Abstract
Background
We aimed to explore the postnatal evolution of ventilation/perfusion ratio (VA/Q) and right-to-left shunt in infants with congenital diaphragmatic hernia (CDH) and whether these indices predicted survival to discharge.
Methods
Retrospective cohort study at King’s College Hospital, London, UK of infants admitted with CDH in 10 years (2011–2021). The non-invasive method of the oxyhaemoglobin dissociation curve was used to determine the VA/Q and shunt in the first 24 h of life, pre-operation, pre-extubation and in the deceased infants, before death.
Results
Eighty-two infants with CDH (71 left-sided) were included with a median (IQR) gestation of 38.1(34.8–39.0) weeks. Fifty-three (65%) survived to discharge from neonatal care. The median (IQR) VA/Q in the first 24 h was lower in the deceased infants [0.09(0.07–0.12)] compared to the ones who survived [0.28(0.19–0.38), p < 0.001]. In the infants who survived, the VA/Q was lower in the first 24 h [0.28 (0.19–0.38)] compared to pre-operation [0.41 (0.3–0.49), p < 0.001] and lower pre-operation compared to pre-extubation [0.48 (0.39–0.55), p = 0.027]. The shunt was not different in infants who survived compared to the infants who did not.
Conclusions
Ventilation-to-perfusion ratio was lower in infants who died in the neonatal period compared to the ones that survived and improved in surviving infants over the immediate postnatal period.
Impact
The non-invasive method of the oxyhaemoglobin dissociation curve was used to determine the ventilation/perfusion ratio VA/Q in infants with congenital diaphragmatic hernia (CDH) in the first 24 h of life, pre-operation, pre-extubation and in the deceased infants, before death.
The VA/Q in the first 24 h of life was lower in the infants who did not survive to discharge from neonatal care compared to the ones who survived.
In the infants who survived, the VA/Q improved over the immediate postnatal period.
The non-invasive calculation of VA/Q can provide valuable information relating to survival to discharge.
Introduction
Congenital diaphragmatic hernia (CDH) is associated with high mortality and respiratory morbidity lasting into childhood and early adulthood.1, 2 Pulmonary hypoplasia is a major determinant of outcomes in infants with CDH.3, 4 Pulmonary vascular structural abnormalities are also well described in CDH with a smaller number of arteries and increased muscular wall thickness.5 Histology and three-dimensional reconstruction have identified prominent bronchopulmonary vascular anastomoses in the lungs of infants who died with severe CDH, with this abnormal development likely representing evidence of intrapulmonary shunting and contributing to the refractory hypoxaemia seen in the severe form of the disease.6
The severity of hypoxaemia is determined by the relationship of ventilation to perfusion (VA/Q) and the extent of intrapulmonary shunting.7 Although VA/Q abnormalities have been reported in surviving CDH children longitudinally,8 these abnormalities have never been explored in the immediate postnatal period, or in infants who did not survive to discharge from neonatal care. The highly specialised and invasive nature of nuclear medicine and histological/post-mortem methods, partly explain this lack of early data in CDH infants. We have recently applied an alternative, non-invasive method to assess the VA/Q and intrapulmonary shunt using the oxyhaemoglobin dissociation curve (ODC) and reported normal values in healthy term infants.9, 10 More so, we described abnormally low VA/Q and high shunt in preterm infants with evolving respiratory disease11 as well as in older children with hepatopulmonary syndrome.12 This method could thus be further utilised to describe oxygenation abnormalities in CDH, as it non-invasively quantifies VA/Q mismatch and right-to-left shunt, both of which are contributing factors to the underlying hypoxaemia in CDH.
We aimed to use the non-invasive ODC method to describe the evolution of VA/Q and shunt abnormalities in infants with CDH during their neonatal stay and compare those early abnormalities in infants who survived to discharge from neonatal care compared to those seen in infants who did not survive.
Methods
Subjects
Newborn infants treated for CDH at King’s College Hospital (KCH) Foundation Trust, London, UK during a 10-year period (1 January 2011–1 January 2021) were included in the study. KCH is a tertiary referral centre for infants with CDH who might benefit from foetal endoscopic tracheal occlusion (FETO); hence, our population includes infants at the severe end of the disease spectrum. The indications for FETO have been previously described.13 The infants were intubated at birth and were ventilated on conventional mechanical ventilation. If the pre-ductal transcutaneous oxygen saturation was below 90% while ventilated with a peak inflation pressure >30 cm H2O the infants were then ventilated with high-frequency oscillation (HFO). Infants were referred for extracorporeal membrane oxygenation (ECMO) following discussion with an ECMO centre, if the echocardiographic assessment was suggestive of significant pulmonary hypertension, which is theoretically a reversible condition.14The infants were deemed stable to undergo an operation when they did not require inhaled nitric oxide (NO), were requiring a fraction of inspired oxygen (FIO2) < 0.50 and were on minimal inotropic support (one inotropic agent, usually dopamine infusion of 5–10 mcg/kg/min). The study was registered as a service evaluation with the Clinical Governance Department of KCH. The Health Research Authority Toolkit of the National Health System, United Kingdom confirmed that the study would not be considered as research and would not require regulatory approval by a research ethics committee.
Calculation of VA/Q and shunt with the oxygen dissociation curve
The method has been previously described in detail.11 The relative position of an individual’s oxyhaemoglobin dissociation curve can be used to calculate the degree of shunt and VA/Q inequality as a non-invasive measure of oxygenation impairment.9 Briefly, right-to-left shunt causes a decrease in arterial oxygen saturation, which cannot be overcome by increasing the amount of inspired oxygen. The level of the right-to-left shunt can be thus quantified by the degree of the depression of the oxyhaemoglobin dissociation curve. A reduction in the VA/Q results in a post-alveolar reduction in the blood oxygen content, which produces a rightward shift of the curve. This shift can be overcome by increasing the fraction of inspired oxygen. The reduction, thus, in VA/Q can be measured by the degree of the right shift of the curve compared to an ideal reference curve (Fig. 1). Similarly, the further downwards an individual’s curve is positioned relative to the ideal curve, the higher that individual’s right-to-left shunt (Fig. 1).15 Using at least two paired values of peripheral oxygen saturation (SpO2) and FIO2 for each infant taken ≤4 h apart, an oxyhaemoglobin dissociation curve was constructed for each subject. Using the paired values of FIO2 and SpO2, the VA/Q and the percentage of right-to-left shunt were calculated at four time endpoints: during the first 24 h of life, in the 12 h before operation (pre-operation), in the 12 h before extubation (pre-extubation) and, in the infants who died, in the last 12 h before death.15 VA/Q and right-to-left shunt were derived using software that derives results for each dataset from a two-compartment model: shunt and VA/Q for a single homogeneous ventilated compartment.16 The contemporaneous haemoglobin value from the arterial blood gas analysis at the time of each assessment was used in the calculations. For ventilated infants treated with NO, the FIO2 was measured and recorded as delivered to the patient from the NO delivery system.
Fig. 1. The oxyhaemoglobin dissociation curve: proportion of haemoglobin saturation versus pressure of inspired oxygen.
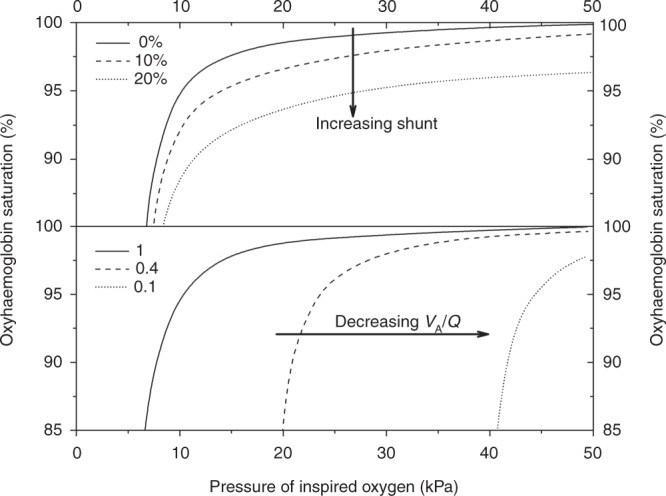
Increasing shunt (upper diagram) displaces the curve downwards and decreasing VA/Q (lower diagram) shifts the curve to the right. The dashed line in the upper diagram represents a subject with a shunt of 10% and the dotted line a subject with a shunt of 20%. The dashed line in the lower diagram represents a subject with a VA/Q of 0.4 and the dotted line a subject with a VA/Q of 0.1. Reproduced from Dassios et al.11
Information from the medical notes
The following information was collected from the medical and surgical notes: observed-to-expected lung-to-head ratio (LHR) at diagnosis,17 FETO procedure (yes/no),18 sex, gestational age (weeks), birth weight (kg), Apgar score at 5 min, left- or right-sided defect, intraoperative confirmation of the liver above the diaphragm (liver up), operated (yes/no), age at surgery (days), pre-operative use of HFO (yes/no), duration of mechanical ventilation (days), preoperative use of NO (yes/no), duration of stay (days), ECMO (yes/no), survival to discharge from neonatal care (yes/no).
Statistics
Data were tested for normality with the Kolmogorov–Smirnov test and found to be non-normally distributed and hence presented as median and interquartile (IQR) range. Differences in the VA/Q and right-to-left shunt were compared at the different time endpoints using the Wilcoxon signed-rank test. The relationship of VA/Q in the first 24 h of life with LHR, duration of mechanical ventilation, and total duration of stay were examined in the infants who survived with the Kendall-tau rank correlation coefficient. Differences in VA/Q, shunt and clinical parameters between infants who survived to discharge from neonatal care and deceased infants were assessed for statistical significance using the Mann–Whitney rank-sum test or Chi-squared test, as appropriate. The factors that were statistically different (p value <0.05) were inserted into a multivariable binary regression model with survival to discharge from neonatal care as the outcome variable. Variables without normal distribution were logarithmically transformed. Multi-collinearity among the independent variables in the regression analysis was assessed by the calculation of the tolerance for the independent variables. The performance of VA/Q in predicting survival to discharge from neonatal care was assessed by receiver operator characteristic (ROC) curve analysis and estimation of the corresponding area under the curve (AUC). One optimal cut-point from the ROC curve was selected to report the corresponding sensitivity and specificity. The cut point was selected on the basis of the optimal combination for the highest values of both sensitivity and specificity.
Statistical analysis was performed using SPSS software (SPSS Inc., Chicago, IL).
Results
During the study period, 104 infants with CDH were treated at the neonatal unit at KCH. Thirteen infants were excluded because of a lack of complete medical and nursing notes. Nine infants were excluded as they were nursed in a FIO2 of 1.0 for the whole duration of their stay and we were thus unable to derive paired values of SpO2 and FIO2. These nine infants all died because of hypoxaemic respiratory failure within the first 24 h of life before they could have surgery. Eighty-two (45 male) infants with a median (IQR) gestational age of 38.1 (34.8–39.0) weeks were included in the study. They had a median (IQR) birth weight of 2.70 (2.23–3.16) kg. The antenatal, demographic, surgical and neonatal characteristics of the included infants are presented in Table 1. The median (IQR) VA/Q on the first day after birth was 0.20 (0.09–0.32) and the shunt was 10 (3–18)%.
Table 1.
Characteristics of the study population.
| Number of infants | 82 | |
|---|---|---|
| Antenatal | Lung to head ratio (%) | 24 (14–36) |
| Antenatal FETO procedure | 28 (34) | |
| Demographics and birth | Male sex | 45 (55) |
| Gestation (weeks) | 38.1 (34.8–39.0) | |
| Birth weight (kg) | 2.70 (2.23–3.16) | |
| Apgar score at 5 min | 8 (6–9) | |
| Surgical | Left-sided hernia | 71 (87) |
| Liver up | 13 (16) | |
| Operated | 54 (66) | |
| Days to surgery (for the operated) | 4 (3–6) | |
| Neonatal | HFO | 41 (50) |
| Days of mechanical ventilation | 6 (3–10) | |
| Nitric oxide | 54 (66) | |
| Duration of stay (days) | 18 (2–31) | |
| Survived to discharge from neonatal care | 53 (65) |
Data are presented as median (interquartile range) or number (percentage).
Fifty-three infants (65%) survived to discharge from neonatal care. The median (IQR) duration of stay in the deceased infants was 2 (1–3) days. The cause of death in all deceased infants was hypoxic respiratory failure secondary to the CDH. The infants who survived had a higher median (IQR) Apgar score at 5 min [8 (7–9)] compared to the infants who did not survive [6 (3–8), p = 0.001] and were less frequently treated with HFO and NO (26 and 43% compared to 90 and 100%, respectively, p < 0.001 for both, Table 2). The infants who survived had a higher median (IQR) VA/Q in the first 24 h [0.28 (0.19–0.37)] compared to the infants who did not survive [0.09 (0.07–0.12), p < 0.001, Fig. 2]. The shunt in the first 24 h was not different in the infants who survived [9 (4–16)%] compared to the infants who did not survive [11 (2–20)%, p = 0.646, Fig. 2]. Three infants had ECMO and all three died in the ECMO referral centre before they could have surgery. Their VA/Q ratios in the first 24 h of life were 0.07, 0.08 and 0.09.
Table 2.
Comparison of demographics, respiratory parameters, VA/Q and shunt in infants who survived to discharge compared to those who did not.
| Survived | Deceased | p value | |
|---|---|---|---|
| Number of infants | 53 (65) | 29 (35) | N/A |
| LHR (%) | 23 (11–33) | 23 (14–35) | 0.552 |
| Antenatal FETO procedure | 18 (34) | 10 (34) | 0.948 |
| Male sex | 29 (55) | 15 (52) | 0.486 |
| Gestation (weeks) | 38.1 (35.2–39.1) | 36.8 (34.2–39.0) | 0.308 |
| Birth weight (kg) | 2.77 (2.26–3.22) | 2.65 (1.94–2.97) | 0.181 |
| Apgar score at 5 min | 8 (7–9) | 6 (3–8) | 0.001 |
| Left-sided hernia | 43 (81) | 26 (90) | 0.933 |
| Liver up | 9 (17) | 4 (14) | 0.555 |
| HFO | 14 (26) | 26 (90) | <0.001 |
| Nitric oxide | 23 (43) | 29 (100) | <0.001 |
| VA/Q in the first 24 h | 0.28 (0.19–0.37) | 0.09 (0.07–0.12) | <0.001 |
| Shunt (%) in the first 24 h | 9 (4–16) | 11 (2–20) | 0.646 |
Data are presented as median (interquartile range) or number (percentage). Mann– Whitney U or Chi-square test as appropriate.
LHR lung-to-head ratio, FETO foetal endoscopic tracheal occlusion, HFO high-frequency oscillation, N/A not applicable.
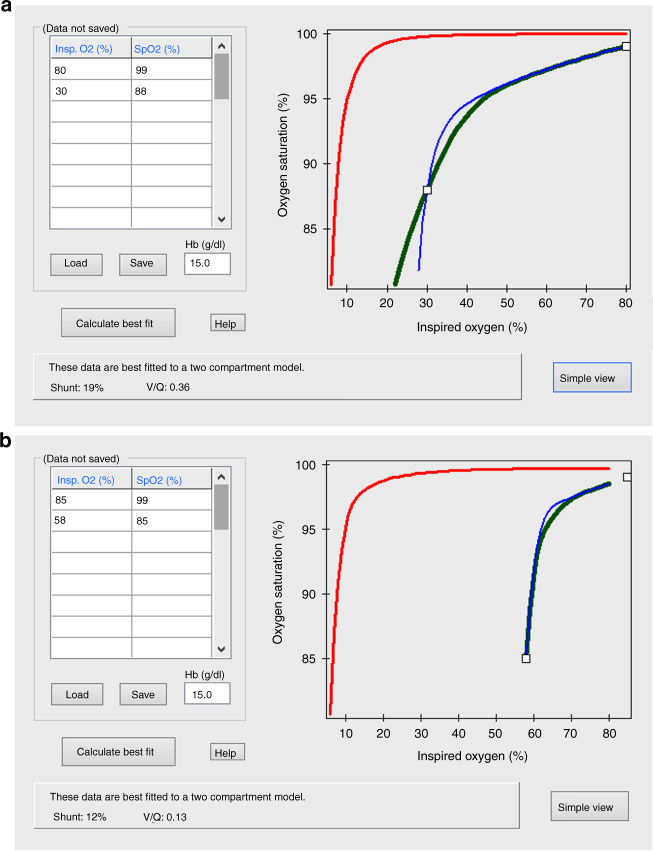
Fig. 2. Survival to discharge from neonatal care.
Representative oxyhaemoglobin dissociation curves of an infant who survived (a) and an infant who did not survive (b). The paired values of FIO2 and SpO2 that were used for the calculations of VA/Q and shunt are presented in the nested table on the left hand side of the figure. The contemporaneous values of the haemoglobin (Hb) concentration are also presented.
In the infants who survived, VA/Q correlated with duration of mechanical ventilation (r = −0.377, p < 0.001) and the total duration of stay (r = -0.279, p = 0.004) but not with the LHR at diagnosis (r = 0.114, p = 0.349).
The evolution of VA/Q in the infants who survived and in the infants who did not survive is presented in Fig. 3. In the infants who survived the median (IQR) VA/Q was lower in the first 24 h [0.28 (0.19–0.38)] compared to pre-operation [0.41 (0.34–0.49), p < 0.001] and lower pre-operation compared to pre-extubation [0.48 (0.39–0.55), p = 0.027]. In the infants who survived, the shunt was not different in the first 24 h [9 (4–16)%] compared to pre-operation [7 (0–13)%, p = 0.064] and not different pre-operation compared to pre-extubation [1 (0–10)%, p = 0.058]. In the infants who did not survive, the median (IQR) VA/Q [0.09 (0.08–0.12)] and shunt [11 (0–20)%] in the first 24 h were not different compared to the VA/Q [0.08 (0.07–0.14), p = 0.398] and shunt [4 (0–19)%, p = 0.337] before they died.
Fig. 3. Ventilation-to-perfusion ratios in infants with CDH who survived to discharge and infants who did not.
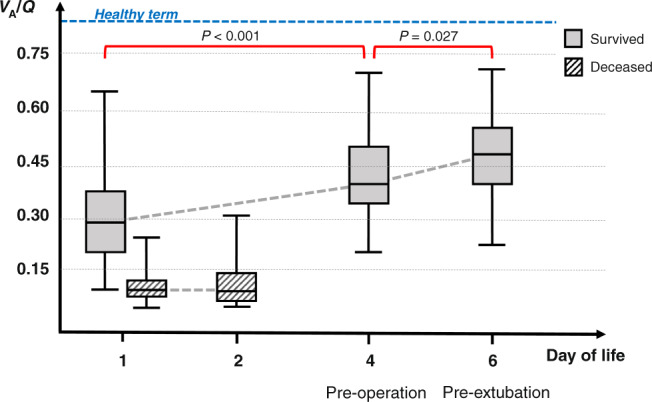
The median duration of stay for the deceased infants was 2 days. The surviving infants were operated at a median age of 4 days and were extubated at a median age of 6 days. A reference line with the mean VA/Q for healthy term infants is also depicted.
Twenty-eight infants (34%) underwent the antenatal FETO procedure. The median (IQR) VA/Q in the first 24 h was not different in infants who underwent the FETO procedure [0.13 (0.09–0.24)] compared to the infants who did not [0.20 (0.09–0.33), p = 0.252]. The median (IQR) shunt in the first 24 h was not different in infants who underwent the FETO procedure [12 (2–22)%] compared to the infants who did not [9 (1–17)%, p = 0.474].
Following multivariable binary regression analysis with survival to discharge as the outcome variable, VA/Q (adjusted p = 0.001) and high-frequency ventilation (adjusted p = 0.003) were significantly related to survival, while Apgar score at 5 min (p = 0.064) was not related to survival. The receiver operator characteristic curve analysis demonstrated that in predicting survival to discharge, the VA/Q in the first 24 h had an AUC of 0.905. A VA/Q in the first 24 h of life >0.15 predicted survival to discharge from neonatal care with 84% sensitivity and 88% specificity (Fig. 4).
Fig. 4.

Receiver operator characteristic curve analysis and estimation of the corresponding area under the curve (AUC) for the performance of VA/Q in the first 24 h of life in predicting survival to discharge from neonatal care.
Discussion
We have demonstrated that in surviving infants with CDH, the relationship of ventilation to perfusion gradually improved over the immediate postnatal period. The infants who did not survive started with severely abnormal ventilation-perfusion relationships, which remained abnormally low, up to their death. We also demonstrated that the ventilation-perfusion ratio in the first day of life predicted mortality in CDH infants with moderate–high sensitivity and specificity. The shunt was not significantly different between infants who survived and infants who did not survive and did not change significantly in the infants who survived during the immediate postnatal period.
Few previous studies have assessed ventilation to perfusion relationships in infants with CDH. Björkman et al. used single-photon emission computed tomography in twelve CDH infants at a mean age of six months and reported varying degrees of ventilation/perfusion abnormalities, which correlated with the presence of pulmonary artery hypertension and duration of ventilation. They concluded that pulmonary hypertension appears to be an important pathophysiological factor related to ventilation/perfusion abnormalities, and that pulmonary hypertension persists into the postoperative neonatal period.19 Similarly, Dao et al. tracked the VA/Q measured by serial nuclear medicine scans in 212 survivors of CDH over 15 years and reported a supra-physiological increase of the average ipsilateral VA/Q over time which was driven by a progressive reduction in perfusion.8 The finding of reduced perfusion as a primary driver of abnormal VA/Q in CDH was confirmed by Weidner et al. who used magnetic resonance imaging to quantify lung perfusion in 2-year-old children following CDH repair and reported that pulmonary blood flow was reduced in the ipsilateral CDH lung.20 Our study is the first to report on the ventilation/perfusion relationships and degree of right-to-left shunt in the early postnatal period that encompasses birth, initial stabilisation, operation and weaning from invasive ventilation. We speculate that the initial low VA/Q in our cohort is explained by a more marked diffusion limitation/ventilation impairment compared to a relatively less abnormal pulmonary perfusion in the context of pulmonary hypoplasia. Furthermore, pulmonary hypertension in the immediate period following birth might also manifest with intracardiac right-to-left shunting which could not occur in the older children of the aforementioned follow-up studies, due to the functional closure of the foramen ovale and the ductus arteriosus shortly after birth. The finding of an improving VA/Q over the first days of life could be explained by lung expansion and improvement of ventilation, which is caused by invasive ventilation and the removal of the intestinal content from the thorax. Another potential explanation for the improving VA/Q might be the gradual reversal of hypoxic pulmonary vasoconstriction which would lead to diversion of blood from poorly ventilated to better-ventilated lung areas.
It should be noted that in absolute terms, the values of VA/Q that we are reporting are markedly abnormal with a median VA/Q of 0.20 in the whole cohort including survivors and non-survivors. These values are comparable to premature infants with severe bronchopulmonary dysplasia21 or pulmonary interstitial emphysema22 and more than four times lower than what would be seen in healthy term infants using the same methodology (median VA/Q: 0.84).9, 10 It was also interesting that although a median shunt of 10% was present in our population, the degree of shunt did not discriminate survivors from non-survivors, nor did it change significantly over the course of neonatal intensive care in the infants who survived to discharge. This might be explained by that right-to-left shunt is the result of pulmonary hypertension due to structural pulmonary blood vessel anomalies. The fixed/structural nature of these abnormalities might explain why the shunt could not be overcome during the initial period of neonatal stay.
We should also note that the nine infants who were excluded because of an unchanged oxygen requirement of 100% for their entire intensive care course, all died. Thus, our reported mortality refers only to the included infants, and the actual overall mortality for the study period is higher.
Compared to other predictors of mortality, the VA/Q demonstrated a high predictive ability with an area under the curve of 0.905. This is superior to the chest radiographic thoracic area we have previously reported which had an AUC of 0.826 and the LHR at diagnosis with an AUC of 0.698.23 We have, however, reported that the mean oxygenation index (OI) can predict mortality with 96% sensitivity and specificity.24 The better performance of the OI might be explained by the ability of the index to be applied in all infants including those nursed in 100% oxygen for the brief period before they die. Although most infants with CDH would have central arterial access in the first days after birth to calculate the OI, the non-invasive VA/Q offers the advantage that it can be calculated without invasive arterial sampling and at any time-point irrespective of the availability of arterial blood gas. Other methods to predict mortality in CDH include the size of the defect at the time of surgery and the need for a patch repair, which have both been associated with higher morbidity and decreased survival.25, 26 These predictors are limited, however, by that, they can only be applied in the infants who survive the surgery. Furthermore, other predictive measures of survival such as the Score for Neonatal Acute Physiology, Version II27 and the Score for Neonatal Acute Physiology Perinatal Extension,28 which are based on general physiological parameters (blood pressure, urine output, lowest temperature, seizures, birth weight and being small for gestational age), are not specifically designed to describe the pathophysiology of hypoxaemia in pulmonary hypoplasia and CDH. Our study presents a simple, non-invasive method that can specifically assess hypoxaemia and one which can predict mortality with moderate–high sensitivity.
One strength of our study is that it was performed in a single, high-volume centre that included a relatively severe population and a high incidence of the FETO procedure for which KCH is a referral centre. Another strength of our method is that the ODC method can directly assess the mechanisms of hypoxaemia (reduced VA/Q and increased right-to-left shunt) which is the main pathophysiological mechanism leading to mortality in CDH. Our conclusions refer to our population based on our local policies, but it would be of interest to establish whether our results could be replicated in a multicentre prospective study or in a different, less severe cohort in centres that do not routinely perform the FETO procedure. We should note that a limitation of our method was that it could not be applied in the severely unwell infants who were nursed in 100% oxygen throughout their brief stay and before they died. This group of infants however does not constitute a prognostic conundrum, as their anticipated course is clear very early in postnatal life. In our study, we used two retrospectively collected paired points of SpO2 and FIO2, whereas some previous neonatal studies have used 3–5 prospective pairs.29, 30 Two pairs, however, have also been used9, 21, 22 as the higher value of SpO2 would predominantly define the degree of shunt and the lower value of SpO2 would adequately define the VA/Q. The retrospective nature of our data, finally, might have left us with fewer paired measurements to calculate the VA/Q and shunt within our narrow time window of 4 h compared to prospectively collected data. The disease is however rare, and a prospective study of a similar number of infants would require a period of approximately ten years to complete, even in a high-volume centre.
In conclusion, we have described that the ventilation to perfusion relationship improved over the immediate postnatal period in surviving infants with congenital diaphragmatic hernia and non-invasive calculation of the ventilation to perfusion ratio in such infants can provide valuable information relating to the anticipated survival to discharge.
Author contributions
T.D. conceived and designed the study and wrote the first version of the manuscript. F.M.S.A.T. acquired the data and contributed to the analysis. E.W. contributed to data acquisition and analysis. M.D. and K.H.N. contributed to study design, data interpretation and critically revised the manuscript. A.G. contributed to study design and administration and substantial critical revision of the manuscript. All authors have approved the final version of the manuscript.
Funding information
E.W. was supported by a grant from the Charles Wolfson Charitable Trust and a non-conditional educational grant from SLE. The research was supported by the National Institute for Health Research (NIHR) Biomedical Research Centre based at Guy’s and St Thomas’ NHS Foundation Trust and King’s College London. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health.
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
Patient consent was not required.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Snoek KG, et al. Congenital diaphragmatic hernia: 10-year evaluation of survival, extracorporeal membrane oxygenation, and foetoscopic endotracheal occlusion in four high-volume centres. Neonatology. 2018;113:63–68. doi: 10.1159/000480451. [DOI] [PubMed] [Google Scholar]
- 2.Coughlin MA, et al. Prenatally diagnosed severe CDH: mortality and morbidity remain high. J. Pediatr. Surg. 2016;51:1091–1095. doi: 10.1016/j.jpedsurg.2015.10.082. [DOI] [PubMed] [Google Scholar]
- 3.Mous, D. S., Kool, H. M., Wijnen, R., Tibboel, D. & Rottier, R. J. Pulmonary vascular development in congenital diaphragmatic hernia. Eur. Respir. Rev.27, 170104 (2018). [DOI] [PMC free article] [PubMed]
- 4.Kotecha S, et al. Congenital diaphragmatic hernia. Eur. Respir. J. 2012;39:820–829. doi: 10.1183/09031936.00066511. [DOI] [PubMed] [Google Scholar]
- 5.Beals DA, Schloo BL, Vacanti JP, Reid LM, Wilson JM. Pulmonary growth and remodeling in infants with high-risk congenital diaphragmatic hernia. J. Pediatr. Surg. 1992;27:997–1001. doi: 10.1016/0022-3468(92)90546-J. [DOI] [PubMed] [Google Scholar]
- 6.Acker SN, et al. Histologic identification of prominent intrapulmonary anastomotic vessels in severe congenital diaphragmatic hernia. J. Pediatr. 2015;166:178–183. doi: 10.1016/j.jpeds.2014.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.West, J. B. & Luks, A. West’s Respiratory Physiology: The Essentials 10th edn (Wolters Kluwer, 2016).
- 8.Dao DT, et al. Longitudinal analysis of ventilation perfusion mismatch in congenital diaphragmatic hernia survivors. J. Pediatr. 2020;219:160.e2–166.e2. doi: 10.1016/j.jpeds.2019.09.053. [DOI] [PubMed] [Google Scholar]
- 9.Dassios T, Ali K, Rossor T, Greenough A. Ventilation/perfusion ratio and right to left shunt in healthy newborn infants. J. Clin. Monit. Comput. 2017;31:1229–1234. doi: 10.1007/s10877-016-9969-7. [DOI] [PubMed] [Google Scholar]
- 10.Dassios T, Ali K, Rossor T, Greenough A. Using the fetal oxyhaemoglobin dissociation curve to calculate the ventilation/perfusion ratio and right to left shunt in healthy newborn infants. J. Clin. Monit. Comput. 2019;33:545–546. doi: 10.1007/s10877-018-0168-6. [DOI] [PubMed] [Google Scholar]
- 11.Dassios T, Curley A, Morley C, Ross-Russell R. Using measurements of shunt and ventilation-to-perfusion ratio to quantify the severity of bronchopulmonary dysplasia. Neonatology. 2015;107:283–288. doi: 10.1159/000376567. [DOI] [PubMed] [Google Scholar]
- 12.Russell-Jones E, Grammatikopoulos T, Greenough A, Dhawan A, Dassios T. Non-invasive assessment of intrapulmonary shunt and ventilation to perfusion ratio in children with hepatopulmonary syndrome before and after liver transplantation. Respir. Med. 2021;180:106372. doi: 10.1016/j.rmed.2021.106372. [DOI] [PubMed] [Google Scholar]
- 13.Ali K, et al. Outcome of Cdh infants following fetoscopic tracheal occlusion - influence of premature delivery. J. Pediatr. Surg. 2013;48:1831–1836. doi: 10.1016/j.jpedsurg.2013.01.049. [DOI] [PubMed] [Google Scholar]
- 14.Yu PT, Jen HC, Rice-Townsend S, Guner YS. The role of ECMO in the management of congenital diaphragmatic hernia. Semin. Perinatol. 2020;44:151166. doi: 10.1053/j.semperi.2019.07.005. [DOI] [PubMed] [Google Scholar]
- 15.Smith HL, Jones JG. Non-invasive assessment of shunt and ventilation/perfusion ratio in neonates with pulmonary failure. Arch. Dis. Child. Fetal Neonatal Ed. 2001;85:F127–F132. doi: 10.1136/fn.85.2.F127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lockwood GG, Fung NL, Jones JG. Evaluation of a computer program for non-invasive determination of pulmonary shunt and ventilation-perfusion mismatch. J. Clin. Monit. Comput. 2014;28:581–590. doi: 10.1007/s10877-014-9554-x. [DOI] [PubMed] [Google Scholar]
- 17.Ruano R, et al. Prediction and probability of neonatal outcome in isolated congenital diaphragmatic hernia using multiple ultrasound parameters. Ultrasound Obstet. Gynecol. 2012;39:42–49. doi: 10.1002/uog.10095. [DOI] [PubMed] [Google Scholar]
- 18.Deprest JA, et al. Randomized trial of fetal surgery for severe left diaphragmatic hernia. N. Engl. J. Med. 2021;385:107–118. doi: 10.1056/NEJMoa2027030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bjorkman KC, et al. Postoperative regional distribution of pulmonary ventilation and perfusion in infants with congenital diaphragmatic hernia. J. Pediatr. Surg. 2011;46:2047–2053. doi: 10.1016/j.jpedsurg.2011.06.042. [DOI] [PubMed] [Google Scholar]
- 20.Weidner M, et al. High temporal versus high spatial resolution in MR quantitative pulmonary perfusion imaging of two-year old children after congenital diaphragmatic hernia repair. Eur. Radiol. 2014;24:2427–2434. doi: 10.1007/s00330-014-3304-9. [DOI] [PubMed] [Google Scholar]
- 21.Dassios T, Kaltsogianni O, Hickey A, Bhat R, Greenough A. Chronology and determinants of respiratory function changes following administration of systemic postnatal corticosteroids in extremely preterm infants. J. Pediatr. 2019;215:17–23. doi: 10.1016/j.jpeds.2019.07.062. [DOI] [PubMed] [Google Scholar]
- 22.Williams E, Dassios T, Clarke P, Chowdhury O, Greenough A. Predictors of outcome of prematurely born infants with pulmonary interstitial emphysema. Acta Paediatr. 2019;108:106–111. doi: 10.1111/apa.14400. [DOI] [PubMed] [Google Scholar]
- 23.Dassios T, et al. Prediction of mortality in newborn infants with severe congenital diaphragmatic hernia using the chest radiographic thoracic area. Pediatr. Crit. Care Med. 2019;20:534–539. doi: 10.1097/PCC.0000000000001912. [DOI] [PubMed] [Google Scholar]
- 24.Tan YW, et al. Prognostic value of the oxygenation index to predict survival and timing of surgery in infants with congenital diaphragmatic hernia. J. Pediatr. Surg. 2019;54:1567–1572. doi: 10.1016/j.jpedsurg.2018.11.014. [DOI] [PubMed] [Google Scholar]
- 25.Brindle ME, Brar M, Skarsgard ED, Canadian Pediatric Surgery Network (CAPSNet). Patch repair is an independent predictor of morbidity and mortality in congenital diaphragmatic hernia. Pediatr. Surg. Int. 2011;27:969–974. doi: 10.1007/s00383-011-2925-1. [DOI] [PubMed] [Google Scholar]
- 26.Congenital Diaphragmatic Hernia Study Group et al. Defect size determines survival in infants with congenital diaphragmatic hernia. Pediatrics. 2007;120:e651–e657. doi: 10.1542/peds.2006-3040. [DOI] [PubMed] [Google Scholar]
- 27.Skarsgard ED, et al. SNAP-II predicts mortality among infants with congenital diaphragmatic hernia. J. Perinatol. 2005;25:315–319. doi: 10.1038/sj.jp.7211257. [DOI] [PubMed] [Google Scholar]
- 28.Chiu LW, et al. SNAPPE II score as a predictor of survival in neonates with congenital diaphragmatic hernia: a single center experience. Eur. J. Pediatr. Surg. 2016;26:316–321. doi: 10.1055/s-0035-1554103. [DOI] [PubMed] [Google Scholar]
- 29.Stoecklin B, et al. Simplified bedside assessment of pulmonary gas exchange in very preterm infants at 36 weeks’ postmenstrual age. Thorax. 2021;76:689–695. doi: 10.1136/thoraxjnl-2020-214659. [DOI] [PubMed] [Google Scholar]
- 30.Svedenkrans J, Stoecklin B, Jones JG, Doherty DA, Pillow JJ. Physiology and predictors of impaired gas exchange in infants with bronchopulmonary dysplasia. Am. J. Respir. Crit. Care Med. 2019;200:471–480. doi: 10.1164/rccm.201810-2037OC. [DOI] [PubMed] [Google Scholar]