Abstract
Interactions of oral streptococci and actinomyces with polymorphonuclear leukocytes (PMNs), mediated by sialic acid- and Gal/GalNAc-reactive adhesins, respectively, result in activation of the PMNs and thereby may contribute to the initiation of oral inflammation. Sialidase treatment of PMNs or HL-60 cells abolished adhesion of Streptococcus gordonii but was required for adhesion of Actinomyces naeslundii. The same effects of sialidase were noted for adhesion of these bacteria to a major 150-kDa surface glycoprotein of either PMNs or undifferentiated HL-60 cells and to a 130-kDa surface glycoprotein of differentiated HL-60 cells. These glycoproteins were both identified as leukosialin (CD43) by immunoprecipitation with a specific monoclonal antibody (MAb). Adhesion of streptococci and actinomyces to a 200-kDa minor PMN surface glycoprotein was also detected by bacterial overlay of untreated and sialidase-treated nitrocellulose transfers, respectively. This glycoprotein was identified as leukocyte common antigen (CD45) by immunoprecipitation with a specific MAb. CD43 and CD45 both possess extracellular mucinlike domains in addition to intracellular domains that are implicated in signal transduction. Consequently, the interactions of streptococci and actinomyces with the mucinlike domains of these mammalian cell surface glycoproteins result not only in adhesion but, in addition, may represent the initial step in PMN activation by these bacteria.
Host receptors for microbial adhesins are not necessarily limited to epithelial cell surfaces but may also be present on other cell types including polymorphonuclear leukocytes (PMNs). Adhesin-mediated encounters between bacteria and PMNs may evoke a series of inflammatory events that have beneficial as well as deleterious consequences (31). Thus, bacterial adhesion may stimulate the production of toxic oxygen intermediates and the release of PMN granule contents. Phagocytosis may ensue, and, in certain cases, the bacteria are killed. This latter response could be of major importance in the early phases of infection since it is initiated in the absence of opsonins. The participation of PMNs in host defense at localized sites within the oral cavity is evident from the severe breakdown of periodontal tissues accompanying impaired PMN function in pathological conditions such as neutropenia or leukemia (56).
Saccharide-specific adhesins are associated with a number of bacteria that colonize the human oral cavity. Thus, Streptococcus gordonii recognizes α2-3-linked sialic-acid termini of salivary mucins and glycoproteins (14, 29, 42, 52), and Actinomyces naeslundii interacts with both Galβ1-3GalNAc- and GalNAcβ1-3Gal-containing cell wall polysaccharides of streptococci that occur in dental plaque (11). The adhesins of these bacteria also interact with surface glycoconjugates of host cells (5, 42, 50) including PMNs, which are activated by encounters with either microorganism. Thus, the interaction of PMNs with S. gordonii stimulates the production of superoxide anions, the release of PMN granule contents, and bacterial phagocytosis (A. L. Sandberg, unpublished observations), and similar biological consequences accompany the interaction of PMNs with A. naeslundii (44). The interaction with A. naeslundii does, however, require prior exposure of the PMNs to sialidase (43), an enzyme produced by actinomyces (13).
Although the specificities of actinomyces and streptococcal adhesins have been defined, little is known about the PMN surface molecules that serve as receptors. In previous studies, in vitro phagocytosis and killing of A. naeslundii by PMNs were blocked by prior incubation of certain Gal/GalNAc-binding plant lectins with the mammalian cells (45). The same plant lectins reacted with a 130-kDa glycoprotein on sialidase-treated nitrocellulose transfers of PMN extracts separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). The present study was initiated to further characterize this glycoprotein, to determine if it also could serve as a receptor for the sialic-acid-reactive adhesin of S. gordonii DL1, and to detect the possible presence of additional PMN glycoproteins that are recognized by these bacterial adhesins.
MATERIALS AND METHODS
Mammalian cells.
The HL-60 promyelocytic leukemia cell line was obtained from the American Type Culture Collection, Rockville, Md., and maintained as a stationary suspension culture at 37°C with 5% CO2 in RPMI-1640 medium (BioWhittaker, Inc,. Walkersville, Md.) supplemented with 20% heat-inactivated fetal calf serum–2 mM l-glutamine–100 U of penicillin–100 μg of streptomycin per ml (Gibco BRL Products, Grand Island, N.Y.). Granulocytic differentiation was induced by culturing the cells at an initial density of 2 × 105 cells/ml for up to 7 days in the presence of 1.25% dimethyl sulfoxide (DMSO) (Sigma Chemical Co., St. Louis, Mo.) (12). PMNs were obtained by Ficoll-Hypaque (Histopaque, Sigma Chemical Co.) separation of human peripheral blood buffy coat cells obtained from the National Institutes of Health (NIH) blood bank. Erythrocytes were lysed with NH4Cl-lysing buffer (B & B Research Laboratories, Inc., Fiskeville, R. I.). Cell numbers and viability were determined by staining with trypan blue (Gibco BRL Products).
Bacteria.
A. naeslundii WVU45 and WVU45M as well as S. gordonii DL1 (Challis) and M5 have been described (10, 23, 52). All bacteria were grown in complex medium, washed three times with Hanks balanced salt solution (BioWhittaker, Inc.), and adjusted to approximately 2 × 109 bacteria per ml (turbidity, 260 Klett units).
Measurement of bacterial adhesion.
PMN or HL-60 cells were washed and resuspended in phosphate-buffered saline (PBS; B & B Research Laboratories, Inc.) containing 1% bovine serum albumin (BSA) and 0.1% sodium azide (Sigma Chemical Co.) (PBS-BSA). Where indicated, aliquots of cell suspension (107 cells/ml) were treated with 0.5 U of sialidase (neuraminidase, Type X from Clostridium perfringens; Sigma Chemical Co.) per ml for 30 min at room temperature. Bacteria (109/ml) were labeled by incubation with 100 μg of fluorescein-5-isothiocyanate (Molecular Probes, Inc., Eugene, Oreg.) in PBS for 30 min at 37°C. Labeled bacteria (25 μl of a 109/ml concentration) and mammalian cells (50 μl of a 107/ml concentration), both in PBS-BSA, were mixed in microtiter wells at room temperature for 30 min. The mammalian cells were washed three times with 150 μl of PBS-BSA to remove the majority of unbound bacteria and resuspended in 250 μl of PBS-BSA containing 5 μg of propidium iodide (Sigma Chemical Co.) per ml to exclude dead cells. Saccharide inhibitors including sialyllactose (N-acetylneuramin-lactose from bovine colostrum), glucuronic acid, lactose, or cellobiose (Sigma Chemical Co.) were added prior to the bacteria and were present throughout the analysis. Flow cytometry was performed with a FACS II (Becton Dickinson Immunocytometry Systems, San Jose, Calif.). The gate for forward-light scatter (equivalent to the size of the cells) was set to exclude detection of unbound bacteria.
Incubation mixtures containing bacteria and mammalian cells were also examined by photomicroscopy. Twenty microliters from the suspension used for FACS analysis was mixed with 150 μl of PBS-BSA and centrifuged in a Cytospin 1 (Shandon Inc., Pittsburgh, Pa.) at 1,000 rpm for 5 min. Slides were stained with Wright Giemsa (Diff-Quick Stain; Baxter Healthcare Corp., Miami, Fla.).
The expression of CR3, the receptor for the inactivated C3b fragment of the third component of complement, iC3b, was determined by incubation with 1 μg of phycoerythrin-conjugated mouse monoclonal antibody (MAb) against human CR3 (clone D12; Becton Dickinson Immunocytometry Systems) in 50 μl of PBS-BSA for 30 min at 4°C. The cells were washed and analyzed by flow cytometry.
Mammalian cell extracts and SDS-PAGE.
PMN or HL-60 cells (5 × 107) were incubated for 30 min in cold PBS containing 2 mM phenylmethylsulfonyl fluoride (PMSF) and 1 mM N-ethylmaleimide (Sigma Chemical Co.), pelleted, flash-frozen on dry ice-ethanol, and stored at −70°C prior to extraction. Pelleted cells were thawed and lysed at a concentration of 108/ml in cold PBS containing 0.2 M n-octylglucoside (Pierce, Rockford, Ill.), 10 μm pepstatin, 0.1 mM leupeptin, 10 μg of aprotinin (Boehringer Mannheim Corp., Indianapolis, Ind.) per ml, 2 mM PMSF, 1 mM N-ethylmaleimide, and 2 mM EDTA (Sigma Chemical Co.). Insoluble material was removed by centrifugation (10 min at 10,000 × g) at 4°C. Protein in supernatants was determined by bicinchoninic acid-protein assay (Pierce) according to the manufacturer's protocol for enhanced detection utilizing BSA as the standard. Cell lysates were subjected to SDS-PAGE on 8 to 16% gradient gels (Novex, San Diego, Calif.). Proteins and glycoproteins were transferred to nitrocellulose in 25 mM Tris–192 mM glycine–20% methanol (pH 8.3) at 6 V/cm (constant voltage) for 18 h at 4°C (54).
Labeling of cell surface glycoconjugates.
Washed PMNs or HL-60 cells at 108/ml were incubated for 30 min in PBS with either 1 mM sodium periodate (Sigma Chemical Co.) at 0°C or 10 mM sodium periodate at room temperature for oxidation of surface sialic acid or total carbohydrates, respectively. Washed cells were labeled for 1 h at room temperature with 30 μg of digoxigenin-3-O-succinyl-ɛ-aminocaproic acid hydrazide hydrochloride (Boehringer Mannheim Corp.) in PBS. Cell lysis, SDS-PAGE, and transfer of glycoproteins to nitrocellulose were performed as described above. Transfers were blocked with 20 mM Tris-HCl–150 mM NaCl (pH 7.6) (TBS) containing 2% nonfat dry milk (Carnation) (TBS-milk) for 1 h and subsequently incubated for 1 h with antidigoxigenin-alkaline phosphatase (Boehringer Mannheim) diluted 1:1,000 in TBS-milk. Transfers were washed three times with TBS and developed with 5-bromo-4-chloro-3-indolyl phosphate (BCIP) and nitroblue tetrazolium (NBT) obtained from Sigma Chemical Co.
Lectin blotting.
Untreated nitrocellulose transfers or transfers incubated with 0.1 U of sialidase in acetate buffer (pH 5.0), containing 10 mM CaCl2 for 2 h at 37°C, were blocked for 1 h at room temperature with TBS containing 2% polyvinyl alcohol (type II, low molecular weight; Sigma Chemical Co.), 0.1% Tween-20 (Bio-Rad Laboratories, Hercules, Calif.), 1 mM CaCl2, 1 mM MgCl2, and 1 mM MnCl2. Transfers were subsequently incubated for 1 h at room temperature with biotinylated Limax flavus agglutinin (LFA), biotinylated peanut agglutinin (PNA), or biotinylated Canavalia ensiformis agglutinin (ConA) (EY Laboratories, Inc., San Mateo, Calif.) at concentrations of 5 μg of LFA or PNA per ml or 50 μg of ConA per ml in blocking buffer. The blots were washed three times in TBS containing 0.1% Tween-20, incubated with 0.2 U of avidin-d-alkaline phosphatase (Vector Laboratories, Inc., Burlingame, Calif.) per ml in the same buffer for 30 min, washed, and developed with BCIP and NBT. Lanes containing fetuin, asialo-fetuin (Sigma Chemical Co.), and thyroglobulin (Boehringer Mannheim Corp.) were included as controls.
Bacterial overlay.
Bacteria at 2 × 109/ml in Hanks balanced salt solution (BioWhittaker, Inc.) were biotin labeled by incubation with sulfosuccinimidyl 6-(biotinamido) hexanoate (NHS-LC)-biotin (Pierce) at 100 μg/ml for 1 h at room temperature (42, 52). Labeling of A. naeslundii WVU45 was performed in the presence of 100 mM lactose. Sialidase-treated or untreated nitrocellulose transfers were blocked in TBS containing 5% BSA, 1 mM CaCl2, 1 mM MgCl2, and 0.02% sodium azide for 4 h at room temperature (36). Labeled bacteria were added to a final concentration of 5 × 108/ml in a total volume of 40 ml (about 1.5 ml of bacterial suspension per cm2 of nitrocellulose membrane). The overlays were incubated overnight at 4°C without mixing and washed four times at room temperature for 5 min on a rotary shaker with TBS containing 0.05% Tween-20, 1 mM CaCl2, 1 mM MgCl2, and 0.02% sodium azide. The blots were then incubated with 0.2 U of avidin-d-alkaline phosphatase (Pierce) per ml in the same buffer for 30 min, washed three times for 5 min, and developed with BCIP and NBT for 5 min.
Western blotting.
Nitrocellulose transfers were blocked in TBS-milk for 1 h and incubated for 1 h with 1 μg of anti-leukosialin MAb (anti-Leu-22, clone L60; Becton Dickinson Immunocytometry Systems) per ml or anti-CD45 MAb (clone HB 10AB2) per ml (22). The blots were washed with TBS, incubated 1 h with horseradish peroxidase-conjugated goat anti-mouse immunoglobulin G antiserum (Bio-Rad Laboratories) in TBS-milk, washed, and developed with 4-chloro-1-naphthol (Pierce).
Immunoprecipitation.
PMNs (107/ml) were surface labeled for 1 h at room temperature with 100 μg of NHS-LC-biotin per ml in PBS containing 2 mM PMSF and washed three times in cold buffer to remove excess label. Extracts of biotinylated or unlabeled cells (5 × 105/ml) were prepared as described above except that 0.5% Triton X-100 (Sigma Chemical Co.) was substituted for n-octylglucoside. All steps of the immunoprecipitation procedure (18) were performed at 4°C. Insoluble material was removed by centrifugation at 10,000 × g. The supernatants (1 ml) were precleared by incubation with 50 μl of dry protein G-Sepharose (GammaBind Plus Sepharose; Pharmacia Biotech, Inc., Piscataway, N.J.) for 1 h. The gel was removed by centrifugation, supernatants were incubated with 3 μg of either an antileukosialin (clone L60) or antileukocyte common antigen MAb (clone HB 10AB2) per ml followed by protein G-Sepharose (25 μl of a 1:1 slurry) to bind immune complexes. The gel was washed twice with 0.1% Triton X-100 in TBS, once with TBS, and once with 0.05 M Tris (pH 6.8) to remove unbound material prior to dissociation of immune complexes and SDS-PAGE.
RESULTS
Bacterial attachment to PMNs and HL-60 cells.
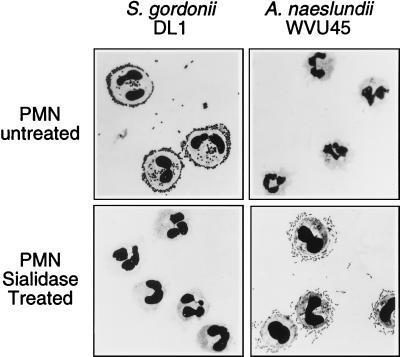
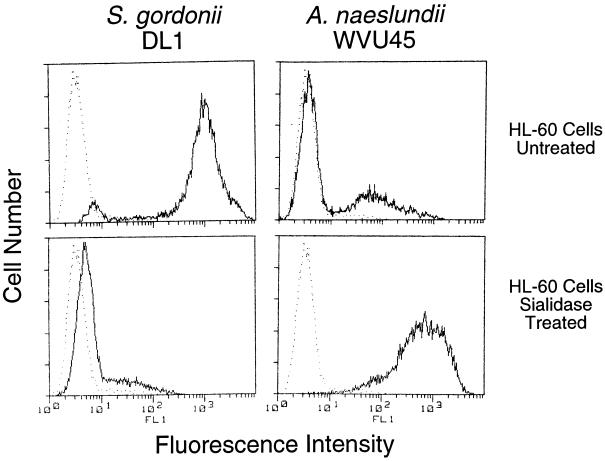
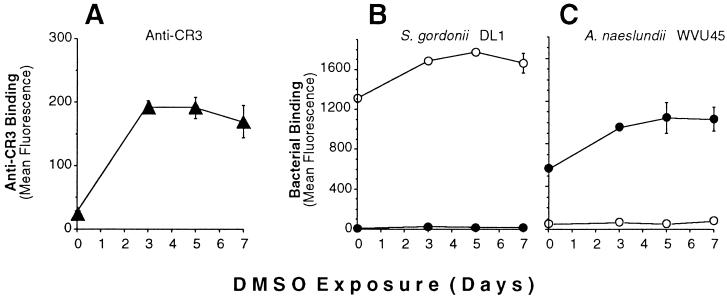
Adhesion of S. gordonii DL1 and A. naeslundii WVU45 to human peripheral blood PMNs was strictly dependent on the presence or absence of sialic acid on the mammalian cells. Thus, streptococci bound to untreated but not sialidase-treated PMNs, whereas actinomyces bound only to the sialidase-treated cells (Fig. 1). Further studies were performed with the human promyelocytic leukemia HL-60 cell line that was induced towards granulocytic differentiation by incubation with DMSO for 5 days. Binding of fluoresceinated bacteria to these cells was determined by flow cytometry. Maximum binding was obtained at a ratio of 50 bacteria per mammalian cell (data not shown). When incubated with fluoresceinated S. gordonii DL1, the majority of HL-60 cells exhibited high fluorescence intensity, thereby indicating extensive bacterial binding (Fig. 2, upper left panel). In contrast, treatment of the HL-60 cells with sialidase resulted in levels of cell-associated fluorescence that approximated those of HL-60 cells alone (Fig. 2, lower left panel). The opposite effect was noted for binding of fluoresceinated A. naeslundii WVU45 in that cell-bound fluorescence shifted from low to high intensities following sialidase treatment of the HL-60 cells (Fig. 2, upper and lower right panels). The untreated HL-60 cells apparently contained a small population of desialylated cells to which A. naeslundii WVU45 bound and S. gordonii DL1 failed to bind (Fig. 2, upper right and left panels, respectively). Analogous binding data were obtained with PMNs isolated from human peripheral blood (data not shown). Potential changes in bacterial binding over the course of DMSO-induced HL-60 cell differentiation were assessed. The increased expression of CR3 is an established marker of differentiation (40). In the present studies, this receptor was significantly up-regulated during exposure of the cells to DMSO (Fig. 3A). Under the same conditions, a modest increase in binding of S. gordonii DL1 to untreated cells (Fig. 3B) and A. naeslundii WVU45 to sialidase-treated cells occurred (Fig. 3C). S. gordonii DL1 and A. naeslundii WVU45 failed to bind to sialidase-treated or untreated cells, respectively.
FIG. 1.
Attachment of S. gordonii DL1 and A. naeslundii WVU45 to PMNs. Untreated or sialidase-treated PMNs were incubated with bacteria and washed. Bacterial binding was determined microscopically on Wright-Giemsa-stained cytospin preparations.
FIG. 2.
Flow cytometric analysis of adhesion of fluoresceinated S. gordonii DL1 and A. naeslundii WVU45 to untreated or sialidase-treated HL-60 cells incubated for 5 days with DMSO. The intensity of cell associated fluorescence (——) indicates the relative bacterial binding. Fluorescence of HL-60 cells in the absence of bacteria (•••••) is included for comparison.
FIG. 3.
Flow cytometric analysis of bacterial binding to HL-60 cells during granulocytic differentiation. HL-60 cells induced to differentiate towards granulocytes by DMSO were harvested at the indicated time intervals and analyzed for (A) the expression of CR3 receptors (▴), (B) binding of fluoresceinated S. gordonii DL1, and (C) binding of fluoresceinated. A. naeslundii WVU45. HL-60 cells were either untreated (○) or treated with sialidase (●).
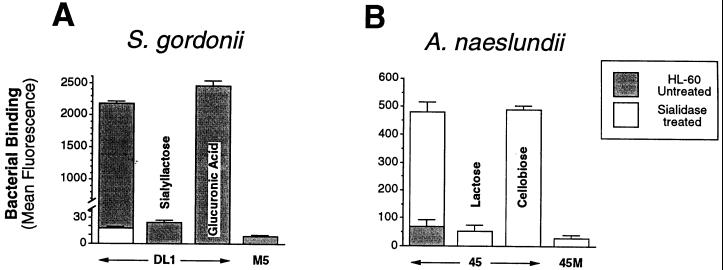
Bacterial binding of both species was saccharide specific. Thus, as shown in Fig. 4A, binding of S. gordonii DL1 to differentiated HL-60 cells was inhibited by sialyllactose, but not by glucuronic acid, another anionic saccharide. S. gordonii M5, a strain that lacks a sialic-acid-reactive adhesin (23, 52), did not adhere to HL-60 cells. Binding of A. naeslundii WVU45 to sialidase-treated differentiated HL-60 cells was inhibited by lactose but not by cellobiose, and a mutant strain, WVU45M, that lacks type 2 fimbriae (10) was not adherent (Fig. 4B). Similar results were obtained utilizing peripheral blood PMNs or undifferentiated HL-60 cells, although in the latter case, initial bacterial binding was lower (data not shown).
FIG. 4.
Flow cytometric analysis of the specificity of bacterial binding to HL-60 cells incubated with DMSO for 5 days. (A) Binding of fluoresceinated S. gordonii DL1 and M5. (B) Binding of fluoresceinated A. naeslundii WVU45 and WVU45M. HL-60 cells were untreated or treated with sialidase. Where indicated, binding was performed in the presence of 10 mM sialyllactose, 10 mM glucuronic acid, 50 mM lactose, or 50 mM cellobiose.
Detection and characterization of sialic acid- and Gal/GalNAc-containing glycoproteins on HL-60 cells and PMNs.
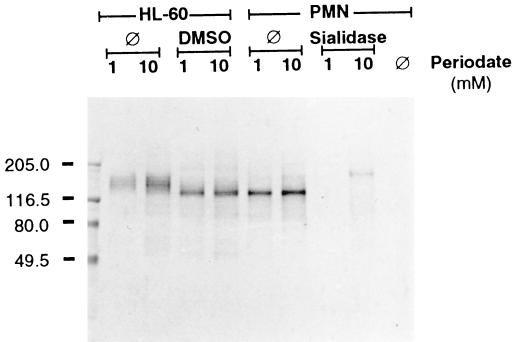
Labeling of sialic acid on undifferentiated HL-60 cells following oxidation with 1 mM sodium periodate revealed a broad prominent band at approximately 150 kDa on nitrocellulose transfers, whereas similar labeling of differentiated HL-60 cells and peripheral blood PMNs identified a band at 130 kDa (Fig. 5). Identically labeled bands were observed following oxidation of total cell surface carbohydrate with 10 mM sodium periodate. As expected, no bands were detected when sialidase-treated PMNs were surface labeled following oxidation with 1 mM periodate, but, significantly, a band appeared at 150 kDa following oxidation of sialidase-treated PMNs with 10 mM sodium periodate. Thus, sialidase treatment of PMNs resulted in a shift in the position of a major cell surface glycoprotein from approximately 130 to 150 kDa.
FIG. 5.
Cell surface labeling of sialoglycoconjugates and total carbohydrates on HL-60 cells and PMNs. HL-60 cells were either undifferentiated (⊘) or incubated with DMSO for 5 days. PMNs were either untreated (⊘) or treated with sialidase. Cells were incubated with 1 mM sodium periodate to oxidize sialic acid or 10 mM periodate to oxidize total carbohydrates prior to labeling with digoxigenin-conjugated hydrazide. Control PMNs were not oxidized (⊘). Cellular extracts (10 μg of protein per lane) were separated by SDS-PAGE and transferred to nitrocellulose. Anti-digoxigenin alkaline phosphatase conjugated antibody was used for detection. Sizes of molecular-mass markers are indicated to the left in kilodaltons.
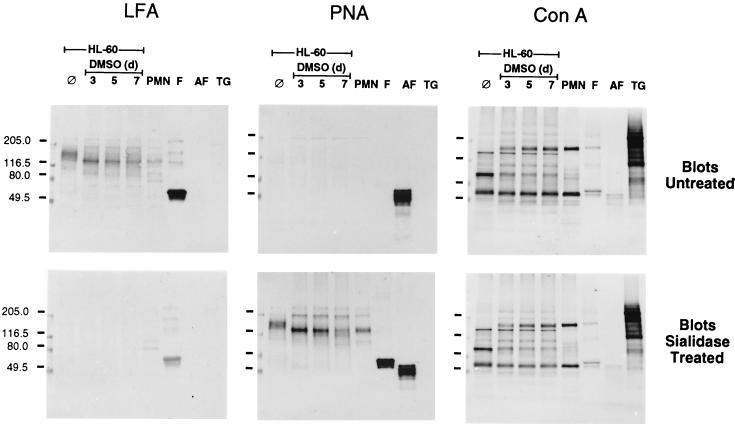
The 150- and 130-kDa surface glycoprotein bands from extracts of HL-60 cells and PMNs were probed for their reactions with selected plant lectins (Fig. 6). LFA, which reacts with α2-3-linked sialic acid (24), detected these glycoproteins on untreated blots and PNA, which is specific for terminal Galβ1-3GalNAc (34), reacted with both bands on sialidase-treated blots. Both LFA and PNA reacted with additional less prominent bands on untreated and sialidase-treated blots, respectively. One such band was seen at approximately 200 kDa. In control lanes, fetuin, which has NeuNAcα2-3Galβ1-3GalNAc termini (48), was recognized by LFA but not by PNA, whereas the reverse pattern was observed with asialo-fetuin. Neither LFA nor PNA reacted with thyroglobulin, an N-glycosylated control glycoprotein (37). The 150- and 130-kDa glycoprotein bands on transfers of HL-60 or PMN cell extracts were not detected by ConA. Instead, ConA reacted with a distinct set of glycoproteins on both untreated and sialidase-treated blots, including one near 150 kDa that may serve as a major receptor for mannose-specific Escherichia coli (38). In control lanes, ConA reacted weakly with both fetuin and asialo-fetuin and strongly with thyroglobulin.
FIG. 6.
Lectin blotting of extracts of HL-60 cells and PMNs. Cellular extracts (5 μg of protein per lane) from HL-60 cells prior to (⊘) and during DMSO-induced differentiation as well as from PMNs were separated by SDS-PAGE. Nitrocellulose transfers were untreated or treated with sialidase prior to incubation with biotinylated LFA, PNA, or ConA. Bound lectins were detected with avidin-d-alkaline phosphatase. Lanes containing either fetuin (F), asialofetuin (AF), or thyroglobulin (TG) (5 μg per lane) were included as controls. Sizes of molecular-mass markers are indicated to the left in kilodaltons.
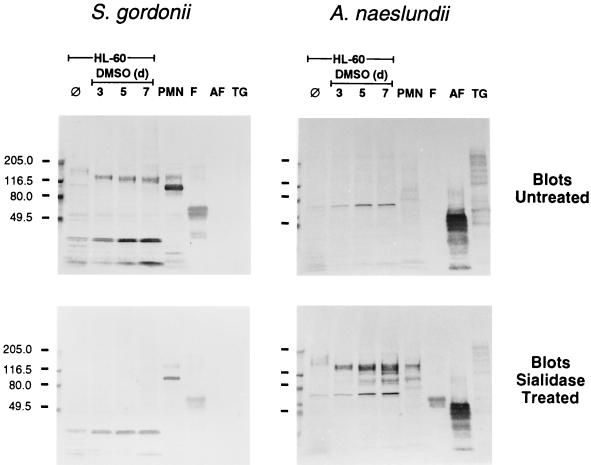
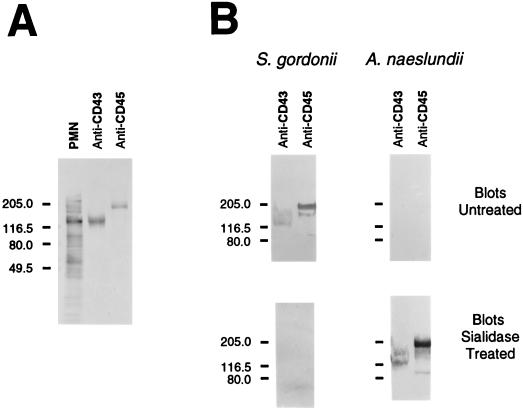
Transfers of HL-60 and PMN extracts were also overlaid with biotinylated bacteria (Fig. 7). S. gordonii DL1, like LFA, bound the 150- and 130-kDa glycoprotein bands on an untreated transfer, and bacterial binding was abolished by pretreatment of an identical blot with sialidase. Adhesion of streptococci to the untreated transfer of PMN extracts also revealed an additional prominent band at 90 kDa as well as bands of lower molecular weights in extracts of HL-60 cells. However, these latter bands were not major surface-labeled glycoproteins (see Fig. 5). A. naeslundii WVU45, like PNA, did not recognize the 150- and 130-kDa glycoproteins on untreated transfers, but this bacterium did recognize these components on transfers pretreated with sialidase. An additional HL-60 component, at approximately 70 kDa, that was not a major cell surface glycoprotein (see Fig. 5) was also detected by A. naeslundii on both untreated and sialidase-treated transfers. In control lanes, S. gordonii bound to fetuin but not to either asialo-fetuin or thyroglobulin, whereas A. naeslundii failed to bind fetuin but bound strongly to asialo-fetuin and weakly to thyroglobulin. Adhesion of S. gordonii to the 150- and 130-kDa glycoprotein bands was inhibited by sialyllactose but not by glucuronic acid, and adhesion of A. naeslundii to the same glycoproteins on sialidase-treated blots was inhibited by lactose but not by cellobiose (results not shown). In addition, 150- and 130-kDa glycoprotein bands were not observed on transfers that were overlaid with either S. gordonii M5, which lacks a sialic acid reactive adhesin, or A. naeslundii WVU45M, which lacks type-2 fimbriae.
FIG. 7.
Bacterial overlays on nitrocellulose transfers of extracts of HL-60 cells and PMNs. Cellular extracts (20 μg of protein per lane) from HL-60 cells prior to (⊘) and during DMSO-induced differentiation as well as from PMNs were separated by SDS-PAGE and transferred to nitrocellulose. Untreated or sialidase-treated transfers were overlaid with either biotinylated S. gordonii DL1 or A. naeslundii WVU45 and washed. Bound biotinylated bacteria were detected with avidin-d-alkaline phosphatase. Lanes containing either fetuin (F), asialofetuin (AF), or thyroglobulin (TG) (5 μg per lane) were included as controls. Sizes of molecular-mass markers are indicated to the left in kilodaltons.
Identification of leukosialin (CD43) and leukocyte common antigen (CD45) as receptors for S. gordonii and A. naeslundii.
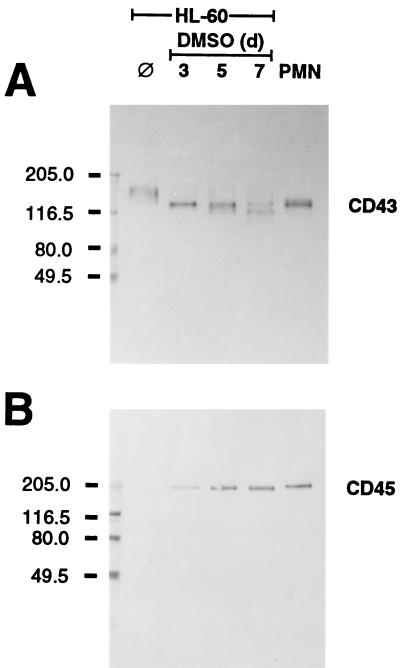
The positions of the 130- and 150-kDa bands were similar to those of leukosialin (CD43), the major O-linked sialoglycoprotein expressed exclusively on leukocytes (15). An anti-leukosialin MAb detected the 130-kDa band in extracts of differentiated HL-60 cells and PMNs (Fig. 8). Moreover, this MAb also revealed a shift in apparent molecular mass of this band from 150 to 130 kDa during HL-60 cell differentiation. Another sialoglycoprotein, leukocyte common antigen (CD45), is also extensively O-glycosylated but is expressed in considerably lower amounts on granulocytes (15, 53). This glycoprotein was detected by anti-CD45 MAb HB 10AB2 as a 200-kDa band in PMN extracts (Fig. 8). Expression of leukocyte common antigen increased over the course of granulocytic differentiation of HL-60 cells.
FIG. 8.
Immunoblots of extracts of HL-60 cells and PMNs. Transfers of extracts (5 μg of protein per lane) of HL-60 cells either prior to (⊘) or during DMSO-induced differentiation or PMNs were incubated with 1 μg of either (A) anti-leukosialin (CD43) MAb or (B) anti-leukocyte common antigen (CD45) MAb and subsequently with horseradish peroxidase-conjugated goat anti-mouse IgG antiserum. Sizes of molecular-mass markers are indicated to the left in kilodaltons.
Extracts of PMNs were immunoprecipitated with MAbs against either leukosialin or leukocyte common antigen. The anti-leukosialin MAb immunoprecipitated the 130-kDa glycoprotein from extracts of PMNs that were surface labeled for amino groups with NHS-LC-biotin prior to extraction (Fig. 9A). A 200-kDa band that was barely discernable in PMN extracts was clearly detected following concentration by immunoprecipitation with the anti-leukocyte common antigen MAb. As shown in Fig. 9B, immunoprecipitated leukosialin and leukocyte common antigen were recognized by both S. gordonii DL1 and A. naeslundii WVU45. However, S. gordonii DL1 bound exclusively to the sialo-forms of these molecules, whereas A. naeslundii WVU45 bound only to the asialo-forms.
FIG. 9.
Identification of leukosialin (CD43) and leukocyte common antigen (CD45) as PMN receptors for S. gordonii DL1 and A. naeslundii WVU45. (A) An extract of biotinylated PMNs and immunoprecipitates prepared with anti-CD43 or anti-CD45 MAbs were subjected to SDS-PAGE and transferred to nitrocellulose. Avidin-d-alkaline phosphatase was used to detect biotinylated proteins present in the extract (PMN) and immunoprecipitates (anti-CD43 or anti-CD45). (B) Immunoprecipitates (anti-CD43 or anti-CD45) from unlabeled cells were separated by SDS-PAGE and transferred to nitrocellulose. Untreated or sialidase-treated transfers were overlaid with either biotinylated S. gordonii DL1 or biotinylated A. naeslundii WVU45. Bound biotinylated bacteria were detected with avidin-d-alkaline phosphatase. Sizes of molecular-mass markers are indicated to the left in kilodaltons.
DISCUSSION
The results of the present investigations clearly indicate that the sialic acid- and Gal/GalNAc-reactive adhesins of S. gordonii and A. naeslundii, respectively, interact with a limited number of PMN surface glycoproteins, the most prominent being leukosialin (CD43). This glycoprotein was previously detected as a 130-kDa band in extracts of sialidase-treated PMNs by various Gal/GalNAc-binding plant lectins whose reactions with PMNs blocked subsequent phagocytosis and killing of A. naeslundii (45). The present results identify the 130-kDa glycoprotein as the major band detected in extracts of PMNs that were surface labeled for sialic acid or total carbohydrate and the only band that reacted with an anti-leukosialin MAb. Of major importance is the finding that this band is also detected by direct binding of actinomyces and streptococci with sialidase requirements identical to those for bacterial binding to PMNs. Thus, sialidase treatment of isolated leukosialin, which presumably removes α2-3-linked sialic-acid termini and exposes Galβ1-3GalNAc, eliminated receptors for S. gordonii and created receptors for A. naeslundii. Adhesion of these bacteria to the sialo- and asialo-forms, respectively, of leukocyte common antigen was also detected but only following concentration of this component from PMN extracts by immunoprecipitation with an anti-CD45 MAb. Thus, both oral streptococci and actinomyces recognized the same PMN surface glycoproteins depending on the presence or absence of sialic acid on these molecules.
Leukosialin in extracts of either peripheral blood PMNs or differentiated HL-60 cells migrated as a 130-kDa glycoprotein whereas leukosialin from undifferentiated HL-60 cells migrated as a 150-kDa glycoprotein. This shift in molecular weight is similar to that previously observed (16) and may reflect an increase in the extent of sialylation of leukosialin during the differentiation process. The binding of S. gordonii and A. naeslundii to HL-60 cells increased somewhat during differentiation but less dramatically than the expression of CR3. However, it is possible that the observed increase in bacterial binding greatly underestimates the increase in receptor expression, since the size of the microorganisms and the multivalency of bacterial attachment preclude quantitation of receptor density. The expression of leukocyte common antigen was enhanced during differentiation and may have contributed to the increase in bacterial binding. It has previously been reported that this antigen is a minor surface component of PMNs (53) and that its expression rapidly increases following stimulation of these cells (51).
Leukosialin and leukocyte common antigen both possess extracellular mucinlike domains, rich in O-linked oligosaccharide chains (7). The dense O-glycosylation confers a rigid rodlike structure on these molecules causing them to project further from the cell surface than other components (7, 49). Consequently, cell surface mucins are ideally situated to serve as receptors and, in addition, may prevent interactions of bacteria with underlying structures. The recognition of cell surface mucins by oral bacteria might be anticipated in view of structural similarities between these molecules and salivary mucins, which also serve as receptors (26, 30).
Adhesion of S. gordonii to untreated PMNs and adhesion of A. naeslundii to sialidase-treated PMNs have similar biological consequences since both bacteria stimulate the respiratory burst, the release of secondary granule contents, and lectinophagocytosis (43, 44). PMN activation is most likely initiated by multivalent interactions of bacterial adhesins with either sialo- or asialo-forms of leukosialin or leukocyte common antigen in a manner analogous to the triggering effects of cross-linking CD43 or CD45 on PMNs by specific MAbs (25, 27, 39). Activation of PMNs by anti-CD43 antibodies is accompanied by proteolytic cleavage and shedding of the CD43 extracellular mucinlike domain (3, 6, 46). It remains to be determined whether shedding of CD43 from PMNs occurs in response to adhesion of S. gordonii or A. naeslundii and conversely, whether shedding of CD43 from PMNs, induced by anti-CD43, affects subsequent adhesion and lectinophagocytosis of these bacteria.
Both leukosialin and leukocyte common antigen are implicated in intracellular signal transduction. The cytoplasmic domain of leukosialin contains three protein kinase C phosphorylation sites (32, 35), and the cytoplasmic domain of leukocyte common antigen has intrinsic protein tyrosine phosphatase activity (9, 55). In addition, activation of T lymphocytes through cross-linking of leukosialin with an anti-CD43 MAb induced the association of leukosialin to Fyn kinase through a putative Src homology 3 binding site of CD43 and the Fyn Src homology 3 domain (33). Leukosialin solubilized from human neutrophils also is associated with tyrosine kinase activity, most of which is attributed to the Src family kinases Lyn and Hck (47). In addition to cell activation and signaling, leukosialin may also be involved in the ingestion of attached bacteria since the cytoplasmic domain of leukosialin and other cell surface sialomucins is linked to cytoskeletal elements (57). This association of bacterial attachment sites with the cytoskeleton appears to be an important facet of phagocytosis (4). Leukosialin and leukocyte common antigen may function independently in regard to bacterial attachment, signal transduction, release of inflammatory mediators, and phagocytosis. Alternatively, these biological consequences may be elicited by a cooperative effect of the two receptors or of either of these receptors with other cell membrane components as has been suggested from studies of Salmonella spp. (4).
Other microbes also recognize the PMN surface glycoproteins that serve as receptors for the adhesins of oral streptococci or actinomyces. For example, influenza A virus (IAV), which binds sialic acid, interacts with a select group of PMN surface glycoproteins that includes leukosialin and leukocyte common antigen (20, 41). Like the interactions of oral actinomyces and streptococci, the interaction of IAV with PMNs stimulates the respiratory burst (28), degranulation (21), and phagocytosis (2). Significantly, IAV also causes deactivation of various PMN metabolic and bactericidal activities, and this effect may be associated with increased susceptibility of IAV patients to secondary bacterial infections (1, 2). Activation and deactivation appear to be triggered, at least in part, by the multivalent interaction of virus particles with cell surface leukosialin as both effects can also be induced by cross-linking leukosialin with specific antibodies or sialic acid-binding lectins such as LFA (8, 19). The multivalent interactions of oral actinomyces and streptococci with PMNs may thus contribute not only to the initiation of inflammation but also to subsequent diminution of PMN bactericidal functions as has been noted by the lazy leukocyte syndrome associated with localized juvenile periodontitis and cyclic neutropenia (17). Clearly, the identification of leukosialin and leukocyte common antigen as PMN receptors for the adhesins of oral streptococci and actinomyces provides a strong basis for further exploration of the signaling processes triggered by these bacteria and their effects on PMN function.
ACKNOWLEDGMENT
We are grateful to R. P. Siraganian for kindly providing the anti-CD45 MAb.
REFERENCES
- 1.Abramson J S, Lewis J C, Lyles D S, Heller K A, Mills E L, Bass D A. Inhibition of neutrophil lysosome-phagosome fusion associated with influenza virus infection in vitro: role in depressed bactericidal activity. J Clin Investig. 1982;69:1393–1397. doi: 10.1172/JCI110580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abramson J S, Mills E L, Giebink G S, Quie P G. Depression of monocyte and polymorphonuclear leukocyte oxidative metabolism and bactericidal capacity by influenza A virus. Infect Immun. 1982;35:350–355. doi: 10.1128/iai.35.1.350-355.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bazil V, Strominger J L. CD43, the major sialoglycoprotein of human leukocytes is proteolytically cleaved from the surface of stimulated lymphocytes and granulocytes. Proc Natl Acad Sci USA. 1993;90:3792–3796. doi: 10.1073/pnas.90.9.3792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bliska J B, Galan J E, Falkow S. Signal transduction in the mammalian cell during bacterial attachment and entry. Cell. 1993;73:903–920. doi: 10.1016/0092-8674(93)90270-z. [DOI] [PubMed] [Google Scholar]
- 5.Brennan M J, Joralmon R A, Cisar J O, Sandberg A L. Binding of Actinomyces naeslundii to glycosphingolipids. Infect Immun. 1987;64:487–489. doi: 10.1128/iai.55.2.487-489.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Campanero M R, Pulido R, Alonso J L, Pivel J P, Pimentel-Muinos F X, Fresno M, Sanchez-Madrid F. Down-regulation by tumor necrosis factor-alpha of neutrophil cell surface expression of the sialophorin CD43 and the hyaluronate receptor CD44 through a proteolytic mechanism. Eur J Immunol. 1991;21:3045–3048. doi: 10.1002/eji.1830211222. [DOI] [PubMed] [Google Scholar]
- 7.Carraway K L, Hull S R. Cell surface mucin-type glycoproteins and mucin-like domains. Glycobiology. 1991;1:131–138. doi: 10.1093/glycob/1.2.131. [DOI] [PubMed] [Google Scholar]
- 8.Cassidy L F, Lyles D S, Abramson J S. Depression of polymorphonuclear leukocyte functions by purified influenza virus hemagglutinin and sialic acid-binding lectins. J Immunol. 1989;142:4401–4406. [PubMed] [Google Scholar]
- 9.Charbonneau H, Tonks N K, Walsh K A, Fischer E H. The leukocyte common antigen (CD45): a putative receptor-linked protein tyrosine phosphatase. Proc Natl Acad Sci USA. 1988;85:7182–7186. doi: 10.1073/pnas.85.19.7182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cisar J O, David V A, Curl S H, Vatter A E. Exclusive presence of lactose-sensitive fimbriae on a typical strain (WVU45) of Actinomyces naeslundii. Infect Immun. 1984;46:453–458. doi: 10.1128/iai.46.2.453-458.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cisar J O, Sandberg A L, Abeygunawardana C, Reddy G P, Bush C A. Lectin recognition of host-like saccharide motifs in streptococcal cell wall polysaccharides. Glycobiology. 1995;5:655–662. doi: 10.1093/glycob/5.7.655. [DOI] [PubMed] [Google Scholar]
- 12.Collins S J, Ruscetti F W, Gallagher R E, Gallo R C. Terminal differentiation of human promyelocytic leukemia cells induced by dimethyl sulfoxide and other polar compounds. Proc Natl Acad Sci USA. 1978;75:2458–2462. doi: 10.1073/pnas.75.5.2458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Costello A H, Cisar J O, Kolenbrander P E, Gabriel O. Neuraminidase-dependent hemagglutination of human erythrocytes by human strains of Actinomyces viscosus and Actinomyces naeslundii. Infect Immun. 1979;26:563–572. doi: 10.1128/iai.26.2.563-572.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Demuth D R, Golub E E, Malamud D. Streptococcal-host interactions: structural and functional analysis of a Streptococcus sanguis receptor for a human salivary glycoprotein. J Biol Chem. 1990;265:7120–7126. [PubMed] [Google Scholar]
- 15.Fukuda M. Leukosialin, a major O-glycan-containing sialoglycoprotein defining leukocyte differentiation and malignancy. Glycobiology. 1991;1:347–356. doi: 10.1093/glycob/1.4.347. [DOI] [PubMed] [Google Scholar]
- 16.Gahmberg K, Nilsson K, Andersson L C. Specific changes in the surface glycoprotein pattern of human promyelocytic leukemic cell line HL-60 during morphologic and functional differentiation. Proc Natl Acad Sci USA. 1979;76:4087–4091. doi: 10.1073/pnas.76.8.4087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Genco R J. Current view of risk factors for periodontal diseases. J Periodontol. 1996;67:1041–1049. doi: 10.1902/jop.1996.67.10.1041. [DOI] [PubMed] [Google Scholar]
- 18.Harlow E, Lane D. Antibodies: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1988. [Google Scholar]
- 19.Hartshorn K L, Daigneault D E, White M R, Tauber A I. Anomalous features of human neutrophil activation by influenza A virus are shared by related viruses and sialic acid-binding lectins. J Leukoc Biol. 1992;51:230–236. doi: 10.1002/jlb.51.3.230. [DOI] [PubMed] [Google Scholar]
- 20.Hartshorn K L, Liou L S, White M R, Kazhdan M M, Tauber J L, Tauber A I. Neutrophil deactivation by influenza A virus: role of hemagglutinin binding to specific sialic acid-bearing cellular proteins. J Immunol. 1995;154:3952–3960. [PubMed] [Google Scholar]
- 21.Henricks P A, van der Tol M E, Verhoef J. Interactions between human polymorphonuclear leukocytes and influenza virus. Scand J Immunol. 1985;22:721–725. doi: 10.1111/j.1365-3083.1985.tb01935.x. [DOI] [PubMed] [Google Scholar]
- 22.Hook W A, Berenstein E H, Zinsser F U, Fischler C, Siraganian R P. Monoclonal antibodies to the leukocyte common antigen (CD45) inhibit IgE-mediated histamine release from human basophils. J Immunol. 1991;147:2670–2676. [PubMed] [Google Scholar]
- 23.Hsu S D, Cisar J O, Sandberg A L, Kilian M. Adhesive properties of viridans streptococcal species. Microb Ecol Health Dis. 1994;7:125–137. [Google Scholar]
- 24.Knibbs R N, Osborne S E, Glick G D, Goldstein I J. Binding determinants of the sialic acid-specific lectin from the slug Limax flavus. J Biol Chem. 1993;268:18524–18531. [PubMed] [Google Scholar]
- 25.Kuijpers T W, Hoogerwerf M, Kujpers K C, Schwartz B R, Harlan J M. Cross-linking of sialophorin (CD43) induces neutrophil aggregation in a CD18-dependent and a CD-18-independent way. J Immunol. 1992;149:998–1003. [PubMed] [Google Scholar]
- 26.Levine M J, Reddy M S, Tabak L A, Loomis R E, Bergey E J, Jones P C, Cohen R E, Stinson M W, Al-Hashimi I. Structural aspects of salivary glycoproteins. J Dent Res. 1987;66:436–441. doi: 10.1177/00220345870660020901. [DOI] [PubMed] [Google Scholar]
- 27.Liles W C, Ledbetter J A, Waltersdorph A W, Klebanoff S J. Crosslinking of CD45 enhances activation of the respiratory burst in response to specific stimuli in human phagocytes. J Immunol. 1995;155:2175–2184. [PubMed] [Google Scholar]
- 28.Mills E L, Debets-Ossenkopp Y, Verbrugh H A, Verhoef J. Inititiation of the respiratory burst of human neutrophils by influenza virus. Infect Immun. 1981;32:1200–1205. doi: 10.1128/iai.32.3.1200-1205.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Murray P A, Levine M J, Tabak L A, Reddy M S. Specificity of salivary-bacterial interactions. II. Evidence for a lectin on Streptococcus sanguis with specificity for a NeuAcα2,3Galβ1,3GalNAc sequence. Biochem Biophys Res Commun. 1982;106:390–396. doi: 10.1016/0006-291x(82)91122-6. [DOI] [PubMed] [Google Scholar]
- 30.Murray P A, Prakobphol A, Lee T, Hoover C I, Fisher S J. Adherence of oral streptococci to salivary glycoproteins. Infect Immun. 1992;60:31–38. doi: 10.1128/iai.60.1.31-38.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ofek I, Goldhar J, Keisari Y, Sharon N. Nonopsonic phagocytosis of microorganisms. Annu Rev Microbiol. 1995;49:239–276. doi: 10.1146/annurev.mi.49.100195.001323. [DOI] [PubMed] [Google Scholar]
- 32.Pallant A, Eskenazi A, Mattei M G, Fournier R E, Carlsson S R, Fukuda M, Frelinger J G. Characterization of cDNAs encoding human leukosialin and localization of the leukosialin gene to chromosome 16. Proc Natl Acad Sci USA. 1989;86:1328–1332. doi: 10.1073/pnas.86.4.1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pedraza-Alva G, Merida L B, Burakoff S J, Rosenstein Y. CD43-specific activation of T cells induces association of CD43 to Fyn kinase. J Biol Chem. 1996;271:27564–27568. doi: 10.1074/jbc.271.44.27564. [DOI] [PubMed] [Google Scholar]
- 34.Pereira M E A, Kabat E A, Lotan R, Sharon N. Immunochemical studies on the specificity of the peanut (Arachis hypogaea) agglutinin. Carbohydr Res. 1976;51:107–118. doi: 10.1016/s0008-6215(00)84040-9. [DOI] [PubMed] [Google Scholar]
- 35.Piller V, Piller F, Fukuda M. Phosphorylation of the major leukocyte surface sialoglycoprotein, leukosialin, is increased by phorbol 12-myristate 13-acetate. J Biol Chem. 1989;264:18824–18831. [PubMed] [Google Scholar]
- 36.Prakobphol A, Murray P A, Fisher S J. Bacterial adherence on replicas of sodium dodecyl sulfate-polyacrylamide gels. Anal Biochem. 1987;164:5–11. doi: 10.1016/0003-2697(87)90359-9. [DOI] [PubMed] [Google Scholar]
- 37.Rawitch A B, Pollock H G, Yang S-X. Thyroglobulin glycosylation: location and nature of the N-linked oligosaccharide units in bovine thyroglobulin. Arch Biochem Biophys. 1993;300:271–279. doi: 10.1006/abbi.1993.1038. [DOI] [PubMed] [Google Scholar]
- 38.Rodriguez-Ortega M, Ofek I, Sharon N. Membrane glycoproteins of human polymorphonuclear leukocytes that act as receptors for mannose-specific Escherichia coli. Infect Immun. 1987;55:968–973. doi: 10.1128/iai.55.4.968-973.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rosenkranz A R, Majdic O, Stockl J, Pickl W, Stockinger H, Knapp W. Induction of neutrophil homotypic adhesion via sialophorin (CD43), a surface sialoglycoprotein restricted to haemopoietic cells. Immunology. 1993;80:431–438. [PMC free article] [PubMed] [Google Scholar]
- 40.Rosmarin A G, Weil S C, Rosner G L, Griffin J D, Arnaout M A, Tenen D G. Differential expression of CD11b/CD18 (Mol) and myeloperoxidase genes during myeloid differentiation. Blood. 1989;73:131–136. [PubMed] [Google Scholar]
- 41.Rothwell S W, Wright D G. Characterization of influenza A virus binding sites on human neutrophils. J Immunol. 1994;152:2358–2367. [PubMed] [Google Scholar]
- 42.Ruhl S, Sandberg A L, Cisar J O. Recognition of immunoglobulin A1 by oral actinomyces and streptococcal lectins. Infect Immun. 1996;64:5421–5424. doi: 10.1128/iai.64.12.5421-5424.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sandberg A L, Mudrick L L, Cisar J O, Brennan M J, Mergenhagen S E, Vatter A E. Type 2 fimbrial lectin-mediated phagocytosis of oral Actinomyces spp. by polymorphonuclear leukocytes. Infect Immun. 1986;54:472–476. doi: 10.1128/iai.54.2.472-476.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sandberg A L, Mudrick L L, Cisar J O, Metcalf J A, Malech H L. Stimulation of superoxide and lactoferrin release from polymorphonuclear leukocytes by the type 2 fimbrial lectin of Actinomyces viscosus T14V. Infect Immun. 1988;56:267–269. doi: 10.1128/iai.56.1.267-269.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sandberg A L, Ruhl S, Joralmon R A, Brennan M J, Sutphin M J, Cisar J O. Putative glycoprotein and glycolipid polymorphonuclear leukocyte receptors for the Actinomyces naeslundii WVU45 fimbrial lectin. Infect Immun. 1995;63:2625–2631. doi: 10.1128/iai.63.7.2625-2631.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schmid K, Hediger M A, Brossmer R, Collins J H, Haupt H, Marti T, Offner G D, Schaller J, Takagaki K, Walsh M T. Amino acid sequence of human plasma galactoglycoprotein: identity with the extracellular region of CD43 (sialophorin) Proc Natl Acad Sci USA. 1992;89:663–667. doi: 10.1073/pnas.89.2.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Skubitz K M, Campbell K D, Skubitz A P. CD43 is associated with tyrosine kinase activity in human neutrophils. J Leukoc Biol. 1998;64:800–802. doi: 10.1002/jlb.64.6.800. [DOI] [PubMed] [Google Scholar]
- 48.Spiro R G, Bhoyroo V D. Structure of the O-glycosidically linked carbohydrate units of fetuin. J Biol Chem. 1974;249:5704–5717. [PubMed] [Google Scholar]
- 49.Strous G J, Dekker J. Mucin-type glycoproteins. Crit Rev Biochem Mol Biol. 1992;27:57–92. doi: 10.3109/10409239209082559. [DOI] [PubMed] [Google Scholar]
- 50.Strömberg N, Karlsson K-A. Characterization of the binding of Actinomyces naeslundii (ATCC 12104) and Actinomyces viscosus (ATCC 19246) to glycosphingolipids using a solid-phase overlay approach. J Biol Chem. 1989;265:11251–11258. [PubMed] [Google Scholar]
- 51.Taetle R, Ostergaard H, Smedsrud M, Trowbridge I. Regulation of CD45 expression in human leukemia cells. Leukemia. 1991;5:309–314. [PubMed] [Google Scholar]
- 52.Takahashi Y, Sandberg A L, Ruhl S, Muller J, Cisar J O. A specific cell surface antigen of Streptococcus gordonii is associated with bacterial hemagglutination and adhesion to α2-3-linked sialic acid-containing receptors. Infect Immun. 1997;65:5042–5051. doi: 10.1128/iai.65.12.5042-5051.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Thomas M L. The leukocyte common antigen family. Annu Rev Immunol. 1989;7:338–369. doi: 10.1146/annurev.iy.07.040189.002011. [DOI] [PubMed] [Google Scholar]
- 54.Towbin H, Staehlin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Biochemistry. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Trowbridge I S, Thomas M L. CD45: an emerging role as a protein tyrosine phosphatase required for lymphocyte activation and development. Annu Rev Immunol. 1994;12:85–116. doi: 10.1146/annurev.iy.12.040194.000505. [DOI] [PubMed] [Google Scholar]
- 56.Van Dyke T E, Hoop G A. Neutrophil function and oral disease. Crit Rev Oral Biol Med. 1990;1:117–133. doi: 10.1177/10454411900010020201. [DOI] [PubMed] [Google Scholar]
- 57.Yonemura S, Nagafuchi A, Sato N, Shoichiro T. Concentration of an integral membrane protein, CD43 (leukosialin, sialophorin), in the cleavage furrow through the interaction of its cytoplasmic domain with actin-based cytoskeletons. J Cell Biol. 1993;120:437–449. doi: 10.1083/jcb.120.2.437. [DOI] [PMC free article] [PubMed] [Google Scholar]