Abstract
To assess the importance of TonB-dependent iron transport systems to growth of Shigella in vivo, a tonB mutant of Shigella dysenteriae was isolated and tested in cultured cells. The tonB mutant invaded epithelial cells, but did not form plaques in confluent monolayers of Henle cells, indicating an inability of this mutant to spread from cell to cell. The rate of intracellular multiplication of the tonB mutant was reduced significantly compared to that of the wild type. The loss of virulence in the tonB mutant was not due to loss of either Shu or Ent, the TonB-dependent systems which allow for transport of heme and ferrienterobactin, respectively. A shuA mutant lacking the outer membrane receptor for heme, an entB mutant defective in enterobactin synthesis, and a shuA entB double mutant each were able to invade cultured cells, multiply intracellularly, and form wild-type plaques. The ability of S. dysenteriae to access iron during intracellular growth was assessed by flow cytometric analysis of an iron- and Fur-regulated shuA-gfp reporter construct. Low levels of green fluorescent protein expression in the intracellular environment were observed in all strains, indicating that iron is available to intracellular bacteria, even in the absence of TonB-dependent iron transport. The failure of the tonB mutant to grow well in an iron-replete intracellular environment suggests that TonB plays a role in addition to heme- and siderophore-mediated iron acquisition in vivo, and this function is required for the intracellular growth and intercellular spread of S. dysenteriae.
Shigella dysenteriae is a facultative intracellular pathogen that causes bacillary dysentery in the human host. The virulence of Shigella is dependent on its ability to invade colonic epithelial cells, escape from the phagosome, multiply intracellularly, and spread to adjacent cells (28). A significant portion of the infective cycle of Shigella is spent in the cytoplasm of the host cell, and survival and replication inside the cell are crucial to the pathogenesis of this organism. Little is known about the intracellular growth environment with respect to the availability of specific nutrients. In order for Shigella to multiply intracellularly, the bacteria must compete successfully for all essential nutrients, including iron. The majority of iron inside the cells is complexed in heme proteins or in the storage protein ferritin (19). The nature of the iron sources available to Shigella growing in the cytoplasm of infected host cells is not known.
Enteric pathogens employ several methods of high-affinity iron acquisition. Many species synthesize and transport siderophores, low-molecular-weight compounds that bind iron(III) with sufficient affinity to remove it from host compounds (29). Another method of iron acquisition is the direct utilization of host iron sources, such as heme, hemoglobin, transferrin, and lactoferrin. S. dysenteriae synthesizes and utilizes the catechol siderophore enterobactin and can grow with heme as a sole iron source (21, 23).
Transport of heme or ferrienterobactin is a high-affinity process that depends on TonB and its accessory proteins, ExbB and ExbD (25). The TonB complex supplies the energy needed for transport of iron complexes across the outer membrane (35). ExbB and ExbD are accessory proteins that function in the stabilization and recycling of the TonB protein (7, 15, 22). TonB spans the periplasmic space and transduces energy from the cytoplasmic membrane to receptors in the outer membrane, allowing the iron complex to be transported across the outer membrane into the periplasm (35). The carboxy terminus of TonB interacts directly with outer membrane receptors involved in iron transport (13). The amino terminus of TonB is thought to anchor the protein in the cytoplasmic membrane and to facilitate its interactions with ExbB (16). Periplasmic binding proteins and inner membrane permeases deliver the ligand to the cytoplasm of the bacterial cell (5). In S. dysenteriae, TonB mediates the transport of heme through the outer membrane receptor ShuA (23). TonB is also required for the transport of the fungal siderophore ferrichrome and has been shown in Escherichia coli to be required for transport of vitamin B12 (4).
The expression of genes involved in iron uptake, including shuA, tonB, and the enterobactin synthesis and transport genes, is controlled at the transcriptional level by the repressor protein Fur (3, 23, 31, 34). Under iron-replete conditions, the Fe2+-Fur complex binds to specific sequences in the promoter and prevents transcription of iron-regulated genes (2). This regulation ensures that the synthesis of iron transport proteins is repressed during growth in high-iron conditions.
The restricted availability of iron within the host and the presence of multiple, perhaps redundant, iron transport systems in S. dysenteriae suggest that iron acquisition systems are needed during infection. To determine if TonB-dependent iron transport is important to the virulence of S. dysenteriae, a series of mutants deficient in one or both high-affinity iron transport systems as well as a strain deficient in all TonB-mediated functions were constructed. The effects of these mutations were tested, and the role of high-affinity iron transport during growth in the intracellular environment was assessed.
MATERIALS AND METHODS
Bacterial strains, plasmids, and media.
Plasmids and bacterial strains used in this study are listed in Table 1. S. dysenteriae strains were routinely grown at 37°C in Luria (L) broth or on Congo Red agar (Trypticase soy broth agar plus 0.01% Congo red dye), and E. coli strains were grown in L broth or on L agar. S. dysenteriae and E. coli strains transformed with plasmids expressing green fluorescent protein (GFP) were grown at 37°C in low-salt L broth (LSLB) containing 5 g of NaCl/liter. When indicated, antibiotics were added at the following concentrations: 250 μg of carbenicillin/ml, 50 μg of kanamycin/ml, 50 μg of chloramphenicol/ml, and 12.5 μg of tetracycline/ml. The iron chelator ethylenediamine di(o-hydroxyphenylacetic acid) (EDDA) was deferrated (37) and added to the media at a concentration sufficient to inhibit growth in L agar containing no additional iron source and 1 mg of EDDA/ml for wild-type strains and 250 μg of EDDA/ml for iron transport mutants. Hemin (8 μM), ferrichrome (2 mM), and FeSO4 (20 μM) were used to supplement media where indicated.
TABLE 1.
Bacterial strains and plasmids
| Strain or plasmid | Relevant characteristic(s) | Source or reference |
|---|---|---|
| Strains | ||
| S. dysenteriae | ||
| SDU378 | Serotype 1 clinical isolate | A. Hartman |
| SDU380 | tonB | This study |
| SDU400 | shuA::mini-Tn10cam | This study |
| SDU402 | entB::Tn5, shuA::mini-Tn10cam | This study |
| E. coli | ||
| AB1515 | E. coli K-12 | C. F. Earhart |
| AB1515.24 | entB::Tn5 | 46 |
| CAG5053 | Hfr; KL208zbc-280::Tn10 | 45 |
| CAG5053entB::Tn5 | entB::Tn5 | This study |
| Plasmids | ||
| pCP410 | E. coli entEB(G)A in pACYC184 | 32 |
| pGTXN3 | Transcriptional fusion vector containing the promoterless mutant 3 gfp gene | 38 |
| pHTL116 | 2.6-kb EcoRV fragment containing shuA cloned into pWKS30 | 50 |
| pHEB1 | 6.6-kb EcoRI fragment of pCP410 cloned into pHTL116 | This study |
| pLR9 | 39-bp dnaY promoter (33) cloned upstream of gfp in pGTXN3 | L. Runyen-Janecky |
| pSc912 | shuA::mini-Tn10cam | 24 |
| pSR2 | 0.8-kb shuA promoter cloned upstream of gfp in pGTXN3 | This study |
| pYUK1 | E. coli tonB in pWSK129Cm | Y. Gleason |
Recombinant DNA techniques.
Standard recombinant DNA methods, including restriction enzyme digests, ligation reactions, and agarose gel electrophoresis were performed as previously described (39). Chromosomal DNA was isolated using the reagent DNAzol (Molecular Research Center) according to the procedure supplied by the manufacturer.
Mutant construction.
SDU380, a spontaneous tonB mutant of wild-type S. dysenteriae SDU378, was isolated by selecting for resistance to 0.25 μg of pirazmonam/ml (18). Pirazmonam was generously provided by S. J. Lucania, Bristol-Myers Squibb Co. The mutation in tonB was confirmed by Western blotting and complementation analysis. SDU400 was generated from SDU378 by allelic exchange with shuA::mini-Tn10cam contained on the plasmid pSc912 (24), which confers sucrose sensitivity. The strain was serially passaged and plated on L agar containing chloramphenicol and 5% sucrose. Colonies were tested for carbenicillin sensitivity and sucrose resistance, indicating loss of pSc912 and recombination of the disrupted gene into the chromosome. The chromosomal shuA mutation was confirmed by PCR.
SDU402, a shuA entB double mutant, was constructed in the SDU400 background as follows. The entB::Tn5 mutation in AB1515.24 was transferred to the Hfr strain CAG5053 by P1 transduction (8). SDU401 was generated by mating SDU400 with CAG5053entB::Tn5. Overnight cultures of CAG5053entB::Tn5 and SDU401 were washed with saline and concentrated 10-fold. Fifty microliters of each culture was mixed together and spread on L agar. After 4 h of incubation, the cells were resuspended in 1 ml of L broth, and dilutions were plated on Congo red agar containing chloramphenicol and kanamycin. Exconjugants were screened for the entB insertion by PCR. An entB mutant of SDU378 was constructed by complementation of SDU402, the shuA entB double mutant, with a plasmid containing shuA, pHTL116. In this way, hemin utilization, but not enterobactin synthesis, was restored to the double mutant.
Bioassays.
Bacterial cultures of wild-type or mutant strains were grown to log phase and added at a concentration of 106 CFU/ml to L agar plus EDDA. The plates were allowed to solidify and then were spotted with 5 μl each of 8 μM hemin, 2 mM ferrichrome, and an overnight culture of E. coli AB1515, an enterobactin-producing strain. The zones of growth around each iron source were measured after 18 to 24 h of incubation at 37°C.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis and Western blotting.
Whole-cell and secreted proteins were separated by electrophoresis on a sodium dodecyl sulfate–12% polyacrylamide gel. Proteins were transferred to nitrocellulose and visualized as described previously (33). The monoclonal anti-TonB antibody was the generous gift of Kathleen Postle (17). Polyclonal monkey convalescent antiserum that recognizes IpaB, IpaC, IpaD, and IcsA was kindly provided by Edwin Oaks (48).
Tissue culture cell invasion and plaque assays.
Henle cell monolayers were cultured in Earle's minimal essential medium–2 mM glutamine–10% fetal calf serum in a 5% CO2 atmosphere at 37°C. Invasion of Henle cells by S. dysenteriae was performed by the procedure of Hale and Formal (9) as described previously (12). Briefly, subconfluent monolayers (10-cm2 plates) were infected with 2 × 108 CFU of bacteria and incubated for 1 h, and then the monolayers were washed and incubated for 1 h in fresh medium containing gentamicin (20 μg/ml). Monolayers were washed and stained using Wright-Giemsa stain. Invasion was expressed as the percentage of Henle cells containing three or more intracellular bacteria. Under these conditions, approximately 40 to 50% of the cells were invaded by the wild-type S. dysenteriae: less than 3% invasion was detected with noninvasive mutants (33). The intracellular multiplication assay was performed as described previously (12). Henle cell monolayers were infected in duplicate as for the invasion assay, and at the indicated time points, bacteria were recovered from one set of infected Henle cells and plated to selective media. The second set was stained and percent invasion was determined. The average number of intracellular bacteria per infected Henle cell was calculated as CFU divided by the number of infected Henle cells. The Henle cell plaque assay was performed according to the method of Oaks et al. (27) and as described previously (12).
Plasmid construction.
The iron- and Fur-regulated shuA-gfp transcriptional fusion was constructed by cloning the shuA promoter upstream of a promoterless gfp gene in pGTXN3 (38). A 749-bp PCR fragment encompassing the shuA promoter was amplified from SDU378 and cloned into the BamHI and SmaI restriction sites of pGTXN3, creating pSR2. The primers used were 5′CCCAGAGATATCGAGGCTTGCA and 5′GCGGATCCATATCCTGACCGTTGGTTCG. Amplification was performed using pfu polymerase (Stratagene Cloning Systems, La Jolla, Calif.) with the following reaction conditions: 30 cycles of 94°C for 45 s, 55°C for 45 s, and 72°C for 2 min. The constitutively active dnaY promoter from pCAT39 (42) was cloned upstream of gfp in pGTXN3 to create pLR9. In order to complement the shuA and entB mutations in SDU402, pHEB1 was constructed by cloning the entEB(G)A genes from pCP410 into the EcoRI restriction site of pHTL116, which contains shuA.
Flow cytometric analysis.
GFP-mediated fluorescence of transformed Shigella strains grown in vitro was measured at an emission wavelength of 511 nm by using a FACSCalibur (Becton Dickinson) fluorescence-activated cell sorter (FACS) with an excitation wavelength of 488 nm. In initial assays, bacteria were grown to late exponential phase in LSLB containing the appropriate antibiotics and EDDA as the iron chelator, when indicated. Following centrifugation, bacteria were resuspended at a concentration of 106 CFU/ml in phosphate-buffered saline (PBS) for FACS analysis. To determine the GFP fluorescence intensities of the bacteria immediately prior to invasion of Henle cells, the invasion assay was performed and bacteria were isolated from tissue culture as follows. After a 30-min incubation with the Henle cells, the supernatant containing extracellular bacteria was removed from the monolayer. The bacteria were pelleted, washed in 1 ml of PBS, and resuspended in 2 ml of PBS prior to FACS analysis. To determine the GFP fluorescence intensities of intracellular bacteria, invasions were allowed to proceed for two additional hours in the presence of gentamicin to kill extracellular bacteria. After the 2-h incubation period, the monolayers were washed five times with PBS, trypsinized, and lysed with deoxycholate, as described for the intracellular multiplication assay. Bacteria were harvested by centrifugation for 2 min at 16,000 × g, washed in 1 ml of PBS, and resuspended in 0.5 ml of PBS for FACS analysis. Bacteria were detected by side scatter and gated. The geometric mean of the fluorescence intensity of this gated population was determined. Fluorescence was measured on a log scale, and values represent the mean of 10,000 gated events for in vitro analysis and 100,000 gated events for invasion assays.
Statistical analysis.
Means and standard deviations for at least three independent measurements were determined for all assays. P values were determined using Student's two-tailed t test.
RESULTS
Iron transport systems in SDU378.
In order to identify high-affinity iron transport systems in the S. dysenteriae clinical isolate SDU378, various iron sources were tested for the ability to stimulate growth of SDU378 under iron-restricted conditions (Table 2). Growth of SDU378 was stimulated by the enterobactin-producing strain AB1515, and SDU378 itself stimulated growth of the E. coli entB mutant AB1515.24, indicating that SDU378 synthesized and used the catechol siderophore enterobactin (Table 2). The production of catechols was confirmed using the Arnow test (1) (data not shown). In the bioassay, the SDU378 ent mutant did not produce growth-stimulatory compounds, indicating that siderophores other than enterobactin were not produced (data not shown). SDU378 also used hemin and the fungal siderophore ferrichrome as iron sources, as has been previously reported for other S. dysenteriae type 1 isolates (23). SDU378 did not use the hydroxamate siderophore aerobactin and was unable to grow using ferritin, lactoferrin, transferrin, hemoglobin, or myoglobin as the sole iron source (data not shown).
TABLE 2.
Phenotypic characterization of S. dysenteriae iron transport system mutants
| Strain | Genotype or phenotype | Synthesis of enterobactinab | Utilization ofa:
|
||
|---|---|---|---|---|---|
| Hemin | Enterobactinc | Ferrichrome | |||
| SDU378 | Wild type | + | + | + | + |
| SDU380 | tonB | + | − | − | − |
| SDU380/pYUK1 | TonB+ | + | + | + | + |
| SDU400 | shuA | + | − | + | + |
| SDU400/pHTL116 | ShuA+ | + | + | + | + |
| SDU402 | shuA entB | − | − | + | + |
| SDU402/pHTL116 | ShuA+entB | − | + | + | + |
| SDU402/pHEB1 | ShuA+ EntB+ | + | + | + | + |
Bioassays were performed as described in Materials and Methods. +, zone of stimulation ≥5 mm. Growth of all strains was stimulated by 20 μl of 10 mM FeSO4.
Enterobactin synthesis was determined by the ability to cross-feed the E. coli entB mutant, AB1515.24.
AB1515 (5 μl of an overnight culture) was used as the enterobactin-producing strain.
Isolation and characterization of SDU378 iron transport mutants.
TonB is required for utilization of siderophores and heme by enteric bacteria (23, 25). In order to assess the effects of a deficiency in all TonB-dependent iron transport systems on growth and virulence, a spontaneous tonB mutant was isolated from wild-type S. dysenteriae SDU378 by selecting for resistance to pirazmonam. The transport of this drug into the bacterial cell is a TonB-dependent process, and this property has been exploited to isolate E. coli tonB mutants (18). A pirazmonam-resistant isolate, SDU380, did not synthesize TonB, as determined by Western blotting (data not shown). SDU380 was unable to grow using hemin, enterobactin, or ferrichrome as the sole iron source (Table 2). A plasmid containing E. coli tonB, pYUK1, restored the ability of this mutant to grow using hemin, enterobactin, and ferrichrome as iron sources (Table 2), confirming that the transport of each of these compounds is TonB dependent and that the mutation is in tonB.
In order to examine the role of individual TonB-dependent pathways in growth and virulence, mutants of SDU378 deficient in the heme transport system and the enterobactin biosynthetic pathway were constructed. SDU400, a shuA mutant of SDU378, was generated by allelic exchange. Bioassays confirmed that SDU400 was deficient in hemin transport only (Table 2). The shuA mutation in SDU400 was complemented by pHTL116, a plasmid carrying the S. dysenteriae shuA gene (Table 2).
SDU402, the shuA entB double mutant, was constructed by moving entB::Tn5 into the SDU400 background by conjugation. SDU402 was unable to transport hemin and did not synthesize enterobactin (Table 2). Complementation of the shuA mutation in SDU402 with pHTL116 restored hemin utilization to SDU402, resulting in a strain defective only in enterobactin production (Table 2). A plasmid containing both the shuA and entB genes, pHEB1, restored the ability of the double mutant to grow using either hemin or enterobactin as a sole iron source (Table 2).
Effect of mutations in iron transport systems on S. dysenteriae invasion and intercellular spread.
To determine the effect of a tonB mutation on invasion and intercellular spread, SDU380 was tested in tissue culture invasion and plaque assays (Table 3). Although SDU380 was as invasive as the wild type, this tonB mutant did not form plaques in confluent monolayers of Henle cells, indicating a defect in intracellular growth or cell-to-cell spread. SDU380 failed to form plaques even when the multiplicity of infection was increased 100-fold relative to the standard multiplicity of infection of 0.01 or when the monolayers were incubated for 6 days instead of 48 to 72 h (data not shown). SDU380 was tested for virulence in the Serény test (44) and did not provoke keratoconjunctivitis in Guinea pigs (data not shown). Supplying tonB on the plasmid pYUK1 restored the plaque-forming phenotype and the ability to cause keratoconjunctivitis to SDU380 (Table 3 and data not shown).
TABLE 3.
Effect of iron transport mutations on Henle cell invasion and plaque formation
| Strain | Phenotype | Percent invasiona | No. of plaquesb |
|---|---|---|---|
| SDU378 | Wild type | 41 ± 3.5 | 107 ± 13 |
| SDU380 | TonB− | 44 ± 11 | 0 |
| SDU380/pYUK1 | TonB+ | 52 ± 6.1 | 112 ± 12 |
| SDU400 | ShuA− | 56 ± 5.5c | 72 ± 7.5d |
| SDU400/pHTL116 | ShuA+ | 52 ± 5.6 | 82 ± 13 |
| SDU402 | ShuA− EntB− | 42 ± 9.3 | 73 ± 11 |
| SDU402/pHTL116 | EntB− | 39 ± 8.5 | 99 ± 3.1 |
| SDU402/pHEB1 | ShuA+ EntB+ | 47 ± 13 | 117 ± 21 |
After a 2-h incubation, Henle cells containing three or more intracellular bacteria were scored as positive for invasion. Values represent the mean ± standard deviation of three independent invasion assays.
Confluent Henle cell monolayers were infected with 104 bacteria per 35-mm-diameter plate. Plaques were counted after 48 h of incubation. The number of plaques reported is the mean ± standard deviation of three independent experiments.
Significantly different (P < 0.0001) compared to the wild type.
Of those strains that produced plaques, only SDU400 was significantly different from the wild type (P < 0.02).
Because TonB is required for the known high-affinity iron transport systems, it appeared likely that the failure of the tonB mutant to form plaques was the result of iron starvation in the intracellular environment. This could be due to loss of all of the TonB-dependent systems or the loss of a specific system, either heme or enterobactin transport, that might be required for plaque formation. Although SDU378 is able to transport the fungal siderophore ferrichrome, and this function is lost in the tonB mutant, ferrichrome-mediated iron acquisition is not believed to be relevant to growth in vivo since ferrichrome is not synthesized by S. dysenteriae or by Henle cells (26). Therefore, strains carrying mutations in heme transport and enterobactin biosynthesis were compared to the tonB mutant. SDU400 and SDU402/pHTL116, the shuA and entB single mutants, were tested in tissue culture assays. Both of these mutant strains were invasive and formed plaques comparable to those formed by the wild type, SDU378 (Table 3). Although the shuA mutant produced a smaller number of plaques, it was slightly more invasive than the wild type. Consistent with their ability to form plaques, both mutants were positive in the Serény test (data not shown). This suggested that the plaque defect in SDU380 was not a result of the loss of one of the known high-affinity iron transport systems that require TonB for function. Surprisingly, the shuA entB double mutant was also invasive, formed wild-type plaques, and was positive in the Serény test (Table 3 and data not shown). The fact that neither heme- nor enterobactin-mediated iron acquisition was required for plaque formation indicates that another iron transport system is functioning intracellularly. TonB could be required for this unidentified iron transport system; alternatively, the inability of the tonB mutant to spread from cell to cell could reflect a function for TonB that is unrelated to iron transport.
A possible explanation for the defect in plaque formation seen with the tonB mutant is improper expression or localization of IcsA. IcsA mediates polymerization of host actin microfilaments and the formation of protrusions from which shigellae move into adjacent cells (41). Localization of IcsA appeared indistinguishable between the tonB mutant and the wild type as determined by indirect immunofluorescence (data not shown), indicating that TonB is not required for IcsA function.
In addition to IcsA, the secretion of IpaB, IpaC, and IpaD is known to be required for intercellular spread (43). Ipa secretion was expected to be normal in the tonB mutant, based on the ability of this strain to invade Henle cells. This was confirmed by Western blot analysis of proteins secreted by the wild-type strain and the tonB mutant (data not shown).
Growth of iron transport mutants.
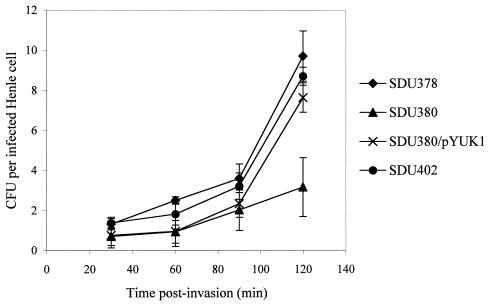
Because a defect in intracellular multiplication can also affect plaque formation, the ability of these strains to grow inside Henle cells was examined using the intracellular multiplication assay. Bacteria were recovered from the infected monolayer at 30, 60, 90, and 120 min postinvasion, and the CFU per infected Henle cell was determined. SDU380 failed to grow well intracellularly compared to the wild type (Fig. 1). Complementation of the tonB mutation restored intracellular growth of SDU380 to wild-type levels, indicating that the tonB mutation was responsible for the reduction in the intracellular growth rate of SDU380. The shuA entB double mutant, SDU402, multiplied intracellularly to the same extent as the wild type (Fig. 1).
FIG. 1.
Intracellular multiplication of S. dysenteriae mutants. The number of intracellular bacteria was determined at each time point by lysing the infected Henle cells and plating the contents to selective media. Henle cells were counted and the percentage of infected cells was determined. Error bars represent the standard deviations for the means of three independent experiments. Differences between the mutants and wild type were not statistically significant (P > 0.05) except for SDU380 compared to the wild type at 120 min (P < 0.01).
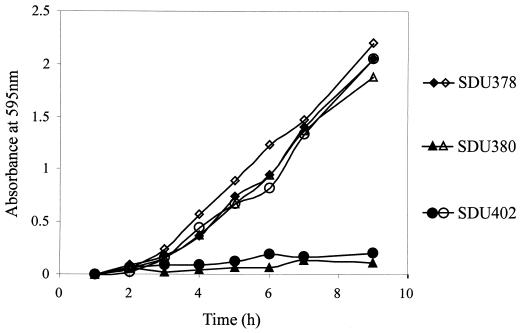
In order to assess the effects of iron limitation on the growth rates of these mutants, in vitro growth assays were performed. Under iron-replete conditions, the mutant strains grew as well as the wild type (Fig. 2). However, when EDDA was added to restrict the available iron in the growth media, SDU380 and SDU402 were unable to grow (Fig. 2). Complementation of the tonB and entB mutations restored growth in iron-restricted media. The shuA mutation had no effect on growth under either condition tested (data not shown). The tonB mutant and the double mutant displayed similar growth phenotypes in high- and low-iron media, suggesting that no additional TonB-dependent iron transport system was functioning under these assay conditions.
FIG. 2.
Growth of S. dysenteriae iron transport system mutants under iron-limiting and iron-replete conditions. L broth containing either 250 μg of EDDA (iron-limiting, closed symbols) per ml or 20 μM FeSO4 (iron replete, open symbols) was inoculated with 2.5 × 106 bacteria per ml and incubated at 37°C. Samples were removed at hourly intervals to determine the optical density at 595 nm. Data points represent the averages of three independent experiments. Standard deviations were less than 25% of the means.
Because high iron levels in vitro were able to restore wild-type growth to the tonB mutant, we determined the effect of increased iron levels in the bacteria or in the Henle cells on intercellular spread of SDU380. The iron available to SDU380 was increased either by supplementing bacterial cultures with 40 μM iron sulfate prior to invasion or by supplementing Henle cell media with saturated transferrin or iron, which has been shown to increase intracellular iron levels (30). None of these treatments restored plaque formation to the tonB mutant (data not shown). Conversely, addition of the iron chelator EDDA to bacterial cultures prior to invasion of Henle cells had no effect on plaque formation with any of the strains tested (data not shown).
Iron-regulated promoter activity in bacteria growing in vitro.
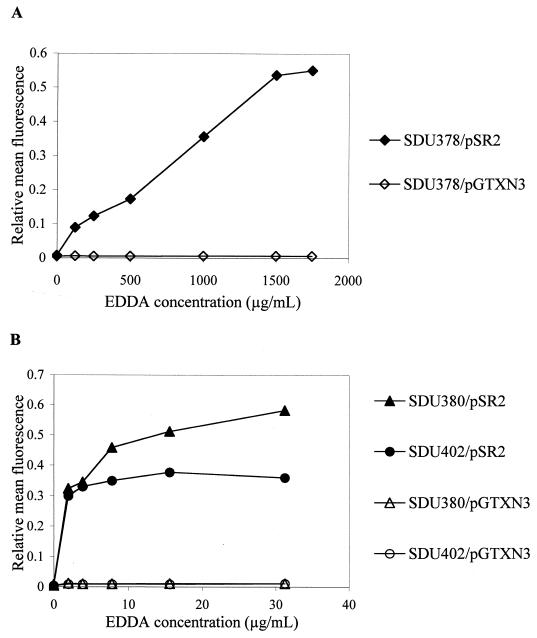
The fact that SDU380 (TonB−) and SDU402 (ShuA− EntB−) exhibited similar growth rates under both high- and low-iron conditions extracellularly but grew at different rates intracellularly, along with the failure of iron supplementation to restore plaque-forming ability to SDU380, suggested that something other than iron limitation was responsible for the defects in intracellular multiplication and plaque formation seen with the tonB mutant. If the intracellular growth defect of the tonB mutant were related to iron transport, then the mutant should show evidence of iron limitation in the intracellular environment. In order to determine whether iron was sufficiently limiting intracellularly to inhibit growth of the tonB mutant, a reporter system that incorporated the Fur-regulated shuA promoter, which is repressed in high-iron conditions, was utilized. The shuA promoter was cloned upstream of a promoterless gfp gene, and the level of fluorescence activity produced by the promoter construct was measured using flow cytometry. This provided an indirect, yet quantitative, measure of iron levels in the bacterial cell and of the availability of iron within the host cell cytoplasm. Each strain was transformed with the shuA-gfp plasmid (pSR2). The same plasmid lacking a promoter (pGTXN3) or containing the constitutive dnaY promoter (pLR9) was included as a negative or positive control, respectively.
To measure the activity of the Fur-regulated promoter in vitro, strains carrying pSR2 were grown in the presence of increasing concentrations of EDDA to limit the availability of iron. A 10- to 50-fold increase in GFP expression was observed when the wild-type strain SDU378 carrying the reporter construct pSR2 was grown under low-iron conditions (Fig. 3A). Likewise, in the tonB mutant, SDU380, and the shuA entB mutant, SDU402, GFP expression from pSR2 was induced 30- to 50-fold in iron-restricted media (Fig. 3B). The concentration of EDDA required for maximum induction of GFP expression in the wild-type strain was 1.5 mg/ml, whereas for SDU380 and SDU402, the maximum level of GFP fluorescence was achieved with 15 and 30 μg of EDDA/ml, respectively, indicating that these mutants were much more sensitive than the wild type to iron starvation. In each of the strains tested, the promoterless gfp gene in pGTXN3 produced a low level of fluorescence activity (mean fluorescence = 4.6), which was nearly identical to the fluorescence level seen with untransformed bacteria (mean fluorescence = 4.3). Expression of GFP driven by the dnaY promoter in pLR9 remained at a high level extra- and intracellularly, regardless of the iron concentration (mean fluorescence = 580.7). These data indicate that expression of shuA-gfp is induced by iron limitation, and thus, GFP production can be used as an indicator of iron availability in bacteria carrying the reporter plasmid pSR2.
FIG. 3.
Induction of GFP expression in vitro following growth under iron-limiting conditions. Wild type (A) or iron transport mutatnts (B) were subcultured 1:200 from an overnight culture into LSLB containing EDDA and grown to late exponential phase. Mean fluorescence values obtained from plasmids pGTXN3 and pSR2 were normalized to values obtained with the constitutive expression plasmid pLR9.
Iron-regulated promoter activity during intracellular growth.
The level of Fur-regulated GFP expression was determined for bacteria growing inside Henle cells and compared to the level of GFP produced by extracellular bacteria. Following a 30-min incubation of bacteria with Henle cells to allow for invasion, the tissue culture media containing extracellular bacteria was removed and GFP-mediated fluorescence was measured. Intracellular bacteria were harvested after 2 h of growth inside the Henle cells, and these samples were likewise analyzed by flow cytometry. Mean fluorescence values of extracellular and intracellular bacteria are shown in Table 4. The wild-type and mutant strains exhibited relatively low levels of intracellular GFP expression from the shuA promoter in pSR2 compared to GFP expression from this plasmid during extracellular growth (Table 4).
TABLE 4.
Comparison of extracellular and intracellular GFP expression by S. dysenteriae strains
| Reporter plasmid and strain | GFP expression in iron-replete culturesa
|
GFP expression in iron-limited cultures
|
||||
|---|---|---|---|---|---|---|
| Mean fluorescence
|
Fold repressiond | Mean fluorescence
|
Fold repression | |||
| Extracellularb | Intracellularc | Extracellular | Intracellular | |||
| pSR2 (shuA-gfp) | ||||||
| SDU378 | 24.5 | 23.2 | 1.1 | 47.2 | 21.9 | 2.2e |
| SDU380 | 60.1 | 24.4 | 2.5 | 122.4f | 21.3 | 5.7e |
| SDU402 | 34.4 | 22.2 | 1.5 | 94.0f | 23.6 | 4.0e |
| pLR9 (dnaY-gfp) | ||||||
| SDU378 | 657.0 | 687.8 | 1.0 | 690.7 | 635.3 | 1.1 |
| SDU380 | 599.7 | 459.3f | 1.3 | 791.5 | 491.9f | 1.6 |
| SDU402 | 818.3 | 692.2 | 1.2 | 820.2 | 637.6 | 1.3 |
Prior to invasion of Henle cells, bacteria were grown to an A595 of 0.4. The culture was divided, and EDDA was added to one set of cultures (iron limited) as indicated, and the bacteria were grown for two additional hours.
Bacteria were incubated with Henle cells to allow for invasion. After 30 min, the supernatant containing the extracellular bacteria was removed and samples were analyzed by flow cytometry.
Following 2 h of intracellular growth, bacteria were harvested from infected Henle cells and samples were analyzed by flow cytometry.
Fold repression is the mean fluorescence of extracellular bacteria divided by the mean fluorescence of intracellular bacteria.
Intracellular expression is significantly lower than extracellular expression (P < 0.01).
Mean fluorescence is significantly different than that of the wild type (P < 0.025).
Bacteria were grown under iron-replete (LSLB) or iron-limiting (LSLB plus EDDA) conditions prior to invasion of Henle cells. When the wild-type and mutant strains were grown in iron-replete media prior to invasion, a slight downregulation of GFP expression was observed for bacteria growing intracellularly (Table 4). GFP-mediated fluorescence was at the same level in the mutant and wild-type strains isolated from the intracellular environment. In each of the strains tested, the promoterless construct pGTXN3 produced the same low level of fluorescence both extra- and intracellularly (mean fluorescence = 4.22) as was observed in vitro. The dnaY promoter in pLR9 produced a constitutively high level of GFP expression preinvasion and during growth in the intracellular environment, indicating that intracellular bacteria were capable of expressing GFP at high levels and that this fluorescence activity was readily detectable (Table 4). The tonB mutant, SDU380, showed lower intracellular expression of dnaY-gfp compared to the wild type (P < 0.001). This may reflect the poor growth or survival of the tonB mutant at 2 h postinvasion.
Bacteria also were pregrown in EDDA prior to invasion to reduce the levels of iron storage that might prevent induction of the Fur-regulated promoter. The wild type and the mutants produced higher levels of GFP fluorescence extracellularly than when the bacteria were grown in iron-replete conditions prior to invasion. Because the two mutants are defective in iron transport, they are more sensitive than the wild type to iron starvation induced by EDDA, resulting in significantly higher expression of shuA-gfp (Table 4). In all three strains, however, expression of GFP by intracellular bacteria was significantly reduced compared to that by the extracellular bacteria. SDU380 and SDU402 showed a 5.7- and a 4-fold repression, respectively, of the Fur-regulated promoter during intracellular growth, while SDU378 displayed a 2.2-fold level of repression. Intracellular expression of GFP from the Fur-regulated promoter remained at the same low level in all the strains, regardless of whether the bacteria were grown under iron-replete or iron-limited conditions prior to invasion. This indicated that S. dysenteriae was not starved for iron in the Henle cell cytoplasm and that the intracellular growth defect of the tonB mutant was not a result of iron starvation.
DISCUSSION
S. dysenteriae is capable of growth in different environments within the human host, including extracellular growth in the lumen of the gut and growth inside epithelial cells. These environments will have different potential iron sources, and it is likely that Shigella uses different iron acquisition systems in each of these environments.
Shigella strains containing mutations in individual iron transport systems have been tested previously for invasion and plaque formation. Neither heme transport in S. dysenteriae (24) nor aerobactin-mediated siderophore transport in Shigella flexneri (20) was found to be essential for invasion or plaque formation. These studies did not rule out a role for high-affinity iron transport in plaque formation, however, because the iron acquisition systems could be functionally redundant. In this study, a tonB mutant and a shuA entB double mutant deficient in both heme transport and siderophore production were constructed and tested. The tonB mutation eliminated all TonB-dependent high-affinity iron transport, while the shuA and entB mutations eliminated TonB-dependent iron acquisition systems thought to be relevant in vivo. Although the tonB mutant was unable to form plaques in cultured cells or to cause keratoconjunctivitis in the Serény test, the shuA entB double mutant formed plaques and was positive in the Serény test. Disruption of both heme- and siderophore-mediated iron transport in SDU402 did not affect virulence, indicating that the loss of both TonB-dependent iron uptake systems was not responsible for the attenuation of the tonB mutant.
Intracellular multiplication is required for a fully virulent phenotype of S. dysenteriae. SDU402 grew inside Henle cells as well as the wild type, but the growth of SDU380 was significantly reduced. This contrasts with the results of in vitro growth assays, in which SDU380 and SDU402 exhibited similar growth rates under both iron-replete and iron-restricted conditions. The fact that the intracellular growth rate of the shuA entB double mutant is normal, but growth of the tonB mutant is defective in this environment, could indicate the presence of another TonB-dependent iron transport system that is required for growth inside Henle cells. However, the addition of excess iron to the media, to the bacteria, and to the cultured cells all failed to compensate for the plaque-minus phenotype of SDU380. The tonB mutant does not appear to be iron-starved while in the intracellular environment, as there is no derepression of a Fur-regulated reporter gene. Therefore, TonB may be required for something other than iron transport in S. dysenteriae. In addition to iron, TonB mediates transport of vitamin B12 through the outer membrane receptor BtuB (6). In order to rule out a possible requirement for vitamin B12 in the intracellular environment, a btuB mutant of SDU378 was constructed and found to have no defect in invasion or plaque formation (our unpublished data). This is consistent with the previous report that btuB mutants of E. coli K-1 and Salmonella enterica serovar Typhimurium were fully virulent in animal assays (40).
Shigella spp. require iron for growth; however, there is no evidence for iron stress when they are growing in the intracellular environment in cultured cells. The shuA entB double mutant, whose growth is inhibited at even modest levels of iron starvation, grows normally inside Henle cells. Furthermore, transcription of an iron-regulated gfp fusion is not induced in the shuA entB mutant in the intracellular environment. This is consistent with our earlier report (10) that the Fur-regulated iuc promoter in S. flexneri is repressed in the intracellular environment, as determined by a chloramphenicol acetyltransferase expression vector. Additionally, protein levels of the aerobactin outer membrane receptor are lower inside cultured cells (10). It is not known how iron is acquired by the tonB mutant and the shuA entB double mutant in the intracellular environment. A TonB-independent iron transport system such as the Feo system of iron(II) uptake (14) could mediate iron acquisition inside host cells. Although it is possible that the Henle cell assays are performed under conditions that are artificially iron replete, the tonB mutant demonstrated an inability to spread intercellularly in the Serény animal model as well as in cultured cells. A positive Serény result with the shuA entB double mutant correlated with the formation of plaques in Henle cell monolayers, indicating that the tissue culture system is able to approximate in vivo conditions in assays measuring cell-to-cell spread.
TonB and TonB-dependent iron transport systems have been shown to contribute to the virulence of several bacterial pathogens, including Vibrio cholerae (11), Haemophilus influenzae (49), S. enterica serovar Typhimurium (47), and Bordetella pertussis (36); however, a function for TonB in virulence that is distinct from its role in iron acquisition has not been previously described. In S. dysenteriae, TonB is absolutely required for virulence. The results of our intracellular multiplication assays indicate that TonB is needed for growth in the intracellular environment. However, TonB does not appear to be required for intracellular iron acquisition. TonB may function in the acquisition of an essential nutrient other than iron, or TonB may be involved in the uptake or export of a compound that is required for virulence.
ACKNOWLEDGMENTS
We thank Antoinette Hartman for performing the Guinea pig Serény tests. We also thank Charles Earhart, Robert Kadner, Ian Molineux, Elizabeth Wyckoff, Laura Runyen-Janecky, and Yuki Gleason for providing strains and plasmids and Kathleen Postle and Edwin Oaks for providing antisera. We thank Bristol-Myers Squibb for supplying pirazmonam. We are especially grateful to Elizabeth Wyckoff for her invaluable advice and for critical reading of the manuscript.
This work was supported by NIH grant AI 16935.
REFERENCES
- 1.Arnow L E. Colorimetric determination of the components of 3,4-dihydroxyphenylalanine tyrosine mixtures. J Biol Chem. 1937;118:531–537. [Google Scholar]
- 2.Bagg A, Neilands J B. Ferric uptake regulation protein acts as a repressor, employing iron (II) as a cofactor to bind the operator of an iron transport operon in Escherichia coli. Biochemistry. 1987;26:5471–5477. doi: 10.1021/bi00391a039. [DOI] [PubMed] [Google Scholar]
- 3.Bagg A, Neilands J B. Molecular mechanism of regulation of siderophore-mediated iron assimilation. Microbiol Rev. 1987;51:509–518. doi: 10.1128/mr.51.4.509-518.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Braun V. Energy-coupled transport and signal transduction through the gram-negative outer membrane via TonB-ExbB-ExbD-dependent receptor proteins. FEMS Microbiol Rev. 1995;16:295–307. doi: 10.1111/j.1574-6976.1995.tb00177.x. [DOI] [PubMed] [Google Scholar]
- 5.Braun V, Hantke K, Koster W. Bacterial iron transport: mechanisms, genetics, and regulation. In: Sigel A, Sigel H, editors. Metal ions in biological systems. Vol. 35. New York, N.Y: Marcel Dekker; 1998. pp. 67–145. [PubMed] [Google Scholar]
- 6.DeVeaux L C, Kadner R J. Transport of vitamin B12 in Escherichia coli: cloning of the btuCD region. J Bacteriol. 1985;162:888–896. doi: 10.1128/jb.162.3.888-896.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fischer E, Gunter K, Braun V. Involvement of ExbB and TonB in transport across the outer membrane of Escherichia coli: phenotypic complementation of exb mutants by overexpressed tonB and physical stabilization of TonB by ExbB. J Bacteriol. 1989;171:5127–5134. doi: 10.1128/jb.171.9.5127-5134.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gerhardt P, Murray R G E, Wood W A, Krieg N P, editors. Methods for general and molecular bacteriology. Washington, D.C.: ASM Press; 1994. pp. 332–334. [Google Scholar]
- 9.Hale T L, Formal S B. Protein synthesis in HeLa or Henle 407 cells infected with Shigella dysenteriae 1, Shigella flexneri 2a, or Salmonella typhimurium W118. Infect Immun. 1981;32:137–144. doi: 10.1128/iai.32.1.137-144.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Headley V, Hong M, Galko M, Payne S M. Expression of aerobactin genes by Shigella flexneri during extracellular and intracellular growth. Infect Immun. 1997;65:818–821. doi: 10.1128/iai.65.2.818-821.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Henderson D P, Payne S M. Vibrio cholerae iron transport systems: role of heme and siderophore iron transport in virulence and identification of a gene associated with multiple iron transport systems. Infect Immun. 1994;62:5120–5125. doi: 10.1128/iai.62.11.5120-5125.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hong M, Gleason Y, Wyckoff E E, Payne S M. Identification of two Shigella flexneri chromosomal loci involved in intercellular spreading. Infect Immun. 1998;66:4700–4710. doi: 10.1128/iai.66.10.4700-4710.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kadner R J. Vitamin B12 transport in Escherichia coli: energy coupling between membranes. Mol Microbiol. 1990;4:2027–2033. doi: 10.1111/j.1365-2958.1990.tb00562.x. [DOI] [PubMed] [Google Scholar]
- 14.Kammler M, Schon C, Hantke K. Characterization of the ferrous iron uptake system of Escherichia coli. J Bacteriol. 1993;175:6212–6219. doi: 10.1128/jb.175.19.6212-6219.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Karlsson M, Hannavy K, Higgins C F. ExbB acts as a chaperone-like protein to stabilize TonB in the cytoplasm. Mol Microbiol. 1993;8:389–396. doi: 10.1111/j.1365-2958.1993.tb01582.x. [DOI] [PubMed] [Google Scholar]
- 16.Karlsson M, Hannavy K, Higgins C F. A sequence-specific function for the N-terminal signal-like sequence of the TonB protein. Mol Microbiol. 1993;8:379–388. doi: 10.1111/j.1365-2958.1993.tb01581.x. [DOI] [PubMed] [Google Scholar]
- 17.Larsen R A, Myers P S, Skare J T, Seachord C L, Darveau R P, Postle K. Identification of TonB homologs in the family Enterobacteriaceae and evidence for conservation of TonB-dependent energy transduction complexes. J Bacteriol. 1996;178:1363–1373. doi: 10.1128/jb.178.5.1363-1373.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Larsen R A, Thomas M G, Wood G E, Postle K. Partial suppression of an Escherichia coli TonB transmembrane domain mutation (delta V17) by a missense mutation in ExbB. Mol Microbiol. 1994;13:627–640. doi: 10.1111/j.1365-2958.1994.tb00457.x. [DOI] [PubMed] [Google Scholar]
- 19.Lash A, Saleem A. Iron metabolism and its regulation. A review. Ann Clin Lab Sci. 1995;25:20–30. [PubMed] [Google Scholar]
- 20.Lawlor K M, Daskaleros P A, Robinson R E, Payne S M. Virulence of iron transport mutants of Shigella flexneri and utilization of host iron compounds. Infect Immun. 1987;55:594–599. doi: 10.1128/iai.55.3.594-599.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lawlor K M, Payne S M. Aerobactin genes in Shigella spp. J Bacteriol. 1984;160:266–272. doi: 10.1128/jb.160.1.266-272.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Letain T E, Postle K. TonB protein appears to transduce energy by shuttling between the cytoplasmic membrane and the outer membrane in Escherichia coli. Mol Microbiol. 1997;24:271–283. doi: 10.1046/j.1365-2958.1997.3331703.x. [DOI] [PubMed] [Google Scholar]
- 23.Mills M, Payne S M. Genetics and regulation of heme iron transport in Shigella dysenteriae and detection of an analogous system in Escherichia coli O157:H7. J Bacteriol. 1995;177:3004–3009. doi: 10.1128/jb.177.11.3004-3009.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mills M, Payne S M. Identification of shuA, the gene encoding the heme receptor of Shigella dysenteriae and analysis of invasion and intracellular multiplication of a shuA mutant. Infect Immun. 1997;65:5358–5363. doi: 10.1128/iai.65.12.5358-5363.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moeck G S, Coulton J W. TonB-dependent iron acquisition: mechanisms of siderophore-mediated active transport. Mol Microbiol. 1998;28:675–681. doi: 10.1046/j.1365-2958.1998.00817.x. [DOI] [PubMed] [Google Scholar]
- 26.Neilands J. Siderophores of bacteria and fungi. Microbiol Sci. 1984;1:9–14. [PubMed] [Google Scholar]
- 27.Oaks E V, Wingfield M E, Formal S B. Plaque formation by virulent Shigella flexneri. Infect Immun. 1985;48:124–129. doi: 10.1128/iai.48.1.124-129.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Parsot C, Sansonetti P J. Invasion and pathogenesis of Shigella infections. Curr Top Microbiol Immunol. 1995;209:25–42. doi: 10.1007/978-3-642-85216-9_2. [DOI] [PubMed] [Google Scholar]
- 29.Payne S M. Iron and virulence in the family Enterobacteriaceae. Crit Rev Microbiol. 1988;16:81–111. doi: 10.3109/10408418809104468. [DOI] [PubMed] [Google Scholar]
- 30.Petrat F, Rauen U, De Groot H. Determination of the chelatable iron pool of isolated rat hepatocytes by digital fluorescence microscopy using the fluorescent probe, phen green SK. Hepatology. 1999;29:1171–1179. doi: 10.1002/hep.510290435. [DOI] [PubMed] [Google Scholar]
- 31.Pettis G S, Brickman T J, McIntosh M A. Transcriptional mapping and nucleotide sequence of the Escherichia coli fepA-fes enterobactin region. J Biol Chem. 1988;35:18857–18863. [PubMed] [Google Scholar]
- 32.Pickett C L, Hayes L, Earhart C F. Molecular cloning of the Escherichla coli K-12 entACGBE genes. FEMS Microbiol Lett. 1984;24:77–80. [Google Scholar]
- 33.Pope L M, Reed K E, Payne S M. Increased protein secretion and adherence to HeLa cells by Shigella spp. following growth in the presence of bile salts. Infect Immun. 1995;63:3642–3648. doi: 10.1128/iai.63.9.3642-3648.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Postle K. Aerobic regulation of the Escherichia coli tonB gene by changes in iron availability and the fur locus. J Bacteriol. 1990;172:2287–2293. doi: 10.1128/jb.172.5.2287-2293.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Postle K. TonB protein and energy transduction between membranes. J Bioenerg Biomembr. 1993;25:591–601. doi: 10.1007/BF00770246. [DOI] [PubMed] [Google Scholar]
- 36.Pradel E, Guiso N, Menozzi F D, Locht C. Bordetella pertussis TonB, a Bvg-independent virulence determinant. Infect Immun. 2000;68:1919–1927. doi: 10.1128/iai.68.4.1919-1927.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rogers H J. Iron-binding catechols and virulence in Escherichia coli. Infect Immun. 1973;7:445–456. doi: 10.1128/iai.7.3.445-456.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Runyen-Janecky L J, Hong M, Payne S M. The virulence plasmid-encoded impCAB operon enhances survival and induced mutagenesis in Shigella flexneri after exposure to UV radiation. Infect Immun. 1999;67:1415–1423. doi: 10.1128/iai.67.3.1415-1423.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 40.Sampson B A, Gotschlich E C. Elimination of the vitamin B12 uptake or synthesis pathway does not diminish the virulence of Escherichia coli K1 or Salmonella typhimurium in three model systems. Infect Immun. 1992;60:3518–3522. doi: 10.1128/iai.60.9.3518-3522.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sansonetti P J. Genetic and molecular basis of epithelial cell invasion by Shigella species. Rev Infect Dis. 1991;13(Suppl. 4):S285–S292. doi: 10.1093/clinids/13.supplement_4.s285. [DOI] [PubMed] [Google Scholar]
- 42.Saxena P, Walker J R. Expression of argU, the Escherichia coli gene coding for a rare arginine tRNA. J Bacteriol. 1992;174:1956–1964. doi: 10.1128/jb.174.6.1956-1964.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schuch R, Sandlin R C, Maurelli A T. A system for identifying post-invasion functions of invasion genes required for the Mxi-Spa type III secretion pathway of Shigella flexneri in intercellular dissemination. Mol Microbiol. 1999;34:675–689. doi: 10.1046/j.1365-2958.1999.01627.x. [DOI] [PubMed] [Google Scholar]
- 44.Serény B. Experimental keratoconjunctivitis shigellosa. Acta Microbiol Acad Sci Hung. 1957;4:367–376. [PubMed] [Google Scholar]
- 45.Singer M, Baker T A, Schnitzler G, Deischel S M, Goel M, Doves W, Jaacks K J, Grossman A D, Erickson J W, Gross C A. A collection of strains containing genetically linked alternating antibiotic resistance elements for genetic mapping of Escherichia coli. Microbiol Rev. 1989;53:1–24. doi: 10.1128/mr.53.1.1-24.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Staab J F, Earhart C F. EntG activity of Escherichia coli enterobactin synthase. J Bacteriol. 1990;172:6403–6410. doi: 10.1128/jb.172.11.6403-6410.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tsolis R M, Baumler A J, Heffron F, Stojiljkovic I. Contribution of TonB- and Feo-mediated iron uptake to growth of Salmonella typhimurium in the mouse. Infect Immun. 1996;64:4549–4556. doi: 10.1128/iai.64.11.4549-4556.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Turbyfill K R, Joseph S W, Oaks E V. Recognition of three epitopic regions on invasion plasmid antigen C by immune sera of Rhesus monkeys infected with Shigella flexneri 2a. Infect Immun. 1995;63:3927–3935. doi: 10.1128/iai.63.10.3927-3935.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Varosik G P, Sanders J D, Cope L D, Muller-Eberhard U, Hansen E J. A functional tonB gene is required for both utilization of heme and virulence expression by Haemophilus influenzae type b. Infect Immun. 1994;62:2470–2477. doi: 10.1128/iai.62.6.2470-2477.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wyckoff E E, Duncan D, Torres A G, Mills M, Maase K, Payne S M. Structure of the Shigella dysenteriae haem transport locus and its phylogenetic distribution in enteric bacteria. Mol Microbiol. 1998;28:1139–1152. doi: 10.1046/j.1365-2958.1998.00873.x. [DOI] [PubMed] [Google Scholar]