Abstract
We reported previously that internalization of Staphylococcus aureus by nonprofessional phagocytes involves an interaction between fibronectin (Fn) binding protein (FnBP) and the host cell, resulting in signal transduction, tyrosine kinase activity, and cytoskeletal rearrangement (K. Dziewanowska, J. M. Patti, C. F. Deobald, K. W. Bayles, W. R. Trumble, and G. A. Bohach, Infect. Immun. 67:4673–4678, 1999). The goal of the present study was to identify the host molecules responsible for uptake of the organism through an interaction with FnBP. First, Fn was required for internalization. Addition of small amounts of exogenous Fn stimulated the uptake of S. aureus by HEp-2 cells, which are deficient in Fn synthesis. Fn antibodies blocked internalization of the organism by MAC-T cell monolayers, a bovine epithelial cell line which expresses Fn. Second, a monoclonal antibody (MAb) specific for β1 integrins dramatically reduced S. aureus invasion, suggesting that the formation of a Fn bridge linking the host cell β1 integrin and FnBP precedes internalization. However, ligand blotting of cell membrane proteins with a functional fragment of FnBP consistently identified an additional ∼55-kDa receptor on both human and bovine epithelial cells. This protein was purified and identified by N-terminal microsequencing as heat shock protein 60 (Hsp60). The interaction between FnBP and Hsp60 also occurred when the whole cells were used. Cell membrane localization of Hsp60 was confirmed by biotinylation with an agent nonpermeable to the cell membrane. Pretreatment of epithelial cells with a MAb specific for eukaryotic Hsp60 significantly reduced internalization of S. aureus. Combined, these results suggest that the FnBP binds directly to both Hsp60 and Fn and is linked to β1 integrins through a Fn bridge. The simultaneous involvement of Fn and two host cell ligands, β1 integrins and Hsp60, suggests that FnBP is a multifunctional adhesin that mediates internalization in a manner similar to that proposed for OpaA, the Neisseria gonorrhoeae FnBP homolog (J. P. M. van Putten, T. D. Duensing, and R. L. Cole, Mol. Microbiol. 29:369–379, 1998).
Bacteria have developed various mechanisms for inducing internalization into nonprofessional phagocytes (8, 9). A shared requirement is that a molecular interaction must occur between a bacterial surface adhesin and a host ligand in the cytoplasmic membrane. This interaction must be of sufficiently high affinity to induce signal transduction through the membrane, resulting in cytoskeletal rearrangements and uptake of the organism (14, 37). For some organisms, a simple model involving the binding of a bacterial adhesin to its host receptor is sufficient to induce uptake. Yersinia species and Listeria monocytogenes are examples of organisms with this pattern of uptake. The adhesins on these facultative intracellular pathogens bind directly to integrins or E-cadherin, respectively, with an affinity that is sufficient to induce internalization (15, 24).
A number of reports have described bacterial surface adhesins, which adhere to host extracellular matrix (ECM) proteins. The ECM binding proteins are termed MSCRAMMS, and Staphylococcus aureus expresses several of these proteins with different ligand specificities (17, 18, 27). We demonstrated previously that the S. aureus surface adhesin responsible for stimulating signal transduction upon uptake by nonprofessional phagocytes is one of its MSCRAMMs, fibronectin (Fn) binding protein (FnBP) (6). Our data were confirmed in two additional publications (22, 28). This finding raised several questions regarding the nature of the molecular interactions at the host cell surface.
Fn is the ECM protein commonly associated with integrins. It is known that Fn is bivalent and can serve as a bridging molecule between FnBP and the host cell integrins (17, 26, 39). Although other bacteria use Fn as a link to adhere to host tissues, the mechanisms by which this linkage could induce internalization are less clear. For example, Tran Van Nhieu and Isberg showed that coating of S. aureus with Fn did not lead to efficient internalization (37). It was proposed that the binding affinity between Fn and integrins was not sufficient to induce uptake. Interestingly, at least one organism, Neisseria gonorrhoeae has overcome this limitation by employing a heparin-containing accessory coreceptor, which is necessary to induce maximal internalization by HEp-2 cells (39).
The present study was initiated to identify potential cellular ligands and other molecular requirements for uptake of S. aureus by nonprofessional phagocytes. Using a variety of methods, we found that FnBP binds directly to heat shock protein 60 (Hsp60) on the membranes of human and bovine epithelial cells. Fn and β1 integrins are also required for maximal uptake. Based on these combined results, a potential model to explain the molecular interactions leading to uptake of S. aureus is proposed.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
The S. aureus strains used in this study are listed in Table 1. All strains were grown in Todd-Hewitt broth (Difco Laboratories) for 18 h at 37°C with aeration, in the presence of antibiotics when necessary. The bacterial cells were grown to stationary phase, collected by centrifugation, and washed with sterile 150 mM phosphate-buffered saline (PBS) (pH 7.2). The washed pellets were resuspended to their original volumes in invasion medium (described below), diluted 1:100 with the same medium, and returned to 37°C for 1 to 2 h to induce logarithmic growth.
TABLE 1.
S. aureus strains used in this study
| Strain | Relevant genotypea | Relevant phenotype or propertiesa | Reference |
|---|---|---|---|
| 8325-4 | fnbA+ fnbB+ | FnBPA+ FnBPB+; NCTC 8325, cured of prophages, plasmid free | 25 |
| DU5883 | fnbA::TcrfnbB::Emr | FnBPA− FnBPB−; isogenic mutant of 8325-4 | 10 |
| DU5883(pFnBPA4) | Apr CmrfnbpA+ | FnBPA+ FnBPB−; DU5883 with multicopy shuttle plasmid carrying wild-type fnbpA | 10 |
| DU5875 | spa::Tcr | Spa−; isogenic mutant of 8325-4 | 23 |
fnbpA and fnbpB refer to the structural genes encoding the two staphylococcal FnBPs, A and B, and Spa refers to staphylococcal protein A.
Cell culture.
An established bovine mammary epithelial cell line (MAC-T) (12) was cultured as described previously (6). Briefly, the cells were grown in high-glucose Dulbecco's modified Eagle's medium (DMEM) (Gibco-BRL), supplemented with 10% heat-inactivated fetal bovine serum (HyClone), insulin (5 μg/ml), hydrocortisone (5 μg/ml), penicillin (100 U/ml), and streptomycin sulfate (100 μg/ml). HEp-2 (ATCC CCL 23) and Caco-2 (ATCC HTB 37) cells were cultured in the same medium except that insulin and hydrocortisone were omitted. The cells were seeded to 24-well culture plates (6 × 104/well) or to 100-mm-diameter culture dishes (2 × 106/dish) (Costar) and incubated at 37°C under 6% CO2. When nearly confluent, the monolayers were washed once with sterile PBS and incubated overnight (at 37°C under 6% CO2) in DMEM (1.0 ml/well).
Internalization assays.
For most bacterial internalization assays, the following standard assay was employed. Confluent cell monolayers (approximately 2.3 × 105 MAC-T cells/well or 4.0 × 105 HEp-2 cells/well) were washed once with DMEM containing 1% bovine serum albumin (BSA) and inoculated with bacteria suspended in the same medium (multiplicity of infection [MOI], 10 to 50). After incubation for 1 h (at 37°C under 6% CO2), the wells were washed once with PBS and 1.0 ml of invasion medium, supplemented with 100 μg of gentamicin per ml, was added to each well. The plates were incubated for an additional 1 h to kill extracellular bacteria. The monolayers were washed four times with sterile PBS, detached from the plates by treatment for 5 min at 37°C with 100 μl of trypsin solution (0.25%) in Hanks' balanced salt solution (HBSS) (GibcoBRL), and then lysed by adding 900 μl of sterile deionized water. The cell lysates were serially diluted 10-fold and plated in triplicate on Todd-Hewitt agar plates to quantify intracellular staphylococci.
To assess internalization in the presence of various potential effectors, the standard assay described above was modified as follows. Prior to inoculation with bacteria, the nearly confluent monolayers were preincubated for 30 min (at 37°C under 6% CO2) in DMEM containing 1% BSA, plus one of the following: bovine Fn (1 to 100 nM) (Calbiochem), protein G-purified immunoglobulin G1 LK-1 murine monoclonal antibody (MAb) specific for eukaryotic Hsp60 (recombinant human Hsp60 was used as the immunogen; the epitope is located between residues 383 and 447 of human Hsp60, so that the antibody identifies Hsp60 in eukaryotes but not in prokaryotes) (StressGen Corp.), bovine Fn antiserum prepared in rabbits (Biogenesis Inc.), nonimmune rabbit serum (Sigma), human β1 integrin ascites prepared in mice (clone P4C10; Gibco-BRL), or nonimmune murine ascites. The cultures were then infected with bacteria and processed by the standard internalization assay method.
An additional modification was made in some experiments to assess the effect of ligand-induced receptor translocation on internalization. The protocol for these experiments was based on the receptor downmodulation method described by Rozdzinski and Tuomanen (30). Wells in 96-well microtiter plates (A1/2; Costar) were coated by adding 50 μl of one of the following reagents dissolved in PBS containing 2 mM CaCl2 and 1 mM MgCl2, (DPBS): LK-1 Hsp60 MAb (10 μg/m), Du-D4 functional FnBP Fn binding fragment (16) (100 μg/ml), or 0.1% BSA. The plates were incubated for 1 h at room temperature and then overnight at 4°C. After the plates were washed three times with DPBS, MAC-T cells (5 × 104) in 100 μl of invasion medium–0.1% BSA, were added to each well. The plates were incubated (at 37°C under 6% CO2) for 1 h to allow attachment and spread of the cells. Subsequently 100 μl of bacterial cell suspension (approximately 3.5 × 105 cells) in DMEM–0.1% BSA was added to achieve a MOI between 20 and 30. The plates were incubated for 30 min at 37°C under 6% CO2 to allow internalization of the bacterial inoculum and then processed by the standard internalization assay method.
SDS-PAGE and blotting.
Proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) using 7.5 or 10% acrylamide gel slabs. Proteins were visualized within the gels by staining with Coomassie blue R-250 or by silver staining.
For some experiments, the resolved proteins were transferred to nitrocellulose (pore size, 0.45 μm; Schleicher & Schuell) or Immobilon P (Millipore Corp.) membranes in a Mini Trans-Blot Electrophoretic Transfer Cell (Bio-Rad), using standard methods, and were detected by immunoblotting or ligand blotting. For ligand blotting, potential membrane receptors were probed with Du-D4 peptide (10 μg per ml of blocking solution [3% BSA, 150 mM Tris HCl (pH 7.5), 150 mM NaCl]) followed by a rabbit polyclonal Du-D4 antiserum. Hsp60 immunoblot analyses were conducted using the LK-1 MAb (see above).
Purification of staphylococcal FnBP.
Wild-type S. aureus expresses two highly conserved forms of FnBP, FnBPA and FnBPB (10). Each is encoded by a separate gene, designated fnbpA and fnbpB, respectively. S. aureus DU5885(pFnBPA4) is a complemented deletion mutant that harbors the fnbpA gene on a recombinant plasmid and overexpresses FnBPA, and it was used to purify the protein for this present study.
S. aureus DU5883(pFnBPA4) was grown in Todd-Hewitt broth plus chloramphenicol (5 μg/ml) to a density of ca. 5 × 107 cells per ml. The cells were collected by centrifugation, washed twice with PBS, and resuspended (12 g of wet weight) in 50 ml of 0.7 M sucrose solution (pH 6.5) containing 0.02 M maleate, 0.02 M MgCl2, 2× Bacto Antibiotic Medium 3 (Difco Laboratories), and 200 μg of lysostaphin (Sigma). The suspension was incubated for 15 to 30 min, until cell wall lysis was complete. The suspension was clarified by centrifugation for 30 min at 14,000 × g and then dialyzed exhaustively against water (4°C). FnBP was partially purified from the retentate by preparative isoelectric focusing in crushed Sephadex G-50, using Ampholine (pH 3.5 to 10.0) (Amersham Pharmacia Biotech AB) (11). Gel fractions were analyzed by SDS-PAGE (10% acrylamide) and immunoblotting with rabbit polyclonal serum specific for FnBP (see above). Fractions containing FnBP with only minor impurities were subjected to further purification (to homogeneity) by precipitation with (NH4)2SO4 (60% saturation). The purified protein was dialyzed against 50 mM ammonium acetate and lyophilized.
Isolation of epithelial cell membrane proteins.
MAC-T and HEp-2 cells were grown to near confluency (2.8 × 107 MAC-T cells/dish and 5.5 × 107 HEp-2 cells/dish) in 100-mm-diameter culture dishes, washed twice with HBSS, and then detached by addition of 1 mM EDTA in HBSS for 20 min at 37°C. The detached cells were washed twice with HBSS–5 mM MgCl2–0.5 mM CaCl2 and were either processed immediately or frozen at −80°C until use.
To isolate crude membrane protein preparations, the cell pellet was suspended in 10 volumes of 10 mM Tris (pH 7.5) containing 1 mM EDTA and protease inhibitor cocktail (Boehringer GmbH, Mannheim, Germany). After being incubated for 20 min on ice, the suspension was transferred to, and lysed using, a Dounce homogenizer. Cell lysis was monitored by light microscopy; 10 strokes were usually sufficient. Cell debris was removed by centrifugation initially for 10 min at 1,000 × g, and the membrane fraction was recovered by centrifugation of the supernatant at 100,000 × g for 1 h at 4°C. Proteins in the membrane fraction pellet (derived from 2 × 107 cells) were dissolved by incubation (3 h at 4°C) in 50 mM HEPES buffer (pH 7.5) containing 100 mM NaCl, 1% Triton X-100, 1% sodium deoxycholate, 1 mM EDTA, and protease inhibitor cocktail. The preparation was clarified by centrifugation for 20 min at 100,000 × g and analyzed by SDS-PAGE and electroblotting or used in ligand affinity experiments.
Identification of potential epithelial cell membrane receptors by using an affinity gel matrix.
Affi-Gel 102 was purchased from Bio-Rad, and purified FnBP (see above) was ligated to the gel as specified by the manufacturer. Typically, 0.25 ml of Affi-Gel 102 and 200 μg of FnBP were used. An aliquot of solubilized membrane proteins (100 μl) derived from 2 × 107 cells was diluted 10-fold with 50 mM HEPES buffer (pH 7.5) containing 100 mM NaCl, 1% Triton X-100, 1% sodium deoxycholate, 1 mM EDTA, and protease inhibitor cocktail. The protein preparation was then mixed with 100 μl of FnBP–Affi-Gel 102 in a 1.7-ml centrifuge tube and incubated for 3 h at 4°C on a rocker. The gel was washed extensively with 50 mM HEPES buffer (pH 7.5) containing 100 mM NaCl, 1% Triton X-100, 1% sodium deoxycholate, 1 mM EDTA, and protease inhibitor cocktail. The bound proteins were dissociated from the gel by adding SDS-PAGE sample buffer and analyzed by SDS-PAGE.
Biotinylation of surface membrane proteins.
Biotinylation reactions were performed on nearly confluent cultures of MAC-T cells or HEp-2 cells. The cells were treated with sulfo-N-hydroxysuccinimide-long-chain spacer arm (sulfo-NHS-LC-biotin; Pierce), which is not permeable to the cell membrane, or with the negative control reagent sulfo-NHS-acetate as recommended by the manufacturer. Membrane proteins from cells exposed to the biotinylation reagents were extracted by the method described above for isolation of membrane proteins. The solubilized and biotinylated membrane proteins were analyzed by SDS-PAGE and electroblotting. For the localization of proteins, the blots were incubated with a streptavidin-alkaline phosphatase conjugate (Sigma) or with specific antibodies.
Microsequencing.
For N-terminal sequencing reactions, proteins were resolved by a double SDS-PAGE separation (20). Crude membrane fractions from MAC-T and HEp-2 cells were resolved first in SDS-PAGE preparative gels (10% acrylamide), and the proteins were localized by staining with Coomassie brilliant blue. The desired protein bands were excised. After the gel had been shrunk by soaking in 50% ethanol–50% stacking gel buffer, the excised band was subjected to an additional electrophoresis step, using an SDS-PAGE gel (7.5% acrylamide). Proteins were electroblotted to Immobilon P membranes (Millipore Corp.) backed by nitrocellulose membranes. Proteins on the Immobilon P membrane were localized by staining with Coomassie brilliant blue, and those on the nitrocellulose membrane were probed with Du-D4 peptide and Du-D4 rabbit antiserum. The protein band colocalizing on both blots was excised from the Immobilon P membrane, and N-terminal sequencing was performed by the Edman procedure using a 477A Sequencer.
Interaction between FnBP and Hsp60 on whole cells.
Nearly confluent MAC-T cell monolayers (in 100-mm-diameter culture dishes) were inoculated with S. aureus DU5883 or S. aureus DU5883(pFnBPA4) at a MOI of 50. After 1 h of incubation at 37°C under 6% CO2, the monolayers were washed three times with HBSS and the cells were lysed by being suspended in 1.5 ml of 50 mM HEPES buffer containing 1% Triton X-100, 1% deoxycholate, 1 mM EDTA, and protease inhibitor cocktail. The lysate was transferred to a 2.0-ml centrifuge tube, and insoluble material was recovered by centrifugation for 10 min at 10,000 × g. The pellet was washed three times with lysing solution, solubilized with SDS-PAGE sample buffer, and analyzed by SDS-PAGE, immunoblotting, or ligand blotting, using Hsp60 MAb or Du-D4 peptide probes, as described above.
RESULTS
A functional fragment of FnBP binds to Hsp60 from epithelial cell membrane fractions.
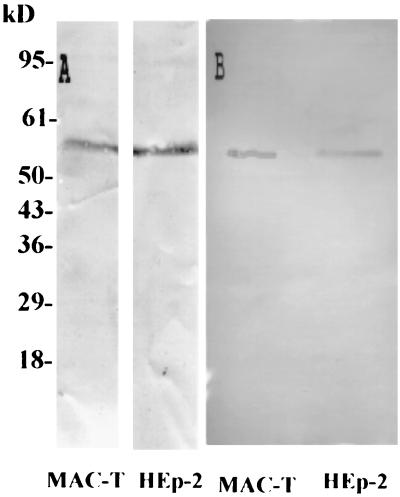
Previous studies in our laboratory have demonstrated that FnBP is essential for efficient internalization within mammary epithelial cells (6). To identify the cell receptor for S. aureus, a ligand-blotting method was employed. Cell membrane proteins, obtained from MAC-T and HEp-2 cells lysates, were resolved by SDS-PAGE (10% acrylamide) and transferred to nitrocellulose. Proteins on the nitrocellulose were probed with Du-D4, a peptide fragment corresponding to the functional ECM binding molecular regions of FnBP. The location of the bound Du-D4 was determined by immunoblotting with Du-D4 rabbit antiserum. This assay revealed that Du-D4 bound significantly to only one major protein band, with an apparent size of ∼55 kDa (Fig. 1A).
FIG. 1.
Ligand blotting for FnBP receptors on epithelial cells. Proteins in MAC-T or HEp-2 cell membrane preparations were resolved by SDS-PAGE and transferred to membranes. The proteins were probed with Du-D4 peptide followed by Du-D4 rabbit antiserum (A) or with Hsp60 MAb (B).
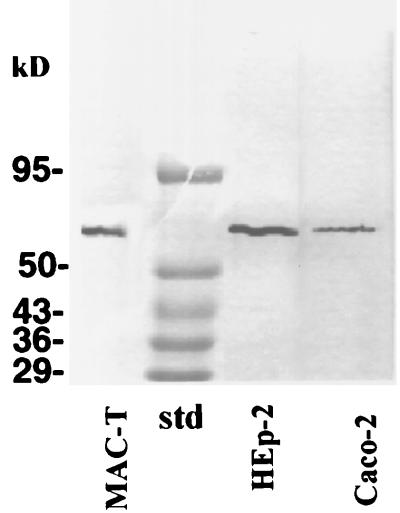
Membrane preparations were obtained from MAC-T, HEp-2, or Caco-2 cells. The partially purified membrane proteins were solubilized and incubated with an affinity gel matrix prepared by ligation of FnBP to Affi-Gel 102. From all three cell types, a protein with an apparent size of ∼55 kDa was retained by the gel (Fig. 2). Control Affi-Gel 102 preparations, derivatized with BSA, did not retain proteins from cell lysates (results not shown).
FIG. 2.
Affinity purification of potential host cell receptors on FnBP–Affi-Gel 102. Solubilized membrane fractions from MAC-T cells, HEp-2 cells, or Caco-2 cells were incubated with FnBP–Affi-Gel 102. The gel was then washed extensively. Proteins on the gel were eluted, analyzed by SDS-PAGE, stained, and in some experiments subjected to N-terminal sequence determinations. Control BSA–Affi-Gel 102 conjugates did not retain proteins from cell lysates (results not shown). std, protein standards.
The identity of this protein was determined by N-terminal microsequencing after being transferred to an Immobilon P membrane. The unambiguous and identical sequence AKDVKFGADA was obtained for the proteins isolated from either MAC-T cells or HEp-2 cells. A search of PIR International Pattern Match, protein databases, and Bioinformatics Tool (www-nbrf.georgetown.edu/pirwww/search/patmatch.html) revealed an exact match with the 10 N-terminal amino acids of human and bovine Hsp60. The identity of the proteins from MAC-T and HEp-2 cells was further confirmed by immunoblotting with the LK-1 Hsp60 MAb (Fig. 1B).
Hsp60 is exposed on the cytoplasmic membrane of epithelial cells.
A potential role for Hsp60 in mediating bacterial adherence and uptake would require its presence on the host cell surface. Although initial experiments (described above) demonstrated the binding of FnBP to Hsp60 in a membrane fraction, it was possible that Hsp60 originated from contamination by a mitochondrial fraction. Thus, to confirm the cell membrane localization and surface exposure of Hsp60, human and bovine epithelial cells were treated with sulfo-NHS-LC-biotin, a biotin derivative which is not permeable to cell membrane. Therefore, any biotinylation observed is attributed to proteins on the cell surface. Furthermore, addition of a biotin tag specifically to the cell surface proteins would be expected to generate two pools of Hsp60 (biotinylated and nonbiotinylated), one derived from the cytoplasmic membrane and the other derived from the intracellular location. As expected, two bands were detected in immunoblots of lysates from surface-biotinylated MAC-T and HEp-2 cells probed with an Hsp60 MAb (Fig. 3). The two bands corresponded to nonbiotinylated Hsp60 (lower band) and its biotinylated derivative (upper band). The latter band is absent in the blot of a control MAC-T cell preparation treated with sulfo-NHS-acetate. These results confirm the cell surface membrane localization of Hsp60 and are consistent with a potential for Hsp60 to interact with FnBP on S. aureus cells.
FIG. 3.
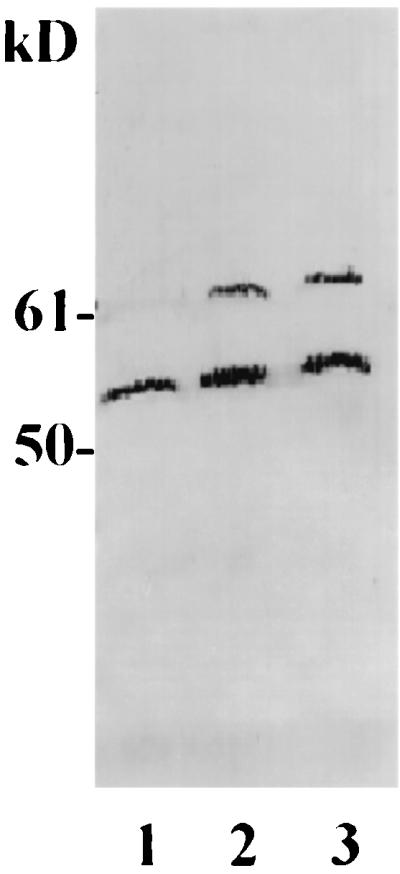
Immunoblot of biotin-tagged and nontagged Hsp60. Intact MAC-T or HEp-2 cells were biotinylated with sulfo-NHS-LC-biotin (lanes 2 and 3) or treated with sulfo-NHS-acetate (for MAC-T cells only) as a negative control (lane 1). Cell lysates were resolved by SDS-PAGE and probed with an Hsp60 MAb.
Interaction of FnBP and Hsp60 on whole cells.
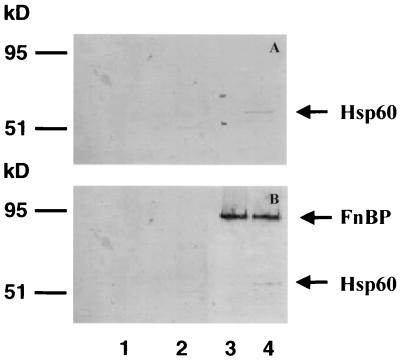
In an experiment to determine the specificity of the Hsp60 interaction with FnBP, MAC-T cell monolayers were incubated with S. aureus DU5883, a mutant deficient in FnBP synthesis, or its isogenic strain complemented with a plasmid harboring fnbpA, the structural gene for FnBPA. The monolayers and bacteria were coincubated for 30 min. The cells were washed to remove nonadherent bacteria, and the MAC-T cells were lysed. The cell lysate was centrifuged to pellet bacterial cells plus any attached MAC-T cell proteins. The resulting pellet was washed exhaustively and analyzed by SDS-PAGE and immunoblotting. The results in Fig. 4 indicate that Hsp60 binds to the surface of intact S. aureus cells expressing FnBP. A protein, which migrated in gels with an apparent size of ∼55 kDa and was identified as Hsp60 by immunoblotting, was consistently detected in cell lysates generated using this technique. This interaction was specific for FnBP since S. aureus DU5883, the FnBP-deficient mutant, did not bind to Hsp60. Similarly, preincubation of S. aureus (pFnBPA4) with Du-D4 antiserum blocked the association of Hsp60 with the bacterial cell surface.
FIG. 4.
FnBP-Hsp 60 interaction on cell surfaces. MAC-T cells were incubated for 30 min with no bacteria (lane 1), S. aureus DU5883 (lane 2), S. aureus DU5883(pFNBPA4) preincubated with Du-D4 rabbit antiserum (lane 3), or S. aureus DU5883(pFNBPA4) (lane 4). After lysis of the MAC-T cells, the residual bacterial cell suspension and attached proteins were recovered by centrifugation and the pellets were solubilized in SDS-PAGE sample buffer, resolved by SDS-PAGE, transferred to a membrane, and probed with Hsp60 MAb (A) or Du-D4 followed by Du-D4 antiserum (B). The protein bands shown in the figure correspond to Hsp 60 (apparent size, ∼55 kDa) (A and B) and S. aureus FnBPA (104 kDa) (B).
Interaction with membrane Hsp60 is required for maximal internalization of S. aureus by epithelial cells.
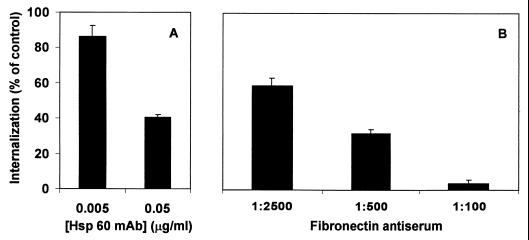
To determine whether interaction of FnBP with Hsp60 could affect internalization of the organism, the effect of a MAb specific for human Hsp60 was assessed. First, MAC-T cell cultures were preincubated with the Hsp60 MAb or a murine acites control and then infected with S. aureus DU5875, a strain expressing FnBP (but not protein A). The results of these experiments are summarized in Fig. 5A and indicate that the addition of the Hsp60 MAb to culture supernatants significantly reduced the efficiency of internalization by the epithelial cells in a dose-dependent manner.
FIG. 5.
Effect of Hsp60 MAb (A) or bovine Fn rabbit antiserum (B) on internalization of S. aureus DU5875 by MAC-T cells. MAC-T cell monolayers were preincubated for 30 min with serum or ascites proteins at the dilution or quantities indicated (MOI = 20). The number of internalized bacteria was determined in a standard internalization assay, and the results are presented as a percentage of the number in identical control cultures treated with nonimmune ascites (A) or normal rabbit serum (B).
Second, we determined the effect of ligand-induced receptor translocation of Hsp60 from the epithelial-cell apical surface. The ability of some membrane proteins to translocate toward their ligands can be used to differentially affect the receptor density on the apical and basal surfaces of cells. As such, it is often possible to reduce the receptor density on the apical membranes of cells by incubating them on surfaces coated with specific ligands or antibodies (30). We predicted that if interaction of Hsp60 with S. aureus FnBP was involved in internalization, translocation of Hsp60 from the apical surfaces of the epithelial cell monolayers should reduce internalization. To address this possibility, MAC-T cells were seeded into wells coated with Hsp60 MAb, Du-D4 peptide (positive control), or BSA (negative control). The results summarized in Table 2 consistently demonstrated, as expected, that Du-D4, the functional fragment of FnBP, reduced internalization by causing translocation of its receptor. A significant reduction was also caused by the Hsp60 antibody, suggesting that an interaction of FnBP with Hsp60 on monolayers is essential for the most efficient internalization of S. aureus.
TABLE 2.
Effect of downmodulating Hsp60 on internalization of S. aureus 8325-4 by MAC-T cells
| Ligand | Internalization (% of control) in:
|
|
|---|---|---|
| Exp I | Exp II | |
| BSA | 100a | 100a |
| Du-D4 | 7.8 ± 2 | 19 ± 2.6 |
| Hsp60 MAb | 19.3 ± 3.1 | 36 ± 3.9 |
100% corresponds to 1.3 × 104 CFU/well (MOI = 15).
Fn and integrins are required for efficient internalization of S. aureus by MAC-T and HEp-2 epithelial cells.
FnBP was initially recognized for its ability to promote adherence to host tissues through its high affinity for Fn. Since integrins also bind Fn and since other bacteria are internalized though β1 integrin signaling (26, 39), it was important to assess the role of Fn in staphylococcal internalization. The effects of adding exogenous Fn varied depending on the cell types used and whether they expressed Fn. For example, initial experiments in which MAC-T cell cultures were first depleted of exogenous Fn and then supplemented with known quantities of Fn showed that internalization was blocked in the presence of very small quantities of exogenous Fn (Fig. 6A). It was suspected that this result was due to saturation of host cell integrins with bovine Fn produced by the MAC-T cells. Supplementing cultures with additional soluble Fn presumably results in its binding to FnBP, making it impossible for the staphylococcal adhesin to interact with Fn on the MAC-T cell surface. We subsequently confirmed this prediction by using two different methods. First, we showed that MAC-T cells express their own endogenous Fn by immunoblot analyses of membrane proteins using Fn antiserum (results not shown). Second, additional experiments showed that uptake of S. aureus by MAC-T cells was reduced in a dose-dependent manner when cultures were preincubated with a commercially available Fn antiserum (Fig. 5B).
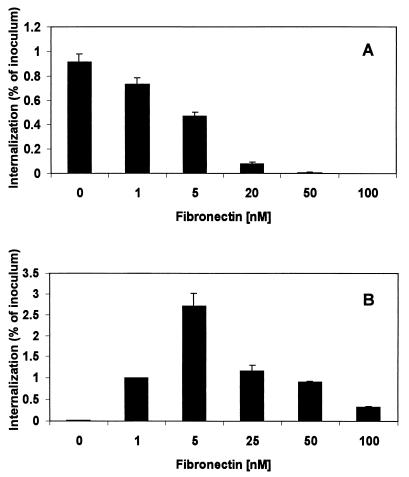
FIG. 6.
Effect of Fn on internalization of staphylococci by HEp-2 or MAC-T cells. Monolayers were incubated for 30 min with Fn, at the concentrations shown, prior to infection with the bacterial suspension (MOI = 25). The number of internalized bacteria was determined by the standard invasion assay, and the results are presented as the percentage of the inoculum used.
Interestingly, a different effect was observed with HEp-2 cells, a cell line noted for lack of Fn expression (26). Experiments using HEp-2 cells showed a clear dose-response effect in which small quantities of Fn (up to 5 nM) stimulated uptake (Fig. 6). These results were consistent with a model in which Fn helps link S. aureus FnBP to the epithelial-cell surface by binding to integrins. Higher Fn concentrations blocked uptake. This effect is consistent with the function of Fn in this system as a bifunctional molecule in which a blocking effect at high concentrations would be due to saturation of the integrin-FnBP system.
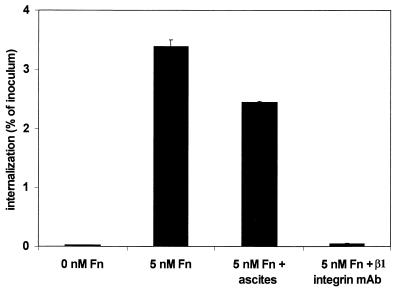
β1 integrins were also required for efficient internalization of S. aureus DU5875 by HEp-2 cells. Preincubation of monolayers with the β1 integrin MAb caused a dramatic reduction in internalization to levels similar to those in the absence of exogenous Fn (Fig. 7). We were unable to demonstrate a similar requirement for integrins with the bovine MAC-T cells since MAbs raised against either bovine or human β1 integrins did not block internalization (results not shown). The reason for this is unclear and is currently a topic of investigation by our laboratories.
FIG. 7.
Effect of β1 integrin MAb on internalization of S. aureus DU5875 by HEp-2 cells. HEp-2 monolayers were preincubated with or without 5 nM Fn plus nonimmune ascites (1:200) or β1 integrin MAb (1:200) for 30 min prior to infection with the bacterial suspension (MOI = 17). The number of internalized bacteria was determined by the standard invasion assay, and the results are presented as the percentage of inoculum used.
DISCUSSION
We previously demonstrated that S. aureus could be internalized by nonprofessional phagocytes through a process requiring FnBP binding to the host cell surface. Our results showed that binding of FnBP to the host cell induced a genistein-sensitive signal transduction and cytoskeletal rearrangement (6), effects similar to those seen with other well-characterized intracellular organisms (29). This present study was performed to identify potential ligands for S. aureus on epithelial cells. Using a variety of techniques, we obtained evidence for a complex interaction at the bacterial and epithelial cell surfaces involving at least four molecules, FnBP, Fn, β1 integrins, and Hsp60. The interactions of FnBP with Fn and integrins have been demonstrated previously and are considered to be crucial for binding to host tissues (17, 26). However, the extension of these observations to defining a role for Fn and integrins in internalization of S. aureus plus the potential contribution of Hsp60 in this process were less obvious prior to the beginning of this study.
To identify host cell ligand proteins that could bind directly to FnBP, we used standard ligand affinity binding techniques that had been used to identify receptors for a number of other intracellular bacterial adhesins (15, 30, 37, 38). The only protein that consistently bound to purified FnBP, cell-associated FnBP, or Du-D4, a functional fragment of FnBP, was bovine or human Hsp60, depending on the cell source. Although the primary sequences of FnBPA and FnBPB are not identical, the predicted functional residues within the region encompassed by the Du-D4 peptide are conserved (17). This suggests that both FnBPs have the ability to interact with Hsp60, although only purified FnBPA was used in the present study. Interestingly, these results are consistent with those of Tompkins et al. (36), who reported the isolation of a 50-kDa protein from human endothelial cells that was capable of binding to S. aureus cells in vitro. Although the protein in that former study was not identified, its properties, including the ability to interact directly with the surface of S. aureus, resembled those of Hsp60 in the present study.
Its direct binding to FnBP, the staphylococcal adhesin responsible for internalization, raises the possibility that Hsp60 is involved in the adherence and internalization processes. In support of this possibility was our demonstration that a MAb raised against Hsp60 significantly reduced internalization of S. aureus by MAC-T cells. Hsp60 is a 57-kDa protein that was originally well characterized because of its function as a molecular chaperonin and its association with the mitochondrial matrix. However, a number of recent reports have documented the presence of Hsp60 not only in the mitochondria but also in several extramitochondrial sites including the cytoplasmic membrane (3, 5, 13, 19, 21, 31, 32, 42). Although many studies have associated the surface expression of Hsp60 with stressed, apoptotic, or transformed cells, membrane Hsp60 is now considered to be typical of eukaryotic cells in general (33, 34).
To be involved in S. aureus internalization by nonprofessional phagocytes, Hsp60 would have to possess several important properties. The first, localization on the cell surface, was confirmed by using several techniques. The topology of Hsp60 in the cytoplasmic membrane is not well understood, but a transmembrane prediction program (Prediction of Transmembrane Regions and Orientation, ISREC Bioinformatics Server, Lausanne, Switzerland [www.ch.embnet.org/cgibin.TMPRED_form.html]) showed that Hsp60 has at least two potential transmembrane helices (results not shown). Although mitochondrial Hsp60 is believed to form heptameric rings (2, 7, 35, 40), it is unclear if membrane Hsp60 aggregates in a similar fashion.
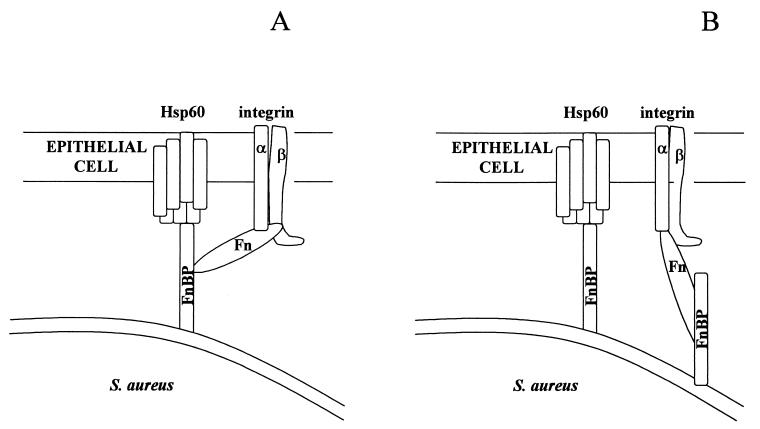
A second requirement for internalization is that Hsp60 should be able to either (i) facilitate signaling by another membrane protein or (ii) to transduce a signal directly. The direct binding of FnBP to Hsp60 does not preclude a potential role for other host ligands or ECM such as Fn and fibrinogen, both of which bind to FnBP (41). In fact, the results of the present study, in addition to the documented ability of Fn and integrins to mediate the adherence of S. aureus (4), suggest that Hsp60 could function as a coreceptor with integrins linked through Fn (Fig. 8A). A similar model has been proposed by van Putten et al. (39) for Neisseria gonorrhoea internalization. OpaA, a gonococcal FnBP, mediates uptake through β1 integrin signaling. The gonococcal model involves a Fn bridge linking OpaA and the integrin, with the Fn linkage stabilized by a host cell glycosaminoglycan coreceptor. The coligand is suspected to bind simultaneously to OpaA and Fn. Binding of bacterial FnBPs to a coligand (i.e., Hsp60 or glycosaminoglycan) would be one potential mechanism for organisms to overcome the low-affinity interaction between Fn and β1 integrins. This notion is consistent with the results of Tran Van Nhieu and Isberg (37) indicating that Fn alone is insufficient to induce the uptake of microorganisms including S. aureus. It was postulated that this inability was due to a low-affinity binding of Fn to the integrin, in contrast to high-affinity bacterial ligands such as Yersinia invasin, which binds directly to integrins.
FIG. 8.
Potential models for FnBP receptor binding leading to internalization. (A) FnBP interacts with integrin and Hsp60 coreceptors through a Fn linkage. (B) FnBP interacts independently with Hsp60 and the Fn-integrin complex.
Another possibility is that the binding of FnBP to Hsp60 occurs independently of β1 integrins (Fig. 8B). This scenario would most probably require a direct or indirect role for Hsp60 in signal transduction. Although Hsp60 has several potential phosphorylation sites and can be phosphorylated by protein kinase A (21), it has not been reported to directly transduce a signal following binding to extracellular ligands. However, Hsp60 is known to control the activity of Src tyrosine kinases. Phosphorylation of E, A, and Y random polymers by c-Src kinase was stimulated 20-fold in the presence of Hsp60 (1). Also, Hsp60 interacts with p21ras, a signaling molecule in the cytoplasmic membrane (5, 13). Whether any of these activities is involved in internalization of S. aureus into epithelial cells is currently under investigation in our laboratories.
ACKNOWLEDGMENTS
This work was supported by USDA (NRICGP) (G.A.B.) and PHS grants AI28401 (G.A.B.) and AI38901 (K.W.B.), the United Diarymen of Idaho (G.A.B.), and the Idaho Agricultural Experiment Station.
We are grateful to Timothy Foster, who supplied the strains used in this study. N-terminal sequencing was performed by Laurey Steinke, Protein Structure Core Facility, University of Nebraska Medical Center, Omaha, Nebr.
ADDENDUM IN PROOF
We recently became aware of a supporting publication (B. Sinha, P. P. François, O. Nüße, M. Foti, O. M. Hartford, P. Vaudaux, T. J. Foster, D. L. Lew, M. Hermann, and K.-H. Krause, Cell. Microbiol. 1:101–117, 1999) in which the authors provided evidence of a role for host cell integrins and fibronectin in internalization of S. aureus by a variety of nonprofessional phagocytes.
REFERENCES
- 1.Abdel-Ghany M, El-Gendy K, Racker E. Control of src kinase activity by activators, inhibitors, and substrate chaperones. Proc Natl Acad Sci USA. 1990;87:7061–7065. doi: 10.1073/pnas.87.18.7061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Azem A, Kessel M, Goloubinoff P. Characterization of a functional GroEL14(GroES7)2 chaperonin hetero-oligomer. Science. 1994;265:653–656. doi: 10.1126/science.7913553. [DOI] [PubMed] [Google Scholar]
- 3.Belles C, Kuhl A, Nosheny R, Carding S R. Plasma membrane expression of heat shock protein 60 in vivo in response to infection. Infect Immun. 1999;67:4191–4200. doi: 10.1128/iai.67.8.4191-4200.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berendt A R, McCormick C J. Use of host adhesion molecules by infectious agents. In: Leendert P C, Issekutz T B, editors. Adhesion molecules in health and disease. New York, N.Y: Marcel Dekker, Inc.; 1997. pp. 347–379. [Google Scholar]
- 5.de Gunzburg J, Riehl R, Weinberg R A. Identification of a protein associated with p21ras by chemical crosslinking. Proc Natl Acad Sci USA. 1989;86:4007–4011. doi: 10.1073/pnas.86.11.4007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dziewanowska K, Patti J M, Deobald C F, Bayles K W, Trumble W R, Bohach G A. Fibronectin binding protein and host cell tyrosine kinase are required for internalization of Staphylococcus aureus by epithelial cells. Infect Immun. 1999;67:4673–4678. doi: 10.1128/iai.67.9.4673-4678.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fenton W A, Horwich A L. GroEL-mediated protein folding. Protein Sci. 1997;6:743–760. doi: 10.1002/pro.5560060401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Finlay B B, Cossart P. Exploitation of mammalian host cell functions by bacterial pathogens. Mol Microbiol. 1997;276:718–725. doi: 10.1126/science.276.5313.718. [DOI] [PubMed] [Google Scholar]
- 9.Goosney D L, Knoechel D G, Finlay B B. Enteropathogenic E. coli, Salmonella, and Shigella: masters of host cell cytoskeletal exploitation. Emerg Infect Dis. 1999;5:216–223. doi: 10.3201/eid0502.990205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Greene C, McDevitt D, Francois P, Vaudaux P E, Lew D P, Foster T J. Adhesion properties of mutants of Staphylococcus aureus defective in fibronectin-binding proteins and studies on the expression of fnb genes. Mol Microbiol. 1995;17:1143–1152. doi: 10.1111/j.1365-2958.1995.mmi_17061143.x. [DOI] [PubMed] [Google Scholar]
- 11.Hovde C J, Marr J C, Hoffmann M L, Hackett S P, Chi Y I, Crum K K, Stevens D L, Stauffacher C V, Bohach G A. Investigation of the role of the disulphide bond in the activity and structure of staphylococcal enterotoxin C1. Mol Microbiol. 1994;13:897–909. doi: 10.1111/j.1365-2958.1994.tb00481.x. [DOI] [PubMed] [Google Scholar]
- 12.Huynh H T, Robitaille G, Turner J D. Establishment of bovine mammary epithelial cells (MAC-T): an in vitro model for bovine lactation. Exp Cell Res. 1991;197:191–199. doi: 10.1016/0014-4827(91)90422-q. [DOI] [PubMed] [Google Scholar]
- 13.Ikawa S, Weinberg R A. An interaction between p21ras and heat shock protein hsp60, a chaperonin. Proc Natl Acad Sci USA. 1992;89:2012–2016. doi: 10.1073/pnas.89.6.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Isberg R R. Discrimination between intracellular uptake and surface adhesion of bacterial pathogens. Mol Microbiol. 1991;252:934–938. doi: 10.1126/science.1674624. [DOI] [PubMed] [Google Scholar]
- 15.Isberg R R, Leong J M. Multiple beta 1 chain integrins are receptors for invasin, a protein that promotes bacterial penetration into mammalian cells. Cell. 1990;60:861–871. doi: 10.1016/0092-8674(90)90099-z. [DOI] [PubMed] [Google Scholar]
- 16.Joh D, Speziale P, Gurusiddappa S, Manor J, Hook M. Multiple specificities of the staphylococcal and streptococcal fibronectin-binding microbial surface components recognizing adhesive matrix molecules. Eur J Biochem. 1998;258:897–905. doi: 10.1046/j.1432-1327.1998.2580897.x. [DOI] [PubMed] [Google Scholar]
- 17.Joh D, Wann E R, Kreikemeyer B, Speziale P, Hook M. Role of fibronectin-binding MSCRAMMS in bacterial adherence and entry into mammalian cells. Matrix Biol. 1999;18:211–223. doi: 10.1016/s0945-053x(99)00025-6. [DOI] [PubMed] [Google Scholar]
- 18.Joh H J, House-Pompeo K, Patti J M, Gurusiddappa S, Hook M. Fibronectin receptors from gram-positive bacteria: comparison of active sites. Biochemistry. 1994;33:6086–6092. doi: 10.1021/bi00186a007. [DOI] [PubMed] [Google Scholar]
- 19.Jones M, Gupta R S, Englesberg E. Enhancement in amount of P1 (hsp60) in mutants of Chinese hamster ovary (CHO-K1) cells exhibiting increases in the A system of amino acid transport. Proc Natl Acad Sci USA. 1994;91:858–862. doi: 10.1073/pnas.91.3.858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Judd R C. Electrophoresis of peptides. Methods Mol Biol. 1994;32:49–57. doi: 10.1385/0-89603-268-X:49. [DOI] [PubMed] [Google Scholar]
- 21.Khan I U, Wallin R, Gupta R S, Kammer G M. Protein kinase A-catalyzed phosphorylation of heat shock protein 60 chaperone regulates its attachment to histone 2B in the T lymphocyte plasma membrane. Proc Natl Acad Sci USA. 1998;95:10425–10430. doi: 10.1073/pnas.95.18.10425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lammers A, Nuijten P J, Smith H E. The fibronectin binding proteins of Staphylococcus aureus are required for adhesion to and invasion of bovine mammary gland cells. FEMS Microbiol Lett. 1999;180:103–109. doi: 10.1111/j.1574-6968.1999.tb08783.x. [DOI] [PubMed] [Google Scholar]
- 23.McDevitt D, Francois P, Vaudaux P, Foster T J. Identification of the ligand-binding domain of the surface-located fibrinogen receptor (clumping factor) Mol Microbiol. 1995;16:895–907. doi: 10.1111/j.1365-2958.1995.tb02316.x. [DOI] [PubMed] [Google Scholar]
- 24.Mengaud J, Ohayon H, Gounon P, Mege R-M, Cossart P. E-cadherin is the receptor for internalin, a surface protein required for entry of L. monocytogenes into epithelial cells. Cell. 1996;84:923–932. doi: 10.1016/s0092-8674(00)81070-3. [DOI] [PubMed] [Google Scholar]
- 25.Novick R. Properties of a cryptic high-frequency transducing phage in Staphylococcus aureus. Virology. 1967;33:155–166. doi: 10.1016/0042-6822(67)90105-5. [DOI] [PubMed] [Google Scholar]
- 26.Ozeri V, Rosenshine I, Mosher D F, Fassler R, Hanski E. Roles of integrins and fibronectin in the entry of Streptococcus pyogenes into cells via protein F1. Mol Microbiol. 1998;30:625–637. doi: 10.1046/j.1365-2958.1998.01097.x. [DOI] [PubMed] [Google Scholar]
- 27.Patti J M, Allen B L, McGavin M J, Hook M. MSCRAMM-mediated adherence of microorganisms to host tissues. Annu Rev Microbiol. 1994;48:585–617. doi: 10.1146/annurev.mi.48.100194.003101. [DOI] [PubMed] [Google Scholar]
- 28.Peacock S J, Foster T J, Cameron B J, Berendt A R. Bacterial fibronectin-binding proteins and endothelial cell surface fibronectin mediate adherence of Staphylococcus aureus to resting human endothelial cells. Microbiology. 1999;145:3477–3486. doi: 10.1099/00221287-145-12-3477. [DOI] [PubMed] [Google Scholar]
- 29.Rosenshine I, Duronio V, Finlay B B. Tyrosine protein kinase inhibitors block invasin-promoted bacterial uptake by epithelial cells. Infect Immun. 1992;60:2211–2217. doi: 10.1128/iai.60.6.2211-2217.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rozdzinski E, Tuomanen E. Interactions of bacteria with leukocyte integrins. Methods Enzymol. 1994;236:333–345. doi: 10.1016/0076-6879(94)36025-1. [DOI] [PubMed] [Google Scholar]
- 31.Soltys B J, Gupta R S. Immunoelectron microscopic localization of the 60-kDa heat shock chaperonin protein (Hsp60) in mammalian cells. Exp Cell Res. 1996;222:16–27. doi: 10.1006/excr.1996.0003. [DOI] [PubMed] [Google Scholar]
- 32.Soltys B J, Gupta R S. Cell surface localization of the 60 kDa heat shock chaperonin protein (hsp60) in mammalian cells. Cell Biol Int. 1997;21:315–320. doi: 10.1006/cbir.1997.0144. [DOI] [PubMed] [Google Scholar]
- 33.Soltys B J, Gupta R S. Mitochondrial-matrix proteins at unexpected locations: are they exported? Trends Biochem Sci. 1999;24:174–177. doi: 10.1016/s0968-0004(99)01390-0. [DOI] [PubMed] [Google Scholar]
- 34.Soltys B J, Gupta R S. Mitochondrial proteins at unexpected cellular locations: export of proteins from mitochondria from an evolutionary perspective. Int Rev Cytol. 1999;194:133–196. doi: 10.1016/s0074-7696(08)62396-7. [DOI] [PubMed] [Google Scholar]
- 35.Todd M J, Viitanen P V, Lorimer G H. Dynamics of the chaperonin ATPase cycle: implications for facilitated protein folding. Mol Microbiol. 1994;265:659–666. doi: 10.1126/science.7913555. [DOI] [PubMed] [Google Scholar]
- 36.Tompkins D C, Hatcher V B, Patel D, Orr G A, Higgins L L, Lowy F D. A human endothelial cell membrane protein that binds Staph ylococcus aureus in vitro. J Clin Investig. 1990;85:1248–1254. doi: 10.1172/JCI114560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tran Van Nhieu G, Isberg R R. Bacterial internalization mediated by beta 1 chain integrins is determined by ligand affinity and receptor density. EMBO J. 1993;12:1887–1895. doi: 10.1002/j.1460-2075.1993.tb05837.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tran Van Nhieu G, Isberg R R. Isolation and identification of eukaryotic receptors promoting bacterial internalization. Methods Enzymol. 1994;236:307–318. doi: 10.1016/0076-6879(94)36023-5. [DOI] [PubMed] [Google Scholar]
- 39.van Putten J P, Duensing T D, Cole R L. Entry of OpaA+ gonococci into HEp-2 cells requires concerted action of glycosaminoglycans, fibronectin and integrin receptors. Mol Microbiol. 1998;29:369–379. doi: 10.1046/j.1365-2958.1998.00951.x. [DOI] [PubMed] [Google Scholar]
- 40.Viitanen P V, Lorimer G H, Seetharam R, Gupta R S, Oppenheim J, Thomas J O, Cowan N J. Mammalian mitochondrial chaperonin 60 functions as a single toroidal ring. J Biol Chem. 1992;267:695–698. [PubMed] [Google Scholar]
- 41.Wann E R, Gurusiddappa S, Hook M. The fibronectin-binding MSCRAMM FnbpA of Staphylococcus aureus is a bifunctional protein that also binds to fibrinogen. J Biol Chem. 2000;275:13863–13871. doi: 10.1074/jbc.275.18.13863. [DOI] [PubMed] [Google Scholar]
- 42.Woodlock T J, Chen X, Young D A, Bethlendy G, Lichtman M A, Segel G B. Association of HSP60-like proteins with the L-system amino acid transporter. Arch Biochem Biophys. 1997;338:50–56. doi: 10.1006/abbi.1996.9798. [DOI] [PubMed] [Google Scholar]