Abstract
Purpose
It is unclear whether or not nonalcoholic fatty liver disease (NAFLD)/metabolic dysfunction–associated fatty liver disease (MAFLD) is related to short sleep duration. A meta-analysis was conducted to determine if inadequate sleep time increased the risk of NAFLD/MAFLD.
Methods
A comprehensive systematic literature review was conducted in the Embase, PubMed, and Cochrane Library databases from inception to August 1, 2022. Studies examining the correlation between inadequate sleep time and the risk of NAFLD/MAFLD were included. We pooled the odds ratios (ORs) and 95% confidence intervals (CIs) using a random-effects model.
Results
This meta-analysis included fifteen studies involving a total of 261,554 participants. In the pooled analysis, short sleep duration was found to be strongly correlated with an increased risk of NAFLD/MAFLD (OR, 1.15; 95% CI, 1.04–1.28; P = 0.01), with a moderate degree of heterogeneity between studies (I2 = 71.92%, Q = 49.87, P < 0.01). The sensitivity analysis suggested that the primary outcome was robust, and there was no significant publication bias.
Conclusion
This meta-analysis indicates that inadequate sleep duration is strongly correlated with an elevated risk of NAFLD/MAFLD. The findings suggest that obtaining an adequate amount of sleep may be useful for preventing NAFLD/MAFLD, which is especially important given the low rate of response to pharmacotherapy.
Supplementary Information
The online version contains supplementary material available at 10.1007/s11325-022-02767-z.
Keywords: Short sleep duration, NAFLD, MAFLD
Introduction
With economic development, a growing number of people have been developing metabolism disorders characterized by insulin resistance (IR). Additionally, nonalcoholic fatty liver disease (NAFLD) has become widespread throughout the world, even in developing countries. The global incidence of NAFLD is approximately 24%, and the prevalence rates in South America and the Middle East are greater than 30% [1, 2]. The heterogeneous pathogenesis of NAFLD and the low response rate to pharmacotherapies make it very difficult to control this disease [3, 4]. To diagnose, classify, and manage fatty liver; conduct clinical trials; and develop new drugs more efficiently, an international fatty liver nomenclature group recommended revising the fatty liver nomenclature from NAFLD to metabolic dysfunction–associated fatty liver disease (MAFLD) [5].
In recent years, long work duration, shift work, academic pressure, increased entertainment, and even the COVID-19 pandemic have resulted in inadequate sleep duration worldwide [6–10]. Sleep duration, sleep pattern, and sleep quality are crucial factors related to human health. Short sleep duration can harm multiple systems by affecting endocrine function [11], metabolism [12], and immune pathways [13]. Many studies have reported that inadequate sleep time is associated with total mortality [14], myocardial infarction [15], infection [13], obesity [16], type 2 diabetes mellitus [17], high blood pressure [18], and asthma. [19] Some studies showed that inadequate sleep time was related to an increased risk of NAFLD/MAFLD [20–22], whereas other studies showed a nonsignificant association or even a reduced risk [23–25]. New studies have been published in the past few years, especially those referring to MAFLD, providing new clinical evidence. Therefore, we performed this meta-analysis to determine the correlation between short sleep time and the risk of NAFLD/MAFLD.
Method
Literature search strategy
We retrieved published studies from the Embase, PubMed, and Cochrane Library electronic databases based on the retrieval strategy outlined in Item S1 in the Supplementary material. The databases were searched from inception to August 1, 2022. Furthermore, we manually searched the reference lists of the original articles and reviews to avert overlooking any pertinent research. Only articles with full texts were reviewed, while meeting abstracts were excluded. There was no language limitation.
Inclusion and exclusion criteria
The criteria for inclusion were as follows: (1) cross-sectional, case–control, or cohort study assessing the relevance between inadequate sleep time and the risk of NAFLD/MAFLD; (2) research provided hazard ratios (HRs), odds ratios (ORs), or risk ratios (RRs) with 95% confidence intervals (CI) or adequate data to determine them; and (3) subjects with longer sleep time were treated as a control group in cohort studies, and subjects without NAFLD/MAFLD were treated as a control group in cross-sectional or case–control research.
The exclusion criteria were as follows: (1) research without a clear definition of short sleep duration, such as just using bedtime to determine sleep duration, (2) publications that have been retracted, and (3) meeting abstracts without full text. The screening of eligible studies was carried out by the two reviewers mentioned above, and disagreements were judged by a third reviewer (Y-D. T.).
If multiple published articles from the same data source were available, we included only the study with the largest sample size. In addition, the cohort and case–control studies were assessed using the Newcastle–Ottawa Scale [26]. Cross-sectional studies were appraised using the modified Newcastle–Ottawa Scale proposed by Herzog et al. [27]. For the original Newcastle–Ottawa Scale, high quality was defined as 7–9 points, moderate quality was defined as 5–6 points, and low quality was defined as 0–4 points. For the modified form, high quality was defined as 8–10 points, moderate quality was defined as 6–7 points, and low quality was defined as 0–5 points.
Data extraction
Two reviewers standardized and extracted detailed information from the studies, including the name of the first author, country of origin, publication year, recruitment of participants, sleep duration category, NAFLD/MAFLD criteria, study design, sample size, confounder adjustment, and adjusted effect estimation with 95% CI.
Statistical analysis
STATA version 17.0 was utilized to perform the statistical analysis. The correlation of inadequate sleep time with the risk of NAFLD/MAFLD was explored by determining the pooled OR and 95% CI. For any study that did not report ORs, we used HRs as estimated ORs [20]. Galbraith plots, the I2 statistic, and Cochran’s Q test were used to explore the between-study heterogeneity. Furthermore, a random-effects model (DerSimonian-Laird method) was utilized to determine the pooled OR if the P value of Cochran’s Q test was < 0.1 and I2 was > 50%; otherwise, a fixed-effects model was utilized to analyze the pooled data. The Z test was conducted to assess the significance of the pooled OR (P < 0.05 was regarded as statistically significant). For studies [21–23, 28, 29] that reported ORs and 95% CIs only by sex, we first computed the pooled ORs and 95% CIs between sexes and put the pooled ORs and 95% CIs into the final meta-analysis between studies using the methods mentioned above. To examine the potential sources of heterogeneity, we carried out subgroup analysis and meta-regression analysis. Moreover, sensitivity analyses, including a leave-one-out procedure and the application of a random-effects model (Hunter-Schmidt method), were conducted to examine the robustness of the results. In addition, an assessment of publication bias was conducted utilizing funnel plots, Egger’s test, and Begg’s test.
Results
Characteristics of the included studies
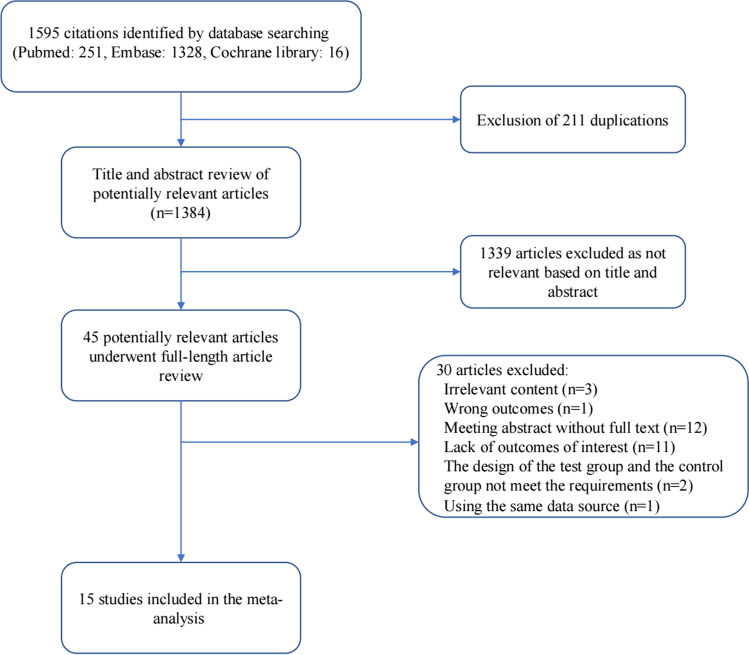
We searched a total of 1595 articles based on the retrieval strategy mentioned above. We excluded 211 duplicates and eliminated another 1339 articles based on relevance after reviewing the titles and abstracts. Then, we reviewed the remaining 45 articles in full-length form. Twelve articles were excluded because they were meeting abstracts without full-length articles available, eleven articles were excluded because they did not address the outcomes of interest, and seven studies were excluded on the basis of other exclusion criteria. Ultimately, 15 studies referring to 261,554 participants were included in the present meta-analysis (Fig. 1). They included subjects from five countries (four from Japan, four from Korea, four from China, two from the USA, and one from Iran). Ten studies directly reported the outcomes of the overall population, whereas five studies only reported the outcomes by sex, so we had to preprocess the data between sexes by the method mentioned above. In addition, three of the 15 studies used the latest nomenclature, i.e., MAFLD. Sleep duration was evaluated by a self-administered questionnaire in all studies. Fatty liver disease was assessed by direct ultrasound or CT examination in 12 studies, whereas it was evaluated indirectly by a composite index in 3 studies. Moreover, all studies reported confounder adjustment and adjusted effect estimates with 95% CIs (Table 1).
Fig. 1.
Flow chart of meta-analysis for exclusion/inclusion of individual articles
Table 1.
Main characteristics of the studies included in this meta-analysis of the association between short sleep duration and NAFLD/MAFLD
| Country | Study design | Sample size | Sex, female | Age (years) | Sleep duration category (h) | Measurement of sleep duration | Definition of NAFLD/MAFLD | Measurement of NAFLD/MAFLD | Confounder adjustment | Quality assessment (Newcastle–Ottawa Scale) | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Kim et al. 2013 [21] | Korean | Cross-sectional | 45,293 | 42.4% | 39.7 ± 5.9 |
Short = ≤ 5 Reference = > 7 |
Self-administered questionnaire |
Fatty liver detected by ultrasonography in the absence of any known causes of chronic liver disease or alcohol intake ≥ 20 g/day |
Liver ultrasound | Age, smoking status, alcohol intake, physical activity, SBP, education level, marital status, presence of job, sleep apnea, loud snoring, and BMI |
Selection: 3 Comparability: 2 Outcome: 3 |
| Imaizumi et al. 2015 [28] | Japan | Cross-sectional | 2172 | 66.3% | 60.9 ± 12.7 |
Short = ≤ 6 Reference = 6–7 |
Self-administered questionnaire |
Fatty liver detected by ultrasonography in the absence of other causes of chronic liver disease or alcoholic consumption ≥ 20 g/day |
Liver ultrasound | Age, smoking status, no breakfast, snacking, regular exercise, and BMI |
Selection: 4 Comparability: 2 Outcome: 3 |
| Miyake et al. 2015 [23] | Japan | Cohort | 2429 | 72.5% | 40.4 ± SD* |
Short = ≤ 6 Reference = 7–8 |
Self-administered questionnaire |
Fatty liver detected by ultrasonography in the absence of other causes of chronic liver disease or alcoholic misuse |
Liver ultrasound | Age, BMI, SBP, TG, HDL-C, FPG, UA, ALT, Cre, snacking habit, and periodic exercise habit |
Selection: 4 Comparability: 2 Outcome: 2 |
| Yu et al. 2015 [30] | Korean | Cross-sectional | 335 | 49.9% | 54.8 ± SD* |
Short = < 5 Reference = ≥ 5 |
Self-administered questionnaire |
Fatty liver detected by abdominal CT in the absence of other causes of chronic liver disease or alcoholic consumption ≥ 140 g/week |
Abdominal CT | Age, sex, exercise, alcohol, smoking, DM, HTN, CVD, and BMI |
Selection: 2 Comparability: 2 Outcome: 2 |
| Liu et al. 2016 [31] | China | Cohort | 8965 | 56.6% | 61.6 ± 7.8 |
Short = < 6 Reference = 7–8 |
Self-administered questionnaire |
Imaging or histologic evidence of hepatic steatosis, and no other cause for secondary hepatic fat accumulation |
Liver ultrasound |
Age, sex, education, marriage, smoking, alcohol consumption, physical activity, diabetes, sleep quality, napping, and BMI |
Selection: 4 Comparability: 2 Outcome: 3 |
| Peng et al. 2017 [32] | China | Cross-sectional | 8559 | 63.7% | 58.5 ± 9.0 |
Short = ≤ 6 Reference = > 9 |
Self-administered questionnaire |
Fatty liver detected by ultrasonography in the absence of other causes of chronic liver disease or alcoholic consumption ≥ 140 g/week for men or ≥ 70 g/week for women |
Liver ultrasound | Age, sex, current smoker, current drinker, physical activity, marital status, habitation status, work status, ALT, HDL-C, LDL-C, TC, HTN, DM, and BMI |
Selection: 4 Comparability: 2 Outcome: 3 |
| Kim et al. 2018 [33] | USA | Cross-sectional | 17,245 | 51.3% | 45.7 ± SD* |
Short = ≤ 5 Reference = ≥ 9 |
Self-administered questionnaire |
NAFLD is defined by US fatty liver index (USFLI) more than 30 in the absence of viral hepatitis or significant alcohol consumption |
US fatty liver index | Age, sex, ethnicity, education level, marital status, economic status, BMI, waist circumference, smoking, diabetes, HTN, and TC |
Selection: 3 Comparability: 2 Outcome: 2 |
| Kim et al. 2019 [25] | Korean | Cohort | 5427 | 51.8% | 50.8 ± SD* |
Short = < 6 Reference = 7–8 |
Self-administered questionnaire | NAFLD is defined by fatty liver index (FLI) more than 60 in the absence of hepatitis history, positive serologic markers for hepatitis B and C or significant alcohol consumption | Fatty liver index | Age, sex, BMI, SBP, DBP, TG, HDL, FDG, physical activity, smoking, daytime napping, and night-time shifting |
Selection: 3 Comparability: 2 Outcome: 2 |
| Okamura et al. 2019 [22] | Japan | Cohort | 12,306 | 52.5% | 42.0 ± SD* |
Short = ≤ 5 Reference = > 7 |
Self-administered questionnaire |
Fatty liver detected by ultrasonography in the absence of viral hepatitis or alcoholic consumption ≥ 140 g/week for men or ≥ 70 g/week for women |
Liver ultrasound | Age, BMI, ALT, TG, HDL-C, SBP, exercise habit, alcohol consumption, smoking, and FPG |
Selection: 4 Comparability: 2 Outcome: 2 |
| Takahashi et al. 2020 [29] | Japan | Cross-sectional | 4828 | 61.4% | 56.2 ± SD* |
Short = < 5 Reference = > 7 |
Self-administered questionnaire |
Fatty liver detected by ultrasonography in the absence of viral hepatitis or alcoholic consumption ≥ 30 g/day for men or ≥ 20 g/day for women |
Liver ultrasound | Age, smoking habits, physical activity, and BMI |
Selection: 3 Comparability: 2 Outcome: 3 |
| Hu et al. 2020 [34] | China | Case–control | 1960 | 72.5% | 59.4 ± SD* |
Short = < 8 Reference = ≥ 8 |
Self-administered questionnaire |
Fatty liver detected by ultrasonography in the absence of other causes of chronic liver disease or alcoholic consumption ≥ 140 g/week for men or ≥ 70 g/week for women |
Liver ultrasound | Age, sex, smoking, drinking, education. Physical activity, work status, SBP, TG, ALT, BMI, bedtime, daytime napping status, sleep quality, and sleep medication use |
Selection: 3 Comparability: 2 Outcome: 2 |
| Weng et al. 2021 [35] | USA | Cross-sectional | 1786 | 50.7% | 50.0 ± 17.9 |
Short = < 6 Reference = ≥ 6 |
Self-administered questionnaire |
MAFLD was diagnosed based on the evidence of hepatic steatosis and any of the following three conditions: over weight/obesity, diabetes mellitus, or metabolic dysfunction |
Fibroscan | Age, gender, race, diabetes, HTN, waist circumstance, overweight, TG, uric acid, HDL-C, and circadian misalignment |
Selection: 4 Comparability: 2 Outcome: 3 |
| Um et al. 2021 [20] | Korean | Cohort | 143,306 | 61.5% | 36.6 ± 6.6 |
Short = ≤ 5 Reference = 7 |
Self-administered questionnaire |
Fatty liver detected by ultrasonography in the absence of other causes of chronic liver disease or alcoholic consumption ≥ 30 g/day for men or ≥ 20 g/day for women |
Liver ultrasound | Age, sex, center, year of screening examination, season, alcohol consumption, smoking, physical activity, total energy intake, marital status, education level, depression, diabetes, HTN, BMI, and waist circumference |
Selection: 4 Comparability: 2 Outcome: 2 |
| Taheri et al. 2021 [36] | Iran | Case–control | 1932 | 60.9% | 49.2 ± 8.8 |
Short = < 5 Reference = > 7 |
Self-administered questionnaire | MAFLD was defined as having a fatty liver index (FLI) ≥ 60 plus at least one of the following: being overweight or obese, having a T2DM diagnosis or having any evidence of metabolic dysregulation, and in the absence of other causes of chronic liver disease or alcoholic misuse | Fatty liver index | Age, sex, educational level, marital status, socioeconomic status, and total energy intake |
Selection: 3 Comparability: 2 Outcome: 2 |
| Yang et al. 2022 [24] | China | Cross-sectional | 5011 | 60.0% | 63.6 ± 10.8 |
Short = < 7 Reference = 7–8 |
Self-administered questionnaire | MAFLD was diagnosed based on ultrasound examination of hepatic steatosis and the presence of any one of the following three criteria: overweight/obesity, presence of diabetes mellitus, or evidence of metabolic dysregulation | Liver ultrasound | Age, sex, drinking, smoking, sedentary time, diet diversity, education level, medication, HTN, diabetes, and obesity |
Selection: 4 Comparability: 2 Outcome: 3 |
Meta-analysis results
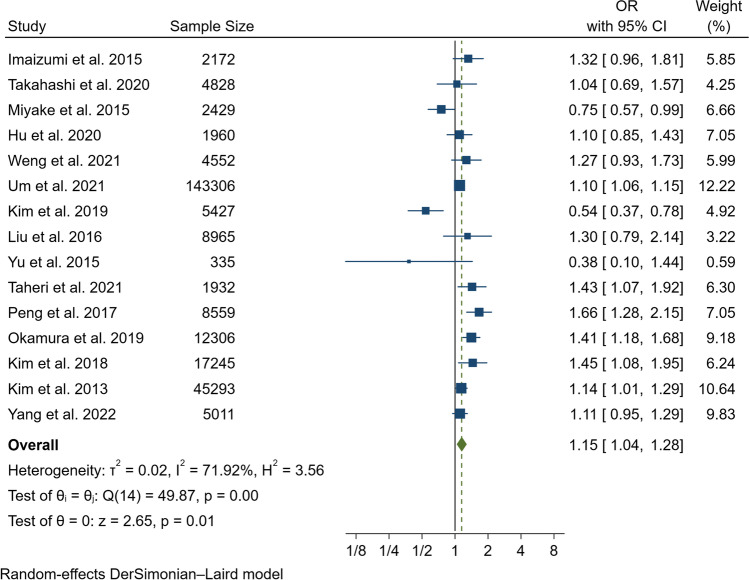
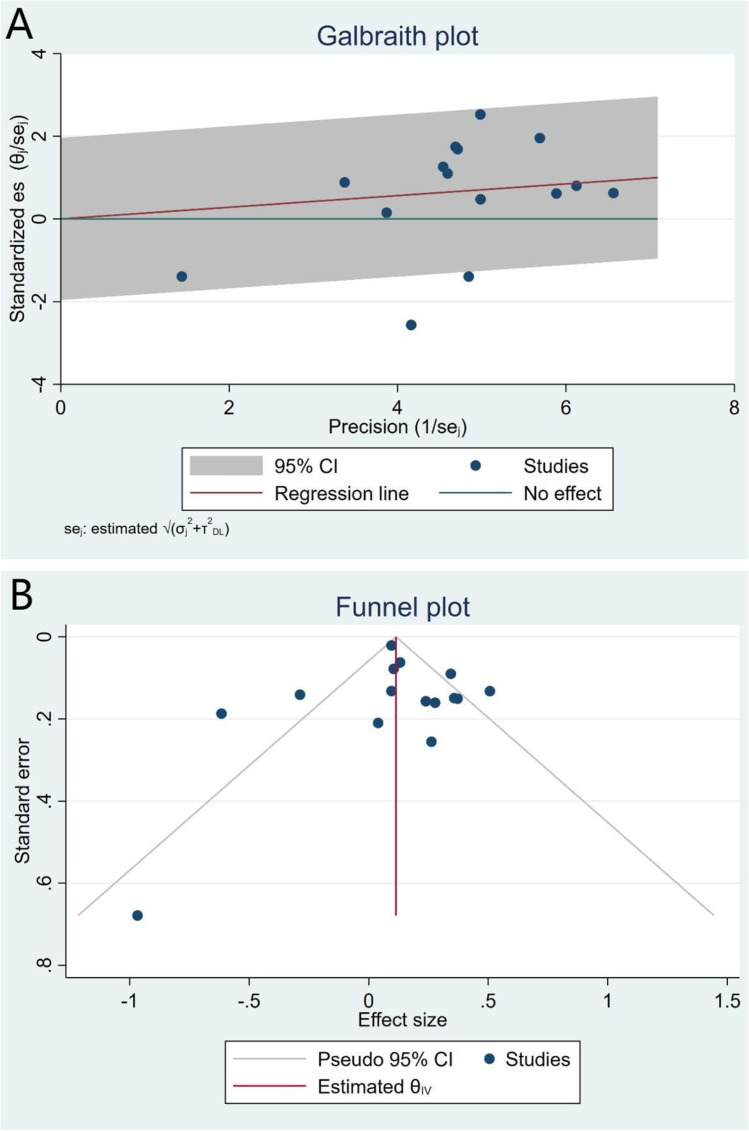
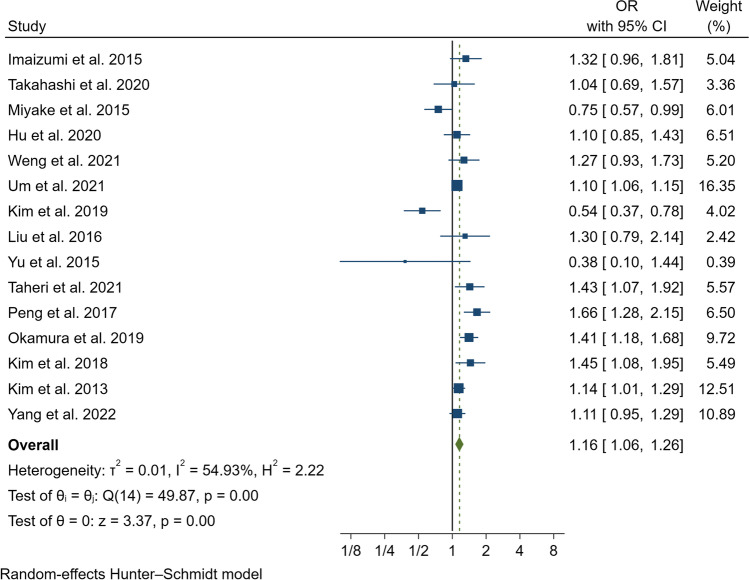
In our pooled analysis, inadequate sleep duration was closely related to an increased risk of NAFLD/MAFLD (OR, 1.15; 95% CI, 1.04–1.28; P = 0.01) with a moderate degree of heterogeneity between studies (I2 = 71.92%, Q = 49.87, P < 0.01) (Fig. 2 and Fig. 3A). The funnel plot was almost symmetrical, and the outcomes of Egger’s test (P = 0.20) and Begg’s test (P = 0.55) indicated no significant publication bias in our meta-analysis (Fig. 3B). In addition, we conducted a subgroup analysis stratified by study type, data preprocessing between genders, nomenclature of fatty liver disease, diagnostic method for fatty liver disease, and definition of short sleep duration. The results of the subgroup analysis did not reveal the source of heterogeneity (Table 2). Therefore, a meta-regression analysis was conducted to determine the possible source of heterogeneity; the covariates included race, mean age, study design, sample size, whether data were preprocessed, definition of inadequate sleep time, method of determining fatty liver disease, nomenclature (MAFLD or NAFLD), and study quality. However, we did not find a potential source of heterogeneity (Supplementary Table 1). In addition, we carried out sensitivity analysis, including excluding one study at a time and using the Hunter-Schmidt model (Figs. 4 and 5). The outcomes of the sensitivity analysis suggested that the primary outcome was robust.
Fig. 2.
Forest plot of the risk of NAFLD/MAFLD associated with short duration of sleep compared to the reference group
Fig. 3.
Galbraith plot (A) and funnel plot (B)
Table 2.
Subgroup analyses to explore source of heterogeneity
| Subgroups | Number of studies | OR (95% CI) | P for heterogeneity |
|---|---|---|---|
| Type of studies | |||
| Cross-sectional | 8 | 1.24 (1.09–1.41) | P = 0.24 |
| Cohort | 5 | 0.98 (0.76–1.26) | |
| Case–control | 2 | 1.24 (0.96–1.61) | |
| Data preprocessing between genders | |||
| Yes | 5 | 1.12 (0.92–1.37) | P = 0.78 |
| No | 10 | 1.16 (1.01–1.34) | |
| Nomenclature of fatty liver disease | |||
| NAFLD | 12 | 1.12 (0.99–1.28) | P = 0.48 |
| MAFLD | 3 | 1.21 (1.04–1.40) | |
| Diagnostic method of fatty liver disease | |||
| Ultrasound or CT imaging | 12 | 1.16 (1.06–1.28) | P = 0.73 |
| Indirect estimation by composite index | 3 | 1.05 (0.58–1.89) | |
| Definition of short sleep duration | |||
| Short sleep defined as less than 5 h | 7 | 1.21 (1.08–1.36) | P = 0.53 |
| Short sleep defined as less than 6 h | 6 | 1.07 (0.76–1.51) | |
| Short sleep defined as less than 7/8 h | 2 | 1.11 (0.97–1.26) | |
Fig. 4.
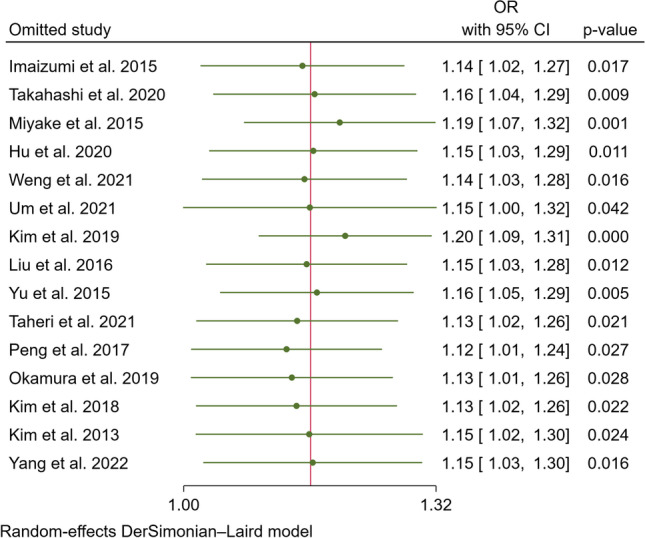
Sensitivity analysis of leaving one out
Fig. 5.
Sensitivity analysis of using Hunter-Schmidt model
Discussion
The present study supplied a comprehensive review of published articles and a quantitative estimation of the correlation between inadequate sleep time and the risk of NAFLD/MAFLD. The findings indicated that inadequate sleep time was closely related to an elevated risk of NAFLD/MAFLD (OR, 1.15; 95% CI, 1.04–1.28; P = 0.01), with a moderate degree of heterogeneity (I2 = 71.92%, Q = 49.87, P < 0.01). There was no significant publication bias, and there was a high level of statistical power owing to the inclusion of over 261,554 participants and the extraction of covariate-adjusted ORs with 95% CIs from every study. In addition, sensitivity analysis showed the robustness of the primary outcome.
It is known that adequate sleep duration and regular circadian rhythm are vital to human health. In recent years, long work duration, shift work, academic pressure, chaos caused by war, and even the COVID-19 pandemic have all led to inadequate sleep duration worldwide. The incidence of inadequate sleep duration is nearly 35% in US juveniles and up to 40% in adults working in healthcare support occupations [6, 37]. Many studies have indicated that inadequate sleep duration could increase the risk of multiple metabolic and immune disorders, including obesity 38, IR 39, type 2 diabetes mellitus40, hypertension [41], hyperlipidemia [44], and elevated C-reactive protein (CRP) and uric acid [45]. Most patients diagnosed with NAFLD have metabolic disorders, with obesity being the most common disorder. Additionally, MAFLD is diagnosed in individuals with liver steatosis and metabolic disorders. Therefore, it is not surprising that short sleep time correlates with the risk of NAFLD/MAFLD. A previous meta-analysis [46] including six studies involving 59,040 participants concluded that short sleep time significantly increased the risk of NAFLD (pooled RR, 1.19; 95% CI, 1.04–1.36; I2 = 0%). Moreover, another meta-analysis [47] assessing the association between insomnia and NAFLD suggested that insomnia was borderline relevant to a higher risk of NAFLD (pooled OR, 1.13; 95% CI, 1.00–1.27; I2 = 62%). Taheri et al. [36] conducted a nested case–control study including 1932 adult subjects, of whom 968 were diagnosed with MAFLD and 964 were controls. The outcomes showed that inadequate sleep time correlated with a 43% increase in the risk of MAFLD. Okamura et al. [22] carried out a historical cohort study involving 12,306 subjects in Japan. Sleep duration was evaluated by a self-administered questionnaire. The primary endpoint was defined as having developed NAFLD at follow-up. The outcomes suggested that short sleep time was closely related to an increased risk of NAFLD. The underlying biological mechanism of the association between inadequate sleep and NAFLD/MAFLD remains unclear. Underlying biological mechanisms may include the following concepts. First, inadequate sleep can activate brain networks related to reward [17], upregulate the ghrelin/leptin ratio [48], and decrease cognitive control and activity in the cerebral cortex [49], which results in food overconsumption, positive energy balance, and obesity. Inadequate sleep time can accelerate the development of insulin resistance by activating sympathetic activity and increasing free fatty acids [50–52]. Obesity and insulin resistance can lead to NAFLD via endoplasmic reticulum stress, mitochondrial defects, and oxidative stress [53, 54]. Specifically, lipotoxicity derived from the excessive release of free fatty acids (FFAs) from disordered and insulin-resistant adipose cells can cause inflammatory activation, hepatic cellular dysfunction, and lipoapoptosis. Second, inadequate sleep can increase the production of proinflammatory factors, such as tumor necrosis factor-α, interleukin-6, and CRP [55]. Elevated inflammatory factors result in superfluous accumulation of lipids in the liver, liver inflammation, and liver fibrosis [56]. Third, the gut microbiota is a potential mediator between short sleep duration and NAFLD/MAFLD [57, 42]. Inadequate sleep duration can lead to gut dysbiosis by activation of the hypothalamic–pituitary–adrenal axis, affecting the natural oscillations of intestinal microbiota and reducing the multiplicity and richness of intestinal microbiota. Gut endothelial barrier dysfunction can result in hepatic inflammation via bacterial translocation. In addition, a variety of metabolic products originating in the gut microbiota may increase NAFLD susceptibility.
This study has limitations that need to be acknowledged. First, all studies included in the meta-analysis used a self-administered questionnaire to evaluate sleep duration, which is somewhat subjective. Nevertheless, some studies have suggested that self-reported sleep time is consistent with parameters obtained by actigraphic monitoring [43]. Second, the definitions of short sleep duration and confounding variables adjusted were different across the studies, which may contribute to the heterogeneity. Third, there was moderate heterogeneity between studies (I2 = 71.92%, Q = 49.87, P < 0.01), and the subgroup analysis and meta-regression did not determine the source of heterogeneity, which suggested that unknown or unmeasured confounding factors might contribute to heterogeneity. Fourth, only Asian and American populations were included in this meta-analysis, which restricts the generalizability of the results to other populations, such as those in Africa or Europe.
Conclusion
This meta-analysis shows that inadequate sleep time is significantly related to an increased risk of NAFLD/MAFLD. Considering the low response rate to pharmacotherapies, obtaining an adequate amount of sleep may be useful for preventing NAFLD/MAFLD.
Supplementary Information
Below is the link to the electronic supplementary material.
Author contribution
JY and KZ conceived the study aims and design, contributed to the systematic review and data extraction, performed the analysis, interpreted the results, and drafted the manuscript. XZ W, YM, and CL S contributed to the data extraction and interpretation of results. WY W and YD T contributed to the study design and the revision of the manuscript.
Funding
This work was supported by National Natural Science Foundation of China (81900272, 81825003, 91957123), National Key R&D Program of China (2020YFC2004705), Research Unit of Medical Science Research Management/Basic and Clinical Research of Metabolic Cardiovascular Diseases from Chinese Academy of Medical Sciences (2021RU003), and Beijing Nova Program from Beijing Municipal Science & Technology Commission (Z201100006820002) funding. The sponsor had no role in the design or conduct of this research.
Data availability
All data generated or analyzed during this study are included in this published article (and its supplementary information files).
Declarations
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Conflict of interest
The authors declare no competing interests.
Footnotes
Jie Yang and Kuo Zhang contributed to the work equally and should be regarded as co-first authors.
Wenyao Wang and Yi-Da Tang contributed to the work equally and should be regarded as co-corresponding authors.
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Jie Yang, Email: yangjie@fuwaihospital.org.
Kuo Zhang, Email: kzhang23@126.com.
Ziwei Xi, Email: xixixiziwei@qq.com.
Yue Ma, Email: 15811184986@139.com.
Chunli Shao, Email: chunlishao@126.com.
Wenyao Wang, Email: wwypumc@126.com.
Yi-Da Tang, Email: tangyida@bjmu.edu.cn.
References
- 1.Younossi Z, Anstee QM, Marietti M, Hardy T, Henry L, Eslam M, George J, Bugianesi E. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. 2018;15(1):11–20. doi: 10.1038/nrgastro.2017.109. [DOI] [PubMed] [Google Scholar]
- 2.Zhou F, Zhou J, Wang W, Zhang XJ, Ji YX, Zhang P, She ZG, Zhu L, Cai J, Li H. Unexpected rapid increase in the burden of NAFLD in China from 2008 to 2018: a systematic review and meta-analysis. Hepatology. 2019;70(4):1119–1133. doi: 10.1002/hep.30702. [DOI] [PubMed] [Google Scholar]
- 3.Friedman SL, Neuschwander-Tetri BA, Rinella M, Sanyal AJ. Mechanisms of NAFLD development and therapeutic strategies. Nat Med. 2018;24(7):908–922. doi: 10.1038/s41591-018-0104-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Younossi Z, Tacke F, Arrese M, Chander Sharma B, Mostafa I, Bugianesi E, Wai-Sun Wong V, Yilmaz Y, George J, Fan J, Vos MB. Global perspectives on nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Hepatology. 2019;69(6):2672–2682. doi: 10.1002/hep.30251. [DOI] [PubMed] [Google Scholar]
- 5.Eslam M, Sanyal AJ, George J. MAFLD: a consensus-driven proposed nomenclature for metabolic associated fatty liver disease. Gastroenterology. 2020;158(7):1999–2014.e1991. doi: 10.1053/j.gastro.2019.11.312. [DOI] [PubMed] [Google Scholar]
- 6.Wheaton AG, Claussen AH. Short sleep duration among infants, children, and adolescents aged 4 months-17 years - United States, 2016–2018. MMWR Morb Mortal Wkly Rep. 2021;70(38):1315–1321. doi: 10.15585/mmwr.mm7038a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lu L, Wang SB, Rao WW, Ungvari GS, Ng CH, Chiu HFK, Zhang J, Kou C, Jia FJ, Xiang YT. Sleep duration and patterns in Chinese older adults: a comprehensive meta-analysis. Int J Biol Sci. 2017;13(6):682–689. doi: 10.7150/ijbs.19695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Galan-Lopez P, Domínguez R, Gísladóttir T, Sánchez-Oliver AJ, Pihu M, Ries F, Klonizakis M (2021) Sleep quality and duration in European adolescents (The AdolesHealth Study): a cross-sectional, quantitative study. Children (Basel) 8(3):188 [DOI] [PMC free article] [PubMed]
- 9.Zhou SJ, Wang LL, Yang R, Yang XJ, Zhang LG, Guo ZC, Chen JC, Wang JQ, Chen JX. Sleep problems among Chinese adolescents and young adults during the coronavirus-2019 pandemic. Sleep Med. 2020;74:39–47. doi: 10.1016/j.sleep.2020.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu Y, Wheaton AG, Chapman DP, Cunningham TJ, Lu H, Croft JB. Prevalence of healthy sleep duration among adults–United States, 2014. MMWR Morb Mortal Wkly Rep. 2016;65(6):137–141. doi: 10.15585/mmwr.mm6506a1. [DOI] [PubMed] [Google Scholar]
- 11.Tobaldini E, Fiorelli EM, Solbiati M, Costantino G, Nobili L, Montano N. Short sleep duration and cardiometabolic risk: from pathophysiology to clinical evidence. Nat Rev Cardiol. 2019;16(4):213–224. doi: 10.1038/s41569-018-0109-6. [DOI] [PubMed] [Google Scholar]
- 12.Spiegel K, Tasali E, Leproult R, Van Cauter E. Effects of poor and short sleep on glucose metabolism and obesity risk. Nat Rev Endocrinol. 2009;5(5):253–261. doi: 10.1038/nrendo.2009.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Besedovsky L, Lange T, Haack M. The sleep-immune crosstalk in health and disease. Physiol Rev. 2019;99(3):1325–1380. doi: 10.1152/physrev.00010.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cappuccio FP, D’Elia L, Strazzullo P, Miller MA. Sleep duration and all-cause mortality: a systematic review and meta-analysis of prospective studies. Sleep. 2010;33(5):585–592. doi: 10.1093/sleep/33.5.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Daghlas I, Dashti HS, Lane J, Aragam KG, Rutter MK, Saxena R, Vetter C. Sleep duration and myocardial infarction. J Am Coll Cardiol. 2019;74(10):1304–1314. doi: 10.1016/j.jacc.2019.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Itani O, Kaneita Y, Murata A, Yokoyama E, Ohida T. Association of onset of obesity with sleep duration and shift work among Japanese adults. Sleep Med. 2011;12(4):341–345. doi: 10.1016/j.sleep.2010.09.007. [DOI] [PubMed] [Google Scholar]
- 17.Antza C, Kostopoulos G, Mostafa S, Nirantharakumar K, Tahrani A. The links between sleep duration, obesity and type 2 diabetes mellitus. J Endocrinol. 2021;252(2):125–141. doi: 10.1530/JOE-21-0155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cappuccio FP, Stranges S, Kandala NB, Miller MA, Taggart FM, Kumari M, Ferrie JE, Shipley MJ, Brunner EJ, Marmot MG. Gender-specific associations of short sleep duration with prevalent and incident hypertension: the Whitehall II Study. Hypertension. 2007;50(4):693–700. doi: 10.1161/HYPERTENSIONAHA.107.095471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hu Z, Song X, Hu K, Ruan Y, Zeng F. Association between sleep duration and asthma in different weight statuses (CHNS 2009–2015) Sleep Breath. 2021;25(1):493–502. doi: 10.1007/s11325-020-02081-6. [DOI] [PubMed] [Google Scholar]
- 20.Um YJ, Chang Y, Jung HS, Cho IY, Shin JH, Shin H, Wild SH, Byrne CD, Ryu S. Sleep duration, sleep quality, and the development of nonalcoholic fatty liver disease: a cohort study. Clin Transl Gastroenterol. 2021;12(10):e00417. doi: 10.14309/ctg.0000000000000417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim CW, Yun KE, Jung HS, Chang Y, Choi ES, Kwon MJ, Lee EH, Woo EJ, Kim NH, Shin H, Ryu S. Sleep duration and quality in relation to non-alcoholic fatty liver disease in middle-aged workers and their spouses. J Hepatol. 2013;59(2):351–357. doi: 10.1016/j.jhep.2013.03.035. [DOI] [PubMed] [Google Scholar]
- 22.Okamura T, Hashimoto Y, Hamaguchi M, Obora A, Kojima T, Fukui M. Short sleep duration is a risk of incident nonalcoholic fatty liver disease: a population-based longitudinal study. J Gastrointestin Liver Dis. 2019;28(1):73–81. doi: 10.15403/jgld.2014.1121.281.alc. [DOI] [PubMed] [Google Scholar]
- 23.Miyake T, Kumagi T, Furukawa S, Hirooka M, Kawasaki K, Koizumi M, Todo Y, Yamamoto S, Tokumoto Y, Ikeda Y, Abe M, Kitai K, Matsuura B, Hiasa Y. Short sleep duration reduces the risk of nonalcoholic fatty liver disease onset in men: a community-based longitudinal cohort study. J Gastroenterol. 2015;50(5):583–589. doi: 10.1007/s00535-014-0989-0. [DOI] [PubMed] [Google Scholar]
- 24.Yang J, Luo S, Li R, Ju J, Zhang Z, Shen J, Sun M, Fan J, Xia M, Zhu W, Liu Y (2022) Sleep factors in relation to metabolic-dysfunction associated fatty liver disease in middle-aged and elderly Chinese. J Clin Endocrinol Metab 107(10):2874–2882 [DOI] [PubMed]
- 25.Kim JH, Jung DH, Kwon YJ, Lee JI, Shim JY. The impact of the sleep duration on NAFLD score in Korean middle-aged adults: a community-based cohort study. Sleep Med. 2019;57:144–150. doi: 10.1016/j.sleep.2019.02.012. [DOI] [PubMed] [Google Scholar]
- 26.Stang A. Critical evaluation of the Newcastle-Ottawa Scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25(9):603–605. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 27.Herzog R, Álvarez-Pasquin MJ, Díaz C, Del Barrio JL, Estrada JM, Gil Á. Are healthcare workers’ intentions to vaccinate related to their knowledge, beliefs and attitudes? A systematic review. BMC Public Health. 2013;13:154. doi: 10.1186/1471-2458-13-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Imaizumi H, Takahashi A, Tanji N, Abe K, Sato Y, Anzai Y, Watanabe H, Ohira H. The association between sleep duration and non-alcoholic fatty liver disease among Japanese men and women. Obes Facts. 2015;8(4):234–242. doi: 10.1159/000436997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Takahashi A, Anzai Y, Kuroda M, Kokubun M, Kondo Y, Ogata T, Fujita M, Hayashi M, Imaizumi H, Abe K, Tanji N, Ohira H. Effects of sleep quality on non-alcoholic fatty liver disease: a cross-sectional survey. BMJ Open. 2020;10(10):e039947. doi: 10.1136/bmjopen-2020-039947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Matenchuk BA, Mandhane PJ, Kozyrskyj AL. Sleep, circadian rhythm, and gut microbiota. Sleep Med Rev. 2020;53:101340. doi: 10.1016/j.smrv.2020.101340. [DOI] [PubMed] [Google Scholar]
- 31.Lockley SW, Skene DJ, Arendt J. Comparison between subjective and actigraphic measurement of sleep and sleep rhythms. J Sleep Res. 1999;8(3):175–183. doi: 10.1046/j.1365-2869.1999.00155.x. [DOI] [PubMed] [Google Scholar]
- 32.Khubchandani J, Price JH. Short sleep duration in working American Adults, 2010–2018. J Community Health. 2020;45(2):219–227. doi: 10.1007/s10900-019-00731-9. [DOI] [PubMed] [Google Scholar]
- 33.Kim D, Kim HJ, Kushida CA, Heo NY, Ahmed A, Kim WR (2018) Short Sleep duration is associated with abnormal serum aminotransferase activities and nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol 16(4):588–590 [DOI] [PubMed]
- 34.Reutrakul S, Van Cauter E. Sleep influences on obesity, insulin resistance, and risk of type 2 diabetes. Metabolism. 2018;84:56–66. doi: 10.1016/j.metabol.2018.02.010. [DOI] [PubMed] [Google Scholar]
- 35.Larcher S, Benhamou PY, Pépin JL, Borel AL. Sleep habits and diabetes. Diabetes Metab. 2015;41(4):263–271. doi: 10.1016/j.diabet.2014.12.004. [DOI] [PubMed] [Google Scholar]
- 36.Gangwisch JE. A review of evidence for the link between sleep duration and hypertension. Am J Hypertens. 2014;27(10):1235–1242. doi: 10.1093/ajh/hpu071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Khubchandani J, Price JH. Short sleep duration in working american adults, 2010–2018. J Community Health. 2020;45(2):219–227. doi: 10.1007/s10900-019-00731-9. [DOI] [PubMed] [Google Scholar]
- 38.St-Onge M-P. Sleep-obesity relation: underlying mechanisms and consequences for treatment. Obes Rev. 2017;18:34–39. doi: 10.1111/obr.12499. [DOI] [PubMed] [Google Scholar]
- 39.Reutrakul S, Van Cauter E. Sleep influences on obesity, insulin resistance, and risk of type 2 diabetes. Metabolism. 2018;84:56–66. doi: 10.1016/j.metabol.2018.02.010. [DOI] [PubMed] [Google Scholar]
- 40.Larcher S, Benhamou P-Y, Pépin J-L, Borel A-L. Sleep habits and diabetes. Diabetes & Metabolism. 2015;41(4):263–271. doi: 10.1016/j.diabet.2014.12.004. [DOI] [PubMed] [Google Scholar]
- 41.Gangwisch JE. A Review of Evidence for the Link Between Sleep Duration and Hypertension. American Journal of Hypertension. 2014;27(10):1235–1242. doi: 10.1093/ajh/hpu071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Matenchuk BA, Mandhane PJ, Kozyrskyj AL. Sleep, circadian rhythm, and gut microbiota. Sleep Medicine Reviews. 2020;53:101340. doi: 10.1016/j.smrv.2020.101340. [DOI] [PubMed] [Google Scholar]
- 43.Lockley SW, Skene DJ, Arendt J. Comparison between subjective and actigraphic measurement of sleep and sleep rhythms. Journal of Sleep Research. 1999;8(3):175–183. doi: 10.1046/j.1365-2869.1999.00155.x. [DOI] [PubMed] [Google Scholar]
- 44.Sabanayagam C, Shankar A. Sleep duration and hypercholesterolaemia: results from the National Health Interview Survey 2008. Sleep Med. 2012;13(2):145–150. doi: 10.1016/j.sleep.2011.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee YC, Son DH, Kwon YJ (2020) U-shaped association between sleep duration, C-reactive protein, and uric acid in Korean women. Int J Environ Res Public Health 17(8):2657 [DOI] [PMC free article] [PubMed]
- 46.Wijarnpreecha K, Thongprayoon C, Panjawatanan P, Ungprasert P. Short sleep duration and risk of nonalcoholic fatty liver disease: a systematic review and meta-analysis. J Gastroenterol Hepatol. 2016;31(11):1802–1807. doi: 10.1111/jgh.13391. [DOI] [PubMed] [Google Scholar]
- 47.Wijarnpreecha K, Thongprayoon C, Panjawatanan P, Ungprasert P. Insomnia and risk of nonalcoholic fatty liver disease: a systematic review and meta-analysis. J Postgrad Med. 2017;63(4):226–231. doi: 10.4103/jpgm.JPGM_140_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Taheri S, Lin L, Austin D, Young T, Mignot E. Short sleep duration is associated with reduced leptin, elevated ghrelin, and increased body mass index. PLoS Med. 2004;1(3):e62. doi: 10.1371/journal.pmed.0010062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Greer SM, Goldstein AN, Walker MP. The impact of sleep deprivation on food desire in the human brain. Nat Commun. 2013;4:2259. doi: 10.1038/ncomms3259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Faber CL, Deem JD, Campos CA, Taborsky GJ, Jr, Morton GJ. CNS control of the endocrine pancreas. Diabetologia. 2020;63(10):2086–2094. doi: 10.1007/s00125-020-05204-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Broussard JL, Chapotot F, Abraham V, Day A, Delebecque F, Whitmore HR, Tasali E. Sleep restriction increases free fatty acids in healthy men. Diabetologia. 2015;58(4):791–798. doi: 10.1007/s00125-015-3500-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen X, Iqbal N, Boden G. The effects of free fatty acids on gluconeogenesis and glycogenolysis in normal subjects. J Clin Invest. 1999;103(3):365–372. doi: 10.1172/JCI5479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mota M, Banini BA, Cazanave SC, Sanyal AJ. Molecular mechanisms of lipotoxicity and glucotoxicity in nonalcoholic fatty liver disease. Metabolism. 2016;65(8):1049–1061. doi: 10.1016/j.metabol.2016.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Caviglia GP, Rosso C, Fagoonee S, Saracco GM, Pellicano R. Liver fibrosis: the 2017 state of art. Panminerva Med. 2017;59(4):320–331. doi: 10.23736/S0031-0808.17.03359-6. [DOI] [PubMed] [Google Scholar]
- 55.Simpson N, Dinges DF. Sleep and inflammation. Nutr Rev. 2007;65(12 Pt 2):S244–252. doi: 10.1301/nr.2007.dec.S244-S252. [DOI] [PubMed] [Google Scholar]
- 56.Luo Y, Lin H. Inflammation initiates a vicious cycle between obesity and nonalcoholic fatty liver disease. Immun Inflamm Dis. 2021;9(1):59–73. doi: 10.1002/iid3.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Safari Z, Gérard P. The links between the gut microbiome and non-alcoholic fatty liver disease (NAFLD) Cell Mol Life Sci. 2019;76(8):1541–1558. doi: 10.1007/s00018-019-03011-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this published article (and its supplementary information files).