role in other RCD processes,[34–36] it suggests that the “intersection” position of lipid peroxidation in RCD is reasonable.[33]
Iron metabolism
The accumulation of intracellular iron due to various conditions, such as increased iron absorption, enhanced release or limited intracellular iron outflow, is capable of promoting the occurrence of ferroptosis through comprehensive pathways.[37] Why is this process iron-dependent? First, the Fenton reaction, which is a non-enzymatic reaction chain, is an indispensable step for the occurrence of ferroptosis: the Fenton reaction can enhance PLOOH production, which is a landmark event of ferroptosis.[38] In addition, iron is an essential component of some enzymes involved in ferroptosis; for example, phospholipid peroxidation metabolism-related LOX and Cytochrome P450 Reductase (POR) need iron to support catalysis, and iron is also vital for a large number of metabolic enzymes involved in the production of ROS in cells.[16] Therefore, metabolic regulators related to iron metabolism, including iron uptake, storage, transport, or degradation, may affect ferroptosis. Among them, the binding of iron regulatory proteins (IRPs) to the iron response element (IRE) in mRNA is the primary pathway regulating the transport, storage, and release of iron,[39,40] and IRE-binding protein 2 (IRE-BP2) is considered to be a very important factor in inducing ferroptosis.[41] Transferrin (TF) can bind to iron in the blood and transport it to various tissues and organs. Research by Gao et al[23] has shown that TF is necessary for inducing ferroptosis, which is also a crucial factor for ferroptosis induced by amino acid starvation. Transferrin receptor (TFR) is a membrane protein that is responsible for the cellular uptake of iron-loaded TF and plays a vital role in mediating ferroptosis.[41] Oncogenic RAS can enrich the iron pool of cells by promoting the function of TFR, thus increasing the sensitivity of cells to ferroptosis induced by erastin.[8] In addition, a study has shown that the accumulation of TFR exists only in the process of ferroptosis, not apoptosis.[42] The degradation of ferritin can be accomplished by selective autophagy mediated by nuclear receptor coactivator 4 (NCOA4), and the knockout of NCOA4 can inhibit ferroptosis induced by erastin in fibroblasts and pancreatic cancer cells by blocking ferritin degradation.[43] In addition, other proteins or regulators related to iron metabolism, such as heme oxygenase 1 (HO1) and prominin-2 (PROM2), may affect the process of ferroptosis.[44–46]
Regulatory Factors of Ferroptosis
At present, the known regulatory signaling pathways related to ferroptosis are very complex, and there are also many regulatory factors. In recent years, FSP1 has become a research hotspot. FSP1, formerly known as apoptosis inducing factor mitochondria associated 2 (AIFM2), was once thought to be a proapoptotic protein[47] Doll et al[48] found for the first time that the inhibitory effect of FSP1 on ferroptosis was not affected by intracellular glutathione, the level or activity of GPX4, long-chain fatty acyl-CoA ligase 4 (ACSL4) expression, or other factors, which shows the particularity of the inhibition mechanism of FSP1 on ferroptosis. Their research showed that this kind of mechanism was mediated by coenzyme Q10 (CoQ10), and FSP1-CoQ10-nicotinamide adenine dinucleotide (NAD[P]H) existed as an independent parallel pathway, which cooperated with the GPX4 pathway and glutathione to restrain phospholipid peroxidation and ferroptosis.[48] Moreover, Bersuker et al[49] found that the expression of FSP1 was positively correlated with ferroptosis resistance in hundreds of cancer cell lines, and they also confirmed that FSP1 is a key component of a non-mitochondrial CoQ10 antioxidant system and works in parallel with the GPX4 pathway. Therefore, FSP1 is a promising candidate for targeting ferroptosis.[48–50] Regulatory factors of ferroptosis are listed in [Table 1].
Table 1.
Regulatory factors of ferroptosis.
| Regulatory factors | Possible pathways | References |
| NRF2, p53, BAP1, BECN1, erastin, RSL3 | System xc− –GPX4 | [14,18,20–22] |
| LOXs, ACSL4, NRF2 | Lipid peroxidation | [30–32,65] |
| IRE-BP2, TF, TFR, NCOA4, HO1, PROM2, NRF2 | Iron metabolism | [8,23,41,43–46,65] |
| FSP1 | FSP1-CoQ10-NAD(P)H | [48,49] |
ACSL4: Long-chain fatty acid–coenzyme A (CoA) ligase 4; BAP1: Breast cancer susceptibility gene 1 (BRCA1) associated protein 1; BECN1: Beclin 1; CoQ10: Coenzyme Q10; FSP1: Ferroptosis suppressor protein 1; GPX4: glutathione peroxidase 4; HO1: Heme oxygenase 1; IRE-BP2: Iron responsive element binding protein 2; LOXs: Lipoxygenases; NAD(P)H: Nicotinamide adenine dinucleotide; NCOA4: Nuclear receptor coactivator 4; NQO1: Quinone oxidoreductase 1; NRF2: Nuclear factor erythroid 2-related factor; PROM2: Prominin-2; RSL3: RAS-selective lethal 3; TF: Transferrin; TFR: Transferrin receptor.
In general, ferroptosis mainly involves three types of pathways: the system xc−–GPX4 pathway, the lipid peroxidation pathway and the iron metabolism pathway. Most other regulatory signals can affect ferroptosis by targeting these three pathways. Strikingly, although we have made great progress in the study of the triggering mechanism of ferroptosis, it is still unclear what kind of mechanism mediates cell death after ferroptosis. Is the cytotoxicity of lipids or the accumulation of ROS dominant? Does it lead to the loss of the integrity of the cell membrane or mitochondrial membrane? Or does it lead to the destruction of some cellular key macromolecules? In our opinion, exploration of the execution mechanism and process of ferroptosis is needed and will provide great contributions to the clinical translation and application of ferroptosis. Next, we discussed the link between ferroptosis and HCC and its application.
Molecular Targets of Ferroptosis in HCC
Currently, research on ferroptosis in the field of cancer has attracted an increasing attention, and one of the main directions is exploring the intricate and close relationship between cancer and ferroptosis. Many studies have shown that various tumor suppressors can regulate the sensitivity of cancer cells to ferroptosis. For example, p53 can enhance the sensitivity of cells to ferroptosis by inhibiting the SLC7A11 subunit and controlling cell metabolism and the redox state;[19,51–53] in contrast, the inhibitory effect of p53 on ferroptosis has been reported.[54,55] Tumor suppressors can also downregulate the expression of SLC7A1.[20] On the other hand, a study has shown that ectopic expression of oncogenic RAS mutants can reduce the sensitivity of rhabdomyosarcoma (RMS)13 cells to ferroptosis.[56] The occurrence of HCC involves alterations in specific gene expression patterns caused by related mutant genes, including mutant RAS, p53, retinoblastoma (Rb), NRF2/ Kelch-like ECH-associated protein 1 (Keap1), and other genes or common changes in signaling pathways.[57]
The p62–Keap1–NRF2 pathway has been the focus of research in recent years. It has been reported that an imbalance in the p62–Keap1–NRF2 pathway can be observed in both HCC patients and mouse liver cancer models.[58,59] p62 is the key component of Mallory–Denk bodies and hyaline granules and is found in most precancerous lesions of the liver and in HCC.[60]NRF2 influences the cellular stress program related to the occurrence and progression of cancer,[61] and participates in the regulation of proliferation of HCC and liver cancer stem cells.[62] Mutations in the NRF2 gene were found in approximately 15% of HCC cases;[63]NRF2 can act as both a tumor suppressor and an oncogene,[64] and the NRF2-mediated antioxidant response pathway is considered to be a “double-edged sword” with respect to the occurrence and development of cancer. Second, in the regulatory network of ferroptosis, the p62–Keap1–NRF2 pathway regulates lipid peroxidation, oxidative stress, and iron metabolism in HCC cells by transcribing downstream target genes such as quinone oxidoreductase 1 (NQO1), HO1, and ferritin heavy chain 1 (FTH1), thus inhibiting ferroptosis.[65] In addition, studies have shown that many steps of the ferroptosis cascade are centered on NRF2.[66–68] Sun et al[65] showed that the inhibition of the p62–Keap1–NRF2 pathway could significantly enhance the anticancer activity of erastin and sorafenib in HCC cells both in vivo and in vitro and finally proved that the p62–Keap1–NRF2 pathway was a key negative regulator of ferroptosis in HCC cells: p62 can competitively bind to Keap1, thus increasing the transcriptional activity and stability of NRF2.[69] Umemura et al[60] showed that the accumulation of p62 is an important prerequisite for the malignant development of precancerous liver lesions, which may occur via the prevention of HCC-initiating cell death induced by oxidative stress. This way of preventing HCC-initiating cell death may be related to ferroptosis inhibition because the accumulation of p62 eventually leads to the non-mutagenic activation of three key cancer drivers including NRF2; however, there is no direct evidence yet. On the other hand, the interaction between autophagy and ferroptosis is still unclear.[70] It has been reported that when autophagy is defective, NRF2 can be activated through the atypical Keap1–NRF2 pathway because p62 autophagy degradation is inhibited.[71] In the occurrence of this series of events, autophagy and ferroptosis seem to be located in upstream and downstream of the p62–Keap1–NRF2 pathway, respectively, and interact with each other on the axis of this pathway. Interestingly, a study reported that NRF2 was the common intermediary of hepatomegaly.[72] We hold the opinion that it is essential for the study of ferroptosis to establish biomarkers that can characterize the occurrence of ferroptosis, which will be of great contribution to our exploration of the relationship between ferroptosis and cancer.
Glutathione S-transferase zeta 1 (GSTZ1) belongs to the glutathione S-transferase (GST) superfamily, which is involved in the catabolism of phenylalanine/tyrosine and catalyzes the isomerization of maleylacetoacetate to fumarylacetoacetate.[73] It has been reported that GSTZ1 shows low expression in HCC, which leads to the accumulation of the metabolite succinylacetone in cells, and activation of the NRF2 pathway, finally promoting the proliferation of HCC cells.[74] Wang et al[73] showed that GSTZ1 was strikingly downregulated in sorafenib-resistant HCC, and the deletion of GSTZ1 promoted the activation of the NRF2 pathway, increased the level of GPX4, and decreased iron levels and lipid peroxidation, which finally inhibited ferroptosis induced by sorafenib.
Metallothionein-1G (MT-1G) belongs to the metallothionein (MT) family, which includes low molecular weight and cysteine-rich proteins, and it exists in mammals as an antioxidant and heavy metal antidote.[75,76] It has been reported that MT-1G can inhibit carcinogenesis,[75] inhibit metastasis and promote differentiation.[77,78] Sun et al[79] found that sorafenib could induce the expression of MT-1G in HCC cells through the activation of NRF2, but not p53 or hypoxia-inducible factor (HIF)-1α, while the inhibition of MT-1G could increase the sensitivity to sorafenib therapy, which finally proved that MT-1G was a negative regulator of ferroptosis in HCC. Their research also found that inhibiting MT-1G could enhance the anticancer activity of sorafenib in vitro and in tumor xenograft models.
The Rb tumor suppressor gene is a key regulatory factor in the cell cycle that is activated in response to numerous anti-mitotic signals and plays a vital role in the G1/S checkpoint.[80] The loss of the Rb function is a key event in the process of HCC.[81–83] A study showed that the cell death level of HCC cells with low Rb expression induced by sorafenib was 2–3 times higher than that of the control group and resulted in complete tumor regression in 50% of sorafenib-treated mice that received HCC cell xenografts with low Rb expression, but the tumors in the control group were stable, and the difference was statistically significant.[84] This study finally proved that the low expression of Rb can effectively promote the induction of HCC cell ferroptosis by sorafenib.
In addition to the regulatory factors mentioned above that affect ferroptosis through participation in iron metabolism,[41–46] recent progress has been made in the identification of regulatory factors that affect ferroptosis by regulating the iron homeostasis of HCC. O-linked N-acetylglucosaminylation (O-GlcNAcylation) is a reversible post-translational modification catalyzed by O-linked N-acetylglucosamine (O-GlcNAc).[85] There is evidence that yes-associated protein (YAP) O-GlcNAcylation can enhance the sensitivity of HCC to ferroptosis by regulating the expression of TFR, which may be a promising treatment for HCC.[86] Ceruloplasmin (CP), a copper-containing protein, belongs to the multicopper oxidase family and can prevent the production of ROS mediated by ferrous ions and is widely regarded as an effective protective agent against antioxidant stress.[87,88] A study has shown that deletion of CP can promote the ferroptosis of HCC cells caused by erastin and RSL3 and can lead to the accumulation of intracellular ferrous ions and lipid ROS, which ultimately proves that CP can suppress ferroptosis by affecting iron homeostasis in HCC.[89]
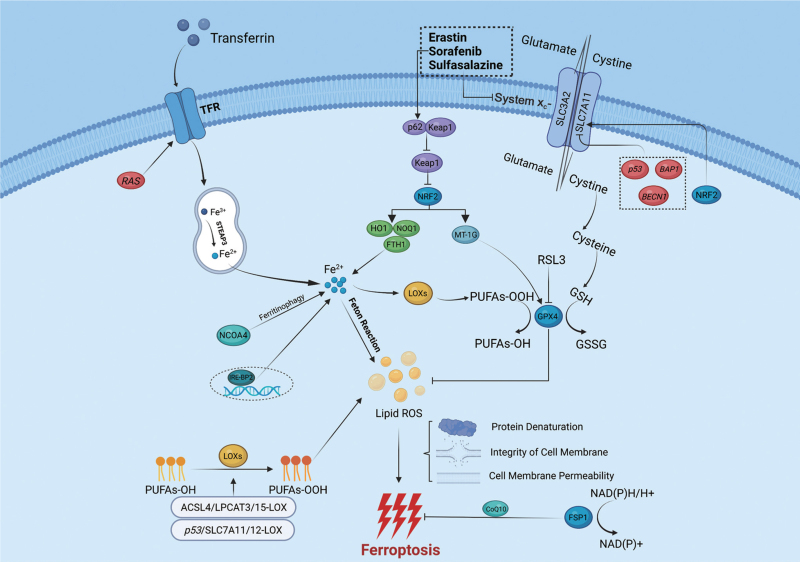
Similarly, the effect of lipid metabolism on ferroptosis is mainly regulated by ACSL4.[32,90] Doll et al[32] showed through oxi-lipidomics analysis that ACSL4 can influence the composition of lipids required for ferroptosis. The main role of ACSL4 in ferroptosis is to ligate coenzyme A to long-chain PUFAs; once inside the membranes, these PUFAs may undergo peroxidation, which is the essential element for ferroptosis.[15,38] In HCC, ACSL4 can restore the lipid metabolism of HCC cells through the c-Myc/sterol regulatory element-binding protein 1 (SREBP1) pathway and then participate in the proliferation and metastasis of HCC.[91] In addition, in recent years, a number of studies have shown that ACSL4 can adequately predict the prognosis of sorafenib-treated HCC patients, especially those who exhibit sorafenib-mediated ferroptosis.[92,93] The molecular mechanisms of ferroptosis and key regulatory pathways are shown in [Figure 1] (created with BioRender.com).
Figure 1.
Molecular mechanisms of ferroptosis and key regulatory pathways. Ferroptosis is directly triggered by iron-dependent lipid peroxidation, through three possible mechanisms: (1) denaturation of related proteins on the cellular membrane; (2) disruption of the integrity of the cellular membrane; and (3) increased permeability of the cellular membrane. Lipid alcohols are oxidized to lipid hydroperoxides in a reaction mediated by LOXs, and eventually participate in lipid peroxidation. Lipid peroxidation requires the Fenton reaction and the overreaction of LOXs; simultaneously, GPX4 can prevent lipid peroxidation by consuming two molecules of GSH. GSH enters the cell through a process mediated by system xc−. Iron is transported to TFR by transferrin, then imported into the cell by TFR, and eventually converted to Fe2+ in endosomes. Subsequently, Fe2+ participates in the Fenton reaction and the synthesis of lipid peroxidation-related enzymes such as LOXs. ACSL4: Long-chain fatty acid–coenzyme A (CoA) ligase 4; BAP1: Breast cancer susceptibility gene 1 (BRCA1) associated protein 1; BECN1: Beclin 1; CoQ10: coenzyme Q10; FSP1: Ferroptosis suppressor protein 1; FTH1: Ferritin heavy chain 1; GPX4: Glutathione peroxidase 4; GSH: Glutathione; GSSG: Oxidized glutathione; HO1: Heme oxygenase-1; IRE-BP2: Iron responsive element binding protein 2; Keap1: Kelch-like ECH-associated protein 1; LOXs: Lipoxygenases; LPCAT3: Lysophospholipid-acyltransferase 3; MT-1G: Metallothionein-1G; NAD(P)H: Nicotinamide adenine dinucleotide; NCOA4: Nuclear receptor coactivator 4; NQO1: Quinone oxidoreductase 1; NRF2: Nuclear factor erythroid 2-related factor; PUFAs: Polyunsaturated fatty acids; ROS: Reactive oxygen species; SLC3A2: Solute carrier family 3 member 2; SLC7A11: Solute carrier family 7 member 11; STEAP3: Six transmembrane epithelial antigen of prostate 3; TFR: Transferrin receptor.
Application of Ferroptosis in HCC
Sorafenib is the first multikinase inhibitor approved for unresectable HCC. It is worth noting that several studies have shown that the mechanism of ferroptosis induced by sorafenib is mediated by non-kinase inhibition.[65,94] On the one hand, sorafenib can inhibit the activity of the system xc− receptor and cause GSH consumption, which eventually leads to ferroptosis.[84] On the other hand, a study showed that the responsive expression of NRF2 increased in HCC cells after treatment with sorafenib, which may be the mechanism of sorafenib resistance in some HCC patients,[65] and the latest research shows that sorafenib may be involved in the regulation of protein phosphorylation signals in the key regulatory pathways as a means of regulating ferroptosis.[95] Recently, research has focused on enhancing the effect of sorafenib on ferroptosis in various ways. Artesunate (ART) itself can induce ROS-mediated cell death by inducing ferritinophagy and disrupting the unstable iron pool in HCC,[96,97] while its combination with sorafenib can produce a synergistic effect to induce ferroptosis.[98] Quiescin sulfhydryl oxidase 1 (QSOX1) can promote ferroptosis induced by sorafenib in HCC by driving the nuclear endosome transport of the epidermal growth factor receptor (EGFR) and restraining the activity of the NRF2.[99] In addition, changes in GSTZ1 and MT-1G expression,[73,79] as mentioned above, can synergize with sorafenib to improve the induction of ferroptosis in tumor cells. However, although a study has shown that ACSL4 can be used as a biomarker for predicting the sensitivity of sorafenib in HCC,[92] we still do not understand the relationship between the kinase inhibition and ferroptosis effects of sorafenib and their dominance in antitumor activity.
However, the bioavailability of sorafenib is limited to a certain extent because of its water solubility and targeting properties. In one study, a novel cascaded copper-based metal-organic framework (MOF) nanocatalyst was constructed using the Hong Kong University of Science and Technology (HKUST) -1 framework by incorporating the cyclooxygenase-2 (COX-2) inhibitor meloxicam (Mel) and sorafenib to improve the treatment effect in HCC.[100] This method can substantially improve the water solubility of sorafenib and effectively regulate the levels of GSH and COX-2 in HCC cells, which provides a new therapeutic strategy. A study constructed sorafenib-loaded MIL-101 (Fe) nanoparticles and combined them with an iRGD peptide containing a tumor-homing motif (RGD) and a tissue-penetrating motif (CendR), which may represent a promising treatment for HCC by inducing ferroptosis.[101] Tang et al[102] introduced manganese-doped mesoporous silica nanoparticles (MMSNs), which could induce the ferroptosis of tumor cells through the consumption of intracellular GSH caused by the degradation of MMSNs. They loaded MMSNs with sorafenib at a drug loading rate of 2.68 ± 0.32% and released the drug into the tumor microenvironment (TME) to achieve GSH inhibition. On the other hand, MMSNs themselves can also consume intracellular GSH, thus achieving synergism. In recent years, the therapeutic strategy of ferroptosis caused by the strong permeability, retention, and targeting of nanomaterials in cancer has been widely studied. Nanotechnology not only enhances the targeting characteristics of tumors but also prolongs the life cycle of ferroptosis inducers,[103] and is thus a very promising field.
In addition to studies on erastin, sorafenib, and RSL3, there have been an increasing number of studies on drugs that induce ferroptosis. It has been reported that disulfiram (DSF) can react with Cu to generate the anticancer metabolite DSF/Cu and inhibit the activity of the p97-dependent proteasome.[104] Ren et al[105] showed that DSF/Cu can seriously disrupt mitochondrial homeostasis, increase the free iron pool, and enhance lipid peroxidation, eventually leading to ferroptosis, and can cooperate with sorafenib to inhibit the NRF2 and mitogen-activated protein kinase (MAPK) pathways. DSF/Cu can also significantly increase the cytotoxicity of sorafenib while inducing ferroptosis in HCC. The nuclear protein 1 (NUPR1) inhibitor ZZW-115 can induce mitochondrial dysfunction in pancreatic cancer and HCC cells, which leads to ferroptosis after the accumulation of ROS. This phenomenon can be inhibited by the Transcription Factor A, Mitochondrial (TFAM).[106] Furthermore, solasonine has been reported to promote ferroptosis in HCC cells by suppressing GPX4 and glutathione synthetase (GSS).[107] Haloperidol can adequately increase GSH levels, ferrous ion levels and lipid peroxidation in cells, thus promoting ferroptosis induced by erastin and sorafenib.[108] Loss of leukemia inhibitory factor (LIF) receptor subunit alpha (LIFR), the gene that encodes the LIF receptor, can induce liver tumorigenesis and resistance to drug-related ferroptosis through upregulation of the iron-sequestering cytokine lipocalin 2 (LCN2) mediated by nuclear factor kappa-B (NF-κB), and this situation can be altered by an LCN2-neutralizing antibody.[109] Yao et al[109] found that this kind of antibody was able to elevate the ferroptosis-inducing and anticancer effects mediated by sorafenib, and they finally proved that co-administration with sorafenib and LCN2-neutralizing antibody had a better therapeutic response in mice bearing HCC patient-derived xenograft tumors.
Immunotherapy has been a great breakthrough in the field of cancer in recent years and has a promising therapeutic effect on malignant tumors, including HCC, and recent studies have begun to explore the role of ferroptosis in cancer immunotherapy and TME. Xu et al[110] have indicated that ferroptosis plays a two-way role in the TME. Specifically, ferroptosis may restrain the activity of antitumor immune cells, including cluster of differentiation 8 (CD8)+ T cells, natural killer (NK) cells, and dendritic cells (DCs),[111–113] leading to a decrease in the antitumor immunity. For example, Ma et al[114] found that CD36 can mediate the uptake of fatty acids in the TME by tumor-infiltrating CD8+ T cells, induce ferroptosis, reduce the production of cytotoxic cytokines, and weaken the antitumor ability, while blocking CD36 can effectively restore the antitumor activity of CD8+ T cells, and the combination with anti-programmed death 1 (PD1) antibodies possessed stronger antitumor efficacy. At the same time, some immunosuppressive immune cells, including M2 tumor-associated macrophages (TAMs), and T regulatory cells (Tregs), are also affected by ferroptosis, which leads to the reversal of immunosuppression.[115,116] A recent study showed that the higher resistance of M1 TAMs to ferroptosis mediated by GPX4 deletion can be used to inhibit the survival of M2 TAMs, and eventually enhance antitumor immunity.[115,117] On the other hand, the effect of ferroptosis on cancer cells also changes the TME, and ferroptotic cancer cells can release immunostimulant signals such as damage-associated molecular pattern (DAMP) signals, so that immune cells can locate cancer cells accurately.[118,119] In addition, ferroptotic cancer cells can induce greater tumor-specific immune responses to enhance the efficacy of immunotherapy.[120] However, ferroptotic cancer cells can also suppress anti-tumor immunity through various mechanisms. Studies have shown that ferroptotic cancer cells are associated with the release of prostaglandin E2 (PGE2), which is a key immunosuppressive factor and capable of inhibiting the activity of antitumor immune cells.[14,121] In short, in view of the complex crosstalk between the TME and ferroptosis, the relationship between ferroptosis and immunotherapy is more than just a synergistic one; it should also be determined according to different immunophenotypes, different induction mechanisms of ferroptosis, and different types of immune cells. At present, the combination of ferroptosis and immunotherapy is relatively rare in HCC. Clinical or preclinical application of ferroptosis in hepatocellular carcinoma are summarized in Table 2.
Table 2.
Clinical or preclinical application of ferroptosis in hepatocellular carcinoma (HCC).
| Treatment | Target | Possible mechanism | Reference |
| DHA | LOXs | By promoting the binding of PEBP1 and 15-LO, and activating LOXs allosterically to induce lipid peroxidation | [30] |
| An LCN2-neutralizing antibody | LCN2 | By targeting the upregulation of LCN2 mediated by loss of LIFR and upregulating Fe2+ and lipid peroxidation levels | [109] |
| Sorafenib | System xc− | By inhibiting system xc− for absorption of cystine, and promoting the depletion of GSH | [123] |
| ART | Ferritin | By inducing ferritinophagy and releasing iron into the cytosol to produce ROS, which can also synergize with sorafenib | [97,98] |
| QSOX1 | NRF2 | By promoting the nuclear endosome transport of EGFR and restraining the activity of NRF2 | [99] |
| A cascaded copper-based nanocatalyst | GSH | By improving the water solubility of sorafenib and increasing the levels of GSH and COX-2 | [100] |
| MMSNs loaded with sorafenib | GSH | By promoting the depletion of GSH and increasing the targeting characteristics of sorafenib, achieving synergism | [102] |
| Co-administration of MIL-101(Fe)@sor NPs with iRGD | GSH; GPX4 | By reducing the levels of GSH and activity of GPX4 | [101] |
| RSL3@O2-ICG NBs | GSH | By promoting the depletion of GSH and damaging mitochondria | [122] |
| DSF/Cu | Iron; NRF2 | By disrupting mitochondrial homeostasis, increasing the iron pool and inhibiting NRF2 pathway for synergizing with sorafenib | [105] |
| Solasonine | GPX4 | By restraining the activity of GPX4 and GSS | [107] |
ART: Artesunate; COX-2: Cyclooxygenase-2; DHA: Dihydroartemisinin; DSF: Disulfiram; EGFR: Epidermal growth factor receptor; GPX4: Glutathione peroxidase 4; GSH: Glutathione; GSS: Glutathione synthetase; ICG: Indocyanine green; LCN2: Lipocalin 2; LIFR: Leukemia inhibitory factor (LIF) receptor subunit alpha; LOXs: Lipoxygenases; MMSNs: Manganese-doped mesoporous silica nanoparticles; NBs: Nanobubbles; NPs: Nanoparticles; NRF2: Nuclear factor erythroid 2-related factor; PEBP1: Phosphatidylethanolamine binding protein 1; QSOX1: Quiescin sulfhydryl oxidase 1; RGD: Tumor-homing motif; ROS: Reactive oxygen species.
Conclusion and Perspective
Ferroptosis is a kind of RCD that involves triggering, execution, and regulation processes; the mechanisms underlying these processes are complex but are mainly mediated by three central axes: system xc−/GPX4, iron metabolism and lipid peroxidation. Nevertheless, the specific mechanism underlying the induction of ferroptosis, such as the mechanism of cell death after triggering ferroptosis and the contributions of these processes, is unclear; these areas need to be further explored to facilitate the clinical translation and application of ferroptosis-related strategies. Moreover, we know little about the relationship between ferroptosis and other types of RCD. Regarding the relationship between ferroptosis and cancer, as far as the current situation is concerned, its role in the occurrence and development of tumors seems to be two-sided, and we need to explore and explain the effects of the related genes and factors on ferroptosis as much as possible, which is a crucial but difficult area. Finally, we described the influence, targets, and clinical applications of ferroptosis in HCC. We have taken a small step toward the application of ferroptosis in HCC. As mentioned previously, ferroptosis is a novel opportunity for treatment in the era of apoptosis resistance, and we should pay more attention to the identification of biomarkers of ferroptosis, which is one of the most important prerequisites for improving ferroptosis assessment and clinical application.
Conflicts of interest
None.
Footnotes
How to cite this article: Cong T, Luo Y, Fu Y, Liu Y, Li Y, Li X. New perspectives on ferroptosis and its role in hepatocellular carcinoma. Chin Med J 2022;135:2157–2166. doi: 10.1097/CM9.0000000000002327
References
- 1.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2021; 71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Sun J, Liu C, Shi J, et al. A novel chemotherapy strategy for advanced hepatocellular carcinoma: a multicenter retrospective study. Chin Med J 2022; doi:10.1097/CM9.0000000000001952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fadeel B, Orrenius S. Apoptosis: a basic biological phenomenon with wide-ranging implications in human disease. J Intern Med 2005; 258:479–517. doi: 10.1111/j.1365-2796.2005.01570.x. [DOI] [PubMed] [Google Scholar]
- 4.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011; 144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 5.Galluzzi L, Vitale I, Aaronson SA, Abrams JM, Adam D, Agostinis P, et al. Molecular mechanisms of cell death: recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ 2018; 25:486–541. doi: 10.1038/s41418-017-0012-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen X, Zeh HJ, Kang R, Kroemer G, Tang DL. Cell death in pancreatic cancer: from pathogenesis to therapy. Nat Rev Gastroenterol Hepatol 2021; 18:804–823. doi: 10.1038/s41575-021-00486-6. [DOI] [PubMed] [Google Scholar]
- 7.Dixon SJ, Lemberg KM, Lamprecht MR, Skouta R, Zaitsev EM, Gleason CE, et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell 2012; 149:1060–1072. doi: 10.1016/j.cell.2012.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang WS, Stockwell BR. Synthetic lethal screening identifies compounds activating iron-dependent, nonapoptotic cell death in oncogenic-RAS-harboring cancer cells. Chem Biol 2008; 15:234–245. doi: 10.1016/j.chembiol.2008.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bridges RJ, Natale NR, Patel SA. System xc(-) cystine/glutamate antiporter: an update on molecular pharmacology and roles within the CNS. Br J Pharmacol 2012; 165:20–34. doi: 10.1111/j.1476-5381.2011.01480.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sato H, Tamba M, Ishii T, Bannai S. Cloning and expression of a plasma membrane cystine/glutamate exchange transporter composed of two distinct proteins. J Biol Chem 1999; 274:11455–11458. doi: 10.1074/jbc.274.17.11455. [DOI] [PubMed] [Google Scholar]
- 11.Brigelius-Flohé R, Maiorino M. Glutathione peroxidases. Biochim Biophys Acta 2013; 1830:3289–3303. doi: 10.1016/j.bbagen.2012.11.020. [DOI] [PubMed] [Google Scholar]
- 12.Ingold I, Berndt C, Schmitt S, Doll S, Poschmann G, Buday K, et al. Selenium utilization by GPX4 is required to prevent hydroperoxide-induced ferroptosis. Cell 2018; 172:409–422. e421. doi: 10.1016/j.cell.2017.11.048. [DOI] [PubMed] [Google Scholar]
- 13.Conrad M, Angeli JP, Vandenabeele P, Stockwell BR. Regulated necrosis: disease relevance and therapeutic opportunities. Nat Rev Drug Discov 2016; 15:348–366. doi: 10.1038/nrd.2015.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang WS, SriRamaratnam R, Welsch ME, Shimada K, Skouta R, Viswanathan VS, et al. Regulation of ferroptotic cancer cell death by GPX4. Cell 2014; 156:317–331. doi: 10.1016/j.cell.2013.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kagan VE, Mao GW, Qu F, Angeli JP, Doll S, Croix CS, et al. Oxidized arachidonic and adrenic PEs navigate cells to ferroptosis. Nat Chem Biol 2017; 13:81–90. doi: 10.1038/nchembio.2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang WS, Kim KJ, Gaschler MM, Patel M, Shchepinov MS, Stockwell BR. Peroxidation of polyunsaturated fatty acids by lipoxygenases drives ferroptosis. Proc Natl Acad Sci U S A 2016; 113:E4966–E4975. doi: 10.1073/pnas.1603244113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ursini F, Maiorino M. Lipid peroxidation and ferroptosis: the role of GSH and GPx4. Free Radic Biol Med 2020; 152:175–185. doi: 10.1016/j.freeradbiomed.2020.02.027. [DOI] [PubMed] [Google Scholar]
- 18.Chen DL, Tavana O, Chu B, Erber L, Chen Y, Baer R, et al. NRF2 is a major target of ARF in p53-independent tumor suppression. Mol Cell 2017; 68:224–232. e224. doi: 10.1016/j.molcel.2017.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jiang L, Kon N, Li TY, Wang SJ, Su T, Hibshoosh H, et al. Ferroptosis as a p53-mediated activity during tumour suppression. Nature 2015; 520:57–62. doi: 10.1038/nature14344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang YL, Shi JJ, Liu XG, Feng L, Gong ZH, Koppula P, et al. BAP1 links metabolic regulation of ferroptosis to tumour suppression. Nat Cell Biol 2018; 20:1181–1192. doi: 10.1038/s41556-018-0178-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Song XX, Zhu S, Chen P, Hou W, Wen QR, Liu J, et al. AMPK-mediated BECN1 phosphorylation promotes ferroptosis by directly blocking system xc(-) activity. Curr Biol 2018; 28:2388–2399. e2385. doi: 10.1016/j.cub.2018.05.094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dixon SJ, Patel DN, Welsch M, Skouta R, Lee ED, Hayano M, et al. Pharmacological inhibition of cystine-glutamate exchange induces endoplasmic reticulum stress and ferroptosis. Elife 2014; 3:e02523.doi: 10.7554/eLife.02523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gao MH, Monian P, Quadri N, Ramasamy R, Jiang XJ. Glutaminolysis and transferrin regulate ferroptosis. Mol Cell 2015; 59:298–308. doi: 10.1016/j.molcel.2015.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tadokoro T, Ikeda M, Ide T, Deguchi H, Ikeda S, Okabe K, et al. Mitochondria-dependent ferroptosis plays a pivotal role in doxorubicin cardiotoxicity. JCI Insight 2020; 5:e132747.doi: 10.1172/jci.insight.132747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gao MH, Yi JM, Zhu JJ, Minikes AM, Monian P, Thompson CB, et al. Role of mitochondria in ferroptosis. Mol Cell 2019; 73:354–363. e353. doi: 10.1016/j.molcel.2018.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bock FJ, Tait SWG. Mitochondria as multifaceted regulators of cell death. Nat Rev Mol Cell Biol 2020; 21:85–100. doi: 10.1038/s41580-019-0173-8. [DOI] [PubMed] [Google Scholar]
- 27.Li DS, Li YS. The interaction between ferroptosis and lipid metabolism in cancer. Signal Transduct Target Ther 2020; 5:108.doi: 10.1038/s41392-020-00216-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yin HY, Xu LB, Porter NA. Free radical lipid peroxidation: mechanisms and analysis. Chem Rev 2011; 111:5944–5972. doi: 10.1021/cr200084z. [DOI] [PubMed] [Google Scholar]
- 29.Chu B, Kon N, Chen DL, Li TY, Liu T, Jiang L, et al. ALOX12 is required for p53-mediated tumour suppression through a distinct ferroptosis pathway. Nat Cell Biol 2019; 21:579–591. doi: 10.1038/s41556-019-0305-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Su Y, Zhao DL, Jin C, Li ZH, Sun SM, Xia SW, et al. Dihydroartemisinin induces ferroptosis in HCC by promoting the formation of PEBP1/15-LO. Oxid Med Cell Longev 2021; 2021:3456725.doi: 10.1155/2021/3456725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stockwell BR, Friedmann Angeli JP, Bayir H, Bush AI, Conrad M, Dixon SJ, et al. Ferroptosis: a regulated cell death nexus linking metabolism, redox biology, and disease. Cell 2017; 171:273–285. doi: 10.1016/j.cell.2017.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Doll S, Proneth B, Tyurina YY, Panzilius E, Kobayashi S, Ingold I, et al. ACSL4 dictates ferroptosis sensitivity by shaping cellular lipid composition. Nat Chem Biol 2017; 13:91–98. doi: 10.1038/nchembio.2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen X, Kang R, Kroemer G, Tang DL. Broadening horizons: the role of ferroptosis in cancer. Nat Rev Clin Oncol 2021; 18:280–296. doi: 10.1038/s41571-020-00462-0. [DOI] [PubMed] [Google Scholar]
- 34.Kang R, Zeng L, Zhu S, Xie YC, Liu J, Wen QR, et al. Lipid peroxidation drives gasdermin D-mediated pyroptosis in lethal polymicrobial sepsis. Cell Host Microbe 2018; 24:97–108. e104. doi: 10.1016/j.chom.2018.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Canli Ö, Alankuş YB, Grootjans S, Vegi N, Hültner L, Hoppe PS. Glutathione peroxidase 4 prevents necroptosis in mouse erythroid precursors. Blood 2016; 127:139–148. doi: 10.1182/blood-2015-06-654194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ran QT, Liang HY, Ikeno Y, Qi WB, Prolla TA. Roberts LJ 2nd, et al. Reduction in glutathione peroxidase 4 increases life span through increased sensitivity to apoptosis. J Gerontol A Bio Sciu Med Sci 2007; 62:932–942. doi: 10.1093/gerona/62.9.932. [DOI] [PubMed] [Google Scholar]
- 37.Chen X, Yu CH, Kang R, Tang DL. Iron metabolism in ferroptosis. Front Cell Dev Biol 2020; 8:590226.doi: 10.3389/fcell.2020.590226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Conrad M, Pratt DA. The chemical basis of ferroptosis. Nat Chem Biol 2019; 15:1137–1147. doi: 10.1038/s41589-019-0408-1. [DOI] [PubMed] [Google Scholar]
- 39.Zhang DL, Ghosh MC, Rouault TA. The physiological functions of iron regulatory proteins in iron homeostasis—an update. Front Pharmacol 2014; 5:124.doi: 10.3389/fphar.2014.00124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gao GF, Li J, Zhang YT, Chang YZ. Cellular iron metabolism and regulation. Adv Exp Med Biol 2019; 1173:21–32. doi: 10.1007/978-981-13-9589-5_2. [DOI] [PubMed] [Google Scholar]
- 41.Bogdan AR, Miyazawa M, Hashimoto K, Tsuji Y. Regulators of iron homeostasis: new players in metabolism, cell death, and disease. Trends Biochem Sci 2016; 41:274–286. doi: 10.1016/j.tibs.2015.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Feng HZ, Schorpp K, Jin J, Yozwiak CE, Hoffstrom BG, Decker AM, et al. Transferrin receptor is a specific ferroptosis marker. Cell Rep 2020; 30:3411–3423. e3417. doi: 10.1016/j.celrep.2020.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hou W, Xie YC, Song XX, Sun XF, Lotze MT. Zeh HJ 3rd, et al. Autophagy promotes ferroptosis by degradation of ferritin. Autophagy 2016; 12:1425–1428. doi: 10.1080/15548627.2016.1187366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang YQ, Chang SY, Wu Q, Gou YJ, Jia LP, Cui YM, et al. The protective role of mitochondrial ferritin on erastin-induced ferroptosis. Front Aging Neurosci 2016; 8:308.doi: 10.3389/fnagi.2016.00308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brown CW, Amante JJ, Chhoy P, Elaimy AL, Liu H, Zhu LJ, et al. Prominin2 drives ferroptosis resistance by stimulating iron export. Dev Cell 2019; 51:575–586. e574. doi: 10.1016/j.devcel.2019.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kwon MY, Park E, Lee SJ, Chung SW. Heme oxygenase-1 accelerates erastin-induced ferroptotic cell death. Oncotarget 2015; 6:24393–24403. doi: 10.18632/oncotarget.5162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wu M, Xu LG, Li XY, Zhai ZH, Shu HB. AMID, an apoptosis-inducing factor-homologous mitochondrion-associated protein, induces caspase-independent apoptosis. J Biol Chem 2002; 277:25617–25623. doi: 10.1074/jbc.M202285200. [DOI] [PubMed] [Google Scholar]
- 48.Doll S, Freitas FP, Shah R, Aldrovandi M, da Silva MC, Ingold I, et al. FSP1 is a glutathione-independent ferroptosis suppressor. Nature 2019; 575:693–698. doi: 10.1038/s41586-019-1707-0. [DOI] [PubMed] [Google Scholar]
- 49.Bersuker K, Hendricks JM, Li ZP, Magtanong L, Ford B, Tang PH, et al. The CoQ oxidoreductase FSP1 acts parallel to GPX4 to inhibit ferroptosis. Nature 2019; 575:688–692. doi: 10.1038/s41586-019-1705-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Han C, Liu YY, Dai RJ, Ismail N, Su WJ, Li B. Ferroptosis and its potential role in human diseases. Front Pharmacol 2020; 11:239.doi: 10.3389/fphar.2020.00239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang SJ, Li D, Ou Y, Jiang L, Chen Y, Zhao Y, et al. Acetylation is crucial for p53- mediated ferroptosis and tumor suppression. Cell Rep 2016; 17:366–373. doi: 10.1016/j.celrep.2016.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bieging KT, Mello SS, Attardi LD. Unravelling mechanisms of p53-mediated tumour suppression. Nat Rev Cancer 2014; 14:359–370. doi: 10.1038/nrc3711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kaiser AM, Attardi LD. Deconstructing networks of p53-mediated tumor suppression in vivo. Cell Death Differ 2018; 25:93–103. doi: 10.1038/cdd.2017.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xie YC, Zhu S, Song XX, Sun XF, Fan Y, Liu JB, et al. The tumor suppressor p53 limits ferroptosis by blocking DPP4 activity. Cell Rep 2017; 20:1692–1704. doi: 10.1016/j.celrep.2017.07.055. [DOI] [PubMed] [Google Scholar]
- 55.Tarangelo A, Magtanong L, Bieging-Rolett KT, Li Y, Ye JB, Attardi LD, et al. p53 suppresses metabolic stress-induced ferroptosis in cancer cells. Cell Rep 2018; 22:569–575. doi: 10.1016/j.celrep.2017.12.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schott C, Graab U, Cuvelier N, Hahn H, Fulda S. Oncogenic RAS mutants confer resistance of RMS13 rhabdomyosarcoma cells to oxidative stress-induced ferroptotic cell death. Front Oncol 2015; 5:131.doi: 10.3389/fonc.2015.00131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nault JC, Cheng AL, Sangro B, Llovet JM. Milestones in the pathogenesis and management of primary liver cancer. J Hepatol 2020; 72:209–214. doi: 10.1016/j.jhep.2019.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Takamura A, Komatsu M, Hara T, Sakamoto A, Kishi C, Waguri S, et al. Autophagy-deficient mice develop multiple liver tumors. Genes Dev 2011; 25:795–800. doi: 10.1101/gad.2016211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bartolini D, Dallaglio K, Torquato P, Piroddi M, Galli F. Nrf2-p62 autophagy pathway and its response to oxidative stress in hepatocellular carcinoma. Transl Res 2018; 193:54–71. doi: 10.1016/j.trsl.2017.11.007. [DOI] [PubMed] [Google Scholar]
- 60.Umemura A, He F, Taniguchi K, Nakagawa H, Yamachika S, Font-Burgada J, et al. p62, upregulated during preneoplasia, induces hepatocellular carcinogenesis by maintaining survival of stressed HCC-initiating cells. Cancer Cell 2016; 29:935–948. doi: 10.1016/j.ccell.2016.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sanghvi VR, Leibold J, Mina M, Mohan P, Berishaj M, Li ZN, et al. The oncogenic action of NRF2 depends on de-glycation by fructosamine-3-kinase. Cell 2019; 178:807–819. e821. doi: 10.1016/j.cell.2019.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sun QK, Zhang ZC, Lu YJ, Liu QY, Xu XL, Xu JB, et al. Loss of xanthine oxidoreductase potentiates propagation of hepatocellular carcinoma stem cells. Hepatology 2020; 71:2033–2049. doi: 10.1002/hep.30978. [DOI] [PubMed] [Google Scholar]
- 63.Gao J, Aksoy BA, Dogrusoz U, Dresdner G, Gross B, Sumer SO, et al. Integrative analysis of complex cancer genomics and clinical profiles using the cbioportal. Sci Signal 2013; 6:pl1–pl1. doi: 10.1126/scisignal.2004088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Menegon S, Columbano A, Giordano S. The dual roles of NRF2 in cancer. Trends Mol Med 2016; 22:578–593. doi: 10.1016/j.molmed.2016.05.002. [DOI] [PubMed] [Google Scholar]
- 65.Sun XF, Ou ZH, Chen RC, Niu XH, Chen D, Kang R, et al. Activation of the p62-Keap1-NRF2 pathway protects against ferroptosis in hepatocellular carcinoma cells. Hepatology 2016; 63:173–184. doi: 10.1002/hep.28251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dai CS, Chen X, Li JB, Comish P, Kang R, Tang DL. Transcription factors in ferroptotic cell death. Cancer Gene Ther 2020; 27:645–656. doi: 10.1038/s41417-020-0170-2. [DOI] [PubMed] [Google Scholar]
- 67.Anandhan A, Dodson M, Schmidlin CJ, Liu PF, Zhang DD. Breakdown of an ironclad defense system: the critical role of NRF2 in mediating ferroptosis. Cell Chem Biol 2020; 27:436–447. doi: 10.1016/j.chembiol.2020.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dodson M, Castro-Portuguez R, Zhang DD. NRF2 plays a critical role in mitigating lipid peroxidation and ferroptosis. Redox Biol 2019; 23:101107.doi: 10.1016/j.redox.2019.101107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ichimura Y, Waguri S, Sou YS, Kageyama S, Hasegawa J, Ishimura R, et al. Phosphorylation of p62 activates the Keap1-Nrf2 pathway during selective autophagy. Mol Cell 2013; 51:618–631. doi: 10.1016/j.molcel.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 70.Zhao YG, Codogno P, Zhang H. Machinery, regulation and pathophysiological implications of autophagosome maturation. Nat Rev Mol Cell Biol 2021; 22:733–750. doi: 10.1038/s41580-021-00392-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Qian H, Chao XJ, Williams J, Fulte S, Li TG, Yang L, et al. Autophagy in liver diseases: a review. Mol Aspects Med 2021; 82:100973.doi: 10.1016/j.mam.2021.100973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.He F, Antonucci L, Yamachika S, Zhang ZC, Taniguchi K, Umemura A, et al. NRF2 activates growth factor genes and downstream AKT signaling to induce mouse and human hepatomegaly. J Hepatol 2020; 72:1182–1195. doi: 10.1016/j.jhep.2020.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wang QJ, Bin C, Xue Q, Gao QZ, Huang AL, Wang K, et al. GSTZ1 sensitizes hepatocellular carcinoma cells to sorafenib-induced ferroptosis via inhibition of NRF2/GPX4 axis. Cell Death Dis 2021; 12:426.doi: 10.1038/s41419-021-03718-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Li JJ, Wang QJ, Yang Y, Lei C, Yang F, Liang L, et al. GSTZ1 deficiency promotes hepatocellular carcinoma proliferation via activation of the KEAP1/NRF2 pathway. J Exp Clin Cancer Res 2019; 38:438.doi: 10.1186/s13046-019-1459-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Si MF, Lang JH. The roles of metallothioneins in carcinogenesis. J Hematol Oncol 2018; 11:107.doi: 10.1186/s13045-018-0645-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Coyle P, Philcox JC, Carey LC, Rofe AM. Metallothionein: the multipurpose protein. Cell Mol Life Sci 2002; 59:627–647. doi: 10.1007/s00018-002-8454-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhang WC, Chin TM, Yang H, Nga ME, Lunny DP, Lim EKH, et al. Tumour-initiating cell-specific miR-1246 and miR-1290 expression converge to promote non-small cell lung cancer progression. Nat Commun 2016; 7:11702.doi: 10.1038/ncomms11702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Arriaga JM, Bravo AI, Mordoh J, Bianchini M. Metallothionein 1G promotes the differentiation of HT-29 human colorectal cancer cells. Oncol Rep 2017; 37:2633–2651. doi: 10.3892/or.2017.5547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sun XF, Niu XH, Chen RC, He WY, Chen D, Kang R, et al. Metallothionein-1G facilitates sorafenib resistance through inhibition of ferroptosis. Hepatology 2016; 64:488–500. doi: 10.1002/hep.28574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Knudsen ES, Knudsen KE. Tailoring to RB: tumour suppressor status and therapeutic response. Nat Rev Cancer 2008; 8:714–724. doi: 10.1038/nrc2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mayhew CN, Carter SL, Fox SR, Sexton CR, Reed CA, Srinivasan SV, et al. RB loss abrogates cell cycle control and genome integrity to promote liver tumorigenesis. Gastroenterology 2007; 133:976–984. doi: 10.1053/j.gastro.2007.06.025. [DOI] [PubMed] [Google Scholar]
- 82.Laurent-Puig P, Zucman-Rossi J. Genetics of hepatocellular tumors. Oncogene 2006; 25:3778–3786. doi: 10.1038/sj.onc.1209547. [DOI] [PubMed] [Google Scholar]
- 83.Anwar SL, Krech T, Hasemeier B, Schipper E, Schweitzer N, Vogel A, et al. Deregulation of RB1 expression by loss of imprinting in human hepatocellular carcinoma. J Pathol 2014; 233:392–401. doi: 10.1002/path.4376. [DOI] [PubMed] [Google Scholar]
- 84.Louandre C, Marcq I, Bouhlal H, Lachaier E, Godin C, Saidak Z, et al. The retinoblastoma (Rb) protein regulates ferroptosis induced by sorafenib in human hepatocellular carcinoma cells. Cancer Lett 2015; 356:971–977. doi: 10.1016/j.canlet.2014.11.014. [DOI] [PubMed] [Google Scholar]
- 85.Chatham JC, Zhang JH, Wende AR. Role of O-linked N-acetylglucosamine protein modification in cellular (patho)physiology. Physiol Rev 2021; 101:427–493. doi: 10.1152/physrev.00043.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhu GQ, Murshed A, Li HJ, Ma J, Zhen N, Ding M, et al. O-GlcNAcylation enhances sensitivity to RSL3-induced ferroptosis via the YAP/TFRC pathway in liver cancer. Cell Death Discov 2021; 7:83.doi: 10.1038/s41420-021-00468-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Vashchenko G, MacGillivray RTA. Multi-copper oxidases and human iron metabolism. Nutrients 2013; 5:2289–2313. doi: 10.3390/nu5072289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bakhautdin B, Goksoy Bakhautdin E, Fox PL. Ceruloplasmin has two nearly identical sites that bind myeloperoxidase. Biochem Biophys Res Commun 2014; 453:722–727. doi: 10.1016/j.bbrc.2014.09.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Shang YX, Luo MY, Yao FP, Wang SK, Yuan ZQ, Yang YF. Ceruloplasmin suppresses ferroptosis by regulating iron homeostasis in hepatocellular carcinoma cells. Cell Signal 2020; 72:109633.doi: 10.1016/j.cellsig.2020.109633. [DOI] [PubMed] [Google Scholar]
- 90.Dixon SJ, Winter GE, Musavi LS, Lee ED, Snijder B, Rebsamen M, et al. Human haploid cell genetics reveals roles for lipid metabolism genes in nonapoptotic cell death. ACS Chem Biol 2015; 10:1604–1609. doi: 10.1021/acschembio.5b00245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chen JR, Ding CF, Chen YH, Hu WD, Yu CK, Peng CH, et al. ACSL4 reprograms fatty acid metabolism in hepatocellular carcinoma via c-Myc/SREBP1 pathway. Cancer Lett 2021; 502:154–165. doi: 10.1016/j.canlet.2020.12.019. [DOI] [PubMed] [Google Scholar]
- 92.Feng J, Lu PZ, Zhu GZ, Hooi SC, Wu Y, Huang XW, et al. ACSL4 is a predictive biomarker of sorafenib sensitivity in hepatocellular carcinoma. Acta Pharmacol Sin 2021; 42:160–170. doi: 10.1038/s41401-020-0439-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Xia HP, Lee KW, Chen JX, Kong SN, Sekar K, Deivasigamani A, et al. Simultaneous silencing of ACSL4 and induction of GADD45B in hepatocellular carcinoma cells amplifies the synergistic therapeutic effect of aspirin and sorafenib. Cell Death Discov 2017; 3:17058.doi: 10.1038/cddiscovery.2017.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lachaier E, Louandre C, Godin C, Saidak Z, Baert M, Diouf M, et al. Sorafenib induces ferroptosis in human cancer cell lines originating from different solid tumors. Anticancer Res 2014; 34:6417–6422. [PubMed] [Google Scholar]
- 95.Werth EG, Rajbhandari P, Stockwell BR, Brown LM. Time course of changes in sorafenib-treated hepatocellular carcinoma cells suggests involvement of phospho-regulated signaling in ferroptosis induction. Proteomics 2020; 20:e2000006.doi: 10.1002/pmic.202000006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Jiang ZY, Wang ZW, Chen L, Zhang C, Liao FY, Wang YW, et al. Artesunate induces ER-derived-ROS-mediated cell death by disrupting labile iron pool and iron redistribution in hepatocellular carcinoma cells. Am J Cancer Res 2021; 11:691–711. [PMC free article] [PubMed] [Google Scholar]
- 97.Sun X, Yan PY, Zou C, Wong YK, Shu YH, Lee YM, et al. Targeting autophagy enhances the anticancer effect of artemisinin and its derivatives. Med Res Rev 2019; 39:2172–2193. doi: 10.1002/med.21580. [DOI] [PubMed] [Google Scholar]
- 98.Li ZJ, Dai HQ, Huang XW, Feng J, Deng JH, Wang ZX, et al. Artesunate synergizes with sorafenib to induce ferroptosis in hepatocellular carcinoma. Acta Pharmacol Sin 2021; 42:301–310. doi: 10.1038/s41401-020-0478-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sun JL, Zhou CH, Zhao Y, Zhang XF, Chen WY, Zhou Q, et al. Quiescin sulfhydryl oxidase 1 promotes sorafenib-induced ferroptosis in hepatocellular carcinoma by driving EGFR endosomal trafficking and inhibiting NRF2 activation. Redox Biol 2021; 41:101942.doi: 10.1016/j.redox.2021.101942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Tian HL, Zhao S, Nice EC, Huang CH, He WF, Zou BW, et al. A cascaded copper-based nanocatalyst by modulating glutathione and cyclooxygenase-2 for hepatocellular carcinoma therapy. J Colloid Interface Sci 2022; 607:1516–1526. doi: 10.1016/j.jcis.2021.09.049. [DOI] [PubMed] [Google Scholar]
- 101.Liu XC, Zhu XY, Qi X, Meng XW, Xu K. Co-administration of iRGD with sorafenib-loaded iron-based metal-organic framework as a targeted ferroptosis agent for liver cancer therapy. Int J Nanomedicine 2021; 16:1037–1050. doi: 10.2147/IJN.S292528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Tang HX, Chen DF, Li CQ, Zheng CH, Wu XD, Zhang Y, et al. Dual GSH-exhausting sorafenib loaded manganese-silica nanodrugs for inducing the ferroptosis of hepatocellular carcinoma cells. Int J Pharm 2019; 572:118782.doi: 10.1016/j.ijpharm.2019.118782. [DOI] [PubMed] [Google Scholar]
- 103.Zafar H, Raza F, Ma SY, Wei YW, Zhang J, Shen Q. Recent progress on nanomedicine-induced ferroptosis for cancer therapy. Biomater Sci 2021; 9:5092–5115. doi: 10.1039/d1bm00721a. [DOI] [PubMed] [Google Scholar]
- 104.Skrott Z, Mistrik M, Andersen KK, Friis S, Majera D, Gursky J, et al. Alcohol-abuse drug disulfiram targets cancer via p97 segregase adaptor NPL4. Nature 2017; 552:194–199. doi: 10.1038/nature25016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ren XY, Li YC, Zhou Y, Hu WY, Yang C, Jing QG, et al. Overcoming the compensatory elevation of NRF2 renders hepatocellular carcinoma cells more vulnerable to disulfiram/copper-induced ferroptosis. Redox Biol 2021; 46:102122.doi: 10.1016/j.redox.2021.102122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Huang C, Santofimia-Castaño P, Liu X, Xia Y, Peng L, Gotorbe C, et al. NUPR1 inhibitor ZZW-115 induces ferroptosis in a mitochondria-dependent manner. Cell Death Discov 2021; 7:269.doi: 10.1038/s41420-021-00662-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Jin MM, Shi CZ, Li T, Wu Y, Hu C, Huang G. Solasonine promotes ferroptosis of hepatoma carcinoma cells via glutathione peroxidase 4-induced destruction of the glutathione redox system. Biomed Pharmacother 2020; 129:110282.doi: 10.1016/j.biopha.2020.110282. [DOI] [PubMed] [Google Scholar]
- 108.Bai T, Wang S, Zhao YP, Zhu RT, Wang WJ, Sun YL. Haloperidol, a sigma receptor 1 antagonist, promotes ferroptosis in hepatocellular carcinoma cells. Biochem Biophys Res Commun 2017; 491:919–925. doi: 10.1016/j.bbrc.2017.07.136. [DOI] [PubMed] [Google Scholar]
- 109.Yao F, Deng YL, Zhao Y, Mei Y, Zhang YL, Liu XG, et al. A targetable LIFR−NF-(B−LCN2 axis controls liver tumorigenesis and vulnerability to ferroptosis. Nat Commun 2021; 12:7333.doi: 10.1038/s41467-021-27452-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Xu HJ, Ye D, Ren ML, Zhang HY, Bi F. Ferroptosis in the tumor microenvironment: perspectives for immunotherapy. Trends Mol Med 2021; 27:856–867. doi: 10.1016/j.molmed.2021.06.014. [DOI] [PubMed] [Google Scholar]
- 111.Poznanski SM, Singh K, Ritchie TM, Aguiar JA, Fan IY, Portillo AL, et al. Metabolic flexibility determines human NK cell functional fate in the tumor microenvironment. Cell metab 2021; 33:1205–1220. e1205. doi: 10.1016/j.cmet.2021.03.023. [DOI] [PubMed] [Google Scholar]
- 112.Zou YL, Palte MJ, Deik AA, Li HX, Eaton JK, Wang WY, et al. A GPX4-dependent cancer cell state underlies the clear-cell morphology and confers sensitivity to ferroptosis. Nat Commun 2019; 10:1617.doi: 10.1038/s41467-019-09277-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Drijvers JM, Gillis JE, Muijlwijk T, Nguyen TH, Gaudiano EF, Harris IS, et al. Pharmacologic screening identifies metabolic vulnerabilities of CD8+ T cells. Cancer Immunol Res 2021; 9:184–199. doi: 10.1158/2326-6066.CIR-20-0384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ma XZ, Xiao LL, Liu LT, Ye LQ, Su P, Bi EG, et al. CD36-mediated ferroptosis dampens intratumoral CD8+ T cell effector function and impairs their antitumor ability. Cell metab 2021; 33:1001–1012. e1005. doi: 10.1016/j.cmet.2021.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kapralov AA, Yang Q, Dar HH, Tyurina YY, Anthonymuthu TS, Kim R, et al. Redox lipid reprogramming commands susceptibility of macrophages and microglia to ferroptotic death. Nat Chem Biol 2020; 16:278–290. doi: 10.1038/s41589-019-0462-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Xu CX, Sun SG, Johnson T, Qi R, Zhang SY, Zhang J, et al. The glutathione peroxidase Gpx4 prevents lipid peroxidation and ferroptosis to sustain Treg cell activation and suppression of antitumor immunity. Cell Rep 2021; 35:109235.doi: 10.1016/j.celrep.2021.109235. [DOI] [PubMed] [Google Scholar]
- 117.Zhu SY, Luo ZY, Li XX, Han X, Shi SL, Zhang T. Tumor-associated macrophages: role in tumorigenesis and immunotherapy implications. J Cancer 2021; 12:54–64. doi: 10.7150/jca.49692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Friedmann Angeli JP, Krysko DV, Conrad M. Ferroptosis at the crossroads of cancer-acquired drug resistance and immune evasion. Nat Rev Cancer 2019; 19:405–414. doi: 10.1038/s41568-019-0149-1. [DOI] [PubMed] [Google Scholar]
- 119.Efimova I, Catanzaro E, Van der Meeren L, Turubanova VD, Hammad H, Mishchenko TA, et al. Vaccination with early ferroptotic cancer cells induces efficient antitumor immunity. J Immunother Cancer 2020; 8:e001369.doi: 10.1136/jitc-2020-001369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Yu B, Choi B, Li WG, Kim DH. Magnetic field boosted ferroptosis-like cell death and responsive MRI using hybrid vesicles for cancer immunotherapy. Nat Commun 2020; 11:3637.doi: 10.1038/s41467-020-17380-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Johnson AM, Kleczko EK, Nemenoff RA. Eicosanoids in cancer: new roles in immunoregulation. Front Pharmacol 2020; 11:595498.doi: 10.3389/fphar.2020.595498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Li YC, Xia J, Shao FC, Zhou Y, Yu JQ, Wu HY, et al. Sorafenib induces mitochondrial dysfunction and exhibits synergistic effect with cysteine depletion by promoting HCC cells ferroptosis. Biochem Biophys Res Commun 2021; 534:877–884. doi: 10.1016/j.bbrc.2020.10.083. [DOI] [PubMed] [Google Scholar]
- 123.Chen YC, Shang HT, Wang CY, Zeng JQ, Zhang ST, Wu BL, et al. RNA-seq explores the mechanism of oxygen-boosted sonodynamic therapy based on all-in-one nanobubbles to enhance ferroptosis for the treatment of HCC. Int J Nanomedicine 2022; 17:105–123. doi: 10.2147/IJN.S343361. [DOI] [PMC free article] [PubMed] [Google Scholar]