Abstract
Primary sensory neurons in dorsal root ganglia (DRG) are wrapped by satellite glial cells (SGCs), and neuron-SGC interaction may affect somatosensation, especially nociceptive transmission. P2-purinergic receptors (P2Rs) are key elements in the two-way interactions between DRG neurons and SGCs. However, because the cell types are in such close proximity, conventional approaches such as in vitro culture and electrophysiologic recordings are not adequate to investigate the physiologically relevant responses of these cells at a population level. Here, we performed in vivo calcium imaging to survey the activation of hundreds of DRG neurons in Pirt-GCaMP6s mice and to assess SGC activation in GFAP-GCaMP6s mice in situ. By combining pharmacologic and electrophysiologic techniques, we investigated how ganglionic purinergic signaling initiated by α,β-methyleneadenosine 5′-triphosphate (α,β-MeATP) modulates neuronal activity and excitability at a population level. We found that α,β-MeATP induced robust activation of small neurons—likely nociceptors—through activation of P2X3R. Large neurons, which are likely non-nociceptive, were also activated by α,β-MeATP, but with a delay. Blocking pannexin 1 channels attenuated the late phase response of DRG neurons, indicating that P2R stimulation may subsequently induce paracrine ATP release, which could further activate cells in the ganglion. Moreover, ganglionic α,β-MeATP treatment in vivo sensitized small neurons and enhanced responses of spinal wide-dynamic-range neurons to subsequent C-fiber inputs, suggesting that modulation via ganglionic P2R signaling could significantly affect nociceptive neuron excitability and pain transmission. Therefore, targeting functional P2Rs within ganglia may represent an important new strategy for pain modulation.
Keywords: Purinergic signaling, Dorsal root ganglion, Satellite glial cell, Calcium imaging, Pain
1. Introduction
As the first relay in the somatosensory pathway, dorsal root ganglion (DRG) neurons are indispensable for transmitting sensory signals to the central nervous system (CNS). These neurons are surrounded by satellite glial cells (SGCs) in ganglia, and neuron-SGC interactions may contribute to sensory processing.11,20 The purinergic system plays an important role in somatosensation, especially in pain.9,35,53,55 Adenosine 5′-triphosphate (ATP) is an endogenous ligand for various P2 purinergic receptors (P2R), including ionotropic P2XRs and metabotropic P2YRs.11,12,55 In DRG, P2X3R and P2X2/3R are expressed mostly in small neurons, which are likely nociceptors, whereas P2X7R is highly expressed in SGCs,9,29,32,40,44 which closely surround the neurons and have bidirectional functional interactions with them.20,55
Only a small proportion of DRG neurons express P2X3R, and they are scattered throughout the ganglion.9,32,40 Moreover, SGCs have small cell bodies and are not electrically excitable.20,47 Thus, traditional electrophysiologic methods cannot efficiently survey enough cells for researchers to identify purinergic-responsive ones or examine complex neuronal and SGC activities in vivo. Both neurons and SGCs display calcium-based excitability,16,20 and measurement of cytosolic calcium levels has been a valuable approach for investigating these cells. The fluorescence intensity of genetically encoded calcium indicator (GCaMP) increases robustly as cytosolic calcium concentration rises, allowing the activities of a large number of cells to be monitored for long periods at single-cell resolution.2,28,49 Accordingly, we generated Pirt-GCaMP6s mice and GFAP-GCaMP6s mice for in vivo calcium imaging of hundreds of DRG neurons and SGCs without ambiguity.15,27,28
The receptors and ion channels on the peripheral and central terminals of DRG neurons have well-characterized roles in sensory transmission.33,38,56 Comparatively, research into the functional implications of these receptors in sensory ganglia is still in its infancy.3,13,20 Because P2Rs have a broad distribution, it has been difficult to formulate strategies of systemic drug administration to selectively target P2Rs that are relevant to nociception, while leaving other processes intact. In this regard, targeting P2Rs in sensory ganglia may avoid this limitation and represent an effective strategy for pain control. In addition, afferent action potentials in peripheral axons may invade neuronal somata and lead to calcium influx through voltage-gated L-type channels, triggering paracrine release of ATP within the ganglia.23,55 Peptides (eg, CGRP) can be released from somata and function as neuromodulators in sensory ganglia.34,54 Yet, it remains unclear how fast-acting neurotransmitters such as ATP modulate population neuronal excitability within the ganglia in vivo.
To that end, we conducted GCaMP6s imaging of hundreds of DRG neurons and SGCs under normal connectivity and performed electrophysiologic recording of spinal wide-dynamic-range (WDR) neurons important to pain transmission.18 Using α,β-methyleneadenosine 5′-triphosphate (α,β-MeATP) as a pharmacologic probe, we examined functions of P2Rs within DRG of intact mice. Our findings begin to resolve important roles of ganglionic P2R signaling in modulating the activities of DRG neurons and SGCs, which can profoundly affect peripheral nociceptive transmission.
2. Materials and methods
2.1. Animals
For in vivo calcium imaging of DRG neurons, we used adult Pirt-GCaMP6s mice (25–35 g, both sexes), which were generated by crossing Pirt-Cre mice with Rosa26-loxP-STOP-loxP-GCaMP6s mice.15,27,28 The Pirt promoter is expressed in >90% of DRG neurons but not in SGCs, neurons, or glial cells of the CNS.26 Because Cre recombinase is controlled by the Pirt promoter, GCaMP6s is expressed specifically in primary sensory neurons in Pirt-GCaMP6s mice. Additionally, because the GFAP promoter is expressed primarily in SGCs in the DRG,47 we generated GFAP-GCaMP6s mice for calcium imaging of SGCs. GFAP-GCaMP6s mice (25–35 g, both sexes) were generated by crossing GFAP-Cre mice with Rosa26-loxP-STOP-loxP-GCaMP6s mice for imaging SGCs (Fig. 1A). For simultaneous calcium imaging of DRG neurons and SGCs, we then crossed Pirt-GCaMP6s mice and GFAP-GCaMP6s mice to generate Pirt:GFAP-GCaMP6s mice, which express GCaMP6s in both neurons and SGCs. We purchased GFAP-Cre mice from Jackson Laboratory [Bar Harbor, ME, B6.Cg-Tg(Gfap-cre)77.6Mvs/2J, Stock No: 024098] and received Rosa26-loxP-STOP-loxP-GCaMP6s mice as a gift from Dr. Dwight E. Bergles in the Solomon H. Snyder Department of Neuroscience, School of Medicine, Johns Hopkins University (Baltimore, MD). All transgenic mice were backcrossed to C57BL/6J mice for at least 10 generations.
Figure 1.

α,β-Methyleneadenosine 5′-triphosphate activates small and large dorsal root ganglion (DRG) neurons sequentially. (A) Strategy for generating conditional Pirt-GCaMP6s, GFAP-GCaMP6s, and Pirt:GFAP-GCaMP6s mice. (B) Setup for in vivo calcium imaging of L4 DRG in anesthetized mice. (C) Representative images showing changes in fluorescence intensity of DRG neurons after ganglionic administration of α,β-MeATP (500 μM) in Pirt-GCaMP6s mice: baseline [frames (F)1–5, 10 seconds/frame], phase I (F6–7), and phase II post-drug (F8–26). Examples of activated neurons are marked by colored circles and arrows. The red-outlined area in the phase II image is shown at higher magnification to the right. DRG neurons were categorized according to somal area as <450 μm2 (small, S), 450 to 700 μm2 (medium, M), and >700 μm2 (large, L). (D) An example of a color-coded image (temporal code, F1–26) showing response to α,β-MeATP. (E) Fluorescence intensity traces (left, F/F0) and heat maps (right) of selected neurons activated by α,β-MeATP in (D). Red arrow indicates the time of drug application. (F) Number of neurons in each subpopulation that were activated by α,β-MeATP (5, 50, and 500 μM, n = 6 mice) in phase I (F6–7) and phase II (F8–26). Data are expressed as mean ± SEM. *P < 0.05, **P < 0.01 vs 5 μM treatment of the same size group; #P < 0.05, ##P < 0.01, ###P < 0.001 vs indicated group or small-neuron group. Two-way mixed-model ANOVA with Dunnett multiple comparisons test. α,β-MeATP, α,β-methyleneadenosine 5′-triphosphate; ANOVA, analysis of variance; GCaMP, genetically encoded calcium indicator.
Electrophysiologic recording of dorsal horn neurons was performed in adult wild-type C57BL/6J mice (25–35 g, both sexes; Jackson Laboratory). Mice were housed in groups of 3 to 5 on a standard 12-hour light/12-hour dark cycle with free access to food and water. All animal work was approved by the Animal Care and Use Committee of Johns Hopkins University and complied with the National Institutes of Health Guide for the Care and Use of Laboratory Animals to ensure minimal animal use and discomfort.
2.2. In vivo calcium imaging
Changes in intracellular calcium concentration were indicated by changes in fluoresce intensity in neurons and SGCs, and were measured by laser-scanning confocal microscopy (Leica TCS SP8; Wetzlar, Germany). Both male and female adult Pirt-GCaMP6s and GFAP-GCaMP6s mice were anesthetized with 1.5% to 2% isoflurane, and the L4 DRG was exposed as described in previous studies.10,27 Mice were positioned prone on a custom-designed microscope stage, and the spinal column was stabilized with a pair of spinal clamps to limit movements during imaging. Epineurium was carefully removed and the DRG was bathed in a pool of extracellular artificial cerebrospinal fluid (ACSF, ~1 mL), which contained 120 mM NaCl, 3 mM KCl, 1.1 mM CaCl2, 10 mM glucose, 0.6 mM NaH2PO4, 0.8 mM MgSO4, and 18 mM NaHCO3.
L4 DRG was then placed under a 25x/0.95 W VISIR 0.17 long-working distance (2.5 mm) water immersion objective lens for imaging (Fig. 1B). Time-lapse z-stacks (frames) of the intact DRG were acquired at 10 seconds/frame and 512 × 512 pixel resolution. Each frame consisted of 10 scans (one scan per second), and 26 frames (260 seconds) were taken for scanning the L4 DRG. A laser wavelength at 488 nm (6% laser power) was used, and the images were acquired at a scan speed of 400 Hz.
To establish the dose–response function, we applied increasing concentrations of α,β-MeATP (5, 50, and 500 μM) at the ganglion, with washout after each dose. High-concentration drug solution (0.1 mL) was infused into the bath (~1 mL ACSF) as one bolus with a pipette to reach the final working concentration as indicated in each experiment. Drug was not washed out until imaging was complete for each dose. After washout with ACSF, we waited for >30 minutes before testing the next higher dose. This interval allows a sufficient recovery from the previous drug action and a complete return of cytosolic fluorescence to pre-drug baseline. Body temperature of the mice was maintained with a heating pad at 36.0 to 37.0°C as monitored by a rectal probe.
2.3. Quantification of calcium imaging
We exported the raw images (Tagged Image File Format) and used ImageJ (National Institutes of Health) and LIF (Leica Microsystems GmbH) to analyze calcium imaging data. Calcium responses were assessed as the increase in green fluorescence intensity of the GCaMP upon binding to intracellular calcium. An experimenter traced the activated cells manually to determine cell size and fluorescence intensity. Fluorescence intensity at the baseline level was taken as F0. Evoked calcium response was expressed as a ratio of the posttreatment fluorescence intensity (F) to basal level. Activation in neurons was defined as an increase in F/F0 ≥1.5-fold.
2.4. In vivo extracellular recording of spinal wide-dynamic-range neurons
We recorded WDR neurons in the spinal cord of mice as described in our previous study.17 Briefly, we anesthetized the mice with 2% isoflurane, performed a laminectomy at vertebral levels T12 to L1, and exposed the spinal lumbar enlargement (segments L3-L5). The L4-L5 segments were continually bathed in a pool of warm ACSF (35–37°C), with the dura mater incised and retracted. Other exposed spinal tissue was covered with warm agar (1.5%). We also exposed the L4 DRG and carefully removed the sheath covering the surface of the DRG (perineurium and epineurium) using fine forceps and scissors under a dissecting microscope. The DRG was bathed in a pool of warm ACSF. Drug solution was applied into the bath to the final working concentration for ganglionic drug treatment. Animals were placed on a pad of circulating hot water to maintain a core body temperature in the normal range (36.0–37.0°C). Extracellular activities of WDR neurons were recorded with fine-tip (<1.0 μm) paralyn-coated tungsten microelectrodes (1–3 mΩ at 1 kHz; Frederick Haer Company). Signals were amplified and filtered (high pass: 300 Hz, low pass: 10 kHz; model DAM80; World Precision Instruments, Inc, Sarasota, FL). Analog data were collected in real time with a computer-based data acquisition and processing system (CED Spike 2, Cambridge, United Kingdom). Wide-dynamic-range cells were identified according to their characteristic responses.17 We quantified the number of WDR neuron action potentials evoked by electrical test stimulation applied at the sciatic nerve.
2.5. Drugs
α,β-Methyleneadenosine 5′-triphosphate lithium salt, A438079 hydrochloride hydrate, A317491 sodium salt hydrate, and probenecid were purchased from Sigma-Aldrich (St. Louis, MO). Other drugs were purchased from Sigma-Aldrich or Tocris Bioscience (Bristol, United Kingdom). Stock solutions were freshly prepared as instructed by the manufacturer.
2.6. Statistical analysis
Data were analyzed with the Prism 8.0 statistical program (GraphPad Software, Inc). The methods for statistical comparisons in each study are given in the figure legends. Data that followed a normal distribution are expressed as mean ± SEM. We randomized animals to the different treatment groups and blinded the experimenter to drug treatment to reduce selection and observation bias. Two-tailed tests were performed, and P < 0.05 was considered statistically significant in all tests.
3. Results
3.1. Ganglionic application of α,β-methyleneadenosine 5′-triphosphate induces early activation of small neurons
Non-noxious and noxious information is transmitted primarily by large and small neurons, respectively, which have different neurochemical properties and transcriptional profiles.25,42 Adenosine 5′-triphosphate is an excitatory transmitter that can be released in DRG from the neuronal soma by membrane depolarization and also from SGCs.20,24,44,55 However, the functional consequences of this activity in vivo are unclear. Accordingly, we first examined the responses of different subpopulations of DRG neurons to ganglionic application of α,β-MeATP, which is stable and has a longer action than ATP.
DRG neurons were categorized into 3 subpopulations with soma areas of <450 (small), 450 to 700 (medium), and >700 (large). Neuronal responses are presented as raw images, traces of calcium transients, and heat maps of changes in cytosolic fluorescence intensity (Figs. 1C–E). In the dose-response study, we applied increasing concentrations of α,β-MeATP (5, 50, and 500 μM) at the ganglion, with washout after each dose and 30 minutes interval before applying the next higher dose. Time course analysis showed that in most neurons, the evoked responses to α,β-MeATP were robust and transient (<1 minute). α,β-Methyleneadenosine 5′-triphosphate increased the fluorescence intensity and the number of activated neurons in different subpopulations of DRG neurons in a concentration-dependent manner (Fig. 1F; Supplemental Fig. S1, available at http://links.lww.com/PAIN/B549). Notably, α,β-MeATP (50, 500 μM) activated more small neurons than large and medium neurons in early phase I (Frames [F] 6–7, 0–20 seconds postdrug; Fig. 1F). The number of large neurons activated by α,β-MeATP (50, 500 μM) was not significantly greater than that of vehicle-treated neurons until phase II (F8–26, 20–210 seconds postdrug).
3.2. α,β-Methyleneadenosine 5′-triphosphate also leads to activation of satellite glial cells
We next monitored responses of SGCs in GFAP-GCaMP6s mice to ganglionic application of ATP and α,β-MeATP. SGCs responded robustly to ganglionic application of ATP (100 μM), as indicated by rising fluorescence intensity in GFAP-GCaMP6s mice (Supplemental Fig. S2a,b, available at http://links.lww.com/PAIN/B549). In some areas, multiple SGCs were seen to aggregate together and form “basket”-like structures that enwrap the neurons, creating the so-called neuron–glial unit.5,14,20
Because SGCs cannot be readily measured as individual cells or be separated into different groups based on morphology (shape and size), we quantified population SGC responses in GFAP-GCaMP6s by measuring the averaged fluorescence intensity in the whole imaging field before (F0) and after (F) drug treatment. We also present example SGC responses in selected areas of interest as images, traces, and heat maps of calcium transients (Figs. 2A and B). Fluorescence intensity was measured for each frame and then plotted against time (Fig. 2C). Ganglionic application of α,β-MeATP (5, 50, and 500 μM) increased fluorescence intensity in SGCs in a concentration-dependent manner (Fig. 2C). Time course analysis showed prolonged excitation of SGCs in response to 50 and 500 μM α,β-MeATP. The increase in fluorescence intensity after 50 and 500 μM α,β-MeATP application (ie, from F6 to F26) was significantly greater than that after vehicle treatment (Fig. 2C). SGC responses to α,β-MeATP lasted longer (>10 frames or >100 seconds, Fig. 2C) than did neuronal responses (Fig. 1E). Thus, α,β-MeATP induced a prolonged activation of SGCs.
Figure 2.

Dose-dependent responses of satellite glial cells (SGCs) to α,β-methyleneadenosine 5′-triphosphate. (A) Representative images illustrate changes in fluorescence intensity of SGCs after ganglionic application of α,β-MeATP (500 μM) in GFAP-GCaMP6s mice: baseline (frames (F) 1–5, 10 seconds/frame), phase I (F6–7), and phase II (F8–26) after drug treatment. For illustrative purposes, 3 areas of interest (AOIs) are marked with colored boxes and numbered. (B) Fluorescence intensity traces (left, F/F0) and heat maps (right) of the 3 AOIs shown in (A) before and after α,β-MeATP (500 μM) treatment. (C) Satellite glial cell responses in GFAP-GCaMP6s mice were quantified by measuring the averaged fluorescence intensity in the whole imaging field before (F0) and after (F) drug treatment. Fluorescence intensity was measured for each frame and plotted against time after ganglionic application of α,β-MeATP (5, 50, and 500 μM, n = 6 mice per dose). Red arrow indicates the time of drug application. Data are expressed as mean ± SEM. ***P < 0.001 vs vehicle; ###P < 0.001 vs indicated group. Two-way repeated measures ANOVA. α,β-MeATP, α,β-methyleneadenosine 5′-triphosphate; ANOVA, analysis of variance; GCaMP, genetically encoded calcium indicator.
3.3. Pretreatment with A317491, A438079, or probenecid inhibits neuronal and satellite glial cell responses to α,β-methyleneadenosine 5′-triphosphate
Because Pirt:GFAP-GCaMP6s mice express GCaMP6s in both DRG neurons and SGCs, their responses can be monitored simultaneously (Fig. 3A). Bath application of α,β-MeATP also induced early activation of small neurons followed by large ones in these mice (Figs. 3A and B). In phase I, α,β-MeATP (50 and 500 μM) activated significantly more small neurons than large neurons (Figs. 3B and C). Activation of SGCs by α,β-MeATP was also observed in Pirt:GFAP-GCaMP6s mice (Figs. 3D–F).
Figure 3.
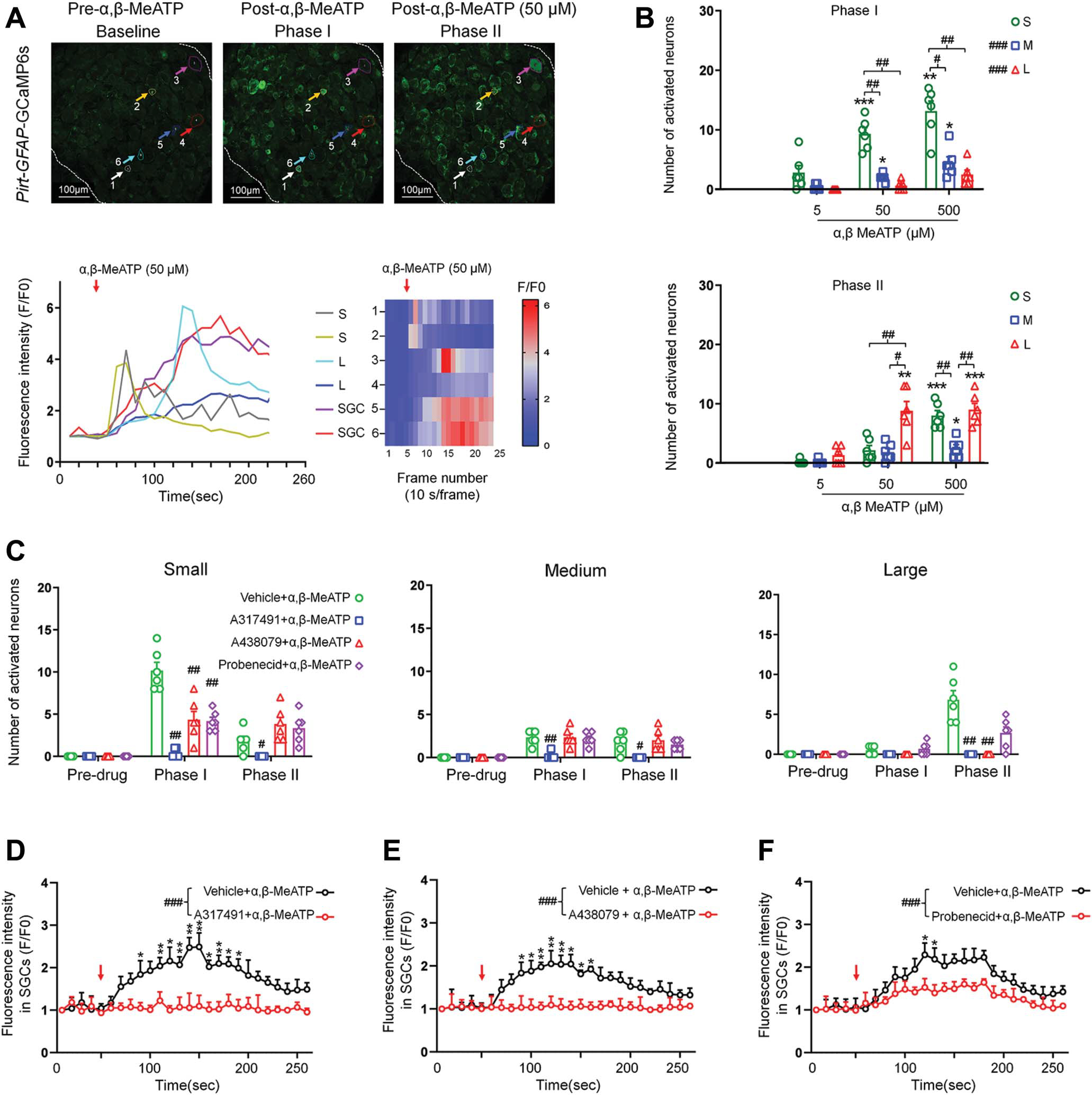
Effects of pretreatment with A317491, A438079, and probenecid on activation of neurons and satellite glial cells (SGCs) by α,β-methyleneadenosine 5′-triphosphate. (A) Upper: Representative images showing DRG neurons and SGCs after ganglionic administration of α,β MeATP (50 μM) in Pirt:GFAP-GCaMP6s mice. Baseline (Frames (F) 1–5, 10 seconds/frame), phase I (F6–7), and phase II post-drug (F8–26). Examples of activated neurons (#1–4) and SGCs (#5–6) are marked with colored circles and arrows. DRG neurons were categorized by somal area as <450 μm2 (small, S), 450 to 700 μm2 (medium, M), and >700 μm2 (large, L). Lower: Fluorescence intensity traces (left, F/F0) and heat maps (right) of DRG neurons and SGCs activated by α,β MeATP. (B) Number of neurons in each subpopulation activated by α,β MeATP (5, 50, and 500 μM, n = 6 mice) in phase I and phase II. Data are expressed as mean ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001 vs 5 μM treatment of the same size group; #P < 0.05, ##P < 0.01, ###P < 0.001 vs indicated group or small-neuron group. Two-way mixed-model ANOVA with Dunnett’s multiple comparisons test. (C) Number of neurons in each subpopulation that were activated by α,β-MeATP (50 μM) after pretreatment with vehicle (ACSF), the P2X3R antagonist A317491 (100 μM), the P2X7R antagonist A438079 (100 μM), or the Panx1 antagonist probenecid (1 mM; n = 6 mice per group). Data are expressed as mean ± SEM. #P < 0.05, ##P < 0.01 vs vehicle 1 α,β-MeATP in the same phase. Two-way mixed-model ANOVA with Tukey multiple comparisons test. (D–F) Average SGC fluorescence intensity produced by α,β-MeATP (50 μM) application after pretreatment with A317491 (100 μM, n = 5 mice, D), A438079 (100 μM, n = 6 mice, E), probenecid (1 mM, n = 6 mice, F), or vehicle (ACSF, n = 6 mice). Fluorescence was measured for each frame and then plotted against time. Red arrow indicates the time of drug application. The curves representing the changes in fluorescence intensity after different drug treatments were compared between groups. Data are expressed as mean ± SEM. *P < 0.05, **P < 0.01 vs antagonist 1 α,β-MeATP; ###P < 0.001 vs indicated group. Two-way mixed-model ANOVA with Tukey multiple comparisons test. α,β-MeATP, α,β-methyleneadenosine 5′-triphosphate; ANOVA, analysis of variance; GCaMP, genetically encoded calcium indicator.
Because α,β-MeATP is a nonselective P2R agonist, we next examined the receptor mechanisms by which α,β-MeATP activates neurons and SGCs. Homomeric P2X3Rs and heteromeric P2X2/3Rs are mostly expressed in small neurons and can be activated by α,β-MeATP.9,32 We pretreated DRG with the P2X3R antagonist A317491 (100 μM), the P2X7R antagonist A438079 (100 μM), or vehicle for 2 minutes before administering α,β-MeATP (50 μM) to Pirt:GFAP-GCaMP6s mice. Different antagonists were tested in different animals. Pretreatment with A317491 completely blocked the responses to α,β-MeATP in all neuronal subpopulations in both phases (Fig. 3C). The activation of small neurons in phase I was substantially reduced by pretreatment with A438079, but it remained significantly higher than predrug baseline, suggesting a partial blockade (Fig. 3C).
Panx1 is a nonselective ion and metabolite channel expressed in both neurons and glial cells and may play an important role in pain.20,24 Because release of ATP is a key consequence of Panx1 channel opening,20,43,46 we also examined whether Panx1-mediated ATP release contributes to activation of neurons and SGCs by α,β-MeATP. The Panx1 blocker probenecid (1 mM) reduced the activation of small neurons in phase I, suggesting that ATP release from DRG cells contributed to the responses (Fig. 3C).
These results indicate that blocking P2X3R inhibits both phase I and phase II responses to α,β-MeATP in different subpopulations of neurons, and that blocking P2X7R and Panx1 preferentially reduces phase I activation of small neurons by α,β-MeATP. Because large neurons do not express high levels of P2X3R and were predominantly activated at late phase, we postulate that their responses may be because of ATP released from small neurons or SGCs during activation of other purinergic receptors (eg, P2X4R) in the early phase. It is unclear why some DRG neurons did not respond to α,β-MeATP, even though their surrounding SGCs showed activation. Neither A317491, nor A438079, nor probenecid alone altered the calcium signal in DRG neurons, suggesting that P2X3R and P2X7R lack tonic activation (Supplemental Fig. S3, available at http://links.lww.com/PAIN/B549).
To quantify SGC calcium responses in Pirt:GFAP-GCaMP6s mice, we measured and averaged the fluorescence intensities in selected areas of interest where SGC signals could be separated from neuronal signals. We then compared the data from predrug and postdrug conditions. SGCs express P2X7R and P2X4R but do not express P2X3R.20,51 In previous in vitro studies, SGCs reportedly did not respond to α,β-MeATP in short-term culture.7,30 Yet, in our in vivo study, we observed that α,β-MeATP activated SGCs in both GFAP-GCaMP6s (Fig. 2) and Pirt:GFAP-GCaMP6s mice (Fig. 3A). We postulate that the initial activation of SGCs by α,β-MeATP may involve some direct, but weak, activation of P2X7R and P2X4R. Their later activation may involve ATP released from neurons activated in the early phase. This notion is supported by our findings that pretreatment with A317491, A438079, or probenecid significantly inhibited α,β-MeATP-induced activation of SGCs (Figs. 3D–F), suggesting the involvement of P2X3R, P2X7R, and Panx1 channels. Because SGCs do not express P2X3R, A317491 may inhibit SGC responses by blocking the activation of small neurons and the subsequent release of ATP in the early phase after α,β-MeATP treatment.
3.4. Ganglionic application of α,β-MeATP sensitizes dorsal root ganglion neurons
Previous studies have suggested that activity of neuronal somata in DRG may modulate the transmission of peripheral afferent inputs.13,39 Therefore, we tested how brief activation of P2R in DRG might alter neuronal responses to subsequent afferent inputs. Brush stimulation alone (~1 Hz, 10 seconds) at the ipsilateral hind paw increased fluorescence intensity primarily in large neurons (Figs. 4A,B), whereas non-noxious heat stimulation (41°C water bath, 10 seconds) activated many small and medium neurons (Figs. 4C,D). The responses evoked by α,β-MeATP in most DRG neurons were transient and declined quickly (<1 minutes). At 5 minutes after α,β-MeATP treatment, the percentage of small and medium neurons in each subpopulation that responded to brush stimulation had significantly increased from predrug level (Fig. 4B, predrug vs postdrug in each subpopulation: small, 8 of 469 vs 88 of 497; medium, 7 of 113 vs 16 of 94; large, 31 of 105 vs 34 of 88 neurons; n = 5 mice). The percentages of small and medium neurons that responded to heat stimulation were also significantly increased from predrug level (Fig. 4D, predrug vs postdrug: small, 68 of 628 vs 156 of 497; medium, 12 of 117 vs 30 of 91; large, 3 of 110 vs 6 of 67 neurons; n = 5 mice).
Figure 4.
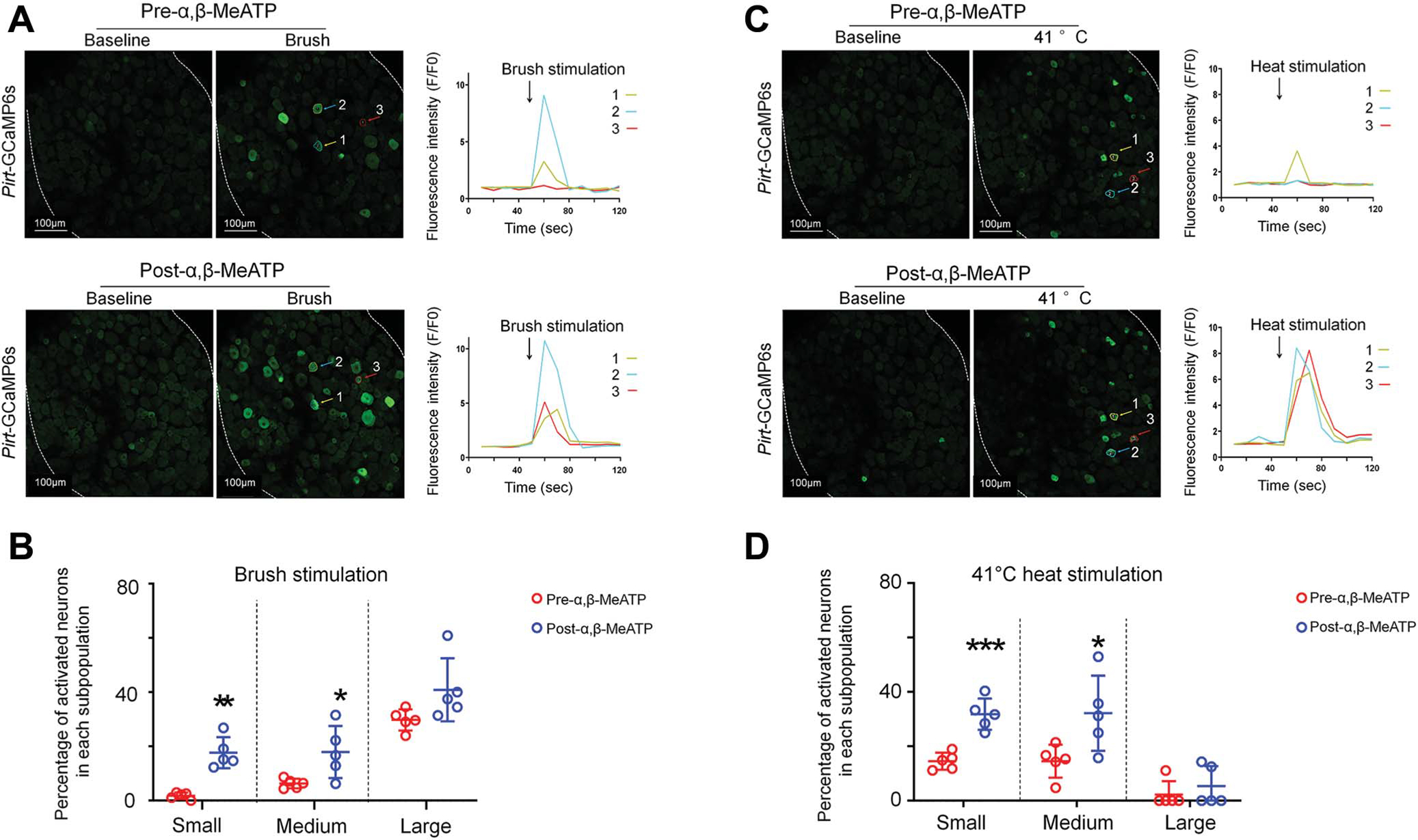
The percentage of dorsal root ganglion neurons that respond to brush and heat stimulation increases after ganglionic application of α,β-methyleneadenosine 5′-triphosphate. (A) Representative images show L4 DRG neuronal fluorescence in response to brush stimulation (~1 Hz for 10 seconds) at the hind paw before and 5 minutes after α,β-MeATP (100 μM) application in Pirt-GCaMP6s mice. Right: Fluorescence intensity traces (F/F0) of the activated neurons marked with colored arrows. (B) Percentage of neurons in each size population that responded to brush stimulation before and after α,β-MeATP treatment (n = 5 mice). DRG neurons were categorized according to somal area as <450 μm2 (small), 450 to 700 μm2 (medium), and >700 μm2 (large). (C) Representative images show neuronal fluorescence in response to heat stimulation of the hind paw (41°C water bath, 10 seconds) before and 10 minutes after α,β-MeATP (100 μM) application. (D) Percentage of neurons in each subpopulation that responded to heat stimulation (n = 5 mice). Data are expressed as mean 6 SEM. *P < 0.05, **P < 0.01, ***P < 0.001 vs predrug (paired t test). α,β-MeATP, α,β-methyleneadenosine 5′-triphosphate; DRG, dorsal root ganglia; GCaMP, genetically encoded calcium indicator.
3.5. Wide-dynamic-range neuron response increases after ganglionic application of α,β-methyleneadenosine 5′-triphosphate
Even with the evidence that α,β-MeATP can act locally in the DRG, a question remains regarding whether intraganglionic purinergic signaling can modulate nociceptive transmission. Because of the pseudo-unipolar topology and T-junctions of DRG neurons, the action potentials traveling from their peripheral axons may bypass the soma and reach the spinal cord through the central branch.1,8 Therefore, it is important to determine whether ganglionic application of α,β-MeATP, which affects the neuronal somata and SGCs, alters nociceptive afferent input into the spinal cord.
Wide-dynamic-range neurons receive both A-fiber and C-fiber inputs and play an important role in spinal nociceptive transmission.17,18,52 We conducted in vivo electrophysiologic recordings of WDR neurons from deep dorsal horn (350–700 μm below the surface) at L4 spinal segment in wild-type C57BL/6J mice (Fig. 5A). Based on the activation thresholds and latencies, responses of WDR neurons to high-intensity electrical test stimulation (3.0 mA, 2 ms) at the sciatic nerve can be separated into A-fiber- and C-fiber-mediated components (Fig. 5B), as shown in our previous studies.17,18 We also exposed the L4 DRG and carefully removed the epineurium. Drug solution (~0.1 mL) was applied into the bath (~1 mL) as one bolus with a pipette and was not washed out until recording was completed. Different drugs were tested in different experiments. The C-component of the WDR neuron response to test stimulation was significantly greater at 10 minutes after α,β-MeATP (100 μM, Figs. 5C and D) was applied to the DRG than it was before α,β-MeATP application, but the A-component was unchanged. The α,β-MeATP-induced increase in C-component (Fig. 5D) was blocked when the ganglion was pretreated with A317491 (100 μM) or A438079 (100 μM) 2 minutes before the agonist. These results suggest that increased activity of DRG neuronal soma increases transmission of nociceptive afferent input to the spinal cord, and that SGCs may contribute to this influence.
Figure 5.

Effects of ganglionic α,β-methyleneadenosine 5′-triphosphate application on spinal wide-dynamic-range (WDR) neuronal responses to electrical test stimulation. (A) Experimental setup for in vivo extracellular recording of WDR neurons and drug application to L4 DRG (inset) in wild-type mice. (B) Example recording shows a WDR neuronal response to high-threshold electrical test stimulation (3.0 mA, 2 ms) at the sciatic nerve before and after ganglionic application of α,β-MeATP (100 μM). A- and C-fiber components (number of action potentials) were separated based on latency. (C and D) Quantification of A-component (C) and C-component (D) response to electrical test stimulation before and 5 minutes after ganglionic application of α,β-MeATP (100 μM) with and without A317491 pretreatment (100 μM, n = 5 mice per group). A317491 (1 mM, 200 μL) was applied to a bath of ~1 mL ACSF 2 minutes before the agonist was added. Data are expressed as mean ± SEM. *P < 0.05, **P < 0.01 vs predrug (paired t test). α,β-MeATP, α,β-methyleneadenosine 5′-triphosphate; DRG, dorsal root ganglia; WDR, wide-dynamic range.
3.6. Probenecid, A317491, and A438079 inhibit dorsal root ganglia neuronal responses
Finally, we investigated the effect of endogenous purinergic signaling on activation of DRG neurons and SGCs by pharmacologically blocking P2Rs and Panx1 one at a time in separate animals. At 10 minutes after application of A438079 or probenecid, but not A317491, the percentage of large neurons that responded to brush stimulation (~1 Hz, 10 seconds) at the hind paw (Figs. 6A–D) was decreased. Heat stimulation (41°C) at the hind paw activated mostly small and medium neurons. Intriguingly, A317491 and probenecid, but not A438079, significantly decreased the percentages of both subpopulations that responded to heat stimulation (Figs. 6E–H). These results suggest that blocking P2X3R, P2X7R, and Panx1 channels in DRG differentially may inhibit different subpopulations of DRG neurons to subsequent peripheral stimulation.
Figure 6.

Ganglionic application of P2R inhibitors reduces the number of dorsal root ganglia neurons that respond to brush and heat stimulation at the hind paw. (A) Representative images show L4 DRG neuronal fluorescence in response to brush stimulation at the hind paw of Pirt-GCaMP6s mice before and 10 minutes after ganglionic application of A438079 (100 μM). (B–D) Quantification of neurons in each subpopulation that responded to brush stimulation before and after A317491 (100 μM, B), A438079 (100 μM, C), or probenecid (1 mM, D) treatment (n = 5 mice per group). (E) Representative images show L4 DRG neuronal fluorescence in response to 41°C heat stimulation at the hind paw of Pirt-GCaMP6s mice before and 10 minutes after ganglionic application of A317491 (100 μM). (F–H) Quantification of neurons in each subpopulation that responded to heat stimulation before and after A317491 (100 μM, F), A438079 (100 μM, G), or probenecid (1 mM, H) treatment (n = 5 mice per group). Data are expressed as mean ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001 vs predrug (paired t test). DRG, dorsal root ganglia; GCaMP, genetically encoded calcium indicator; P2R, P2-purinergic receptor.
4. Discussion
Studies of the roles of the purinergic system in pain are ever-expanding. By using in vivo calcium imaging, we demonstrated that ganglionic application of α,β-MeATP induces robust responses of neurons and SGCs. Importantly, α,β-MeATP increased neuronal excitability and nociceptive signaling, as shown in both calcium imaging of DRG neurons and electrophysiologic recordings of spinal WDR neurons. Thus, functional P2Rs are present in DRG of intact mice, are important for transmission of nociceptive signals, and have key roles in pain modulation. These findings extend the knowledge of P2R in pain beyond sensory transduction at the nerve terminals and suggest that targeting them in sensory ganglia may also represent an effective strategy for pain modulation.
Ganglionic application of α,β-MeATP activated predominately small neurons initially, followed by more large neurons in the late phase. This sequential activation is unlikely attributable to drug diffusion-related changes in the concentration profile because α,β-MeATP (~0.1 mL) was infused quickly into the bath (~1 mL). With the short diffusion distance and small bath volume, it would reach the final working concentration within seconds, much earlier than the start of phase II (20 seconds). Because different sizes of neurons are scattered throughout the DRG, a drug should affect them nearly simultaneously in a given region. Accordingly, the order in which neurons are activated is apparently governed by their neurochemical properties, not by physical factors (eg, diffusion, location, size). α,β-MeATP induced concentration-dependent activation of DRG neurons. Although the responses in most neurons to α,β-MeATP were transient, and we delayed testing of the next dose for >30 minutes to allow sufficient recovery, we cannot completely exclude the possibility that repeated drug treatment may alter the sensitivity of purinergic receptors and thus neuronal response to the subsequent doses.
P2X3Rs and heteromeric P2X2/3Rs are the main P2XRs expressed in small neurons.9,21,29,36 Pretreatment with A317491 blocked the activation of small and medium neurons by α,β-MeATP in the early phase, suggesting an important role of P2X3R.41 Large neurons express P2X4R but do not express high levels of P2X3R.9,29,31,40 The small number of large neurons activated in the early phase suggests that α,β-MeATP has minimal effect on P2X4R. This finding supports a previous report that the EC50 of α,β-MeATP to activate medium to large neurons (66 μM) is much higher than that to excite small ones (10 μM) in rat DRG.50
Our results also indicate the presence of complex interactions involving several cell types and receptors that we have attempted to summarize in a graphic form (Fig. 7). The action of α,β-MeATP is known to be brief because of strong P2X3R desensitization. Membrane depolarization can induce ATP release from neuronal somata through both Ca2+-dependent and Ca2+-independent mechanisms.23,39,54 Probenecid, a selective Panx1 blocker,6,45 reduced the activation of large neurons in phase II, suggesting that the late neuron responses may be partially caused by ATP release via Panx1 channel opening during phase I (Fig. 7A). The slower activation of P2X7R on SGCs by α,β-MeATP may also contribute to ATP release, although that would require a very high α,β-MeATP concentration. The detailed mechanisms remain to be examined.
Figure 7.

Hypothetical model illustrating drug actions on dorsal root ganglia neurons and satellite glial cells (SGCs). (A) Activation of neurons and SGCs after ganglionic application of α,β-MeATP. 1. α,β-Methyleneadenosine 5′-triphosphate strongly activates P2X3Rs, which are expressed primarily in small neurons. α,β-Methyleneadenosine 5′-triphosphate may weakly activate large neurons, which express P2X4R and low levels of P2X3R, and may also activate SGCs. The action of α,β-MeATP is brief because of strong desensitization, and α,β-MeATP is less potent than ATP at P2X3R. 2. Activation of small neurons leads to Panx1 opening and release of ATP from neurons to the extracellular space. 3. ATP can strongly activate large neurons through P2X4R and P2X3R. ATP also activates SGCs through P2X7R and P2YR. 4. Activation of SGCs leads to opening of Panx1 channels and release of ATP. 5. Glial ATP further activates both small and large neurons. 6. Activation of large neurons may lead to release of ATP from Panx1 channels. This process ends rapidly as ATP is degraded or cleared out of the extracellular space. Neurons and SGCs return to the resting state, and small neurons become sensitized to subsequent stimulation. (B) Diagram illustrates the sequential activation of small neurons, SGCs, and large neurons after α,β-MeATP treatment. α,β-MeATP, α,β-methyleneadenosine 5′-triphosphate.
The neuron and its attendant SGCs, so called “neuron–glial units,”5,14,20 can communicate bidirectionally through diffusible (eg, ATP, glutamate) and nondiffusible mechanisms.19,20,22 P2X7R is selectively expressed in SGCs and is insensitive to α,β-MeATP,32,41,44 with EC50 >300 μM.4 Yet, α,β-MeATP robustly activated SGCs in vivo, and the effect was blocked by pretreatment with A438079, a P2X7R antagonist. A previous sniffer patch recording study showed that ATP was released from vesicles in DRG neurons.19,55 Thus, P2X7R on SGCs might be activated by ATP released from small neurons in phase I. This possibility is supported by our findings that α,β-MeATP induced early activation of small neurons followed by SGCs, and that SGC activation was blocked by P2X3R antagonist A317491. Collectively, these findings suggest that P2R signaling mediates dynamic interactions between SGCs and DRG neurons in vivo. The results may partially explain the late phase of neuronal activation by α,β-MeATP, in which ATP became dominant and activated both neurons and SGCs (Figs. 7A and B).
In DRG, activation of SGC P2X7Rs may mediate the release of ATP and TNF-α,11,48,55 which in turn potentiate P2X3R-mediated currents and increase neuronal excitability. Upon activation, SGCs may also participate in calcium waves that spread to neurons via gap junctions and P2Rs.22,55 Another salient finding of our study is that ganglionic purinergic signaling modulated neuronal excitability and nociceptive inputs into the spinal cord. Brush stimulation primarily activates large neurons, but it activated more small neurons after ganglionic application of α,β-MeATP, suggesting an increase in neuronal excitability. More importantly, WDR neurons also exhibited an increased C-component in response to electrical stimulation after ganglionic application of α,β-MeATP. Thus, increased DRG neuronal responses correlated with increased spinal nociceptive transmission. Our finding that pretreatment with A317491 or A438079 blocked the increase in WDR neuronal responses induced by α,β-MeATP confirmed these receptor mechanisms. Collectively, these results suggest that ganglionic P2X3R and P2X7R have an important role in the signaling cascade that leads to increased spinal nociceptive transmission.
P2X3R and Panx1 channel blockers also inhibited DRG and WDR neuronal responses to peripheral stimulation, suggesting that endogenous paracrine purinergic signaling contributes to modulation of nociceptive processing. One possible scenario is that action potentials invade neuronal somata and open low-voltage-activated calcium channels, leading to an increase in intracellular calcium. This process does not involve activation of P2Rs; hence, initial neuronal activation by peripheral stimulation was not affected by blockade of P2X3R or Panx1 in sensory ganglia. Yet, the ATP released from activated neurons via Panx1 channels may excite other neurons and SGCs. Although the concentration of endogenous ATP may be low in DRG and is difficult to measure, paracrine ATP can still be highly effective owing to the small extracellular space and close proximity of the cells. This action appeared to be important for neuronal responses to subsequent stimulation.
State-of-the-art in vivo calcium imaging enabled us to monitor intracellular calcium transients of many hundreds of DRG neurons simultaneously as they were activated by physiologic stimuli delivered to receptive fields. Monitoring a large population of neurons and SGCs in situ allows characterization of their activities within the natural environment. This has a considerable advantage over isolated ganglia, making the findings more physiologically relevant than those of previous in vitro studies. Although GCaMP6s is highly sensitive to changes in intracellular calcium level, calcium transients have slower kinetics than electrical signals. Because CGaMP6s has low temporal resolution and a long recovery from the fluorescent state, and the scanning speed of in vivo calcium imaging is slow, this method may not be adequate for capturing fast and transient changes in neuronal firing or identifying neurons that adapt quickly to repetitive stimuli. Thus, combining electrophysiologic recording and calcium imaging in intact DRG will be valuable in future studies.
In summary, the current findings illustrate that functional P2Rs are present in sensory ganglia of intact mice and that ganglionic P2R signaling profoundly modulates activities of DRG neurons and SGCs. Sensory information is generally thought to be modulated and integrated only in the CNS. Here, we demonstrated that P2Rs in DRG can also play an important role in modulating neuronal excitability and thus nociceptive processing. We further propose that a cascade of early cellular activation by exogenous P2R agonists is followed by secondary paracrine action of endogenous ATP, but the details remain to be examined in future studies. Chemical messengers, including ATP, secreted by neurons and SGCs in sensory ganglia may play a role in the genesis and maintenance of chronic pain.3,13,20,22 Dorsal root ganglion neurons and SGCs undergo neurochemical and functional changes after injury, and altered purinergic signaling is an important mechanism underlying pathological pain.20,35,37 Accordingly, purinergic receptors on neuronal somata and SGCs may represent important targets for therapeutic interventions that could avoid central side effects.
Supplementary Material
Acknowledgments
The authors thank Claire F. Levine, MS, ELS (Scientific Editor, Department of Anesthesiology and Critical Care Medicine, the Johns Hopkins University), for editing the manuscript.
Funding:
This study was conducted at the Johns Hopkins University and was supported by grants NS110598 (Y.G.), NS070814 (Y.G.), and NS117761 (Y.G.) from the National Institutes of Health (Bethesda, Maryland). This study was subsidized by the Lerner Family Fund for Pain Research and a grant from the Neurosurgery Pain Research Institute at the Johns Hopkins University (Y.G.). This work was facilitated by the Pain Research Core funded by the Blaustein Fund and the Neurosurgery Pain Research Institute at the Johns Hopkins University. M. Hanani was supported by the Israel Science Foundation (1297/18) and the United States-Israel Binational Science Foundation (BSF, 2019076). Funders had no role in study design, data collection, or data interpretation, or in the decision to submit the work for publication.
Footnotes
Conflict of interest statement
The authors have no conflicts of interest to declare.
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Appendix A. Supplemental digital content
Supplemental digital content associated with this article can be found online at http://links.lww.com/PAIN/B549.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s Web site (www.painjournalonline.com).
References
- [1].Amir R, Devor M. Electrical excitability of the soma of sensory neurons is required for spike invasion of the soma, but not for through-conduction. Biophysical J 2003;84:2181–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Anderson M, Zheng Q, Dong X. Investigation of pain mechanisms by calcium imaging approaches. Neurosci Bull 2018;34:194–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Belzer V, Hanani M. Nitric oxide as a messenger between neurons and satellite glial cells in dorsal root ganglia. Glia 2019;67:1296–307. [DOI] [PubMed] [Google Scholar]
- [4].Bianchi BR, Lynch KJ, Touma E, Niforatos W, Burgard EC, Alexander KM, Park HS, Yu H, Metzger R, Kowaluk E, Jarvis MF, van Biesen T. Pharmacological characterization of recombinant human and rat P2X receptor subtypes. Eur J Pharmacol 1999;376:127–38. [DOI] [PubMed] [Google Scholar]
- [5].Boesmans W, Hao MM, Fung C, Li Z, Van den Haute C, Tack J, Pachnis V, Vanden Berghe P. Structurally defined signaling in neuro-glia units in the enteric nervous system. Glia 2019;67:1167–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Burma NE, Bonin RP, Leduc-Pessah H, Baimel C, Cairncross ZF, Mousseau M, Shankara JV, Stemkowski PL, Baimoukhametova D, Bains JS, Antle MC, Zamponi GW, Cahill CM, Borgland SL, De Koninck Y, Trang T. Blocking microglial pannexin-1 channels alleviates morphine withdrawal in rodents. Nat Med 2017;23:355–60. [DOI] [PubMed] [Google Scholar]
- [7].Ceruti S, Fumagalli M, Villa G, Verderio C, Abbracchio MP. Purinoceptor-mediated calcium signaling in primary neuron-glia trigeminal cultures. Cell calcium 2008;43:576–90. [DOI] [PubMed] [Google Scholar]
- [8].Chao D, Zhang Z, Mecca CM, Hogan QH, Pan B. Analgesic dorsal root ganglionic field stimulation blocks conduction of afferent impulse trains selectively in nociceptive sensory afferents. PAIN 2020;161:2872–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Chen CC, Akopian AN, Sivilotti L, Colquhoun D, Burnstock G, Wood JN. A P2X purinoceptor expressed by a subset of sensory neurons. Nature 1995;377:428–31. [DOI] [PubMed] [Google Scholar]
- [10].Chen TW, Wardill TJ, Sun Y, Pulver SR, Renninger SL, Baohan A, Schreiter ER, Kerr RA, Orger MB, Jayaraman V, Looger LL, Svoboda K, Kim DS. Ultrasensitive fluorescent proteins for imaging neuronal activity. Nature 2013;499:295–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Chen Y, Zhang X, Wang C, Li G, Gu Y, Huang LY. Activation of P2X7 receptors in glial satellite cells reduces pain through downregulation of P2X3 receptors in nociceptive neurons. Proc Natl Acad Sci U S A 2008;105:16773–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Cisneros-Mejorado A, Perez-Samartin A, Gottlieb M, Matute C. ATP signaling in brain: release, excitotoxicity and potential therapeutic targets. Cell Mol Neurobiol 2015;35:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Du X, Hao H, Yang Y, Huang S, Wang C, Gigout S, Ramli R, Li X, Jaworska E, Edwards I, Deuchars J, Yanagawa Y, Qi J, Guan B, Jaffe DB, Zhang H, Gamper N. Local GABAergic signaling within sensory ganglia controls peripheral nociceptive transmission. J Clin Invest 2017;127:1741–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Durham PL, Garrett FG. Development of functional units within trigeminal ganglia correlates with increased expression of proteins involved in neuron-glia interactions. Neuron glia Biol 2010;6:171–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Gao X, Han S, Huang Q, He S, Ford NC, Zheng Q, Chen Z, Yu S, Dong X, Guan Y. Calcium imaging in population of dorsal root ganglion neurons unravels novel mechanisms of visceral pain sensitization and referred somatic hypersensitivity. PAIN 2021;162:1068–81. [DOI] [PubMed] [Google Scholar]
- [16].Grienberger C, Konnerth A. Imaging calcium in neurons. Neuron 2012; 73:862–85. [DOI] [PubMed] [Google Scholar]
- [17].Guan Y, Borzan J, Meyer RA, Raja SN. Windup in dorsal horn neurons is modulated by endogenous spinal mu-opioid mechanisms. J Neurosci 2006;26:4298–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Guan Y, Liu Q, Tang Z, Raja SN, Anderson DJ, Dong X. Mas-related G-protein-coupled receptors inhibit pathological pain in mice. Proc Natl Acad Sci U S A 2010;107:15933–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Hanani M Satellite glial cells in sensory ganglia: from form to function. Brain Res Brain Res Rev 2005;48:457–76. [DOI] [PubMed] [Google Scholar]
- [20].Hanani M, Spray DC. Emerging importance of satellite glia in nervous system function and dysfunction. Nat Rev Neurosci 2020;21:485–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].He JJ, Wang X, Liang C, Yao X, Zhang ZS, Yang RH, Fang D. Wnt5b/Ryk-mediated membrane trafficking of P2X3 receptors contributes to bone cancer pain. Exp Neurol 2020;334:113482. [DOI] [PubMed] [Google Scholar]
- [22].Huang LY, Gu Y, Chen Y. Communication between neuronal somata and satellite glial cells in sensory ganglia. Glia 2013;61:1571–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Huang LY, Neher E. Ca(21)-dependent exocytosis in the somata of dorsal root ganglion neurons. Neuron 1996;17:135–45. [DOI] [PubMed] [Google Scholar]
- [24].Iglesias R, Shraer N, Suadicani SO, Belzer V, Hanstein R, Hanani M, Spray DC, Hanani M. Gap junctions, pannexins and pain. Glia 2019;695:46–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Julius D, Basbaum AI. Molecular mechanisms of nociception. Nature 2001;413:203–10. [DOI] [PubMed] [Google Scholar]
- [26].Kim AY, Tang Z, Liu Q, Patel KN, Maag D, Geng Y, Dong X. Pirt, a phosphoinositide-binding protein, functions as a regulatory subunit of TRPV1. Cell 2008;133:475–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Kim YS, Anderson M, Park K, Zheng Q, Agarwal A, Gong C, YoungL Saijilafu, He S, LaVinka PC, Zhou F, Bergles D, Hanani M, Guan Y, Spray DC, Dong X. Coupled activation of primary sensory neurons contributes to chronic pain. Neuron 2016;91:1085–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Kim YS, Chu Y, Han L, Li M, Li Z, LaVinka PC, Sun S, Tang Z, Park K, Caterina MJ, Ren K, Dubner R, Wei F, Dong X. Central terminal sensitization of TRPV1 by descending serotonergic facilitation modulates chronic pain. Neuron 2014;81:873–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Kobayashi K, Yamanaka H, Noguchi K. Expression of ATP receptors in the rat dorsal root ganglion and spinal cord. Anat Sci Int 2013;88:10–16. [DOI] [PubMed] [Google Scholar]
- [30].Kushnir R, Cherkas PS, Hanani M. Peripheral inflammation upregulates P2X receptor expression in satellite glial cells of mouse trigeminal ganglia: a calcium imaging study. Neuropharmacology 2011;61:739–46. [DOI] [PubMed] [Google Scholar]
- [31].Lalisse S, Hua J, Lenoir M, Linck N, Rassendren F, Ulmann L. Sensory neuronal P2RX4 receptors controls BDNF signaling in inflammatory pain. Sci Rep 2018;8:964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Lewis C, Neidhart S, Holy C, North RA, Buell G, Surprenant A. Coexpression of P2X2 and P2X3 receptor subunits can account for ATP-gated currents in sensory neurons. Nature 1995;377:432–5. [DOI] [PubMed] [Google Scholar]
- [33].Lin King JV, Emrick JJ, Kelly MJS, Herzig V, King GF, Medzihradszky KF, Julius D. A cell-penetrating scorpion toxin enables mode-specific modulation of TRPA1 and pain. Cell 2019;178:1362–74 e1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Liu T, Shang SJ, Liu B, Wang CH, Wang YS, Xiong W, Zheng LH, Zhang CX, Zhou Z. Two distinct vesicle pools for depolarization-induced exocytosis in somata of dorsal root ganglion neurons. J Physiol 2011;589:3507–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Magni G, Riccio D, Ceruti S. Tackling chronic pain and inflammation through the purinergic system. Curr Med Chem 2018;25:3830–65. [DOI] [PubMed] [Google Scholar]
- [36].North RA. Molecular physiology of P2X receptors. Physiol Rev 2002;82:1013–67. [DOI] [PubMed] [Google Scholar]
- [37].North RA. P2X receptors. Philosophical Trans R Soc Lond Ser B Biol Sci 2016:371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Osteen JD, Herzig V, Gilchrist J, Emrick JJ, Zhang C, Wang X, Castro J, Garcia-Caraballo S, Grundy L, Rychkov GY, Weyer AD, Dekan Z, Undheim EA, Alewood P, Stucky CL, Brierley SM, Basbaum AI, Bosmans F, King GF, Julius D. Selective spider toxins reveal a role for the Nav1.1 channel in mechanical pain. Nature 2016;534:494–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Ouyang K, Zheng H, Qin X, Zhang C, Yang D, Wang X, Wu C, Zhou Z, Cheng H. Ca21 sparks and secretion in dorsal root ganglion neurons. Proc Natl Acad Sci U S A 2005;102:12259–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Petruska JC, Cooper BY, Gu JG, Rau KK, Johnson RD. Distribution of P2X1, P2X2, and P2X3 receptor subunits in rat primary afferents: relation to population markers and specific cell types. J Chem Neuroanat 2000;20:141–62. [DOI] [PubMed] [Google Scholar]
- [41].Prado FC, Araldi D, Vieira AS, Oliveira-Fusaro MC, Tambeli CH, Parada CA. Neuronal P2X3 receptor activation is essential to the hyperalgesia induced by prostaglandins and sympathomimetic amines released during inflammation. Neuropharmacology 2013;67:252–8. [DOI] [PubMed] [Google Scholar]
- [42].Renthal W, Tochitsky I, Yang L, Cheng YC, Li E, Kawaguchi R, Geschwind DH, Woolf CJ. Transcriptional reprogramming of distinct peripheral sensory neuron subtypes after axonal injury. Neuron 2021;108:128–144.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Sandilos JK, Chiu YH, Chekeni FB, Armstrong AJ, Walk SF, Ravichandran KS, Bayliss DA. Pannexin 1, an ATP release channel, is activated by caspase cleavage of its pore-associated C-terminal autoinhibitory region. J Biol Chem 2012;287:11303–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Serrano A, Mo G, Grant R, Pare M, O’Donnell D, Yu XH, Tomaszewski MJ, Perkins MN, Seguela P, Cao CQ. Differential expression and pharmacology of native P2X receptors in rat and primate sensory neurons. J Neurosci 2012;32:11890–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Silverman W, Locovei S, Dahl G. Probenecid, a gout remedy, inhibits pannexin 1 channels. Am J Physiol Cell Physiol 2008;295:C761–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Sorge RE, Trang T, Dorfman R, Smith SB, Beggs S, Ritchie J, Austin JS, Zaykin DV, Vander MH, Costigan M, Herbert TA, Yarkoni-Abitbul M, Tichauer D, Livneh J, Gershon E, Zheng M, Tan K, John SL, Slade GD, Jordan J, Woolf CJ, Peltz G, Maixner W, Diatchenko L, Seltzer Z, Salter MW, Mogil JS. Genetically determined P2X7 receptor pore formation regulates variability in chronic pain sensitivity. Nat Med 2012;18:595–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Souza GR, Talbot J, Lotufo CM, Cunha FQ, Cunha TM, Ferreira SH.Fractalkine mediates inflammatory pain through activation of satellite glial cells. Proc Natl Acad Sci U S A 2013;110:11193–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Suadicani SO, Cherkas PS, Zuckerman J, Smith DN, Spray DC, Hanani M. Bidirectional calcium signaling between satellite glial cells and neurons in cultured mouse trigeminal ganglia. Neuron Glia Biol 2010;6:43–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Tian L, Hires SA, Mao T, Huber D, Chiappe ME, Chalasani SH, Petreanu L, Akerboom J, McKinney SA, Schreiter ER, Bargmann CI, Jayaraman V, Svoboda K, Looger LL. Imaging neural activity in worms, flies and mice with improved GCaMP calcium indicators. Nat Methods 2009;6:875–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Ueno S, Tsuda M, Iwanaga T, Inoue K. Cell type-specific ATP-activated responses in rat dorsal root ganglion neurons. Br J Pharmacol 1999;126:429–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Villa G, Fumagalli M, Verderio C, Abbracchio MP, Ceruti S. Expression and contribution of satellite glial cells purinoceptors to pain transmission in sensory ganglia: an update. Neuron Glia Biol 2010;6:31–42. [DOI] [PubMed] [Google Scholar]
- [52].Zain M, Bonin RP. Alterations in evoked and spontaneous activity of dorsal horn wide dynamic range neurons in pathological pain: a systematic review and analysis. PAIN 2019;160:2199–209. [DOI] [PubMed] [Google Scholar]
- [53].Zhang C, Medzihradszky KF, Sanchez EE, Basbaum AI, Julius D. Lys49 myotoxin from the Brazilian lancehead pit viper elicits pain through regulated ATP release. Proc Natl Acad Sci U S A 2017;114:E2524–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Zhang C, Zhou Z. Ca(21)-independent but voltage-dependent secretion in mammalian dorsal root ganglion neurons. Nat Neurosci 2002;5: 425–30. [DOI] [PubMed] [Google Scholar]
- [55].Zhang X, Chen Y, Wang C, Huang LY. Neuronal somatic ATP release triggers neuron-satellite glial cell communication in dorsal root ganglia. Proc Natl Acad Sci U S A 2007;104:9864–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Zhao J, Lin King JV, Paulsen CE, Cheng Y, Julius D. Irritant-evoked activation and calcium modulation of the TRPA1 receptor. Nature 2020;585:141–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


