Highlights
-
•
Walking reduces pre-stimulus alpha power and increases N1 amplitude.
-
•
Pre-stimulus alpha predicts N1, P3, and behavior but not post-stimulus alpha.
-
•
Post-stimulus alpha power is less modulated by the stimulus during walking.
-
•
The effect of walking on post-stimulus alpha is dependent on stimulus features.
Keywords: Alpha power, Attention, Free walking, Mobile EEG, Visual evoked potentials, Orientation discrimination
Abstract
Walking influences visual processing but the underlying mechanism remains poorly understood. In this study, we investigated the influence of walking on pre-stimulus and stimulus-induced visual neural activity and behavioural performance in a discrimination task while participants were standing or freely walking. The results showed dissociable pre- and post-stimulus influences by the movement state. Walking was associated with a reduced pre-stimulus alpha power, which predicted enhanced N1 and decreased P3 components during walking. This pre-stimulus alpha activity was additionally modulated by time on the task, which was paralleled by a similar behavioural modulation. In contrast, the post-stimulus alpha power was reduced in its modulation due to stimulus onset during walking but showed no evidence of modulation by time on the task. Additionally, stimulus parameters (eccentricity, laterality, distractor presence significantly influenced post-stimulus alpha power, whereas the visually evoked components showed no evidence of such an influence. There was further no evidence of a correlation between pre-stimulus and post stimulus alpha power. We conclude that walking has two dissociable influences on visual processing: while the walking induced reduction in alpha power suggests an attentional state change that relates to visual awareness, the post-stimulus influence on alpha power modulation indicates changed spatial visual processing during walking.
1. Introduction
Animal studies of visual perception during locomotion have shown motor state related changes in both early and late visual cortical activities (Ayaz et al., 2013; Busse et al., 2017; Erisken et al., 2014; Mineault et al., 2016; Niell and Stryker, 2010). In recent years, there is also an increase in human electrophysiological research using various movement tasks (e.g. cycling, treadmill walking, and freely walking) investigating the influence of locomotion on different stages of visual processing (Bullock et al., 2015, 2017; Cao and Händel, 2019; Chen et al., 2022; Dodwell et al., 2021; Garrett et al., 2021; Gramann et al., 2010; Ladouce et al., 2016; Wagner et al., 2014). The visual processing stages can be roughly divided into an early and a late processing stage, where the early stage is related to the processing of the physical properties of stimuli and the late stage is related to the cognitive extraction of stimulus information (e.g. discrimination and identification).
For early sensory processing, in line with the animal work that observed a movement related increase in firing rates in the visual cortex (Dipoppa et al., 2018; Kaneko et al., 2017; Niell and Stryker, 2010; Vinck et al., 2015), a similar increase of early visual evoked potentials (VEPs) during locomotion in humans was reported recently using mobile EEG (electroencephalogram). For instance, both P1 component (peak ∼ 130 ms) and Ppc component (peak: ∼ 150 ms) have been found to be larger during cycling compared to resting state (Bullock et al., 2015; Dodwell et al., 2021). A robust N1 (peak∼180 ms) component enhancement was further observed during free walking (Chen et al., 2022). In a slightly later time window, studies have revealed a reduction in the P3 component due to movement. Bradford et al. (2019) found that walking led to a decreased P3 using a visual oddball task. A similar P3 reduction was found by Nenna et al. (2020) using a discrimination task as well as a target detection task (Protzak et al. (2021). Even though divergent visual stimuli were used in the above-listed studies, walking consistently affected the evoked VEPs, i.e. walking led to increased early visual responses and reduced later visual responses at ∼300 ms. However, the source of the movement-related modulation in sensory processing remains unclear. In the current study, we tested the idea that a general change in alpha oscillations due to movement state can account for the changes in VEPs. Based on the above-mentioned movement-related studies, we specifically focused on the N1 component peaking at ∼180 ms and the P3 component peaking at ∼ 300 ms.
Previous animal studies have shown that the modulation of early visual cortex by walking speed can be independent of visual input as it persists in complete darkness (Dipoppa et al., 2018; Erisken et al., 2014; Keller et al., 2012). Similarly, a recent walking study in humans found a movement related alpha power decrease in both light and darkness (Cao et al., 2020). This suggests that the activity changes introduced by walking have a non-visual basis which can be reflected by alpha power changes. Indeed, the alpha power decrease during walking is a robust effect and has been shown by several groups (Ehinger et al., 2014; Lin et al., 2014; Peterson and Ferris, 2018; Storzer et al., 2016; Yokoyama et al., 2021). Albeit the fact that the alpha modulation can be independent of the visual input, a recent finding indicated that the walking related alpha power is associated with a change in the spatial distribution of visual input processing during walking (Cao and Händel, 2019). This could indicate that alpha marks a processing state which is modified by walking. In the study at hands we therefore asked if the alpha activity, as modulated by walking, predicts the changed early VEPs following sensory input. Indeed, in previous studies a correlation between pre-stimulus alpha power and the early VEPs has been described (Brandt and Jansen, 1991; De Blasio and Barry, 2013; Roberts et al., 2014). While the influence of walking on continuous alpha power as well as early VEPs is known, a relationship between these processes has not been established during walking. This however would constitute an important step in understanding how walking influences early sensory processing.
A second important aspect was to test if the walking induced change in processing is based on a general modulation of state or if it is input specific. If general in nature, the walking induced effect should already be found before stimulus onset and be similar for every stimulus manipulation. In other words, there should be no interaction between stimulus features and movement state. In contrast, the change in the response to sensory input might be specific to certain aspects of sensory processing. In this case, one should find a significant interaction between stimulus features and movement state, given that the relevant features are indeed included. In order to test this, we manipulated the stimulus with respect to target location, distractor presence and eccentricity. These manipulations were chosen because of the involvement of alpha activity in distractor inhibition (Clayton et al., 2015; Foxe and Snyder, 2011; Händel et al., 2011; Schroeder et al., 2018; Wostmann et al., 2019) and the differential effect of walking on neural and behavioural measures over the visual field (Cao and Händel, 2019). The main aim of the study was to understand the relationship between the movement state induced changes in ongoing alpha activity and the amplitude modulation of VEPs. However, in order to further establish this alpha activity as a marker of state, we additionally investigated another state change that is experienced during the course of an experiment. To this end, we analysed the change in alpha power as well as the behavior over the whole period of the experiment.
To investigate the nature of the effect of movement state on early sensory processing, electrophysiological data and behavioural performance were collected while participants performed a line orientation discrimination task (Fig. 1) during free walking vs. standing using AR glasses and mobile EEG. Pre-stimulus and post-stimulus neural responses were both investigated as a comparison. We replicated previous findings of an enhanced N1 and a reduced P3 component during walking but further showed that these components were predicted by the pre-stimulus alpha power. Additionally, no evidence was found that the effect of walking was dependent on the stimulus-related factors (target location, distractor presence and eccentricity), therefore suggesting that walking can induce a rather general change in the internal state. Alpha power, which we interpret as a marker of the internal state, was also modulated by time on the task and may be related to a learning effect. This was independently found during movement and stationarity. Besides the general movement related change in state, we found a second, stimulus specific influence of walking. Stimulus induced alpha power showed less modulation during walking throughout the task but was particularly influenced by eccentricity and distractor presence, which was neither predicted by pre-stimulus alpha power nor modulated by time on the task. Our findings indicate dissociable pre- and post-stimulus influences of walking on visual processing. One is marked by pre-stimulus alpha power and predicts the amplitude of early sensory responses to any visual input. The other is marked by stimulus induced alpha power and is specific for visual features.
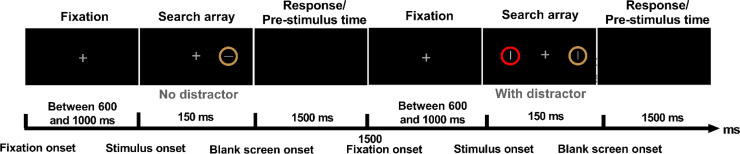
Fig. 1.
Two possible trial sequences. Every trial started with a fixation interval (duration randomly selected between 600 and 1000 ms) followed by the visual search array (150 ms) that included a yellow circle (the target) with a gray line inside. The target was either displayed alone (“no distractor” manipulation, shown here in the first trial) or accompanied by a distractor which was a gray line inside either a red or a green circle (“with distractor” manipulation, shown here in the second trial). Stimulus manipulations further included the target location (left vs. right) and stimulus eccentricity (1.3°, 9° and 16°). Two examples with an eccentricity of 9° are shown here. After the visual search array, a blank screen was presented for 1500 ms before the start of the next trial, during which participants should report the line orientation within the yellow circle with a keypress as fast and as accurately as possible. Please note that the background is not actually black as participants saw the visual input superimposed on the real-world scene with AR glasses.
2. Materials and methods
2.1. Participants
30 healthy adults (21 females, 9 males; age: M = 25.37, SD = 3.88) with normal or corrected-to-normal visual acuity were recruited from a local participant pool via the SONA system. All participants gave informed consent before the experiment, and they were compensated with 10 euros per hour after the experiment. The experimental protocol was approved by the Research Ethics Committee at the University of Würzburg and complied with the Declaration of Helsinki and the European data protection law (GDPR). All measures also complied with the COVID related hygiene safety concept for Psychological Experiments in the Summer Semester 2020 at the Institute for Psychology (Division for Cognitive Psychology) of Wuerzburg University.
2.2. Stimuli and procedure
Participants performed a line orientation discrimination task in two movement states (stand vs. walk). The stimulus manipulation included the target location (left vs. right), distractor presence (“with distractor” (red/green) vs. “no distractor”) and stimulus eccentricity (1.3°, 9° and 16°). There was an equal number of trials (32) for each possible combination of stimulus manipulation (36) which resulted in a total of 1152 trials. Trials were presented randomly except for the movement state manipulation, which was grouped in four blocks (each block contained one sub-block of standing and another sub-block of walking, with each sub-block including 144 trials). In the “with distractor” manipulation, the visual search array consisted of a yellow circle marking the target and an opposite green or red circle marking the distractor. There was a gray line inside each circle with a horizontal or vertical orientation (balanced between trials). We manipulated the distractor to be either red or green as the two colours can both work as distractors (Chen et al., 2022). In the “no distractor” manipulation, only a target, i.e. a yellow circle with a gray line inside, was included. All circles were 2.2° in diameter with the eccentricity (the distance between the fixation cross and the center of the circle) being 1.3°, 9° or 16° The two circles in the “with distractor” manipulation were always presented in equal eccentricity. Participants were asked to report the line orientation inside the yellow target circle. Half of the participants used their right hand and the other half used their left hand for the response. Responses were collected using a handheld response box, which has two buttons for indicating the line orientation as vertical or horizontal (thumb press and middle finger press; counterbalanced between participants for response mapping). Both accuracy and reaction time were emphasized as the goal to the participants. Two examples of possible event sequences within a trial are illustrated in Fig. 1. All stimuli were presented via a pair of augmented reality glasses with a 60 Hz refresh rate (DreamWorld AR, Dream Glass 4 K edition; San Mateo, CA). Participants could see through the transparent glasses while the task related stimuli were projected onto the real-world scene (as if floating in the air).
The experiment was conducted in a dim and spacious room of approximately 5 × 6 m. Participants could walk around freely in the room. The dim environment helped to keep the environmental light relatively constant despite the movement. The experiment was conducted in four blocks, each containing a standing and a walking sub-block. Over the experiment, all manipulations were balanced between the walking and standing. Trials were randomly placed within the blocks. In the standing manipulation, participants were asked to stand still while doing the task. In the walking manipulation, participants were asked to walk freely at a normal speed while doing the task. Prior to the formal testing, the experimenter showed the participants the normal walking speed (∼0.8 m/s) and made sure they could follow the required speed by providing feedback in an approximately 1 min practice session. Half of the participants were first tested with the standing manipulation in each block and the other half with the walking manipulation first. A self-paced short break was given in between blocks. During the whole experiment, the experimenter visually monitored the walking speed making sure there was no significant pace change.
2.3. Data recording
EEG data were collected using a Smarting mobile EEG system (mBrainTrain LLC, Serbia), which has 24 recording electrodes with a sampling rate of 500 Hz. Among the 24 electrodes, 6 electrodes (3 electrodes for each eye: one below and one above the eye, one to the outer canthus) were used for electrooculogram (EOG) recording, which were included in the independent component analysis (ICA) for removing eye movement artefacts. Among the remaining 18 electrodes used for EEG recording, 2 electrodes on the two earlobes were used for possible re-referencing, and the other 16 electrodes were distributed according to the standard 10–20 EEG system including the following electrodes: AFz, F3, Fz, F4 (frontal); C3, Cz, C4, CPz (central); P3, P4, P7, P8, Pz, POz (posterior); O1, O2 (occipital). The common mode sense active electrode placed between Fz and Cz was used for online reference. The mobile EEG system has the EEG signal amplifier and data transmitter integrated into a little box (82 × 51 × 12 mm; 60 gs), which was attached to the back of the EEG cap. The EEG and EOG data were transmitted via Bluetooth. Stimulus triggers were generated with the software Lab Streaming Layer (https://github.com/sccn/labstreaminglayer), which was also used for collecting and synchronizing the other streams of data (EEG, behavioural data). Stimulus generation and presentation were controlled by MATLAB (The Mathworks Inc, R2019b) with the Psychtoolbox add-on (Kleiner et al., 2007). A Dell laptop (model: Latitude E7440) was used for the implementation of the study, which was carried by the participants in a rucksack during the experiment. Therefore, participants were free to move without restriction during the testing.
2.4. Behavioural analysis
2.4.1. Subject exclusion
One participant was excluded due to data transmission error during the experiment. For the remaining 29 participants, a correct response was registered if participants correctly reported the line orientation within 1500 ms from stimulus onset. The accuracy was computed as the ratio of correct response trials to total trials. Reaction time data (from stimulus onset to response) were only calculated for correct response trials. Data from 3 participants were excluded from further analysis because of low accuracy (< 0.60). The remaining 26 participants all had an accuracy over 0.70 (M = 0.87, SD = 0.07).
2.4.2. Main behavioural analysis
The sensitivity measure d’ was used, which was calculated as d’ = Z(Hit rate) – Z(False alarm rate). We calculated d’ separately for targets in the left visual field and targets in the right visual field. Horizontal lines were defined as the signal, and vertical lines were defined as the noise. Hit rate was the ratio of correct response trials to the signal, and false alarm rate was the ratio of incorrect response to the noise. Extreme false alarm rates of 0 were replaced with 0.5/n, and extreme hit rates of 1 were replaced with (n − 0.5)/n (where n is the trial number in each condition) (Macmillan and Kaplan, 1985; Stanislaw and Todorov, 1999).
A four-way (movement state: standing vs. walking; target location: left vs. right; distractor presence: with vs. no distractor; eccentricity: 1.3° vs. 9° vs. 16°) repeated-measures ANOVA was performed with the d’ and the mean reaction time as the dependent variable. Throughout the manuscript, a Greenhouse-Geisser correction was performed for ANOVA results where necessary. Statistical results are reported as significant when the p value was below 0.05.
2.5. EEG - ERP analysis
Among the 26 participants included in the behavioural analysis, the EEG data from 1 participant were incomplete because of strong artefacts in the occipital channels (P7, O1 and O2) which could not be fixed. The remaining data from the 25 participants were analysed with the Fieldtrip toolbox (Oostenveld et al., 2011) and in-house scripts using Matlab (The MathWorks Inc., USA). Because of the low frequency noise, a band-pass filter between [1 30] Hz using a windowed sinc FIR filter was used. In order to exclude potential artificial effects due to the use of a 1 Hz lower bound in signal filtering, all the analyses were also performed with a lower high pass cut-off of 0.1 Hz, which led to qualitatively similar results but were noisier (Supporting information S1). We therefore reported the results obtained with the 1 Hz lower bound filter. The filtered data were then epoched into trials ([−1000 1500] ms, with stimulus presentation at time 0). Artefact rejection was implemented in two steps. First, trials were visually inspected using the fieldtrip function ft_rejectvisual, and trials with excessive noise were manually excluded based on the variance across channels. Second, the principal component analysis was performed to reduce the spatial dimensionality of the EEG data to 16, which was followed by an ICA to correct for eye movements, heartbeat, and muscle related artefacts. An average of 12.17 trials (SD = 8.21; out of the total 1152 trials) and 2.77 artefact components (SD = 1.52; out of the total 16 components) were rejected. To demonstrate the robustness of the reulsts obtained with the above pre-processing, we also showed all main EEG results with data filtered between [1 100] Hz and with artefact subspace reconstruction before ICA (Supporting information S2). The EEG data were baseline-corrected by applying a 600 ms pre-stimulus (averaged over −600 to 0 ms) absolute baseline to each trial. The grand average ERP was calculated using 4 electrodes: P7, P8, O1 and O2. The time windows of the N1 and P3 components were selected by using a 50 ms window centring the component peak.
2.6. EEG – time-frequency analysis
To evaluate the temporal evolution of alpha power, single-trial power (2 to 30 Hz) was computed based on the multi-taper-convolution method (‘mtmconvol’ in Fieldtrip) which uses a sliding window (500 ms, in steps of 50 ms starting from −1000 ms to 1500 ms, resulting in a frequency resolution of 2 Hz) with a Hanning taper. The grand average alpha power was averaged over [8 −14] Hz, the range of which covered 22 out of 25 participants’ peak alpha frequency (individual peak: M = 10.88, SD = 1.17). The 3 remaining participants had no visible alpha peak). The same four occipital electrodes as chosen for the ERP analysis (P7, P8 O1 and O2) were used in related alpha power analyses. Alpha power from three time windows was selected for analysis. To test whether the walking related modulation of the stimulus induced alpha changes is influenced by the features of the visual input, we chose a time window of 300 to 450 ms after stimulus onset, as it shows the biggest difference in alpha power between standing and walking via visual inspection. Only for the stimulus induced alpha analysis, we applied a relative baseline correction ([−600 0] ms) using log transformation. This was done to get rid of the movement state related difference in baseline alpha power. The pre-stimulus alpha was selected between 1000 and 1200 ms after the stimulus onset. Note that this pre-stimulus time window was relative to the next trial. The fixation alpha was selected between −200 and 0 ms relative to the stimulus onset.
2.7. Within-participants correlational analyses between alpha power, VEPs and behavior
In order to evaluate the relationship between alpha power, N1, P3, and behavioural performance, within-participants correlation analyses were performed. For each participant (n = 25), the Pearson correlation coefficient (r) was calculated between relevant measures (e.g. alpha power and N1) over the 36 manipulations (i.e. 2 movement states by 2 target locations by 3 distractor scenarios (red/green distractor, no distractor) by 3 stimulus eccentricities; 32 trials in each manipulation). In each manipulation, the relevant measures were calculated as the average over 32 trials. The resulting 25 r values (Fisher's z-transformed) were subject to a one sample t-test against 0 to check statistical significance A significant within-participants correlation was assumed given a t-test result of p < 0.05.
We tested three different alpha power types. The stimulus induced alpha power ([300 450] ms, baseline corrected), the pre-stimulus alpha power ([1000 1200] ms) and the alpha power during the fixation period ([−200 0] ms). For the pre-stimulus alpha power, trials with a reaction time > 1000 ms were excluded to exclude the potential influence of motor response on alpha power (excluded trials in each manipulation: M = 1.54, SD =1.87).
2.8. Modulation of alpha power, VEPs and behavior over time on the task
In order to evaluate the modulation of the alpha power, VEPs and behavioural responses over time, the 32 trials within each of the 36 manipulations were ordered based on the time of presentation and then averaged over all manipulations. In this way, trials from 1 to 32 followed the order of presentation during the testing, however, would not represent an exact time point. Therefore, trial 3 would always be after trial 2 in all manipulations, but trial 3 of manipulation A could be presented before trial 2 of manipulation B. Nevertheless, the correlation between the trial number (time) and the tested variables (pre-stimulus alpha, fixation alpha, stimulus induced alpha, N1 and P3, d’, and reaction time) can reveal time dependent changes.
3. Results
3.1. Behaviour results
3.1.1. Walking led to a decreased d’
To check how movement states interact with target location, distractor presence and eccentricity, the d’ data were entered into a four-way (movement state: standing vs. walking; target location: left vs. right; distractor presence: with distractor vs. no distractor; eccentricity: 1.3° vs. 9° vs. 16°) repeated-measures ANOVA. The main effect of movement state (F(1,25) = 4.77, p = 0.04) showed a higher d’ for the standing condition (M = 3.10, SD = 0.60) than for the walking condition (M = 2.92, SD = 0.43). The main effect of distractor presence (F(1,25) = 5.99, p = 0.02) showed a higher d’ when there was no opposite distractor (M = 3.10, SD = 0.49) compared with when there was an opposite distractor (M = 2.92, SD = 0.54), indicating a successful manipulation of the distraction effect. The main effect of eccentricity (F(2,50) = 8.06, p = 0.002) showed that 16° eccentricity was associated with a lower d’ than the other two eccentricities (1.3°: M = 3.10, SD = 0.50; 9°: M = 3.10, SD = 0.53; 16°: M = 2.83, SD = 0.55). We also observed a significant interaction effect between target location and distractor presence (F(1,25) = 5.82, p = 0.02). In addition, the interaction between movement state, distractor presence, and eccentricity was also significant (F(2,50) = 3.50, p = 0.04), which can be found in Fig. 2a. Complete test statistics are listed in Table 1.
Fig. 2.
Behavioural performance averaged over target location. Data shown for the d’(a) and the reaction time (b) for each manipulation during standing (black bars) and walking (green bars).
Table 1.
Results of multifactorial repeated measures ANOVA with d’ and reaction time data.
| d’ |
Reaction time |
|||||
|---|---|---|---|---|---|---|
| Parameter | d.f. | F | p-value | F | p-value | |
| Movement state(MS) | 1, 25 | 4.77 | 0.04* | 1.24 | 0.28 | |
| Target location(TL) | 1, 25 | 2.25 | 0.15 | 16.04 | < 0.001⁎⁎ | |
| Distractor presence(DP) | 1, 25 | 5.99 | 0.02* | 114.98 | < 0.001⁎⁎ | |
| Eccentricity(EC) | 2, 50 | 8.06 | 0.003* | 85.35 | < 0.001⁎⁎ | |
| MS × TL | 1, 25 | 0.45 | 0.51 | 0.02 | 0.89 | |
| MS × DP | 1, 25 | 0.44 | 0.52 | 0.07 | 0.79 | |
| MS × EC | 2, 50 | 0.33 | 0.71 | 0.31 | 0.73 | |
| TL × DP | 1, 25 | 5.82 | 0.02* | 0.29 | 0.60 | |
| TL × EC | 2, 50 | 0.76 | 0.44 | 1.48 | 0.24 | |
| DP × EC | 2, 50 | 2.08 | 0.15 | 1.25 | 0.29 | |
| MS × TL × DP | 1, 25 | 1.04 | 0.32 | 5.33 | 0.03* | |
| MS × TL × EC | 2, 50 | 1.99 | 0.15 | 1.21 | 0.31 | |
| MS × DP × EC | 2, 50 | 3.50 | 0.04* | 0.97 | 0.38 | |
| TL × DP × EC | 2, 50 | 1.54 | 0.23 | 0.46 | 0.62 | |
| MS × TL × DP × EC | 2, 50 | 2.60 | 0.09 | 0.03 | 0.97 | |
Significant effects are indicated in bold.
p<0.05,.
p<0.001.
The same analysis using reaction time as dependent variable again revealed a significant main effect of target location (F(1,25) = 16.04, p < 0.001), with a faster response for the right targets (M = 0.60, SD = 0.11) than for the left targets (M = 0.62, SD = 0.11). The main effect of distractor presence was also significant (F(1,25) = 11.5, p < 0.001), and the reaction time was faster when there was no opposite distractor (M = 0.60, SD = 0.11) compared to when there was one (M = 0.63, SD = 0.11), again indicating a successful manipulation of the distraction effect. Also the main effect of eccentricity was significant (F(2,50) = 85.35, p < 0.001), with the large eccentricity leading to a significantly slower response compared to the smaller two eccentricities (1.3°: M = 0.59, SD = 0.10; 9°: M = 0.60, SD = 0.11, 16°: M = 0.65, SD = 0.11). In contrast to d’, there was no significant main effect of movement state on reaction time (F(1,25) = 1.24, p = 0.28). However, a significant interaction effect between movement state, target location and distractor presence was found (F(1,25) = 5.33, p = 0.03). No other interaction effects were statistically significant. Complete test statistics are listed in Table 1.
Taken together, the d’ data showed a reduction in behavioural performance during walking. The interaction between movement state, distractor presence and eccentricity also indicated a changed spatial visual processing during walking. Reaction time largely followed the effects of d’ but showed no main effect of movement state but an interaction between movement state, target location and distractor presence indicating an input specific effect of walking on the behavioural output.
3.2. EEG results
3.2.1. Walking led to an early internal-state related processing change
Enhanced N1 response during walking
The amplitude of N1 component was averaged over occipital electrodes (O1, O2, P7 and P8). A four-way (movement state: standing vs. walking; target location: left vs. right; distractor presence: with distractor vs. no distractor; eccentricity: 1.3° vs. 9° vs. 16°) repeated-measures ANOVA with the N1 amplitude ([170 220] ms) showed a significant main effect of movement state (F(1,24) = 11.2, p = 0.002) (Fig. 3a-c). The N1 component was larger during walking (M = - 4.48, SD = 3.01) than during standing (M = −3.14, SD = 2.62). The main effect of distractor presence was also significant (F(1, 24) = 4.45, p = 0.045). A stronger N1 was found during “with distractor” manipulation (M = - 4.09, SD =3.19) than during “no distractor” manipulation (M = −3.80, SD = 3.07). No other main effects or interaction effects were found statistically significant. To be noted, the N1 enhancement during walking did not interact with any stimulus-related manipulation, including the interaction between movement state and target location (F(1,24) = 0.03, p = 0.95), the interaction between movement state and distractor presence (F(1,24) = 0.01, p = 0.91) as well as the interaction between movement state and eccentricity (F(2,48) = 0.93, p = 0.38). In summary, we found an enhanced N1 response due to walking and due to distractor presence. Importantly, no evidence was found supporting that the effect of movement was dependent on any external stimulus-related manipulation (target location, distractor presence, and eccentricity).
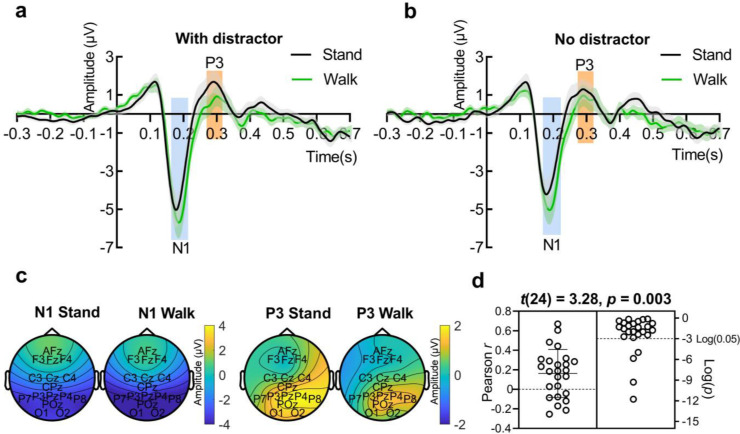
Fig. 3.
N1 enhancement and P3 decrease during walking. The grand average ERP during standing (black line) and during walking (green line) for (a) “with distractor” manipulation and (b) “no distractor” manipulation. Data are presented as mean ± SEM (standard error of the mean). (c) The topography of the N1 ([170 220] ms) and P3 component ([270 320] ms) for standing and walking separately. (d) Within each participant (n = 25), the correlation between the N1 and the P3 component was tested among all 36 manipulations based on the average amplitude of 32 trials in each manipulation. A significant one-sample t-test between the Pearson r value (Z-transformed) and 0 (n = 25) indicates a positive correlation between N1 and P3 component. Pearson r data correspond to the left y-axis while the p value are plotted on a log scale corresponding to the right y-axis. Each circle represents a participant.
Reduced P3 response during walking
The posterior P3 component was also tested as to its modulation by movement state, target location, distractor presence, and eccentricity. A four-way (movement state: standing vs. walking; target location: left vs. right; distractor presence: with distractor vs. no distractor; eccentricity: 1.3° vs. 9° vs. 16°) repeated-measures ANOVA with the P3 amplitude ([270 320] ms) showed only a significant main effect of movement state (F(1,24) = 7.28, p = 0.01). The P3 component was larger during standing (M = 1.33, SD = 2.60) than during walking (M = 0.75, SD = 2.57) (Fig. 3a-c). No other effects were significant. Similar to the N1 component, no evidence for the dependence of the movement effect on any external stimulus-related manipulation (target location, distractor presence, and eccentricity) was found, as the interaction between movement state and target location (F(1,24) = 2.40, p = 0.10), the interaction between movement state and distractor presence (F(1,24) = 1.12, p = 0.30), and the interaction between movement state and eccentricity (F(2,48) = 0.99, p = 0.37) all did not reach statistical significance.
The enhanced N1 component and decreased P3 component during walking all did not show any evidence of an interaction with external stimulus manipulation, indicating that the two movement state related effects may have a common origin. To this end, we checked whether the two components were correlated. The within-participant test based on manipulations showed a significant positive correlation between N1 and P3 component (one-sample t-test between each participant's r value and zero: t(24) = 3.28, p = 0.003) (Fig. 3d). When testing the behavioural outcome, we found neither a significant within-participants correlation between the N1 amplitude and reaction time (t(24) = - 0.89, p = 0.38) or d’ (t(24) = - 0.49, p = 0.63), nor between the P3 amplitude and reaction time (t(24) = 0.52, p = 0.61) or d’(t(24) = - 0.38, p = 0.70).
3.2.2. Pre-stimulus alpha power was reduced during walking and linked to reaction time, N1 and P3 component
In order to check whether the N1 and P3 changes induced by walking were driven by a general movement-induced state change, we compared the non-baseline corrected pre-stimulus ([1000 1200] ms) and fixation ([−200 0] ms) alpha power (averaged between [8–14] Hz) between standing and walking. Alpha power was taken from the same electrodes (P7, P8, O1 and O2) as chosen for the VEPs and the relationship with VEPs was also analysed. For pre-stimulus alpha power, the results showed a decrease in alpha power during walking (M = 17.62, SD = 12.01) compared to standing (M = 24.37, SD = 21.39) (t(25) = 3.21, p = 0.004) (Fig. 4a). The topography of the pre-stimulus alpha power showed a difference between standing and walking over posterior-occipital electrodes (Fig. 4b). We then checked whether the pre-stimulus alpha power was linked to behavioural responses. The within-participants correlation analysis showed a significant correlation between pre-stimulus alpha power and the reaction time (one-sample t-test between each participant's r value and zero: t(24) = −3.27, p = 0.003), with stronger pre-stimulus alpha power linked to a faster response (Fig. 4d). The correlation between pre-stimulus alpha power and d’ was not significant (t(24) = −1.11, p = 0.27). The same comparison between standing and walking was performed for alpha power during the fixation time window ([−200 0] ms). However, the fixation alpha power neither differed significantly between standing and walking (t(24) = −0.58, p = 0.57) (Fig. 4c), nor was correlated with the reaction time (t(24) = −1.03, p = 0.32).
Fig. 4.
Pre-stimulus alpha power correlated with reaction time and predicted the N1 and P3 components. (a) EEG power spectra of the pre-stimulus time window ([1000 1200] ms) for standing (black line) and walking (green line). Data are presented as mean ± SEM (standard error of the mean) (left panel). Alpha power (averaged between [8 −14] Hz) during walking was significantly decreased compared to standing (right panel). (b) The topography of the difference between standing and walking (stand-walk) during the pre-stimulus time window. (c) EEG power spectra of the fixation time window ([−200 0] ms) for standing (black line) and walking (green line). (d) Within each participant (n = 25), the correlation between the pre-stimulus alpha power and the reaction time was tested among all 36 manipulations based on the average amplitude of 32 trials in each manipulation. A significant one-sample t-test between the r value (z-transformed) and 0 (n = 25) indicates a prevalent negative correlation between pre-stimulus alpha power and reaction time. The original Pearson r data correspond to the left y axis while the p values (log scale) correspond to the right y axis. (e) Same as in d but between pre-stimulus alpha power and the N1 component. (f) Same as in d but between pre-stimulus alpha power and the P3 component.
We further checked whether the movement state-related decrease in pre-stimulus alpha power could predict the N1 and P3 components. The correlations between the pre-stimulus alpha power and the next trial's N1 and P3 components were first examined (based on all 36 manipulations). As a result, the pre-stimulus alpha power significantly predicted the N1 component (t-test between each participant's r value and zero: t(24) = 2.17, p = 0.04) and the P3 component (t(24) = 2.35, p = 0.03) (Fig. 4e, f); The same correlations were also checked for standing and walking separately. Since the within-participants correlation was performed based on the manipulations, the correlation analysis for standing and walking separately led to only 18 sub-manipulations for each participant and each movement state. The correlation between the pre-stimulus alpha power and P3 component was significant for walking (t(24) = 2.28, p = 0.03) but did not reach statistical significance for standing (t(24) = 1.61, p = 0.12). The correlation between pre-stimulus alpha and the N1 component for standing (t(24) = 1.70, p = 0.10) and walking (t(24) = 1.13, p = 0.27) did not reach significance. However, this probably was due to the reduction in the availability of data when testing separately for each movement state. Same reason might explain the non-significant result when testing for a correlation between pre-stimulus alpha power and reaction time for standing (t(24) = −0.46, p = 0.65) and walking (t(24) = −0.75, p = 0.46) separately.
In addition, although we did not find a significant difference between standing and walking in the alpha power during fixation, we nevertheless checked its relationship with the N1 and P3 components. There was no indication that fixation alpha power was correlated with the N1 component (t(24) = 0.46, p = 0.64) or the P3 component (t(24) = 0.35, p = 0.73). To summarize, the pre-stimulus alpha power significantly differed between standing and walking and predicted the N1 and P3 components as well as reaction time.
3.3. Modulation of alpha power, VEPs and behavior over time on the task
The overall pre-stimulus alpha power showed a trend to increase with time on the task during both standing and walking (Supporting information S2). To statistically test whether alpha power changeed over time on the task, we investigated how alpha power (fixation and pre-stimulus) and behavioural responses changed from the first trial (1) to the last trial (32). As shown in Fig. 5, pre-stimulus alpha power increased with time during both standing (r = 0.88, p < 0.001) and walking (r = 0.87, p < 0.001) (Fig. 5a). The increase in alpha power with time corresponded to a behavioural improvement over time: reaction time decreased with time for both standing (r = 0.57, p < 0.001) and walking (r = 0.67, p < 0.001) (Fig. 5c); d’ increased with time for both standing (r = 0.49, p = 0.004) and walking (r = 0.75, p < 0.001) (Fig. 5d). However, the fixation alpha power only increased with time during standing (r = 0.79, p < 0.001), but not during walking (r = - 0.01, p = 0.68) (Fig. 5b).
Fig. 5.
The modulation of alpha power, VEPs and behavioural responses over time on the task. (a) Averaged over all manipulations, the pre-stimulus alpha power ([1000 1200] ms) increased with time during both standing (black line) and walking (green line). Each dot represents a trial (1 to 32 trials, ordered by time on the task). (b) The fixation alpha power ([−200 0] ms) increased with time during standing but not during walking. (c) The reaction time became faster with time similarly for standing and walking. (d) The d’ also increased with time similarly for standing and walking. Data are presented as mean ± SEM (standard error of the mean).
To check the validity of the time effect, the modulation by time on the N1 and P3 components was also examined. Since pre-stimulus alpha power can predict the N1 and P3 component, we found as expected, that N1 decreased during standing (r = 0.50, p = 0.004) and walking (r = 0.44, p = 0.01) as time increased; P3 increased during standing (r = 0.47, p = 0.01) and walking (r = 0.52, p = 0.002) as time increased.
3.4. Stimulus related alpha desynchronisation was smaller during walking and dependent on distractor presence and eccentricity
Alpha power at posterior-occipital electrodes is believed to reflect visual attentional processing with a special role in distractor suppression (Foxe and Snyder, 2011; Händel et al., 2011; van Diepen, Miller, Mazaheri, and Geng, 2016; Van Dijk, Schoffelen, Oostenveld, and Jensen, 2008). Therefore, we investigated the difference of the baseline corrected post-stimulus alpha power (induced by stimulus onset) between standing and walking with respect to spatial attention, distractor suppression and eccentricity of attentional focus. Posterior baseline-corrected alpha power was taken from the same electrodes as used for the ERP analysis (P7, P8, O1 and O2). The time window that demonstrated the clearest alpha power difference between standing and walking was identified through visual inspection of an average over all manipulations ([300 450] ms). The corresponding topography showed an alpha decrease over posterior electrodes (Fig. 6c). A four-way (movement state: standing vs. walking; target location: left vs. right; distractor presence: with distractor vs. no distractor; eccentricity: 1.3° vs. 9° vs. 16°) repeated-measures ANOVA was performed with the average alpha power in [300 450] ms. The results revealed a significant main effect of movement state (F(1,24) = 4.43, p = 0.045), with standing (M = −2.48, SD = 1.50) showing a stronger alpha power decrease than walking (M = −2.07, SD = 1.04) (Fig. 6a, b). However, please note that this comparison was circular, as the time window and electrodes were selected based on this difference. All other comparisons from the ANOVA are valid. A significant interaction effect between movement state and distractor presence was observed (F(1,24) = 7.02, p = 0.01), showing that alpha power during walking was significantly modulated by the distractor presence while there was no influence of the distractor during standing (Fig. 6d). When more closely investigating the significant interaction between movement state and eccentricity (F(2,48) = 4.13, p = 0.03), we found that particularly at an eccentricity of 1.3°showd a difference of alpha power between two movement states (Fig. 6d). No other effects were significant.
Fig. 6.
The stimulus related alpha desynchronisation was smaller during walking and affected by distractor presence and eccentricity. (a) Baseline corrected alpha power (averaged over [8 −14] Hz, and P7, P8, O1 and O2 electrodes) for standing (black) and walking (green line) for “with distractor” and (b) “no distractor” manipulation. The time between [300 450] ms (marked in orange) showed the strongest difference in alpha power between standing and walking. Data are presented as mean ± SEM (standard error of the mean). (c) The topography of alpha power ([300 450] ms) during standing (left panel) and walking (right panel). (d) The interplay between alpha power and movement state (stand vs. walk), distractor presence (with distractor vs. no distractor) and eccentricity (1.3° vs. 9° vs.16°) is shown. During walking, alpha power was modulated by distractor presence and eccentricity as indicated by significant interactions.
We further investigated whether the stimulus induced alpha power ([300 450] ms) reflected a similar process as the early sensory N1 and P3 amplitude change which was predicted by pre-stimulus alpha power. A within-participant correlation between stimulus induced alpha power and pre-stimulus alpha power was performed. A one-sample t-test between each participant's r value and zero did not indicate a prevalent correlation (t(24) = −1.72, p = 0.10). Additionally, we did not find an indication for a correlation between stimulus induced alpha and the N1 (t(24) = - 0.96, p = 0.35), or the P3 component (t(24) = 0.06, p = 0.95). The stimulus induced alpha power was not modulated by time during both standing (r = - 0.25, p = 0.17) and walking (r = - 0.30, p = 0.10). In summary, we found that the stimulus induced alpha power was less modulated by the stimulus onset during walking compared to standing, and at the same time more strongly influenced by distractor absence and stimulus eccentricity. This stimulus induced alpha power modulation may index a later processing that is distinct from the processing indexed by the pre-stimulus alpha power, N1 and P3 components.
4. Discussion
While previous studies have indicated a change in early visual processing during walking, our aim was to investigate whether this change is related to a movement induced general state-change indicated by reduced pre-stimulus alpha activity. We confirmed an enhanced N1 and a reduced P3 component due to walking and replicated the alpha power reduction effect during walking. No evidence was found that the walking related N1 enhancement and P3 reduction were dependent on stimulus-related manipulations (target location, distractor presence and eccentricity). Importantly, we found that the amplitude of the early VEPs could be predicted by pre-stimulus alpha power. In contrast, the stimulus induced alpha power could not be predicted by pre-stimulus alpha power, but showed a stimulus feature specific modulation due to movement. Over the course of the experiment, pre-stimulus alpha power but not stimulus induced alpha power increased, while reaction time decreased and d’ increased. Interestingly, the effect of time on task was found similarly for walking and standing suggesting a comparable effect of learning during standing and walking. Overall, the findings suggest that the alpha power change found during pre-stimulus time can mark different internal states but is clearly distinct from stimulus induced alpha indexing specific sensory input processing. The findings will be discussed in detail below.
4.1. Enhanced N1 and reduced P3 during walking are linked to an internal-state related processing change
In the current study, we replicated the movement induced change of early VEPs as previously reported in humans (Chen et al., 2022). The increase in the N1 component is likely related to the increased firing rates in the visual cortex as found in animal studies (Bullock et al., 2015; Dodwell et al., 2021; Niell and Stryker, 2010; Vinck et al., 2015). Besides the walking related increase, the study at hands found no evidence that the N1 component was affected by stimulus manipulation except for the main effect of distractor presence. The presence of a distractor led to an increased N1 component, which is likely due to the doubling of sensory input as compared to when no distractor was presented. Could an increased sensory input also explain the enhanced N1 component during walking? This is very unlikely as walking adds sensory noise that is not temporally linked to the stimulus onset and therefore should not affect the averaged signal. The P3 decrement during walking was also in line with previous studies showing a decrease in P3 in visual tasks performed during movement (Bradford et al., 2019; De Sanctis, Butler, Malcolm, and Foxe, 2014; Nenna et al., 2020; Richardson et al., 2022). In the current study, again, no evidence was found that P3 component was dependent on distractor presence or stimulus eccentricity. This indicates that N1 and P3 share some functionality as they are correlated in amplitude and are both affected by the movement state. Importantly, the lack of interaction between movement state and stimulus features suggests that the change in state due to walking is not specific for certain visual input, but reflects a general change in the way visual input is processed early in the hierarchy of visual processing.
In line with previous studies, we further found a change in ongoing alpha activity during the pre-stimulus time with respect to the movement state. That alpha power is reduced during movement has been reported repeatedly (Ehinger et al., 2014; Lin et al., 2014; Peterson and Ferris, 2018; Storzer et al., 2016). To be more specific, an alpha decrease was found during treadmill walking compared to standing (Lin et al., 2014) as well as during walking compared to stationary cycling (Storzer et al., 2016). Ehinger et al. (2014) reported an alpha suppression specifically during the turning movement in a VR set-up. Previous careful analysis has demonstrated that a change in alpha power during walking can be independent of visual input: Cao and colleagues (Cao et al., 2020) have found that walking in both light and darkness led to a reduced alpha power, which is in line with the animal work (Erisken et al., 2014; Keller et al., 2012). The independence of the modulation of alpha activity from visual input again suggests a general state difference between stationarity and locomotion.
Given the known movement related change in alpha activity (independent of visual input) and the described modulation of VEPs, we investigated whether the pre-stimulus alpha power would predict the strength of visual input processing, indexed by N1 and P3. We found a significant positive correlation between pre-stimulus alpha power and N1 as well as P3, showing that smaller pre-stimulus alpha power led to a stronger N1 and a smaller P3 amplitude. Previous studies have shown that pre-stimulus oscillatory activity can modulate ERP components in various ways and, depending on the type of oscillation (zero or non-zero mean), can either lead to a reduction in amplitude of all early ERP components or an amplification in late responses (Iemi et al., 2019; Roberts et al., 2014; Zazio et al., 2022). We found a specific increase only in N1 and P3. Additionally, alpha power affecting the VEP did not directly precede the stimulus onset since pre-stimulus alpha but not alpha during fixation correlated with the VEP component. We therefore conclude, that the walking induced change in alpha power influenced specific visual processing steps. The active inhibition of alpha power might be one mechanism through which walking affects such early visual responses. Whether walking has other ways to modulate visual responses is a question awaiting further studies.
Can we further interpret this movement related state change? Since alpha power has been shown to mark inhibitory processes modulated by attention (Bacigalupo and Luck, 2019; Foxe and Snyder, 2011; Händel et al., 2011; Hanslmayr et al., 2011; Sauseng et al., 2005; Thut et al., 2006; Yamagishi et al., 2003), the walking induced modulation of alpha could suggest an attentional process. Additionally, a change in N1 and P3 has been shown to be induced by attentional manipulations (Fedota et al., 2012; Hong et al., 2017; Kapanci et al., 2019; Liebherr et al., 2021; Polich and Bondurant, 1997; Slagter et al., 2016; Vogel and Luck, 2000; Wascher et al., 2009). Following the interpretation of the previously reported link between pre-stimulus alpha and visually evoked components in stationary setups (De Blasio and Barry, 2013), the modulation due to walking might be related to an attentional state change. Note that the attentional state does not refer to ‘directed’ or ‘selective’ attention affecting specific spatial or other features of the input.
Notably, we also found a link between the pre-stimulus alpha power and the reaction time but such correlation was not observed with the d’. In a recent finding, using a letter detection task and additionally including awareness ratings of the target, higher pre-stimulus power predicted lower visual awareness ratings but not discrimination accuracy (Benwell et al., 2021). The work replicated their previous finding of a link between pre-stimulus alpha power and perceptual awareness but not objective performance (accuracy) in a landmark task (Benwell et al., 2018). The specific link between alpha power and awareness might well explain the correlation between perceptual outcome and pre-stimulus alpha power in certain visual illusions (Handel and Jensen, 2014) or detection tasks (Van Dijk et al., 2008). However, awareness may not necessarily lead to an improvement of every aspect of performance. In our study, the lower alpha power during walking may be associated with a higher awareness, but the task probed the orientation discrimination performance. This might explain why the d’ was not correlated with pre-stimulus alpha power or N1 amplitude and why walking showed no improvement in the task related discrimination despite the significant increase in N1 amplitude. Pre-stimulus alpha and the correlated N1 amplitude might however show a correlation with RT based on their link to awareness of the stimulus. Indeed, studies using location detection or change detection tasks found that subjective awareness was associated with an early negative posterior component around 180–280 ms (VAN, visual awareness negativity) which has similar feature as the N1 component in the current study (Koivisto and Grassini, 2016; Koivisto and Revonsuo, 2003).
In summary, we would like to suggest that walking introduces a reduction in alpha activity, marking a disinhibition of cortical activities thereby leading to a stronger N1 component as response to visual input. This modulation (alpha and N1) does not affect spatial or feature based attention but rather increases perceptual awareness. The movement related change in state and the respective change in alpha activity and N1 are therefore not necessarily related to the behavioral outcome of a discrimination task but might well predict awareness. An indicator is the correlation of pre-stimulus alpha power with RT and the absence of a slowing of RT during walking, that might have been expected to parallel the reduction in d’ in the walking condition.
4.2. Pre-stimulus alpha power is modulated over time
Investigating the effect of time on task, we found another state change marked by pre-stimulus alpha power. Over the course of the experiment, pre-stimulus alpha power increased, while reaction time decreased and d’ increased. Similar changes over time have been described before: Toosi et al. (2017) showed a significant increase in pre-stimulus alpha power from early to the late trials in the predictable discrimination blocks. The increase was also accompanied by an increase in accuracy (see their Fig. 3) and was interpreted as a consequence of perceptual learning. A similar increase in power with training was also found in somatosensory alpha (Brickwedde et al., 2019), and the alpha power was interpreted as neural marker of perceptual learning efficiency. Indeed, the link between alpha power and learning has been indicated even earlier and was also suggested to be independent of stimulus features (e.g. predictable or not, trained or not). Bays et al. (2015) found an increase in pre-stimulus alpha power in both trained stimuli and untrained stimuli, suggesting automaticity in perceptual learning. Our finding of the time modulation on pre-stimulus alpha power with unpredictable and non-cued stimuli is also in line with the stimuli independent learning hypothesis (Bays et al., 2015).
Importantly, the effect of time on task was found during both standing and walking, indicating that independent of the interpretation of this timing effect, the improvement in a task over time was present throughout different movement states. This suggests that movement has no detrimental effect on learning. However, interestingly, there was one difference in the effect of time on alpha power between the movement conditions. While during walking, the correlation was only visible for the pre-stimulus period, during the standing manipulation, such correlation was also significant for the fixation period. This could mean that during walking we do not change our response to the initial stimulus appearance. While during non-movement manipulation, we adapt all responses to the task. In other words, while during stationarity, we allow the neural response to visual onset (as the post-stimulus alpha likely marks attention towards the input) to adapt with time, but the response to visual input during walking will not be modulated but kept constant no matter what visual task is additionally executed. Ecologically, this interpretation makes sense as it is likely always advisable to react to visual input when walking so as not to run into objects or to modify the path according to the new information.
4.3. Stimulus induced alpha power is less modulated during walking and accompanied with a significant modulation by distractor presence and eccentricity
Besides the pre-stimulus alpha activity, we also investigated the alpha activity induced by the target onset. We found an overall reduced modulation of induced alpha activity during walking. One reason might be that the overall alpha power is lower during walking than standing, which might in turn lead to less modulation by sensory input. However, surprisingly, the exogenous factors related to the stimulus (distractor presence and eccentricity) had a significant effect on alpha power modulation during walking while they had little effect on alpha modulation during standing. Particularly, the eccentricity of 1.3° and “no distractor” manipulation showed a strongly reduced modulation of alpha power during walking and the greatest difference to the standing manipulation. The effect of eccentricity indicates that during later visual processing stages, the visual input in the foveal area is less processed than in peripheral areas while walking. That the spatial distribution of attention (or preferred input processing) is different during movement has already been indicated by our previous work, showing that the spatial distribution of visual input processing, as can be modulated via spatial attention, is shifted towards the periphery while walking (Cao and Händel, 2019). In addition, the present study showed that for the central foveal area, there was a large difference in alpha power modulation dependent on the presence of a distractor. Distractor presence will increase the amount of visual input (2 stimuli vs. 1 stimulus). However, this is unlikely the explanation as the difference in input did not affect all eccentricities. However, the perceptual trend does not consistently follow the stimulus induced alpha power change. Strong modulation in induced alpha power goes along with better performance when comparing walking vs standing and also the effect of eccentricity (best for 9°) follows the modulation strength (strongest for 9°) but only during walking. During standing this relationship cannot be observed so clearly. Especially the positive influence of distractor absence on d' cannot be found as increased alpha modulation in the data. This finding suggests that alpha power modulation due to stimulus onset cannot be generalized over stimulus features as behavioral predictor.
One might ask whether the visual flow that was visible through the AR glasses attracted attention during walking and thereby introduced the walking related changes. Regarding the VEP, the optic flow was not time locked to stimulus onset and therefore not additive. Additionally, one would not expect the observed increase in amplitude if attention was directed away from the relevant (VEP introducing) stimulus. Changes in alpha power might be introduced by a general change in attention away from the stimulus. However, our previous study has shown, that the alpha reduction during walking is independent from visual input as it persists in complete darkness (Cao et al., 2020). Pre-stimulus alpha power is therefore unlikely introduced by the optic flow but might represent a change in attentional state independent from visual input. The present data further suggested that the pre-stimulus alpha and the stimulus induced alpha power reflect distinct processes as they showed no correlation and alpha power showed no significant difference between movement conditions once the fixation dot appeared. Was the walking related decrease in modulation of stimulus induced alpha power caused by the optic flow visible during stimulus presentation? Previous work indeed suggested increased peripheral visual processing (possibly equivalent to a shift of the attentional focus towards the periphery) during walking (Cao and Händel, 2019), which was also accompanied by a decreased alpha power during stimulation. In this previous study, we did not completely avoid optic flow, but could show through a target detection task that attention was not drawn towards the visible motion introduced by walking. We therefore conclude that also in the current study the reduced modulation of stimulus induced alpha power during walking was unlikely introduced by reduced attention towards the stimulus due to optic flow.
5. Conclusion
The current study showed that the established amplitude increase in N1 due to walking can be predicted by pre-stimulus alpha power, which is also significantly modulated by movement. We argue that the pre-stimulus alpha power indicates a general state change which is introduced by walking. This state change might be related to the awareness of sensory input but not to the processing of the input features, as no evidence was found that the N1 amplitude was affected by stimulus features like eccentricity which might influence discrimination sensitivity measured with d’. However,the pre-stimulus alpha power and the amplitude of N1 were related to the RT. We additionally strengthen the interpretation of pre-stimulus alpha as an indicator of internal states by showing a positive effect of time to be present in alpha power (and N1) and the behavioural performance. This further adds to the important observation that a learning processes existed in both movement states Overall, the finding supports the idea of a movement dependent state in visual processing marked by ongoing alpha activity. However, we additionally found that stimulus induced alpha power was affected by walking dependent on stimulus features like distractor presence and eccentricity. Our work therefore indicates two dissociable movement-related influences on sensory processing. One is based on an ongoing reduction in alpha power due to walking which increases early VEPs to sensory input such as N1. The second influence is marked by stimulus induced alpha power which is specific for the central visual field and when no distracting input is present, indicating a visual spatial change during walking. Our study provides novel insights as to the underlying mechanism of movement induced early sensory enhancement in visual processing. We hypothesize that it is a state change related to sensory awareness which, as we show, is clearly dissociable from influences of walking on stimulus induced processes.
Funding
This study was supported by a starting grant from the European Research Council awarded to BH (grant number 677819) and a scholarship from China Scholarship Council (grant number 201908060012) awarded to XC.
CRediT authorship contribution statement
Xinyu Chen: Conceptualization, Methodology, Investigation, Resources, Data curation, Formal analysis, Writing – original draft, Visualization. Liyu Cao: Conceptualization, Methodology, Software, Validation, Resources, Data curation, Formal analysis, Writing – original draft, Writing – review & editing, Visualization, Supervision. Barbara F Haendel: Conceptualization, Methodology, Writing – review & editing, Visualization, Supervision, Project administration, Funding acquisition.
Declaration of Competing Interest
None.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.neuroimage.2022.119757.
Appendix. Supplementary materials
Availability of data and code
The data and data analysis code are available at figshare (10.6084/m9.figshare.19403390.v2).
References
- Ayaz A., Saleem A.B., Scholvinck M.L., Carandini M. Locomotion controls spatial integration in mouse visual cortex. Curr. Biol. 2013;23(10):890–894. doi: 10.1016/j.cub.2013.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacigalupo F., Luck S.J. Lateralized suppression of alpha-band EEG activity as a mechanism of target processing. J. Neurosci. 2019;39(5):900–917. doi: 10.1523/Jneurosci.0183-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bays B.C., Visscher K.M., Le Dantec C.C., Seitz A.R. Alpha-band EEG activity in perceptual learning. J. Vis. 2015;15(10):7. doi: 10.1167/15.10.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benwell C.S.Y., Coldea A., Harvey M., Thut G. Low pre-stimulus EEG alpha power amplifies visual awareness but not visual sensitivity. Eur. J. Neurosci. 2021 doi: 10.1111/ejn.15166. [DOI] [PubMed] [Google Scholar]
- Benwell C.S.Y., Keitel C., Harvey M., Gross J., Thut G. Trial-by-trial co-variation of pre-stimulus EEG alpha power and visuospatial bias reflects a mixture of stochastic and deterministic effects. Eur. J. Neurosci. 2018;48(7):2566–2584. doi: 10.1111/ejn.13688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford C., Lukos J.R., Passaro A., Ries A., Ferris D.P. Effect of locomotor demands on cognitive processing. Sci. Rep. 2019;9(1):1–12. doi: 10.1038/s41598-019-45396-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt M.E., Jansen B.H. The relationship between prestimulus-alpha amplitude and visual evoked potential amplitude. Int. J. Neurosci. 1991;61(3–4):261–268. doi: 10.3109/00207459108990744. [DOI] [PubMed] [Google Scholar]
- Brickwedde M., Kruger M.C., Dinse H.R. Somatosensory alpha oscillations gate perceptual learning efficiency. Nat. Commun. 2019;10(1):263. doi: 10.1038/s41467-018-08012-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullock T., Cecotti H., Giesbrecht B. Multiple stages of information processing are modulated during acute bouts of exercise. Neuroscience. 2015;307:138–150. doi: 10.1016/j.neuroscience.2015.08.046. [DOI] [PubMed] [Google Scholar]
- Bullock T., Elliott J.C., Serences J.T., Giesbrecht B. Acute exercise modulates feature-selective responses in human cortex. J. Cogn. Neurosci. 2017;29(4):605–618. doi: 10.1162/jocn_a_01082. [DOI] [PubMed] [Google Scholar]
- Busse L., Cardin J.A., Chiappe M.E., Halassa M.M., McGinley M.J., Yamashita T., Saleem A.B. Sensation during active behaviors. J. Neurosci. 2017;37(45):10826–10834. doi: 10.1523/JNEUROSCI.1828-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao L., Chen X., Händel B.F. Overground walking decreases alpha activity and entrains eye movements in humans. Front. Hum. Neurosci. 2020;14 doi: 10.3389/fnhum.2020.561755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao L., Händel B. Walking enhances peripheral visual processing in humans. PLoS Biol. 2019;17(10) doi: 10.1371/journal.pbio.3000511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X., Cao L., Haendel B.F. Differential effects of walking across visual cortical processing stages. Cortex. 2022;149:16–28. doi: 10.1016/j.cortex.2022.01.007. [DOI] [PubMed] [Google Scholar]
- Clayton M.S., Yeung N., Cohen Kadosh R. The roles of cortical oscillations in sustained attention. Trends Cogn. Sci. 2015;19(4):188–195. doi: 10.1016/j.tics.2015.02.004. [DOI] [PubMed] [Google Scholar]
- De Blasio F.M., Barry R.J. Prestimulus alpha and beta determinants of ERP responses in the Go/NoGo task. Int. J. Psychophysiol. 2013;89(1):9–17. doi: 10.1016/j.ijpsycho.2013.04.018. [DOI] [PubMed] [Google Scholar]
- De Sanctis P., Butler J.S., Malcolm B.R., Foxe J.J. Recalibration of inhibitory control systems during walking-related dual-task interference: a mobile brain-body imaging (MOBI) study. Neuroimage. 2014;94:55–64. doi: 10.1016/j.neuroimage.2014.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dipoppa M., Ranson A., Krumin M., Pachitariu M., Carandini M., Harris K.D. Vision and locomotion shape the interactions between neuron types in mouse visual cortex. Neuron. 2018;98(3):602–615. doi: 10.1016/j.neuron.2018.03.037. e608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodwell G., Liesefeld H.R., Conci M., Muller H.J., Tollner T. EEG evidence for enhanced attentional performance during moderate-intensity exercise. Psychophysiology. 2021;58(12):e13923. doi: 10.1111/psyp.13923. [DOI] [PubMed] [Google Scholar]
- Dodwell G., Liesefeld H.R., Conci M., Müller H.J., Töllner T. EEG evidence for enhanced attentional performance during moderate-intensity exercise. Psychophysiology. 2021:e13923. doi: 10.1111/psyp.13923. [DOI] [PubMed] [Google Scholar]
- Ehinger B.V., Fischer P., Gert A.L., Kaufhold L., Weber F., Pipa G., Konig P. Kinesthetic and vestibular information modulate alpha activity during spatial navigation: a mobile EEG study. Front. Hum. Neurosci. 2014;8:71. doi: 10.3389/fnhum.2014.00071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erisken S., Vaiceliunaite A., Jurjut O., Fiorini M., Katzner S., Busse L. Effects of locomotion extend throughout the mouse early visual system. Curr. Biol. 2014;24(24):2899–2907. doi: 10.1016/j.cub.2014.10.045. [DOI] [PubMed] [Google Scholar]
- Fedota J.R., McDonald C.G., Roberts D.M., Parasuraman R. Contextual task difficulty modulates stimulus discrimination: electrophysiological evidence for interaction between sensory and executive processes. Psychophysiology. 2012;49(10):1384–1393. doi: 10.1111/j.1469-8986.2012.01455.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foxe J.J., Snyder A.C. The role of alpha-band brain oscillations as a sensory suppression mechanism during selective attention. Front. Psychol. 2011;2 doi: 10.3389/fpsyg.2011.00154. doi:ARTN 15,4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrett J., Bullock T., Giesbercht B. Tracking the contents of spatial working memory during an acute bout of aerobic exercise. J. Cogn. Neurosci. 2021;33(7):1271–1286. doi: 10.1162/jocn_a_01714. [DOI] [PubMed] [Google Scholar]
- Gramann K., Gwin J.T., Bigdely-Shamlo N., Ferris D.P., Makeig S. Visual evoked responses during standing and walking. Front. Hum. Neurosci. 2010;4 doi: 10.3389/fnhum.2010.00202. doi:ARTN 20,2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Händel B.F., Haarmeier T., Jensen O. Alpha oscillations correlate with the successful inhibition of unattended stimuli. J. Cogn. Neurosci. 2011;23(9):2494–U2552. doi: 10.1162/jocn.2010.21557. [DOI] [PubMed] [Google Scholar]
- Handel B.F., Jensen O. Spontaneous local alpha oscillations predict motion-induced blindness. Eur. J. Neurosci. 2014;40(9):3371–3379. doi: 10.1111/ejn.12701. [DOI] [PubMed] [Google Scholar]
- Hanslmayr S., Gross J., Klimesch W., Shapiro K.L. The role of alpha oscillations in temporal attention. Brain Res. Rev. 2011;67(1–2):331–343. doi: 10.1016/j.brainresrev.2011.04.002. [DOI] [PubMed] [Google Scholar]
- Hong X., Wang Y., Sun J., Li C., Tong S. Segregating top-down selective attention from response inhibition in a spatial cueing Go/NoGo task: an ERP and source localization study. Sci. Rep. 2017;7(1):9662. doi: 10.1038/s41598-017-08807-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iemi L., Busch N.A., Laudini A., Haegens S., Samaha J., Villringer A., Nikulin V.V. Multiple mechanisms link prestimulus neural oscillations to sensory responses. Elife. 2019;8 doi: 10.7554/eLife.43620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko M., Fu Y., Stryker M.P. Locomotion induces stimulus-specific response enhancement in adult visual cortex. J. Neurosci. 2017;37(13):3532–3543. doi: 10.1523/JNEUROSCI.3760-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapanci T., Merks S., Rammsayer T.H., Troche S.J. On the relationship between P3 latency and mental ability as a function of increasing demands in a selective attention task. Brain Sci. 2019;9(2) doi: 10.3390/brainsci9020028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller G.B., Bonhoeffer T., Hubener M. Sensorimotor mismatch signals in primary visual cortex of the behaving mouse. Neuron. 2012;74(5):809–815. doi: 10.1016/j.neuron.2012.03.040. [DOI] [PubMed] [Google Scholar]
- Kleiner, M., Brainard, D., & Pelli, D. (2007). What's new in Psychtoolbox-3?
- Koivisto M., Grassini S. Neural processing around 200ms after stimulus-onset correlates with subjective visual awareness. Neuropsychologia. 2016;84:235–243. doi: 10.1016/j.neuropsychologia.2016.02.024. [DOI] [PubMed] [Google Scholar]
- Koivisto M., Revonsuo A. An ERP study of change detection, change blindness, and visual awareness. Psychophysiology. 2003;40(3):423–429. doi: 10.1111/1469-8986.00044. [DOI] [PubMed] [Google Scholar]
- Ladouce S., Donaldson D.I., Dudchenko P.A., Ietswaart M. Understanding minds in real-world environments: toward a mobile cognition approach. Front. Hum. Neurosci. 2016;10:694. doi: 10.3389/fnhum.2016.00694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebherr M., Corcoran A.W., Alday P.M., Coussens S., Bellan V., Howlett C.A., Bornkessel-Schlesewsky I. EEG and behavioral correlates of attentional processing while walking and navigating naturalistic environments. Sci. Rep. 2021;11(1):22325. doi: 10.1038/s41598-021-01772-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y.P., Wang Y., Wei C.S., Jung T.P. Assessing the quality of steady-state visual-evoked potentials for moving humans using a mobile electroencephalogram headset. Front. Hum. Neurosci. 2014;8:182. doi: 10.3389/fnhum.2014.00182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macmillan N.A., Kaplan H.L. Detection theory analysis of group data: estimating sensitivity from average hit and false-alarm rates. Psychol. Bull. 1985;98(1):185–199. https://www.ncbi.nlm.nih.gov/pubmed/4034817 Retrieved from. [PubMed] [Google Scholar]
- Mineault P.J., Tring E., Trachtenberg J.T., Ringach D.L. Enhanced spatial resolution during locomotion and heightened attention in mouse primary visual cortex. J. Neurosci. 2016;36(24):6382–6392. doi: 10.1523/JNEUROSCI.0430-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nenna F., Do C.T., Protzak J., Gramann K. Alteration of brain dynamics during dual-task overground walking. Eur. J. Neurosci. 2020 doi: 10.1111/ejn.14956. [DOI] [PubMed] [Google Scholar]
- Niell C.M., Stryker M.P. Modulation of visual responses by behavioral state in mouse visual cortex. Neuron. 2010;65(4):472–479. doi: 10.1016/j.neuron.2010.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oostenveld R., Fries P., Maris E., Schoffelen J.M. FieldTrip: open source software for advanced analysis of MEG, EEG, and invasive electrophysiological data. Comput. Intell. Neurosci. 2011 doi: 10.1155/2011/156869. doi:Artn 15,686,9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson S.M., Ferris D.P. Differentiation in theta and beta electrocortical activity between visual and physical perturbations to walking and standing balance. eNeuro. 2018;5(4) doi: 10.1523/ENEURO.0207-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polich J., Bondurant T. P300 sequence effects, probability, and interstimulus interval. Physiol. Behav. 1997;61(6):843–849. doi: 10.1016/s0031-9384(96)00564-1. [DOI] [PubMed] [Google Scholar]
- Protzak J., Wiczorek R., Gramann K. Peripheral visual perception during natural overground dual-task walking in older and younger adults. Neurobiol Agin. 2021;98:146–159. doi: 10.1016/j.neurobiolaging.2020.10.009. [DOI] [PubMed] [Google Scholar]
- Richardson D.P., Foxe J.J., Mazurek K.A., Abraham N., Freedman E.G. Neural markers of proactive and reactive cognitive control are altered during walking: a mobile brain-body imaging (MoBI) study. Neuroimage. 2022;247 doi: 10.1016/j.neuroimage.2021.118853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts D.M., Fedota J.R., Buzzell G.A., Parasuraman R., McDonald C.G. Prestimulus oscillations in the alpha band of the EEG are modulated by the difficulty of feature discrimination and predict activation of a sensory discrimination process. J. Cogn. Neurosci. 2014;26(8):1615–1628. doi: 10.1162/jocn_a_00569. [DOI] [PubMed] [Google Scholar]
- Sauseng P., Klimesch W., Stadler W., Schabus M., Doppelmayr M., Hanslmayr S., Birbaumer N. A shift of visual spatial attention is selectively associated with human EEG alpha activity. Eur. J. Neurosci. 2005;22(11):2917–2926. doi: 10.1111/j.1460-9568.2005.04482.x. [DOI] [PubMed] [Google Scholar]
- Schroeder S.C.Y., Ball F., Busch N.A. The role of alpha oscillations in distractor inhibition during memory retention. Eur. J. Neurosci. 2018;48(7):2516–2526. doi: 10.1111/ejn.13852. [DOI] [PubMed] [Google Scholar]
- Slagter H.A., Prinssen S., Reteig L.C., Mazaheri A. Facilitation and inhibition in attention: functional dissociation of pre-stimulus alpha activity, P1, and N1 components. Neuroimage. 2016;125:25–35. doi: 10.1016/j.neuroimage.2015.09.058. [DOI] [PubMed] [Google Scholar]
- Stanislaw H., Todorov N. Calculation of signal detection theory measures. Behav. Res. Methods Instrum. Comput. 1999;31(1):137–149. doi: 10.3758/bf03207704. [DOI] [PubMed] [Google Scholar]
- Storzer L., Butz M., Hirschmann J., Abbasi O., Gratkowski M., Saupe D., Dalal S.S. Bicycling and walking are associated with different cortical oscillatory dynamics. Front. Hum. Neurosci. 2016;10:61. doi: 10.3389/fnhum.2016.00061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thut G., Nietzel A., Brandt S.A., Pascual-Leone A. alpha-Band electroencephalographic activity over occipital cortex indexes visuospatial attention bias and predicts visual target detection. J. Neurosci. 2006;26(37):9494–9502. doi: 10.1523/Jneurosci.0875-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toosi T., Tousi E.K., Esteky H. Learning temporal context shapes prestimulus alpha oscillations and improves visual discrimination performance. J. Neurophysiol. 2017;118(2):771–777. doi: 10.1152/jn.00969.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Diepen R.M., Miller L.M., Mazaheri A., Geng J.J. The role of alpha activity in spatial and feature-based attention. eNeuro. 2016;3(5) doi: 10.1523/ENEURO.0204-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dijk H., Schoffelen J.M., Oostenveld R., Jensen O. Prestimulus oscillatory activity in the alpha band predicts visual discrimination ability. J. Neurosci. 2008;28(8):1816–1823. doi: 10.1523/Jneurosci.1853-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinck M., Batista-Brito R., Knoblich U., Cardin J.A. Arousal and locomotion make distinct contributions to cortical activity patterns and visual encoding. Neuron. 2015;86(3):740–754. doi: 10.1016/j.neuron.2015.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel E.K., Luck S.J. The visual N1 component as an index of a discrimination process. Psychophysiology. 2000;37(2):190–203. doi: 10.1017/S0048577200981265. [DOI] [PubMed] [Google Scholar]
- Wagner J., Solis-Escalante T., Scherer R., Neuper C., Muller-Putz G. It's how you get there: walking down a virtual alley activates premotor and parietal areas. Front. Hum. Neurosci. 2014;8:93. doi: 10.3389/fnhum.2014.00093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wascher E., Hoffmann S., Sanger J., Grosjean M. Visuo-spatial processing and the N1 component of the ERP. Psychophysiology. 2009;46(6):1270–1277. doi: 10.1111/j.1469-8986.2009.00874.x. [DOI] [PubMed] [Google Scholar]
- Wostmann M., Alavash M., Obleser J. Alpha oscillations in the human brain implement distractor suppression independent of target selection. J. Neurosci. 2019;39(49):9797–9805. doi: 10.1523/JNEUROSCI.1954-19.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagishi N., Callan D.E., Goda N., Anderson S.J., Yoshida Y., Kawato M. Attentional modulation of oscillatory activity in human visual cortex. Neuroimage. 2003;20(1):98–113. doi: 10.1016/S1053-8119(03)00341-0. [DOI] [PubMed] [Google Scholar]
- Yokoyama H., Kaneko N., Masugi Y., Ogawa T., Watanabe K., Nakazawa K. Gait-phase-dependent and gait-phase-independent cortical activity across multiple regions involved in voluntary gait modifications in humans. Eur. J. Neurosci. 2021;54(12):8092–8105. doi: 10.1111/ejn.14867. [DOI] [PubMed] [Google Scholar]
- Zazio A., Ruhnau P., Weisz N., Wutz A. Pre-stimulus alpha-band power and phase fluctuations originate from different neural sources and exert distinct impact on stimulus-evoked responses. Eur. J. Neurosci. 2022;55(11–12):3178–3190. doi: 10.1111/ejn.15138. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data and data analysis code are available at figshare (10.6084/m9.figshare.19403390.v2).