Abstract
Malaria is among the tropical diseases that cause the most deaths in Africa. Around 500,000 malaria deaths are reported yearly among African children under the age of five. Chloroquine (CQ) is a low-cost antimalarial used worldwide for the treatment of Plasmodium vivax malaria. Due to resistance mechanisms, CQ is no longer effective against most malaria cases caused by P. falciparum. The World Health Organization recommends artemisinin combination therapies for P. falciparum malaria, but resistance is emerging in Southeast Asia and some parts of Africa. Therefore, new medicines for treating malaria are urgently needed. Previously, our group identified the 4-aminoquinoline DAQ, a CQ analog containing an acetylenic bond in its side chain, which overcomes CQ resistance in K1 P. falciparum strains. In this work, the antiplasmodial profile, drug-like properties, and pharmacokinetics of DAQ were further investigated. DAQ showed no cross-resistance against standard CQ-resistant strains (e.g., Dd2, IPC 4912, RF12) nor against P. falciparum and P. vivax isolates from patients in the Brazilian Amazon. Using drug pressure assays, DAQ showed a low propensity to generate resistance. DAQ showed considerable solubility but low metabolic stability. The main metabolite was identified as a mono N-deethylated derivative (DAQM), which also showed significant inhibitory activity against CQ-resistant P. falciparum strains. Our findings indicated that the presence of a triple bond in CQ-analogues may represent a low-cost opportunity to overcome known mechanisms of resistance in the malaria parasite.
Keywords: Malaria, Chloroquine, Resistance, ACTs, DAQ
Graphical abstract
Highlights
-
•
4-aminoquinoline antimalarial candidates containing a triple bond did not show in vitro cross-resistance against CQ-resistant strains.
-
•
DAQ showed a low propensity to generate resistant mutants in vitro after 90 days of drug pressure.
-
•
DAQ was active against Plasmodium falciparum and P. vivax field isolates from Brazilian Amazon.
-
•
DAQM, the main metabolite of DAQ, also potently inhibited both CQ-sensitive and CQ-resistant strains.
1. Introduction
Malaria is a tropical disease with high incidence in South America, Africa and Southeast Asia. There were more than 240 million human malaria cases and 620,000 deaths reported in 2020, caused by six different species of Plasmodium P. falciparum, P. vivax, P. malariae, P. ovale wallikeri, P. ovale curtisi and P. knowlesi) (World Health Organization (WHO), 2021a). P. falciparum is the prevalent species in Africa, and the most lethal, while P. vivax is widespread throughout tropical regions of the world. The Plasmodium species have a complex life cycle in the human body, following the bite of infected female Anopheles mosquitoes (Antinori et al., 2012). Once in human blood, the sporozoite forms of the parasite enter liver cells, where they mature to schizont forms. In the case of P. vivax and P. ovale, they may remain dormant as hypnozoites in liver cells, which may lead to a relapse of malaria weeks or years after the infection. Mature schizonts burst and release merozoites, where they mature to merozoites and multiply until they rupture the cells. Some merozoites may develop into the sexual forms of the parasite, known as gametocytes, and then be transferred to the mosquitoes, where they finally develop into sporozoites, reinitiating the cycle (Aly et al., 2009).
Chloroquine (CQ) is a low-cost 4-aminoquinoline derivative developed in the 1940s that targets the asexual forms of the parasite in RBCs. CQ or Artemisinin Combination Therapies (ACTs) are recommended by the World Health Organization (WHO) to treat uncomplicated blood-stage malaria caused by P. vivax, P. malariae, P. ovale and P. knowlesi in areas with CQ-susceptible infections (World Health Organization (WHO), 2021b). CQ was widely used against P. falciparum, but the parasite developed resistance, and the use of CQ against this species is currently limited to the regions where the strains are still CQ-sensitive, such as Mesoamerica. WHO recommends ACTs for P. falciparum malaria in most parts of the world, but emerging cases of resistance in Southeast Asia and Africa are driving the search for new effective antimalarials (Noisang et al., 2019). P. falciparum parasites with reduced in vivo susceptibility to artemisinin derivatives have also been identified in western Cambodia. These findings threaten global efforts to control and eliminate malaria (Dondorp et al., 2009; Phyo et al., 2012; Projectfalciparum, 2016; Thriemer et al., 2014).
Despite P. falciparum resistance, CQ remains extensively used, due in part to its low cost, high accessibility, and manufacturing capacity across the globe. Malaria mostly affects low- and middle-income countries, and, therefore, more accessible treatments are needed. Ferroquine (FQ), a CQ analog with high barrier for P. falciparum resistance, is currently in late-stage clinical trials as part of combination therapies, although it has some liabilities. For instance, the ferrocene group in FQ significantly increases the lipophilicity compared to CQ, resulting in >500-fold decreased solubility in PBS buffer (pH 7.4) (Charman et al., 2020). Moreover, a recent study demonstrated that FQ reduces the exposure of artefenomel, a front-runner candidate for inclusion in combination with FQ, in milk-based formulations (Salim et al., 2019).
In 1969, Singh and co-workers showed that unsaturated CQ analogues, including DAQ (Fig. 1), have in vivo antimalarial activity against P. berghei, and reduced toxicity in mice at concentrations as high as 640 mg/kg (Singh et al., 1969). The antimalarial activity of acetylenic CQ analogues against CQ-resistant strains remained unexplored until our group reported in 2018 that DAQ is active against K1 strain (Aguiar et al., 2018).
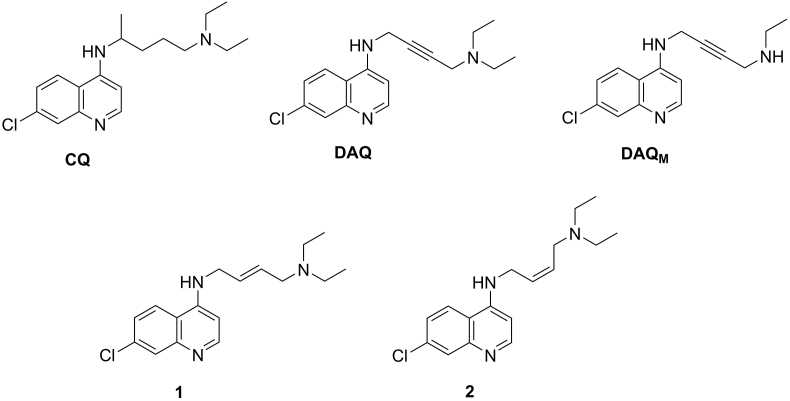
Fig. 1.
Chemical structures of CQ and compounds DAQ, DAQM, 1 and 2.
In this work, the antimalarial activities of DAQ and its major metabolite, DAQM, were assessed against other CQ-resistant strains: Dd2, IPC 4912 and RF12. Moreover, DAQ was tested against human isolates from patients infected with P. falciparum or P. vivax in the Brazilian Amazon. Finally, we performed in vitro absorption, distribution, metabolism, excretion, and toxicity (ADMET) assays, and evaluated the pharmacokinetics (PK) of DAQ in mice.
2. Methods
2.1. Compounds
Compounds DAQ, DAQM, 1 and 2 were synthesized by ChemPartner (https://chempartner.com/). The chemical structures of the compounds investigated herein are indicated in Fig. 1. Analytical reports for these compounds are available in Supporting Information (Reports S1).
SYBR green I growth inhibition assay.
Continuous in vitro cultures of P. falciparum were kept as described by Trager and Jensen, using strains 3D7, Dd2, K1 and IPC 4912 (Trager and Jensen, 1976). The strains were obtained as a donation from EIB resources (https://www.beiresources.org/). The Dd2, K1 and IPC 4912 strains show resistance to the antimalarials CQ and pyrimethamine. In addition, strain IPC 4912 has shown resistance to artemisinin and when exposed to dihydroartemisinin gave a ring-stage survival assay (RSA0-3h) value of 49.3%. Cultures were maintained at a low-oxygen atmosphere (5% O2, 5% CO2, 90% N2) in a humidified environment at 37 °C. The parasites were cultivated in a 2% hematocrit suspension of O+ human red blood cells, in RPMI 1640 medium supplemented with 25 mM Hepes (pH 7.4), 21 mM sodium bicarbonate, 11 mM D-glucose, 3.7 mM hypoxanthine, 40 μg/mL of penicillin-streptomycin, and 0.5% (w/v) Albumax. Parasitemia was assessed by microscopy through exams of daily blood smears.
P. falciparum cultures were synchronized by treatment with a solution of 5% sorbitol for 10 min (Lambros and Vanderberg, 1979). Compounds were diluted to a stock concentration of 20 mM in 100% DMSO before the experiments and maintained at −20 °C. Compound inhibitory potencies were determined using the SYBR green I phenotypic assay (Smilkstein et al., 2004). Briefly, in 96-well plates, 180 μL of a parasite suspension in the ring-stage form at 0.5% parasitemia and 2% hematocrit were incubated for 72 h with 20 μL of 10-fold concentrated serial dilutions of each compound. The test concentration of the compounds ranged from 0.75 to 1000 nM. CQ was used as a positive control for inhibition against all P. falciparum strains. The plates were kept at 37 °C and a low oxygen atmosphere (5% O2, 5% CO2 and 90% N2). RBCs were used as a negative control and infected erythrocytes (iRBCs) without antimalarials were added as a positive control for parasite growth. Non-positive and negative controls were added to each plate. The growth medium was then removed after 72 h of incubation, and the RBCs were resuspended in PBS buffer (116 mM NaCl, 10 mM Na2HPO4, 3 mM KH2PO4). A diluted solution of SYBR green I DNA Stain was added in a lysis buffer (20 mM Tris, 5 mM EDTA, 0.008% (m/v) saponin, and 0.08% (m/v) Triton X-100, at pH 7.5) to induce hemolysis. The plates were incubated for an additional 30 min, after which the fluorescence of the plate was measured (absorption and emission wavelengths of 485 nm and 535 nm, respectively). Fluorescence intensity was analyzed in terms of parasite viability as compared to controls, using GraphPad Prism version 8. Concentration-response curves were built using nonlinear regression analysis, and half-maximal inhibitory concentration (IC50) values were determined for each compound. At least 3 experiments were performed to calculate the mean IC50 value and standard deviation.
2.2. Lactate dehydrogenase (LDH) growth inhibition assay
Parasite inoculum of 2% hematocrit with 0.25% parasitemia was prepared from asynchronous culture (5–7% parasitemia with ≥70% rings) in RPMI media containing 5% Albumax. The parasite inoculum was added to each well already containing 2.5 μl of compound/vehicle and plates were shaken for 10 s to ensure mixing and finally incubated at 37 °C for 72 h in an atmosphere of 5% CO2, 5% O2, 90% N2. After 72 h of incubation, plates were frozen at −80 °C overnight and then thawed at 21 °C for 5 h. To evaluate PfLDH activity, 70 μl of freshly prepared reaction mix containing 143 mM sodium L-lactate, 143 μM 3-acetyl pyridine adenine dinucleotide (APAD), 178.75 μM Nitro Blue tetrazolium chloride (NBT), diaphorase (2.83 U/ml), 0.7% Tween 20, 100 mM Tris-HCl pH 8.0 was added into each well of the incubation plate mentioned above. Plates were shaken to ensure mixing. Plates are placed in a dark cupboard for 20 min. Once a color reaction is observed from yellow to purple, absorbance at 650 nm was recorded in a plate reader (Spectramax M5) after 20 min of incubation (within the linear range of PfLDH activity) at 21 °C (Gamo et al., 2010). The assay was performed at TCG Life Sciences (TCGLS: https://www.tcgls.com/) and the Medicines for Malaria Venture (MMV) with compound vials provided by ChemPartner (https://chempartner.com/). This assay was performed in duplicates for DAQ and DAQM. CQ was tested 8 times against the 3D7 strain, and in duplicates against the Dd2 and RF12 strains.
2.3. Schizont maturation test
Human blood samples were collected from patients in the region of Porto Velho, Rondônia, Brazil, and the standard Schizont Maturation Test (SMT) was performed as described by Rieckmann and co-workers (Rieckmann et al., 1978) at the Research Center for Tropical Medicine (CEPEM – RO). The study complied with all relevant ethical regulations, under the Ethics Committee CAAE 61442416.7.0000.0011. Briefly, blood samples were collected in heparinized tubes and centrifuged (600 g, 5 min). The RBC fraction was collected and filtered through a cellulose column for residual white blood cell removal. Hematocrit was adjusted to 2% using either complete medium RPMI 1640 medium plus 10% AB human serum, with P. falciparum cultures; or the McCoy's 5A medium plus 20% AB human serum, with P. vivax samples. Next, the resulting suspension was distributed onto 96-well plates with the pre-diluted compounds. Twelve dilutions of each compound were prepared, in addition to untreated control wells, using non-supplemented culture medium. The experiment was carried out in monoplicate. The plate was then incubated at 37 °C, with periodic monitoring of parasite morphology using thick smears. Upon reaching ≥40% schizonts in the control wells, thick smears were prepared for microscopic evaluation of the growth inhibitory activity. Schizont percentages were analyzed for 200 observed parasites for each compound concentration and used as a measure of inhibition relative to control wells (considered as 100% growth). IC50 values were calculated independently against each patient sample, and median IC50 values were used to represent compound potencies.
2.4. Selection of resistant strain
The 3D7 strain was cloned using the microdilution technique, to obtain a single parasite progeny. Then, inocula of 105 to 108 parasites were subjected to drug pressure from DAQ using 10-fold the IC50 for the 3D7 strain previously determined in the SYBR green assay (Smilkstein et al., 2004). The use of 3D7 clones and 10-fold the IC50 of antimalarials for in vitro selection studies has been previously reported in literature (Cowell et al., 2018; Ross et al., 2014). The experiments were carried out in triplicate, to obtain clones from different culture plates. The morphology of the parasites was observed by optical microscopy, and drug pressure was interrupted as soon as parasites were not observed in the culture. Then, when the culture reached 5% of parasitemia, drug pressure was reestablished (10-fold the IC50 value). This cycle of drug pressure was performed during 90 consecutive days.
2.5. ADME assays
In vitro Absorption, Distribution, Metabolism and Excretion (ADME) assays were performed by ChemPartner, at pH 7.4, in duplicate, using liquid chromatography–mass spectrometry (LC–MS/MS) for compound detection. These assays are described as follows: (i) Thermodynamic solubility was performed in 100 mM phosphate buffer, using a targeted test concentration of 4 mg/mL of each compound's powder. These solutions were shaken (1000 revolutions per minute, rpm) for 1 h, then equilibrated over night at room temperature; (ii) Permeability (time points: 0 and 90 min; compound concentration = 10 μM) was measured using a Caco-2 cell line (American Type Culture Collection, ATCC HTB-37) at 37 °C (Lea et al., 2015). Atenolol and metoprolol, compounds with low and high permeability, respectively, were used as control groups (Chen et al., 2017; Incecayir et al., 2013); (iii) Plasma Protein Binding (PPB) (time points: 0 and 5 h; compound concentration = 1 μM) was determined by equilibrium dialysis using a high-throughput dialysis device in human and CD-1 mouse plasmas. Percentage of bound compound was calculated as: 100 × ([Donor]5h - [Receiver]5h)/[Donor]5h. Warfarin, a compound with high PPB in both mouse and human plasma, and quinidine, a compound with high PPB in human plasma and moderate PPB in mouse plasma, were used as control groups (Mungall et al., 1984); (iv) Plasma stability (time points: 0, 5, 15, 30, 45 and 60 min; compound concentration = 2 μM) was measured following compound incubation at 37 °C in human and CD-1 mouse plasmas; (v) Liver microsomal stability/clearance (time points: 0, 5, 15, 30 and 45 min; compound concentration = 1 μM) was measured in human and CD-1 mouse liver microsomes at 37 °C, and root square deviation (RSD) values were calculated per time point (n = 2, RSD <10% of measured mean values of remaining compound's percentage). Ketanserin, a compound with high in vitro clearance in both human and mouse liver microsomes, was used as a control (Bonn et al., 2016). Different control groups and compound concentrations were used in these ADME assays based on historical control settings by ChemPartner.
In vitro metabolite identification was conducted after incubating DAQ (parent compound, final concentration 10 μM) with human, mouse, and monkey hepatocytes at 37 °C, 5% CO2 in HI hepatocyte medium. The samples taken at 0 min and 30 min were quenched by acetonitrile and analyzed using AB SCIEX X500B-QTOF system. LC-UV-MS extract ion chromatograms (EIC) at 0 min and 30 min were compared to identify major putative metabolites. The MS/MS spectra of DAQ and metabolites were obtained during positive-ion electrospray. The possible chemical structures of the metabolites were deduced based on their MS/MS spectra and retention time.
2.6. hERG inhibition, CYP inhibition panel and mini-Ames test
Assays for in vitro exploratory toxicology were performed by ChemPartner, as follows: (i) The automated patch (QPatch) was used in two CHO cell lines (Chinese hamster ovary cell) for determining inhibition of the human Ether-à-go-go-Related Gene (hERG) (Sorota et al., 2005). Six compound concentrations were used for IC50 determination (0.1, 0.3, 1, 3, 10 and 30 μM). Compound testing was repeated on two different cells to make sure the standard deviation of the results for each concentration was less than 10. Cisapride (C4740-10 mg, Sigma) was used as a positive control. In addition, an independent hERG experiment (n = 1) was performed in collaboration with MMV/TCGLS using the Invitrogen PredictorTM hERG Fluorescence Polarization Assay, with 1% DMSO as vehicle and an incubation time of 4 h at 20-25 °C (Piper et al., 2008); (ii) Inhibition of CYP450 proteins (1A2, 3A4, 2B6, 2C8, 2C9, 2C19 and 2D6) was determined using human liver microsomes. Eight compound concentrations ranging from 0 to 10 μM were used for IC50 determination against each CYP450 protein; (iii) The mutagenicity assay (mini-Ames test) was performed with five Salmonella typhimurium strains (TA97a, TA98, TA100, TA102 and TA1535) (Flamand et al., 2001). The assay was conducted in both the presence and absence of rat liver S9 mixture (Aroclor 1254 induced, Moltox 11–101) along with DMSO and positive controls in duplicate using 6-well plates. The following positive controls were used: Fenaminosulf (2.0 μg/well, CAS 140-56-7, AccuStandard, P–058NB-250) for TA97a without S9 activation, 2-Nitrofluorene (2.0 μg/well, CAS 607-57-8, Aldrich, N16754) for TA98 without S9 activation, sodium azide (NaN3, 2.0 μg/well, CAS 26628-22-8, Sigma Aldrich, S8032) for TA100 and TA1535 without S9 activation, methyl methanesulfonate (1.0 μL/well, CAS 66-27-3, Aldrich, 129925) for TA102 without S9 activation, 2-aminoanthracene (20.0 μg/well, CAS 613-13-8, Aldrich, A38800) for TA97a with S9 activation, 2-aminofluorene (10.0 and 100.0 μg/well, CAS 153-78-6, Aldrich, A55500) for TA98, TA100 and TA102 with S9 activation and cyclophosphamide (200.0 μg/well, CAS 6055-19-2, Jiangsu Shengdi, 69390D) for TA1535 with S9 activation. Five concentrations ranging from 62.5 to 1000.0 μg/well were tested for DAQ.
2.7. In vivo pharmacokinetics assays
In vivo pharmacokinetics (PK) experiments were performed by ChemPartner. Concentrations of DAQ and its primary metabolite DAQM were measured in plasma following intravenous (IV, 10 mg/kg) or oral (PO, 20 mg/kg) administrations in male CD1 mice (9 mice per route of administration), with sampling at 0.5, 1, 2, 4, 8, 24 and 48 h post dosing.
3. Results
We found that DAQ is a low nanomolar inhibitor of CQ-sensitive and CQ-resistant strains of P. falciparum (Table 1, S1 and S2). The resistance index (RI) values for DAQ ranged from 1.1 to 1.5, demonstrating that DAQ showed no significant cross-resistance with 3D7 in either SYBR green I or LDH growth inhibition assays (Table S2). Moreover, we synthesized and assessed the inhibitory activity of three new CQ analogues (Compounds DAQM, 1 and 2, Fig. 1). Compounds 1 and 2 are ethylenic analogues and geometric isomers (trans and cis isomers, respectively) and they showed cross-resistance with CQ when tested against the CQ-resistant Dd2 strain (IC50 values of 1468 ± 120 nM and 262 ± 30 nM, respectively, Fig. S1). Therefore, the replacement of the triple bond of DAQ with the double bond of Compounds 1 and 2 had a significant impact in the inhibitory properties against the CQ-resistant Dd2 strain. DAQM, DAQ's primary metabolite (Table S3), was a potent inhibitor of CQ-sensitive and CQ-resistant strains (IC50 values ranging from 23 nM to 91 nM) (Table 1) (Valderramos and Fidock, 2006).
Table 1.
IC50 inhibition data of CQ and acetylenic compounds against P. falciparum strains according to the growth inhibition assay.
| Compounds | Growth Inhibition Assay |
P. falciparum IC50 (nM) |
|||
|---|---|---|---|---|---|
| 3D7 | Dd2 | IPC 4912 | RF12 | ||
| DAQ | SYBR green I | 43 ± 5 | 51 ± 2 | 49 ± 3 | NT |
| LDH | 53 ± 9 | 68 ± 1 | NT | 79 ± 10 | |
| DAQM | SYBR green I | 34 ± 4 | 61 ± 8 | NT | NT |
| LDH | 23 ± 2 | 91 ± 9 | NT | 86 ± 11 | |
| CQ | SYBR green I | 15 ± 2 | 2313 ± 475 | 139 ± 5 | NT |
| LDH | 21 ± 1 | 211 ± 16 | NT | 205 ± 6 | |
NT: Not tested. The SYBR green I growth inhibition assay was performed in triplicates for DAQ, DAQM and CQ. The LDH growth inhibition assay for DAQ and DAQM was performed in duplicates. CQ was tested 8 times against the 3D7 strain using the LDH assay, and in duplicates against the Dd2 and RF12 strains. Individual IC50 values are reported in Table S1.
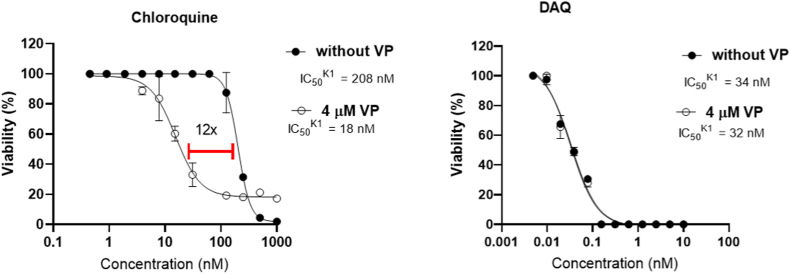
One of the potential CQ-resistance mechanisms has been related to CQ-resistance transporter PfCRT, an efflux membrane transporter of Plasmodium's digestive vacuole (Valderramos and Fidock, 2006). We assessed DAQ's and CQ's inhibitory activities against the K1 strain (CQ-resistant) in the presence and absence of verapamil, a known PfCRT inhibitor. The assessed IC50 values for CQ with and without verapamil were 19 ± 6 nM and 252 ± 114 nM nM, respectively, demonstrating that the incubation with verapamil increased by > 10-fold the inhibitory activity of CQ (Fig. 2 for representative experiment, Table S4 for data of three independent assays). By contrast, the inhibitory activity of DAQ (IC50 = 33 ± 5 nM) was unaffected by the presence of the PfCRT inhibitor (IC50 = 36 ± 5 nM) (Fig. 2, Table S4).
Fig. 2.
Representative graph for IC50 measure of DAQ against K1 CQ-resistant strain, with the addition of verapamil at 4 μM (open circle) and without verapamil (closed circle). CQ was used as a control. Data for two other independent experiments are provided in Table S4.
DAQ's potential to generate resistant mutants was investigated in vitro by incubating it, at 10-fold the IC50 value, with blood parasites of P. falciparum clone 3D7 in four different inocula of 105 to 108 parasites. No parasite growth was observed after 90 days of drug pressure (Fig. 3). Therefore, DAQ did not induce the generation of resistant mutants in vitro.
Fig. 3.
Incubation of 10-fold IC50 concentration of DAQ against 3D7 P. falciparum strain over 90 days (D1 to D90). The Y-axis refers to increasing number of parasites. Drug pressure, represented as purple rectangles, was applied at D1, and then during every day in which parasitemia was further observed. *At day 30 of the experiment with 106 parasites, only one out of three vials showed parasites above the limit of detection, and this vial was then submitted to drug pressure. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
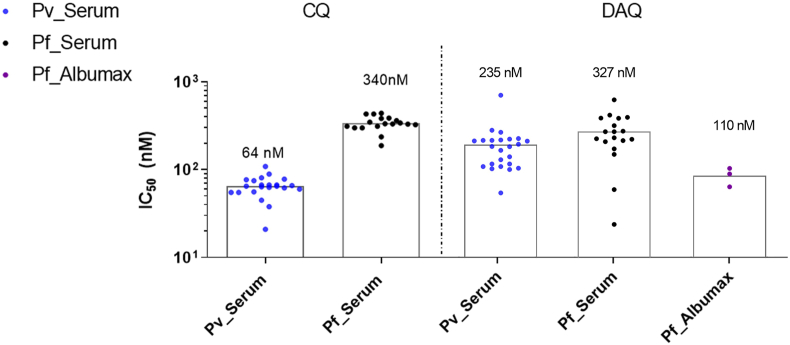
The antimalarial activity of DAQ was further investigated against P. falciparum blood isolates from three patients in Porto Velho (Amazon, Brazil), in media supplemented with Albumax, using a similar protocol to the in vitro inhibitory assays against 3D7 strains. The median IC50 values for DAQ against P. falciparum isolates was 110 nM (Fig. 4). This is ∼2-fold the IC50 value of DAQ against the 3D7 strain (Table 1), thereby verifying that DAQ retains a pronounced inhibitory activity against P. falciparum field isolates.
Fig. 4.
Median IC50 values of DAQ and CQ against P. vivax (Pv) and P. falciparum (Pf) field isolates from patients in Amazon, Brazil, in media supplemented with either 20% of human serum or Albumax. The number of P. falciparum isolates supplemented with Albumax (n = 3) was lower than isolates supplemented with human serum (n = 18) due to the challenges in recruiting patients infected with P. falciparum malaria during Covid19 pandemic.
DAQ's IC50 was also measured against P. vivax isolates from 24 patients from the Brazilian Amazon. Ex vivo cultures of P. vivax were supplemented with 20% human serum, due to the challenge of cultivating these parasites in Albumax-containing media. For comparison, DAQ was tested against 18 P. falciparum field isolates supplemented with 20% human serum. DAQ showed median IC50 values of 327 nM and 235 nM against P. falciparum and P. vivax isolates, respectively (Fig. 4). Therefore, no significant cross-resistance was observed in these field isolates. CQ was used as a control, with measured IC50 value of 64 nM against P. vivax isolates and 340 nM against P. falciparum.
The assessed IC50 values for DAQ in human isolates in culture media supplemented with human serum were ∼3-fold greater than the ones determined against P. falciparum isolates supplemented with Albumax (110 nM). To investigate if the culture media affect DAQ's antimalarial activity, the IC50 values of DAQ were measured against P. falciparum 3D7 strains cultivated in media supplemented with Albumax or 20% human serum. DAQ's IC50 against 3D7 strains in media supplemented with human serum ranged from 173 nM to 352 nM, which are 7 to 14-fold greater than in media supplemented with Albumax (IC50 = 25 nM) (Fig. S2). This finding may be related to DAQ's property of binding extensively to human plasma proteins (PPBhuman = 97.5%), which is higher than literature values reported for CQ (PPBhuman = 46.0%) and FQ (95.9%) (Charman et al., 2020).
To further characterize DAQ as a candidate for new antimalarial drug discovery, inhibition of hERG and CYP proteins was assessed, as well as the compound's physicochemical and ADMET properties, mutagenic potential, and in vivo PK profile. DAQ's IC50 against hERG (IC50,Exp. 1 = 7.3 μM; IC50,Exp. 2 = 7.0 μM) were within 2-fold of CQ's inhibition values (IC50,Exp. 1 = 12.1 μM, IC50,Exp. 2 = 7.5 μM, Table 2). DAQ was tested against a panel of seven CYP protein isoforms (CYP 1A2, 2B6, 2C8, 2C9, 2C19, 2D6 and 3A4). The results indicated that DAQ is not a strong inhibitor of the main CYP isoforms (IC50 > 10 μM). DAQ is highly soluble and moderately permeable (Table 2). DAQ's ability to induce mutagenic effects were evaluated in five strains of Salmonella typhimurium: TA97a, TA98, TA100, TA102 and TA1535. DAQ did not induce a positive increase in the mean number of revertants per well in these strains, even at DAQ concentrations as high as 1000 μg/well (Table S5).
Table 2.
DAQ's and DAQM's hERG, ADME and PK.
| DAQ | DAQM | Controls | ||
|---|---|---|---|---|
| hERG inhibition (IC50) | CQ | Cisapride | ||
| QPatch (2 different cells, fitting curve – Report S2) | 7.3 μM | 12.2 μM | 12.1 μM | 0.02 μM |
| Fluorescence polarization | 7.0 μM | 5.3 μM | 7.5 μM | – |
| CYP inhibition panel (IC50)a | >10 μM | NTb | – | |
| Thermodynamic solubility (PBS, pH 7.4) | 1210 ± 0.02 μM | 1350 ± 0.02 μM | – | |
| Permeability (Caco2) | Atelonol | Metoprolol | ||
| PappA-B | (15 ± 0.07) x 10-6 cm/s | (14 ± 0.06) x 10-6 cm/s | (0.26 ± 0.07) x 10-6 cm/s | (35 ± 0.07) x 10-6 cm/s |
| PappB-A | (11 ± 0.05) x 10-6 cm/s | (11 ± 0.09) x 10-6 cm/s | (0.57 ± 0.05) x 10-6 cm/s | (29 ± 0.01) x 10-6 cm/s |
| Plasma stability (t = 0–60min) | >60 minc | 158.7 min | – | |
| Plasma protein binding (PPB) | Warfarin | Quinidine | ||
| PPB mouse | 76.4% | 84.5% | 95.1% | 82.7% |
| PPB human | 97.5% | NT | 99.4% | 92.2% |
| DAQ's abundance in hepatocytes after 30min | ||||
| Mouse | <15% | NT | – | |
| Monkey | 39% | NT | – | |
| Human | 55% | NT | – | |
| Clearance (Clint) in liver microsomes (LM) | Ketanserin | |||
| mouse (MLM) | 2741 mL/min/kg | 273 mL/min/kg | 445 mL/min/kg | |
| human (HLM) | 159 mL/min/kg | 8 mL/min/kg | 52 mL/min/kg | |
| PK (t1/2, mice) | ||||
| PO | 2.1 h | 4.7 h | – | |
| IV | 2.9 h | 4.8 h | – | |
ADME assays by ChemPartner performed in duplicates.
CYP 1A2, 2B6, 2C8, 2C9, 2C19, 2D6 and 3A4.
NT: Not Tested.
% of remaining DAQ was near 100% at t = 60 min.
DAQ is quickly metabolized in mouse hepatocytes, and to a lesser extent in monkey and human hepatocytes (Table 2), similar to what has been reported for CQ and FQ (Daher et al., 2006). Low metabolic stability was also noted in human and mouse liver microsomes (159 mL/min/kg). In line with these observations, the half-life of DAQ was 2.1 h in mice (Table 2, Fig. 5). DAQ's major metabolite (DAQM) results from N-deethylation (Fig. S3 and Table S3) and is more stable than the parent compound, with a half-life of 4.7 h and much lower clearance in human liver microsomes (8 mL/min/kg). Considering that DAQ is highly active in vivo, with parasitemia reduction close to 100% in 11 days post infection, we hypothesize that DAQM is also active and plays a key role in killing the parasites, as also observed for metabolites of CQ and FQ (Aderounmu, 1984; Aguiar et al., 2018; Daher et al., 2006).
Fig. 5.
Mean plasma concentration-time profiles of DAQ after single IV (10 mg/kg) or PO (20 mg/kg) dose to male CD1 mice (9 mice per route of administration). The concentration of the mono N-deethylated metabolite (DAQM) was also determined in the same experiment.
4. Discussion
Since its discovery in the 1940s, CQ has been a pivotal agent in the fight against malaria. Although resistant cases were reported as early as the late 1950s, CQ remains widely used against non-resistant Plasmodium strains around the world, partially due to its low cost and low toxicity (Taylor and White, 2004). Herein, we reported that CQ analogues containing an acetylenic group (DAQ and DAQM) potently inhibit CQ-resistant strains of the parasite. Like CQ, these acetylenic analogues are simple to synthesize and should be able to be manufactured at low cost.
DAQ did not show cross-resistance against a wide range of CQ-resistant P. falciparum strains (Table 1). In a previous study, DAQ was shown to inhibit β-hematin formation, generating a complex with heme that is toxic to different Plasmodium species (Aguiar et al., 2018). This is in line with one of the proposed antimalarial mechanisms of action for CQ (Sullivan et al., 1996). DAQ and CQ have two amino groups that can be protonated under acidic conditions, which may facilitate their accumulation in the digestive vacuole of the parasite, where these compounds can interact with heme groups resulting from hemoglobin degradation. The major difference between CQ and DAQ is in the rigid, linear side chain of the latter, which may impact the interactions between DAQ and potential protein targets involved with parasite's resistance pathways (Valderramos and Fidock, 2006). The mono N-deethylated analog of DAQ, DAQM, did not show cross-resistance against CQ-resistant P. falciparum strains. On the other hand, CQ analogues with ethylenic groups in their lateral chains (Compounds 1 and 2) did show cross-resistance with CQ-resistant strains (Fig. S1). Therefore, the linear geometry of the lateral chain conferred by sp hybridization of the carbon atoms of DAQ and DAQM seems to be an important structural feature to improve the resistance-fighting potential.
One known mechanism of resistance to CQ involves increased efflux from the digestive vacuole of the parasite by membrane transporters such as PfCRT (De et al., 1996). Here we have shown that the IC50 of CQ against CQ-resistant K1 strain is ∼12-fold lower in the presence of verapamil, a known inhibitor of PfCRT (Table S4). Our hypothesis is that high concentrations of verapamil in K1 strain reduce the efflux of CQ by PfCRT. However, DAQ's IC50 is not affected by the presence of verapamil, which suggests that DAQ is not competing with verapamil for efflux mechanisms induced by the parasite's membrane transporters. More experiments are needed to test the hypothesis that DAQ is not a substrate of PfCRT, e.g., (a) using isogenic parasite cell lines expressing either the wild-type PfCRT or the CQ-resistant associated PfCRT isoforms and (b) expressing PfCRT in a heterologous system and measuring the ability of DAQ to inhibit [3H]CQ transport via PfCRT (Pulcini et al., 2015).
The drug pressure experiment indicated that DAQ's potency remained the same over the course of 90 days of treatment. Moreover, no parasites were observed at day 90 (with drug pressure being applied at day 40). By contrast, using another protocol for the generation of resistant strains, it was demonstrated that CQ induced the emergence of resistant parasites in an inocula of 6 × 108 (Cooper et al., 2002). These findings suggest that DAQ has low propensity to select resistant clones in vitro, which makes this compound an attractive candidate for a malaria eradication program. However, it is important to mention the need to evaluate other protocols for the generation of resistant strains in vitro, e.g., using the resistant clone Dd2 and 3xIC90 for compound concentration (Duffey et al., 2021).
The potential of CQ analogues to overcome CQ-resistance has already been reported for FQ, a 4-aminoquinoline containing a more lipophilic ferrocene group in its lateral chain (Biot et al., 2011). FQ is in late-stage clinical trials as part of combination therapies with other drugs with different mechanisms of action. One of the advantages of FQ combination therapies is FQ's long blood apparent terminal half-life in humans (>15 days) (Supan et al., 2012). This is due to the fact that FQ is slowly converted to its mono N-demethylated metabolite, SSR97213 (Adoke et al., 2021; McCarthy et al., 2016). Our in vitro clearance studies show that DAQ is rapidly converted to its mono N-deethylated metabolite in mouse hepatocytes (DAQM) and with a somewhat slower rate in human hepatocytes (Table 2). Similar to FQ's main metabolite, DAQM is also a potent inhibitor of CQ-resistant P. falciparum strains, which may explain why DAQ is active against P. berghei infected mice (Aguiar et al., 2018). DAQM itself is more slowly metabolized than the parent DAQ, in mice PK studies and in mice liver microsomes. This may partially account for the long period of efficacy of DAQ in mouse malaria models. We predict a longer half-life of DAQM in humans due to its much lower in vitro clearance in human liver microsomes (Clint_human = 8 mL/min/kg; Clint_mice = 273 mL/min/kg, Table 2).
The ferrocene center increases the lipophilicity of FQ compared to CQ; in addition, it reduces FQ's solubility in phosphate buffered saline (PBS) buffer by > 500-fold and in fasted state simulated intestinal fluid (FaSSIF) by > 8-fold. FQ is highly soluble (>4600 μM) in biologically relevant fed state simulated intestinal fluids (FeSSIF) and fasted state simulated gastric fluid (FaSSGF) (Charman et al., 2020). However, FQ reduces the solubility of artefenomel in milk-based formulations intended for pediatric use (Salim et al., 2019). In contrast to FQ, DAQ and DAQM overcome resistance mechanisms without significantly increasing lipophilicity relative to CQ and are highly soluble in aqueous solvents (thermodynamic solubility in PBS buffer >1200 μM). This high solubility could facilitate their use in combination therapies. Additional experiments are required to determine DAQ's solubility in different media (e.g., FaSSIF, FeSSIF and FaSSGF) as well as DAQ's effect on the solubility of other potential drug candidate partners.
Other properties of DAQ and DAQM support their further investigation as potential anti-malarial drugs. Both compounds showed minimal inhibition of hERG at concentrations up to 5 μM, similar to CQ. For comparison, FQ showed a hERG IC50 of 2 μM, and its mono N-demethylated major metabolite an IC50 of 183 nM (WHO Evidence Review Group Meeting, 2017). We have previously shown that the selectivity index for DAQ (ratio between maximum lethal dose, MLD50, and IC50) was ∼3-fold greater than that observed for CQ using a mammalian cell line (BGM-VN, African green monkey kidney) (Aguiar et al., 2018). Herein, we determined that DAQ is not a strong inhibitor of seven human, drug metabolizing CYP proteins (IC50 > 10 μM). A preliminary in vitro evaluation of DAQ in the mini-Ames test against five different strains of Salmonella typhimurium induced no reverse mutations even at the highest concentration tested (1000 μg/well). In vivo exploratory toxicity studies are yet to be performed by our group, but Singh et al. showed that single large doses of DAQ (C = 640 mg/kg) increased the survival of P. berghei infected mice by > 9 days. DAQ did not cause deaths within 2–5 days post infection. Comparatively, high-doses of CQ (C = 160 mg/kg and 320 mg/kg) killed 3/5 and 5/5 P. berghei infected mice within 2–5 days post infection, respectively (Singh et al., 1969). Hence, the preliminary profiling of DAQ suggested that the compound showed minimal toxic effects on a mammalian cell, is a weak inhibitor of hERG and CYP proteins, and was well tolerated in an in vivo model of the disease.
Recent reports have drawn attention to the emergence of P. falciparum resistance to ACTs (Nsanzabana, 2019). In parallel, Chu & White have reported slowly emerging P. vivax resistance to CQ in South America, Africa and Southeast Asia (Chu and White, 2021). The most used therapies against P. vivax are CQ-based (usually in combination with the 8-aminoquinoline primaquine), except in Indonesia, Sabah and Papua New Guinea, where CQ is no longer efficacious against this species. Ex vivo studies are important to determine geographic regions where different species of Plasmodium may be less susceptible to specific malaria treatments. For instance, Van Schalkwyk and co-workers showed CQ was equipotent against malaria samples of different Plasmodium species from travelers returning to the United Kingdom (van Schalkwyk et al., 2021). In the Brazilian Amazon, ex vivo studies have showed that P. falciparum malaria is less sensitive to CQ treatments than P. vivax malaria (Aguiar et al., 2014). We demonstrated that DAQ is a potent inhibitor of both P. vivax and P. falciparum isolates from Amazon. Therefore, the development of DAQ as a drug candidate has the potential to replace CQ as an alternative treatment for endemic areas with CQ-resistant parasite species.
In conclusion, DAQ and DAQM are potent inhibitors of P. falciparum resistant strains with attractive pharmacokinetic and toxicological properties, and simple synthetic routes. Moreover, they are candidates for more effective combination therapies against the emerging P. vivax resistance worldwide.
Declaration of competing interest
WAC is currently employed at Novartis, however, all his efforts mentioned in this manuscript are a result of his work/collaboration with UCSF and collaborators in Brazil.
Acknowledgements
All authors thank the UCSF Catalyst Program for supporting ADMET and PK studies with DAQ and analogues. The Fiocruz (Oswaldo Cruz Foundation, Brazil) team has been highly supportive for technical feedback on intellectual property matter (INPI: BR 10 2019 018557 0, PCT/BR 2020/050351), with special mentions to Ana Paula Granato Ribeiro and Cristina Lima Carrara Carvalho. All authors are also appreciative of MMV's collaboration for IC50 determination of DAQ and analogues against different strains of P. falciparum, as well as hERG IC50 evaluation, through a partnership with TGC Life Sciences. MRM thanks CNPq for research fellowships. AESS thanks Capes for scholarship. ASP was recently awarded the Scientist of Our State by FAPERJ (201.186/2022). He also thanks to the fellowship of research productivity granted by the national council for scientific and technological development (310166/2020-9). ACCA and RVCG thank FAPESP for financial support (processes: 2019/19708-0; CEPID CIBFar grant 2013/07600-3, and 2020/12904-5). AUK thanks CNPQ for a Senior Fellowship. Finally, all authors would kindly thank interns and the advisors from the UCSF Catalyst Program for supporting our team on defining a Target Product Profile for DAQ and its analogues. Special thanks for constructive feedback provided by Jenna Pellegrino, Alison Maxwell and Emilio Ramos.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijpddr.2022.10.003.
Contributor Information
Antoniana U. Krettli, Email: antoniana.krettli@fiocruz.br.
Anna Caroline C Aguiar, Email: annaccaguiar@gmail.com.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- Aderounmu A.F. In vitro assessment of the antimalarial activity of chloroquine and its major metabolites. Ann. Trop. Med. Parasitol. 1984;78:581–585. doi: 10.1080/00034983.1984.11811868. [DOI] [PubMed] [Google Scholar]
- Adoke Y., Zoleko-Manego R., Ouoba S., Tiono A.B., Kaguthi G., Bonzela J.E., Duong T.T., Nahum A., Bouyou-Akotet M., Ogutu B., Ouedraogo A., Macintyre F., Jessel A., Laurijssens B., Cherkaoui-Rbati M.H., Cantalloube C., Marrast A.C., Bejuit R., White D., Wells T.N.C., Wartha F., Leroy D., Kibuuka A., Mombo-Ngoma G., Ouattara D., Mugenya I., Phuc B.Q., Bohissou F., Mawili-Mboumba D.P., Olewe F., Soulama I., Tinto H., Ramharter M., Nahum D., Zohou H., Nzwili I., Ongecha J.M., Thompson R., Kiwalabye J., Diarra A., Coulibaly A.S., Bougouma E.C., Kargougou D.G., Tegneri M., Castin Vuillerme C., Djeriou E., Ansary A.F., Group, the F.S. A randomized, double-blind, phase 2b study to investigate the efficacy, safety, tolerability and pharmacokinetics of a single-dose regimen of ferroquine with artefenomel in adults and children with uncomplicated Plasmodium falciparum malaria. Malar. J. 2021;20:222. doi: 10.1186/s12936-021-03749-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguiar A.C.C., Murce E., Cortopassi W.A., Pimentel A.S., Almeida M.M.F.S., Barros D.C.S., Guedes J.S., Meneghetti M.R., Krettli A.U. Chloroquine analogs as antimalarial candidates with potent in vitro and in vivo activity. Int. J. Parasitol. Drugs drug Resist. 2018;8:459–464. doi: 10.1016/j.ijpddr.2018.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguiar A.C.C., Pereira D.B., Amaral N.S., De Marco L., Krettli A.U. Plasmodium vivax and Plasmodium falciparum ex vivo susceptibility to anti-malarials and gene characterization in Rondônia, West Amazon, Brazil. Malar. J. 2014;13:73. doi: 10.1186/1475-2875-13-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aly A.S.I., Vaughan A.M., Kappe S.H.I. Malaria parasite development in the mosquito and infection of the mammalian host. Annu. Rev. Microbiol. 2009;63:195–221. doi: 10.1146/annurev.micro.091208.073403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antinori S., Galimberti L., Milazzo L., Corbellino M. Biology of human malaria plasmodia including Plasmodium knowlesi. Mediterr. J. Hematol. Infect. Dis. 2012;4 doi: 10.4084/MJHID.2012.013. e2012013–e2012013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biot C., Nosten F., Fraisse L., Ter-Minassian D., Khalife J., Dive D. The antimalarial ferroquine: from bench to clinic. Parasite. 2011;18:207–214. doi: 10.1051/parasite/2011183207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonn B., Svanberg P., Janefeldt A., Hultman I., Grime K. Determination of human hepatocyte intrinsic clearance for slowly metabolized compounds: comparison of a primary hepatocyte/stromal cell Co-culture with plated primary hepatocytes and HepaRG. Drug Metab. Dispos. 2016;44:527. doi: 10.1124/dmd.115.067769. LP – 533. [DOI] [PubMed] [Google Scholar]
- Charman S.A., Andreu A., Barker H., Blundell S., Campbell A., Campbell M., Chen G., Chiu F.C.K., Crighton E., Katneni K., Morizzi J., Patil R., Pham T., Ryan E., Saunders J., Shackleford D.M., White K.L., Almond L., Dickins M., Smith D.A., Moehrle J.J., Burrows J.N., Abla N. An in vitro toolbox to accelerate anti-malarial drug discovery and development. Malar. J. 2020;19:1. doi: 10.1186/s12936-019-3075-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X., Slättengren T., de Lange E.C.M., Smith D.E., Hammarlund-Udenaes M. Revisiting atenolol as a low passive permeability marker. Fluids Barriers CNS. 2017;14:30. doi: 10.1186/s12987-017-0078-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu C.S., White N.J. The prevention and treatment of Plasmodium vivax malaria. PLoS Med. 2021;18 doi: 10.1371/journal.pmed.1003561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper R.A., Ferdig M.T., Su X.-Z., Ursos L.M.B., Mu J., Nomura T., Fujioka H., Fidock D.A., Roepe P.D., Wellems T.E. Alternative mutations at position 76 of the vacuolar transmembrane protein PfCRT are associated with chloroquine resistance and unique stereospecific quinine and quinidine responses in<em>Plasmodium falciparum</em> mol. Pharmacology. 2002;61(35) doi: 10.1124/mol.61.1.35. LP – 42. [DOI] [PubMed] [Google Scholar]
- Cowell A.N., Istvan E.S., Lukens A.K., Gomez-Lorenzo M.G., Vanaerschot M., Sakata-Kato T., Flannery E.L., Magistrado P., Owen E., Abraham M., LaMonte G., Painter H.J., Williams R.M., Franco V., Linares M., Arriaga I., Bopp S., Corey V.C., Gnädig N.F., Coburn-Flynn O., Reimer C., Gupta P., Murithi J.M., Moura P.A., Fuchs O., Sasaki E., Kim S.W., Teng C.H., Wang L.T., Akidil A., Adjalley S., Willis P.A., Siegel D., Tanaseichuk O., Zhong Y., Zhou Y., Llinás M., Ottilie S., Gamo F.-J., Lee M.C.S., Goldberg D.E., Fidock D.A., Wirth D.F., Winzeler E.A. Mapping the malaria parasite druggable genome by using in vitro evolution and chemogenomics. Science. 2018;359:191–199. doi: 10.1126/science.aan4472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daher W., Pelinski L., Klieber S., Sadoun F., Meunier V., Bourrié M., Biot C., Guillou F., Fabre G., Brocard J., Fraisse L., Maffrand J.-P., Khalife J., Dive D. In vitro metabolism of ferroquine (SSR97193) in animal and human hepatic models and antimalarial activity of major metabolites on Plasmodium falciparum. Drug Metab. Dispos. 2006;34:667–682. doi: 10.1124/dmd.104.003202. [DOI] [PubMed] [Google Scholar]
- De D., Krogstad F.M., Cogswell F.B., Krogstad D.J. Aminoquinolines that circumvent resistance in Plasmodium falciparum in vitro. Am. J. Trop. Med. Hyg. 1996;55:579–583. doi: 10.4269/ajtmh.1996.55.579. [DOI] [PubMed] [Google Scholar]
- Dondorp A.M., Nosten F., Yi P., Das D., Phyo A.P., Tarning J., Lwin K.M., Ariey F., Hanpithakpong W., Lee S.J., Ringwald P., Silamut K., Imwong M., Chotivanich K., Lim P., Herdman T., An S.S., Yeung S., Singhasivanon P., Day N.P.J., Lindegardh N., Socheat D., White N.J. Artemisinin resistance in Plasmodium falciparum malaria. N. Engl. J. Med. 2009;361:455–467. doi: 10.1056/NEJMoa0808859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffey M., Blasco B., Burrows J.N., Wells T.N.C., Fidock D.A., Leroy D. Assessing risks of Plasmodium falciparum resistance to select next-generation antimalarials. Trends Parasitol. 2021;37:709–721. doi: 10.1016/j.pt.2021.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flamand N., Meunier J., Meunier P., Agapakis-Caussé C. Mini mutagenicity test: a miniaturized version of the Ames test used in a prescreening assay for point mutagenesis assessment. Toxicol. Vitr. an Int. J. Publ. Assoc. with BIBRA. 2001;15:105–114. doi: 10.1016/s0887-2333(01)00003-0. [DOI] [PubMed] [Google Scholar]
- Gamo F.-J., Sanz L.M., Vidal J., de Cozar C., Alvarez E., Lavandera J.-L., Vanderwall D.E., Green D.V.S., Kumar V., Hasan S., Brown J.R., Peishoff C.E., Cardon L.R., Garcia-Bustos J.F. Thousands of chemical starting points for antimalarial lead identification. Nature. 2010;465:305–310. doi: 10.1038/nature09107. [DOI] [PubMed] [Google Scholar]
- Incecayir T., Tsume Y., Amidon G.L. Comparison of the permeability of metoprolol and labetalol in rat, mouse, and Caco-2 cells: use as a reference standard for BCS classification. Mol. Pharm. 2013;10:958–966. doi: 10.1021/mp300410n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambros C., Vanderberg J.P. Synchronization of Plasmodium falciparum erythrocytic stages in culture. J. Parasitol. 1979;65:418–420. [PubMed] [Google Scholar]
- Lea T. In: Verhoeckx K., Cotter P., Lopez-Expósito I., Kleiveland C., Lea T., Mackie A., Requena T., Swiatecka D., Wichers H., editors. 2015. Caco-2 cell line; pp. 103–111. Cham (CH) [DOI] [PubMed] [Google Scholar]
- McCarthy J.S., Rückle T., Djeriou E., Cantalloube C., Ter-Minassian D., Baker M., O'Rourke P., Griffin P., Marquart L., Hooft van Huijsduijnen R., Möhrle J.J. A Phase II pilot trial to evaluate safety and efficacy of ferroquine against early Plasmodium falciparum in an induced blood-stage malaria infection study. Malar. J. 2016;15:469. doi: 10.1186/s12936-016-1511-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mungall D., Wong Y.Y., Talbert R.L., Crawford M.H., Marshall J., Hawkins D.W., Ludden T.M. Plasma protein binding of warfarin: methodological considerations. J. Pharmacol. Sci. 1984;73:1000–1001. doi: 10.1002/jps.2600730738. [DOI] [PubMed] [Google Scholar]
- Noisang C., Prosser C., Meyer W., Chemoh W., Ellis J., Sawangjaroen N., Lee R. Molecular detection of drug resistant malaria in Southern Thailand. Malar. J. 2019;18:275. doi: 10.1186/s12936-019-2903-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nsanzabana C. Resistance to artemisinin combination therapies (ACTs): do not forget the partner drug. Trav. Med. Infect. Dis. 2019;4 doi: 10.3390/tropicalmed4010026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phyo A.P., Nkhoma S., Stepniewska K., Ashley E.A., Nair S., McGready R., ler Moo C., Al-Saai S., Dondorp A.M., Lwin K.M., Singhasivanon P., Day N.P.J., White N.J., Anderson T.J.C., Nosten F. Emergence of artemisinin-resistant malaria on the western border of Thailand: a longitudinal study. Lancet (London, England) 2012;379:1960–1966. doi: 10.1016/S0140-6736(12)60484-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper D.R., Duff S.R., Eliason H.C., Frazee W.J., Frey E.A., Fuerstenau-Sharp M., Jachec C., Marks B.D., Pollok B.A., Shekhani M.S., Thompson D.V., Whitney P., Vogel K.W., Hess S.D. Development of the predictor HERG fluorescence polarization assay using a membrane protein enrichment approach. Assay Drug Dev. Technol. 2008;6:213–223. doi: 10.1089/adt.2008.137. [DOI] [PubMed] [Google Scholar]
- Project, M.P. falciparum C. Genomic epidemiology of artemisinin resistant malaria. Elife. 2016;5 doi: 10.7554/eLife.08714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulcini S., Staines H.M., Lee A.H., Shafik S.H., Bouyer G., Moore C.M., Daley D.A., Hoke M.J., Altenhofen L.M., Painter H.J., Mu J., Ferguson D.J.P., Llinás M., Martin R.E., Fidock D.A., Cooper R.A., Krishna S. Mutations in the Plasmodium falciparum chloroquine resistance transporter, PfCRT, enlarge the parasite's food vacuole and alter drug sensitivities. Sci. Rep. 2015;5 doi: 10.1038/srep14552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieckmann K.H., Campbell G.H., Sax L.J., Mrema J.E. Drug sensitivity of plasmodium falciparum. An in-vitro microtechnique. Lancet (London, England) 1978;1:22–23. doi: 10.1016/s0140-6736(78)90365-3. [DOI] [PubMed] [Google Scholar]
- Ross L.S., Gamo F.J., Lafuente-Monasterio M.J., Singh O.M.P., Rowland P., Wiegand R.C., Wirth D.F. In vitro resistance selections for Plasmodium falciparum dihydroorotate dehydrogenase inhibitors give mutants with multiple point mutations in the drug-binding site and altered growth. J. Biol. Chem. 2014;289:17980–17995. doi: 10.1074/jbc.M114.558353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salim M., Khan J., Ramirez G., Murshed M., Clulow A.J., Hawley A., Ramachandruni H., Beilles S., Boyd B.J. Impact of ferroquine on the solubilization of artefenomel (OZ439) during in vitro lipolysis in milk and implications for oral combination therapy for malaria. Mol. Pharm. 2019;16:1658–1668. doi: 10.1021/acs.molpharmaceut.8b01333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh T., Stein R.G., Biel J.H. Antimalarials. Unsaturation in chloroquine side chain and antimalarial activity. J. Med. Chem. 1969;12 doi: 10.1021/jm00303a005. [DOI] [PubMed] [Google Scholar]
- Smilkstein M., Sriwilaijaroen N., Kelly J.X., Wilairat P., Riscoe M. Simple and inexpensive fluorescence-based technique for high-throughput antimalarial drug screening. Antimicrob. Agents Chemother. 2004;48:1803–1806. doi: 10.1128/AAC.48.5.1803-1806.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorota S., Zhang X.-S., Margulis M., Tucker K., Priestley T. Characterization of a hERG screen using the IonWorks HT: comparison to a hERG rubidium efflux screen. Assay Drug Dev. Technol. 2005;3:47–57. doi: 10.1089/adt.2005.3.47. [DOI] [PubMed] [Google Scholar]
- Sullivan D.J.J., Gluzman I.Y., Russell D.G., Goldberg D.E. On the molecular mechanism of chloroquine's antimalarial action. Proc. Natl. Acad. Sci. U.S.A. 1996;93:11865–11870. doi: 10.1073/pnas.93.21.11865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Supan C., Mombo-Ngoma G., Dal-Bianco M.P., Ospina Salazar C.L., Issifou S., Mazuir F., Filali-Ansary A., Biot C., Ter-Minassian D., Ramharter M., Kremsner P.G., Lell B. Pharmacokinetics of ferroquine, a novel 4-aminoquinoline, in asymptomatic carriers of Plasmodium falciparum infections. Antimicrob. Agents Chemother. 2012;56:3165–3173. doi: 10.1128/AAC.05359-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor W.R.J., White N.J. Antimalarial drug toxicity. Drug Saf. 2004;27:25–61. doi: 10.2165/00002018-200427010-00003. [DOI] [PubMed] [Google Scholar]
- Thriemer K., Hong N. Van, Rosanas-Urgell A., Phuc B.Q., Ha D.M., Pockele E., Guetens P., Van N. Van, Duong T.T., Amambua-Ngwa A., D'Alessandro U., Erhart A. Delayed parasite clearance after treatment with dihydroartemisinin-piperaquine in Plasmodium falciparum malaria patients in central Vietnam. Antimicrob. Agents Chemother. 2014;58:7049–7055. doi: 10.1128/AAC.02746-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trager W., Jensen J.B. Human malaria parasites in continuous culture. Science. 1976;193:673–675. doi: 10.1126/science.781840. [DOI] [PubMed] [Google Scholar]
- Valderramos S.G., Fidock D.A. Transporters involved in resistance to antimalarial drugs. Trends Pharmacol. Sci. 2006;27:594–601. doi: 10.1016/j.tips.2006.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Schalkwyk D.A., Moon R.W., Duffey M., Leroy D., Sutherland C.J. Ex vivo susceptibility to new antimalarial agents differs among human-infecting Plasmodium species. Int. J. Parasitol. Drugs Drug Resist. 2021;17:5–11. doi: 10.1016/j.ijpddr.2021.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO Evidence Review Group Meeting 2017. https://www.who.int/malaria/mpac/mpac-mar2017-erg-cardiotoxicity-report-session2.pdf The cardiotoxicity of antimalarials [WWW Document]. URL.
- World Health Organization (WHO) vol. 6. World Health Organization; Geneva PP - Geneva: 2021. World Malaria Report 2021, Angewandte Chemie International Edition; pp. 951–952. 11. [Google Scholar]
- World Health Organization (WHO) 2021. https://www.who.int/publications/i/item/guidelines-for-malaria WHO Guidelines for malaria [WWW Document]. URL.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.