Abstract
Background Interthalamic adhesion (ITA) or massa intermedia is a midline rod-like neural structure interconnecting the medial surfaces of two thalami. Its absence is considered as a midline defect associated with schizophrenia spectrum disorder. The present study aimed to determine the prevalence, location, and dimensions of the ITA in South Asian brains.
Materials and Methods One hundred midsagittal sections of adult cadaveric brains were examined for the presence or absence of ITAs, their location about the lateral wall of the third ventricle, and their dimensions.
Results ITA was found in 86 sections. In two cases, it was double. There was no significant relationship between the incidence of ITAs and sex ( p > 0.05). The ITA was most commonly located in the anterosuperior quadrant. The horizontal diameter was 4.61 ± 1.17 mm, and the vertical diameter was 3.10 ± 0.78 mm. In all cases, the horizontal diameter was longer than the vertical. The average area of the ITA was significantly larger in females (17.56 ± 5.26 mm 2 ) than in males (13.62 ± 5.22 mm 2 ) ( p = 0.025).
Conclusion Presence of ITA is common in South Asian brains, with usual location in the anterosuperior quadrant of the lateral wall of the third ventricle. The cross-sectional area of the ITA was significantly larger in females than in males. No correlation was found between the surface area of the ITA and the length of the third ventricle.
Keywords: massa intermedia, neurosurgery, schizophrenia, thalamus, third ventricle
Introduction
The interthalamic adhesion (ITA) or massa intermedia (MI) is a neuroanatomical mass composed of commissural fibers, interconnect the medial surfaces of two thalami across the third ventricular cavity, 1 2 behind the interventricular foramen of Monro. 3 Among mammals, in humans it is comparatively less developed and includes only the nucleus reuniens and part of the nucleus rhomboideus. 4 5 It can even be absent 6 or duplicated on rare occasions. 7 8 During the early embryonic stage, the ITA appears concurrently with the thalami. As the thalami grow, they gradually approach each other to meet over a variable area, known as the ITA. Owing to the pressure of cerebrospinal fluid (CSF), it elongates as a bar of neural tissue. 9
The actual function of the ITA in humans and consequent symptoms due to its absence are mostly unrevealed. Cognizance of its morphology, size, shape, and topography is critical in neurosurgery and neuroradiology. In humans, the ITA is vital owing to its substantial variability 5 6 and its possible role in dopaminergic regulation of the limbic system as described in cat model. 10 ITA is more likely to be absent in male schizophrenics compared with the female. 11 Association have been reported between ITA size differences and neuropsychological measures of attention functioning in healthy females 1 and reported as more prevalent and prominent in females. 12 13 Overall, most researchers brace the conviction that commissural fibers within the ITA interconnects the limbic and cognitive processing networks shows sexual dimorphism in morphology and prevalence. Studying ITA using in vivo neuroimaging techniques is helpful to understand its anatomical connectivity. 1 14 However, identifying ITA on acquired images 11 15 16 17 is very challenging, specifically in younger individuals with narrow third ventricles and “kissing” thalami. 2 Studies on cadaveric or corpse brains can help to make out the prevalence, detailed structure, and location of this minute structure in detail. Probabilistic tractography is a recnet advanced technique to know the patterns of the fiber passing through the ITA and their connectivity. Very few studies 3 7 have described the morphology, morphometry, and topographic location of ITA on the lateral wall of the third ventricle in midsagittal sections of the brain. However, to date, no such data on South Asian ethnicity is available. With this background, the present study was undertaken, primarily to determine the prevalence, location, and morphology of ITA in South Asian cadaveric brains and to determine any correlation between ITA size and sex and third ventricle.
Materials and Methods
This cross-sectional observational study was done in the department of anatomy over a period of 3 years. Fifty adult human cadaveric brains (38 male and 12 female) aged at death approximately 45 to 65 years were fixed in a basic suspension of formalin (10%) for 4 weeks to achieve complete and uniform fixation. Cadavers with a history of head injury and visible brain abnormalities were excluded from the study. Formalin fixation causes brain shrinkage. 18 To compensate this shrinkage effect, 10% formol solution (the actual amount of formaldehyde dissolved in 10% formalin is only 3.7–4.0%) was used, as this can be considered a standard method of fixation. Subsequently, the brains were meticulously sectioned with a brain knife in the midsagittal plane through the body of the corpus callosum, the interhemispheric fissure, the septum pellucidum, the cavity of the third ventricle, and the cerebral aqueduct. The ITA, connecting the medial surfaces of two thalami, was cut thereby and features were noted on these midsagittal sections and recorded.
Medial surfaces of the sagittal sections were looked for the presence or absence of the ITA. When present, the shapes of the ITAs were noted and photographed for record. Sections with absence/duplication of ITA were excluded from further study.
Subsequently, to determine the topographic location of the ITA, a coordinate system was drawn on the lateral wall of the third ventricle (viewed from its medial aspect). The X -axis (abscissa) was defined as a straight line connecting the superior most point of the anterior commissure (CA) to the inferior most end of the posterior commissure (CP). The Y -axis (ordinate) intercepted the midpoint of this CA-CP line. 3 This coordinate system divides the lateral wall of the third ventricle into four quadrants. The position of the ITA was described according to the location of its center in one of those quadrants ( Fig. 1 ).
Fig. 1.

Coordinate system on the medial surface of the sagittal section of brain. The X -axis: straight line connecting the superior most point of the anterior commissure (CA) and the inferior most point of the posterior commissure (CP). The Y -axis: line intercepted the midpoint of the CA-CP line ( X -axis). A, anterior; P, posterior; S, superior; I, inferior.
To estimate its size (midsagittal cross-sectional area), the horizontal (HD) and vertical (VD) diameters were measured using digital calipers (Mitutoyo 12”/300 mm, 0.01 mm resolution), and the cross-sectional area (SA) was computed. HD was measured as the anteroposterior length of the ITA at the level of the posterior point of the interventricular foramen (of Monro). VD was measured as the vertical tangential length of the ITA passing through its center. The SA calculated by this procedure was correlated with the distance between the CA and CP (CA-CP interval) to establish whether it depends on the length of the third ventricle.
Results
An ITA was present in 43 of the 50 brains studied (86%). Among the 38 males, it was present in 34 (89.47%), and among the 12 females, it was present in 9 (75.00%). It was absent in 7 brains (14.00%) ( Fig. 2 ), 4 males (10.52%) and 3 females (25.00%). There was no significant relationship between the prevalence of the ITA and sex ( p > 0.05). Among the 43 cases, the ITA was single in 42 (96.97%) and doubled in one (3.03%) ( Fig. 3 ). ITAs of different shapes were found, oval or elliptical (primary diameter or length was anteroposterior) as the most common, followed by circular and triangular or irregular as least common ( Fig. 4 ). In two-thirds of the cases, the ITA was located entirely or mainly in the anterosuperior quadrant. In the rest of the cases, it was found in the center, or in the antero- or posteroinferior quadrant of the lateral wall of the third ventricle. Table 1 depicts the morphometric data of the ITA with their statistical analysis. None of the variables showed significant difference between male and female except for surface area (SA) of the ITA.
Fig. 2.

Absence of interthalamic adhesion (ITA). CA, anterior commissure; CP, posterior commissure; A, anterior; P, posterior; S, superior; I, inferior.
Fig. 3.
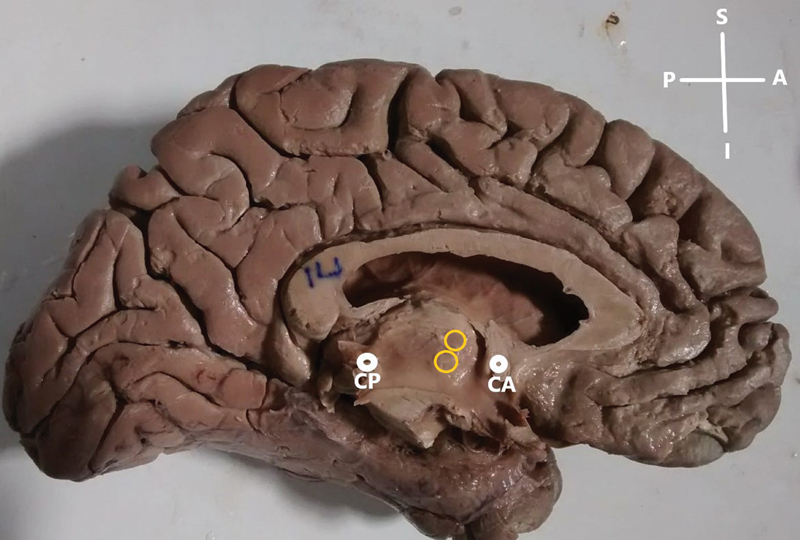
Duplication of interthalamic adhesion (ITA). CA, anterior commissure; CP, posterior commissure; A, anterior; P, posterior; S, superior; I, inferior.
Fig. 4.

Various shapes of the ITA. ( A ) Oval; ( B ) circular; ( C ) triangular.
Table 1. The morphometric data of the ITA.
| Measured Variables | Mean (in mm) | Range | p -Value | ||
|---|---|---|---|---|---|
| Male | Female | Male | Female | ||
| Horizontal diameter (HD) | 4.51 ± 1.13 | 4.63 ± 1.19 | 2.23–2.33 | 2.31–2.37 | > 0.05 |
| Vertical diameter (VD) | 3.07 ± 0.63 | 3.13 ± 0.83 | 1.63–3.78 | 1.98–5.31 | > 0.05 |
| Surface area (SA) | 12.17 ± 4.57 | 14.49 ± 5.23 | 3.63–18.80 | 4.57–22.58 | 0.025 |
| CA-CP distance | 18.46 ± 4.22 | 17.34 ± 6.94 | 11.2–22.12 | 9.66–24.7 | > 0.05 |
Abbreviations: CA, anterior commissure; CP, posterior commissure; ITA, interthalamic adhesion.
Discussion
The morphology, function, and clinical relevance of ITA have received very little attention in anatomy textbooks and the published literature. The ITA becomes apparent about the second trimester of gestation along with several features of the ventricular system and as the age advances, the capacity of the third ventricle increases leading into ITA shrinkage. 18 Although the specific function of the ITA is yet to be established, it is presumed that it can affect the flow and pressure distribution of CSF within the cavity of the third ventricle. 19 According to Snyder et al, 20 the ITA, together with other midline structures, participates in forming the central neural circuits responsible for the processing of attention and information.
It also regulates electrocortical activities, distribution of epileptiform discharges over the cerebral hemispheres, in the temporal and limbic regions of the cerebrum, more obvious in males than females. 13 21
Various studies reported conflicting results on sex predilection of its presence and absence. 1 15 22 Allen and Gorski 22 studied 100 healthy cadaveric brains and reported that the ITA was more frequent in females (78.00%) than males (68.00%). In contrast, we found it more frequently in males than females; however, the difference was nonsignificant ( p > 0.05). Sexual dimorphism in the presence of the ITA could be associated with aberrant neurodevelopmental phenomenon, more commonly seen in males. 23
The absence of ITA varies widely in the normal human population (2.30–31.7%). An extensive cadaveric study done on 921 brains, showed absence of ITA in 31.70% cases. 5 Park et al 24 studied on Korean brains, and reported the absence of the ITA in 11.60%. A meta-analysis by Trzesniak et al 17 reported that the absence of the ITA ranges from 2.30 to 22.30%. The wide variation could be attributed to differences in the type of study (cadaveric or neuroimaging), ethnicity of the populations under study, and sample size. Neuroimaging studies usually incorporate a wide age range, while cadaveric studies tend to be skewed toward an older population. While some reports 1 2 17 have not reported decline in the prevalence of ITA across age, other studies done by Rabl, 5 Trzesniak et al, 17 and Rosales et al 18 have shown a decrease in the prevalence of ITA with age. Thus, it remains plausible that whether age plays a role in determining ITA prevalence or not. A comprehensive longitudinal study across all decades of life may help to get the answer.
Erbağci et al 25 reported that the ITA was more often absent in schizophrenia patients than in healthy individuals. However, such an association has not been clinically established with certainty.
Most previous studies have reported sexual dimorphism in the absence of the ITA in various populations. A definite sexual dimorphism in the absence of the ITA was reported by Nopoulos et al 15 and Ceyhan et al 26 (13.56% vs. 32.08% and 6.00% vs. 23.10%, respectively). Allen and Gorski 22 also revealed that men were less likely to have an ITA than women, suggesting potentially underlying the sex difference in cognitive function. 27 In contrary, we found more females without ITA than males ( p < 0.05). This result could be due to the lesser number of female brains than male or to the race and ethnicity of the specimens. Additionally, Erbağci et al 25 and Haghir et al 27 tried to establish an association between the absence of the ITA and schizophrenia but found no such association.
Although the specific role of the ITA in humans is uncertain, animal studies have proven its role in regulating dopamine release from the basal ganglia. 11 Schizophrenic patients have more pronounced surges of inactivity of the dopaminergic system in the mesolimbic pathway but diminished activity in the mesocortical pathway. This background indicates that the absence of the ITA could be an early risk factor associated with the future manifestation of schizophrenia. 11 26
The association between the absence of ITA and psychiatric illness has been extensively reported in the literature. Takahashi et al 11 reported significantly smaller amygdala in schizophrenic patients without ITA than those with ITA. While these findings are preliminary, they indicate ITA's crucial role in connecting interhemispheric limbic systems. This, in turn, suggests lack of ITA may be an independent risk factor for limbic-mediated psychiatric conditions. On the contrary, de Souza Crippa et al 28 reported that the ITA was absent just as often in patients as in controls, even though absence was more common in male versus female schizophrenic patients.
Only a few reports of duplication of the ITA have been described in the literature. In few cases, it is not clear whether the duplication is actual or whether there is a single fenestrated ITA. 7 8 In our study, we found only one case (2%) with duplication of the ITA in Indian brains similar to the findings of Malobabić et al 7 in Serbian brains. Pavlović et al 3 studied 43 Serbian brains and reported duplication of the ITA in 3%. Samra and Cooper 29 studied 32 brains and reported duplication in four. ITA duplication is a congenital abnormality, and does not require routine medical or surgical attention. Moreover, the long-term prognosis in such patients remains unclear.
Neurosurgeons performing endoscopic ventriculostomy frequently encounter with the variations in topography and size of the ITA. Particularly, enlarged ITA in patients with neural tube defects (e.g., myelomeningocele) can obstruct the visualization of the floor of the third ventricle while performing third ventriculostomy for hydrocephalus. 30 Therefore, the exact position of the ITA is crucial for preoperative planning.
Pavlović et al 3 studied 43 Serbian brains and located the ITA using the CA-CP line system. In most brains, it was in the anterosuperior quadrant, but only in two (5.13%) cases were it entirely located in that quadrant; in the rest, it encroached into other quadrants. In 16 (41.02%) cases, it was chiefly located in the anterosuperior quadrant with caudal extension into the posterosuperior. In 11 (28.21%), it was equally located in the anterosuperior and posterosuperior quadrants, and in 10 (25.64%) it was located almost in the center of the lateral wall of the third ventricle.
In our study, the location of the ITA was determined by constructing a coordinate system on the lateral wall of the third ventricle using the CA-CP line, as Pavlović et al 3 did. The ITA lay predominantly in the anterosuperior quadrant, consistent with the findings of Malobabić et al. 7 In the specimen with double ITAs, both were located entirely within the anterosuperior compartment. Contrary to the findings of Pavlović et al, 3 we did not report any ITA extending from the anterosuperior quadrant to any other, such might be due to their smaller size.
The average cross-sectional area (SA) of the ITA we reported ( Table 1 ) is close to the value reported by Malobabić et al 7 who measured ITA sizes in 50 brains and recorded an average SA of 13.1 mm 2 (range 1.50–34.00 mm 2 ). In a similar study by Pavlović et al 3 on 41 Serbian brains, the average SA was 29.81 ± 12.49 mm 2 (range 3.53–56.50 mm 2 ). Pavlović et al 3 found most of the SAs between 20.00 and 40.00 mm 2 , while we found them mostly between 10.00 and 20.00 mm 2 . Sen et al 31 reported that the mean transverse diameter of the ITA was longer in males than females, whereas the vertical and anteroposterior diameters were larger in females. Most researchers have found larger SAs of the ITAs in females than males, 26 32 even though the female brain is smaller. The larger ITA could enable females to counterbalance the overall brain size differences. 22 We also found a significant difference between the SAs of females and males ( Table 1 ). Although the sex difference in SA is well established, there is no literature describing the timing at which this differentiation originates. It is believed that differences in ITA morphology can account for sexual dimorphism in cognitive functions and cerebral lateralization.
Females having on average a larger ITA 3 12 and larger right orbitofrontal cortices 33 shows robust connectivity between ITA and lateral orbitofrontal cortex in the right hemisphere. Additionally, Damle et al 1 and Borghei et al 12 have reported more substantial attention scores in females with ITA.
We attempted to correlate SA of ITA with third ventricle size. However, neither such relation was reported previously, 3 7 nor we found one. A few researchers attempted to correlate the development of the ITA with size of the thalamus or brain weight, but again no relationship could be established. 34
Clinical Relevance
To date, the role of ITA (MI) in the human brain has not been established. Recent studies suggest its presence may play a role in normal human neurocognitive function. 1 There is growing evidence that ITA is likely a midline white matter conduit, responsible for interhemispheric connectivity, similar to other midline commissures. Probabilistic tractography shows that the fibers passing through ITA act as a conduit for broad frontal lobe interhemispheric connectivity. 2 So, knowing its morphology, variability in topographic location, and SA/linear dimensions is of interest in neurosurgery. A combination of modern imaging techniques with morphological examination of postmortem brain specimens might be considered the best possible way to read up on this miniature neuroanatomical structure.
Conclusion
Presence of ITA is common in the South Asian brains, with usual location in the anterosuperior quadrant of the lateral wall of the third ventricle. The cross-sectional area of the ITA was significantly larger in females than males. No correlation was found between the surface area of the ITA and the length of the third ventricle.
Acknowledgments
The authors sincerely thank those who donated their bodies to science so that anatomical research could be performed. Results from such study can potentially increase humankind's overall knowledge that can then improve patient care. Therefore, these donors and their families deserve our highest gratitude.
Conflict of Interest None declared.
Ethical Approval
Ethical approval obtained from Institutional Ethics Committee of All India Institute of Medical Sciences, Rishikesh, vide letter no: AIIMS/IEC/21/474 dated 02–09–2021.
Authors' Contributions
A.P. contributed to project development, data collection, manuscript writing; K.S.R. contributed to manuscript writing, editing and critical revision; A.A. contributed to manuscript editing critical revision. All authors approve the final version of the manuscript.
References
- 1.Damle N R, Ikuta T, John M. Relationship among interthalamic adhesion size, thalamic anatomy and neuropsychological functions in healthy volunteers. Brain Struct Funct. 2017;222(05):2183–2192. doi: 10.1007/s00429-016-1334-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Borghei A, Kapucu I, Dawe R, Kocak M, Sani S. Structural connectivity of the human massa intermedia: a probabilistic tractography study. Hum Brain Mapp. 2021;42(06):1794–1804. doi: 10.1002/hbm.25329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pavlović M N, Jovanović I D, Ugrenović S Z. Position and size of massa intermedia in Serbian brains. Folia Morphol (Warsz) 2020;79(01):21–27. doi: 10.5603/FM.a2019.0046. [DOI] [PubMed] [Google Scholar]
- 4.Peele T L. New York: McGraw-Hill; 1977. The Neuroanatomical Basis for Clinical Neurology. [Google Scholar]
- 5.Rabl R. Studies on the structure of the massa intermedia of the thalamus opticus [in German] J Hirnforsch. 1958;4(01):78–112. [PubMed] [Google Scholar]
- 6.Carpenter M B.Core Text of Neuroanatomy. 4th ed Baltimore: Williams and Wilkins; 1996:49–50 [Google Scholar]
- 7.Malobabić S, Puskas L, Blagotić M. Size and position of the human adhaesio interthalamica. Gegenbaurs Morphol Jahrb. 1987;133(01):175–180. [PubMed] [Google Scholar]
- 8.Tubbs R S, Smyth M D, Oakes W J, Blount J P. Duplication of the massa intermedia in a child. Pediatr Neurosurg. 2004;40(01):42–43. doi: 10.1159/000076578. [DOI] [PubMed] [Google Scholar]
- 9.Collins P. London: Churchill Livingstone; 1995. Embryology and development; pp. 91–329. [Google Scholar]
- 10.Chéramy A, Romo R, Godeheu G, Glowinski J. Effects of electrical stimulation of various midline thalamic nuclei on the bilateral release of dopamine from dendrites and nerve terminals of neurons in the nigro-striatal dopaminergic pathways. Neurosci Lett. 1984;44(02):193–198. doi: 10.1016/0304-3940(84)90080-6. [DOI] [PubMed] [Google Scholar]
- 11.Takahashi T, Suzuki M, Zhou S Y. Prevalence and length of the adhesio interthalamica in schizophrenia spectrum disorders. Psychiatry Res. 2008;164(01):90–94. doi: 10.1016/j.pscychresns.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 12.Borghei A, Cothran T, Brahimaj B, Sani S. Role of massa intermedia in human neurocognitive processing. Brain Struct Funct. 2020;225(03):985–993. doi: 10.1007/s00429-020-02050-5. [DOI] [PubMed] [Google Scholar]
- 13.Trzesniak C, Linares I M, Coimbra ÉR. Adhesio interthalamica and cavum septum pellucidum in mesial temporal lobe epilepsy. Brain Imaging Behav. 2016;10(03):849–856. doi: 10.1007/s11682-015-9461-x. [DOI] [PubMed] [Google Scholar]
- 14.Kochanski R B, Dawe R, Kocak M, Sani S. Identification of stria medullaris fibers in the massa intermedia using diffusion tensor imaging. World Neurosurg. 2018;112:e497–e504. doi: 10.1016/j.wneu.2018.01.066. [DOI] [PubMed] [Google Scholar]
- 15.Nopoulos P, Swayze V, Flaum M, Ehrhardt J C, Yuh W T, Andreasen N C. Cavum septi pellucidi in normals and patients with schizophrenia as detected by magnetic resonance imaging. Biol Psychiatry. 1997;41(11):1102–1108. doi: 10.1016/S0006-3223(96)00209-0. [DOI] [PubMed] [Google Scholar]
- 16.Shimizu M, Fujiwara H, Hirao K.Structural abnormalities of the adhesio interthalamica and mediodorsal nuclei of the thalamus in schizophrenia Schizophr Res 2008101(1-3):331–338. [DOI] [PubMed] [Google Scholar]
- 17.Trzesniak C, Kempton M J, Busatto G F. Adhesio interthalamica alterations in schizophrenia spectrum disorders: a systematic review and meta-analysis. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35(04):877–886. doi: 10.1016/j.pnpbp.2010.12.024. [DOI] [PubMed] [Google Scholar]
- 18.Rosales R K, Lemay M J, Yakovley P I. The development and involution of massa intermedia with regard to age and sex. J Neuropathol Exp Neurol. 1968;27(01):166. [PubMed] [Google Scholar]
- 19.Cheng S, Tan K, Bilston L E. The effects of the interthalamic adhesion position on cerebrospinal fluid dynamics in the cerebral ventricles. J Biomech. 2010;43(03):579–582. doi: 10.1016/j.jbiomech.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 20.Snyder P J, Bogerts B, Wu H, Bilder R M, Deoras K S, Lieberman J A. Absence of the adhesio interthalamica as a marker of early developmental neuropathology in schizophrenia: an MRI and postmortem histologic study. J Neuroimaging. 1998;8(03):159–163. doi: 10.1111/jon199883159. [DOI] [PubMed] [Google Scholar]
- 21.Hirayasu Y, Wada J A. N-methyl-D-aspartate injection into the massa intermedia facilitates development of limbic kindling in rats. Epilepsia. 1992;33(06):965–970. doi: 10.1111/j.1528-1157.1992.tb01745.x. [DOI] [PubMed] [Google Scholar]
- 22.Allen L S, Gorski R A. Sexual dimorphism of the anterior commissure and massa intermedia of the human brain. J Comp Neurol. 1991;312(01):97–104. doi: 10.1002/cne.903120108. [DOI] [PubMed] [Google Scholar]
- 23.Raz S, Goldstein R, Hopkins T L. Sex differences in early vulnerability to cerebral injury and their neurodevelopmental implications. Psychobiology (Austin Tex) 1994;22:244–253. [Google Scholar]
- 24.Park I A, Lee H Y, Chung I H, Han Y P, Shin T S. A morphologic study of interthalamic adhesions in Korean brains. Clin Anat. 1993;6:33–36. [Google Scholar]
- 25.Erbağci H, Yildirim H, Herken H, Gümüşburun E.A magnetic resonance imaging study of the adhesio interthalamica in schizophrenia Schizophr Res 200255(1-2):89–92. [DOI] [PubMed] [Google Scholar]
- 26.Ceyhan M, Adapınar B, Aksaray G, Ozdemir F, Colak E. Absence and size of massa intermedia in patients with schizophrenia and bipolar disorder. Acta Neuropsychiatr. 2008;20(04):193–198. doi: 10.1111/j.1601-5215.2008.00296.x. [DOI] [PubMed] [Google Scholar]
- 27.Haghir H, Mokhber N, Azarpazhooh M R, Haghighi M B, Radmard M. A magnetic resonance imaging study of adhesio interthalamica in clinical subtypes of schizophrenia. Indian J Psychiatry. 2013;55(02):135–139. doi: 10.4103/0019-5545.111450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.de Souza Crippa J A, Zuardi A W, Busatto G F. Cavum septum pellucidum and adhesio interthalamica in schizophrenia: an MRI study. Eur Psychiatry. 2006;21(05):291–299. doi: 10.1016/j.eurpsy.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 29.Samra K A, Cooper I S. Radiology of the massa intermedia. Radiology. 1968;91(06):1124–1128. doi: 10.1148/91.6.1124. [DOI] [PubMed] [Google Scholar]
- 30.Kandel E I. Moscow: Meditsina; 1981. Functional and Stereotaxic Neurosurgery [Russian translation] [Google Scholar]
- 31.Sen F, Ulabay H, Ozeksi P, Sargon M F, Tascioglu A B. Morphometric measurements of the thalamus and interhemispheric adhesion by MR imaging. Neuroanat. 2005;4:10–12. [Google Scholar]
- 32.Luders E, Gaser C, Narr K L, Toga A W. Why sex matters: brain size independent differences in gray matter distributions between men and women. J Neurosci. 2009;29(45):14265–14270. doi: 10.1523/JNEUROSCI.2261-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Welborn B L, Papademetris X, Reis D L, Rajeevan N, Bloise S M, Gray J R. Variation in orbitofrontal cortex volume: relation to sex, emotion regulation and affect. Soc Cogn Affect Neurosci. 2009;4(04):328–339. doi: 10.1093/scan/nsp028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Agarwal N, Rambaldelli G, Perlini C. Microstructural thalamic changes in schizophrenia: a combined anatomic and diffusion weighted magnetic resonance imaging study. J Psychiatry Neurosci. 2008;33(05):440–448. [PMC free article] [PubMed] [Google Scholar]


