Abstract
Background:
Cell competition is an important feature in pancreatic cancer (PC) progression, but the underlying mechanism remains elusive. This study aims to explore the role of exosomes derived from normal pancreatic ductal epithelial cells involved in PC progression.
Methods:
PC cells and pancreatic stellate cells (PSCs) were treated with exosomes isolated from pancreatic ductal epithelial cells. Cell proliferation was assessed by CCK8 assays. Cell migration and invasion were assessed by Transwell assays. PC and matched adjacent non-tumor tissue specimens were obtained from 46 patients pathologically diagnosed with PC at Peking University First Hospital from 2013 to 2017. Tissue miR-485-3p and p21-activated kinase-1 (PAK1) expression was examined by real-time polymerase chain reaction (RT-PCR), and the relationship of the two was analyzed using Pearman's product-moment correlation. The clinical significance of miR-485-3p was analyzed using the Chi-square test, Wilcoxon rank-sum test, and Fisher exact probability, respectively. The binding of miR-485-3p to PAK1 5′-untranslated region (5′-UTR) was examined by luciferase assay. PC cells were xenografted into nude mice as a PC metastasis model.
Results:
Exosomes from pancreatic ductal epithelial cells suppressed PC cell migration and invasion as well as the secretion and migration of PSCs. MiR-485-3p was enriched in the exosomes of pancreatic ductal epithelial cells but deficient in those of PC cells and PSCs, in accordance with the lower level in PSCs and PC cells than that in pancreatic ductal cells. And the mature miR-485-3p could be delivered into these cells by the exosomes secreted by normal pancreatic duct cells, to inhibit PC cell migration and invasion. Clinical data analysis showed that miR-485-3p was significantly decreased in PC tissues (P < 0.05) and was negatively associated with lymphovascular invasion (P = 0.044). As a direct target of miR-485-3p, PAK1 was found to exert an inhibitory effect on PC cells, and there was a significantly negative correlation between the expression levels of miR-485-3p and PAK1 (r = −0.6525, P < 0.0001) in PC tissues. Moreover, miR-485-3p could suppress PC metastasis in vivo by targeting p21-activated kinase-1.
Conclusions:
Exosomal miR-485-3p delivered by normal pancreatic ductal epithelial cells into PC cells inhibits PC metastasis by directly targeting PAK1. The restoration of miR-485-3p by exosomes or some other vehicle might be a novel approach for PC treatment.
Keywords: Pancreatic neoplasms, Cell competition, Exosomes, miR-485-3p, p21-activated kinase-1
Introduction
Cell competition describes the competitive growth and survival of different cell types and the newly emerging unfit cells within tissues, which has been recognized as an important feature in organ homeostasis,[1–3] maintenance of immune function,[4] and tumor development.[4,5] Our previous study based on laser capture microdissection revealed that pancreatic cancer (PC) cells, pancreatic stellate cells (PSCs), and normal pancreatic ductal epithelial cells were the three main components in PC tissues.[6] However, it remains unclear whether normal pancreatic ductal epithelial cells with a high proportion within PC tissues could show competition against PC cells and PSCs.
Exosomes are nanosized extracellular vesicles released by almost all types of cells and have been recognized as messengers of cell-to-cell communication.[7,8] Ribonucleic acid (RNA) sequencing analysis has shown a diverse collection of exosomal RNA species in human plasma samples, among which microRNAs (miRNAs) were the most abundant.[9] Exosomal miRNAs are found to be widely involved in tumor development, while previous studies mainly focused on the role of exosomes derived from tumor cells or stromal cells.[10–13] Recently, Zheng et al[14] reported that exosomal long non-coding RNA PTENP1 derived from normal bladder cells could inhibit the biological malignant behavior of cancer cells, indicating that cell competition mediated by exosome may play an important role in cancer progression. The effects and mechanisms of cell competition in PC progression remain unclear. Therefore, the present study aimed to investigate whether miRNAs released by exosomes derived from normal pancreatic ductal epithelial cells could suppress PC progression.
Methods
Clinical specimens and ethical approval
PC and matched adjacent non-tumor tissue specimens were obtained from 46 patients who were pathologically diagnosed with PC at Peking University First Hospital (Beijing, China) from 2013 to 2017. All specimens were stored in liquid nitrogen immediately after resection. The study was conducted in accordance with the Declaration of Helsinki and was approved by the local ethics committee of Peking University First Hospital (No. 201933). Informed written consent was obtained from all patients before their enrollment in this study.
Cell culture and transfection
Human PSCs were isolated from resected PC tissues and cultured as previously described.[10,15] Human pancreatic normal epithelial cell line hTERT-HPNE and PC cell lines (AsPC-1, BxPC-3, MIA PaCa-2, PANC-1, Patu8988, T3M4) were purchased from the American Type Culture Collection (Manassas, USA). hTERT-HPNE cells were cultured in 75% Dulbecco's modified eagle medium (DMEM) without glucose (Corning, New York, USA) and 25% Medium M3 Base (Incell Corporation, Texas, USA) containing 10 ng/mL human recombinant epidermal growth factor (Amyjet Scientific, Wuhan, China), 5.5 mmol/L D-glucose (MedChemExpress, Shanghai, China), 750 ng/mL puromycin (Beyotime, Shanghai, China), and 5% Fetal bovine serum (FBS) (Corning). PC cell lines were cultured in RPMI-1640 (Gibco, California, USA) or DMEM (Corning) containing 10% FBS. All cell lines were cultured at 37°C in a humid atmosphere with 5% CO2.
The lentiviral vector pCDH/MSCV/MCS/EF1/GFP/Puro for PAK1 overexpression was constructed by SyngenTech (Beijing, China). Other plasmids were purchased from GenePharma (Shanghai, China). The hsa-miR-485-3p mature sequence (miR-485-3p mimic GTCATACACGG CTCTCCTCTCT) and the scramble sequence (miR-NC TT CTCCGAACGTGTCACGT) were cloned into pGLV10/U6/RFP/Puro lentivirus vector. The miR-485-3p inhibitor sequence (Inh-miR-485-3p GATCCAGAGAGGAGAGCC GTGTATGACCGATAGAGAGGAGAGCCGTGTATGA CACCGGTAGAGAGGAGAGCCGTGTATGACTCACA GAGAGGAGAGCCGTGTATGACTTTTTTGAATT) and the scramble sequence (Inh-NC TTCTCCGAACGTGT CACGT) were cloned into pGLV3/H1/GFP/Puro lentivirus vector.
Lentivirus was produced via 293T cells seeded in 6-cm dishes using lipofectamine 3000 (Invitrogen, California, USA) according to the manufacturer's instructions. Transfection reagent was prepared in two parts: one was the 250 μL mixture of opti-MEM (Gibco) and 10 μg total plasmids, the other was the mixture of 15 μL lipofectamine 3000 and 235 μL opti-MEM. To construct PC cells that express PAK1 stably, PC cells were cultured in a medium containing lentivirus and polybrene (Beyotime) for 24 h and were selected with 2 μg/mL puromycin.
Exosome isolation and characterization
Exosomes were isolated from PSCs, hTERT-HPNE, and PC cell lines using the ultracentrifugation method as previously described or ExoQuick-tc kits (SBI, California, USA).[16,17] The morphological characteristics of exosomes were observed and imaged using a transmission electron microscope (FEI, Oregon, USA). The particle size of exosomes was analyzed by Beijing Enze Kangtai Biological Technology (Beijing, China).
Transwell migration and invasion assays
BxPC-3, PANC-1 cells, and PSCs (2 × 104 cells/well) were suspended in FBS-free medium and seeded in the top chambers of 24-well Transwell inserts (Corning). For invasion assays, the top chambers were pre-coated with 100 μL of Matrigel Matrix (1:7 dilution, Corning, New York, USA) according to the manufacturer's instructions. The lower chambers contained 600 μL of culture medium supplemented with 10% FBS as the chemoattractant. All the cells were cultured under standard conditions for 36 to 48 h. Cells that migrated or invaded through the polycarbonate membranes were fixed in 4% paraformaldehyde (Solar-bio, Beijing, China), washed with phosphate-buffered saline (PBS) (Solarbio, Beijing, China), stained with 0.1% crystal violet (Solarbio), imaged, and counted under a microscope (Olympus, Tokyo, Japan).
Immunofluorescence
Exosomes were fluorescently labeled with PKH-67 (BestBio, Shanghai, China) at 37°C for 30 min according to the manual. Excessive PKH-67 was removed with Exosome Spin Columns (MW 3000) (Thermo Fisher Scientific, Massachusetts, USA) following previously published protocols.[10] Then PKH-67-labeled exosomes were mixed with PSCs, BxPC-3, or PANC-1 in confocal dishes (Nest, Wuxi, China) under standard conditions for 8 to 12 h. Next, the cells were fixed, permeabilized, blocked, stained in DAPI (Beyotime), and imaged using a laser scanning confocal microscope (Leica, Wetzlar, Germany).
Real-time polymerase chain reaction (RT-PCR)
RNA isolation and real-time PCR wereperformed following our previously published protocols.[10] The primers for U6 and miR-485-3p were purchased from RiboBio (Guangzhou, China). Other primers were purchased from TSINGKE (Beijing, China): GAPDH, Forword primer 5′- GTATTGGGCGCCT GGTCACC-3′, Reverse primer 5′-CGCTCCTGGAAGATGGT-GATGG-3′; PAK1, Forword primer 5′- CAGCCCCTCCGATGAGAAATA-3′, Reverse primer 5′-CAAAACCGACATGAATTG TGTGT-3′; Pre-miR-485-3p, Stem loop primer 5′-GTCGTATCCAGTGCGTGTCG TGGAGTCGGCAATTGACCACTGGATACGACTAAAAG-3′, Forword primer 5′- GCCGA GTGGAGAGAGGCTGGC-3′, Reverse primer 5′-CAGTGCGTGTCGTGGAGT-3′; Pri-miR-485-3p, Forword primer 5’- CGAGTCATACACGGCTCTCC-3′, Reverse primer 5′- TACCTTGGAAGCAGCACTGG-3′. U6 and GAPDH served as the endogenous controls for miRNA and mRNA, respectively. Each sample was examined in triplicate, and all data were analyzed using the 2−ΔΔCT method.
Western blotting and immunohistochemistry (IHC)
Western blotting and immunohistochemistry analyses were performed as previously described.[18] Antibodies for PAK1 (ab172730), TSG101 (ab125011) were purchased from Abcam (Cambridge, MA, USA); anti-CD9 (#13174) were purchased from Cell Signaling Technology (Beverly, MA, USA); anti-CD63 (AF1471) was purchased from Beyotime; and β-actin (bs-0061R) antibody was obtained from Bioss (Beijing, China).
CCK-8 assay
The cell viability of PSCs and PC cells was detected using Cell Counting kit-8 (Dojindo, Kyushu, Japan). PC cells and PSCs were, respectively, seeded at 5000 cells/well and 10,000 cells/well in 96-well plates (Nest, Wuxi, China) in triplicate. One hundred microliters of FBS-free medium containing 10 μL WST-8 reagent were added into each well and the plates were incubated at 37°C for 1 h, and the optical absorbance at 450 nm was measured using a microplate reader (Bio-Rad Laboratories, California, USA).
Enzyme linked immunosorbent assay (ELISA)
The levels of fibronectin 1 (FN1), Col I, and fibroblast activation protein alpha (FAP) in PSCs cell supernatant were detected by using Human FN ELISA Kit (No. E-EL-H0179c, Elabscience, Wuhan, China), Human Col I ELISA Kit (No. JM-03329H1, JINGMEI, Jiangsu, China), and Human FAPα ELISA Kit (No. EH3037, FineTest, Wuhan, China) according to the manufacturer's instructions.
Dual-luciferase reporter assay
The reporter plasmids containing 5′UTR fragment of PAK1 harboring wild-type or mutant miR-485-3p binding sites were constructed by SyngenTech (Beijing, China). Then, PANC-1 cells were cultured in 96-well plates and co-transfected with 50 nmol/L miR-485-3p mimic or negative control and 100 ng of either reporter plasmid using Lipofectamine 3000. PANC-1 cells were lysed 48 h after transfection, and the luciferase activity of each group was measured according to the manufacturer's instructions (Promega, Wisconsin, USA). All experiments were independently performed three times.
Animal model
BxPC-3 cells transfected with luci-miR-NC, luci-miR-485-3p mimic, luci-Inh-NC, and luci-Inh-miR-485-3p were harvested to prepare single cell suspensions. An equal number of BxPC-3 cells (1 × 106 cells in 100 μL of PBS) from each group was injected into the lateral tail vein of 4-week-old nude mice (BALB/c-nu) (n = 5). Potential metastases in the mice were detected and photographed with an IVIS Spectrum in-vivo imaging system (Perkin Elmer, Massachusetts, USA) at 35 days after injection. Then, all the mice were euthanized, and the lungs were collected for further analysis. All animal studies were approved and supervised by the Ethics Committee for Animal Studies at Peking University First Hospital.
Statistical analysis
Statistical analysis was carried out using SPSS 19.0 (SPSS, Chicago, IL, USA) and GraphPad Prism 8 (GraphPad, La Jolla, CA, USA). Categorical data and hierarchical data were reckoned using percentages and counts of cases to be described. The correlations between varieties of clinical data and miR-485-3p were ascertained using Chi-square test, Wilcoxon test, or Fisher exact test for comparison. The relationships between the expression of miR-485-3p and PAK1 were arrived at using paired sample test and Pearson correlation analysis. Independent-sample t test and non-parametric test were used to compare two groups; Bonferroni or Dunnett t test were used for pairwise comparisons, which were applied using continuous variables. All tests were performed using a two-sided test. Factors with P < 0.05 were considered statistically significant.
Results
Exosomes of hTERT-HPNE cells suppressed PC cell migration and invasion
To explore exosome-mediated cell competition among different cell clones within PC tissues, we isolated the exosomes from non-transformed immortalized normal pancreatic duct cell line hTERT-HPNE (ExohTERT-HPNE) and observed typical exosomal morphology and particle size distribution [Supplementary Figure 1A-B]. The detection of exosomal markers CD9, CD63, and TSG101 confirmed the isolation of ExohTERT-HPNE [Supplementary Figure 1C]. To examine the pickup of ExohTERT-HPNE by the PC cell lines BxPC-3 and PANC-1, ExohTERT-HPNE was labeled with PKH-67, and the internalization in PC cells was captured by laser scanning confocal microscopy [Supplementary Figure 1D]. These results demonstrated the isolation of ExohTERT-HPNE and its internalization into PC cells.
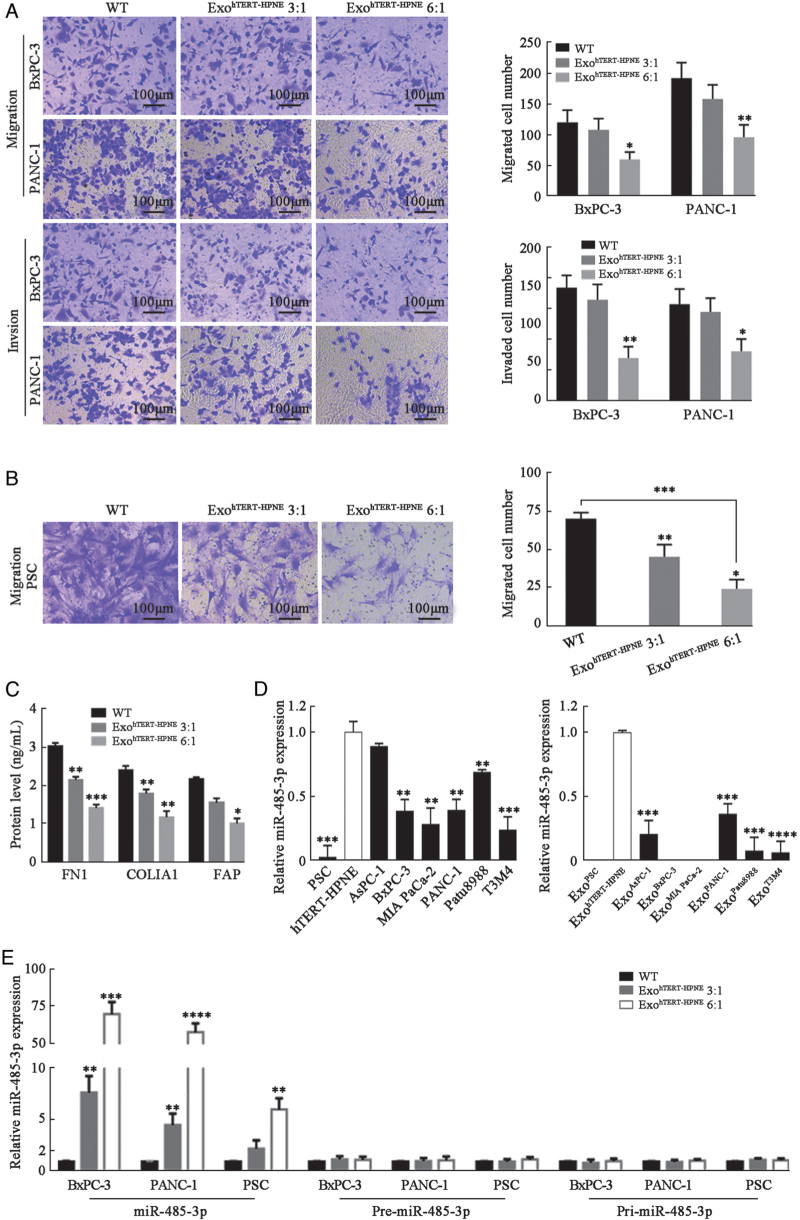
Next, BxPC-3 and PANC-1 cells were treated with ExohTERT-HPNE. CCK8 assay showed that PC cell proliferation could not be inhibited by ExohTERT-HPNE [Supplementary Figure 1E], but Transwell migration and invasion assays showed that ExohTERT-HPNE suppressed PC cell migration and invasion [Figure 1A]. These data suggest that hTERT-HPNE cells could release exosomes to inhibit PC cell migration and invasion.
Figure 1.
Suppressive effects of ExohTERT-HPNE on PC cells and PSCs through delivering mature miR-485-3p into the corresponding cells. (A) Transwell migration and invasion assays of BxPC-3 and PANC-1 cells treated with or without ExohTERT-HPNE (bar: 100 μm). Cells were imaged at 200 × magnification in five random fields and counted using ImageJ software. (B) Transwell migration assay of PSCs treated with or without ExohTERT-HPNE (bar: 100 μm). (C) FN1, COLIA1, and FAP levels in cell supernatant of PSCs treated with or without ExohTERT-HPNE assessed by ELISA. (D) Detection of miR-485-3p in exosomes of hTERT-HPNE, PSCs, and PC cells, as well as in the corresponding parental cells using RT-PCR. MiR-485-3p was not detected in ExoPSC, ExoBxPC-3, and ExoMIAPaCa-2. U6 served as endogenous reference. (E) Mature miR-485-3p and its precursors (pre-miR-485-3p, pri-miR-485-3p) of PSCs and PC cells were detected by RT-PCR. U6 and GAPDH, respectively, served as endogenous reference of miRNA and mRNA. ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001,∗∗∗∗P < 0.0001. COLIA1: Collagen type alpha 1 chain; ELISA: Enzyme linked immunosorbent assay; FAP: Fibroblast activation protein alpha; FN1: Fibronectin 1; miRNAs: microRNAs; PC: Pancreatic cancer; PSCs: Pancreatic stellate cells; RT-PCR: Real-time PCR; WT: Wild type.
Exosomes of hTERT-HPNE cells suppressed PSC migration and secretion
As an important cell component within PC tissues, PSCs contribute to the malignant phenotype of PC cells.[19,20] First, we examined the internalization of PKH-67-labeled ExohTERT-HPNE in PSCs [Supplementary Figure 1D]. CCK8 assays showed that cell proliferation of PSCs was not affected by ExohTERT-HPNE [Supplementary Figure 1E], but cell migration of PSCs was significantly suppressed by ExoTERT-HPNE [Figure 1B]. It is well known that activated PSCs secrete a large amount of fibronectin and collagen to maintain the stroma of PC.[20–22] FAP is an important immunosuppressive factor within the PC microenvironment.[23] We found that the secretion of FN1, collagen type alpha 1 chain (COLIA1), and FAP were downregulated in PSCs treated by ExohTERT-HPNE [Figure 1C]. Taken together, these results suggest that hTERT-HPNE cells could release exosomes to inhibit cell migration and secretion of PSCs without affecting cell proliferation.
Exosomal miR-485-3p was downregulated in those of PC cells and PSCs
To explore the inhibitory mechanisms of ExohTERT-HPNE on PC cells and PSCs, we collected ExohTERT-HPNE and ExoBxPC-3 to conduct miRNA sequencing (Supplementary Exo-miRNA sequencing data). After incorporating the miRNA sequencing data of PSC-derived exosomes (ExoPSC) reported by Takikawa et al[16] into the present study, we noticed that miR-485-3p was absent both in ExoPSC and ExoBxPC-3. Exosomes of PSCs, hTERT-HPNE and six PC cell lines (AsPC-1, BxPC-3, MIA PaCa-2, PANC-1, Patu8988, and T3M4 cells) were all collected, and the results confirmed that exosomal miR-485-3p was deficient in PC cells and PSCs compared with hTERT-HPNE [Figure 1D and Supplementary Figure 2). The miR-485-3p expression was also detected in the corresponding parental cells, and the results showed that miR-485-3p was expressed at a lower level in PSCs and PC cells compared with pancreatic ductal cells [Figure 1D]. We further detected miR-485-3p expression in 46 paired PC and adjacent non-tumor tissues by RT-PCR to determine its clinical significance. Clinical data analysis showed that miR-485-3p was decreased in 78.3% (36/46) of PC tissues, and was negatively associated with lymphovascular invasion (P = 0.044) [Table 1].
Table 1.
Correlation analysis between tissue miR-485-3p levels and clinicopathological features of 46 PC patients (n).
| miR-485-3p expression | ||||
| Variables | Number of cases (n = 46) | Low (n = 36) | High (n = 10) | P value |
| Age | 0.240 | |||
| < 60 years | 13 | 12 | 1 | |
| ≥60 years | 33 | 24 | 9 | |
| Gender | 0.480 | |||
| Male | 28 | 23 | 5 | |
| Female | 18 | 13 | 5 | |
| Location | 1.000 | |||
| Head | 31 | 24 | 7 | |
| Other | 15 | 12 | 3 | |
| Tumor size | 0.719 | |||
| <4cm | 27 | 22 | 5 | |
| ≥4cm | 19 | 14 | 5 | |
| Histologic grade | 1.000 | |||
| Well/moderate | 32 | 25 | 7 | |
| Poor | 14 | 11 | 3 | |
| Regional lymph nodes | 0.667 | |||
| N0 | 22 | 16 | 6 | |
| N1 | 19 | 16 | 3 | |
| N2 | 5 | 4 | 1 | |
| Perineural invasion | 0.056 | |||
| Negative | 14 | 8 | 6 | |
| Positive | 32 | 28 | 4 | |
| Lymphovascular invasion | 0.044 | |||
| Negative | 34 | 24 | 10 | |
| Positive | 12 | 12 | 0 | |
Based on the American Joint Committee on Cancer Staging System for Pancreatic Adenocarcinoma (8th Edition). PC: Pancreatic cancer.
PANC-1 and BxPC-3 cell lines were chosen to conduct further experiments. ExohTERT-HPNE was added to the medium of PC cells (BxPC-3, PANC-1) and PSCs for 12 h. ExohTERT-HPNE and recipient cells were collected to detect the expression of mature miR-485-3p and precursor RNA. The results showed that ExohTERT-HPNE directly delivered mature miR-485-3p into PC cells and PSCs [Figure 1E and Supplementary Figure 3] rather than stimulating its production. Collectively, these results suggest that exosomal miR-485-3p is downregulated in PC cells and PSCs, and the mature miR-485-3p could be delivered into these cells by the exosomes secreted by normal pancreatic duct cells.
MiR-485-3p regulated PC cell migration and invasion
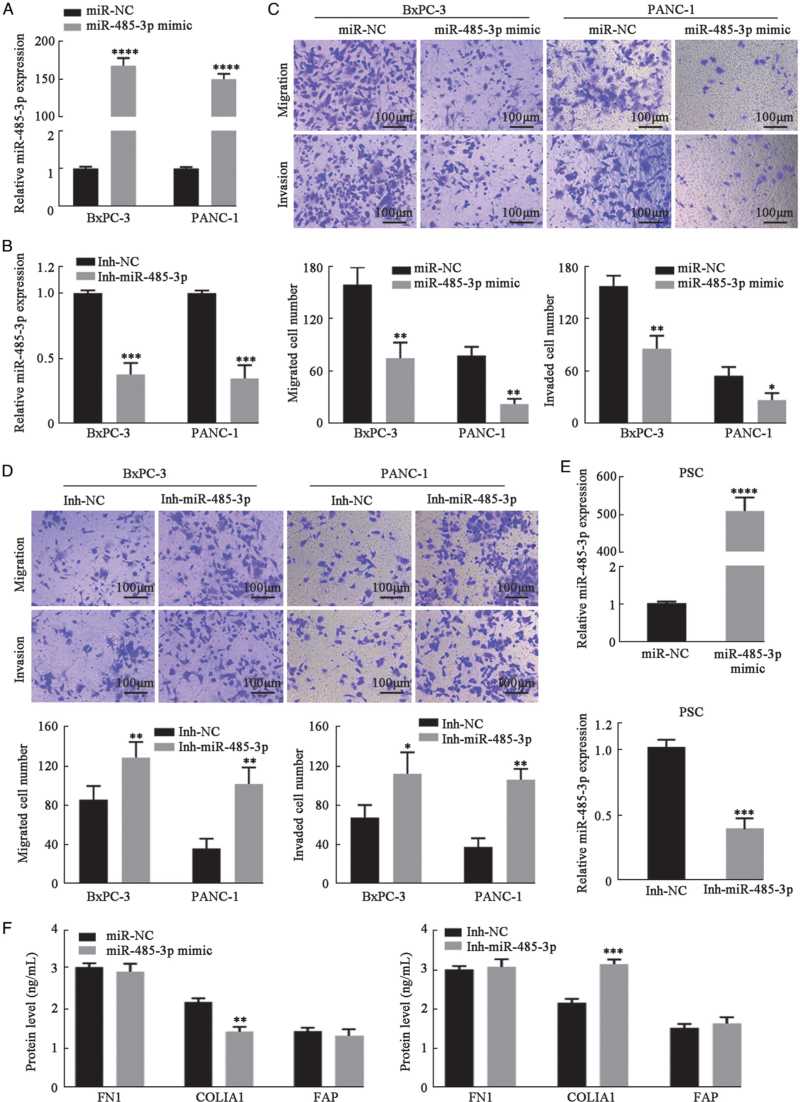
To investigate the role of miR-485-3p in PC metastasis, we used BxPC-3 and PANC-1 cells as models and established stable miR-485-3p-overexpressing cells (miR-485-3p mimic), miR-485-3p-silenced cells (Inh-485-3p), and control cells (miR-NC and Inh-NC) [Figure 2A,B]. PC cells overexpressing miR-485-3p were less likely to migrate or invade compared with control cells [Figure 2C]. In contrast, silencing of miR-485-3p in PC cells increased cell migration and invasion [Figure 2D].
Figure 2.
MiR-485-3p regulated PC cell migration and Invasion. (A, B) miR-485-3p was overexpressed and silenced In BxPC-3 and PANC-1 cells. (C, D) Transwell migration and Invasion assays in BxPC-3 and PANC-1 cells with overexpression or silencing of miR-485-3p (bar: 100 μm). The migrated or invaded cells were imaged at 200 × magnification in five random fields and counted using ImageJ software. (E) MiR-485-3p was overexpressed and silenced in PSCs. (F) FN1, COLIA1, and FAP levels in cell supernatant of PSCs transfected with miR-485-3p mimic or Inh-miR-485-3p were assessed by ELISA. ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001,∗∗∗∗P < 0.0001. COLIA1: Collagen type alpha 1 chain; ELISA: Enzyme linked immunosorbent assay; FAP: Fibroblast activation protein alpha; FN1: Fibronectin 1; PC: Pancreatic cancer; PSCs: Pancreatic stellate cells.
Next, miR-485-3p was also overexpressed or silenced in PSCs [Figure 2E]. No significant changes in migration compared to control PSCs were found [Supplementary Figure 4]. COLIA1 secreted by PSCs changed slightly with the overexpression or silencing of miR-485-3p [Figure 2F]. Taken together, miR-485-3p could regulate PC cell migration and invasion but did not markedly affect PSCs.
Upregulation of miR-485-3p in hTERT-HPNE cells enhanced the inhibitory effect of ExohTERT-HPNE on PC cell migration and invasion
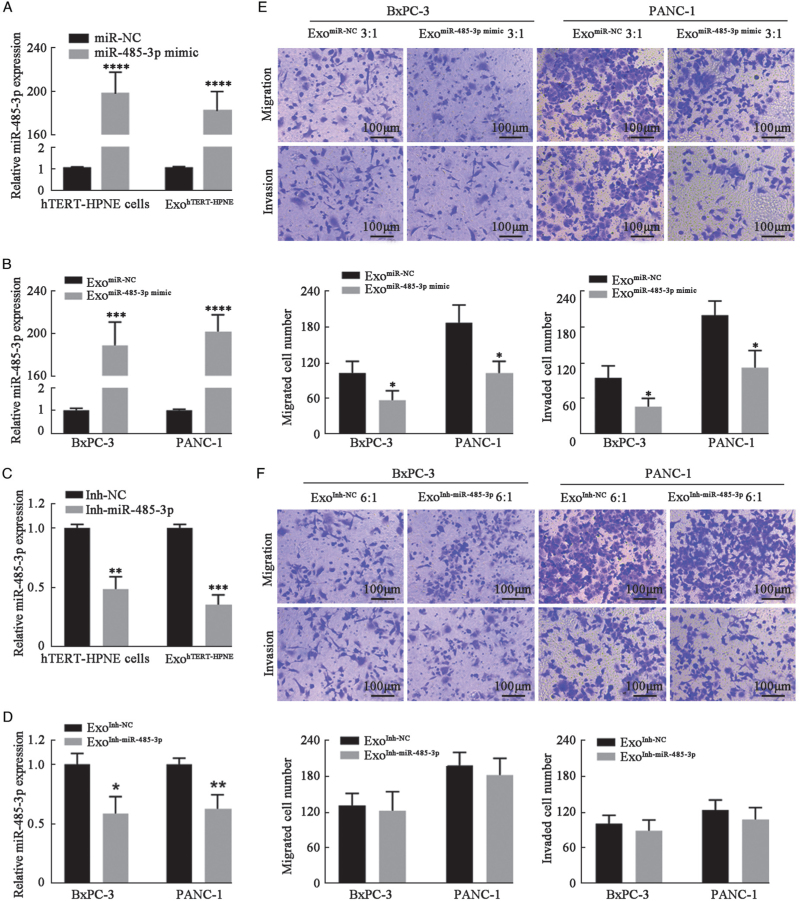
To explore the contribution of miR-485-3p to the inhibitory effects of ExohTERT-HPNE on PC cell migration and invasion, hTERT-HPNE cells were transfected with miR-485-3p mimic [Figure 3A] or its inhibitor [Figure 3C] and then exosomes were successively enriched, including ExomiR-NC, ExomiR-485-3pmimic, ExoInh-NC, and ExoInh-miR-485-3p. BxPC-3 and PANC-1 cells were treated with these exosomes for 48 h, and the cells were collected to confirm the changes of miR-485-3p expression [Figure 3B,D].
Figure 3.
Upregulating miR-485-3p enhanced the inhibitory effect of ExohTERT-HPNE on PC cell migration and invasion. (A, C) miR-485-3p was overexpressed and silenced In hTERT-HPNE cells and their exosomes. U6 served as endogenous reference. (B, D) The expression levels of miR-485-3p in each group were detected by RT-PCR. U6 served as endogenous reference. (E, F) Transwell migration and invasion assays in BxPC-3 and PANC-1 cells with overexpression or silencing of exosomal miR-485-3p (bar: 100 μm). ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001,∗∗∗∗P < 0.0001. PC: Pancreatic cancer; RT-PCR: Real-time PCR.
Transwell migration and invasion assays showed that overexpression of miR-485-3p enhanced the inhibitory effect of ExohTERT-HPNE on PC cell migration and invasion [Figure 3E]. However, the knockdown of miR-485-3p could not abolish the inhibitory effect of ExohTERT-HPNE [Figure 3F]. These results suggest that exosomal miR-485-3p from hTERT-HPNE cells plays an important role in the inhibition of PC cell migration and invasion.
MiR-485-3p directly modulated PAK1 to suppress PC metastasis
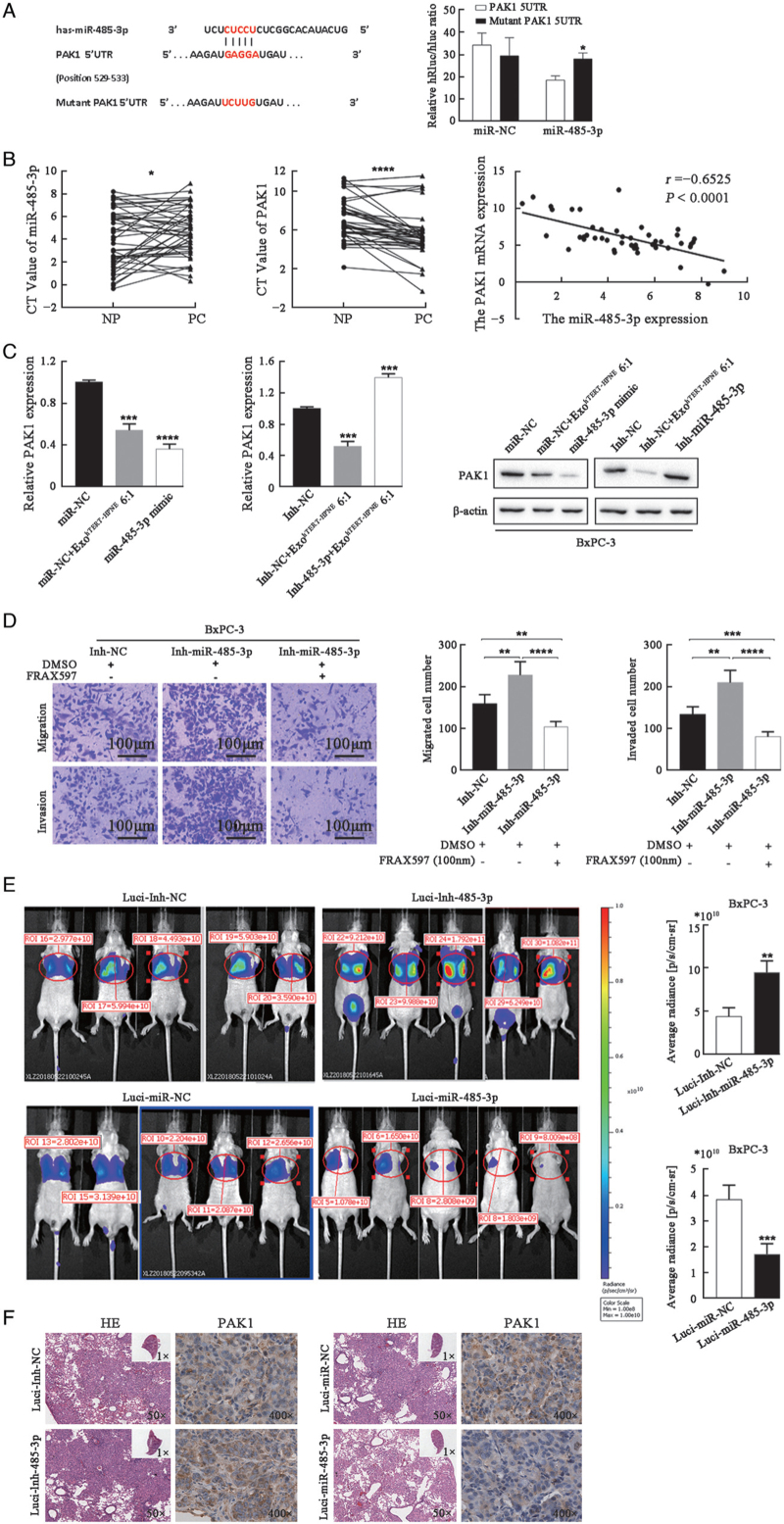
To elucidate the mechanism by which miR-485-3p inhibited PC cell migration and invasion, we conducted bioinformatics analysis to identify its potential targets. The miRNA target database miRWalk suggested the presence of a putative miR-485-3p binding site in the 5′UTR of PAK1 [Figure 4A, left]. Furthermore, a dual-luciferase reporter assay validated that PAK1 was a target of miR-485-3p in PANC-1 cells [Figure 4A, right]. Then, the expression of miR-485-3p and PAK1 in 46 pairs of human PC tissues and adjacent normal tissues was detected by RT-PCR. The results showed that the level ofmiR-485-3p was significantly decreased while PAK1 was increased in PC tissues, and there was a significantly negative correlation between their expression levels (r = −0.6525, P < 0.0001, Figure 4B). In addition, both ExohTERT-HPNE and transfection of miR-485-3p mimic could decrease the PAK1 expression in either transcription or protein level in BxPC-3 cells, while transfection of Inh-miR-485-3p increased the PAK1 expression in BxPC-3 cells [Figure 4C].
Figure 4.
MiR-485-3p directly modulates PAK1 to suppress PC metastasis. (A) Dual-luciferase reporter assay in PANC-1 cells transfected with vectors containing 5′UTR of PAK1 harboring miR-485-3p binding sites or corresponding mutated sequences. (B) MiR-485-3p and PAK1 expression in 46 PC tissues and adjacent normal tissues (NP) were normalized to U6 and GAPDH, and PAK1 expression was significantly correlated with miR-485-3p expression. (C) The transcription and protein levels of PAK1 in BxPC-3 cells treated with ExohTERT-HPNE and transfected with miR-485-3p mimic or Inh-miR-485-3p were analyzed by RT-PCR and Western blot. GAPDH and β-actin served as endogenous reference. (D) MiR-485-3p silencing BxPC-3 cells and control cells were exposed to DMSO or 100 nM FRAX597 for 48 h and subjected to Transwell migration and invasion assays (bar: 100 μm). (E) Bioluminescence images of mice in each group. The average fluorescence radiance of mice was compared. (F) Representative H&E staining of pulmonary metastatic lesions and IHC staining of PAK1 in the lungs. ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001,∗∗∗∗P < 0.0001. IHC: Immunohistochemistry; PC: Pancreatic cancer; RT-PCR: Real-time PCR; DMSO: Dimethylsulfoxide; CT: Cycle threshold.
To prove the functional interaction of PAK1 and miR-485-3p, the small molecule PAK1 inhibitor FRAX59719 (MedChemExpress, Shanghai, China) was used. While silencing of miR-485-3p promoted BxPC-3 cell migration and invasion, these effects were abrogated by FRAX597 [Figure 4D].
To verify the role of miR-485-3p in PC metastasis in vivo, BxPC-3 cells transfected with different vectors (luci-miR-NC, luci-miR-485-3p, luci-Inh-NC, and luci-Inh-485-3p) were injected into the lateral tail vein of nude mice. After 5 weeks, fluorescence radiance was detected in nude mice using an IVIS Spectrum in-vivo imaging system [Figure 4E]. The lungs were dissected for IHC analysis at the endpoint to evaluate PC metastasis to the lungs. miR-485-3p overexpression effectively decreased PAK1 levels and inhibited PC metastasis, while miR-485-3p silencing increased PAK levels and promoted PC metastasis [Figure 4F]. Taken together, these data indicate that miR-485-3p could suppress PC metastasis in vivo by targeting PAK1.
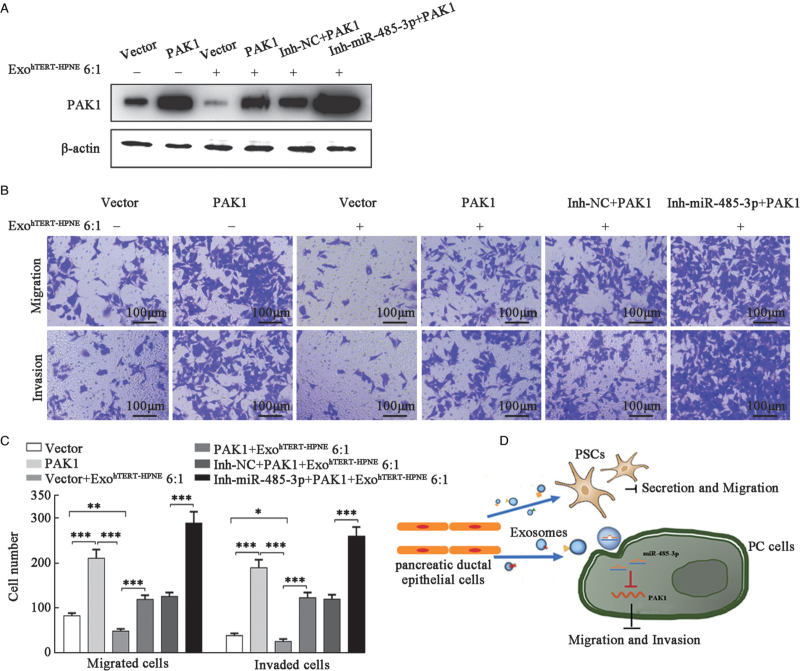
MiR-485-3p mediated the inhibitory effect of ExohTERT-HPNE on PC cell migration and invasion via PAK1
To confirm that hTERT-HPNE cells derived exosomal miR-485-3p suppressed PC cell migration and invasion by decreasing PAK1, a lenti-PAK1/BxPC-3 cell line was established to overexpress PAK1 in a stable manner, and the cells were treated with ExohTERT-HPNE in the absence or presence of miR-485-3p inhibitor (Inh-miR-485-3p) or negative control inhibitor (Inh-NC). Western blot analysis confirmed that Inh-miR-485-3p could augment PAK1 overexpression in PAK1/BxPC-3 stable cells [Figure 5A]. Transwell migration and invasion assays showed that PAK1 overexpression restored cell migration and invasion inhibited by ExohTERT-HPNE, and the effect could be augmented by Inh-miR-485-3p [Figure 5B,C].
Figure 5.
miR-485-3p mediated inhibitory effects of ExohTERT-HPNE on PC metastasis. (A) BxPC-3/PAK1 cells were treated with ExohTERT-HPNE and/or Inh-miR-485-3p, and the PAK1 protein levels were detected by Western blot. β-actin served as loading control. (B) Transwell migration and invasion assays were conducted in each group. (C) Quantitative analysis of BxPC-3 cell migration and invasion. (D) The model of cell competition in PC tissues. ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001,∗∗∗∗P < 0.0001. PC: Pancreatic cancer; PSCs: Pancreatic stellate cells.
These data indicate that the inhibitory effect of ExohTERT-HPNE on PC cell migration and invasion may be mediated by the targeting of PAK1 by miR-485-3p [Figure 5D].
Discussion
In this study, we demonstrated the cell competition phenomenon in PC tissues, a resultant in which normal pancreatic ductal epithelial cells could release exosomes to suppress the malignant behaviors of PC cells and PSCs.
Moreover, we elucidated that exosomal miR-485-3p from normal human pancreatic ductal epithelial cells mediated cell competition by targeting PAK1 in PC cells.
Increasing studies reveal the epithelial defense against cancer phenomenon, under which normal epithelial cells often eliminate newly emerging transformed cells by squeezing these cells and affecting their metabolism to promote apical elimination.[24–27] However, transformed cells, especially those with TP53, KRAS mutations, often acquire malignant phenotypes to survive, such as entosis.[28] In addition, the activation of PSCs precedes the tumorigenesis during the development of PC, which has been confirmed to enhance the malignancy of PC cells.[29–33] Our results proved that in human PC, the normal pancreatic ductal cells suppressed PC cell migration and invasion as well as the migration and secretion of PSCs, but did not affect their proliferation, at least partially via exosomes. Furthermore, we demonstrated that miR-485-3p was especially expressed in ExohTERT-HPNE rather than in exosomes of PC cells and PSCs, and the mature miR-485-3p could be delivered into PC cells and PSCs directly by ExohTERT-HPNE.
MiR-485-3p has been proved to exert anti-cancer effects in several types of cancers,[34–37] but its role in PC remains to be elucidated. In the current study, we confirmed that miR-485 was significantly decreased in both PC tissues and cell lines. And clinical data analysis showed that miR-485-3p was associated with perineural and lymphovascular invasion in PC patients. Moreover, transfection of miR-485-3p mimic inhibited PC cell migration and invasion, and the miR-485-3p inhibitor promoted PC cell migration and invasion. The upregulation of miR-485-3p in ExohTERT-HPNE enhanced the inhibitory effects on PC cells, while the knockdown of miR-485-3p did not abolish the suppression of ExohTERT-HPNE, suggesting that miR-485-3p may not be the only molecule to mediate the role of ExohTERT-HPNE on PC cells. In addition, the overexpression and knockdown of miR-485-3p did not affect migration of PSCs, and so there must be other molecules in ExohTERT-HPNE mediating the inhibitory effects of ExohTERT-HPNE on PSCs. This aspect requires further investigation.
Next, we explored the regulatory mechanism of miR-485-3p in PC cells and identified PAK1 as its target in PC cells.
PAK1 is an important non-receptor serine/threonine kinase that serves as a downstream activator of various oncogenic signaling pathways.[38–40] PAK1 expression is upregulated in PC compared to normal tissues.[41,42] The inhibition of PAK1 by inhibitor or the upstream molecule could suppress PC metastasis, PC cell proliferation and drug resistance, and immunosuppressive microenvironment.[43–45] In this study, we constructed BxPC-3 cells stably overexpressing PAK1 and demonstrated that miR-485-3p suppressed PC cell invasion in vitro and inhibited lung metastasis of xenografted PC cells in a mouse model by targeting PAK1.
Collectively, we proposed a model for normal pancreatic ductal epithelial cells to exhibit cell competition against PC cells and PSCs in PC tissues. In particular, exosomal miR-485-3p delivered by normal pancreatic ductal epithelial cells into PC cells inhibits PC metastasis by directly targeting PAK1. The restoration of miR-485-3p by exosomes or some other vehicle might be a novel approach for PC treatment.
Funding
This study was supported by grants from the National Natural Science Foundation of China (No. 82171722, No. 81871954), the Beijing Natural Science Foundation (No. 7212111), and the Peking University Medicine Fund of Fostering Young Scholar's Scientific & Technological Innovation supported by “the Fundamental Research Funds for the Central Universities” (BMU2018PY026).
Conflicts of interest
None.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Footnotes
How to cite this article: Li M, Zhou J, Zhang Z, Li J, Wang F, Ma L, Tian X, Mao Z, Yang Y. Exosomal miR-485-3p derived from pancreatic ductal epithelial cells inhibits pancreatic cancer metastasis through targeting PAK1. Chin Med J 2022;135:2326–2337. doi: 10.1097/CM9.0000000000002154
Mingzhe Li and Jiaxin Zhou contributed equally to this work.
Supplemental digital content is available for this article.
References
- 1.Baker NE. Author correction: emerging mechanisms of cell competition. Nat Rev Genet 2020; 21:683–697. doi: 10.1038/s41576-020-00283-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Merino MM, Rhiner C, Lopez-Gay JM, Buechel D, Hauert B, Moreno E. Elimination of unfit cells maintains tissue health and prolongs lifespan. Cell 2015; 160:461–476. doi: 10.1016/j.cell.2014.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Martins VC, Busch K, Juraeva D, Blum C, Ludwig C, Rasche V, et al. Cell competition is a tumour suppressor mechanism in the thymus. Nature 2014; 509:465–470. doi: 10.1038/nature13317. [DOI] [PubMed] [Google Scholar]
- 4.Waclaw B, Bozic I, Pittman ME, Hruban RH, Vogelstein B, Nowak MA. A spatial model predicts that dispersal and cell turnover limit intratumour heterogeneity. Nature 2015; 525:261–264. doi: 10.1038/nature14971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reinfeld BI, Madden MZ, Wolf MM, Chytil A, Bader JE, Patterson AR, et al. Cell-programmed nutrient partitioning in the tumour microenvironment. Nature 2021; 593:282–288. doi: 10.1038/s41586-021-03442-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ma L, Tian X, Wang F, Zhang Z, Du C, Xie X, et al. The long noncoding RNA H19 promotes cell proliferation via E2F-1 in pancreatic ductal adenocarcinoma. Cancer Biol Ther 2016; 17:1051–1061. doi: 10.1080/15384047.2016.1219814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schuh CMAP, Cuenca J, Alcayaga-Miranda F, Khoury M. Exosomes on the border of species and kingdom intercommunication. Transl Res 2019; 210:80–98. doi: 10.1016/j.trsl.2019.03.008. [DOI] [PubMed] [Google Scholar]
- 8.An Y, Lin S, Tan X, Zhu S, Nie F, Zhen Y, et al. Exosomes from adipose-derived stem cells and application to skin wound healing. Cell Prolif 2021; 54:e12993.doi: 10.1111/cpr.12993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang X, Yuan T, Tschannen M, Sun Z, Jacob H, Du M, et al. Characterization of human plasma-derived exosomal RNAs by deep sequencing. BMC Genomics 2013; 14:319.doi: 10.1186/1471-2164-14-319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li M, Guo H, Wang Q, Chen K, Marko K, Tian X, et al. Pancreatic stellate cells derived exosomal miR-5703 promotes pancreatic cancer by downregulating CMTM4 and activating PI3K/Akt pathway. Cancer Lett 2020; 490:20–30. doi: 10.1016/j.canlet.2020.06.009. [DOI] [PubMed] [Google Scholar]
- 11.Patel GK, Khan MA, Bhardwaj A, Srivastava SK, Zubair H, Patton MC, et al. Exosomes confer chemoresistance to pancreatic cancer cells by promoting ROS detoxification and miR-155-mediated suppression of key gemcitabine-metabolising enzyme, DCK. Br J Cancer 2017; 116:609–619. doi: 10.1038/bjc.2017.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Soltész B, Buglyó G, Németh N, Szilágyi M, Pos O, Szemes T, et al. The role of exosomes in cancer progression. Int J Mol Sci 2021; 22:12204.doi: 10.3390/ijms230 10008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lima LG, Ham S, Shin H, Chai EPZ, Lek ESH, Lobb RJ, et al. Tumor microenvironmental cytokines bound to cancer exosomes determine uptake by cytokine receptor-expressing cells and biodistribution. Nat Commun 2021; 12:3543.doi: 10.1038/s41467-021-23946-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zheng R, Du M, Wang X, Xu W, Liang J, Wang W, et al. Exosome-transmitted long non-coding RNA PTENP1 suppresses bladder cancer progression. Mol Cancer 2018; 17:143.doi: 10.1186/s12943-018-0880-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meng FT, Huang M, Fan FF, Shao F, Wang C, Huang Q. A modified method for isolating human quiescent pancreatic stellate cells. Cancer Manag Res 2019; 11:1533–1539. doi: 10.2147/CMAR.S192354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Takikawa T, Masamune A, Yoshida N, Hamada S, Kogure T, Shimosegawa T. Exosomes derived from pancreatic stellate cells: microRNA signature and effects on pancreatic cancer cells. Pancreas 2017; 46:19–27. doi: 10.1097/MPA.0000000000000722. [DOI] [PubMed] [Google Scholar]
- 17.Kugeratski FG, Hodge K, Lilla S, McAndrews KM, Zhou X, Hwang RF, et al. Quantitative proteomics identifies the core proteome of exosomes with syntenin-1 as the highest abundant protein and a putative universal biomarker. Nat Cell Biol 2021; 23:631–641. doi: 10.1038/s41556-021-00693-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang Z, Wang F, Du C, Guo H, Ma L, Liu X, et al. BRM/SMARCA2 promotes the proliferation and chemoresistance of pancreatic cancer cells by targeting JAK2/STAT3 signaling. Cancer Lett 2017; 402:213–224. doi: 10.1016/j.canlet.2017.05.006. [DOI] [PubMed] [Google Scholar]
- 19.Vonlaufen A, Phillips PA, Xu Z, Goldstein D, Pirola RC, Wilson JS, et al. Pancreatic stellate cells and pancreatic cancer cells: an unholy alliance. Cancer Res 2008; 68:7707–7710. doi: 10.1158/0008-5472.CAN-08-1132. [DOI] [PubMed] [Google Scholar]
- 20.Amrutkar M, Aasrum M, Verbeke CS, Gladhaug IP. Secretion of fibronectin by human pancreatic stellate cells promotes chemoresistance to gemcitabine in pancreatic cancer cells. BMC Cancer 2019; 19:596.doi: 10.1186/s12885-019-5803-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Erdogan B, Ao M, White LM, Means AL, Brewer BM, Yang L, et al. Cancer-associated fibroblasts promote directional cancer cell migration by aligning fibronectin. J Cell Biol 2017; 216:3799–3816. doi: 10.1083/jcb.201704053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Olivares O, Mayers JR, Gouirand V, Torrence ME, Gicquel T, Borge L, et al. Collagen-derived proline promotes pancreatic ductal adenocarcinoma cell survival under nutrient limited conditions. Nat Commun 2017; 8:16031.doi: 10.1038/ncomms16031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Feig C, Jones JO, Kraman M, Wells RJ, Deonarine A, Chan DS, et al. Targeting CXCL12 from FAP-expressing carcinoma-associated fibroblasts synergizes with anti-PD-L1 immunotherapy in pancreatic cancer. Proc Natl Acad Sci U S A 2013; 110:20212–20217. doi: 10.1073/pnas.1320318110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kon S, Ishibashi K, Katoh H, Kitamoto S, Shirai T, Tanaka S, et al. Cell competition with normal epithelial cells promotes apical extrusion of transformed cells through metabolic changes. Nat Cell Biol 2017; 19:530–541. doi: 10.1038/ncb3509. [DOI] [PubMed] [Google Scholar]
- 25.Maruyama T, Fujita Y. Cell competition in mammals – novel homeostatic machinery for embryonic development and cancer prevention. Curr Opin Cell Biol 2017; 48:106–112. doi: 10.1016/j.ceb.2017.06.007. [DOI] [PubMed] [Google Scholar]
- 26.Hogan C, Dupré-Crochet S, Norman M, Kajita M, Zimmermann C, Pelling AE, et al. Characterization of the interface between normal and transformed epithelial cells. Nat Cell Biol 2009; 11:460–467. doi: 10.1038/ncb1853. [DOI] [PubMed] [Google Scholar]
- 27.Kajita M, Sugimura K, Ohoka A, Burden J, Suganuma H, Ikegawa M, et al. Filamin acts as a key regulator in epithelial defence against transformed cells. Nat Commun 2014; 5:4428.doi: 10.1038/ncomms5428. [DOI] [PubMed] [Google Scholar]
- 28.Hayashi A, Yavas A, McIntyre CA, Ho YJ, Erakky A, Wong W, et al. Genetic and clinical correlates of entosis in pancreatic ductal adenocarcinoma. Mod Pathol 2020; 33:1822–1831. doi: 10.1038/s41379-020-0549-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sousa CM, Biancur DE, Wang X, Halbrook CJ, Sherman MH, Zhang L, et al. Pancreatic stellate cells support tumour metabolism through autophagic alanine secretion. Nature 2016; 536:479–483. doi: 10.1038/nature19084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang HC, Lin YL, Hsu CC, Chao YJ, Hou YC, Chiu TJ, et al. Pancreatic stellate cells activated by mutant KRAS-mediated PAI-1 upregulation foster pancreatic cancer progression via IL-8. Theranostics 2019; 9:7168–7183. doi: 10.7150/thno.36830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ferdek PE, Jakubowska MA. Biology of pancreatic stellate cells-more than just pancreatic cancer. Pflugers Arch 2017; 469:1039–1050. doi: 10.1007/s00424-017-1968-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sherman MH. Stellate cells in tissue repair, inflammation, and cancer. Annu Rev Cell Dev Biol 2018; 34:333–355. doi: 10.1146/annurev-cellbio-100617-062855. [DOI] [PubMed] [Google Scholar]
- 33.Shi Y, Gao W, Lytle NK, Huang P, Yuan X, Dann AM, et al. Author correction: targeting LIF-mediated paracrine interaction for pancreatic cancer therapy and monitoring. Nature 2019; 569:131–135. doi: 10.1038/s41586-021-04176-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lai J, Xin J, Fu C, Zhang W. CircHIPK3 promotes proliferation and metastasis and inhibits apoptosis of renal cancer cells by inhibiting MiR-485-3p. Cancer Cell Int 2020; 20:248.doi: 10.1186/s12935-020-01319-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lou C, Xiao M, Cheng S, Lu X, Jia S, Ren Y, et al. MiR-485-3p and miR-485-5p suppress breast cancer cell metastasis by inhibiting PGC-1alpha expression. Cell Death Dis 2016; 7:e2159.doi: 10.1038/cddis.2016.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu J, Liu X, Li R. LncRNA SNHG6 enhances the radioresistance and promotes the growth of cervical cancer cells by sponging miR-485-3p. Cancer Cell Int 2020; 20:424.doi: 10.1186/s12935-020-01448-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang Q, Liu MJ, Bu J, Deng JL, Jiang BY, Jiang LD, et al. miR-485-3p regulated by MALAT1 inhibits osteosarcoma glycolysis and metastasis by directly suppressing c-MET and AKT3/mTOR signalling. Life Sci 2021; 268:118925.doi: 10.1016/j.lfs.2020.118925. [DOI] [PubMed] [Google Scholar]
- 38.Wang K, Baldwin GS, Nikfarjam M, He H. p21-activated kinase signalling in pancreatic cancer: new insights into tumour biology and immune modulation. World J Gastroenterol 2018; 24:3709–3723. doi: 10.3748/wjg.v24.i33.3709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Feng X, Zhang H, Meng L, Song H, Zhou Q, Qu C, et al. Hypoxia-induced acetylation of PAK1 enhances autophagy and promotes brain tumorigenesis via phosphorylating ATG5. Autophagy 2020; 17:723–742. doi: 10.1080/15548627.2020.1731266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li B, Jia R, Li W, Zhou Y, Guo D, Teng Q, et al. PAK1 mediates bone marrow stromal cell-induced drug resistance in acute myeloid leukemia via ERK1/2 signaling pathway. Front Cell Dev Biol 2021; 9:686695.doi: 10.3389/fcell.2021.686695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang J, Zhu Y, Chen J, Yang Y, Zhu L, Zhao J, et al. Identification of a novel PAK1 inhibitor to treat pancreatic cancer. Acta Pharm Sin B 2019; 10:603–614. doi: 10.1016/j.apsb.2019.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Symeonidis N, Lambropoulou M, Pavlidis E, Anagnostopoulos C, Tsaroucha A, Kotini A, et al. PAK1 expression in pancreatic cancer: clinicopathological characteristics and prognostic significance. Clin Med Insights Oncol 2019; 13:1179554919831990.doi: 10.1177/1179554919831990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang K, Zhan Y, Huynh N, Dumesny C, Wang X, Asadi K, et al. Inhibition of PAK1 suppresses pancreatic cancer by stimulation of anti-tumour immunity through down-regulation of PD-L1. Cancer Lett 2019; 472:8–18. doi: 10.1016/j.canlet.2019.12.020. [DOI] [PubMed] [Google Scholar]
- 44.Xu S, Lei SL, Liu KJ, Yi SG, Yang ZL, Yao HL. circSFMBT1 promotes pancreatic cancer growth and metastasis via targeting miR-330-5p/PAK1 axis. Cancer Gene Ther 2020; 28:234–249. doi: 10.1038/s41417-020-00215-2. [DOI] [PubMed] [Google Scholar]
- 45.Yeo D, He H, Patel O, Lowy AM, Baldwin GS, Nikfarjam M. FRAX597, a PAK1 inhibitor, synergistically reduces pancreatic cancer growth when combined with gemcitabine. BMC Cancer 2016; 16:24.doi: 10.1186/s12885-016-2057-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.