Abstract
Schistosomiasis is a major neglected tropical disease mainly caused by Schistosoma haematobium, S. japonicum and S. mansoni, and results in the greatest disease burden. Mass drug administration (MDA) with praziquantel (PZQ), a single drug only available for the disease, has played a vital role in schistosomiasis control. Therefore, any possibility of selection of the parasites for PZQ resistance or low sensitivity may hamper the 2030's target of global disease elimination. We had experimentally demonstrated the long-term survival and reproductive potential of single-sex (of either sex) S. japonicum infections in definitive hosts mice. What has not yet been adequately addressed is whether the long live single-sex schistosomes remain sensitive to PZQ, and what reproduction potential for those schistosomes surviving treatment may have. We therefore performed experimental mice studies to explore the treatment effectiveness of PZQ (at total doses of 200 or 400 mg/kg, corresponding to the sub-standard or standard treatment doses in humans) for single-sex S. japonicum aged three months old. The results showed that no treatment efficiency was observed on female schistosomes, whereas on male schistosomes only at PZQ 400 mg/kg a significant higher efficiency in reducing worm burdens was observed. Moreover, either schistosome males or females surviving PZQ treatment remained their reproduction potential as normal. The results indicate that long (i.e., three months) live single-sex S. japonicum can easily survive the current treatment strategy, and moreover, any schistosomes, if with PZQ resistance or low sensitivity, could be easily transmitted in nature. Therefore, in order to realize the target for the national and the global schistosomiasis elimination, there is undoubtedly a great need for refining PZQ administration and dosage, looking for alternative therapies, and/or developing vaccines against schistosome.
Keywords: S. japonicum, Single-sex infection, Praziquantel, Treatment efficiency
Graphical abstract
Highlights
-
•
PZQ efficiency of three-month-old single-sex S. japonicum was tested on mice.
-
•
No treatment efficiency was observed on females at total doses PZQ 200 or 400 mg/kg.
-
•
Significance in reducing worm burdens was observed on males only at PZQ 400 mg/kg.
-
•
Survived schistosome females or males remained their normal reproduction potential.
-
•
S. japonicum with PZQ resistance or low sensitivity could be easily transmitted.
1. Introduction
About two billion of the poorest people in the world are infected with parasitic worms, and among which schistosomiasis, a major neglected tropical disease mainly caused by Schistosoma haematobium, S. japonicum and S. mansoni, results in the greatest disease burden (King, 2019) with an estimate of 1.4–3.3 million disability-adjusted life years (DALYs) annually (Lo et al., 2022). In recent years, the disease even spread into Europe (Boissier et al., 2015, 2016). S. japonicum is endemic mainly in China, the Philippines and parts of Indonesia. In the past 70 years, the integral control efforts within China have seen great success in schistosomiasis control, with the infection prevalence in both humans and livestock having been reduced to a much lower level (Zhang et al., 2020). Consequently, in 2014 the government set the target for transmission interruption and elimination of the disease at the country level by 2030 (Lei et al., 2015). However, China still faces many challenges, including the zoonotic nature of the parasite, the most pathogenic schistosome species, the wide distribution of the intermediate host snail habitats, and, in particular, only one drug available for treatment and the reported divert sensitivities of the drug for schistosomes (Coles et al., 1987; He et al., 2001; Colley et al., 2017).
Praziquantel (PZQ) is currently the choice for treatment of schistosomiasis. Mass drug administration (MDA) with PZQ (40 mg/kg of bodyweight) has been strongly advocated by WHO for the control of schistosomiasis morbidity and transmission through periodic and targeted treatment administered to human populations at risk of infection (Webster et al., 2014). For example, in 2019 an estimate of at least 236.6 million people required preventive treatment for schistosomiasis (WHO, https://www.who.int/en/news-room/fact-sheets/detail/schistosomiasis. Accessed on July 3, 2022). The estimate could be expected to increase greatly as the new WHO guidelines are to expand preventive chemotherapy programs from school-aged children only to entire communities, to lower the prevalence threshold to initiate preventive chemotherapy more equitably, and to implement more frequent treatment in high-risk settings (Lo et al., 2022). However, reliance on a single drug available for the disease affecting over two million people worldwide raises great concerns about selection of the parasites for PZQ resistance (Cioli et al., 2014). Indeed, schistosomes with reduced sensitivity to PZQ have been easily generated in the laboratory (Fallon and Doenhoff, 1994; Coeli et al., 2013), and several studies reported their findings of the reduced efficacy of PZQ treatment of human schistosomiasis in the field (Coles et al., 1987; Webster et al., 2014). Moreover, the reduced susceptibility to PZQ has not been limited to schistosomes, as fully reviewed by (Norbury et al., 2022).
The worry of PZQ resistance (or low sensitivity) may be further exacerbated by the fact that there was a deferential sensitivity between schistosome males and females, or between paired and unpaired. Schistosomes are dioecious. Prior experiments have shown that the in vitro EC50 for female S. mansoni was 11.66 (95%CI: 6.4–21.0) μg/ml, significantly higher than for male S. mansoni 0.95 (95%CI: 0.3–2.9), and moreover, single-sex male S. mansoni aged seven weeks old had an in vivo ED50 of PZQ 198 mg/kg, whereas single-sex females had an ED50 of 1107 mg/kg, both significantly higher than that of their dual-sex infection counterparts (Pica-Mattoccia and Cioli, 2004). This may be pertinent here in terms of potential emergence of PZQ resistance since single-sex schistosome infections in final hosts may have existed at a large scale and are particularly likely to increase over time in line with enhanced MDA efforts, although there is currently no validated and proved approaches for detection of single-sex schistosome infection (Lu et al., 2018). We had recently experimentally demonstrated the long-term survival and reproductive potential of single-sex (of either sex) S. japonicum infections in definitive hosts mice (Lu et al., 2021). What has not yet been adequately addressed is whether single-sex S. japonicum, who have lived within a final host for a long term, for example, for up to three months, remains sensitive to PZQ, and moreover, what reproduction potential for those parasites surviving treatment may have. We therefore performed experimental mice studies aimed to explore the treatment effectiveness of PZQ (at single oral doses of 400 and 200 mg/kg, corresponding to the standard and the sub-standard treatment doses in humans, respectively (Xu, 1996)) for single-sex S. japonicum infections, and, in particular, to investigate any production potential for the schistosomes post treatment.
2. Materials and methods
2.1. Schistosoma japonicum cercariae
S. japonicum cercariae originated in a hilly area in Anhui province of China, where wild rodents have been considered to serve as main reservoirs for the parasite (Lu et al., 2010; Rudge et al., 2013). No drug administration has, due to logistic difficulty, ever been performed on wild rodents infected with schistosomes. We performed field surveys for infected Oncomelania hupensis hupensis snails in 2020 and 2021. Infected snails with S. japonicum were identified by using the cercarial shedding method (Chinese patent: ZL2019212680818), which is based on a 24-cell culture plate and very field-applicable. We used infection-worm recovery method (Shi et al., 2014) to determine the sex of schistosome cercariae from each infected snail. Briefly, a mouse was infected with about 100 cercariae shed from a single snail. Five weeks post infection, infected mice were euthanized and dissected for worms (i.e., schistosome males only, females only, or paired worms), and were then identified with schistosome male infection only, female only, or dual-sex infection. Consequently, the sex of cercariae of the corresponding snail was determined.
Only the infected snails with schistosome males only or females only were used for the following two experiments. In 2020, we obtained 28 infected snails with schistosome females only and 33 with males only. In 2021, we obtained 30 infected snails with schistosome females only and 38 with males only.
2.2. Experiment schedule
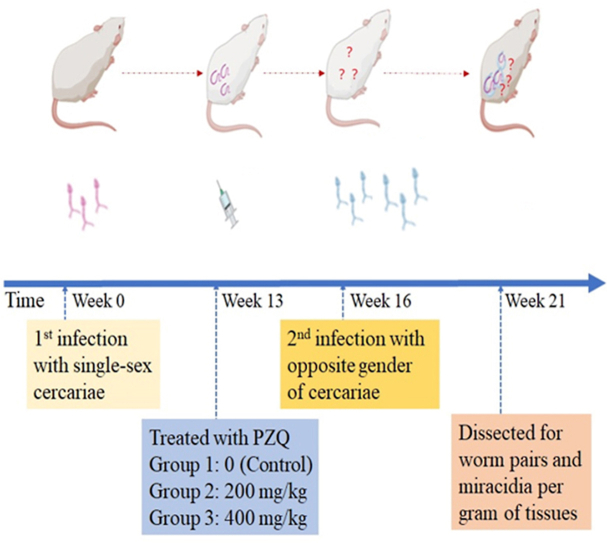
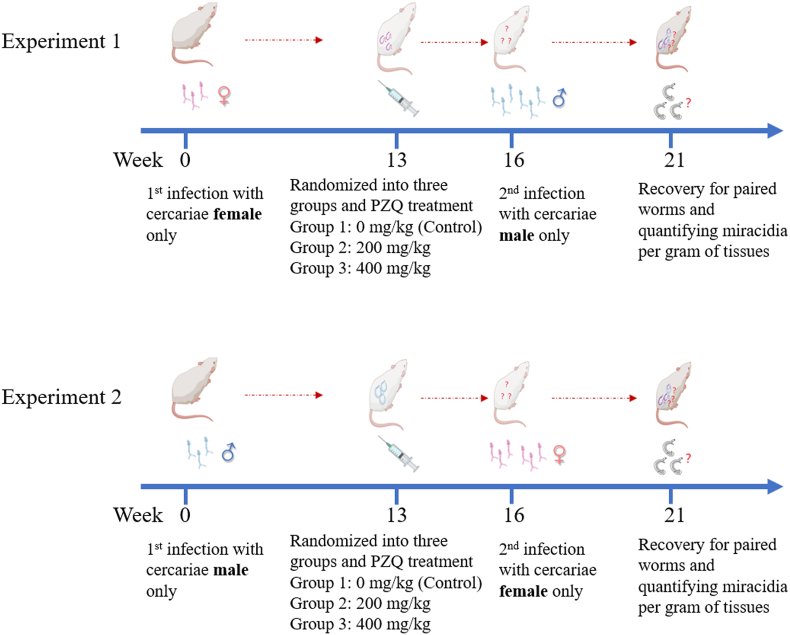
Two experiments were performed, as outlined in Fig. 1. In the first experiment, laboratory mice each were exposed to a quantified number of S. japonicum cercariae female only at primary infection, and then were randomly divided into three groups, i.e., PZQ 0 mg/kg (Control), PZQ 200 mg/kg and PZQ 400 mg/kg. Thirteen weeks later, each mouse received a treatment at a single oral dose of PZQ 0, 200 or 400 mg/kg in 2% cremophor EL according to its assigned group. Three weeks post treatment each mouse was at the second exposure to cercariae male only, which far outnumbered female cercariae used at primary infection with the purpose to ensure any female schistosomes surviving previous treatment to have a chance to pair and mate with a male partner. In the second experiment, except for the converse regarding the primary infection with cercariae male only and the second infection with cercariae female only, all other aspects including group assignment and PZQ treatments were performed at the same time schedule as in the first experiment. Five weeks post second infection, each mouse in both experiments was euthanized, and dissected for recovering adult worm pairs and for counting offspring produced by worms.
Fig. 1.
Experimental design for treatment efficiency of single-sex Schistosoma japonicum on mice at total doses of PZQ 0 (Control), 200, or 400 mg/kg. Two experiments were performed. In Experiment one, mice each were first infected with S. japonicum cercariae female only (i.e., exposed to female cercariae for 20 min), and then randomized into three groups (PZQ 0, 200, or 400 mg/kg). Thirteen weeks post primary infection PZQ treatment (at total doses of PZQ 0, 200, or 400 mg/kg in 2% cremophor EL) was orally administered to the mice. Three weeks post treatment the mice each received a second infection with S. japonicum cercariae male only (i.e., exposed to male cercariae for 20 min), which outnumbered cercariae female used in the primary infection. Experiment two was performed at the same time schedule as the first experiment. Mice each were first infected with S. japonicum cercariae male only, then randomized into three groups and administered with PZQ treatment (at total doses of PZQ 0, 200, or 400 mg/kg in 2% cremophor EL), and finally received a second infection with cercariae female only. Five weeks post the second infection, all experimented mice in both experiments were euthanized and dissected, and from each of them paired adult worms were recovered and resultant miracidia quantified.
All dissected mice were carefully examined for paired adult worms. The livers of mice were weighed, and replicate sections of the liver were used to quantify miracidia hatched from tissue eggs. We calculated two indexes to measure the PZQ sensitivity of single-sex schistosomes: 1) the survival rate of single-sex schistosomes within a mouse post treatment, measured as the number of worm pairs recovered from the mouse divided by the number of single-sex cercariae used at the primary infection; 2) the offspring production potential of single-sex schistosomes within a mouse post treatment, calculated as the number of miracidia per worm pair (Lu et al., 2021).
Statistical analyses of data were performed using a Kruskal-Wallis (K-W) Chi-squared test, and if necessary, further multiple comparisons between groups were conducted with a Bonferroni correction. All ICR mice were female at age of eight weeks old at the start of experiments and were purchased from the laboratory center of Soochow University. The procedures of the care and use of all experimental animals were in accordance with the Regulations for the Administration of Affairs Concerning Experimental Animals (Ministry of Science and Technology, China, 2004). The research protocols were reviewed and approved by the Ethical Committee of Soochow University (No. 81971957). The study was conducted in compliance with the ARRIVE guidelines.
3. Results
In the first experiment, as seen in Table 1, three groups of mice (i.e., Control, PZQ 200 and PZQ 400 mg/kg) received a primary infection of an average female cercariae of 38.90, 38.00 and 40.18, respectively. Among three groups the numbers of female cercariae used were comparable, and the average adult worm pairs finally recovered were 28.60, 25.25 and 26.00 per mouse, respectively. The mean survival rates of female schistosomes post treatment were respectively 0.74, 0.67 and 0.65, and the mean numbers of miracidia per worm pair were respectively 151.29, 148.93 and 118.18. No significant difference was observed among three groups in the survival rate of female schistosomes (K-W test, Chi-squared = 2.66, df = 2, P = 0.26), nor in the number of miracidia per worm pair (K-W test, Chi-squared = 1.32, df = 2, P = 0.52). See Table 2.
Table 1.
Average female cercariae used in 1st primary infection and average adult worm pairs recovered among three groups of mice in Experiment one.
| Group | No. mice | No. female cercariae used in 1st infection per mouse |
No. adult worm pairs recovered per mouse |
||
|---|---|---|---|---|---|
| Mean, SD | M, Range | Mean, SD | M, Range | ||
| Control | 10 | 38.90, 6.03 | 38.50, 31-48 | 28.60, 5.70 | 28.00, 21-43 |
| PZQ 200 mg/Kg | 12 | 38.00, 5.41 | 36.50, 30-45 | 25.25, 6.21 | 25.50, 15-36 |
| PZQ 400 mg/Kg | 11 | 40.18, 8.41 | 37.00, 26-57 | 26.00, 6.87 | 26.00, 12-35 |
Table 2.
Survival of female schistosomes post treatment and their reproduction potential.
| Group | No. mice | Survival rate of female schistosomes post treatment |
Production potential of females post treatment (No. miracidia per worm pair) |
||
|---|---|---|---|---|---|
| Mean, SD | Median, Range | Mean, SD | Median, Range | ||
| Control | 10 | 0.74, 0.14 | 0.78, 0.51–0.91 | 151.29, 88.69 | 148.90, 44.61–316.94 |
| PZQ 200 mg/Kg | 12 | 0.67, 0.17 | 0.66, 0.36–1.00 | 148.93, 95.70 | 123.61, 46.41–375.95 |
| PZQ 400 mg/Kg | 11 | 0.65, 0.14 | 0.65, 0.46–0.88 | 118.18, 93.17 | 100.65, 32.27–378.86 |
| Kruskal-Wallis Test | Chi-squared = 2.66, df = 2, P = 0.26 | Chi-squared = 1.32, df = 2, P = 0.52 | |||
In the second experiment, as seen in Table 3, three groups of mice (i.e., Control, PZQ 200 and PZQ 400 mg/kg) received a primary infection of an average male cercariae of 43.00, 43.58 and 44.69, respectively. Among three groups no significant difference was observed in number of male cercariae used. The average adult worm pairs finally recovered were 27.00, 19.33 and 3.08 per mouse, respectively. The mean survival rates of male schistosomes post treatment was respectively 0.62, 0.45 and 0.06, and the mean numbers of miracidia per worm pair were respectively 138.42, 243.05 and 24.85. A significant difference was observed among groups in the survival rate of male schistosomes (K-W test, Chi-squared = 21.23, df = 2, P < 0.0001) but not in the number of miracidia per worm pair (K-W test, Chi-squared = 3.41, df = 2, P = 0.1814). After performing multiple comparisons with Bonferroni correction, a significantly lower survival rate of male schistosomes was observed in PZQ 400 mg/kg group than in either Control (K-W test, Chi-squared = 17.45, df = 1, P < 0.0001) or PZQ 200 mg/kg group (K-W test, Chi-squared = 13.13, df = 1, P = 0.0003). See Table 4.
Table 3.
Average male cercariae used in 1st primary infection and average worm pairs recovered among three groups of mice in Experiment two.
| Group | No. mice | No. male cercariae used in 1st infection per mouse |
No. worm pairs recovered per mouse |
||
|---|---|---|---|---|---|
| Mean, SD | Median, Range | Mean, SD | Median, Range | ||
| Control | 12 | 43.00, 5.80 | 44, 27-49 | 27.00, 8.64 | 28.5, 9-36 |
| PZQ 200 mg/Kg | 12 | 43.58, 5.26 | 42, 34-54 | 19.33, 13.22 | 20.5, 1-41 |
| PZQ 400 mg/Kg | 13 | 44.69, 5.66 | 45, 30-51 | 3.08, 7.11 | 0, 0-23 |
Table 4.
Survival of male schistosomes post treatment and their reproduction potential.
| Group | No. mice | Survival rate of male schistosomes post treatment |
Production potential of male schistosomes post treatment (No. miracidia per worm pair) |
||
|---|---|---|---|---|---|
| Mean, SD | Median, Range | Mean, SD | Median, Range | ||
| Control | 12 | 0.62, 0.17 | 0.65, 0.24–0.84 | 138.42, 97.49 | 119.39, 16.53–340.81 |
| PZQ 200 mg/Kg | 12 | 0.45, 0.31 | 0.45, 0.02–0.93 | 243.05, 284.42 | 100.64, 17.02–1020.07 |
| PZQ 400 mg/Kg* | 13 | 0.06, 0.15 | 0.00, 0.00–0.49 | 80.75, 121.40 | 8.075, 13.56–262.69 |
| Kruskal-Wallis Test | Chi-squared = 21.23, df = 2, P < 0.0001 | Chi-squared = 3.41, df = 2, P = 0.1814 | |||
Note: *, Multiple comparisons showed significant difference only between PZQ 400 mg/kg and either Control (K-W test, Chi-squared = 17.45, df = 1, P < 0.0001) or PZQ 200 mg/kg (Chi-squared = 13.13, df = 1, P = 0.0003) in terms of survival rate of male schistosomes post treatment.
4. Discussion
We have previously argued that single-sex schistosome infections in final hosts are predicted to become more common when the prevalence of the parasites in the environment decreases, as in response to recent increases in successful control programs (Lu et al., 2018), and had experimentally proved, contrary to prior belief, an extended but not reduced (i.e., within one year) survival and reproductive potential following single-sex S. japonicum infections of either gender (Lu et al., 2021). In S. japonicum endemic areas, humans usually get infected in summer and then will be treated or test-treated in winter (Balen et al., 2007). Therefore, in this research we performed further experiments to investigate the treatment efficiency for three months old single-sex (female only or male only) S. japonicum in definitive hosts (mice) at two different doses (i.e., PZQ 200 and 400 mg/kg, corresponding to the sub-standard and the standard treatment in humans, respectively). The results showed that no treatment efficiency was observed on female schistosomes aged three months old, whereas on male schistosomes of the same age only a significant higher efficiency in reducing worm burdens was observed at PZQ 400 mg/kg. Moreover, either S. japonicum males or females surviving PZQ treatment remained their reproduction potential as those without treatment.
PZQ is currently the only choice for schistosomiasis treatment, and its effect against platyhelminths is to constantly stimulate worm activity and then cause worm body contraction and cortical damage (Harder et al., 1987; Xiao, 2005). The PZQ efficacy is also related to the total administered PZQ dose (Liang et al., 2003; Cioli et al., 2004; Pica-Mattoccia and Cioli, 2004; Abla et al., 2017). In our previous meta-analyses of praziquantel efficacy of S. japonicum in mice (Yu et al., 2021) PZQ had significantly reduced worm burden, and with the increase of the total dose from PZQ 37.5 to 2000 mg/kg, the anti-schistosome effect significantly increased. We also noted that at the total dose of PZQ 300–600 mg/kg, which is an approximate equivalent to the single oral dosage of 40 mg/kg in humans (Xu, 1996) currently recommended by WHO, over 70% worms were killed, and at total PZQ 100–300 mg/kg, over 50% worms were killed, all showing the high efficiency of PZQ treatment in worm burden reduction in experimented mice. However, in all the included studies, experimented animals were infected with dual-sex schistosomes infection, and moreover, there was no information on the sex ratio of schistosome cercariae used in experiments. This could bias the results and then mislead any conclusions made. In this work we tested the in vivo efficiency of PZQ 200 and 400 mg/kg on S. japonicum female only or male only within mice. We saw no reductions in worm burden when administered at a total dose of PZQ 200 mg/kg for either sex schistosome infections. At a total dose of PZQ 400 mg/kg, a significant reduction in survival rate of schistosomes was observed on only male schistosome infections. Our results did show the sexual difference regarding the treatment efficiency, in consistence with the in vitro experiment in which the value of EC50 for female S. mansoni was significantly higher than for males (Pica-Mattoccia and Cioli, 2004). However our observed worm reduction were not in agreement with the in vivo work on S. mansoni with single-sex infections. In the latter, infected mice with single-sex (male only or female only) S. mansoni aged 7 weeks old were observed to have a significant reduction in worm burden at doses of PZQ 250 or 500 mg/kg, when compared to the control group (i.e., PZQ 0 mg/kg) (see Table 1 in (Pica-Mattoccia and Cioli, 2004)). This could be mainly due to the existence of different PZQ sensitivity between schistosome species, as showed both in the laboratory (Xiao et al., 2018) and in the field (Levecke et al., 2020). Even among different S. japonicum isolates there was a significant difference in PZQ sensitivity (Yue et al., 1988).
In our work, we noted that in the second experiment at the total dose of PZQ 400 mg/kg four out of 13 mice were found to harbor paired adult worms post treatment, among which the survival rate of schistosome males ranged from 2% to 49%. A recent human S. mansoni infection trial (Langenberg et al., 2020) reported that after a single PZQ 40 mg/kg dose of treatment 12 weeks after exposure to male schistosomes, about 43% of infected volunteers were believed to still harbor live worms as detected with worm antigen positive in their sera, indicating that a part of unpaired male schistosomes might have been able to survive a standard drug treatment. The substantial variation of schistosome survival among mice reported in our work could probably arise from the heterogeneity of parasites among infected snail individuals sampled from the field. This would warrant further research on any genetic difference underlying low sensitivity or possible resistance of parasites, as the recent research on S. mansoni have confirmed a transient receptor potential (Sm.TRPMPZQ) channel (Smp_246790) underlying PZQ sensitivity (Le Clec'h et al., 2021; Park et al., 2021).
Development of resistance to PZQ treatments is a major threat to the future control of schistosomes. It is recognized that the exposure of parasites to sub-curative doses of drugs promotes the development of parasite resistance. For example, S. mansoni with resistance or reduced susceptibility to PZQ were easily generated by exposing snails harboring the parasite to low doses of PZQ (Couto et al., 2011), or on experimented mice after a few generations of sub-curative dose selection (Fallon and Doenhoff, 1994; Sanchez et al., 2019). Such development progress of PZQ resistance could even be facilitated for those parasites surviving standard or normal curative doses, plus their normal reproduction potential in the case of S. japonicum reported here. An example is clearly demonstrated by (Lamberton et al., 2017) on S. mansoni, as the resistant isolates reported and tested in their laboratory had arisen from infected humans who had received three rounds of treatments each at PZQ 40–60 mg/kg. In our study here, S. japonicum males or females, surviving either a sub-standard or standard PZQ treatment, hold on the same reproduction potential as those schistosomes without treatment. This was in contrast with the research on S. mansoni (Lamberton et al., 2017), in which in vivo praziquantel treatment even with low doses (i.e., PZQ 25 or 50 mg/kg) significantly reduced fecundity in surviving worms across four laboratory-passaged generations. This highlights the possible transmission ease with which S. japonicum with drug resistance or reduced sensitivity, if there is, could occur.
Compared to infections in final hosts with balance dual-sex schistosomes, imbalance infections (or single-sex infections) would be more likely to occur in the field, due to the high proportions of snails with single-sex schistosomes in the field (Shi et al., 2014). This may lead to extra males or females living in the form of unpaired during their lifetime or along with paired worms within a final host. Paired worms have been shown to be more susceptible to PZQ as reported in all animal experiments to date (Yu et al., 2021) and be more susceptible than single-sex schistosomes (Pica-Mattoccia and Cioli, 2004). Therefore, it would be predicted that the single-sex unpaired schistosomes in humans or animals surviving treatment might serve as an alternative reservoir for transmission, if they later have a chance to pair and mate an opposite gender of schistosome and produce offspring.
In our study we did not set a paralleled group of mice to explore the treatment efficiency of PZQ on dual-sex schistosome infections. There are two main explanations. Firstly, there were many experiments on this kind of dual-sex infections and the accumulated evidence had shown the high efficiency of drug treatment (see (Yu et al., 2021) for synthesized results). In addition, as we wanted to test the treatment efficiency of PZQ on single-sex S. japonicum who had lived for a long term (e.g., three months), it seemed impossible to have the mice, infected with dual-sex schistosome infections and then heavily ill, wait so long. Another limitation was that we did not test more PZQ dosages to explore the effective dose for female S. japonicum.
To conclude, schistosomes are dioecious, and single-sex schistosome infections within definitive hosts are normal in the field. Long (over a season) live single-sex S. japonicum within definitive hosts (mice) can easily survive the treatment even at a standard PZQ dose, and moreover the reproduction potential of surviving schistosomes did not decrease. Therefore, in order to realize the target for the national and the global schistosomiasis elimination, there is undoubtedly a great need for refining PZQ administration and dosage, looking for alternative therapies, and/or developing vaccines against schistosomes.
Declarations of competing interest
None.
Acknowledgements
The authors and/or their research are currently funded by the National Science Foundation of China (to DL, No. 81971957) and by a Zoonoses and Emerging Livestock Systems (International Research Consortium on Animal Health) research grant (combined BBSRC, MRC, ESRC, NERC, DSTL and DFID: BB/S013822/1 to JPW). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Contributor Information
Joanne P. Webster, Email: jowebster@rvc.ac.uk.
Da-Bing Lu, Email: Ludabing@suda.edu.cn.
References
- Abla N., Keiser J., Vargas M., Reimers N., Haas H., Spangenberg T. Evaluation of the pharmacokinetic-pharmacodynamic relationship of praziquantel in the Schistosoma mansoni mouse model. PLoS Neglected Trop. Dis. 2017;11 doi: 10.1371/journal.pntd.0005942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balen J., Zhao Z.Y., Williams G.M., McManus D.P., Raso G., Utzinger J., Zhou J., Li Y.S. Prevalence, intensity and associated morbidity of Schistosoma japonicum infection in the Dongting Lake region, China. Bull. World Health Organ. 2007;85:519–526. doi: 10.2471/BLT.06.034033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boissier J., Grech-Angelini S., Webster B.L., Allienne J.F., Huyse T., Mas-Coma S., Toulza E., Barre-Cardi H., Rollinson D., Kincaid-Smith J., Oleaga A., Galinier R., Foata J., Rognon A., Berry A., Mouahid G., Henneron R., Mone H., Noel H., Mitta G. Outbreak of urogenital schistosomiasis in Corsica (France): an epidemiological case study. Lancet Infect. Dis. 2016;16:971–979. doi: 10.1016/S1473-3099(16)00175-4. [DOI] [PubMed] [Google Scholar]
- Boissier J., Mone H., Mitta G., Bargues M.D., Molyneux D., Mas-Coma S. Schistosomiasis reaches Europe. Lancet Infect. Dis. 2015;15:757–758. doi: 10.1016/S1473-3099(15)00084-5. [DOI] [PubMed] [Google Scholar]
- Cioli D., Botros S.S., Wheatcroft-Francklow K., Mbaye A., Southgate V., Tchuente L.A., Pica-Mattoccia L., Troiani A.R., El-Din S.H., Sabra A.N., Albin J., Engels D., Doenhoff M.J. Determination of ED50 values for praziquantel in praziquantel-resistant and -susceptible Schistosoma mansoni isolates. Int. J. Parasitol. 2004;34:979–987. doi: 10.1016/j.ijpara.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Cioli D., Pica-Mattoccia L., Basso A., Guidi A. Schistosomiasis control: praziquantel forever? Mol. Biochem. Parasitol. 2014;195:23–29. doi: 10.1016/j.molbiopara.2014.06.002. [DOI] [PubMed] [Google Scholar]
- Coeli R., Baba E.H., Araujo N., Coelho P.M., Oliveira G. Praziquantel treatment decreases Schistosoma mansoni genetic diversity in experimental infections. PLoS Neglected Trop. Dis. 2013;7 doi: 10.1371/journal.pntd.0002596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coles G.C., Bruce J.J., Kinoti G.K., Mutahi W.T., Dias L.C.S., Rocha R.S., Katz N. The potential for drug resistance in schistosomiasis. Parasitol. Today. 1987;3:349. doi: 10.1016/0169-4758(87)90121-9. [DOI] [PubMed] [Google Scholar]
- Colley D.G., Andros T.S., Campbell C.H., Jr. Schistosomiasis is more prevalent than previously thought: what does it mean for public health goals, policies, strategies, guidelines and intervention programs? Infect. Dis. of Poverty. 2017;6:63. doi: 10.1186/s40249-017-0275-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couto F.F., Coelho P.M., Araujo N., Kusel J.R., Katz N., Jannotti-Passos L.K., Mattos A.C. Schistosoma mansoni: a method for inducing resistance to praziquantel using infected Biomphalaria glabrata snails. Mem. Inst. Oswaldo Cruz. 2011;106:153–157. doi: 10.1590/s0074-02762011000200006. [DOI] [PubMed] [Google Scholar]
- Fallon P.G., Doenhoff M.J. Drug-resistant schistosomiasis: resistance to praziquantel and oxamniquine induced in Schistosoma mansoni in mice is drug specific. Am. J. Trop. Med. Hyg. 1994;51:83–88. doi: 10.4269/ajtmh.1994.51.83. [DOI] [PubMed] [Google Scholar]
- Harder A., Andrews P., Thomas H. Praziquantel: mode of action. Biochem. Soc. Trans. 1987;15:68–70. doi: 10.1042/bst0150068. [DOI] [PubMed] [Google Scholar]
- He Y.X., Salafsky B., Ramaswamy K. Host-parasite relationships of Schistosoma japonicum in mammalian hosts. Trends Parasitol. 2001;17:320–324. doi: 10.1016/s1471-4922(01)01904-3. [DOI] [PubMed] [Google Scholar]
- King A. Only vaccines can eradicate parasitic worms. Nature. 2019;575:S54. doi: 10.1038/d41586-019-03643-9. [DOI] [PubMed] [Google Scholar]
- Lamberton P.H.L., Faust C.L., Webster J.P. Praziquantel decreases fecundity in Schistosoma mansoni adult worms that survive treatment: evidence from a laboratory life-history trade-offs selection study. Infect. Dis. of Poverty. 2017;6:110. doi: 10.1186/s40249-017-0324-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langenberg M.C.C., Hoogerwerf M.A., Koopman J.P.R., Janse J.J., Kos-van Oosterhoud J., Feijt C., Jochems S.P., de Dood C.J., van Schuijlenburg R., Ozir-Fazalalikhan A., Manurung M.D., Sartono E., van der Beek M.T., Winkel B.M.F., Verbeek-Menken P.H., Stam K.A., van Leeuwen F.W.B., Meij P., van Diepen A., van Lieshout L., van Dam G.J., Corstjens P., Hokke C.H., Yazdanbakhsh M., Visser L.G., Roestenberg M. A controlled human Schistosoma mansoni infection model to advance novel drugs, vaccines and diagnostics. Nat. Med. 2020;26:326–332. doi: 10.1038/s41591-020-0759-x. [DOI] [PubMed] [Google Scholar]
- Le Clec'h W., Chevalier F.D., Mattos A.C.A., Strickland A., Diaz R., McDew-White M., Rohr C.M., Kinung'hi S., Allan F., Webster B.L., Webster J.P., Emery A.M., Rollinson D., Djirmay A.G., Al Mashikhi K.M., Al Yafae S., Idris M.A., Mone H., Mouahid G., LoVerde P., Marchant J.S., Anderson T.J.C. Genetic analysis of praziquantel response in schistosome parasites implicates a transient receptor potential channel. Sci. Transl. Med. 2021;13 doi: 10.1126/scitranslmed.abj9114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei Z.L., Zhang L.J., Xu Z.M., Dang H., Xu J., Lv S., Cao C.L., Li S.Z., Zhou X.N. [Endemic status of schistosomiasis in People's Republic of China in 2014] Zhongguo Xue Xi Chong Bing Fang Zhi Za Zhi. 2015;27:563–569. [PubMed] [Google Scholar]
- Levecke B., Vlaminck J., Andriamaro L., Ame S., Belizario V., Degarege A., Engels D., Erko B., Garba A.D., Kaatano G.M., Mekonnen Z., Montresor A., Olliaro P., Pieri O.S., Sacko M., Sam-Wobo S.O., Tchuem Tchuente L.A., Webster J.P., Vercruysse J. Evaluation of the therapeutic efficacy of praziquantel against schistosomes in seven countries with ongoing large-scale deworming programs. Int. J. Parasitol.: Drugs Drug Resist. 2020;14:183–187. doi: 10.1016/j.ijpddr.2020.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Y.S., Dai J.R., Zhu M.C., Li H.J., Xu M., Xu Y.L., Hang P.Y., Ccoles G., Jdoenhoff M. Studies on resistance of schistosoma to praziquantel III response of cercariae of praziquantel-resistance and -susceptible Schistosoma mansoni to praziquantel. Chinese J. Schistosomiasis Control. 2003:12–16. [Google Scholar]
- Lo N.C., Bezerra F.S.M., Colley D.G., Fleming F.M., Homeida M., Kabatereine N., Kabole F.M., King C.H., Mafe M.A., Midzi N., Mutapi F., Mwanga J.R., Ramzy R.M.R., Satrija F., Stothard J.R., Traore M.S., Webster J.P., Utzinger J., Zhou X.N., Danso-Appiah A., Eusebi P., Loker E.S., Obonyo C.O., Quansah R., Liang S., Vaillant M., Murad M.H., Hagan P., Garba A. Review of 2022 WHO guidelines on the control and elimination of schistosomiasis. Lancet Infect. Dis. 2022 doi: 10.1016/S1473-3099(22)00221-3. [DOI] [PubMed] [Google Scholar]
- Lu D.B., Deng Y., Ding H., Liang Y.S., Webster J.P. Single-sex schistosome infections of definitive hosts: implications for epidemiology and disease control in a changing world. PLoS Pathog. 2018;14 doi: 10.1371/journal.ppat.1006817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu D.B., Wang T.P., Rudge J.W., Donnelly C.A., Fang G.R., Webster J.P. Contrasting reservoirs for Schistosoma japonicum between marshland and hilly regions in Anhui, China - a two-year longitudinal parasitological survey. Parasitology. 2010;137:99–110. doi: 10.1017/S003118200999103X. [DOI] [PubMed] [Google Scholar]
- Lu D.B., Yu Q.F., Zhang J.Y., Sun M.T., Gu M.M., Webster J.P., Liang Y.S. Extended survival and reproductive potential of single-sex male and female Schistosoma japonicum within definitive hosts. Int. J. Parasitol. 2021;51:887–891. doi: 10.1016/j.ijpara.2021.03.005. [DOI] [PubMed] [Google Scholar]
- Norbury L.J., Shirakashi S., Power C., Nowak B.F., Bott N.J. Praziquantel use in aquaculture - current status and emerging issues. Int. J. Parasitol.: Drugs Drug Resist. 2022;18:87–102. doi: 10.1016/j.ijpddr.2022.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S.K., Friedrich L., Yahya N.A., Rohr C.M., Chulkov E.G., Maillard D., Rippmann F., Spangenberg T., Marchant J.S. Mechanism of praziquantel action at a parasitic flatworm ion channel. Sci. Transl. Med. 2021;13 doi: 10.1126/scitranslmed.abj5832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pica-Mattoccia L., Cioli D. Sex- and stage-related sensitivity of Schistosoma mansoni to in vivo and in vitro praziquantel treatment. Int. J. Parasitol. 2004;34:527–533. doi: 10.1016/j.ijpara.2003.12.003. [DOI] [PubMed] [Google Scholar]
- Rudge J.W., Webster J.P., Lu D.B., Wang T.P., Fang G.R., Basanez M.G. Identifying host species driving transmission of schistosomiasis japonica, a multihost parasite system, in China. Proc. Natl. Acad. Sci. U. S. A. 2013;110:11457–11462. doi: 10.1073/pnas.1221509110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez M.C., Cupit P.M., Bu L., Cunningham C. Transcriptomic analysis of reduced sensitivity to praziquantel in Schistosoma mansoni. Mol. Biochem. Parasitol. 2019;228:6–15. doi: 10.1016/j.molbiopara.2018.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi H.P., Lu D.B., Shen L., Shi T., Gu J. Single- or mixed-sex Schistosoma japonicum infections of intermediate host snails in hilly areas of Anhui, China. Parasitol. Res. 2014;113:717–721. doi: 10.1007/s00436-013-3700-0. [DOI] [PubMed] [Google Scholar]
- Webster J.P., Molyneux D.H., Hotez P.J., Fenwick A. The contribution of mass drug administration to global health: past, present and future. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2014;369 doi: 10.1098/rstb.2013.0434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao S.H. Development of antischistosomal drugs in China, with particular consideration to praziquantel and the artemisinins. Acta Trop. 2005;96:153–167. doi: 10.1016/j.actatropica.2005.07.010. [DOI] [PubMed] [Google Scholar]
- Xiao S.H., Sun J., Chen M.G. Pharmacological and immunological effects of praziquantel against Schistosoma japonicum: a scoping review of experimental studies. Infect. Dis. of Poverty. 2018;7:9. doi: 10.1186/s40249-018-0391-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu S.Y. second ed. People's Medical Publishing House; Beijing, China: 1996. Pharmacological Experiment Methodology. [Google Scholar]
- Yu Q.F., Zhang J.Y., Sun M.T., Gu M.M., Zou H.Y., Webster J.P., Lu D.B. In vivo praziquantel efficacy of Schistosoma japonicum over time: a systematic review and meta-analysis. Acta Trop. 2021;222 doi: 10.1016/j.actatropica.2021.106048. [DOI] [PubMed] [Google Scholar]
- Yue W., Xu X., Mei J., Xiao S.H. Observation on susceptibility of different geographic Schistosoma japonicum to praziquantel in mice. Chin. Pharmacol. Bull. 1988;4:355–357. [Google Scholar]
- Zhang L.J., Xu Z.M., Dang H., Li Y.L., Lu S., Xu J., Li S.Z., Zhou X.N. Endemic status of schistosomiasis in People's Republic of China in 2019. Chinese J. Schistosomiasis Control. 2020;32:551–558. doi: 10.16250/j.32.1374.2020263. [DOI] [PubMed] [Google Scholar]