Abstract
Background
A 3rd COVID-19 vaccination is currently recommended for patients under immunosuppression. However, a fast decline of antibodies against the SARS-CoV-2 receptor-binding domain (RBD) of the spike protein has been observed. Currently it remains unclear whether immunosuppressive therapy affects kinetics of humoral and cellular immune responses.
Methods
50 patients under immunosuppression and 42 healthy controls (HCs) received a 3rd dose of an mRNA-based vaccine and were monitored over a 12-weeks period. Humoral immune response was assessed 4 and 12 weeks after 3rd dose. Antibodies were quantified using the Elecsys Anti-SARS-CoV-2 Spike immunoassay against the receptor-binding domain (RBD) of the spike protein. SARS-CoV-2-specific T cell responses were quantified by IFN-γ ELISpot assays. Adverse events, including SARS-CoV-2 infections, were monitored over a 12-week period.
Results
At week 12, reduced anti-RBD antibody levels were observed in IMID patients as compared to HCs (median antibody level 5345 BAU/ml [1781–10,208] versus 9650 BAU/ml [6633–16,050], p < 0.001). Reduction in relative antibody levels was significantly higher in IMID patients as compared to HCs at week 12 (p < 0.001). Lowest anti-RBD antibody levels were detected in IMID patients who received biological disease-modifying anti-rheumatic drugs (DMARDs) or a combination therapy with conventional synthetic and biological DMARDs. Number of SARS-CoV-2-specific T cells against wildtype and Omicron variants remained stable over 12 weeks in IMID patients. No serious adverse events were reported.
Conclusion
Due to a fast decline in anti-RBD antibodies in IMID patients an early 4th vaccination should be considered in this vulnerable group of patients.
Keywords: SARS-CoV-2, Vaccination, COVID-19, Immunosuppression
1. Introduction
COVID-19 vaccination is one of the most critical elements to combat the SARS-CoV-2 pandemic. Humoral as well as cellular immune responses thereby contribute to vaccination response [1]. Reduced antibody levels against the receptor-binding domain (RBD) of the spike protein have been reported in patients with immune-mediated inflammatory diseases (IMID) as compared to healthy controls (HCs) after primary vaccination [[2], [3], [4]]. Immunosuppressive therapy with conventional synthetic (cs) and biological (b) disease-modifying anti-rheumatic drugs (DMARDs) can diminish vaccination response in IMID patients [5]. Here, responses were lowest in patients receiving tumor necrosis factor (TNF) inhibitor, combination therapy with methotrexate, JAK inhibitors or abatacept [[6], [7], [8], [9]]. In addition, a decline of anti-RBD antibody levels have been described in healthy individuals and, more pronounced, in patients under immunosuppression after primary vaccination, which likely corresponds to a waning in clinical protection and vaccine effectiveness [4,[10], [11], [12]]. Consequently, a 3rd COVID-19 vaccination is authorized by the European Medicines Agency (EMA), the U.S. Food and Drug Administration (FDA) and accordingly has been recommended by the Center of Disease Control as well as the European Alliance of Associations for Rheumatology (EULAR) and the American College of Rheumatology (ACR) [[13], [14], [15]]. We and others recently reported an impaired immune response to a 3rd vaccination in IMID patients as compared to HCs [4,16]. Especially with the Omicron variant of concern, lower neutralizing capacity and a faster waning of vaccine response was reported [17]. However, it remains unclear whether immunosuppressive therapy affects stability of a humoral and cellular immune response over time. We therefore performed an open-label extension trial which revealed a fast decline of anti-RBD antibodies over a period of 12 weeks in immunosuppressed IMID patients as compared to HC while cellular immune response was preserved.
2. Methods
2.1. Trial design and participants
In this open-label extension phase, individuals were invited to participate in a 12 weeks observational period after a 3rd COVID-19 vaccination. As described previously, in the main open-label prospective clinical trial, participants received a 3rd dose of a COVID-19 vaccine using either BNT162b2 (30 μg dose) or mRNA-1273 (100 μg dose) [16]. Briefly, IMID patients under immunomodulatory therapy as well as HCs (age ≥18 years) with an anti-RBD antibody level of <1500 BAU/ml (binding antibody units per milliliter) after primary COVID-19 vaccination were included. Only participants who were vaccinated with an mRNA vaccine (BNT162b2 or mRNA-1273) were eligible. Main exclusion criteria included previous infection with SARS-CoV-2, known allergies to vaccine compounds or previous B-cell depleting therapies, due to the different mode of action [[18], [19], [20]]. A detailed trial protocol can be found in the supplementary appendix. The trial was registered in the European Clinical Trials Database (EudraCT No.: 2021-002693-10) on the July 15, 2021.
2.2. Procedures
Vaccine immune responses were assessed one week (cellular) or 4 weeks (humoral) after the 3rd COVID-19 vaccination. In the herein presented extension phase, humoral and cellular immunity was assessed 12 weeks after vaccination. All obtained serum samples were stored below −70 °C at the Biobank of the Medical University of Vienna, which provides centralized preparation and storage of biomaterial with certified quality management (International Organization for Standardization (ISO) 9001:2015) [21]. Peripheral blood mononuclear cells (PBMCs) were isolated one and 12 weeks after the 3rd dose using density gradient centrifugation. Samples were stored in the vapor phase of liquid nitrogen.
The study procedures were performed in accordance with Good Clinical Practice guidelines and the Declaration of Helsinki. The trial protocol was approved by the ethics committee (No.: 1583/2021) of the Medical University of Vienna and competent authorities approved the trial protocol. All participants provided their written informed consent before enrollment. All individuals were recruited at the Division of Rheumatology and all trial visits were conducted in a single center (Vienna General Hospital). The first participant entered the extension phase on November 2nd 2021 and the last completed the 12-week follow-up February 21st 2022.
2.3. Anti-SARS-CoV-2 antibody testing
Antibodies to the receptor-binding domain (RBD) of the viral spike (S) protein were quantified using an Elecsys Anti-SARS-CoV-2 S immunoassay as previously described [22,23]. The assays detection range was between 0.4 and 2500.0 BAU/mL, thus samples exceeding this range were approximated by manual predilutions up to a value of 750,000 BAU/mL. All samples were analyzed on a Cobas e801 (Roche Diagnostics, Rotkreuz, Switzerland) at the Department of Laboratory Medicine, Medical University of Vienna (certified acc. to ISO 9001:2015 and accredited acc. to ISO 15189:2012).
2.4. T-cell responses
PepMix SARS-CoV-2 peptide pools for the wild type (WT) virus and the Omicron variant were acquired from JPT (Berlin, Germany). Peptides were dissolved in dimethyl sulfoxide was used to dissolve peptide and further diluted in AIM-V medium for use in enzyme-linked immunosorbent spot (ELISpot) assays as described previously [24,25].
To perform the ex vivo T-cell IFN-γ ELISpot assay, participants’ PBMC samples from week one and 12 after the 3rd COVID-19 vaccine dose were thawed from the vapor phase of liquid nitrogen and processed on the same day.
2.5. Statistical analysis
All participants who completed the extension phase were included into the analysis. Antibody concentrations and cellular responses were assessed either as absolute numbers and as fold change between week one/four and week 12. A Wilcoxon signed rank test was performed to assess differences of antibody levels between groups. A paired Wilcoxon signed rank test was used for matched observations. Correlation between humoral and cellular immune responses were calculated using Spearman's rank correlation coefficient. All additional analyses were performed in a descriptive manner. R version 4.2.1 (R Development Core Team. Vienna, Austria) was utilized for the entire statistical analysis, using the packages: “tidyverse”, “ggpubr” and “tableone”.
2.6 Role of the funding source
Laboratory testing was provided the Vienna General Hospital and the Medical University of Vienna. Otherwise, there was no specific funding or grant for this research from any funding agency in the public, commercial or not-for-profit sectors. The funders had no role in considering the study design or in the collection, analysis, or interpretation of data, the writing of the report, or the decision to submit the article for publication.
3. Results
3.1. Patient characteristics
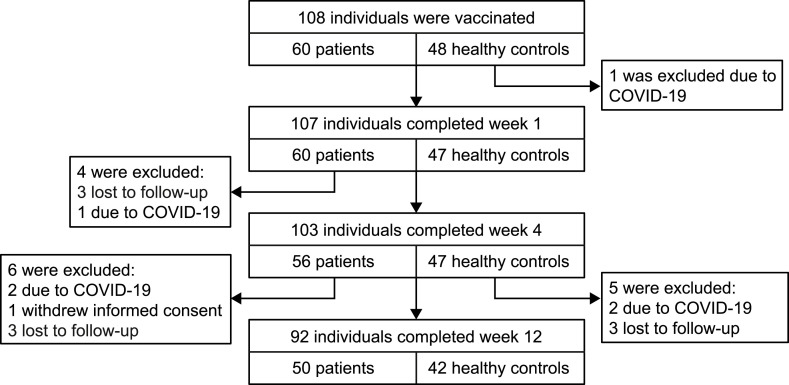
Overall, 103 individuals completed week 4 of the main trial. During the extension phase, 6 healthy controls (HCs) and 5 patients were excluded, with reasons being COVID-19 infections (2 HCs, 2 patients), withdrawal (1 patient) or loss to follow up (3 HCs, 3 patients) (Fig. 1 ). A total of 92 individuals, 50 patients and 42 healthy controls completed the extension phase at week 12. Patient and HCs characteristics are presented in Table 1 . Disease entities in the patient cohort consisted of inflammatory arthritis (44%), connective tissue disease (34%), vasculitis (14%), and other patients receiving immunosuppressive therapy (8%) ( Supplementary Table 1 ).
Fig. 1.
Trial flow diagram.
Table 1.
Characteristics of patients and healthy controls who completed week 12. Data are presented as n (%) or mean ± standard deviation (SD).
| Healthy controls | Patients | |
|---|---|---|
| N | 42 | 50 |
| Age | 48.9 (±14.5) | 57.9 (±16.0) |
| Sex: male | 20 (47.6%) | 11 (22.0%) |
| Primary vaccination compound | ||
| BNT162b2 | 41 (97.6%) | 46 (92.0%) |
| mRNA-1273 | 1 (2.4%) | 4 (8.0%) |
| 3rdvaccination compound | ||
| BNT162b2 | 41 (97.6%) | 45 (90.0%) |
| mRNA-1273 | 1 (2.4%) | 5 (10.0%) |
| SARS-CoV-2-S antibodies at week 4 (BAU/ml) | 22,191 (±14,512) | 15,951 (±16,508) |
3.2. Humoral immune response
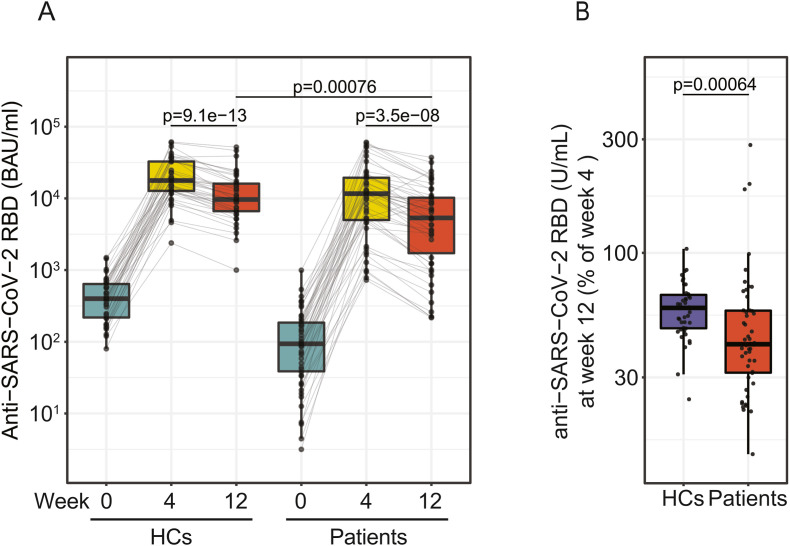
Anti-RBD antibody levels were compared between IMID patients and HCs after the 3rd vaccination. At week 12, antibody concentrations were significantly higher in HCs than IMID patients (9650 BAU/ml [6633–16,050] vs. 5345 BAU/ml [1781–10,208], p < 0.001) (Fig. 2 A). Analysis of anti-RBD antibody levels at week 12 relative to week 4 showed that IMID patients at week 12 retained 41% [31%–57%] and HCs 58% [48%–67%] of week 4 antibody levels, suggesting a pronounced waning of anti-RBD antibodies in IMID patients (Fig. 2B). No association of absolute or relative antibody waning with age could be observed ( Supplemental Fig. 1 ).
Fig. 2.
Humoral immune response at week 4 after 3rdvaccination. A) Antibody levels to the receptor-binding domain (RBD) of the viral spike (S) protein in patients (n = 50) and healthy controls (n = 42) at screening (week 0), week 4 and week 12 after the 3rd vaccination. B) Relative anti-RBD antibody abundance at week 12 (week 4 = 100%) in patients and HCs.
3.3. Cellular immune response
Cellular immune response was determined one and 12 weeks after 3rd COVID-19 vaccination by ELISpot assay. In 25 HCs and 28 IMID patients both timepoints could be obtained. Participant characteristics are provided in Supplementary Table 2.
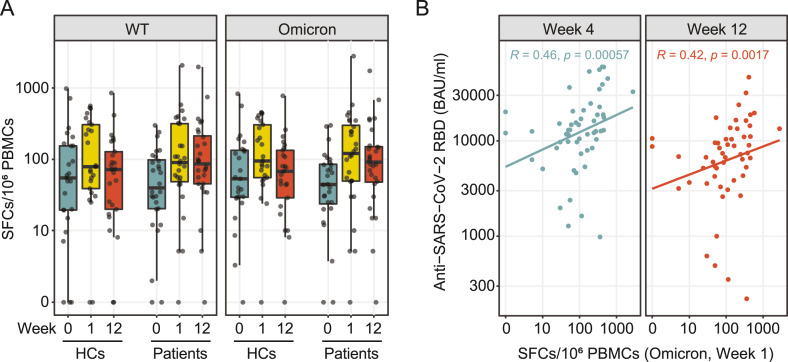
No explicit decrease in SARS-CoV-2-specific spot-forming cells (SFCs) was observed in IMID patients after restimulation with WT and Omicron peptides 12 weeks after 3rd vaccination (WT: median, week 1: 91 [49−316] vs. week 12: 87, [46−214] per 106 SFCs; Omicron: median, week 1: 122 [ 50−301] vs. week 12: 92 [48−151] per 106 SFCs), suggesting a stable cellular immune response over a period of 12 weeks (Fig. 3 A). A significant correlation was observed between SARS-CoV-2 spike-specific T-cells and anti-RBD antibodies at week 4 and week 12, which was detectable in patients as well as HCs, indicating the importance of a cellular immune response for antibody production and stability (Fig. 3B and Supplemental Fig. 2).
Fig. 3.
SARS-CoV-2-specific T-cell response. SARS-CoV-2-specific T-cell responses were determined by ELISpot assay from peripheral blood mononuclear cells (PBMCs) stimulated with wild type (WT) and Omicron spike subunit S1 and S2 peptide pools at week 0, 1 and 12 after the 3rd vaccination. A) Ex vivo IFN-γ ELISpot results of 28 patients and 25 HCs for SARS-CoV-2 WT and Omicron peptide pools at week 0, week 1 and week 12 post vaccination. B) Correlation of average SFCs/106 PBMCs for Omicron peptide pools at week 1 with antibody levels to the receptor-binding domain (RBD) of the viral spike (S) protein at week 4 and 12 post vaccination.
3.4. Effects of immunosuppressive therapy on humoral immune response
Of the 50 patients 25 individuals received a treatment with csDMARDs, 14 with bDMARDs, 10 combination treatment of csDMARDs and bDMARDs and 1 patient received a glucocorticoid mono treatment ( Supplementary Table 3 ).
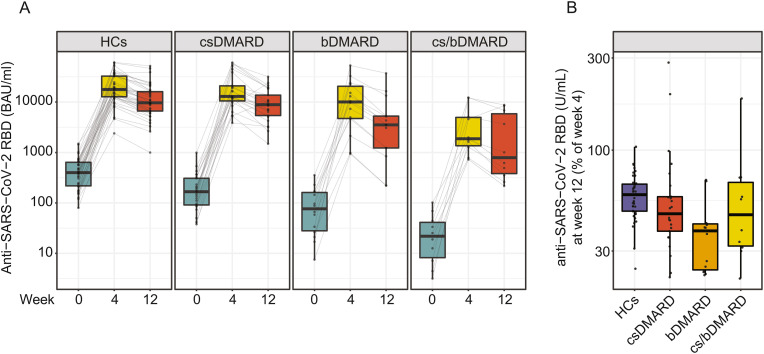
Anti-RBD antibody levels were compared in IMID patients who received either cs DMARDs, bDMARDs or a combination treatment of csDMARDs and bDMARDs 4 and 12 weeks after the 3rd vaccination. Decreased levels of anti-RBD antibody levels were observed for IMID patients, in particular under bDMARD or combination therapy (Fig. 4 A). Analysis of anti-RBD antibody levels at week 12 relative to week 4 showed that IMID patients under csDMARD therapy retained 47% [38%–57%], IMID patients under bDMARD therapy retained 38% [24%–41%] and IMID patients under combination therapy retained 47% [32%–68%] of their peak antibody levels, suggesting a pronounced waning of anti-RBD antibodies in IMID patients under immunosuppressive therapy as compared to HCs (59% [48%–67%]) (Fig. 4B).
Fig. 4.
Treatment-specific response to a 3rdvaccination. A) Antibody levels to the receptor-binding domain (RBD) of the viral spike (S) protein at week 0, 4 and 12 in HCs (42), patients with csDMARD (25), bDMARD (14) or csDMARD/bDMARD combination treatment (10). B) Relative anti-RBD antibody abundance at week 12 (week 4 = 100%) in patients and HCs for the indicated treatments.
3.5. Safety
Between week 4 and week 12, no serious adverse events occurred. Two IMID patients and two HCs were tested positive for SARS-CoV-2 between week 4 and 12 and were subsequently excluded from analysis. All had a mild disease course not requiring hospitalization.
4. Discussion
In this open-label extension study we investigated the stability of a humoral and cellular immune response after a 3rd COVID-19 vaccination in IMID patients compared to HCs. While cellular immune response was largely maintained, IMID patients under immunosuppressive therapy showed a rapid decrease of anti-RBD antibody levels over a 12-weeks period.
It has widely been accepted that anti-RBD antibody levels correlate to clinical protection of SARS-CoV-2 infections and that virus neutralization requires sufficient antibody levels [[26], [27], [28]]. Previous reports depict, that IMID patients have an impaired immune response to primary COVID-19 vaccination leading to reduced antibody levels when compared to healthy individuals [[2], [3], [4]]. Based on these data the Center of Disease Control (CDC) and the World Health Organization (WHO) recommended a 3rd vaccination for immunocompromised individuals, which is in line with statements from the American College of Rheumatology (ACR) and European Alliance of Associations for Rheumatology (EULAR) [29,30]. Patients under immunosuppressive therapy exhibit more pronounced antibody waning than healthy controls after the second dose [8,9,11]. Similarly, we observed reduced anti-RBD antibody levels in IMID patients 12 weeks after 3rd vaccination. Our data imply treatment strategy as the most important factor for antibody decrease. Stratification of patients based on their treatment showed that patients under bDMARD or combination of bDMARD and csDMARD therapy have the lowest anti-RBD antibody levels with the highest relative decrease compared to week 4. The data are in line with recently published reports, that show a negative effect of csDMARD therapy, bDMARD therapy or combination therapy on the efficacy of a 2nd and 3rd COVID-19 vaccination, but also on the stability of the vaccination [3,4,7,16].
Cellular immune responses are essential for immunogenicity after COVID-19 vaccination [1]. However, data on cellular immune response in IMID patients after COVID-19 vaccinations are scarce. We observed an induction of a cellular immune response one week after a 3rd COVID-19 vaccination, which could be maintained over a period of 12 weeks after restimulation with WT peptides. There was only a slight decrease of the cellular response to Omicron peptides highlighting the relevance of a third vaccination for currently circulating variants of concern. SARS-CoV-2-specific T cells for both WT and Omicron variants correlated with anti-RBD antibody levels at week 4 and 12, supporting the importance of a cellular immune response for antibody development.
Several concerns have been raised regarding adverse events of an additional COVID-19 vaccination with respect to pronounced side effects as well as disease flares [31]. Also in a three months assessment, no disease flares have been reported in IMID patients and no severe adverse events in both groups, emphasizing the safety of a 3rd vaccination in IMID patients.
The mRNA-1273 vaccine in the full dose (100 μg) is supposed to be more immunogenic compared to BNT162b2 in healthy individuals [32,33]. In this extension phase only two patients were vaccinated with RNA-1273, which diminishes the risk of a vaccine-induced bias between the two cohorts on the one hand, but does not allow a comparison of the two vaccination compounds as a 3rd vaccination.
Although this investigation addresses stability of a humoral and cellular immune response of a 3rd vaccination in IMID patients as compared to HCs in a controlled setting, there are some limitations: a significant effect of different treatment strategies was observed, however a higher number of patients will be required to draw solid conclusions on the action of individual DMARD-containing therapies on antibody stability. Additional data are needed to further understand disease-specific effects on the stability of a humoral immune response.
Together our data support that a 3rd vaccination is safe in IMID patients. However, particularly in patients under immunosuppressive therapy a timely 4th vaccination should be considered.
Funding
Medical University of Vienna.
Author contribution
DS, HR, PM, DM, MB and DA contributed to the study design. FK, DM, ES, LG, TH, TD, IG, and MB recruited participants, performed patient visits and recorded data. PH was relevant for sample handling and contributed to cellular assays. TP and HH performed antibody measurements. FK and DM performed data analysis and HR, DS, LXH and MB reviewed the data analysis. All authors contributed to the manuscript writing. MB acts as guarantor.
Ethical approval
The trial protocol was approved by competent authorities and the ethics committee of the Medical University of Vienna (No.: 1583/2021).
Declaration of competing interest
DM reports support for meeting attendances from Pfizer and consultation fees from AstraZeneca; JS reports about grants, consulting and personal fees from AbbVie, Amgen, AstraZeneca, Astro, Bristol-Myers Squibb, Celltrion, Gilead-Galapagos, Janssen, Lilly, Pfizer, R-Pharma, Samsung, Sanofi, Chugai, Merck Sharp & Dohme, Novartis-Sandoz Roche, Samsung and UCB and grants from Abbvie, AstraZeneca, Lilly, Novartis, and Roche; HR received speaker fees from Gilead, Merck Sharp and Pfizer and travel support from Janssen; HH received grants from Glock Health, BlueSky Immunotherapies and Neutrolis; DA received grants, speaker fees, or consultancy fees from Abbvie, Amgen, Galapagos, Lilly, Janssen, Merck, Novartis, Pfizer, Sandoz, and Sanofi; RK reports consulting fees from AstraZeneca, Takeda Pharma, MEDahead and Janssen Cilag and speaker fees from Otsuka; ES received travel support from Pfizer, Bristol-Myers Squibb and Boehringer-Ingelheim and speaker fees from Lilly; MB reports about personal fees from Eli-Lilly. All other authors declare no competing interests.
Acknowledgements
We thank all participants of the study. Special thanks go to Martina Durechova, Daffodil Dioso, Elyza Raymundo and Michael Zauner for their support. We thank Brigitte Meyer, Birgit Niederreiter, Carl-Walter Steiner, Ursula Sinzinger, Patrick Mucher and Sylvia Taxer for their assistance.
Handling editor: M.E. Gershwin
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jaut.2022.102981.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
Data availability
Data will be made available on request.
References
- 1.Sette A., Crotty S. Adaptive immunity to SARS-CoV-2 and COVID-19. Cell. 2021;184:861–880. doi: 10.1016/j.cell.2021.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Furer V., Eviatar T., Zisman D., Peleg H., Paran D., Levartovsky D., Zisapel M., Elalouf O., Kaufman I., Meidan R., Broyde A., Polachek A., Wollman J., Litinsky I., Meridor K., Nochomovitz H., Silberman A., Rosenberg D., Feld J., Haddad A., Gazzit T., Elias M., Higazi N., Kharouf F., Shefer G., Sharon O., Pel S., Nevo S., Elkayam O. Immunogenicity and safety of the BNT162b2 mRNA COVID-19 vaccine in adult patients with autoimmune inflammatory rheumatic diseases and in the general population: a multicentre study. Ann. Rheum. Dis. 2021;80:1330–1338. doi: 10.1136/annrheumdis-2021-220647. [DOI] [PubMed] [Google Scholar]
- 3.Wieske L., van Dam K.P.J., Steenhuis M., Stalman E.W., Kummer L.Y.L., van Kempen Z.L.E., Killestein J., Volkers A.G., Tas S.W., Boekel L., Wolbink G.J., van der Kooi A.J., Raaphorst J., Löwenberg M., Takkenberg R.B., D'Haens G.R.A.M., Spuls P.I., Bekkenk M.W., Musters A.H., Post N.F., Bosma A.L., Hilhorst M.L., Vegting Y., Bemelman F.J., Voskuyl A.E., Broens B., Sanchez A.P., van Els C.A.C.M., de Wit J., Rutgers A., de Leeuw K., Horváth B., Verschuuren J.J.G.M., Ruiter A.M., van Ouwerkerk L., van der Woude D., Allaart R.C.F., Teng Y.K.O., van Paassen P., Busch M.H., Jallah P.B.P., Brusse E., van Doorn P.A., Baars A.E., Hijnen D.J., Schreurs C.R.G., van der Pol W.L., Goedee H.S., Keijzer S., Keijser J.B.D., Boogaard A., Cristianawati O., Ten Brinke A., Verstegen N.J.M., Zwinderman K.A.H., van Ham S.M., Kuijpers T.W., Rispens T., Eftimov F. T2B! Immunity against SARS-CoV-2 study group, Humoral responses after second and third SARS-CoV-2 vaccination in patients with immune-mediated inflammatory disorders on immunosuppressants: a cohort study. Lancet Rheumatol. 2022 doi: 10.1016/S2665-9913(22)00034-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Simon D., Tascilar K., Fagni F., Kleyer A., Krönke G., Meder C., Dietrich P., Orlemann T., Mößner J., Taubmann J., Mutlu M.Y., Knitza J., Kemenes S., Liphardt A.-M., Schönau V., Bohr D., Schuster L., Hartmann F., Minopoulou I., Leppkes M., Ramming A., Pachowsky M., Schuch F., Ronneberger M., Kleinert S., Hueber A.J., Manger K., Manger B., Atreya R., Berking C., Sticherling M., Neurath M.F., Schett G. Intensity and longevity of SARS-CoV-2 vaccination response in patients with immune-mediated inflammatory disease: a prospective cohort study. Lancet Rheumatol. 2022;4 doi: 10.1016/S2665-9913(22)00191-6. e614–e625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haberman R.H., Herati R., Simon D., Samanovic M., Blank R.B., Tuen M., Koralov S.B., Atreya R., Tascilar K., Allen J.R., Castillo R., Cornelius A.R., Rackoff P., Solomon G., Adhikari S., Azar N., Rosenthal P., Izmirly P., Samuels J., Golden B., Reddy S.M., Neurath M.F., Abramson S.B., Schett G., Mulligan M.J., Scher J.U. Methotrexate hampers immunogenicity to BNT162b2 mRNA COVID-19 vaccine in immune-mediated inflammatory disease. Ann. Rheum. Dis. 2021;80:1339–1344. doi: 10.1136/annrheumdis-2021-220597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Syversen S.W., Jyssum I., Tveter A.T., Tran T.T., Sexton J., Provan S.A., Mjaaland S., Warren D.J., Kvien T.K., Grødeland G., Nissen-Meyer L.S.H., Ricanek P., Chopra A., Andersson A.M., Kro G.B., Jahnsen J., Munthe L.A., Haavardsholm E.A., Vaage J.T., Lund-Johansen F., Jørgensen K.K., Goll G.L. Arthritis & Rheumatology; 2022. Immunogenicity and Safety of Standard and Third-Dose SARS–CoV-2 Vaccination in Patients Receiving Immunosuppressive Therapy. n/a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mandl P., Tobudic S., Haslacher H., Karonitsch T., Mrak D., Nothnagl T., Perkmann T., Radner H., Sautner J., Simader E., Winkler F., Burgmann H., Aletaha D., Winkler S., Blüml S. Response to SARS-CoV-2 vaccination in systemic autoimmune rheumatic disease depends on immunosuppressive regimen: a matched, prospective cohort study. Ann. Rheum. Dis. 2022 doi: 10.1136/annrheumdis-2021-221788. [DOI] [PubMed] [Google Scholar]
- 8.Le Moine C., Soyfoo M.S., Mekkaoui L., Dahma H., Tant L. Waning humoral immunity of SARS-CoV-2 vaccination in a rheumatoid arthritis cohort and the benefits of a vaccine booster dose. Clin. Exp. Rheumatol. 2022 doi: 10.55563/clinexprheumatol/ti3tvu. [DOI] [PubMed] [Google Scholar]
- 9.Frey S., Chiang T.P.-Y., Connolly C.M., Teles M., Alejo J.L., Boyarsky B.J., Christopher-Stine L., Werbel W.A., Massie A.B., Segev D.L., Paik J.J. Antibody durability 6 months after two doses of SARS-CoV-2 mRNA vaccines in patients with rheumatic and musculoskeletal disease. Lancet Rheumatol. 2022 doi: 10.1016/S2665-9913(21)00417-3. 0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Andrews N., Tessier E., Stowe J., Gower C., Kirsebom F., Simmons R., Gallagher E., Thelwall S., Groves N., Dabrera G., Myers R., Campbell C.N.J., Amirthalingam G., Edmunds M., Zambon M., Brown K., Hopkins S., Chand M., Ladhani S.N., Ramsay M., Lopez Bernal J. Duration of protection against mild and severe disease by covid-19 vaccines. N. Engl. J. Med. 2022;386:340–350. doi: 10.1056/NEJMoa2115481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Levin E.G., Lustig Y., Cohen C., Fluss R., Indenbaum V., Amit S., Doolman R., Asraf K., Mendelson E., Ziv A., Rubin C., Freedman L., Kreiss Y., Regev-Yochay G. Waning immune humoral response to BNT162b2 covid-19 vaccine over 6 months. N. Engl. J. Med. 2021;385:e84. doi: 10.1056/NEJMoa2114583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goldberg Y., Mandel M., Bar-On Y.M., Bodenheimer O., Freedman L., Haas E.J., Milo R., Alroy-Preis S., Ash N., Huppert A. Waning immunity after the BNT162b2 vaccine in Israel. N. Engl. J. Med. 2021;385:e85. doi: 10.1056/NEJMoa2114228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.FDA, Coronavirus (COVID-19) Update . FDA; 2021. FDA Authorizes Additional Vaccine Dose for Certain Immunocompromised Individuals.https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-additional-vaccine-dose-certain-immunocompromised accessed. [Google Scholar]
- 14.Clinical Guidance for COVID-19 Vaccination. CDC; 2022. https://www.cdc.gov/vaccines/covid-19/clinical-considerations/interim-considerations-us.html accessed. [Google Scholar]
- 15.EMA Comirnaty, Spikevax . European Medicines Agency; 2021. EMA Recommendations on Extra Doses Boosters.https://www.ema.europa.eu/en/news/comirnaty-spikevax-ema-recommendations-extra-doses-boosters accessed. [Google Scholar]
- 16.Kartnig F., Mrak D., Simader E., Tobudic S., Radner H., Mandl P., Göschl L., Hommer N., Mayer M., Hofer P., Hummel T., Deimel T., Geßl I., Puchner A., Kerschbaumer A., Thalhammer R., Handisurya A., Kain R., Winkler S., Smolen J.S., Stiasny K., Perkmann T., Haslacher H., Aberle J.H., Aletaha D., Heinz L.X., Sieghart D., Bonelli M. Safety and immunogenicity of a third COVID-19 vaccination in patients with immune-mediated inflammatory diseases compared with healthy controls. Ann. Rheum. Dis. 2022 doi: 10.1136/ard-2022-222682. [DOI] [PubMed] [Google Scholar]
- 17.Kim W.-J., Choi S.-H., Park J.Y., Song J.S., Chung J.-W., Choi S.T. SARS-CoV-2 Omicron escapes mRNA vaccine booster-induced antibody neutralisation in patients with autoimmune rheumatic diseases: an observational cohort study. Ann. Rheum. Dis. 2022 doi: 10.1136/ard-2022-222689. [DOI] [PubMed] [Google Scholar]
- 18.Bonelli M.M., Mrak D., Perkmann T., Haslacher H., Aletaha D. SARS-CoV-2 vaccination in rituximab-treated patients: evidence for impaired humoral but inducible cellular immune response. Ann. Rheum. Dis. 2021 doi: 10.1136/annrheumdis-2021-220408. annrheumdis-2021-220408. [DOI] [PubMed] [Google Scholar]
- 19.Mrak D., Tobudic S., Koblischke M., Graninger M., Radner H., Sieghart D., Hofer P., Perkmann T., Haslacher H., Thalhammer R., Winkler S., Blüml S., Stiasny K., Aberle J.H., Smolen J.S., Heinz L.X., Aletaha D., Bonelli M. SARS-CoV-2 vaccination in rituximab-treated patients: B cells promote humoral immune responses in the presence of T-cell-mediated immunity. Ann. Rheum. Dis. 2021;80:1345–1350. doi: 10.1136/annrheumdis-2021-220781. [DOI] [PubMed] [Google Scholar]
- 20.Mrak D., Simader E., Sieghart D., Mandl P., Radner H., Perkmann T., Haslacher H., Mayer M., Koblischke M., Hofer P., Göschl L., Kartnig F., Deimel T., Kerschbaumer A., Hummel T., Kornek B., Thalhammer R., Stiasny K., Winkler S., Smolen J.S., Aberle J.H., Aletaha D., Heinz L.X., Bonelli M. Immunogenicity and safety of a fourth COVID-19 vaccination in rituximab-treated patients: an open-label extension study. Ann. Rheum. Dis. 2022 doi: 10.1136/ard-2022-222579. annrheumdis-2022-222579. [DOI] [PubMed] [Google Scholar]
- 21.Haslacher H., Gerner M., Hofer P., Jurkowitsch A., Hainfellner J., Kain R., Wagner O.F., Perkmann T. Usage data and scientific impact of the prospectively established fluid bioresources at the hospital-based MedUni wien Biobank. Biopreserv. Biobanking. 2018;16:477–482. doi: 10.1089/bio.2018.0032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Higgins V., Fabros A., Kulasingam V. Quantitative Measurement of Anti-SARS-CoV-2 Antibodies: Analytical and Clinical Evaluation. J. Clin. Microbiol. 2021;59(4) doi: 10.1128/JCM.03149-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Perkmann T., Perkmann-Nagele N., Koller T., Mucher P., Radakovics A., Marculescu R., Wolzt M., Wagner O.F., Binder C.J., Haslacher H. Anti-spike protein assays to determine SARS-CoV-2 antibody levels: a head-to-head comparison of five quantitative assays. Microbiol. Spectr. 2021 doi: 10.1128/Spectrum.00247-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bonelli M., Mrak D., Tobudic S., Sieghart D., Koblischke M., Mandl P., Kornek B., Simader E., Radner H., Perkmann T., Haslacher H., Mayer M., Hofer P., Redlich K., Husar-Memmer E., Fritsch-Stork R., Thalhammer R., Stiasny K., Winkler S., Smolen J.S., Aberle J.H., Zeitlinger M., Heinz L.X., Aletaha D. Additional heterologous versus homologous booster vaccination in immunosuppressed patients without SARS-CoV-2 antibody seroconversion after primary mRNA vaccination: a randomised controlled trial. Ann. Rheum. Dis. 2022 doi: 10.1136/annrheumdis-2021-221558. [DOI] [PubMed] [Google Scholar]
- 25.Mrak D., Sieghart D., Simader E., Tobudic S., Radner H., Mandl P., Göschl L., Koblischke M., Hommer N., Wagner A., Mayer M., Schubert L., Hartl L., Kozbial K., Hofer P., Kartnig F., Hummel T., Kerschbaumer A., Deimel T., Puchner A., Gudipati V., Thalhammer R., Munda P., Uyanik-Ünal K., Zuckermann A., Novacek G., Reiberger T., Garner-Spitzer E., Reindl-Schwaighofer R., Kain R., Winkler S., Smolen J.S., Stiasny K., Fischer G.F., Perkmann T., Haslacher H., Zeitlinger M., Wiedermann U., Aberle J.H., Aletaha D., Heinz L.X., Bonelli M. Heterologous vector versus homologous mRNA COVID-19 booster vaccination in non-seroconverted immunosuppressed patients: a randomized controlled trial. Nat. Commun. 2022;13:5362. doi: 10.1038/s41467-022-33036-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Khoury D.S., Cromer D., Reynaldi A., Schlub T.E., Wheatley A.K., Juno J.A., Subbarao K., Kent S.J., Triccas J.A., Davenport M.P. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat. Med. 2021;27:1205–1211. doi: 10.1038/s41591-021-01377-8. [DOI] [PubMed] [Google Scholar]
- 27.Gilbert P.B., Montefiori D.C., McDermott A.B., Fong Y., Benkeser D., Deng W., Zhou H., Houchens C.R., Martins K., Jayashankar L., Castellino F., Flach B., Lin B.C., O'Connell S., McDanal C., Eaton A., Sarzotti-Kelsoe M., Lu Y., Yu C., Borate B., van der Laan L.W.P., Hejazi N.S., Huynh C., Miller J., El Sahly H.M., Baden L.R., Baron M., De La Cruz L., Gay C., Kalams S., Kelley C.F., Andrasik M.P., Kublin J.G., Corey L., Neuzil K.M., Carpp L.N., Pajon R., Follmann D., Donis R.O., Koup R.A. Immune assays Team, moderna, inc. Team, coronavirus vaccine prevention network (CoVPN)/Coronavirus efficacy (COVE) Team, United States GOVERNMENT (USG)/COVPN BIOSTATISTICS TEAM, immune correlates analysis of the mRNA-1273 COVID-19 vaccine efficacy clinical trial. Science. 2022;375:43–50. doi: 10.1126/science.abm3425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cromer D., Steain M., Reynaldi A., Schlub T.E., Wheatley A.K., Juno J.A., Kent S.J., Triccas J.A., Khoury D.S., Davenport M.P. Neutralising antibody titres as predictors of protection against SARS-CoV-2 variants and the impact of boosting: a meta-analysis. Lancet Microbe. 2022;3 doi: 10.1016/S2666-5247(21)00267-6. e52–e61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Landewé R.B.M., Kroon F.P.B., Alunno A., Najm A., Bijlsma J.W., Burmester G.-R.R., Caporali R., Combe B., Conway R., Curtis J.R., Elkayam O., Gossec L., Heijstek M.W., Haupt L., Iagnocco A., Isaacs J.D., Juhász I.Á., Makri S., Mariette X., McInnes I.B., Mehta P., Mueller-Ladner U., Schulze-Koops H., Smolen J.S., Wiek D., Winthrop K.L., Navarro-Compán V., Machado P.M. EULAR recommendations for the management and vaccination of people with rheumatic and musculoskeletal diseases in the context of SARS-CoV-2: the November 2021 update. Ann. Rheum. Dis. 2022 doi: 10.1136/annrheumdis-2021-222006. [DOI] [PubMed] [Google Scholar]
- 30.Curtis J.R., Johnson S.R., Anthony D.D., Arasaratnam R.J., Baden L.R., Bass A.R., Calabrese C., Gravallese E.M., Harpaz R., Kroger A., Sadun R.E., Turner A.S., Williams E.A., Mikuls T.R. American College of Rheumatology guidance for COVID-19 vaccination in patients with rheumatic and musculoskeletal diseases: version 4. Arthritis Rheumatol. 2022;74 doi: 10.1002/art.42109. e21–e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vojdani A., Kharrazian D. Potential antigenic cross-reactivity between SARS-CoV-2 and human tissue with a possible link to an increase in autoimmune diseases. Clin. Immunol. 2020;217 doi: 10.1016/j.clim.2020.108480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Steensels D., Pierlet N., Penders J., Mesotten D., Heylen L. Comparison of SARS-CoV-2 antibody response following vaccination with BNT162b2 and mRNA-1273. JAMA. 2021;326:1533–1535. doi: 10.1001/jama.2021.15125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Munro A.P.S., Janani L., Cornelius V., Aley P.K., Babbage G., Baxter D., Bula M., Cathie K., Chatterjee K., Dodd K., Enever Y., Gokani K., Goodman A.L., Green C.A., Harndahl L., Haughney J., Hicks A., van der Klaauw A.A., Kwok J., Lambe T., Libri V., Llewelyn M.J., McGregor A.C., Minassian A.M., Moore P., Mughal M., Mujadidi Y.F., Murira J., Osanlou O., Osanlou R., Owens D.R., Pacurar M., Palfreeman A., Pan D., Rampling T., Regan K., Saich S., Salkeld J., Saralaya D., Sharma S., Sheridan R., Sturdy A., Thomson E.C., Todd S., Twelves C., Read R.C., Charlton S., Hallis B., Ramsay M., Andrews N., Nguyen-Van-Tam J.S., Snape M.D., Liu X., Faust S.N., Riordan A., Ustianowski A., Rogers C.A., Hughes S., Longshaw L., Stockport J., Hughes R., Grundy L., Jones L.T., Guha A., Snashall E., Eadsforth T., Reeder S., Storton K., Munusamy M., Tandy B., Egbo A., Cox S., Ahmed N.N., Shenoy A., Bousfield R., Wixted D., Gutteridge H., Mansfield B., Herbert C., Holliday K., Calderwood J., Barker D., Brandon J., Tulloch H., Colquhoun S., Thorp H., Radford H., Evans J., Baker H., Thorpe J., Batham S., Hailstone J., Phillips R., Kumar D., Westwell F., Makia F., Hopkins N., Barcella L., Mpelembue M., Dabagh M., Lang M., Khan F., Adebambo O., Chita S., Corrah T., Whittington A., John L., Roche S., Wagstaff L., Farrier A., Bisnauthsing K., Serafimova T., Nanino E., Cooney E., Wilson-Goldsmith J., Nguyen H., Mazzella A., Jackson B., Aslam S., Bawa T., Broadhead S., Farooqi S., Piper J., Weighell R., Pickup L., Shamtally D., Domingo J., Kourampa E., Hale C., Gibney J., Stackpoole M., Rashid-Gardner Z., Lyon R., McDonnell C., Cole C., Stewart A., McMillan G., Savage M., Beckett H., Moorbey C., Desai A., Brown C., Naker K., Qureshi E., Trinham C., Sabine C., Moore S., Hurdover S., Justice E., Smith D., Plested E., Silva C.F.D., White R., Robinson H., Cifuentes L., Morshead G., Drake-Brockman R., Kinch P., Kasanyinga M., Clutterbuck E.A., Bibi S., Stuart A.S., Shaw R.H., Singh M., Champaneri T., Irwin M., Khan M., Kownacka A., Nabunjo M., Osuji C., Hladkiwskyj J., Galvin D., Patel G., Mouland J., Longhurst B., Moon M., Giddins B., Alves C.P.D., Richmond L., Minnis C., Baryschpolec S., Elliott S., Fox L., Graham V., Baker N., Godwin K., Buttigieg K., Knight C., Brown P., Lall P., Shaik I., Chiplin E., Brunt E., Leung S., Allen L., Thomas S., Fraser S., Choi B., Gouriet J., Freedman A., Perkins J., Gowland A., Macdonald J., Seenan J.P., Starinskij I., Seaton A., Peters E., Singh S., Gardside B., Bonnaud A., Davies C., Gordon E., Keenan S., Hall J., Wilkins S., Tasker S., James R., Seath I., Littlewood K., Newman J., Boubriak I., Suggitt D., Haydock H., Bennett S., Woodyatt W., Hughes K., Bell J., Coughlan T., van Welsenes D., Kamal M., Cooper C., Tunstall S., Ronan N., Cutts R., Dare T., Yim Y.T.N., Whittley S., Ricamara M., Hamal S., Adams K., Baker H., Driver K., Turner N., Rawlins T., Roy S., Merida-Morillas M., Sakagami Y., Andrews A., Cordeiro L.G., Stokes M., Ambihapathy W., Spencer J., Parungao N., Berry L., Cullinane J., Presland L., Ross-Russell A., Warren S., Baker J., Oliver A., Buadi A., Lee K., Haskell L., Romani R., Bentley I., Whitbred T., Fowler S., Gavin J., Magee A., Watson T., Nightingale K., Marius P., Summerton E., Locke E., Honey T., Lingwood A., de la Haye A., Elliott R.S., Underwood K., King M., Davies-Dear S., Horsfall E., Chalwin O., Burton H., Edwards C.J., Welham B., Garrahy S., Hall F., Ladikou E., Mullan D., Hansen D., Campbell M., Santos F.D., Habash-Bailey H., Lakeman N., Branney D., Vamplew L., Hogan A., Frankham J., Wiselka M., Vail D., Wenn V., Renals V., Ellis K., Lewis-Taylor J., Magan J., Hardy A., Appleby K. Safety and immunogenicity of seven COVID-19 vaccines as a third dose (booster) following two doses of ChAdOx1 nCov-19 or BNT162b2 in the UK (COV-BOOST): a blinded, multicentre, randomised, controlled, phase 2 trial. Lancet. 2021;398:2258–2276. doi: 10.1016/S0140-6736(21)02717-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.