Abstract
BACKGROUND:
Little is known about policies and practices for patients undergoing Transcatheter Aortic Valve Replacement (TAVR) who have a documented preference for Do Not Resuscitate (DNR) status at time of referral. We investigated how practices across TAVR programs align with goals of care for patients presenting with DNR status.
METHODS:
Between June and September 2019, we conducted semi-structured interviews with TAVR coordinators from 52/73 invited programs (71%) in Washington and California (TAVR volume >100/year:34%; 50–99:36%; 1–50:30%); 2 programs reported no TAVR in 2018. TAVR coordinators described peri-procedural code status policies and practices and how they accommodate patients’ goals of care. We used data from the Society of Thoracic Surgeons/American College of Cardiology Transcatheter Valve Therapy Registry, stratified by programs’ DNR practice, to examine differences in program size, patient characteristics and risk status, and outcomes.
RESULTS:
Nearly all TAVR programs (48/50: 96%) addressed peri-procedural code status, yet only 26% had established policies. Temporarily rescinding DNR status until after TAVR was the norm (78%), yet time frames for reinstatement varied (38% <48 hours post-TAVR; 44% 48 hours-to-discharge; 18% >30 days post-discharge). For patients with fluctuating code status, no routine practices for discharge documentation were well-described. No clinically substantial differences by code status practice were noted in Society of Thoracic Surgeons Predicted Risk of Mortality risk score, peri-procedural or in-hospital cardiac arrest, or hospice disposition. Six programs maintaining DNR status recognized TAVR as a palliative procedure. Among programs categorically reversing patients’ DNR status, rationale for differing lengths of time to reinstatement reflect divergent views on accountability and reporting requirements.
CONCLUSIONS:
Marked heterogeneity exists in management of peri-procedural code status across TAVR programs, including timeframe for reestablishing DNR status post-procedure. These findings call for standardization of DNR decisions at specific care points (before/during/after TAVR) to ensure consistent alignment with patients’ health-related goals and values.
Keywords: Transcatheter Aortic Valve Replacement, Do Not Resuscitate, peri-procedural, palliative care, policy
Introduction
Transcatheter Aortic Valve Replacement (TAVR) improves survival of patients with severe aortic stenosis at prohibitive surgical risk and has revolutionized the treatment of valvular heart disease.1–3 With widespread adoption in the United States for nearly a decade,4–6 and recent extension to lower risk populations,7–9 TAVR volumes now exceed surgical aortic valve replacement.10
Many patients presenting for evaluation prior to TAVR have Do Not Resuscitate (DNR) orders or other advance directives limiting use of life-sustaining therapies. Management of DNR status is not addressed in current guidelines10 or guidance documents for TAVR,11–13 despite recommendations from professional societies and the Centers for Medicare & Medicaid Services promoting shared decision-making prior to TAVR.10, 11 Little is known about programs’ policies and procedures surrounding DNR status in this context.
Approaches to code status before, during and after TAVR are of particular importance for several reasons. First, the increasing prevalence of older adults in the United States portends higher demand for TAVR in the coming decades with greater documentation of DNR preference.14, 15 Second, most older adults with severe aortic stenosis present for TAVR evaluation to improve quality of life,16 requiring a nuanced understanding of their treatment goals to assess the appropriateness of peri-procedural cardiopulmonary resuscitation (CPR). Only 7% of octogenarians report prolonged life as the primary reason for undergoing TAVR, with many instead prioritizing improvements in functional status, maintenance of independence, and palliation of severe symptoms.16 Lastly, older adults with severe aortic stenosis are often considered to be at elevated risk of adverse outcomes due to increased prevalence of frailty and comorbidities.17–19 Evidence suggests these patients are at risk of adverse outcomes and decreased quality of life not only post-procedurally,20, 21 but also following CPR for in-hospital cardiac arrest.22–28
In this study, we interviewed TAVR program coordinators to examine current policies and management practices pertaining to peri-procedural DNR status and their supporting rationales.
Methods
We conducted a mixed methods study evaluating data from semi-structured interviews with TAVR coordinators in Washington and California hospitals and data from The Society of Thoracic Surgeons (STS) / American College of Cardiology (ACC) Transcatheter Valve Therapy (TVT) Registry. We selected coordinators as key informants given their leading roles in the identification, management and monitoring of TAVR candidates, and in reporting outcomes to mandated registries. Participants were recruited via electronic mail through the ACC. Interested coordinators completed 30–60-minute phone interviews between June and September 2019, which were recorded and transcribed for analysis.
We used a set of standardized demographic questions to characterize participants’ educational backgrounds and program sizes, then asked open-ended questions about formal policies and informal practices concerning peri-procedural DNR using an interview guide (Supplemental Material). We also asked coordinators to share copies of any formal policies for review. We did not explicitly ask coordinators about their own views of policies and practices surrounding peri-procedural code status. The University of Washington Institutional Review Board approved this protocol.
Study investigators (GMB, AK, JMS) used directed content analysis29, 30 to address the following research questions: 1) how many and what size programs had either ‘formal’ policies (written documents issued by division, department or hospital) and/or ‘informal’ practices (unwritten understanding of common practices) addressing peri-procedural code status; 2) how program policies and practices elicited and incorporated patients’ goals, values, and preferences; and 3) methods for determining eligibility for patients with DNR status referred for TAVR.
The coding team inductively created a codebook characterizing peri-procedural code status practices. We independently identified and then collaboratively compared coded excerpts to identify patterns across programs. We examined patterns by program size based on the number of TAVRs performed in 2018: large (≥100); mid-size (50–99); or small (1–50), and by their peri-procedural DNR practices. We focused on rationales used for adoption of DNR practices, as well as language used to reconcile discordant views between formal policies and informal practices.
From these analyses, we identified five approaches to managing peri-procedural status: 1) maintaining DNR status, 2) reversing DNR status but reinstating within 48 hours of procedure, 3) reversing DNR status but reinstating prior to discharge, 4) reversing DNR status but reinstating 30 days post-procedure, and 5) no consistent practice with respect to DNR status (Figure 1). We also obtained aggregated, unadjusted patient characteristics, peri-procedural risk status, and outcomes data across all programs from the TVT Registry, stratified by peri-procedural DNR practice.
Figure 1.
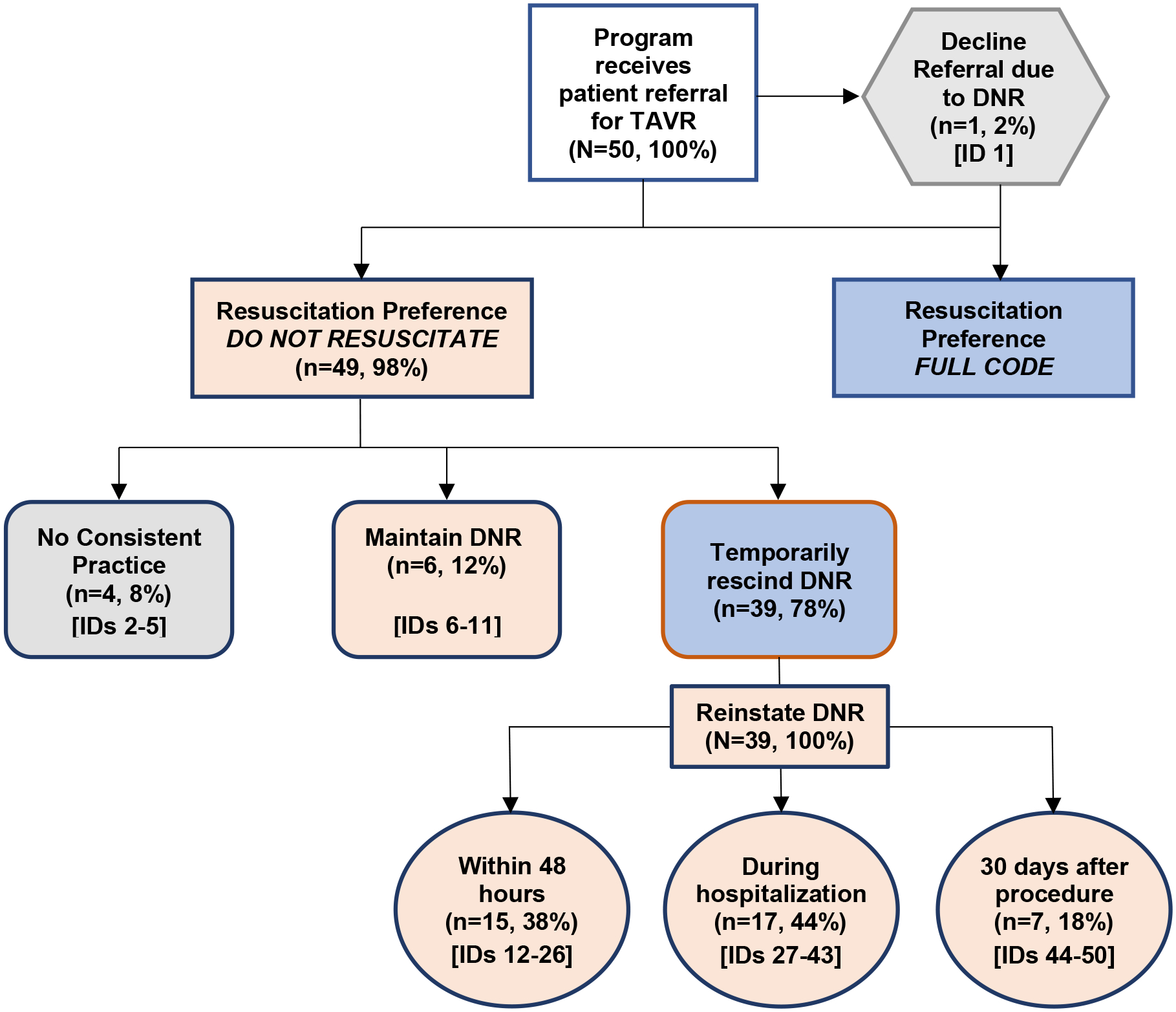
Transcatheter Aortic Valve Replacement (TAVR) Program Peri-procedural Code Status Practices
Results
Cohort Description and Code Status Policies
Of the 73 TAVR coordinators invited, 52 were interviewed (71% response rate) and 50 were included in the analysis (two did not report any TAVRs to the Registry in 2018). Among the coordinators who declined, most stated that their administration would not allow their participation; others cited time as their limiting factor. More than one-third (38%) of participants were nurse practitioners or equivalent, 16% were Masters-level nurses, and 46% were registered nurses. Three (6%) coordinators were male. Participants represented programs of all sizes: large (34%), mid-size (36%), and small (30%). Program geographic distribution was 56% urban; 38% suburban; and 6% rural; 50% were associated with academic medical centers.
Nearly all (48/50: 96%) programs reported routinely addressing code status during TAVR eligibility screening. Figure 1 shows the distribution of programs by their approach to peri-procedural code status. Six programs (12%) reported performing TAVR with maintenance of DNR status. One program (2%) declined referral due to DNR status and 4 programs (8%) described no consistent practice. The remaining 39 (78%) participants reported that their programs routinely temporarily reverse or suspend DNR solely for the purpose of performing TAVR. However, time frames for DNR reinstatement varied widely, with 15 (38%) programs reinstating DNR within 48 hours of the procedure, 17 (44%) reinstating DNR between 48 hours and hospital discharge, and 7 (18%) waiting until 30-days post-TAVR.
Roughly one-quarter of participants (13/50:26%) cited formal policies addressing peri-procedural code status. Eight programs shared their policies for review. Seven of the eight policies addressed the importance of providing patients with an updated Physician Orders for Life Sustaining Treatment (POLST) form at the time of discharge to reinstate their DNR preferences, however this was routinely done by only one program. Only two programs reported practices reflecting their institutions’ formal policies. The content of these two policies differed substantially; one maintained the patient’s DNR status, and the other automatically suspended it for the procedure.
Program and Patient Characteristics
Figure 2 shows the distribution of DNR practices by program size, geographic location, and teaching affiliation; none of these differences were statistically significant (p=0.219). Registry data (Table 1) show that the patient populations treated are similar across programs despite differences in DNR practices. The mean patient age across TAVR programs was 80.4 ± 9.1 years and 55% were men. Unadjusted pre-procedure risk assessment scores were also similar across groups, with mean STS Predicted Risk Of Mortality (PROM) scores ranging from lowest of 6.1 ± 5.3 for those remaining DNR to highest of 6.8 ± 5.9 for those who reinstate DNR by hospital discharge. Patients’ unadjusted outcomes (Table 2) were also similar, with very low rates of in-hospital cardiac arrest (1.5%−2%) and in-hospital death ≤ 2% across categories of program size. In-cath lab death was low, ranging from 1 to 5 (0.2–0.5%) patients. Among patients discharged alive, hospice referral was rare, ranging from 0 to 3 (0.0–0.4%) patients.
Figure 2.

DNR Peri-procedural Code Status Practice by TAVR Program Size, Location and Teaching Program
Table 1:
Patient Characteristics and Procedural Risk of those undergoing TAVR among 50 Programs in Washington and California States by Designated Code Status Practice in 2018
| PATIENT CHARACTERISTIC | CODE STATUS PRACTICE1 | |||||
|---|---|---|---|---|---|---|
| Remain DNR (n=1019) | Temporarily Rescind and Reinstate DNR | No consistent practice/ only full code(n=321) | Total (N=4943) | |||
| within 48 hours of TAVR (n=1148) | 48 hours - hospital discharge (n=1672) | after 30 days post TAVR (n=783) | ||||
| Age in years (mean, SD) | 80.0 (9.3) | 80.5 (8.9) | 81.0 (9.0) | 78.9 (9.4) | 82.1 (8.5) | 80.4 (9.1) |
| Male (n, %) | 605 (59.4) | 597 (52.0) | 924 (55.3) | 407 (52.0) | 179 (55.8) | 2712 (54.9) |
| White, non-Hispanic (n, %) | 841 (82.5) | 891 (77.6) | 1303 (77.9) | 719 (91.8) | 261 (81.3) | 4015 (81.2) |
| Medicare (n, %) | 887 (87.0) | 878 (76.5) | 1482 (88.6) | 688 (87.9) | 257 (80.1) | 4192 (84.8) |
| Comorbid Conditions (n, %) | ||||||
| Diabetes | 352 (34.5) | 437 (38.1) | 632 (37.8) | 318 (40.6) | 107 (33.3) | 1846 (37.3) |
| Currently on Dialysis | 53 (5.2) | 54 (4.7) | 84 (5.0) | 30 (3.8) | 15 (4.7) | 236 (4.8) |
| Home Oxygen | 46 (4.5) | 73 (6.4) | 101 (6.0) | 51 (6.5) | 16 (5.0) | 287 (5.8) |
| Prior Stroke or TIA | 153 (15.0) | 204 (17.8) | 296 (17.7) | 130 (16.6) | 52 (16.2) | 835 (16.9) |
| Prior CABG | 162 (15.9) | 150 (13.1) | 261 (15.6) | 125 (16.0) | 45 (14.0 | 743 (15.0) |
| Prior ICD | 23 (2.3) | 34 (3.0) | 45 (2.7) | 30 (3.8) | 11 (3.4) | 143 (2.9) |
| Prior Permanent Pacemaker | 143 (14.0) | 129 (11.2) | 221 (13.2) | 88 (11.2) | 53 (16.5 | 634 (12.8) |
| Hostile Chest | 30 (2.9) | 31 (2.7) | 129 (7.7) | 47 (6.0) | 12 (3.7) | 249 (5.0) |
| Pre-Procedure Risk Status | ||||||
| STS-PROM score, % (mean, SD) | 6.1 (5.3) | 6.4 (5.0) | 6.8 (5.9) | 6.1 (4.8) | 6.4 (5.2) | 6.4 (5.4) |
| KCCQ-12 Score (mean, SD)2 | 45.5 (25.9) | 44.2 (25.5) | 50.6 (24.3) | 48.7 (23.6) | 45.8 (26.1) | 47.5 (25.1) |
| 5 Meter Walk Test in sec (mean, SD) | 7.2 (2.4) | 8.3 (4.3) | 8.1 (4.1) | 7.1 (3.3) | 9.5 (23.6) | 7.9 (6.9) |
| Unable to walk3 (n, %) | 216 (21.2) | 205 (17.9) | 211 (12.6) | 100 (12.8) | 33 (10.3) | 765 (15.5) |
| Heart Failure within 2 weeks4 (n, %) | 906 (88.9) | 846 (73.7) | 1362 (81.5) | 650 (83.0) | 203 (63.2) | 3967 (80.3) |
| NYHA Class IV (n, %) | 133 (13.1) | 129 (11.2) | 180 (10.8) | 116 (14.8) | 24 (7.5) | 582 (11.8) |
| Other Cardiac Procedure within 30 days | 45 (4.4) | 196 (17.1) | 173 (10.3) | 96 (12.3) | 24 (7.5) | 534 (10.8) |
| Porcelain Aorta (n, %) | 15 (1.5) | 60 (5.2) | 49 (2.9) | 77 (9.8) | 4 (1.2) | 205 (4.1) |
| Procedural Acuity (n, %) | ||||||
| Non-Elective Status | 149 (14.6) | 121 (10.5) | 191 (11.4) | 107 (13.7) | 22 (6.9) | 590 (11.9) |
| Cardiac Arrest within 24 hours prior | 1 (0.1) | 6 (0.5) | 4 (0.2) | 3 (0.4) | 0 (0.0) | 14 (0.3) |
For patients with >1 TAVR in 2018, the first TAVR was used. All denominators include missing (<0.5% of total) unless otherwise specified.
For KCCQ-12 Score, N=936, 1077, 1635, 619 for columns B-E, respectively.
Among those who tried to complete a 5 Meter Walk Test
There is physician documentation or report that the patient has been in a state of heart failure within the past 2 weeks.
Table 2:
Unadjusted Procedural Risk Status and Clinical Outcomes of Patients undergoing TAVR among 50 Programs in Washington and California States by Designated Code Status Practice in 2018
| PROCEDURAL RISK & OUTCOME | CODE STATUS PRACTICE1 | Total (N=4943) | ||||
|---|---|---|---|---|---|---|
| Remain DNR (n=1019) | Temporarily Rescind and Reinstate DNR | No consistent practice/ only full code (n=321) | ||||
| within 48 hours of TAVR (n=1148) | 48 hours - hospital discharge (n=1672) | after 30 days post TAVR (n=783) | ||||
| Procedural Risk Status (n, %) | ||||||
| On Inotropes during Procedure | 291 (28.6) | 471 (41.0) | 433 (25.9) | 276 (35.2) | 38 (11.8) | 1509 (30.5) |
| Conversion to Open Heart Surgery | 5 (0.5) | 7 (0.6) | 10 (0.6) | 5 (0.6) | 0 (0.0) | 27 (0.5) |
| Use of Cardio-Pulmonary Bypass | 9 (0.9) | 6 (0.5) | 8 (0.5) | 4 (0.5) | 1 (0.3) | 28 (0.6) |
| Clinical Outcome2 (n, %) | ||||||
| In-hospital Cardiac Arrest | 17 (1.7) | 17 (1.5) | 31 (1.9) | 16 (2.0) | 5 (1.6) | 86 (1.7) |
| In-Cath Lab Death | 5 (0.5) | 3 (0.3) | 4 (0.2) | 2 (0.3) | 1 (0.3) | 15 (0.3) |
| In-hospital Death | 21 (2.1) | 16 (1.4) | 22 (1.3) | 16 (2.0) | 2 (0.6) | 77 (1.6) |
| Stroke within 30 days | 24 (2.4) | 27 (2.4) | 49 (2.9) | 22 (2.8) | 2 (0.6) | 124 (2.5) |
| New Requirement for Dialysis within 30 days | 4 (0.4) | 5 (0.5) | 6 (0.4) | 1 (0.1) | 0 (0.0) | 16 (0.3) |
| Reintervention within 30 days | 115 (11.3) | 157 (13.7) | 210 (12.6) | 100 (12.8) | 36 (11.2) | 618 (12.5) |
| Rehospitalization within 30 days | 55 (5.4) | 90 (7.8) | 144 (8.6) | 66 (8.4) | 25 (7.8) | 380 (7.7) |
| 30-day KCCQ-12 mean (SD) | 76.9 (22.1) | 76.3 (22.5) | 79.0 (21.2) | 72.2 (22.8) | 73.9 (22.6) | 76.7 (22.1) |
| 30-day Death (unadjusted) | 24 (2.4) | 29 (2.5) | 42 (2.5) | 23 (2.9) | 4 (1.2) | 122 (2.5) |
| Hospice Referral | 2 (0.2) | 2 (0.2) | 2 (0.1) | 3 (0.4) | 0 (0.0) | 9 (0.2) |
For patients with >1 TAVR in 2018, the first TAVR was used. All denominators include missing (<0.5% of total) unless otherwise specified.
Clinical outcomes are unadjusted. Stroke, Rehospitalization, Reintervention, 30-day Death, New requirement for dialysis <5% missing; KCCQ <30% missing; Hospice no missing.
Duration and Documentation of Code Status Reversal
The qualitative data identify what factors different programs consider in determining how to manage DNR status. Table 3 includes representative quotes across practices (the [ID] numbers indicate different coordinators and peri-procedural code status practice). About three-quarters of coordinators (78%) reported that their programs would not perform TAVR on patients without DNR reversal (prior to, during, or following consultation), though this type of exclusion was typically cited as a rare event (Table 3–A). In at least one case, patients were excluded from consideration for TAVR without having DNR reversal prior to consultation. One coordinator justified the program’s practice based on effort and expense:
We do not perform TAVRs on patients who are no-code status. It’s not a walk in the park. It’s an expense. Why would you have [TAVR] if you did not want to survive?
[12]
Table 3:
Participant Explanations for Variations in Policies and Practices related to TAVR Peri-procedural Code Status
|
A. Cancel TAVR “The expectation is that all patients are ‘full codes’ for the procedure and hospitalization. If the patient is refusing [to have the DNR removed], there is a discussion with them about it. If we know up front about it, there would be a discussion if the patient should even have the TAVR, and that came up yesterday. The patient then consented to have the DNR removed, and agreed to have CPR, temporary intubation, and medication. Or the TAVR would’ve been cancelled.” [Program 27] |
|
B. Maintain DNR
“For the most part, that means that these patients don’t want to be converted to ‘open’ under any circumstances. But a lot of them are OK if maybe they go hypotensive or brady, or they need compression or medication during the procedure… So, I guess we tailor it.“ [Program 6] “There are many older patients that have DNR, but they don’t have a terminal diagnosis. We’re not going to totally exclude them–it would be a DNR if something happened on the table. … I think if they have a DNR in place, and express no open chest, resuscitation, anything, then we respect that. We’re not going to resuscitate someone with DNR in place.” [Program 7] “No, it is not [reversed]. …Typically, they go in DNR. …About 20 patients in the past year.” [Program 8] |
|
C. Negotiate meaning/purpose of DNR and code status “It’s not a ‘full code, no code’ world in our hospital, we have ‘no intubation/no meds’, …’meds/no intubation’, …’do intubation/no meds’ …we do everybody full code, and then if anything should happen during the procedure, we come out and speak to the family and ask how they want to proceed.” [Program 13] “I’ve found that …their understanding of [their DNR from 2013 is] if they are found down…and they are going to be brain dead, they don’t want to be resuscitated. Their biggest fear is being in a vegetative state and being on life support, unable to have quality of life. I don’t think they perceive DNR status as when you come in for elective or routine health care—I don’t think they think ‘it counts.’” [Program 45] “Because sometimes they are DNR for [an]other underlying condition that they wouldn’t want treatment for, separate from their [treatable] valve disorder.” [Program 46] |
|
D. Rescind DNR and reinstate after the procedure Within 24–48 Hours “We still move forward … DNR is held while they’re in [procedure] and reactivated after [in PACU].” [Program 14] “I think [code status does not revert] in PACU, but once they’re admitted as an inpatient and up in the unit. …I’m sure a day goes by without anyone ‘hey, you’re full code’ and ‘no, I was DNR’. So, the nurse has to get the doctor to officially change it back.” [The patient might go up to the floor as full code if the provider doesn’t readdress it with the patient?] “Yes.” [Program 15] “Yes, [they’re asked to change to full code] for the first 24 to 28 hours post-procedure.” [What happened for patients who declined to do so?] “Then the goals-of-care discussion continues, and we can refer back to their primary care physician or cardiologist…” [Program 16] Duration of the hospital stay “[DNR is] reversible for the procedure. …What we found is that the patients that are DNR status, but good enough to go through the procedure, they generally have some other issues going on, so we try to address those issues and see if this is actually what they want – or do they feel like a burden to everybody? We discuss the whole psycho-social issues with them. [They remain full code] just during hospital stay.” [Program 28] Often [most] times…, patients remain full code throughout their hospitalization.” [Program 29] After 30 days “I think it’s more financial than anything, and that has to do with the Registry data, anything that has to do within that 30-days scope really is part of ‘the program hit’. We try to maintain that 30 days, because after that 30 days, it’s not an ‘at fault’ issue. If a patient gets into a car accident on day 28, that death gets blamed on the procedure. That’s the rationale.” [Program 47] “30 days–that’s just a number the team decided on…” [Program 46] |
|
E. Documentation of DNR status after reinstatement “It is not in the computer – we just talk to [the patients] about it.” [Program 17] “I think the majority remain full code after the procedure, but there’s not clear documentation of that anywhere. It’s just a verbal understanding between the team and patient and family.” [Program 31] “I don’t believe it’s changed [in the record] …It’s on their record it’s DNR when they get out.” [Program 14] “I would say no, [not included in discharge summary] …No, [not readdressed at 30-day visit.]” [Program 27] |
[square brackets] indicate interviewer comments/edits for clarity; program numbers identify speakers.
In contrast, 6 programs (12%) allowed patients to remain DNR during TAVR. Coordinators reported that these patients were referred for TAVR to address symptoms and understood the procedure to be more compatible with comfort-oriented care:
TAVR is …more of a way to improve quality of life than quantity, I mean it’s great we get the quantity, …and I think that’s the way that a lot of our patients view it as well. Just because you don’t want compressions or open-heart surgery doesn’t mean you’re not open to having a procedure.
[6]
With this goal in mind, many described discussing what DNR means to the patient and what interventions they would be willing to undergo in the event of an arrest (Table 3–B, 3–C).
Rescind and Reinstate DNR
Programs that reinstated DNR within 48 hours cited peri-procedural risk and safe transfer to the post-anesthesia care unit (Table 3–D). Those that maintained a status of ‘full code’ until discharge cited the possibility of untoward events in the immediate post-procedural period; however, one commented on the fluidity of code status, especially for older adults with co-morbid conditions:
It’s for the length of their stay. Their code status is their code status unless it gets changed. So, it could be ever-changing, and on some patients, it is ever-changing…
[13]
Registry reporting requirements were cited by those waiting until 30 days post-procedure:
If they are going to invest in it, they have to invest in it for 30 days…. for outcomes for TVT and STS registries.
[44]
Despite stated practices around reinstating DNR status, no routine practices for documenting code status at the time of discharge were described. Some coordinators cited relying instead on pre-procedural POLST forms (Table 3–E).
Limited Life Expectancy
Despite guideline recommendations to perform TAVR only if life expectancy is greater than one year,8 some coordinators reported otherwise. Ten programs (20%) reported having performed TAVR, and 16 programs (32%) reported a willingness to perform TAVR, for patients with life expectancy less than one year. Improved quality of life and symptom relief were the primary reasons offered for pursuing TAVR in this situation; undergoing chemotherapy and other solid organ transplantation were other reasons. Reporting requirements, lack of reimbursement, risk of adverse outcomes, and availability of balloon valvuloplasty were cited as reasons against this practice.
Discussion
Nearly all TAVR programs (96%) reported routinely addressing peri-procedural code status through informal practices, yet there was considerable variation among programs regarding how code status is managed, and few programs reported having formal policies in place. More than three quarters of programs require temporary suspension of DNR status prior to TAVR. Among programs that rescind DNR status, the rationales offered for three time periods for reinstatement reflected different perceptions about external regulatory frameworks and accountability for adverse outcomes.
It is not clear what clinical differences between programs contribute to the observed variability in practice. TVT Registry data do not support the notion that patients with DNR status be excluded from consideration for TAVR, since in-hospital cardiac arrest and mortality within 30 days were similar among peri-procedural code status groups. This contrasts with other studies, where DNR status was strongly associated with mortality,31 and cardiac arrest emerged from machine learning models as a significant factor in predicting in-hospital mortality following TAVR.32
Frailty is common among older adults undergoing TAVR17, and a rapidly growing body of evidence suggests a dismal prognosis for frail patients following in-hospital CPR, with survival to discharge ranging from 0 to 4.8% versus 26% to 31% in frail vs. non-frail patients, respectively.24–26 Whether such patients should proceed to TAVR as ‘full code’ may depend on their specific motivations, circumstances and preferences.17, 18, 33 Our findings suggest a need for clinicians to gain a better understanding of how TAVR fits into patients’ goals of care and values, and whether symptom relief and palliation can be reasonably achieved through this intervention.34–36
Our findings also imply that routine reversal of DNR status prior to TAVR may be partly motivated by considerations unrelated to patient preferences and characteristics, such as requirements to report outcomes like all-cause mortality to the STS/ACC TVT Registry. Such concerns are not unique to TAVR; ‘surgical buy-in’ has long been seen as a prerequisite to invasive procedures as well as a barrier to treatment limitations in the perioperative setting.37, 38 Lack of ‘surgical buy-in’ has been thought to contribute to surgeons’ unwillingness to operate, hesitancy to pursue comfort care strategies, and shifting of responsibility for outcomes to the patient.37 Indeed, 54% of surgeons in a prior study would refuse to operate on patients whose advance directives placed limits on postoperative care.38 However, the targeted focus on 30-day surgical outcomes has come under scrutiny and is giving way to more patient-centered decision-making processes focused on goal-concordant surgical care.39, 40
Our study indicates significant variability in practices surrounding re-instatement of DNR status following TAVR. The finding that most programs suspend DNR for some time but do not routinely document when DNR is reinstated presents a clear opportunity for improvement. If patients’ DNR orders are suspended or modified for TAVR, it is necessary to ensure that their prior code status is re-established in a timely and well-documented manner to avoid unwanted treatment.41
Standardization of DNR policies is not addressed in current guidelines10 or guidance documents for TAVR.11–13, 42 By contrast, the American Society of Anesthesiologists’ (ASA) and American College of Surgeons (ACS) guidelines for the care of patients with DNR status have addressed automatic DNR suspension as conflicting with patients’ rights to self-determination.33, 41, 43 These societies advocate for discussion of the appropriateness of resuscitation based on a patient’s specific circumstances and preferences prior to anesthesia and surgery. In 2019, the ACS launched the Geriatric Surgery Verification Quality Improvement Program, which established standards for shared decision-making, assessment of geriatric-specific vulnerabilities, and interdisciplinary care planning.44 These standards recommend that surgeons discuss code status in all patients 75 years of age or greater and clarify preferences for life-sustaining therapies. Such practices acknowledge frequent overlap and compatibility between palliative care and procedural interventions, of which TAVR is a clear example.40
The goal-directed approach articulated in the ASA and ACS guidelines41, 43 derives from accepted ethical precepts supporting patients’ rights to self-determination and reflects widespread efforts to deliver care tailored to patients’ goals and values. This approach may be especially well-suited to older adults undergoing TAVR, since many are motivated by concerns about their quality of life and also tend to be at higher risk of complications following CPR.16, 27, 36 A recent study demonstrates the importance of understanding how patients make sense of apparent contradictions in preferences: Burkle et al. found that 92% of patients with pre-existing DNR orders expected discussions about DNR status to occur prior to operations, but 57% felt that preoperative DNR orders should be suspended during their elective surgical cases.45 Excluding patients from consideration for TAVR in this manner, without addressing underlying reasons for DNR status, risks making decisions to pursue TAVR unilateral, rather than shared. Yet, 78% of coordinators in our study said their programs would not perform TAVR on candidates with a standing DNR order.
Clinicians who refer older adults with DNR status for TAVR can prepare them by setting expectations for possibly suspending their DNR and discussing goals for peri-procedural resuscitation, especially for those whose goals of care are palliative. Given the variability in practice and different interpretations of what code status stands for in the context of TAVR, developing standard practices for assessing goals, suspending DNR if appropriate, and clear procedures for reinstatement and documentation are needed. A broader conversation among clinicians who care for patients with aortic stenosis, including primary care, geriatrics, cardiology, cardiac surgery, anesthesiology, and palliative care about how TAVR can be palliative, life-prolonging or both among older persons is needed, and should lead to the development of a multi-society expert decision clinical pathway. Multi-disciplinary heart teams responsible for reviewing TAVR candidacy are best positioned to implement any proposed standardization of the peri-procedural approach to code status for patients undergoing TAVR and probably should include representation from geriatrics and palliative medicine.46
Our study has several limitations. Resources limited our capacity to interview TAVR coordinators outside of Washington and California, thus we cannot say how wider geographic differences might affect policies and practices. However, given coordinators’ demonstrated depth of understanding and range of views represented in this study, we do not believe geography limits generalizability. Most coordinators who declined participation stated that their administrations would not allow their participation in this study; while this creates unavoidable selection bias, it also suggests a need for a broader platform to discuss issues surrounding peri-procedural code status. Finally, the study did not seek patient or family perspectives—a critical area for future research.
Our study suggests current approaches to DNR orders prior to TAVR are frequently in tension with longstanding ethical frameworks supporting shared decision-making and patient-centered approaches to use of life-sustaining therapies in the peri-procedural setting. These findings indicate a need to determine best practices and standardize approaches to code status in patients undergoing TAVR.
Supplementary Material
Key Points.
Peri-procedural code status was a concern addressed by nearly all Transcatheter Aortic Valve Replacement (TAVR) programs, yet few had established policies in place for those with a documented preference for Do Not Resuscitate (DNR) status at the time of referral.
Most programs require patients with DNR status revert to full code status at the time of TAVR; one program excluded patients with DNR status from TAVR consideration. Time frames for reinstating DNR status varied and included 48 hours post-procedure, total hospital stay and/or after 30 days. Post-TAVR code status was rarely documented at discharge.
No consensus exists among TAVR programs about the appropriate timing for reinstating DNR status and assuring that current documentation reflects patients’ preferences. Clinicians who refer older adults with DNR status for TAVR can prepare them by setting expectations for possibly suspending DNR status and documenting goals for peri-procedural resuscitation and TAVR.
Why does this matter?
Our findings indicate a need for standardization of DNR decisions before, during and after TAVR to ensure consistent longitudinal alignment with patients’ health-related goals and values.
Acknowledgements
We acknowledge Tammy Hu for her assistance with recruitment and transcription.
Conflict of Interest:
The authors declare that there is no conflict of interest. There are the following industry relationships: Abbott consulting and research grant (WBB); Amgen research grant (EY); Boston Scientific consulting and research grant (WBB), teaching (JW); Clocktree Advisory Board (EY); Edwards Lifesciences teaching (JW); Idorsia consulting (WBB); Genetech Advisory Board (EY); Medtronic consulting (WBB); and V Wave consulting (WBB).
Sponsor’s Role:
The sponsor had no role in the design, methods, subject recruitment, data collections, analysis or preparation of the paper.
Funding:
This work has been supported by the American College of Cardiology and National Heart, Lung, and Blood Institute (Bethesda, Maryland; T32HL125195-04) and Veterans Administration Office of Academic Affiliations (Advanced Geriatrics Research Fellowship); none had any role in the design or conduct of the study, in the collection, analysis or interpretation of the data, or in the preparation of the manuscript. The Society of Thoracic Surgery (STS) / American College of Cardiology (ACC) Transcatheter Valve Therapy (TVT) Registry reviewed and approved the manuscript. The opinions stated here reflect those of the authors and do not represent the official views of the Society of Thoracic Surgeons, the American College of Cardiology or the STS/ACC TVT Registry.
Footnotes
AGS.21 Presidential Poster
References
- 1.Leon MB, Smith CR, Mack M, et al. , Transcatheter Aortic Valve Implantation for Aortic Stenosis in Patients Who Cannot Undergo Surgery. N Engl J Med, 2010. 363(17): p. 1597–1607. [DOI] [PubMed] [Google Scholar]
- 2.Smith CR, Leon MB, Mack MJ, et al. , Transcatheter versus Surgical Aortic-Valve Replacement in High-Risk Patients. N Engl J Med, 2011. 364(23): p. 2187–98. [DOI] [PubMed] [Google Scholar]
- 3.American College of Cardiology’s Cardiosmart: A decision aid for treatment options for severe aortic stenosis for patients deciding between TAVR and surgery. November 19, 2021]; Available from: https://www.cardiosmart.org/topics/aortic-stenosis/assets/decision-aid/choosing-between-tavr-and-surgery.
- 4.Leon MB, Smith CR, Mack MJ, et al. , Transcatheter or Surgical Aortic-Valve Replacement in Intermediate-Risk Patients. N Engl J Med, 2016. 374(17): p. 1609–20. [DOI] [PubMed] [Google Scholar]
- 5.Reardon MJ, Van Mieghem NM, Popma JJ, et al. , Surgical or Transcatheter Aortic-Valve Replacement in Intermediate-Risk Patients. N Engl J Med, 2017. 376(14): p. 1321–1331. [DOI] [PubMed] [Google Scholar]
- 6.Mack MJ, Leon MB, Smith CR, et al. , 5-year outcomes of transcatheter aortic valve replacement or surgical aortic valve replacement for high surgical risk patients with aortic stenosis (PARTNER 1): a randomised controlled trial. The Lancet, 2015. 385(9986): p. 2477–2484. [DOI] [PubMed] [Google Scholar]
- 7.Mack MJ, Leon MB, Thourani VH, et al. , Transcatheter Aortic-Valve Replacement with a Balloon-Expandable Valve in Low-Risk Patients. N Engl J Med, 2019. 380(18): p. 1695–1705. [DOI] [PubMed] [Google Scholar]
- 8.Popma JJ, Deeb GM, Yakubov SJ, et al. , Transcatheter Aortic-Valve Replacement with a Self-Expanding Valve in Low-Risk Patients. N Engl J Med, 2019. 380(18): p. 1706–1715. [DOI] [PubMed] [Google Scholar]
- 9.Coylewright M, Forrest JK, McCabe JM, et al. , TAVR in Low-Risk Patients: FDA Approval, the New NCD, and Shared Decision-Making. J Am Coll Cardiol, 2020. 75(10): p. 1208–1211. [DOI] [PubMed] [Google Scholar]
- 10.Otto CM, Nishimura RA, Bonow RO, et al. , 2020 ACC/AHA Guideline for the Management of Patients With Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation, 2021. 143(5): p. e72–e227. [DOI] [PubMed] [Google Scholar]
- 11.Decision memo for transcatheter aortic valve replacement (TAVR) (CAG-00430R. 2019. [Accessed August 14, 2021]; Available from: https://www.cms.gov/medicare-coverage-database/details/ncd-details.aspx?NCDid=355.
- 12.Nishimura RA, O’Gara PT, Bavaria JE, et al. , 2019 AATS/ACC/ASE/SCAI/STS Expert Consensus Systems of Care Document: A Proposal to Optimize Care for Patients With Valvular Heart Disease: A Joint Report of the American Association for Thoracic Surgery, American College of Cardiology, American Society of Echocardiography, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol, 2019. 73(20): p. 2609–2635. [DOI] [PubMed] [Google Scholar]
- 13.Bavaria JE, Tommaso CL, Brindis RG, et al. , 2018 AATS/ACC/SCAI/STS Expert Consensus Systems of Care Document: Operator and Institutional Recommendations and Requirements for Transcatheter Aortic Valve Replacement: A Joint Report of the American Association for Thoracic Surgery, American College of Cardiology, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol, 2019. 73(3): p. 340–374. [DOI] [PubMed] [Google Scholar]
- 14.Palmer MK, Jacobson M, and Enguidanos S, Advance Care Planning For Medicare Beneficiaries Increased Substantially, But Prevalence Remained Low. Health Aff (Millwood), 2021. 40(4): p. 613–621. [DOI] [PubMed] [Google Scholar]
- 15.Brinkman-Stoppelenburg A, Rietjens JA, and van der Heide A, The effects of advance care planning on end-of-life care: a systematic review. Palliat Med, 2014. 28(8): p. 1000–25. [DOI] [PubMed] [Google Scholar]
- 16.Coylewright M, Palmer R, O’Neill ES, et al. , Patient-defined goals for the treatment of severe aortic stenosis: a qualitative analysis. Health Expect, 2016. 19(5): p. 1036–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Afilalo J, Lauck S, Kim DH, et al. , Frailty in Older Adults Undergoing Aortic Valve Replacement: The FRAILTY-AVR Study. J Am Coll Cardiol, 2017. 70(6): p. 689–700. [DOI] [PubMed] [Google Scholar]
- 18.Kim DH, Afilalo J, Shi SM, et al. , Evaluation of Changes in Functional Status in the Year After Aortic Valve Replacement. JAMA Intern Med, 2019. 179(3): p. 383–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Don CW and Dean LS, Balloon aortic valvuloplasty to stabilize patients prior to aortic valve replacement: strategy of the future or a bridge to the past? Catheter Cardiovasc Interv, 2013. 82(4): p. 638–9. [DOI] [PubMed] [Google Scholar]
- 20.Arnold SV, Spertus JA, Vemulapalli S, et al. , Association of Patient-Reported Health Status With Long-Term Mortality After Transcatheter Aortic Valve Replacement: Report From the STS/ACC TVT Registry. Circ Cardiovasc Interv, 2015. 8(12): p. e002875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arnold SV, Cohen DJ, Dai D, et al. , Predicting Quality of Life at 1 Year After Transcatheter Aortic Valve Replacement in a Real-World Population. Circ Cardiovasc Qual Outcomes, 2018. 11(10): p. e004693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaiser HA, Saied NN, Kokoefer AS, et al. , Incidence and prediction of intraoperative and postoperative cardiac arrest requiring cardiopulmonary resuscitation and 30-day mortality in non-cardiac surgical patients. PLoS One, 2020. 15(1): p. e0225939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kazaure HS, Roman SA, Rosenthal RA, et al. , Cardiac arrest among surgical patients: an analysis of incidence, patient characteristics, and outcomes in ACS-NSQIP. JAMA Surg, 2013. 148(1): p. 14–21. [DOI] [PubMed] [Google Scholar]
- 24.Wharton C, King E, and MacDuff A, Frailty is associated with adverse outcome from in-hospital cardiopulmonary resuscitation. Resuscitation, 2019. 143: p. 208–211. [DOI] [PubMed] [Google Scholar]
- 25.Ibitoye SE, Rawlinson S, Cavanagh A, et al. , Frailty status predicts futility of cardiopulmonary resuscitation in older adults. Age Ageing, 2021. 50(1): p. 147–152. [DOI] [PubMed] [Google Scholar]
- 26.Fernando SM, McIsaac DI, Rochwerg B, et al. , Frailty and associated outcomes and resource utilization following in-hospital cardiac arrest. Resuscitation, 2020. 146: p. 138–144. [DOI] [PubMed] [Google Scholar]
- 27.Mowbray FI, Manlongat D, Correia RH, et al. , Prognostic association of frailty with post-arrest outcomes following cardiac arrest: A systematic review and meta-analysis. Resuscitation, 2021. 167: p. 242–250. [DOI] [PubMed] [Google Scholar]
- 28.Truog RD, “Do-Not-Resuscitate” Orders during Anesthesia and Surgery. Anesthesiology, 1991. 74: p. 606–608. [DOI] [PubMed] [Google Scholar]
- 29.Hsieh HF and Shannon SE, Three approaches to qualitative content analysis. Qual Health Res, 2005. 15(9): p. 1277–88. [DOI] [PubMed] [Google Scholar]
- 30.Tong A, Sainsbury P, and Craig J, Consolidated criteria for reporting qualitative research (COREQ): a 32-item checklist for interviews and focus groups. Int J Qual Health Care, 2007. 19(6): p. 349–57. [DOI] [PubMed] [Google Scholar]
- 31.Patel K, Sinvani L, Patel V, et al. , Do-Not-Resuscitate Orders in Older Adults During Hospitalization: A Propensity Score-Matched Analysis. J Am Geriatr Soc, 2018. 66(5): p. 924–929. [DOI] [PubMed] [Google Scholar]
- 32.Hernandez-Suarez DF, Kim Y, Villablanca P, et al. , Machine Learning Prediction Models for In-Hospital Mortality After Transcatheter Aortic Valve Replacement. JACC Cardiovasc Interv, 2019. 12(14): p. 1328–1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Allen MB, Bernacki RE, Gewertz BL, et al. , Beyond the Do-not-resuscitate Order: An Expanded Approach to Decision-making Regarding Cardiopulmonary Resuscitation in Older Surgical Patients. Anesthesiology, 2021. [DOI] [PubMed] [Google Scholar]
- 34.Kirkpatrick JN, Hauptman PJ, and Goodlin SJ, Bundling Informed Consent and Advance Care Planning in Chronic Cardiovascular Disease: We Need to Talk. JAMA Intern Med, 2015. 175(1): p. 5–6. [DOI] [PubMed] [Google Scholar]
- 35.Baz L, Wiesel M, Mobius-Winkler S, et al. , Depression and anxiety in elderly patients with severe symptomatic aortic stenosis persistently improves after transcatheter aortic valve replacement (TAVR). Int J Cardiol, 2020. 309: p. 48–54. [DOI] [PubMed] [Google Scholar]
- 36.Dharmarajan K, Foster J, Coylewright M, et al. , The medically managed patient with severe symptomatic aortic stenosis in the TAVR era: Patient characteristics, reasons for medical management, and quality of shared decision making at heart valve treatment centers. PLoS One, 2017. 12(4): p. e0175926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schwarze ML, Bradley CT, and Brasel KJ, Surgical “buy-in”: the contractual relationship between surgeons and patients that influences decisions regarding life-supporting therapy. Crit Care Med, 2010. 38(3): p. 843–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Redmann AJ, Brasel KJ, Alexander CG, et al. , Use of advance directives for high-risk operations: a national survey of surgeons. Ann Surg, 2012. 255(3): p. 418–23. [DOI] [PubMed] [Google Scholar]
- 39.Schwarze ML, Brasel KJ, and Mosenthal AC, Beyond 30-day mortality: aligning surgical quality with outcomes that patients value. JAMA Surg, 2014. 149(7): p. 631–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lilley EJ, Cooper Z, Schwarze ML, et al. , Palliative Care in Surgery: Defining the Research Priorities. Ann Surg, 2018. 267(1): p. 66–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ethical guidelines for the anesthesia care of patients with do-not-resuscitate orders or other directives that limit treatment. Accessed September 26, 2021]; Available from: https://www.asahq.org/standards-and-guidelines/ethical-guidelines-for-the-anesthesia-care-of-patients-with-do-not-resuscitate-orders-or-other-directives-that-limit-treatment.
- 42.Yu S, Fabbro M 2nd, and Aljure O, Expert Consensus Systems of Care Proposal to Optimize Care for Patients With Valvular Heart Disease Review of the 2019 Document for the Cardiac Anesthesiologist. J Cardiothorac Vasc Anesth, 2020. 34(9): p. 2476–2483. [DOI] [PubMed] [Google Scholar]
- 43.American College of Surgeons Committee on Ethics Statement on Advance Directives by Patients: “Do Not Resuscitate” in the Operating Room. 2014. October 28, 2021]; Available from: https://www.facs.org/about-acs/statements/19-advance-directives. [PubMed]
- 44.American College of Surgeons: Geriatric Surgery Verification Program. October 28, 2021]; Available from: https://www.facs.org/quality-programs/geriatric-surgery. [DOI] [PubMed]
- 45.Burkle CM, Swetz KM, Armstrong MH, et al. , Patient and doctor attitudes and beliefs concerning perioperative do not resuscitate orders: anesthesiologists’ growing compliance with patient autonomy and self determination guidelines. BMC Anesthesiol, 2013. 13(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Krishnaswami A, Bernacki GM, Bhatt DL. Geriatric and Palliative Care Specialists as Valued Members of the Multidisciplinary Heart Team. Am J Med. 2022. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


