Abstract
Activation of the Wnt/β-catenin pathway regulates gene expression by promoting the formation of a β-catenin–T-cell factor (TCF) complex on target enhancers. In addition to TCFs, other transcription factors interact with the Wnt/β-catenin pathway at different levels to produce tissue-specific patterns of Wnt target gene expression. The transcription factor SOX9 potently represses many Wnt target genes by downregulating β-catenin protein levels. Here, we find using colony formation and cell growth assays that SOX9 surprisingly promotes the proliferation of Wnt-driven colorectal cancer (CRC) cells. In contrast to how it indirectly represses Wnt targets, SOX9 directly co-occupies and activates multiple Wnt-responsive enhancers in CRC cells. Our examination of the binding site grammar of these enhancers shows the presence of TCF and SOX9 binding sites that are necessary for transcriptional activation. In addition, we identify a physical interaction between the DNA-binding domains of TCFs and SOX9 and show that TCF-SOX9 interactions are important for target gene regulation and CRC cell growth. Our work demonstrates a highly context-dependent effect of SOX9 on Wnt targets, with the presence or absence of SOX9-binding sites on Wnt-regulated enhancers determining whether they are directly activated or indirectly repressed by SOX9.
Keywords: Wnt signaling, Wnt pathway, colorectal cancer, gene transcription, transcription factor, enhancers
Abbreviations: ChIP-seq, chromatin immunoprecipitation sequencing; CRC, colorectal cancer; DOX, doxycycline; FBS, fetal bovine serum; qPCR, quantitative PCR; TF, transcription factor; WRE, Wnt-regulated element
Transcriptional regulation by the Wnt/β-catenin pathway is essential for metazoan development and homeostasis (1), and the transcription factors (TFs) of the T-cell factor/lymphoid enhancer factor family (TCF/LEF family or TCFs) are major effectors of this signaling cascade (2). Clusters of TCF-binding sites are characteristic of many Wnt-regulated elements (WREs), the cis-regulatory DNA elements that drive Wnt-responsive transcription (3). WRE activation is triggered by the Wnt-dependent stabilization and accumulation of the protein β-catenin, which is recruited to WREs by TCFs. β-catenin recruits coactivators to induce Wnt target gene transcription (2, 4). Signaling by Wnt proteins performs many essential developmental functions that do not involve transcriptional regulation through β-catenin (5, 6). For brevity, we use the phrases “Wnt signaling” and “Wnt target gene” in this study to refer to Wnt signaling through β-catenin and genes regulated by the Wnt/β-catenin pathway.
While TCFs mediate Wnt target gene activation across cell types, there is great spatiotemporal diversity in the identity of Wnt target genes, with different tissues expressing unique cell type–specific Wnt transcriptional programs. This specificity is thought to arise from interactions between TCFs and other TFs that help determine cell fate and identity. Many non-TCF TFs bind to WREs and affect Wnt target gene expression, but a comprehensive molecular understanding of how these TFs generate target gene specificity is still incomplete (7, 8). One well-studied example is TFs of the CDX family, which bind to several WREs and recruit TCFs to them (9, 10). Their ability to form complexes with TCFs is essential for the activation of several Wnt target genes (11).
Another group of developmentally important TFs that modulate Wnt target gene expression is the SOX family of proteins. While some SOX family members directly bind to TCFs and β-catenin and promote the expression of specific Wnt targets (12, 13, 14, 15), other SOX proteins are known to repress Wnt/β-catenin signaling, with SOX9 being the most intensively studied. SOX9 has essential functions in the development of cartilage and the skeletal system, and mutations in SOX9 are associated with sex reversal and severe skeletal deformities in humans (16, 17). SOX9 is also well known for its role in promoting testis formation during mammalian development (18). Genetic studies have shown that testis or ovary commitment in mammalian gonadal development involves a mutual opposition between the Wnt/β-catenin pathway, which promotes ovarian development and inhibits testis fate, and SOX9, which inhibits the Wnt/β-catenin pathway to promote testes growth (19). In several different mammalian cell lines, the overexpression of SOX9 causes a reduction in Wnt transcriptional readouts and in β-catenin protein levels (20, 21, 22, 23). Recent work from our group found that SOX9 promoted β-catenin degradation by transcriptionally activating the Notch pathway coactivator MAML2, which associates with β-catenin (22).
Although primarily characterized as a repressor of Wnt/β-catenin target genes, correlative evidence hints at a more complex relationship between SOX9 and Wnt/β-catenin signaling in the intestinal epithelium and in colorectal cancer (CRC). The villi of the intestinal epithelium consist of short lived terminally differentiated cells that are continuously replenished from a population of intestinal epithelial stem cells. The proliferation of these stem cells and the differentiation of the Paneth cells, which surround and metabolically support them, are both dependent on Wnt/β-catenin signaling (24, 25). Paneth cells express high levels of SOX9 (26), and the loss of either SOX9 or Wnt signaling prevents Paneth cell differentiation (24, 27, 28). Additionally, SOX9 overexpression in Wnt-dependent CRC lines induces the expression of many genes characteristic of Paneth cells (29). Some of these are also directly activated by Wnt signaling (29, 30, 31), suggesting the possibility of SOX9 and Wnt signaling working together to activate gene expression. Understanding the relationship between SOX9 and Wnt signaling in this context requires an understanding of the regulatory logic of target gene WREs regulated by both factors.
Aberrant Wnt/β-catenin pathway activation in intestinal stem cells leads to uncontrolled proliferation, and consequently, activating mutations in the Wnt/β-catenin pathway are major drivers of CRC (4, 32). Additionally, mutations that increase the activity of WREs that regulate oncogenes such as MYC are also associated with increased cancer risk (33, 34, 35, 36). Many Wnt-dependent CRC lines and primary CRC samples also show high levels of SOX9 expression (37, 38), and recent reports suggest that SOX9 promotes stemness and survival of some CRC lines (29, 39, 40). The findings of SOX9 promoting Paneth cell fate and CRC cell survival are at odds with models of SOX9 as a Wnt/β-catenin pathway repressor.
In this study, we interrogate the relationship between SOX9 and Wnt signaling in regulating gene expression. In contrast to its previously known role as a repressor of Wnt/β-catenin signaling, we show that SOX9 and Wnt signaling work together to promote the growth and survival of CRC cells. Among the target genes activated by Wnt/β-catenin signaling and SOX9 is the oncogene MYC. In CRC cells, SOX9 is associated with many WREs, including several cancer risk-associated enhancers of MYC. Our characterization of the cis-regulatory grammar that allows SOX9 to activate Wnt target genes reveals the presence of SOX9-binding sites in the c-myc-335 WRE, which regulate enhancer activity along with TCF sites. A similar regulatory logic is also seen in the promoters of Defa5 and Defa6, two markers of Paneth cells, that are synergistically upregulated under high Wnt, high SOX9 conditions. With reporter mutagenesis and synthetic reporters, we show that the combination of TCF- and SOX-binding sites is necessary and sufficient for synergistic activation by Wnt and SOX9. Mechanistically, we show that SOX9 directly binds to TCFs. Through a novel SOX9 separation-of-function mutant that is defective in TCF binding, we show that the activation of WREs and CRC cell growth both require not just the activities of TCFs and SOX9 but also the activity of a TCF-SOX9 complex. Our work demonstrates that in addition to its role as a repressor of the Wnt/β-catenin pathway, SOX9 works together with the Wnt/β-catenin pathway to activate a subset of Wnt target genes.
Results
Both SOX9 and Wnt signaling promote the growth and survival of CRC cells
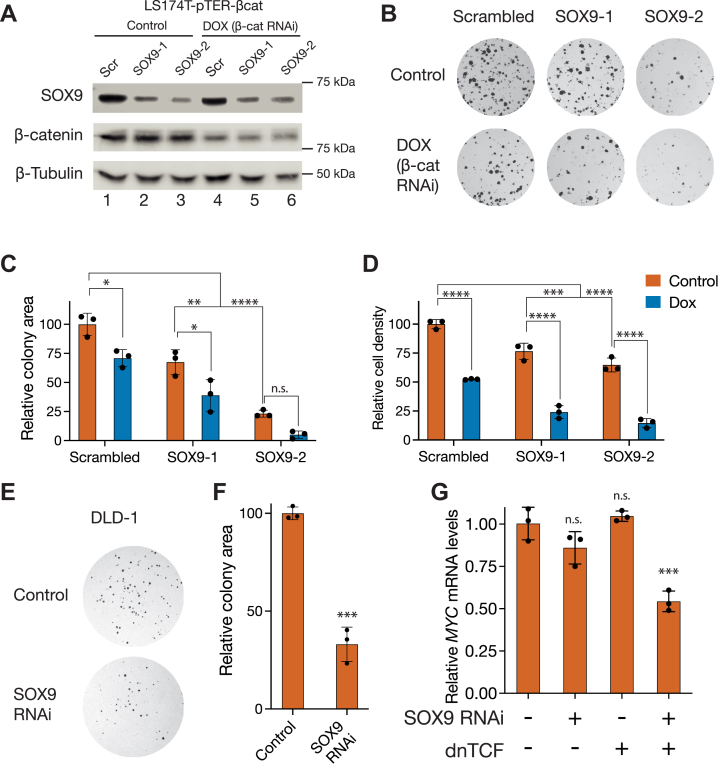
To explore the roles of SOX9 and Wnt/β-catenin signaling in CRC, we tested the consequences of depleting SOX9 and/or β-catenin in LS174T CRC cells. These cells contain an activating mutation in β-catenin (S45F) and are dependent on Wnt signaling for their growth and survival (41, 42). We used LS174T cells containing a doxycycline (DOX)-inducible β-catenin shRNA expression cassette (41) (referred to as LS174T-pTER-β-cat cells) and transduced them with constructs expressing either nontargeting (Scrambled) or SOX9-targeting shRNAs (Table S1). Western blots showed reduced SOX9 expression in the two SOX9 shRNA lines (Fig. 1A, lanes 2,3). This reduction of SOX9 protein levels was not accompanied by an upregulation of β-catenin protein, as would be expected if SOX9 was promoting β-catenin turnover (Fig. 1A, lanes 2,3). This suggested that SOX9 does not repress β-catenin levels in these cells. SOX9 protein levels were not affected by β-catenin depletion, suggesting that SOX9 is not a target of Wnt signaling in these cells (Fig. 1A, lanes 1,4).
Figure 1.
Wnt signaling and SOX9 work together to promote the growth and proliferation of colorectal cancer cells.A, Western blots showing depletion of SOX9 and β-catenin in LS174T colorectal cancer cells by RNAi. Cells were stably transduced with constructs expressing either a scrambled (Scr) or two independent SOX9-targeting shRNAs (SOX9-1, SOX9-2) and then treated with DOX for 24 h to deplete β-catenin. B, representative images showing reduced colony formation in LS174T cells after knockdown of SOX9 and β-catenin. Cells were treated in similar conditions as in (A) and then seeded at a density of 1000 cells/well. C, quantification of the area occupied by the colonies shown in (B). D, RNAi against β-catenin and SOX9 reduces the growth rate of LS174T cells. Cells were treated with DOX for 48 h, following which relative cell numbers were measured using an MTT assay. E, reduced colony formation in DLD-1 colorectal cancer cells after SOX9 knockdown. Cells were transfected with either a control plasmid or one expressing a SOX9 targeting shRNA and plated at a density of 500 cells/well. F, quantification of the area occupied by the colonies shown in (E). G, RT-qPCR data showing MYC mRNA levels in DLD-1 cells after transfection with plasmids expressing dominant negative TCF (dnTCF), SOX9 RNAi, or both. mRNA levels were measured with RT-qPCR 24 h after transfection. In (C, D, F, G), each bar represents mean ± SD from three biological replicates (N = 3). ns p > 0.05, ∗p < 0.05,∗∗p < 0.01, ∗∗∗p < 0.001. ∗∗∗∗p < 0.0001. p-values were in (C and D) were calculated by two-way ANOVA followed by Tukey’s multiple comparisons test, in F using unpaired two-tailed t test, and in G using one-way ANOVA followed by Dunnett’s test. DOX, doxycycline; MTT, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; RT-qPCR, reverse transcription quantitative PCR.
To understand whether SOX9 and Wnt signaling worked together to promote the survival of LS174T-pTER-β-cat cells, we tested for a SOX9 requirement in a colony formation assay with and without DOX-induced β-catenin depletion. Knocking down SOX9 alone resulted in fewer colonies, and the combined depletion of β-catenin and SOX9 reduced the clonogenicity of these cells even further (Fig. 1, B and C). We then tested the impact of Wnt signaling and SOX9 on the growth rate of these cells. We treated cells with or without DOX for 48 h and measured metabolic activity as a proxy for cell number using an 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. The depletion of β-catenin or SOX9 led to slower growth, and combined depletion of β-catenin and SOX9 reduced growth 4- to 5-fold (Fig. 1D). To ensure that our findings were not specific to LS174T cells, we also tested the role of SOX9 in promoting the survival of DLD-1 cells. DLD-1 cells also have elevated levels of Wnt signaling, but unlike LS174T cells, express a WT β-catenin protein and a truncated form of the destruction complex component APC (43). In DLD-1 cells, SOX9 depletion caused a 3-fold reduction in colony formation (Fig. 1, E and F). Just like in LS174T cells, SOX9 depletion did not cause an upregulation of β-catenin protein levels, and the inhibition of Wnt signaling through a dominant negative TCF (dnTCF) construct (44) did not reduce SOX9 protein levels (Fig. S1A).
We then examined the importance of Wnt signaling and SOX9 across a panel of CRC lines using data from the Cancer Cell Line Encyclopedia (CCLE) (45, 46). First, we examined gene expression data and correlated it with dependency scores calculated from CRISPR KO experiments using the Chronos algorithm (47). As expected in CRC, 49/53 lines examined showed high β-catenin (CTNNB1) expression and dependence based on our cutoffs (Fig. S1B). A similar analysis for SOX9 revealed that 38/53 lines showed high SOX9 expression and SOX9-dependent growth (Fig. S1C). Dependence on SOX9 was highly correlated with β-catenin-dependence, with 40/56 lines being highly dependent on both β-catenin and SOX9 (Fig. S1D). As a negative control, we looked at SOX17, which represses Wnt targets and inhibits the proliferation of CRC cells (13). Just 2/56 lines showed a dependence on both genes (Fig. S1E). Across all cancers in the CCLE database (n = 1070), β-catenin was the seventh-most correlated gene with SOX9 in terms of essentiality (Fig. S1F). In conjunction with our experimental data, these analyses strongly indicated that Wnt signaling and SOX9 working together was a general feature of CRC.
Since both Wnt signaling and SOX9 are major regulators of transcription, we wondered whether SOX9 could cooperate with the Wnt/β-catenin pathway to activate a common transcriptional program in CRC. One of the best-characterized Wnt targets in the context of CRC is the MYC oncogene (48), which is regulated by several WREs associated with CRC risk (33). In DLD-1 cells, MYC transcripts were downregulated by the combination of SOX9 knockdown and Wnt inhibition by dnTCF (Fig. 1G).
SOX9 directly binds and regulates the CRC risk-associated c-myc-335 WRE
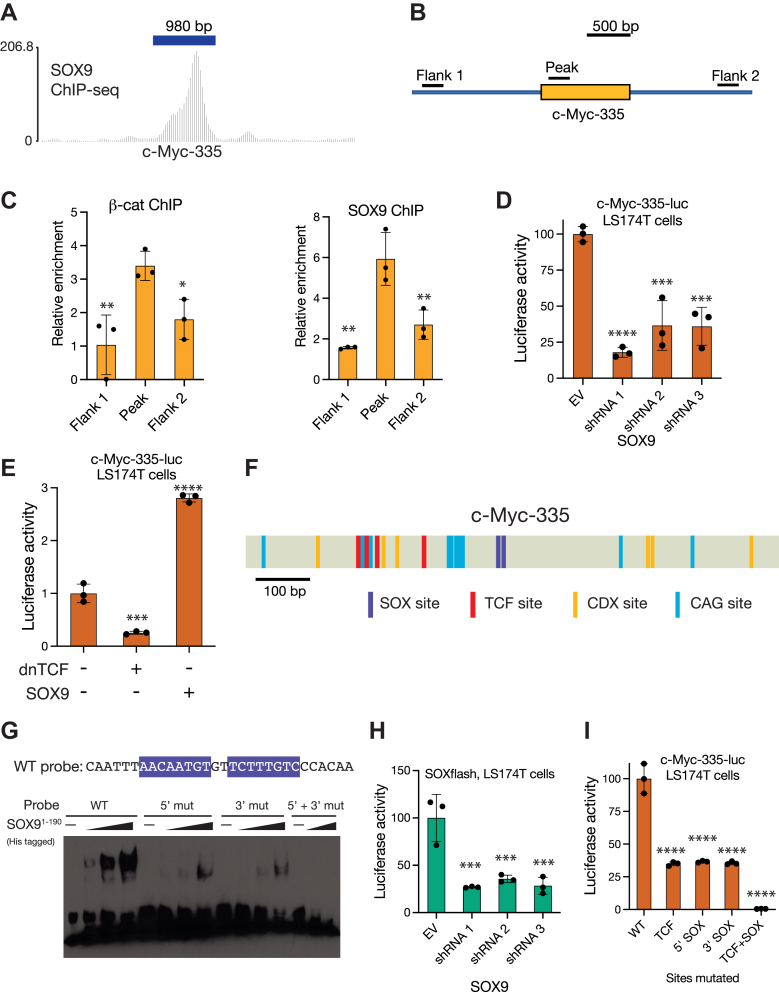
After finding that MYC transcript levels were upregulated by Wnt signaling and SOX9, we wanted to study the regulatory interactions that allowed SOX9 to activate this Wnt target. One of the best-characterized WREs regulating MYC is the c-myc-335 enhancer containing a SNP (rs6983267) that increases CRC risk in humans and is regulated by TCF activity (11, 49, 50). Examination of a previously published SOX9 chromatin immunoprecipitation sequencing (ChIP-seq) dataset (51) showed that c-myc-335 was bound by SOX9 in HT-29 CRC cells (Fig. 2A). We verified this result in LS174T cells by performing ChIP using antibodies against β-catenin and SOX9. To detect ChIP enrichment, we compared quantitative PCR (qPCR) signals from primers located inside the enhancer to those from two primer sets flanking the enhancer (Fig. 2B) and found that β-catenin and SOX9 were both enriched at the enhancer (Fig. 2C).
Figure 2.
The colorectal cancer risk associated c-myc-335 WRE requires the direct binding of TCFs and SOX9 for its activation.A, ChIP-seq tracks showing the binding of SOX9 to the c-myc-335 locus in HT29 colorectal cancer cells. Dataset from (51). B, cartoon showing positions of primers used for ChIP-qPCR analysis of the c-myc-335 locus in LS174T colorectal cancer cells. C, ChIP-qPCR showing β-catenin, and SOX9 are enriched at the c-myc-335 locus in LS174T cells. ChIP signals were quantified by qPCR using primers shown in (B). D, RNAi against SOX9 reduces c-myc-335 reporter activity in LS174T cells. Cells were transfected with the reporter plasmid and plasmids encoding shRNAs against SOX9 or an empty vector (pSUPER). Luciferase activity was assayed 48 h post transfection. E, luciferase assay showing the effect of dnTCF and SOX9 overexpression on c-myc-335 reporter activity in LS174T cells. Cells were transfected with the reporter and plasmids encoding dnTCF, Flag-SOX9, or an empty vector (pcDNA3.1). Luciferase activity was assayed 24 h post transfection. F, cartoon of the c-myc-335 enhancer showing positions of the newly identified SOX-binding sites (purple) along with previously characterized TCF, CDX, and CAG sites (11). G, EMSA showing a recombinant SOX9 fragment containing the DNA-binding domain specifically binds to the newly identified binding sites on c-myc-335, and mutations in these sites abolish SOX9 binding. H, luciferase assay showing the effect of SOX9 RNAi on the activity of a synthetic reporter containing three tandem copies of the SOX9-binding sites from c-myc-335. Reporter was measured similarly as in (D). I, luciferase assay showing the effect of TCF and SOX site mutations on c-myc-335 reporter activity. WT and mutant reporters were transfected into LS174T cells and luciferase activity was assayed 24 h post transfection. In (C–E, H, I), each bar represents mean ± SD from three biological replicates (N = 3). ns p > 0.05, ∗p < 0.05,∗∗p < 0.01, ∗∗∗p < 0.001. ∗∗∗∗p < 0.0001. p-values were calculated using one-way ANOVA followed by Dunnett’s test. ChIP, chromatin immunoprecipitation; EMSA, electrophoretic mobility shift assay; qPCR, quantitative PCR; RT-qPCR, reverse transcription quantitative PCR.
Previous work from our group had generated a luciferase reporter driven by c-myc-335, which showed Wnt-dependent activity (11). In LS174T cells, we found that RNAi-mediated SOX9 depletion reduced c-myc-335 reporter activity (Fig. 2D). Wnt/β-catenin pathway inhibition with dnTCF repressed, while SOX9 overexpression activated the c-myc-335 reporter (Fig. 2E). In combination with the ChIP data, these results were consistent with a model of SOX9 directly binding and activating this enhancer in conjunction with TCFs and β-catenin.
To understand how SOX9 was recruited to this enhancer, we searched its sequence for potential SOX9-binding sites. Our previous work on the cis-regulatory logic of c-myc-335 had identified four TCF-binding sites, two sites bound by CDX proteins, and five repeated motifs, we named CAG sites, all of which contributed to enhancer activity (11). Using SOX9-binding site data from the JASPAR database, we scanned the c-myc-335 sequence for putative clusters of SOX9-binding sites. This search identified a pair of putative SOX-binding sites located adjacently in an inverted repeat orientation (Figs. 2F and S1A). The ability of SOX9 to dimerize and bind DNA is an important part of its ability to activate some of its transcriptional targets (52), and the binding sites in c-myc-335 were in the correct orientation to be bound by a SOX9 dimer. We confirmed the ability of SOX9 to bind to these predicted sites using a gel-shift assay. A purified His-tagged recombinant SOX9 fragment containing the DNA-binding domain (SOX91-190) induced a robust gel shift in a probe containing the WT SOX-binding sites and mutating either or both SOX9-binding sites in the probe attenuated the gel shift (Figs. 2G and S2B).
To functionally verify these SOX9-binding sites, we created a synthetic reporter containing multimerized copies of the SOX-binding sites upstream of a minimal promoter. The activity of this reporter, named “SOXflash,” was SOX9 dependent, with reduced activity in LS174T cells upon RNAi-mediated SOX9 depletion (Fig. 2H). We then tested the contribution of SOX sites for WRE activation by mutating them in the c-myc-335-luc reporter. Previously, we found that mutating the four TCF sites caused a reduction in enhancer activity (11). Mutating either one of the SOX sites caused a reduction in enhancer activity comparable to mutating the four TCF-binding sites. Combined mutation of the TCF and SOX sites caused a nearly 200-fold reduction in enhancer activity (Fig. 2I). BLAST searches of the mouse and rat genomes showed that MYC enhancer regions in both species corresponding to human c-myc-335 also contained TCF- and SOX9-binding sites (Fig. S2C). Strikingly, the sequences of three out of the four TCF sites and one of the SOX9-binding sites are identical in humans, mice, and rats, suggesting that this mode of regulation is conserved among mammals.
SOX9 is important for activating a subset of WREs in cancer cells
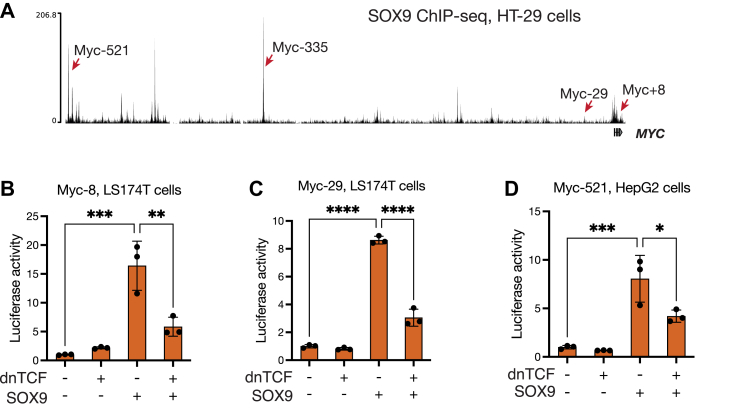
Our analysis of the c-myc-335 WRE revealed that it was occupied by both TCFs and SOX9 and that its activity was dependent on both TCF- and SOX9-binding sites. While the c-myc-335 WRE is well known for its role in CRC risk, the MYC gene is surrounded by several WREs implicated in the misregulation of MYC in many different kinds of cancer (33). Our examination of HT-29 SOX9 ChIP-seq data (51) identified multiple putative SOX9-bound enhancers around the MYC locus (Fig. 3A). Comparing these regions with previously characterized WREs of MYC, we identified three WREs that overlap with SOX9-bound regions (Figs. 3A, S3–S5 and S6A) (33, 53, 54, 55). Analogous to c-myc-335, we named these regions Myc+8, Myc-29, and Myc-521, based on the distance in kilobases between the enhancers and the transcription start site of the MYC gene.
Figure 3.
SOX9 and Wnt together regulate a multitude of WREs in cancer cells.A, SOX9 ChIP-seq tracks showing the MYC gene locus and the positions of cancer risk-associated WREs bound by SOX9 in HT29 cells. Dataset generated by (51). B–D, luciferase assays showing the effect of overexpressing dnTCF, SOX9, or both on the activity of the Myc+8, Myc-29, and Myc-521 reporters. Indicated cell lines were transfected with reporters and protein expression constructs 24 h before being assayed for luciferase activity. Each bar represents mean ± SD from three biological replicates (N = 3). ns p > 0.05, ∗p < 0.05,∗∗p < 0.01, ∗∗∗p < 0.001. ∗∗∗∗p < 0.0001. p-values were calculated using one-way ANOVA followed by Dunnett’s test. ChIP-seq, chromatin immunoprecipitation sequencing; WRE, Wnt-regulated element.
To confirm that these enhancers were jointly regulated by Wnt signaling and SOX9, we generated luciferase reporters by cloning these regions upstream of a minimal promoter and a luciferase coding sequence. In LS174T cells, both Myc+8 and Myc-29 were activated by SOX9 overexpression, which was silenced by the overexpression of the Wnt/β-catenin target repressor dnTCF (Fig. 3, B and C). We then tested Myc-521 in HepG2 hepatocellular carcinoma cells, which express a truncated form of β-catenin resulting in elevated Wnt signaling (56). Like Myc+8 and Myc-29, Myc-521 enhancer activity was stimulated by SOX9 overexpression and inhibited by additional dnTCF overexpression (Fig. 3D). These results suggest that SOX9 is a cofactor important for the activation of multiple MYC WREs in cancer cells.
Our findings raised the question of whether the positive role of SOX9 in WRE regulation was confined to MYC or whether other Wnt target genes in CRC were also activated by SOX9. We examined SOX9-bound regions in the HT-29 SOX9 ChIP-seq dataset (51) around Wnt-regulated genes from a HT-29 cell RNA-seq dataset (57). Of the top 5 high-confidence Wnt targets identified by this study, three genes (NOTUM, SP5, and LARGE2) had significant SOX9 peaks near their promoters and gene bodies, while no significant enrichment was seen near the other two (NKD1, TGFB3) (Fig. S6B). These findings suggest that SOX9 binds and regulates a subset of WREs in CRC cells.
Synergistic activation of Paneth cell markers by Wnt signaling and SOX9
To identify WREs regulated by Wnt signaling and SOX9 in the context of normal development, we examined genes expressed specifically in Paneth cells, which require both Wnt signaling and SOX9 for their differentiation (24, 27, 28). Transcript levels of two human Paneth cell marker genes, Defensin alpha 5 (Defa5) and Defensin alpha 6 (Defa6), have been recently shown to be upregulated in CRC cells by SOX9 overexpression (29). The promoters of two human Paneth cell markers, Defensin alpha 5 (Defa5) and Defensin alpha 6 (Defa6), are known to contain multiple TCF-binding sites, and transcriptional reporters made from these promoters are activated by Wnt signaling in cell culture (30, 31). We were interested in whether these Paneth cell WREs were also directly regulated by TCFs and SOX9.
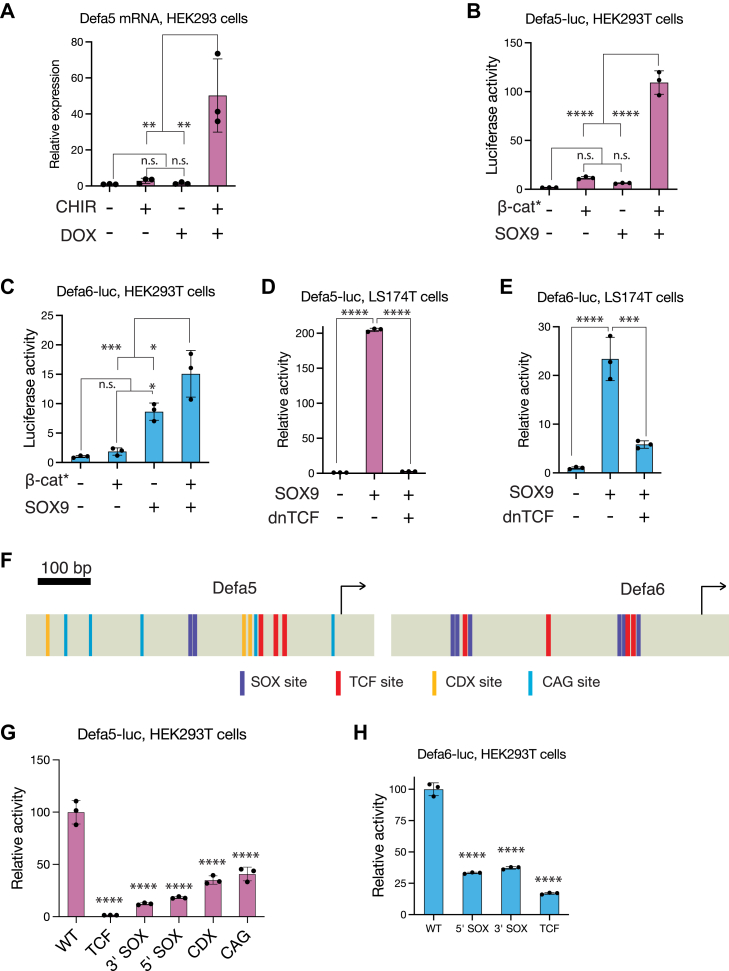
To test whether Defa5 was coregulated by SOX9 and the Wnt/β-catenin pathway, we examined its expression in a HEK293 cell line with a DOX-inducible SOX9 overexpression construct (DOX-SOX9 cells) (22). We found that Defa5 mRNA levels were synergistically upregulated under high-Wnt and high-SOX9 levels induced by DOX treatment in conjunction with the β-catenin destruction complex inhibitor CHIR-99021 (Fig. 4A). There was no detectable Defa6 mRNA expression in these cells. We then cloned the promoters of both Defa5 and Defa6 into the pGL4.10 promoterless luciferase reporter plasmid to generate transcriptional reporters for both genes (referred to as Defa5-luc and Defa6-luc). We tested the response of both reporters to SOX9 overexpression and Wnt/β-catenin pathway stimulation by the overexpression of a stabilized β-catenin (containing the S33Y mutation, referred to as β-catenin∗) (58). Defa5-luc was not significantly activated by β-catenin∗ or SOX9 overexpression individually but showed synergistic activation in the presence of both (Fig. 4B). Defa6-luc was activated by SOX9 overexpression but showed maximal activity under high-Wnt and high-SOX9 conditions similar to those found in Paneth cells (Fig. 4C). In LS174T cells, which already have high levels of Wnt signaling, SOX9 overexpression activated Defa5 and Defa6 promoter activity, which was repressed by inhibiting Wnt signaling with dnTCF (Fig. 4, D and E).
Figure 4.
SOX9 and Wnt signaling synergistically drive Paneth cell defensin activity through TCF and SOX binding sites.A, RT-qPCR data showing induction of Defa5 mRNA levels under high Wnt, high SOX9 levels. DOX-SOX9 HEK293 cells (22) were treated with CHIR-99021 to activate Wnt signaling and DOX to activate SOX9 overexpression. Transcript levels were measured 24 h after treatment. B, induction of Defa5-luc reporter activity by Wnt signaling and SOX9 in HEK293T cells. C, induction of Defa6-luc reporter activity by Wnt signaling and SOX9 in HEK293T cells. D, activation of Defa5-luc reporter activity by SOX9 overexpression and repression by dnTCF overexpression in LS174T cells. E, activation of Defa6-luc reporter activity by SOX9 overexpression and repression by dnTCF overexpression in LS174T cells. F, cartoons of the promoters of Defa5 and Defa6 showing the locations of TCF, SOX, CDX, and CAG sites. G, loss of Defa5-luc reporter activity upon mutation of TCF, SOX, CDX, or CAG sites. H, loss of Defa6-luc reporter activity upon mutation of TCF or SOX sites. For (B–E, G, and H), cells were transfected with indicated reporters and plasmids expressing stabilized β-catenin (β-catenin∗), Flag-SOX9, dnTCF, or empty vector (pcDNA3.1). Luciferase activity was measured 24 h post transfection. Each bar in (A–E, G, H) represents mean ± SD from three biological replicates (N = 3). ns p > 0.05, ∗p < 0.05,∗∗p < 0.01, ∗∗∗p < 0.001. ∗∗∗∗p < 0.0001. p-values in (A–C) were calculated by one-way ANOVA followed by Tukey’s test, and in (D, E, G, H) using one-way ANOVA followed by Dunnett’s test. DOX, doxycycline; RT-qPCR, reverse transcription quantitative PCR.
We then probed the cis-regulatory logic of the Defa5/6 promoters. Computational searches for TF-binding sites identified putative TCF- and SOX-binding sites in the promoters of Defa5/6, in addition to the CDX and CAG sites identified in several other WREs (Figs. 4F, S7, A and B). We tested the importance of these sites by mutating the TCF, SOX, CDX, and CAG sites in Defa5 and the TCF and SOX sites in Defa6. Measurements of reporter activity in HEK293T cells under high Wnt and high SOX9 conditions (Fig. 4, G and H) and in LS174T cells with SOX9 overexpression (Fig. S7, C and D) showed that all four sets of motifs were essential for WRE activity. Put together, these results indicate that in addition to working together to direct Paneth cell fate, Wnt signaling and SOX9 continue to be important after differentiation by directly activating genes expressed in Paneth cells using a regulatory logic similar to that seen in SOX9-activated oncogenic enhancers of MYC.
TCF- and SOX-binding sites are sufficient for synergistic enhancer activation by Wnt signaling and SOX9
While we found no evidence of SOX9 repressing the Wnt/β-catenin pathway in LS174T and DLD-1 cells, SOX9 is known to potently repress WRE expression in HEK293T cells (22), making the identification of WREs that were activated synergistically by Wnt signaling and SOX9 in HEK293T cells very striking. Previous work from our group characterized CREAX, a distal Wnt-responsive enhancer of the Axin2 Wnt target gene and found that enhancer activity was mediated by a combination of TCF, CDX, and CAG sites (11). However, CREAX lacks SOX9-binding sites and is repressed by SOX9 overexpression (22). While the Defa5/6 and c-myc-335 WREs also contain TCF, CDX, and CAG sites, they were set apart from CREAX by the presence of SOX9-binding sites. This suggested that the cis-regulatory grammar of these WREs was the sole determinant of whether they were activated or repressed by SOX9.
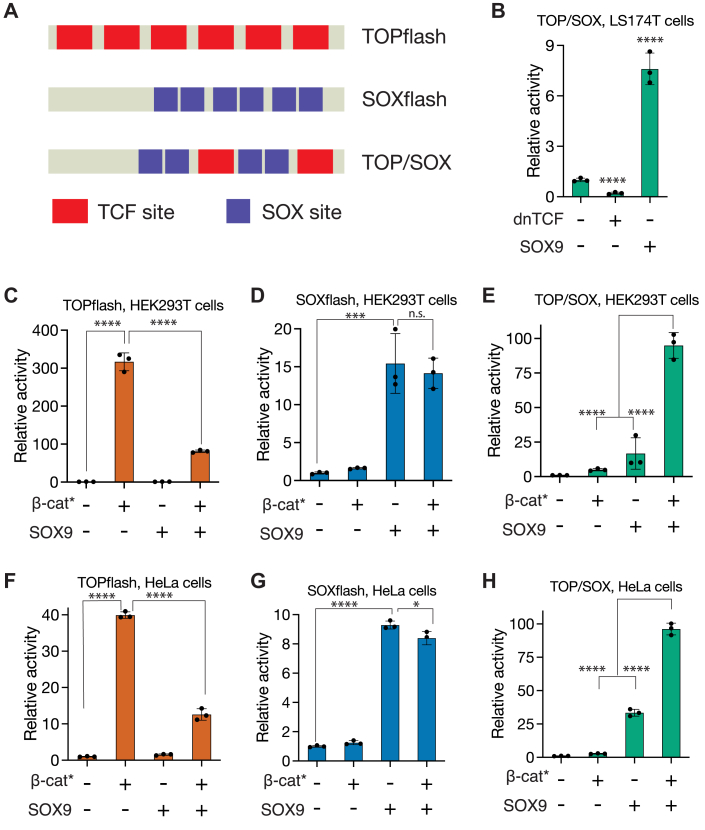
To directly test this idea, we generated a synthetic enhancer with a combination of TCF- or SOX9-binding sites. Reporters containing only multimerized TCF-binding sites (TOPflash) are robustly activated by Wnt signaling (59), and similar reporters containing SOX9-binding sites (SOXflash) show SOX9-dependent enhancer activity (Fig. 2H). Our new reporter, named TOP/SOX, contains two TCF and two pairs of SOX9-binding sites in the dimer-binding orientation (Figs. 5A and S8). In LS174T cells, TOP/SOX-luc was repressed by dnTCF and activated by SOX9 overexpression, mimicking the expression pattern of the c-myc-335 enhancer (compare Figs. 5B and 2E).
Figure 5.
TCF and SOX binding sites are sufficient for synergistic enhancer activation by Wnt signaling and SOX9.A, cartoons of synthetic enhancers containing combinations of TCF and SOX binding sites. TOPflash contains only TCF sites, SOXflash contains only SOX sites, and TOP/SOX contains both TCF and SOX sites. B, TOP/SOX is repressed by dnTCF overexpression and activated by SOX9 overexpression in LS174T cells. C–H, relative activity levels of TOPflash, SOXflash, and TOP/SOX in HEK293T (C–E), and HeLa cells (F–H) upon stimulation of Wnt signaling, SOX9 overexpression, or both. Cells were transfected with indicated reporters and overexpression constructs, and luciferase activity was assayed 24 h later. Each bar represents mean ± SD from three biological replicates (N = 3). ns p > 0.05, ∗p < 0.05,∗∗p < 0.01, ∗∗∗p < 0.001. ∗∗∗∗p < 0.0001. p-values were calculated using one-way ANOVA followed by Dunnett’s test.
We then compared the response of TOPflash, SOXflash, and TOP/SOX in HEK293T and HeLa cells to the overexpression of β-catenin∗, SOX9, or both. As expected, TOPflash activity was robustly induced by β-catenin∗ and repressed by SOX9 overexpression (Fig. 5C). In contrast, SOXflash was robustly activated by SOX9 overexpression and not significantly affected by β-catenin∗ (Fig. 5D). Strikingly, TOP/SOX was maximally activated by the combined overexpression of both β-catenin∗ and SOX9 (Fig. 5E). A similar pattern of expression was seen in HeLa cells, with TOP/SOX showing a qualitatively different expression pattern to TOPflash and SOXflash (Fig. 5, F–H). The conditions that cause dramatic reductions in TOPflash activity are identical to those that result in maximal activation of TOP/SOX (Fig. 5, C and F). Our data demonstrate that within a single cell type, the presence or absence of SOX9-binding sites can determine whether a WRE is repressed or activated by SOX9.
TCFs and SOX9 directly interact through non-DNA contacting residues in their DNA-binding domains
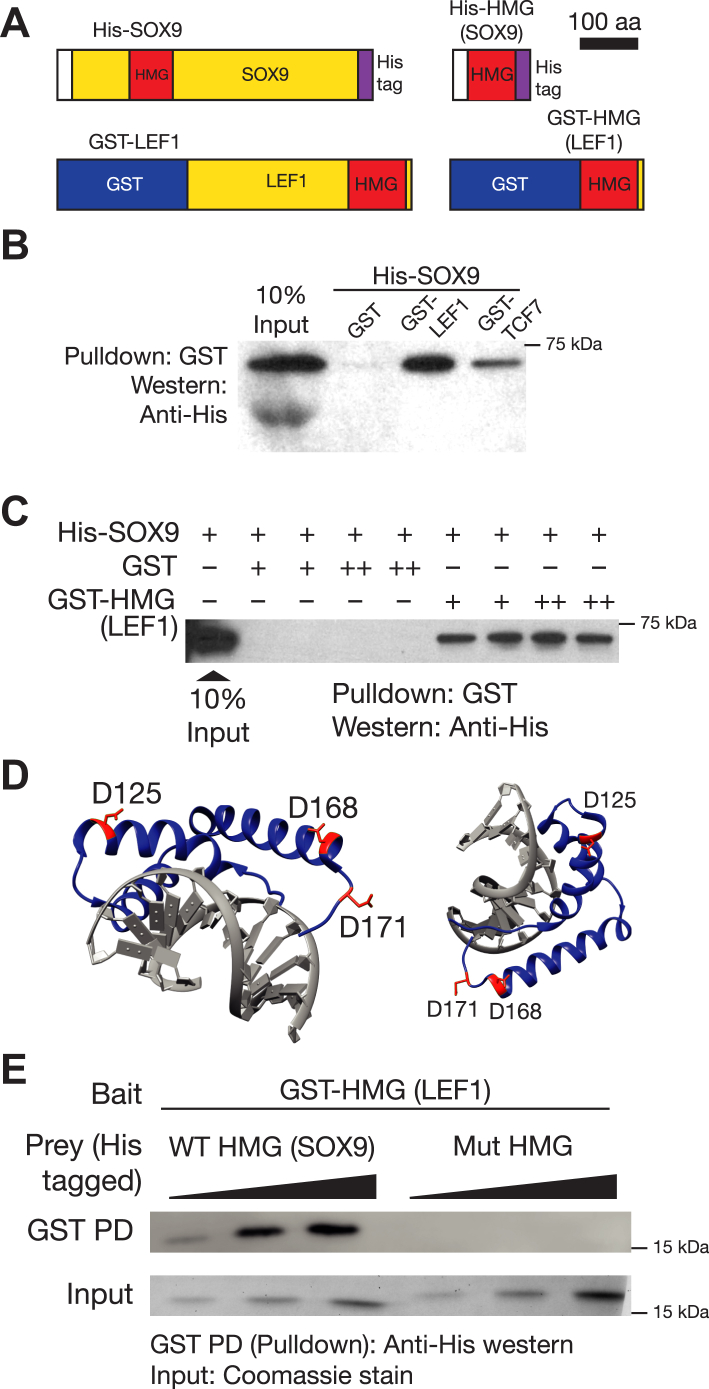
Multiple SOX family members have been shown to complex with TCFs through their DNA-binding HMG domains (12), and we wondered if the same was true in the case of SOX9. We tested the ability of purified recombinant glutathione-S-transferase (GST)-tagged LEF1 and TCF7 to interact with His-tagged SOX9 in a pull-down assay, with GST as a negative control. Full-length SOX9 directly bound to both LEF1 and TCF7 in vitro (Fig. 6, A and B). A GST-tagged fragment of LEF1 containing just the HMG domain (Fig. 6A) also robustly pulled down full-length SOX9 (Fig. 6C), suggesting that like other TCF-SOX protein interactions, TCF-SOX9 interactions were also mediated by HMG domains. To eliminate the possibility that this interaction was scaffolded by residual bacterial DNA in our purified protein preparations, we tested the impact of Mnase treatment and found no effect on the interaction between the HMG domains of LEF1 and SOX9 (Fig. S9A). This confirmed that the HMG domain of SOX9 could directly interact with the HMG domain of TCFs, independent of DNA binding.
Figure 6.
SOX9 directly binds to TCFs through non-DNA contacting residues in its DNA-binding HMG domain.A, cartoons of GST-tagged LEF1 and His-tagged SOX9 fragments used for pull-down experiments. B, GST pull down showing direct binding of SOX9 to LEF1 and TCF7. C, GST pull down showing binding of full-length SOX9 to the HMG domain of LEF1. D, two views of a crystal structure of the SOX9 HMG domain (PDB ID: 4EUW). Amino acid residues mutated to abolish TCF binding are colored red with their side chains shown. E, mutation of three amino acids in SOX9’s HMG domain (D125, D168, and D171) abolishes the TCF-SOX interaction. Purified His-tagged proteins were pulled down using glutathione beads with indicated GST-tagged proteins as bait for (B, C, and E). GST was used as negative control. PDB, Protein Data Bank.
To test the importance of TCF/SOX9 interactions in gene regulation, we wanted to generate a separation-of-function mutant of SOX9 that retained its ability to bind to DNA but could not bind to TCFs. An analysis of a crystal structure of the HMG domain of SOX9 complexed with DNA (Protein Data Bank ID: 4EUW) revealed the presence of multiple charged residues on the outer surface of the HMG domain. Working off the hypothesis that the TCF/SOX9 interaction was mediated by charged residues whose side chains pointed away from the DNA, we tested constructs containing charge swap mutations for their ability to bind to the HMG domain of LEF1. We found that a SOX9 HMG domain fragment containing mutations changing three aspartate (acidic) residues in SOX9 (D125, D168, and D171) to lysines (basic) showed no detectable binding to the HMG domain of LEF1 (Fig. 6, D and E). These results suggested that TCFs and SOX9 bound to each other through salt bridges mediated by non-DNA contacting residues on their HMG domains. They also provided us with a reagent that could be used to test the role of TCF/SOX9 protein–protein interactions in gene regulation. The interacting residues were highly conserved across vertebrate orthologs of SOX9 (Fig. S9B), suggesting that TCF-SOX9 interactions may be important for gene regulation across many species. Within human SOX proteins, the three residues were highly conserved (identical or conserved mutation) in the SOXE (SOX8,9,10) and SOXC (SOX4,11,12) subfamilies, with limited conservation in other family members (Fig. S10). We examined the correlation between the conservation of these residues and the ability of SOX proteins to suppress or synergistically activate targets in a Wnt/β-catenin dependent manner. Only one of the residues is conserved in SOX2 and SOX7, while none of them are conserved in SRY (Fig. S11A). When overexpressed in HEK293T cells, SOX9, SOX2, SRY, and SOX7 could all suppress β-catenin∗-driven TOPflash reporter activity (Fig. S11, B and C). However, only SOX9 was able to synergistically activate the Defa5-luc reporter along with β-catenin∗ (Fig. S11D).
TCF/SOX9 interactions are essential for synergistic enhancer activation and cancer cell survival
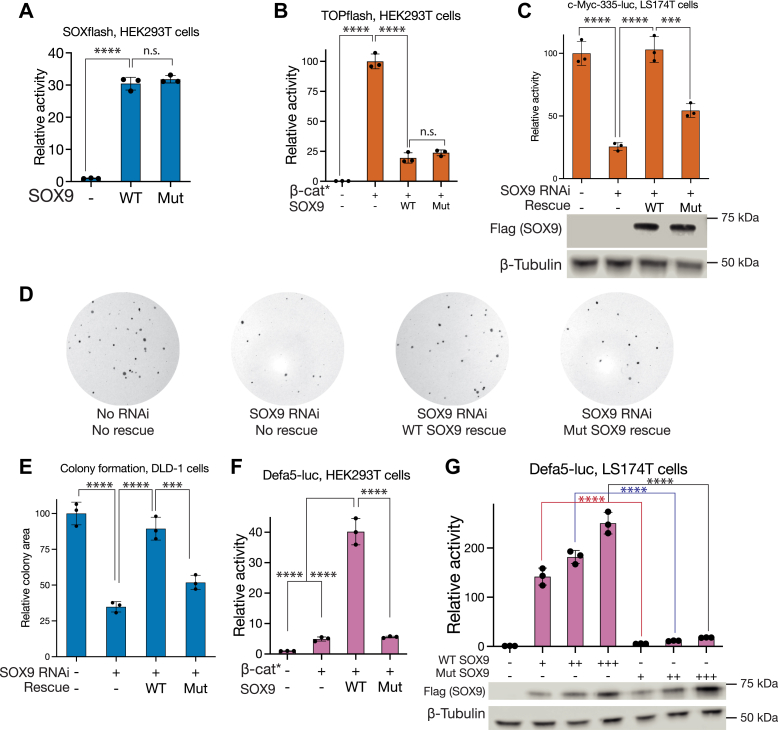
Before using our novel mutant to test the importance of TCF/SOX9 interactions for gene regulation, we wanted to ensure that the mutations did not compromise the ability of SOX9 to perform its TCF-independent functions. First, we compared the ability of WT and D125,168,171K mutant (Mut) SOX9 to activate the SOXflash reporter in HEK293T cells. Transfection of identical amounts of plasmid expressing WT or Mut SOX9 produced similar activation of SOXflash (Fig. 7A). The ability of SOX9 to repress Wnt reporters such as TOPflash involves the transcriptional activation of the SOX9 target gene MAML2 (22). In HEK293T cells, both WT and mutant suppressed β-catenin∗-induced TOPflash activation to similar levels (Fig. 7B). These experiments suggested that the ability of SOX9 to enter the nucleus, complex with DNA, and mediate transcriptional activation was not affected by the HMG domain mutations.
Figure 7.
TCF/SOX interactions are essential for WRE activity and colorectal cancer cell growth.A, luciferase assay showing that SOXflash activity can be driven by both WT and TCF interaction-deficient (Mut) SOX9. B, luciferase assay showing both WT and Mut SOX9 are equally capable of suppressing TOPflash activation by β-catenin∗. C, luciferase assay showing that WT SOX9 but not Mut can rescue the loss of c-myc-335 reporter activity caused by SOX9 RNAi in LS174T cells. Western blots show relative expression levels of WT and Mut SOX9. D, representative images showing that WT SOX9 but not Mut can rescue the loss of colony formation caused by SOX9 RNAi in DLD-1 cells. E, quantification of the area occupied by colonies in (D). F and G, luciferase assays showing that Defa5-luc can be activated by WT but not Mut SOX9 in HEK293T and LS174T cells. Western blots in (G) show relative expression levels of WT and Mut SOX9. Each bar represents mean ± SD from three biological replicates (N = 3). ns p > 0.05, ∗p < 0.05,∗∗p < 0.01, ∗∗∗p < 0.001. ∗∗∗∗p < 0.0001. p-values were calculated using one-way ANOVA followed by Dunnett’s test for (A) and Tukey’s test for all other panels. WRE, Wnt-regulated element.
We then compared the ability of WT and Mut SOX9 to drive c-myc-335 enhancer activity. While the overexpression of WT SOX9 could rescue the loss of reporter activity caused by SOX9 RNAi, overexpression of Mut SOX9 at similar levels only resulted in a partial rescue (Fig. 7C). Similarly, overexpression of WT but not Mut SOX9 rescued the colony formation defect in DLD-1 cells caused by SOX9 RNAi (Fig. 7, D and E). In the case of Defa5-luc, the overexpression of Mut SOX9 along with β-catenin∗ failed to produce the synergistic activation shown by WT SOX9 in HEK293T cells (Fig. 7F). In LS174T cells, overexpression of Mut SOX9 produced a very attenuated activation of the Defa5-luc reporter when compared to similar levels of WT SOX9 overexpression (Fig. 7G). Finally, Mut SOX9 also showed a compromised ability to activate the Defa6-luc reporter in concert with β-catenin∗ in HEK293T cells (Fig. S12A), and its overexpression in LS174T cells resulted in reduced Defa6-luc reporter activation when compared to WT SOX9 (Fig. S12B). Put together, these results suggest that a combination of cis-regulatory grammar and TCF/SOX9 protein–protein interactions is essential for activating a Wnt/SOX9 target gene program that allows Paneth cell gene expression and the growth and survival of CRC cells.
Discussion
How the interplay between signaling-dependent developmental regulators like the Wnt/β-catenin pathway and lineage-determining transcription factors such as SOX proteins results in spatiotemporally diverse patterns of gene expression is a fundamental question in developmental biology. Prior to this study, models of SOX9’s role in Wnt signaling characterized SOX9 as a Wnt/β-catenin pathway repressor, primarily influenced by work on the mammalian sex determination pathway and cell culture studies that examined the effects of SOX9 overexpression on Wnt signaling (12). In conjunction with several recent studies (29, 39, 40), our findings demonstrate a highly context-specific role for SOX9 in CRC, in which it promotes the growth and survival of CRC cells (Fig. 1). This transcriptional program involves SOX9 directly binding and regulating a subset of WREs (Figs. 2 and 3). Findings of SOX9 activating Paneth cell markers suggest a role for Wnt signaling and SOX9 cooperating to activate gene expression during both normal and cancerous growth (Fig. 4). Whether SOX9 activates or represses a given WRE is determined by the presence or absence of SOX9-binding sites (Fig. 5), and the ability of SOX9 to complex with TCFs is integral to its ability to activate Wnt targets (Figs. 6 and 7). In addition to the well-characterized binding between β-catenin and TCFs (60), SOX9 and β-catenin have also been reported to interact in coimmunoprecipitation and protein–protein binding assays (20, 61). While our data do not directly address whether these proteins form a trimeric complex, it is worth noting that the β-catenin–binding site on TCFs is at the N terminus (60), distinct from the HMG domain that we found is sufficient for binding SOX9 (Fig. 6, C and E). Further study is needed to elucidate the biochemical relationship between TCFs, SOX9, and β-catenin on chromatin when they activate specific Wnt targets.
Previous work on SOX9 identified a naturally occurring shorter isoform of SOX9, named miniSOX9, that was expressed in many CRC lines (61). When overexpressed, miniSOX9 inhibited SOX9-driven reporter activity and also alleviated SOX9’s inhibition of Wnt readouts, leading to a model of miniSOX9 as a naturally occurring dominant negative variant that allowed the SOX9 gene to stimulate cancer growth by inhibiting the ability of full-length SOX9 to repress Wnt signaling (61). However, this model was not tested directly with loss-of-function experiments. Our data show that SOX9 does not repress the Wnt/β-catenin pathway in CRC cells at endogenous levels of expression (Figs. 1A and S1A). Additionally, overexpression of full-length SOX9 was sufficient to rescue the loss of colony formation after SOX9 KD (Fig. 7, D and E) targeting the 5′-UTR that is shared by full-length and WT SOX9 (61). These results establish a role for the full-length SOX9 protein in promoting cancer growth by transcriptional activation.
Our examination of the regulatory logic of SOX9-activated WREs revealed the presence of functionally important TCF- and SOX9-binding sites without any easily discernible binding site grammar (Figs. 2 and 4). While the validated TCF- and SOX-binding sites in c-myc-335 and the Defa5 promoter are separated by several dozen nucleotides, they are more tightly clustered in the Defa6 promoter (Figs. 2F and 4F). Similarly, the predicted TCF- and SOX9-binding sites in Myc+8, Myc-29, and Myc-521 also do not follow a readily observable pattern of binding site grammar. Alignments of the four enhancers of MYC across humans, mice, and rats indicate that while some of the predicted TCF- and SOX9-binding sites are phylogenetically conserved, each species also has unique and nonconserved predicted TCF/SOX9-binding sites (Figs. S2–S5). This supports the view that there is little constraint on the positioning of these sites. A similar comparison was not possible for Defa5/6 due to the high species-specific evolutionary divergence of the Defensin gene family (62). Together with our synthetic reporter experiments (Fig. 5), this suggests that the mere presence or absence of SOX-binding sites is sufficient to discriminate between activation and repression of WREs by SOX9. While some of the SOX9-binding sites characterized in this study are in the correct orientation to be bound by SOX9 dimers, others are singular binding sites. Previous work has shown that the ability of SOX9 to dimerize is crucial for its role in some contexts such as chondrogenesis, but not in others such as the mammalian sex determination pathway (52, 63). In this study, we only tested the effect of individual SOX-binding site mutations in the context of c-myc-335 and Defa5, which have a single pair of SOX9-binding sites each. While these data might indicate a role for SOX9 dimerization in Wnt target gene regulation, more mutagenesis experiments and experiments using dimerization-deficient mutants are required to test it directly.
Previous work has shown that when overexpressed, SOX9 inhibits WREs in a dose-dependent manner. SOX9 overexpression results in the transcriptional upregulation of MAML2, which promotes β-catenin degradation (22). At the same time, overexpression of SOX9 in CRC cells with high levels of Wnt signaling activates a Paneth cell–like gene expression program (29). While intestinal stem cells retain their ability to proliferate and differentiate in the absence of SOX9 (27, 28), they do show changes in their gene expression patterns after SOX9 removal (64). The requirement of SOX9 for proliferation in these cell types has led to the idea that a “critical dose” of SOX9 may be important for intestinal stem and cancer cell proliferation (38, 65). Our findings support this model and provide a molecular basis for it. The ability of SOX9 to promote the expression of a subset of Wnt target genes makes it an essential regulator of growth and proliferation in these cells. Genes coregulated by Wnt signaling and SOX9 may also show differential sensitivity to SOX9 levels. For example, the c-myc-335 enhancer showed high activity at the endogenous SOX9 levels seen in LS174T CRC cells and was only modestly activated by SOX9 overexpression (Fig. 2E). In contrast, the promoters of Defa5/6, which are active in Paneth cells that express higher levels of SOX9, were significantly activated by SOX9 overexpression (Fig. 4D). Our finding that Wnt/SOX9-activated enhancers are most active under conditions that lead to a partial inhibition of WREs lacking SOX9-binding sites (Fig. 5) may provide a mechanistic explanation for the “critical dose” model, with higher levels of SOX9 shutting down WRE activity through β-catenin degradation, and lower levels of SOX9 preventing the activation of some WREs, leading to reduced proliferation under both reduced and increased SOX9 levels.
While hyperactivated Wnt signaling has long been known as a driver of CRC, its important role in driving the homeostasis of many different adult tissues has proved an important roadblock for the use of Wnt/β-catenin pathway inhibition as a cancer therapy (1, 32, 66, 67, 68). Our identification of the importance of SOX9-TCF interactions as a driving force for Wnt target gene activation in CRC may allow the development of novel therapeutics for CRC that disrupt the TCF-SOX9 interaction. Short peptides that mimic TF-TF interaction domains have been used in the past to successfully and specifically disrupt TF binding in vivo (69). Similar peptides may allow the specific inhibition of Wnt signaling in cancer cells without depleting the many Wnt-dependent stem cell populations found in the body.
Experimental procedures
Cell culture and transfection
Cell lines were grown at a temperature of 37 °C with 5% CO2. HEK293T (ATCC, CRL-3216) and DLD-1 (ATCC, CCL-221) cells were grown in Dulbecco's modified Eagle’s medium (Gibco, 11995065) supplemented with 10% fetal bovine serum (FBS) and penicillin/streptomycin/glutamine (Gibco, 10378016). HepG2 cells were a kind gift from Dr Jun Wu (University of Michigan). The LS174T-derived pTer-β-catenin-RNAi stable line (pTer-β-cat) was a kind gift from Dr Xi He (Boston Children’s Hospital, Harvard Medical School. LS174T cells (ATCC, CL-188); their derivatives and HepG2 cells were grown in minimum essential medium (Gibco, 11095080) with 10% FBS and penicillin/streptomycin/glutamine. Tetracycline-screened FBS (Cytiva, SH30071.03T) was used to culture pTer-β-cat cells and their derivatives. pTer-β-cat cell media was supplemented with 100 μg/ml Zeocin (InvivoGen, ant-zn-05) to select for the β-catenin RNAi cassette.
Lentiviral supernatants were produced at the University of Michigan Vector Core. To generate cells stably expressing nontargeting (scrambled) or SOX9-targeting shRNAs (details in Table S1), cells were incubated with viral supernatants overnight. Cells were thereafter maintained in 1 μg/ml puromycin to select for the Scrambled or SOX9 shRNA expressing cassette.
Transient transfections of HEK293T, HepG2, and LS174T cells were done with PEI MAX (Polysciences, 24765-1). DLD-1 cells were transfected with Lipofectamine 2000 (Invitrogen, 11668030) according to manufacturer’s instructions.
Plasmids
SOX9 RNAi plasmids used to generate stable LS174T cell lines were from the pGIPZ library and purchased from the University of Michigan Vector Core (details in Table S1). shRNA constructs used for transient transfection experiments were cloned into the pSUPER vector (Oligoengine, VEC-pBS-0002) (details in Table S2). Empty pSUPER plasmid was used as negative control. The c-myc-335 locus was amplified via PCR using human Jurkat cell genomic DNA as a template and cloned into XhoI/BglII sites in pGL4.23 (Promega, E8411). dnTCF (70) and Flag-SOX9 (22) constructs have been described previously. TF-binding site mutations in c-myc-335 were generated using a combination of Gibson assembly and site-directed PCR mutagenesis. Details of mutations are specified in Table S3. Myc+8, Myc-29, and Myc-521 reporters were amplified from HEK293T cell genomic DNA and cloned into pGL4.23 between the XhoI and HindIII restriction sites. The sequence of the region between these sites is shown in Figs. S3–S5. Human β-catenin∗ containing the S33Y mutation was cloned into the pcDNA3.1 vector and was a gift from Dr Eric Fearon, University of Michigan. Defa5/6 reporters were PCR amplified from human genomic DNA and cloned into the pGL4.10 promoterless luciferase vector (Promega, E6651) between the XhoI and KpnI restriction sites. gBlock fragments containing mutations in TF-binding sites were ordered from IDT and cloned into these plasmids to generate site mutant reporters. Details of mutations are specified in Table S4. Oligonucleotides containing fragments of the TOP/SOX reporter sequence were ordered from IDT, annealed together, and cloned into the pGL4.23 vector. The sequence of the synthetic reporter is shown in Fig. S8. GST-tagged TCF7 has been previously described (11). GST-tagged LEF1 was generated similarly by cloning the LEF1 ORF between the BamHI/NotI sites of pGEX-6p-1. His-tagged SOX9 was generated by cloning the ORF into the BamHI/SalI sites of pET-52b. HMG domain fragments of LEF1 and SOX9 were PCR amplified and cloned into pGEX-6p-1 and pET-52b between the same restriction sites used to make the full-length expression constructs. D125,168,171K mutations were introduced in the His-HMG and Flag-SOX9 expression plasmids using PCR with mismatched primers followed by Gibson assembly. To create a Flag-SOX2 expression plasmid, the Flag-SOX9 expression plasmid was restriction digested with BamHI and XhoI. SOX2 complementary DNA was PCR amplified from a tagged SOX2 ORF clone (Origene, RC200757) using primers containing BamHI and XhoI sites. The PCR product was digested with the two enzymes and ligated into the vector. A sequence encoding the Flag-SRY construct was ordered as a gBlock from IDT and cloned between the BamHI/XhoI sites of pcDNA3.1. The SOX7 expression plasmid was a kind gift from Prof. Injune Kim, Graduate School of Medical Science and Engineering, Korea Advanced Institute of Science and Technology.
Colony formation assays
Cells were plated in 12-well plates and transfected or treated with DOX for the indicated amounts of time. Ultrapure water (solvent) was used as negative control for DOX experiments. Following this, they were resuspended in the appropriate cell culture media after treatment with 0.25% Trypsin-EDTA. Cell numbers were determined for each condition using a hemocytometer. Cells were then diluted to obtain the final concentrations reported in each experiment and then seeded in triplicates onto 6-well plates. They were allowed to grow undisturbed for 12 to 14 days until colonies were easily visible to the naked eye. Colonies were fixed with methanol for 20 min, following which they were stained with crystal violet staining solution (0.5% (w/v) crystal violet, 20% methanol) for 10 min. They were then rinsed with distilled water, dried overnight, and imaged.
The area occupied by the colonies was quantified using Fiji (71). Each well was defined as a circular region of interest and then cropped out. Wells were individually thresholded and the fraction of the area occupied by the wells was measured.
MTT assay for cell number
MTT assays were carried out using Cell Proliferation Kit I (Roche, 11465007001). Cells expressing scrambled or SOX9 shRNAs were plated onto 48-well plates with and without DOX at a density of 30,000 cells/well. Serial dilutions of cells were plated to determine a standard curve. Forty-eight hours later, 20 μl of MTT labeling reagent was added to each well. About 200 μl of solubilization solution was added 4 h later. Absorbance was measured at 565 nm using a Tecan Infinite 200 plate reader. Relative cell densities of the different samples and treatments were then calculated based on the standard curve. All measurements were done with biological triplicates.
Reverse transcription quantitative PCR for transcript quantification
RNA was extracted using the Rneasy mini kit (Qiagen, 74104). Reverse transcription was then performed using Superscript III Reverse Transcriptase (Invitrogen, 18080093). The Power SYBR Green PCR master mix (Applied Biosystems, 4368577) was used to perform PCRs using a CFX Connect Real-Time PCR Detection System (Bio-Rad, 1855201). Mean and SDs of transcript levels were quantified using the delta-delta Ct method using G6PD transcript levels for normalization. All measurements were done with biological triplicates. Primers are shown in Table S5.
ChIP
ChIP was performed and signals were quantified using qPCR as previously described (11). SOX9 ChIP was done using an anti-SOX9 antibody (Sigma, AB5535). For β-catenin ChIP, cells were first treated with the protein-protein crosslinker EGS (ethylene glycol bis(succinimidyl succinate)) (Thermo Scientific, 21565) at a concentration of 12.5 mM (70), following which ChIP was performed using an antibody targeting active β-catenin (CST, 8814S). One set of primers for qPCR (Table S6) was designed to amplify inside the c-myc-335 enhancer region and two primer pairs were designed to amplify flanking regions outside the enhancer for comparison. ChIP signals from each of these primers were compared to the signal from a control region to quantify enrichment. Primers used for ChIP are shown in Table S6.
ChIP-seq data analysis
ChIP-seq data analyzed in this study was generated by a previous study (51). Publicly available data were downloaded from GEO (accession number: GSE63629). The data file GSM1554224_HT29_SOX9_antibody.bar was converted into bedgraph format using USeq (72). ChIP-seq track visualizations were generated using pyGenomeTracks (73).
Computational analysis of TF-binding sites
TCF, CDX, and CAG sites were computationally annotated using FIMO (74) as previously described (11). Initial identification of SOX9-binding sites was based on the SOX9 MA0077.1 motif file from the JASPAR database (75). To ensure the identification of potential SOX9 dimeric binding sites, searches were repeated with lowered thresholds to look for lower scoring sites in the correct orientation located adjacent to high-scoring sites.
Luciferase assays and analysis
Luciferase assays were done with firefly luciferase reporter plasmids and constitutively expressed renilla luciferase as an internal transfection control. Luciferase activity was measured using Promega’s Dual Luciferase Assay system (Promega, E1910) according to manufacturer’s protocols. Firefly/renilla ratio was used as the measure of activity for each well. All assays were done in triplicates, following which the mean and SD of firefly/renilla ratios were calculated for each experimental condition. To calculate relative luciferase activity, a basal condition was selected (specified in each figure) and the ratios of all conditions were expressed in proportion to it.
Electrophoretic mobility shift assay
His-tagged SOX91-190 was expressed and purified as indicated later. Binding reactions were carried out in binding buffer (10 mM Tris-Cl pH 7.5, 5 mM MgCl2, 50 mM KCl, 1 mM DTT, 2.5% glycerol, and 0.5% NP-40), 50 ng/ml poly dI•dC, 40 fmol biotinylated oligonucleotides (IDT). The reactions were then allowed to sit on ice for 5 min, then at room temperature for 15 min. The reactions were then loaded onto a pre-run 6% TBE polyacrylamide gel followed by transfer to a Biodyne B positively charged membrane (Pall life sciences, 60208). Membranes were developed using a Light Shift chemiluminescent EMSA kit (Thermo Scientific, 20148).
Recombinant protein expression and purification
GST and His-tagged protein expression constructs were expressed and purified as previously described (11). Protein expression was induced with 1 mM IPTG and cells were collected 3 to 4 h later. All constructs except for the His-tagged mutant SOX9 HMG were grown using the BL21(DE3) Escherichia coli strain (Thermo Scientific, EC0114). The mutant HMG construct was expressed and purified in the Rosetta(DE3) strain (Novagen, 70954).
GST pull-down assays
The concentration of purified recombinant proteins was measured using Bradford’s reagent (Bio-Rad, 5000202). About 0.25 to 2 μg of each protein was used in a binding reaction. Binding reactions were carried out in a binding buffer containing 25 mM Hepes pH 7.5, 12.5 mM MgCl2, 300 mM KCl, 0.1% NP-40, 1 mg/ml bovine serum albumin, 1 mM DTT. GST and His-tagged proteins were mixed in binding buffer to a total volume of 200 μl and incubated in a rotator for 1 h at 4 °C. Glutathione-sepharose (Thermo-Scientific, 25236) were washed thrice in binding buffer and then added to binding reactions at a packed bead volume of 10 μl per reaction. After another 1 h incubation at 4 °C with rotation, beads were washed thrice in washing buffer (25 mM Hepes pH 7.5, 12.5 mM MgCl2, 400 mM KCl, 0.5% NP-40) and eluted by boiling for 5 min in 2× SDS buffer (100 mM Tris-Cl pH 6.8, 4% SDS, 0.2% bromophenol blue, 20% glycerol). Samples were then analyzed by SDS-PAGE followed by Western blotting.
Western blotting
Western blotting was performed after SDS-PAGE as previously described (11). Western blots were probed with the following antibodies: anti-Flag-HRP (Sigma–Aldrich, A8592), anti-SOX9 (Sigma–Aldrich, AB5535), anti-β-catenin (BD Biosciences, 610154), anti-β-tubulin (Proteintech, 66240-1-Ig), anti-His (Cytiva, 27-4710-01).
Protein structure visualization
Protein structures were downloaded from RSC PDB and visualized with UCSF ChimeraX (76), developed by the Resource for Biocomputing, Visualization, and Informatics at the University of California, San Francisco, with support from National Institutes of Health R01-GM129325 and the Office of Cyber Infrastructure and Computational Biology, National Institute of Allergy and Infectious Diseases.
Data collection and analysis
Cell culture experiments were performed in biological triplicates. Cells in different wells or plates were independently treated with drugs or transfected as indicated for each experiment and subsequently assayed independently. To ensure reproducibility, all experiments were repeated at least three different times with similar results. Data were then analyzed and graphed using Graphpad Prism 9 (GraphPad Software Inc). The specific statistical tests used to calculate p-values are indicated in the figure legends.
Data availability
The data supporting the findings in this study are available within the article and supplemental information. All reagents described in this study are available upon request.
Supporting information
This article contains supporting information (51).
Conflict of interest
The authors declare that they have no conflicts of interest with the contents of this article.
Acknowledgments
The authors would like to thank Haidar A. Haidar and Dr Abhishek Sinha for contributing to the initial stages of this project, and Aarti Kejriwal and Matthew Benson for generating some of the constructs used in this study.
Author contributions
A.-B. R., P. E. B., and K. M. C. conceptualization; A.-B. R. and K. M. C. methodology; A.-B. R. software; A.-B. R. validation; A.-B. R. formal analysis; A.-B. R., P. E. B., and K. A. investigation; A.-B. R. data curation; A.-B. R. writing–original draft; P. E. B., K. A., and K. M. C. writing–review & editing; A.-B. R. and K. A. visualization; A.-B. R. supervision; K. M. C. project administration; A.-B. R. and K. M. C. funding acquisition.
Funding and additional information
This work was supported by grants from the US National Institute of Health (R01 GM108468 to K. M. C.); National Cancer Institutes of Health under Award Number P30CA046592 (to K. M. C.); University of Michigan M-cubed (to K. M. C.); University of Michigan Department of Molecular, Cellular, and Developmental Biology (Biosciences fellowship to A-B. R.); and the Rackham Graduate School, University of Michigan (One-Term Dissertation Fellowship and a Graduate Student Research Grant to A-B. R.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Edited by Brian Strahl
Supporting information
References
- 1.Clevers H., Loh K.M., Nusse R. An integral program for tissue renewal and regeneration: Wnt signaling and stem cell control. Science. 2014;346 doi: 10.1126/science.1248012. [DOI] [PubMed] [Google Scholar]
- 2.Ramakrishnan A.-B., Cadigan K.M. Wnt target genes and where to find them. F1000Res. 2017;6:746. doi: 10.12688/f1000research.11034.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Archbold H.C., Yang Y.X., Chen L., Cadigan K.M. How do they do Wnt they do?: regulation of transcription by the Wnt/β-catenin pathway. Acta Physiol. 2012;204:74–109. doi: 10.1111/j.1748-1716.2011.02293.x. [DOI] [PubMed] [Google Scholar]
- 4.Clevers H., Nusse R. Wnt/β-Catenin signaling and disease. Cell. 2012;149:1192–1205. doi: 10.1016/j.cell.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 5.Acebron S.P., Niehrs C. β-Catenin-Independent roles of Wnt/LRP6 signaling. Trends Cell Biol. 2016;26:956–967. doi: 10.1016/j.tcb.2016.07.009. [DOI] [PubMed] [Google Scholar]
- 6.Yang Y., Mlodzik M. Wnt-frizzled/planar cell polarity signaling: cellular orientation by facing the wind (Wnt) Annu. Rev. Cell Dev. Biol. 2015;31:623–646. doi: 10.1146/annurev-cellbio-100814-125315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nakamura Y., Hoppler S. Genome-wide analysis of canonical Wnt target gene regulation in Xenopus tropicalis challenges β-catenin paradigm. genesis. 2017;55 doi: 10.1002/dvg.22991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Söderholm S., Cantù C. The WNT/β-catenin dependent transcription: a tissue-specific business. WIREs Mech. Dis. 2021;13 doi: 10.1002/wsbm.1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lewis A., Freeman-Mills L., de la Calle-Mustienes E., Giráldez-Pérez R.M., Davis H., Jaeger E., et al. A polymorphic enhancer near GREM1 influences bowel cancer risk through differential CDX2 and TCF7L2 binding. Cell Rep. 2014;8:983–990. doi: 10.1016/j.celrep.2014.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Verzi M.P., Hatzis P., Sulahian R., Philips J., Schuijers J., Shin H., et al. TCF4 and CDX2, major transcription factors for intestinal function, converge on the same cis-regulatory regions. Proc. Natl. Acad. Sci. U. S. A. 2010;107:15157–15162. doi: 10.1073/pnas.1003822107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ramakrishnan A.-B., Chen L., Burby P.E., Cadigan K.M. Wnt target enhancer regulation by a CDX/TCF transcription factor collective and a novel DNA motif. Nucl. Acids Res. 2021;49:8625–8641. doi: 10.1093/nar/gkab657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kormish J.D., Sinner D., Zorn A.M. Interactions between SOX factors and Wnt/β-catenin signaling in development and disease. Dev. Dyn. 2010;239:56–68. doi: 10.1002/dvdy.22046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sinner D., Kordich J.J., Spence J.R., Opoka R., Rankin S., Lin S.-C.J., et al. Sox17 and Sox4 differentially regulate β-catenin/T-cell factor Activity and proliferation of colon carcinoma cells. Mol. Cell. Biol. 2007;27:7802–7815. doi: 10.1128/MCB.02179-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mukherjee S., Chaturvedi P., Rankin S.A., Fish M.B., Wlizla M., Paraiso K.D., et al. Sox17 and β-catenin co-occupy Wnt-responsive enhancers to govern the endoderm gene regulatory network. eLife. 2020;9 doi: 10.7554/eLife.58029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mukherjee S., Luedeke D.M., McCoy L., Iwafuchi M., Zorn A.M. SOX transcription factors direct TCF-independent WNT/β-catenin responsive transcription to govern cell fate in human pluripotent stem cells. Cell Rep. 2022;40 doi: 10.1016/j.celrep.2022.111247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lefebvre V. In: Current Topics in Developmental Biology. Vertebrate Skeletal Development. 133. Olsen B.R., editor. Academic Press; Cambridge, MA: 2019. Seven - roles and regulation of SOX transcription factors in skeletogenesis; pp. 171–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lefebvre V., Angelozzi M., Haseeb A. SOX9 in cartilage development and disease. Curr. Opin. Cell Biol. 2019;61:39–47. doi: 10.1016/j.ceb.2019.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sekido R., Lovell-Badge R. Sex determination involves synergistic action of SRY and SF1 on a specific Sox9 enhancer. Nature. 2008;453:930–934. doi: 10.1038/nature06944. [DOI] [PubMed] [Google Scholar]
- 19.Nicol B., Yao H.H.-C. Gonadal identity in the absence of pro-testis factor SOX9 and pro-ovary factor beta-catenin in Mice1. Biol. Reprod. 2015;93:1–12. doi: 10.1095/biolreprod.115.131276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Akiyama H., Lyons J.P., Mori-Akiyama Y., Yang X., Zhang R., Zhang Z., et al. Interactions between Sox9 and β-catenin control chondrocyte differentiation. Genes Dev. 2004;18:1072–1087. doi: 10.1101/gad.1171104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sellak H., Wu S., Lincoln T.M. KLF4 and SOX9 transcription factors antagonize β-catenin and inhibit TCF-activity in cancer cells. Biochim. Biophys. Acta (Bba) - Mol. Cell Res. 2012;1823:1666–1675. doi: 10.1016/j.bbamcr.2012.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sinha A., Fan V.B., Ramakrishnan A.-B., Engelhardt N., Kennell J., Cadigan K.M. Repression of Wnt/β-catenin signaling by SOX9 and Mastermind-like transcriptional coactivator 2. Sci. Adv. 2021;7 doi: 10.1126/sciadv.abe0849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Topol L., Chen W., Song H., Day T.F., Yang Y. Sox9 inhibits Wnt signaling by promoting β-catenin phosphorylation in the nucleus. J. Biol. Chem. 2009;284:3323–3333. doi: 10.1074/jbc.M808048200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van Es J.H., Jay P., Gregorieff A., van Gijn M.E., Jonkheer S., Hatzis P., et al. Wnt signalling induces maturation of Paneth cells in intestinal crypts. Nat. Cell Biol. 2005;7:381–386. doi: 10.1038/ncb1240. [DOI] [PubMed] [Google Scholar]
- 25.van Es J.H., Haegebarth A., Kujala P., Itzkovitz S., Koo B.-K., Boj S.F., et al. A critical role for the Wnt effector Tcf4 in adult intestinal homeostatic self-renewal. Mol. Cell Biol. 2012;32:1918–1927. doi: 10.1128/MCB.06288-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Blache P., van de Wetering M., Duluc I., Domon C., Berta P., Freund J.-N., et al. SOX9 is an intestine crypt transcription factor, is regulated by the Wnt pathway, and represses the CDX2 and MUC2 genes. J. Cell Biol. 2004;166:37–47. doi: 10.1083/jcb.200311021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bastide P., Darido C., Pannequin J., Kist R., Robine S., Marty-Double C., et al. Sox9 regulates cell proliferation and is required for Paneth cell differentiation in the intestinal epithelium. J. Cell Biol. 2007;178:635–648. doi: 10.1083/jcb.200704152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mori–Akiyama Y., van den Born M., van Es J.H., Hamilton S.R., Adams H.P., Zhang J., et al. SOX9 is required for the differentiation of Paneth cells in the intestinal epithelium. Gastroenterology. 2007;133:539–546. doi: 10.1053/j.gastro.2007.05.020. [DOI] [PubMed] [Google Scholar]
- 29.Liang X., Duronio G.N., Yang Y., Bala P., Hebbar P., Spisak S., et al. An enhancer-driven stem cell–like program mediated by SOX9 blocks intestinal differentiation in colorectal cancer. Gastroenterology. 2022;162:209–222. doi: 10.1053/j.gastro.2021.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wehkamp J., Wang G., Kübler I., Nuding S., Gregorieff A., Schnabel A., et al. The Paneth cell α-defensin deficiency of ileal crohn’s disease is linked to Wnt/Tcf-4. J. Immunol. 2007;179:3109–3118. doi: 10.4049/jimmunol.179.5.3109. [DOI] [PubMed] [Google Scholar]
- 31.Beisner J., Teltschik Z., Ostaff M.J., Tiemessen M.M., Staal F.J.T., Wang G., et al. TCF-1-mediated Wnt signaling regulates Paneth cell innate immune defense effectors HD-5 and -6: Implications for crohn’s disease. Am. J. Physiol. - Gastrointest. Liver Physiol. 2014;307:G487–G498. doi: 10.1152/ajpgi.00347.2013. [DOI] [PubMed] [Google Scholar]
- 32.Gregorieff A., Clevers H. Wnt signaling in the intestinal epithelium: From endoderm to cancer. Genes Dev. 2005;19:877–890. doi: 10.1101/gad.1295405. [DOI] [PubMed] [Google Scholar]
- 33.Dave K., Sur I., Yan J., Zhang J., Kaasinen E., Zhong F., et al. Mice deficient of Myc super-enhancer region reveal differential control mechanism between normal and pathological growth. eLife. 2017;6 doi: 10.7554/eLife.23382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Konsavage W.M., Yochum G.S. The myc 3′ Wnt-responsive element suppresses colonic tumorigenesis. Mol. Cell. Biol. 2014;34:1659–1669. doi: 10.1128/MCB.00969-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tuupanen S., Turunen M., Lehtonen R., Hallikas O., Vanharanta S., Kivioja T., et al. The common colorectal cancer predisposition SNP rs6983267 at chromosome 8q24 confers potential to enhanced Wnt signaling. Nat. Genet. 2009;41:885–890. doi: 10.1038/ng.406. [DOI] [PubMed] [Google Scholar]
- 36.Wright J.B., Brown S.J., Cole M.D. Upregulation of c-MYC in cis through a large chromatin loop linked to a cancer risk-associated single-nucleotide polymorphism in colorectal cancer cells. Mol. Cell. Biol. 2010;30:1411–1420. doi: 10.1128/MCB.01384-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Matheu A., Collado M., Wise C., Manterola L., Cekaite L., Tye A.J., et al. Oncogenicity of the developmental transcription factor Sox9. Cancer Res. 2012;72:1301–1315. doi: 10.1158/0008-5472.CAN-11-3660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Prévostel C., Blache P. The dose-dependent effect of SOX9 and its incidence in colorectal cancer. Eur. J. Cancer. 2017;86:150–157. doi: 10.1016/j.ejca.2017.08.037. [DOI] [PubMed] [Google Scholar]
- 39.Xu Y., Zhang X., Hu X., Zhou W., Zhang P., Zhang J., et al. The effects of lncRNA MALAT1 on proliferation, invasion and migration in colorectal cancer through regulating SOX9. Mol. Med. 2018;24:52. doi: 10.1186/s10020-018-0050-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhou T., Wu L., Ma N., Tang F., Yu Z., Jiang Z., et al. SOX9-activated FARSA-AS1 predetermines cell growth, stemness, and metastasis in colorectal cancer through upregulating FARSA and SOX9. Cell Death Dis. 2020;11:1–15. doi: 10.1038/s41419-020-03273-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.van de Wetering M., Oving I., Muncan V., Fong M.T.P., Brantjes H., Leenen D. van, et al. Specific inhibition of gene expression using a stably integrated, inducible small-interfering-RNA vector. EMBO Rep. 2003;4:609–615. doi: 10.1038/sj.embor.embor865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li V.S.W., Ng S.S., Boersema P.J., Low T.Y., Karthaus W.R., Gerlach J.P., et al. Wnt signaling through inhibition of β-catenin degradation in an intact Axin1 complex. Cell. 2012;149:1245–1256. doi: 10.1016/j.cell.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 43.Kishida S., Yamamoto H., Ikeda S., Kishida M., Sakamoto I., Koyama S., et al. Axin, a negative regulator of the Wnt signaling pathway, directly interacts with adenomatous polyposis coli and regulates the stabilization of β-catenin∗. J. Biol. Chem. 1998;273:10823–10826. doi: 10.1074/jbc.273.18.10823. [DOI] [PubMed] [Google Scholar]
- 44.van de Wetering M., Sancho E., Verweij C., de Lau W., Oving I., Hurlstone A., et al. The β-catenin/TCF-4 complex imposes a crypt progenitor phenotype on colorectal cancer cells. Cell. 2002;111:241–250. doi: 10.1016/s0092-8674(02)01014-0. [DOI] [PubMed] [Google Scholar]
- 45.Barretina J., Caponigro G., Stransky N., Venkatesan K., Margolin A.A., Kim S., et al. The Cancer Cell Line Encyclopedia enables predictive modelling of anticancer drug sensitivity. Nature. 2012;483:603–607. doi: 10.1038/nature11003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ghandi M., Huang F.W., Jané-Valbuena J., Kryukov G.V., Lo C.C., McDonald E.R., et al. Next-generation characterization of the cancer cell line Encyclopedia. Nature. 2019;569:503–508. doi: 10.1038/s41586-019-1186-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dempster J.M., Boyle I., Vazquez F., Root D.E., Boehm J.S., Hahn W.C., et al. Chronos: a cell population dynamics model of CRISPR experiments that improves inference of gene fitness effects. Genome Biol. 2021;22:343. doi: 10.1186/s13059-021-02540-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Van der Flier L.G., Sabates–Bellver J., Oving I., Haegebarth A., De Palo M., Anti M., et al. The intestinal Wnt/TCF signature. Gastroenterology. 2007;132:628–632. doi: 10.1053/j.gastro.2006.08.039. [DOI] [PubMed] [Google Scholar]
- 49.Tomlinson I., Webb E., Carvajal-Carmona L., Broderick P., Kemp Z., Spain S., et al. A genome-wide association scan of tag SNPs identifies a susceptibility variant for colorectal cancer at 8q24.21. Nat. Genet. 2007;39:984–988. doi: 10.1038/ng2085. [DOI] [PubMed] [Google Scholar]
- 50.Zanke B.W., Greenwood C.M., Rangrej J., Kustra R., Tenesa A., Farrington S.M., et al. Genome-wide association scan identifies a colorectal cancer susceptibility locus on chromosome 8q24. Nat. Genet. 2007;39:989–994. doi: 10.1038/ng2089. [DOI] [PubMed] [Google Scholar]
- 51.Shi Z., Chiang C.-I., Labhart P., Zhao Y., Yang J., Mistretta T.-A., et al. Context-specific role of SOX9 in NF-Y mediated gene regulation in colorectal cancer cells. Nucl. Acids Res. 2015;43:6257–6269. doi: 10.1093/nar/gkv568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Coustry F., Oh C., Hattori T., Maity S.N., Crombrugghe B. de, Yasuda H. The dimerization domain of SOX9 is required for transcription activation of a chondrocyte-specific chromatin DNA template. Nucl. Acids Res. 2010;38:6018–6028. doi: 10.1093/nar/gkq417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sotelo J., Esposito D., Duhagon M.A., Banfield K., Mehalko J., Liao H., et al. Long-range enhancers on 8q24 regulate c-Myc. Proc. Natl. Acad. Sci. U. S. A. 2010;107:3001–3005. doi: 10.1073/pnas.0906067107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yochum G.S., Cleland R., Goodman R.H. A genome-wide screen for β-catenin binding sites identifies a downstream enhancer element that controls c-myc gene expression. Mol. Cell Biol. 2008;28:7368–7379. doi: 10.1128/MCB.00744-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yochum G.S., Sherrick C.M., MacPartlin M., Goodman R.H. A β-catenin/TCF-coordinated chromatin loop at MYC integrates 5′ and 3′ Wnt responsive enhancers. Proc. Natl. Acad. Sci. U. S. A. 2010;107:145–150. doi: 10.1073/pnas.0912294107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Coste A. de L., Romagnolo B., Billuart P., Renard C.-A., Buendia M.-A., Soubrane O., et al. Somatic mutations of the β-catenin gene are frequent in mouse and human hepatocellular carcinomas. Proc. Natl. Acad. Sci. U. S. A. 1998;95:8847–8851. doi: 10.1073/pnas.95.15.8847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dietinger V., García de Durango C.R., Wiechmann S., Boos S.L., Michl M., Neumann J., et al. Wnt-driven LARGE2 mediates laminin-adhesive O-glycosylation in human colonic epithelial cells and colorectal cancer. Cell Commun. Signal. 2020;18:102. doi: 10.1186/s12964-020-00561-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stamos J.L., Weis W.I. The β-catenin destruction complex. Cold Spring Harb. Perspect. Biol. 2013;5:a007898. doi: 10.1101/cshperspect.a007898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Molenaar M., van de Wetering M., Oosterwegel M., Peterson-Maduro J., Godsave S., Korinek V., et al. XTcf-3 transcription factor mediates β-catenin-induced Axis formation in Xenopus embryos. Cell. 1996;86:391–399. doi: 10.1016/s0092-8674(00)80112-9. [DOI] [PubMed] [Google Scholar]
- 60.Cadigan K.M., Waterman M.L. TCF/LEFs and Wnt signaling in the nucleus. Cold Spring Harb. Perspect. Biol. 2012;4:a007906. doi: 10.1101/cshperspect.a007906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Abdel-Samad R., Zalzali H., Rammah C., Giraud J., Naudin C., Dupasquier S., et al. MiniSOX9, a dominant-negative variant in colon cancer cells. Oncogene. 2011;30:2493–2503. doi: 10.1038/onc.2010.621. [DOI] [PubMed] [Google Scholar]
- 62.Patil A., Hughes A.L., Zhang G. Rapid evolution and diversification of mammalian α-defensins as revealed by comparative analysis of rodent and primate genes. Physiol. Genomics. 2004;20:1–11. doi: 10.1152/physiolgenomics.00150.2004. [DOI] [PubMed] [Google Scholar]
- 63.Bernard P., Tang P., Liu S., Dewing P., Harley V.R., Vilain E. Dimerization of SOX9 is required for chondrogenesis, but not for sex determination. Hum. Mol. Genet. 2003;12:1755–1765. doi: 10.1093/hmg/ddg182. [DOI] [PubMed] [Google Scholar]
- 64.Sato T., van Es J.H., Snippert H.J., Stange D.E., Vries R.G., van den Born M., et al. Paneth cells constitute the niche for Lgr5 stem cells in intestinal crypts. Nature. 2011;469:415–418. doi: 10.1038/nature09637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Roche K.C., Gracz A.D., Liu X.F., Newton V., Akiyama H., Magness S.T. SOX9 maintains reserve stem cells and preserves radioresistance in mouse small intestine. Gastroenterology. 2015;149:1553–1563.e10. doi: 10.1053/j.gastro.2015.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nusse R. Wnt signaling in disease and in development. Cell Res. 2005;15:28–32. doi: 10.1038/sj.cr.7290260. [DOI] [PubMed] [Google Scholar]
- 67.Nusse R., Clevers H. Wnt/β-Catenin signaling, disease, and emerging therapeutic modalities. Cell. 2017;169:985–999. doi: 10.1016/j.cell.2017.05.016. [DOI] [PubMed] [Google Scholar]
- 68.Polakis P. Wnt signaling in cancer. Cold Spring Harb. Perspect. Biol. 2012;4:a008052. doi: 10.1101/cshperspect.a008052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Baar M.P., Brandt R.M.C., Putavet D.A., Klein J.D.D., Derks K.W.J., Bourgeois B.R.M., et al. Targeted apoptosis of senescent cells restores tissue homeostasis in response to chemotoxicity and aging. Cell. 2017;169:132–147.e16. doi: 10.1016/j.cell.2017.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Schuijers J., Mokry M., Hatzis P., Cuppen E., Clevers H. Wnt-induced transcriptional activation is exclusively mediated by TCF/LEF. EMBO J. 2014;33:146–156. doi: 10.1002/embj.201385358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Schindelin J., Arganda-Carreras I., Frise E., Kaynig V., Longair M., Pietzsch T., et al. Fiji: an open-source platform for biological-image analysis. Nat. Met. 2012;9:676–682. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nix D.A., Courdy S.J., Boucher K.M. Empirical methods for controlling false positives and estimating confidence in ChIP-Seq peaks. BMC Bioinform. 2008;9:523. doi: 10.1186/1471-2105-9-523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lopez-Delisle L., Rabbani L., Wolff J., Bhardwaj V., Backofen R., Grüning B., et al. pyGenomeTracks: reproducible plots for multivariate genomic datasets. Bioinformatics. 2021;37:422–423. doi: 10.1093/bioinformatics/btaa692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Grant C.E., Bailey T.L., Noble W.S. Fimo: scanning for occurrences of a given motif. Bioinformatics. 2011;27:1017–1018. doi: 10.1093/bioinformatics/btr064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fornes O., Castro-Mondragon J.A., Khan A., van der Lee R., Zhang X., Richmond P.A., et al. Jaspar 2020: Update of the open-access database of transcription factor binding profiles. Nucl. Acids Res. 2020;48:D87–D92. doi: 10.1093/nar/gkz1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pettersen E.F., Goddard T.D., Huang C.C., Meng E.C., Couch G.S., Croll T.I., et al. UCSF ChimeraX: structure visualization for researchers, educators, and developers. Protein Sci. 2021;30:70–82. doi: 10.1002/pro.3943. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data supporting the findings in this study are available within the article and supplemental information. All reagents described in this study are available upon request.