Abstract
Introduction:
Internal auditory canal (IAC) diverticula, also known as IAC cavitary lesions or anterior cupping of the IAC, observed in otopathologic specimens and high-resolution computed tomography (CT) scans of the temporal bone are thought to be related to otosclerosis. Herein, we examined the utility of CT scans in identifying diverticula and determined whether IAC diverticula are associated with otosclerosis on otopathology.
Methods:
105 consecutive specimens were identified from the National Temporal Bone Hearing and Balance Pathology Resource Registry. Inclusion criteria included the availability of histological slides and post-mortem specimen CT scans. Exclusion criteria included cases with severe post-mortem changes or lesions causing bony destruction of the IAC.
Results:
Ninety-seven specimens met criteria for study. Of these, 42% of the specimens were from male patients and the average age of death was 77 years old (SD=18). IAC diverticula were found in 48 specimens, of which 46% were identified in the CT scans. The mean area of the IAC diverticula was 0.34mm2. The sensitivity and specificity of detecting IAC diverticula based on CT were 77% and 63% respectively. Overall, 27% of specimens had otosclerosis. Histologic IAC diverticula were more common in specimens with otosclerosis than those without (37.5% vs 16%, p=0.019). Cases with otosclerosis had a greater mean histological diverticula area compared to non-otosclerosis cases (0.69 mm2 vs 0.14 mm2, p=0.001).
Conclusion:
IAC diverticula are commonly found in otopathologic specimens with varied etiologies, but larger diverticula are more likely to be associated with otosclerosis. The sensitivity and specificity of CT scans to detect IAC diverticula are limited.
Keywords: otosclerosis, otopathology, temporal bone pathology, conductive hearing loss, sensorineural hearing loss, diverticula, apple bite
Introduction
Otosclerosis is a common cause of acquired hearing loss and is characterized by abnormal remodeling of endochondral bone in the otic capsule.1–3 Typically, otosclerosis presents from the third to fifth decade of life with conductive hearing loss (CHL),2,4,5 which is caused by spreading of the lesion across the stapedial annular ligament that results in fixation the stapes (fenestral otosclerosis).1–3 However, it may also surround the cochlea and parts of the labyrinth (cochlear otosclerosis), causing mixed or sensorineural hearing loss.6,7
The estimated incidence of clinical otosclerosis in the Caucasian population is about 0.1%, though postmortem histological evidence in individuals without clinical symptoms have been reported in up to 11%.8 Histologically, otosclerosis consists of islands of bony remodeling with enlarged pseudovascular spaces, highly acidophilic new bone deposition, increased cellularity and occasionally osteoclasts.4,9 Even though these histological changes are most commonly located along the area anterior to the oval window, otosclerosis have also been reported in other locations of the inner ear, such as the round window, cochlear apex, cochlear aqueduct, semicircular canals, within the stapes footplate and anterior wall of the internal auditory canal (IAC).1 In fact, several small histologic case series have referred to this latter finding, also known as IAC diverticula, as a form of “cavitary otosclerosis”.10–12 At our institution, the anterior IAC diverticula is sometimes referred to as the “apple bite” lesion due to the erosive appearance in the anterior canal wall.
There are several tools for evaluating otosclerosis clinically, including otoscopy, tuning forks, behavioral audiometry, and imaging. Although the accuracy of diagnosing otosclerosis without high resolution temporal bone (TB) CT scan is quite high, this is the imaging modality of choice when imaging is included in the preoperative evaluation of otosclerosis.1,2,13 Further, CT scans may be helpful in determining the extent of the disease.1,2,13 Commonly used imaging findings for otosclerosis diagnosis include changes in the width of the oval window and sclerotic or hypodense foci on or around footplate, thickening, irregular or hypodense footplate and demineralized areas in temporal bone.14–18 Additionally, the aforementioned IAC diverticula has been also described in CT scans of patients with otosclerosis19–22 and suggested to be related to the severity of the disease.23 A recent otopathological study by Muelleman et al.24 found that when otosclerosis involved the IAC, there was a concomitant IAC diverticula in 70%. However, IAC diverticula have been also described in high resolution CT scans of patients without radiographic evidence of fenestral or retrofenestral otosclerosis20, and even reported in histologically normal temporal bones from subjects with normal hearing.24
Taken together, while these lesions have been identified in both otopathology specimens and CT scans, their etiology, prevalence and clinical implications are not completely understood. Previous otopathological studies on IAC diverticula have been largely based in small cohorts10–12, or limited to cases with known IAC otosclerosis.24 Additionally, the sensitivity and specificity of IAC diverticula as a radiologic marker of otosclerosis has never been specifically explored. Thus, this study aimed to (1) determine whether IAC diverticula are associated with otosclerosis on otopathology and (2) compare TB histologic sections and CT scans to evaluate the sensitivity and specificity of CT scans in identifying diverticula.
Methods
Subjects
The National Temporal Bone Hearing and Balance Pathology Resource Registry was searched for cases. One hundred and five consecutive temporal bone specimens from the Massachusetts Eye and Ear Otopathology Laboratory collection were identified. The inclusion criteria were the availability of histologic slides and CT scans. The exclusion criteria were severe post-mortem changes or bony destruction of the IAC due to pathology, such as benign or malignant tumors.
Histological Methods
Temporal bones were prepared via fixation in formalin, decalcification in ethylenediaminetetraacetate (EDTA) or trichloroacetic acid (TCA), dehydration in alcohols, and embedment in celloidin. Axial sections were cut 20 μm thick, and every 10th section was stained with hematoxylin and eosin (H&E) and mounted on a glass slide.25 Slides were then examined using light microscopy for otosclerosis and IAC diverticula. Otopathology raters were blinded to the clinical history. Two otopathology raters reviewed together and reported consensus results. Otosclerosis was diagnosed if there was any histologic evidence of the disease,9 including early or mild otosclerosis. Diverticula was defined as any cavitary lesion – regardless of size – on the anterolateral side of the IAC. Size was measured by identifying the slide with a diverticulum’s largest cross-sectional area and measuring that area using ImageJ software (ImageJ version 1.51; The National Institutes of Health, Bethesda, MD; available at: http://imagej.nih.gov/ij/).
CT Scan Evaluation
High resolution CT imaging (40-slice MDCT SOMATOM Sensation; Siemens, Erlangen, Germany; 64-slice MDCT Discovery 750 HD, General Electric, Milwaukee, WI) of the TBs was performed after extraction and prior to histologic preparation. Images were obtained as follows: 40-slice MDCT scanner with 120 kVp and 320 mAs, 0.6 mm slice thickness with 0.1 mm overlap; 64-slice MDCT with 120 kVp, 10 mAs, 0.6 mm slice thickness with 0.3 mm overlap. CT scans were then reviewed retrospectively by a neuroradiologist who was blinded to the histologic evaluations. Diverticula were defined as any low-density outpouching on the anterolateral wall of the IAC, regardless of size.
Statistical Analysis
To evaluate the efficacy of CT scanning in detecting IAC diverticula, sensitivity and specificity values were calculated. Histology was used as a reference for the “true presence” or “true absence” of IAC diverticula. A chi-squared test of independence was used to evaluate the correlation between IAC diverticula presence (determined histologically) and otosclerosis. The mean histological diverticula area of case with and without otosclerosis was compared using the Mann-Whitney U test. A p-value <.05 was considered statistically significant.
Results
Demographics
Ninety-seven out of 105 TB specimens met the inclusion and exclusion criteria. Eight TBs were excluded due to severe post-mortem artifacts or bony destruction of the IAC. The mean age of death was 77 years old (SD ±18) and 42% of TB’s came from male patients.
Histopathology
There were 48 cases (49%) with IAC diverticula present and 49 cases (51%) with diverticula absent (Table 1). The mean diverticula area was 0.34mm2 (SD: ±0.60, range: 0.02–3.07mm2). Photomicrographs of horizontal sections of TBs from representative cases are shown in Figures 1–3. Histological evidence of otosclerosis was found in 26 out of the 97 specimens (27%).
Table 1.
IAC diverticula in cases with and without otosclerosis
| IAC diverticula on histology | IAC diverticula on CT review | |||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Present | Not Present | Total | Present | Not Present | Total | |
| Otosclerosis | ||||||
| Present | 18 | 8 | 26 | 13 | 13 | 26 |
| Not present | 30 | 41 | 71 | 32 | 39 | 71 |
| Total | 48 | 49 | 97 | 45 | 52 | 97 |
IAC, Internal auditory canal; CT, Computed tomograpghy
Figure 1. Representative CT and temporal bone histologic sections illustrating patients with an IAC diverticulum (yellow arrow).
(A, B) A left temporal bone from a 58-year-old male without otosclerosis. (C, D) A left temporal bone from a 91-year-old female with otosclerosis(*).
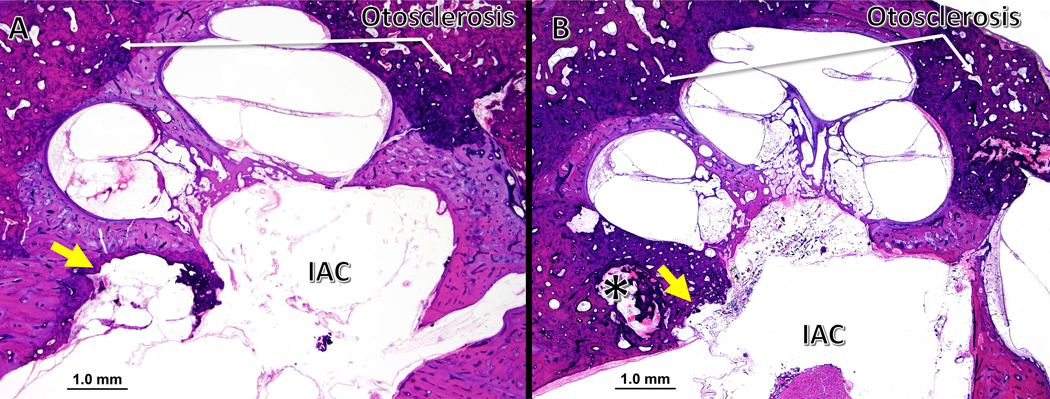
Figure 3. IAC diverticula in temporal bones with otosclerosis.
(A) A right temporal bone from a 91-year-old female with clinical and histological evidence of otosclerosis. There is a large diverticulum (yellow arrow) completely surrounded by a thin layer otosclerotic bone. (B) A right temporal bone from an 89-year-old male, also with clinical and histological diagnosis of otosclerosis. There is a much larger otosclerotic foci involving the cochlea, with a shallower diverticulum (yellow arrow). An additional cavitary lesion (*) is adjacent to the IAC diverticulum, and associated with the same focus of otosclerosis.
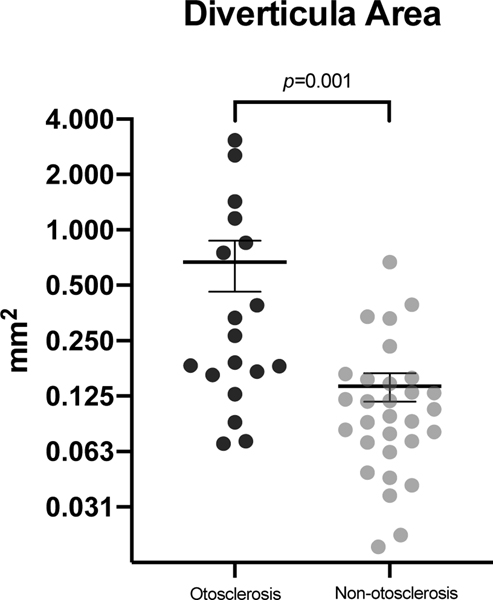
In patients with histologically identified IAC diverticula, the rate of otosclerosis was 37.5%, which was significantly higher than the rate of 16% for those without diverticula (chi squared test of independence, p=0.019). Of all patients with diverticula identified histologically, those with concurrent otosclerosis typically had larger diverticula (Mann-Whitney U test, p=0.001). The diverticula mean area for cases with otosclerosis was 0.69 mm2 (SD ±0.88 mm2), whereas non-otosclerosis cases had a mean area of 0.14 mm2 (SD ±0.13 mm2) (Figure 4).
Figure 4. Scatter plot with diverticula area in otosclerosis and non-otosclerosis cases.
Cases with otosclerosis had a greater mean diverticula area compared to non-otosclerosis cases (p=0.001). The horizontal lines and error bars represent the mean and standard error of the mean, respectively.
CT Imaging
CT review identified 45 patients (46%) with diverticula, of which 70% had evidence of IAC diverticula on histology. The sensitivity and specificity of identifying IAC diverticula on CT for specimens with histologically confirmed IAC diverticula was 77% and 63%, respectively (Table 2). Thirteen out of the 45 cases with diverticula identified on CT review had histological evidence of otosclerosis (Table 1). The sensitivity and specificity of CT in identifying IAC diverticula that were associated with otosclerosis was 50% and 55%, respectively. The mean histological diverticula area for those patients with otosclerosis that had diverticula identified by CT was 0.65 mm2 (SD ±0.85 mm2), which was significantly greater than those without otosclerosis (mean= 0.13 mm2, SD ±0.15 mm2; Mann-Whitney U test, p<0.001).
Table 2.
Diagnostic efficacy of CT scan in identifying diverticula
| Statistic | Estimate | 95% Confidence Interval |
|---|---|---|
|
| ||
| Sensitivity | 77.08 | 62.69 – 87.97 |
| Specificity | 63.27 | 48.29 – 76.58 |
Discussion
Our study identified IAC diverticula in 49% of histologic specimens, though there was variability in shape and size. In our study, CT did not appear to be a sensitive or specific modality for detecting IAC diverticula, especially when the diverticula were small. However, cases with diverticula on CT scan and concurrent otosclerosis presented with larger diverticula size on histology compared to those without otosclerosis. Also, the rate of otosclerosis in specimens with IAC diverticula in the histopathological analysis was significantly higher than the rate in the control group without diverticula.
Previous radiological studies have already linked these cavitary lesions on the anterior wall of the IAC to otosclerosis.19–22,26 In a retrospective study by Puac et al.,21 35% of cases with otosclerotic lesions were reported to have a cavitary lesion. In another prospective study by Wang et al.26, IAC diverticula was significantly more frequent in both fenestral and retrofenestral otosclerosis ears, with an incidence of 39.3%. Their results are consistent with our findings. However, our study also showed that the presence of IAC diverticula on imaging is not sensitive nor specific enough to diagnose cases with otosclerosis, as they may be still present in normal ears or associated with other pathologies. Additionally, our findings suggest that the presence of small IAC diverticula on imaging is less likely to be associated with otosclerosis.
Other studies have suggested that these cavitary lesions may represent a normal anatomic variant.13,27 In another recent study by Pippin et al.20, IAC diverticula were usually described as an isolated CT scan finding, and rarely occurred alongside otosclerosis, which conflicts with our findings. One possible explanation for this discrepancy is that their study was entirely based on CT scans rather than histologic slides to identify diverticula,20 and small diverticula were probably missed. Although there was variability in IAC diverticula shape and size in our study, we provide evidence of an association between large IAC diverticula and otosclerosis.
There are limitations that should be considered when interpreting the results of this study. Although the present study is among the largest otopathological study comparing randomly selected cases with and without IAC diverticula, it is important to consider that individuals with severe hearing or balance disorders are more motivated than other members of the population to donate their TBs for scientific study, leading to a skewed subject pool. Therefore, we cannot infer the population-wide prevalence of IAC diverticula from this current study. Additionally, even though audiometric evaluation was not assessed in our study, conclusions on how diverticula could affect hearing in our sample would likely be confounded by the higher rate of hearing loss and diverse otological histories from patients who donate their TBs. In fact, recent studies have failed to demonstrate a relationship between the presence and/or size of an IAC diverticulum and the degree of hearing loss, suggesting that hearing loss in this population may be a product of sampling bias.21,28 Nevertheless, further studies with a healthy control group are needed to better understand how IAC diverticula may influence on hearing.
Taken together, our study supports the notion that while IAC diverticula may be more commonly associated with otosclerosis, they are not pathognomonic. Also, even though CT scan does not appear sensitive or specific modality to identify IAC diverticula in our study, the presence of a diverticulum on CT, particularly when large, may be an indicator, but not a definitive marker, of otosclerosis. Nevertheless, we suggest that TB CT scan evaluations include a survey for IAC diverticula, particularly when ordered in the setting of clinical suspicion for otosclerosis.
Conclusion
Histopathologic analysis of human TBs indicates that IAC diverticula, although not pathognomonic, are more commonly found in the setting of otosclerosis, especially when large. While the sensitivity and specificity of CT scans to detect IAC diverticula is limited, identification of a large IAC diverticula may be an indicator of otosclerosis.
Figure 2. IAC diverticula in temporal bones without otosclerosis.
(A) A left temporal bone from a 88-year-old male with a large IAC diverticulum. (B) A smaller, more rounded diverticulum is seen in the right temporal bone from a 90-year-old female.
Funding:
This work was supported by a grant from the National Institutes of Health (NIDCD U24DC013983).
Disclosures are listed below:
Jenny Chen: Grace Medical (patent pending)
Elliott Kozin: Desktop Metal (scientific advisor), Grace Medical (patent pending)
Hinrich Staecker: MedEl (surgical advisory board), Rescue Hearing (shareholder)
Michael McKenna: Akouos Inc (co-founder and CMO)
Alicia Quesnel: Frequency Therapeutics (sponsored research agreement), Grace Medical (sponsored research agreement, patent pending), Alcon (consulting)
Footnotes
No authors have direct conflicts of interest.
References
- 1.Schuknecht HF. Pathology of the ear. Philadelphia: Lea & Febiger, 1993. [Google Scholar]
- 2.Quesnel A, McKenna M. Otosclerosis. In: Sataloff RT, Lalwani AK, eds. Sataloff’s Comprehensive Textbook of Otolaryngology: Head & Neck Surgery: Otology/Neurotology/Skull Base Surgery: JP Medical Ltd, 2015:231–242. [Google Scholar]
- 3.Cureoglu S, Schachern PA, Ferlito A, Rinaldo A, Tsuprun V, Paparella MM. Otosclerosis: etiopathogenesis and histopathology. Am J Otolaryngol 2006; 27:334–340. [DOI] [PubMed] [Google Scholar]
- 4.Karosi T, Sziklai I. Etiopathogenesis of otosclerosis. Eur Arch Otorhinolaryngol 2010; 267:1337–1349. [DOI] [PubMed] [Google Scholar]
- 5.Roland P, Samy R. Otosclerosis. In: Bailey BJ, Johnson JT, Newlands SD, eds. Head & neck surgery--otolaryngology. Philadelphia, PA: Lippincott Williams & Wilkins, 2006:2126–2137. [Google Scholar]
- 6.Schuknecht HF. Cochlear otosclerosis. An intractable absurdity. J Laryngol Otol Suppl 1983; 8:81–83. [PubMed] [Google Scholar]
- 7.Ishai R, Halpin CF, Shin JJ, McKenna MJ, Quesnel AM. Long-term Incidence and Degree of Sensorineural Hearing Loss in Otosclerosis. Otol Neurotol 2016; 37:1489–1496. [DOI] [PubMed] [Google Scholar]
- 8.Declau F, Van Spaendonck M, Timmermans JPet al. Prevalence of otosclerosis in an unselected series of temporal bones. Otol Neurotol 2001; 22:596–602. [DOI] [PubMed] [Google Scholar]
- 9.Quesnel AM, Ishai R, McKenna MJ. Otosclerosis: Temporal Bone Pathology. Otolaryngol Clin North Am 2018; 51:291–303. [DOI] [PubMed] [Google Scholar]
- 10.Makarem AO, Linthicum FH. Cavitating otosclerosis. Otol Neurotol 2008; 29:730–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Makarem AO, Hoang TA, Lo WW, Linthicum FH Jr., Fayad JN. Cavitating otosclerosis: clinical, radiologic, and histopathologic correlations. Otol Neurotol 2010; 31:381–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schuknecht HF, Kirchner JC. Cochlear otosclerosis: fact or fantasy. Laryngoscope 1974; 84:766–782. [DOI] [PubMed] [Google Scholar]
- 13.Gulya AJ, Gulya AJ. Gulya and Schuknecht’s anatomy of the temporal bone with surgical implications. New York: Informa Healthcare, 2007. [Google Scholar]
- 14.Valvassori GE. Imaging of otosclerosis. Otolaryngol Clin North Am 1993; 26:359–371. [PubMed] [Google Scholar]
- 15.Veillon F, Riehm S, Emachescu Bet al. Imaging of the windows of the temporal bone. Semin Ultrasound CT MR 2001; 22:271–280. [DOI] [PubMed] [Google Scholar]
- 16.Swartz JD, Faerber EN, Wolfson RJ, Marlowe FI. Fenestral otosclerosis: significance of preoperative CT evaluation. Radiology 1984; 151:703–707. [DOI] [PubMed] [Google Scholar]
- 17.Mafee MF, Henrikson GC, Deitch RLet al. Use of CT in stapedial otosclerosis. Radiology 1985; 156:709–714. [DOI] [PubMed] [Google Scholar]
- 18.Naumann IC, Porcellini B, Fisch U. Otosclerosis: incidence of positive findings on high-resolution computed tomography and their correlation to audiological test data. Ann Otol Rhinol Laryngol 2005; 114:709–716. [DOI] [PubMed] [Google Scholar]
- 19.Bou-Assaly W, Mukherji S, Srinivasan A. Bilateral cavitary otosclerosis: a rare presentation of otosclerosis and cause of hearing loss. Clin Imaging 2013; 37:1116–1118. [DOI] [PubMed] [Google Scholar]
- 20.Pippin KJ, Muelleman TJ, Hill J, Leever J, Staecker H, Ledbetter LN. Prevalence of Internal Auditory Canal Diverticulum and Its Association with Hearing Loss and Otosclerosis. AJNR Am J Neuroradiol 2017; 38:2167–2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Puac P, Rodriguez A, Lin HC et al. Cavitary Plaques in Otospongiosis: CT Findings and Clinical Implications. AJNR Am J Neuroradiol 2018; 39:1135–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shim YJ, Bae YJ, An GS et al. Involvement of the Internal Auditory Canal in Subjects With Cochlear Otosclerosis: A Less Acknowledged Third Window That Affects Surgical Outcome. Otol Neurotol 2019; 40:e186–e190. [DOI] [PubMed] [Google Scholar]
- 23.Hoeberigs M, Postma A, Waterval J, Stokroos R, Stadler A. Prevalence of anterior internal auditory canal ‘‘diverticulum’’ on high resolution CT in patients with otosclerosis Proceedings of the Radiological Society of North America 2012 Scientific Assembly and Annual Meeting. Chicago, Illinois, 2012. [Google Scholar]
- 24.Muelleman T, Maxwell AK, Lopez Iet al. Histopathologic Characteristics of Internal Auditory Canal Diverticula. Otol Neurotol 2019; 40:e653–e656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Merchant S. Methods of Removal, Preparation and Study. In: Merchant S, Nadol J, eds. Schuknecht’s Pathology of the Ear. Shelton, CT: People’s Medical Pub. House-USA Inc, 2010:3–51. [Google Scholar]
- 26.Wang F, Yoshida T, Shimono Met al. Significance of internal auditory canal diverticula in ears with otosclerosis. Acta Otolaryngol 2018; 138:1066–1069. [DOI] [PubMed] [Google Scholar]
- 27.Mihal DC, Feng Y, Kodet ML, Lohse CM, Carlson ML, Lane JI. Isolated Internal Auditory Canal Diverticula: A Normal Anatomic Variant Not Associated with Sensorineural Hearing Loss. AJNR Am J Neuroradiol 2018; 39:2340–2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Muelleman TJ, Pippin K, Shew Met al. The Size of Internal Auditory Canal Diverticula Is Unrelated to Degree of Hearing Loss. Laryngoscope 2020; 130:1011–1015. [DOI] [PubMed] [Google Scholar]