Abstract
Advances in immunotherapy, including immune checkpoint inhibitors (ICIs), have transformed the standard of care of many types of cancer including melanoma. ICIs have improved the overall outcome of melanoma patients; however, a significant proportion of patients suffer from primary or secondary tumor resistance. Therefore, there is an urgent need to develop predictive biomarkers to better select patients for ICI therapy. Numerous biomarkers that predict response of melanoma to ICIs have been investigated, including biomarker signatures based on genomics or transcriptomics. Most of these predictive biomarkers have not been systematically evaluated across different cohorts to determine the reproducibility of these signatures in metastatic melanoma. We evaluated 28 previously published predictive biomarkers of ICIs based on gene expression signatures in 8 previously published studies with available RNA-sequencing data in public repositories. We found that signatures related to IFN-γ-responsive genes, T and B cell markers and chemokines in the tumor immune microenvironment are generally predictive of response to ICIs in these patients. In addition, we identified that these predictive biomarkers have higher predictive values in on-treatment samples as compared to pre-treatment samples in metastatic melanoma. The most frequently overlapping genes among the top 18 predictive signatures were CXCL10, CXCL9, PRF1, RANTES, IFNG, HLA-DRA, GZMB and CD8A. From gene set enrichment analysis and cell type deconvolution, we estimated that the tumors of responders were enriched with infiltrating cytotoxic T-cells and other immune cells and the upregulation of genes related to interferon-γ signaling. Conversely, the tumors of non-responders were enriched with stromal related cell types such as fibroblasts and myofibroblasts, as well as enrichment with T helper 17 cell types across all cohorts. In summary, our approach of validating and integrating multi-omics data can help guide future biomarker development in the field of ICIs and serve the quest for a more personalized therapeutic approach for melanoma patients.
INTRODUCTION
Despite the significant recent advances in the systemic therapy of melanoma, it remains a lethal disease for a large proportion of patients. It is estimated that there will be about 99,780 newly diagnosed invasive melanoma cases and 7,650 projected deaths in the United States in 20221. For the past decade, several immune checkpoint inhibitors (ICIs) have transformed the standard of care of many types of cancer including melanoma. Ipilimumab, a human anti-cytotoxic T-lymphocyte-associated antigen 4 antibody (anti-CTLA-4) represents the first ICI approved by the U.S. FDA for the treatment of melanoma in 20112. Following this approval, monoclonal antibodies against programmed death 1 (anti-PD-1) pembrolizumab3 and nivolumab4 were subsequently approved in 2014 for melanoma. Similarly, the combination of ipilimumab and nivolumab was approved for melanoma in 20155. Most recently, the fixed dose combination of relatlimab (anti-Lymphocyte Activating 3, anti-LAG3) and nivolumab was approved6. These ICIs have demonstrated substantial benefits as systemic treatment options in the advanced, neoadjuvant and adjuvant disease settings of melanoma. However, only a subset of patients achieves long-term survival when treated with these new agents. For example, at 6.5 years the overall survival rates with ipilimumab plus nivolumab, nivolumab, and ipilimumab were 49%, 42% and 23%, respectively, as reported in the CheckMate-067 pivotal trial7. Furthermore, ICIs are associated with significant toxicities that are primarily immune mediated and have high economic costs. However, we have no clinical grade reliable biomarkers in the clinic to accurately predict benefits and risks. Therefore, it is paramount to develop biomarkers that can guide patients to a specific optimal therapeutic treatment based on predicted ability to respond and save patients the unwanted toxicities and costs for those with no predicted benefits.
Numerous biomarkers that predict response of melanoma to ICIs have been investigated, including potentially predictive immune related biomarker signatures generated within the tumor microenvironment (TME) and the peripheral circulation8. Biomarker signatures based on genomic or transcriptomic data have been especially promising. For genomics data, these predictive biomarkers include tumor mutational burden (TMB)9, neoantigen load10, genotypes of HLA-I10,11, T-cell repertoire12, aneuploidy (also known as somatic copy number alterations, SCNAs)13, and germline variations14. On the other hand, predictive biomarkers derived from transcriptomics data included tumor oncogene expression signatures such as genes related to MYC15, WNT/ß-catenin 16,17 or RAS18 signaling; or gene expression profiles within the tumor immune microenvironment (TIME) such as interferon-γ (IFN-γ) responsive genes19, chemokines20,21, major histocompatibility complex (MHC) class I and II22, cytotoxic T-cell and T-cell effector23,24 gene expression markers that have been reported to be predictive of ICI response in metastatic melanoma. However, most of these predictive biomarkers, especially the gene expression-based signatures, have not been systematically evaluated across different cohorts to determine the reproducibility of these signatures in metastatic melanoma.
In this study, we focused our evaluation on 28 previously published predictive biomarkers of ICIs based on gene expression signatures. We included 531 patients with advanced melanoma treated with ICIs and belonging to 15 cohorts across 8 previously published studies with available RNA-sequencing data in public repositories. We further compared the performance of these predictive biomarkers between pre-treatment and on-treatment tumor biopsies in 5 patient cohorts. We found that gene expression-based signatures developed from IFN-γ-responsive genes and T-cell markers in the TME are generally predictive of ICIs responders in these patient cohorts. Conversely, gene signatures involved in tumor oncogene signaling or stroma primarily relate to tumor suppressive elements and tend to be associated with non-responders to ICIs. Finally, we also identified that these predictive biomarkers have higher predictive values, as measured by area under the receiver operating curves, in on-treatment samples as compared to pre-treatment samples in metastatic melanoma.
MATERIALS AND METHODS
Data Sets, Treatment Cohorts and RNA-seq Data
We collected eight previously published RNA-sequencing data sets including 15 cohorts of melanoma patients treated with immunotherapy from public repositories. These data sets included: Gide et al25 (n = 91, four cohorts), Hugo et al26 (n = 26, one cohort), Lee et al27 (n = 78, two cohorts), Liu et al28 (n = 122, two cohorts), Riaz et al29 (n = 98, two cohorts), Van Allen et al30 (n = 41, one cohort), Du et al31 (n = 50, two cohorts) and Lauss et al32 (n = 25, one cohort). We considered as responders, patients who had a RECIST criteria Complete Response (CR) or Partial Response (PR). Patients with RECIST criteria Stable Disease (SD) or Progressive Disease (PD) were classified as non-responders. Previously processed RNA-seq data were downloaded from the repositories (Table 1), and the quantified values were transformed as log2(CPM+1), log2(TPM+1), or log2(FPKM + 1). Supplementary Table 1 provides the link to the public repositories.
Table 1:
Summary of the 15 cohorts across 8 studies used in this study.
| Data Set |
Cohort | Description | Treatment(s) | Pre / On |
# Patients |
Responders | Non- Responders |
REF |
|---|---|---|---|---|---|---|---|---|
| Gide | Gide_Pre_PD1_CTLA4 | 91 Samples treated with anti PD-1 or anti PD-1 CTLA4 31 Female Samples : 60 Male samples Median age = 61 |
Ipilimumab Nivolumab Pembrolizumab | Pre | 32 | 21 | 11 | 25 |
| Gide_Pre_PD1 | Pembrolizumab Nivolumab | Pre | 41 | 19 | 22 | |||
| Gide_On_PD1_CTLA4 | Ipilimumab Nivolumab Pembrolizumab | On | 9 | 5 | 4 | |||
| Gide_On_PD1 | Nivolumab Pembrolizumab | On | 9 | 4 | 5 | |||
| Hugo | HugoLo_IPRES_2016 | 26 Samples 25 Pre-anti PD1 treatment 1 On anti PD1 treatment 8 Female Samples : 18 Male Samples Median Age = 61 M Stage 21 M1c : 3 M1b : 1 M1a : 1 M0 |
Pembrolizumab | Pre /On |
26 | 13 | 13 | 26 |
| Lee | Lee_Pre | RNA seq analysis of 78 tumors from 55 patients. 28 paired pre and on treatment biopsies and 50 unpaired biopsies. |
Pembrolizumab Nivolumab |
Pre | 43 | 22 | 21 | 27 |
| Lee_On | Pembrolizumab Nivolumab | On | 35 | 6 | 29 | |||
| Liu | Liu_Naive | 122 Samples 51 Female : 71 Male M Stage 91 M1c : 14 M1b : 10 M0 : 7 M1a |
Pembrolizumab Nivolumab | Pre | 74 | 31 | 43 | 28 |
| Liu_Prog | Pembrolizumab Nivolumab | On | 48 | 17 | 31 | |||
| Riaz | Riaz_Pre | Patients were stratified based on whether they had received previous ICI therapy with Ipilimumab. 98 samples were then treated with Nivolumab to characterize the genomic changes of pre and post therapy. |
Nivolumab | Pre | 49 | 10 | 39 | 29 |
| Riaz_On | Nivolumab | On | 49 | 10 | 39 | |||
| Van Allen | VanAllen_antiCTLA4_2015 | Patient Details Median Age = 61 13 Female : 28 Male M Stage 29 M1c : 8 M1b : 3 M1a : 1 M0 |
Ipilimumab | Pre | 41 | 8 | 33 | 30 |
| Du | Du_Pre | 42 paired pre and on treatment tumor samples. 8 unpaired pre and on treatment tumor samples. |
Ipilimumab Nivolumab Pembrolizumab | Pre | 19 | 6 | 13 | 31 |
| Du_On | Ipilimumab Nivolumab Pembrolizumab | On | 31 | 5 | 26 | |||
| Lauss | Lauss | Majority of samples obtained from lymph node or subcutaneous metastases. All patients were previously treated with other immunotherapies like IL-2 and anti-CTLA4. | Adoptive T-cell therapy | Pre | 25 | 10 | 15 | 32 |
Generating Gene Expression Signature Scores
We surveyed the previously published predictive biomarkers based on gene expression signatures in the literature (Table 2 and Supplementary Table 2). For each published gene signature, we collected the gene names and harmonized using the NCBI Entrez gene number. To quantify the published gene expression score, we first transformed the gene expressions across samples in a study into a Z-score. Next, we averaged the standardized Z-score across the number of genes in the signature as previously described33. This score is used to compare responders and non-responders of ICIs in a particular study based on the area under the receiver operating curve (AUROC) as previously described34. A perfect classifier will have an AUROC = 1, whereas a random classifier will have an AUROC = 0.5. Mann-Whitney U test was performed in comparing the two groups and p<0.05 was deemed as statistically significant.
Table 2:
Summary of the 28 predictive gene signatures evaluated in this study.
| Gene Signature Name |
Description | Number of Genes |
Reference |
|---|---|---|---|
| Angiogenesis | A signature is constructed from genes which are highly co-expressed in the angiogenesis pathway. | 16 | 34 |
| Chaurio | A set of genes that reflect T and B cell responses in human cancer. | 7 | 39 |
| Chemokine | Chemokine gene set. | 12 | 20,21 |
| Davoli | A signature based on scoring the immune infiltration based on gene expression profile. | 7 | 13 |
| effector_t | Effector T cell gene set | 6 | 24 |
| Glycolysis | This signature is related to the expression of glucose transporters and genes related to the metabolism of glucose. | 30 | 34 |
| gMDSC | The granulocytic myeloid-derived suppressor cell signature contains markers for neutrophils and chemokines which are ligands for the neutrophils. | 43 | 34 |
| Hypoxia | This signature was developed based on the transcriptional response of various cell types to hypoxia. | 20 | 34 |
| ifng-effector | IFNγ and T effector gene set | 8 | 23 |
| ifng18 | 18 gene IFNγ immune gene set | 18 | 19 |
| ifng6 | 6 gene IFNγ associated gene set | 6 | 19 |
| Impres | IMPRES score based on logical comparison of quantile-normalized expression of pre-defined checkpoint gene pairs. | 15 | 48 |
| Ipi-neo | A gene signature that reflects a proinflammatory tumor microenvironment and elements of T and B cell interplay. | 32 | 38 |
| MHC-I | 6 Major histocompatibility complex (MHC) class I genes. | 6 | 22 |
| MHC-II | 13 Major histocompatibility complex (MHC) class II genes. | 13 | 22 |
| mMDSC | The monocytic myeloid-derived suppressor cell signature contains markers of the monocytic morphology. | 209 | 34 |
| MYC | The Myc signature is an oncogenic signature focused on MYC pathway activation. | 32 | 34 |
| NRS | The neoadjuvant response signature (NRS) including genes involved in T cell activation, adaptive immune response, and T cell migration. | 68 | 49 |
| Ock | A gene signature predictor based around Bayesian compound covariate predictor algorithm. Stratifies patients into a responder/non-responder. | 105 | 50 |
| Pan | A gene panel of 18 DNA damage repair genes, developed to predict response to ICI. | 18 | 51 |
| Proliferation | A marker that emphasizes genes related to the cell cycle and proliferation rates in cell lines. | 227 | 34 |
| Ras | The Ras signature focuses on prediction of the response against PI3K and RAS inhibitors. | 11 | 34 |
| Roh | A gene signature that combines cytolytic markers, HLA molecules, IFNγ pathway genes, chemokines, and adhesions molecules. | 41 | 52 |
| Rooney | A gene signature based around the expression of two key cytolytic effectors. | 2 | 53 |
| Stroma | This signature is based on the infiltration of stromal cells into the tumor. | 51 | 34 |
| TIP Hot | Genes that serve as a marker of 'hot' (inflamed) tumors. | 12 | 54 |
| TLS | Tertiary lymphoid structures gene signature. | 9 | 55 |
| WNT | A signature developed to predict WNT mutations within human tumor datasets. | 13 | 34 |
Gene Set Enrichment Analysis
Gene set enrichment analysis (GSEA)35 was performed by comparing the responders versus non-responders in each data set. We used two gene sets in this study, the Hallmark gene sets obtained from MSigDB36 to interrogate the enrichment of pathways in the responders versus non-responders data. In order to further deconvolute the cell types in the bulk transcriptomics, we used gene sets obtained from TIMEx37 in comparing the responder vs non-responder samples in each data set. TIMEx is a novel deconvolution method for bulk transcriptomics based on scRNA-seq signatures. TIMEx currently contains 37 cell type signatures, which are classified into 4 main cell groups (malignant, immune, stromal and other), 14 major myeloid and lymphoid immune cell lineages (e.g. conventional CD4+ T cells), 15 minor immune cell lineages (e.g. naïve CD4, effector CD4) and 4 stromal cell types. We used TIMEx cell type signatures to deconvolute the bulk RNA-seq in this study. To estimate the p-value of the gene sets and cell types, we performed permutation analysis of 1000 gene sets. Gene sets with a false discovery rate q-value <0.1 were deemed as significant. We also performed GSEA using KEGG and REACTOME gene sets.
RESULTS
Melanoma Patient Cohorts treated with Immune Checkpoint Inhibitors
In this study, we analyzed 531 melanoma patients collected from 15 cohorts across 7 studies25-31 with RNA-seq data available for patients treated with monotherapy anti-PD-1/PD-L1/anti-CTLA4 or combination of anti-PD-1 plus anti-CTLA-4. We also included one Adoptive T-cell therapy in this study32 as another immunotherapy treated cohort to test the general predictive value of the gene signatures. Among these data sets, five cohorts from four studies25,27,29,31 have pre-treatment and on-treatment cohorts, which were investigated separately in this study. Table 1 summarizes the patient cohorts used in this study.
Performance of the Published Predictive Gene Expression Signatures in the Data Sets
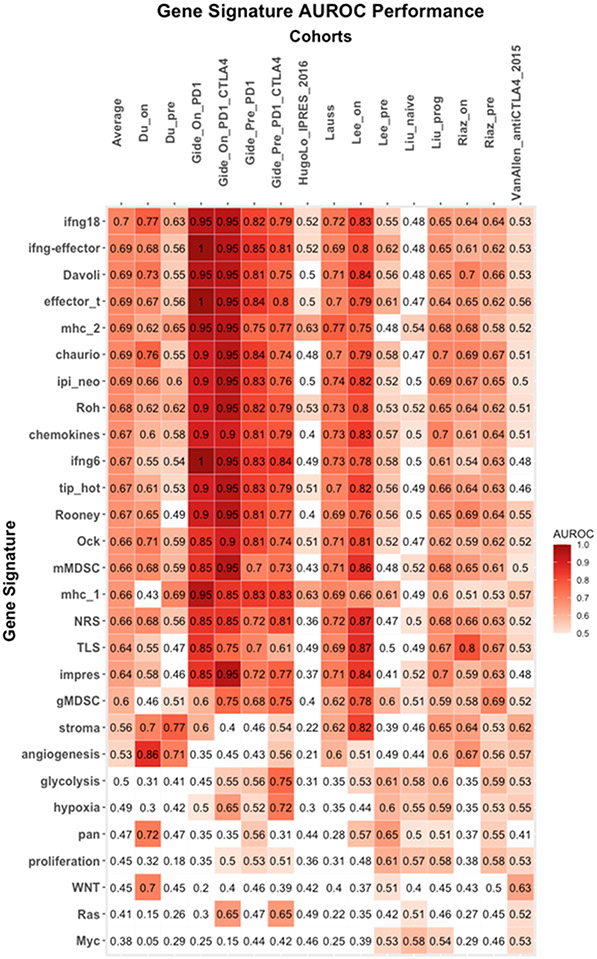
Here, we collected 28 previously published predictive gene signatures that have been associated with responses to ICI immunotherapy. Table 2 summarizes the predictive gene signatures evaluated in this study. To evaluate the previously published gene expression signatures in predicting ICI responses, we tested these signatures on the 15 cohorts. We used area under the receiver operating curve (AUROC) as the metric to evaluate the predictive power of these signatures. Figure 1 summarizes the performance of the 28 published gene expression signatures on the 15 cohorts. In this evaluation, 18 predictive gene signatures achieved AUROC > 0.6; 3 gene signatures have AUROC between 0.5 to 0.6, and 7 predictive gene signatures have AUROC < 0.5.
Figure 1: AUROC performance of the 28 predictive gene signatures across 15 melanoma ICIs- cohorts.
Heatmap depicting the AUROC of the 28 predictive gene signatures evaluated across the 15 cohorts across 8 studies where columns and rows represent cohorts and signatures, respectively. The “average” column represents the average AUROC for a particular gene signature across all cohorts. The signatures are ranked by the average AUROC from the highest to the lowest predictive value.
There are seven gene signatures that performed equally well in these cohorts with average AUROC > 0.69. The IFNγ-18 gene signature19 achieved an average AUROC of 0.70 (range: 0.48 – 0.95). The remaining six gene signatures achieved the same average AUROC of 0.69 and include the IFNγ/effector T-cell signature23 (range: 0.48 – 1), the Ipi-neo signature38 (range: 0.5 – 0.95), the Davoli cytotoxic immune signature13 (range:0.48 - 0.95), the T-cell effector signature (range: 0.47 – 1), the Chaurio immune signature39 (range: 0.47 – 0.95) and the MHC II signature22 (range: 0.47 – 0.95). No statistical significance different between these seven gene signatures in AUROC across the data sets.
From the results, we noticed that there is no correlation between the number of genes in the predictive gene expression signatures to the average AUROC in these cohorts (Supplementary Figure 1). This suggests that the content of the genes that can capture the tumor immune microenvironment is more important in the predictive power of these signatures than the number of genes included in the signature. In addition, on average, we observed that the evaluated gene expression signatures performed poorly in two cohorts: Hugo26 (average AUROC = 0.44) and Liu_naive28 (average AUROC = 0.50). This may be related to the heterogeneity of the patients included in these two cohorts.
Correlations of Gene Signatures
To interrogate the common themes across these predictive gene signatures, we performed Pearson’s correlation analysis of the average AUROC across the data sets. Notably, there are two main clusters from the pairwise correlation plot as illustrated in Figure 2. One cluster contains the predictive gene signatures with average AUROC ≥ 0.6 (n = 18 gene signatures), which are enriched for gene signatures involved in immune activation (e.g. cytotoxic T cells, IFNγ, chemokines and MHC I/II). The other cluster is made up by gene signatures with average AUROC < 0.6 (n = 10 gene signatures), enriched with gene signatures related to the intra-signaling of cellular cells (e.g. MYC, RAS, WNT signaling or DNA Damage Repair genes) or related to stroma (e.g. hypoxia, angiogenesis, stroma/EMT/TGF-ß signaling). The latter cluster suggests that these gene signatures predict non-responders of ICIs, potentially representing the immune suppressive elements in the tumor samples.
Figure 2: Correlation of the 28 predictive gene signatures.
Pearson’s correlation of the 28 predictive gene signatures across all 15 cohorts based on AUROC. The heatmap illustrated the two clusters, the “immune active” and the “immune suppressive” clusters.
Overlapping genes in pro-inflammatory/cytotoxic T-cell/IFNγ activation
We next investigated the overlapping genes found in the 18 gene signatures related to immune activation. There were 91 genes present in at least two predictive gene signatures (Figure 3). The most frequent gene was CXCL10 which was present in 9 predictive gene signatures. CXCL10, together with CXCL9 (present in 8 signatures) and CXCL11 (present in 4 signatures) are commonly known as the “inflammatory” chemokines, which are induced by interferon (IFN)-γ and bind to CXC chemokine receptor 3 (CXCR3) to mediate the immune response through the activation and recruitment of leukocytes such as T cells, natural killer (NK) cells and monocytes. PRF1 (perforin 1), another important player in leukocyte activation and recruitment is found in 8 of the immune activation predictive signatures. CCL5 (C-C chemokine ligand 5), also known as Regulated upon Activation, Normal T-cell Expressed, and Secreted (RANTES), another “inflammatory” chemokine in the CCL5/CCR5 axis is found in 7 predictive gene signatures. The following four genes: IFNG (interferon-γ), HLA-DRA (major histocompatibility complex, class II, DR alpha), GZMB (Granzyme B) and CD8A (Cluster of Differentiation 8A), were included in six predictive gene signatures. Notably, two cytotoxic T lymphocyte marker genes, GZMB and CD8A, were included in the predictive gene signatures. Taken together, the most common genes shared between the 18 predictive gene signatures involved genes related to inflammatory chemokines, activation of T-cells and interferon-γ signaling.
Figure 3: Common genes in the 18 immune activation gene signatures.
The top panel represents the occurrence of a gene in more than two times in the 18 immune activation gene signatures. The bottom panel shows the genes present in a particular gene signature.
Gene signatures have higher predictive power in On-treatment data sets.
Next, we evaluated the predictive power of the 18 immune activation gene signatures in five cohorts of data with pre-treatment and on-treatment patients. Interestingly, as illustrated in Figure 4, we observed that these immune activation gene signatures on average have higher predictive power for responders to ICIs in the on-treatment as compared to the pre-treatment patients. This may suggest the dynamic changes of the tumor immune microenvironment in patients after receiving therapy could assist in better predicting responses to ICIs.
Figure 4: Comparison of 18 immune activation gene signatures in pre-treatment vs on-treatment cohorts.
The top panel shows the 18 immune activation gene signatures in the five on-treatment cohorts. The bottom panel shows the same 18 immune activation gene signatures in the five pre-treatment cohorts. As illustrated in the figure, the gene signatures have higher predictive value (based on AUROC) in the on-treatment as compared to the pre-treatment cohort.
Tumors of responders to ICIs were enriched with interferon, inflammatory and immune-related gene sets
To further dissect the immune pathways involved in predicting responses to ICIs in treated melanoma patients, we performed gene set enrichment analysis (GSEA) using the Hallmark gene sets in responders versus non-responders for the five pre-treatment and on-treatment cohorts. In both the pre-treatment and on-treatment cohorts, the tumors of responders were enriched with genes related to interferon, inflammation, and immune activation. In contrast, tumors of non-responders to ICIs were enriched with genes related to MYC, oxidative phosphorylation, myogenesis, and cell cycle related genes. This is consistent with our earlier findings related to the gene signatures enriched with immune suppressive elements. Similar GSEA results were also observed by using KEGG (Supplementary Table 2) and REACTOME (Supplementary Table 3) gene sets.
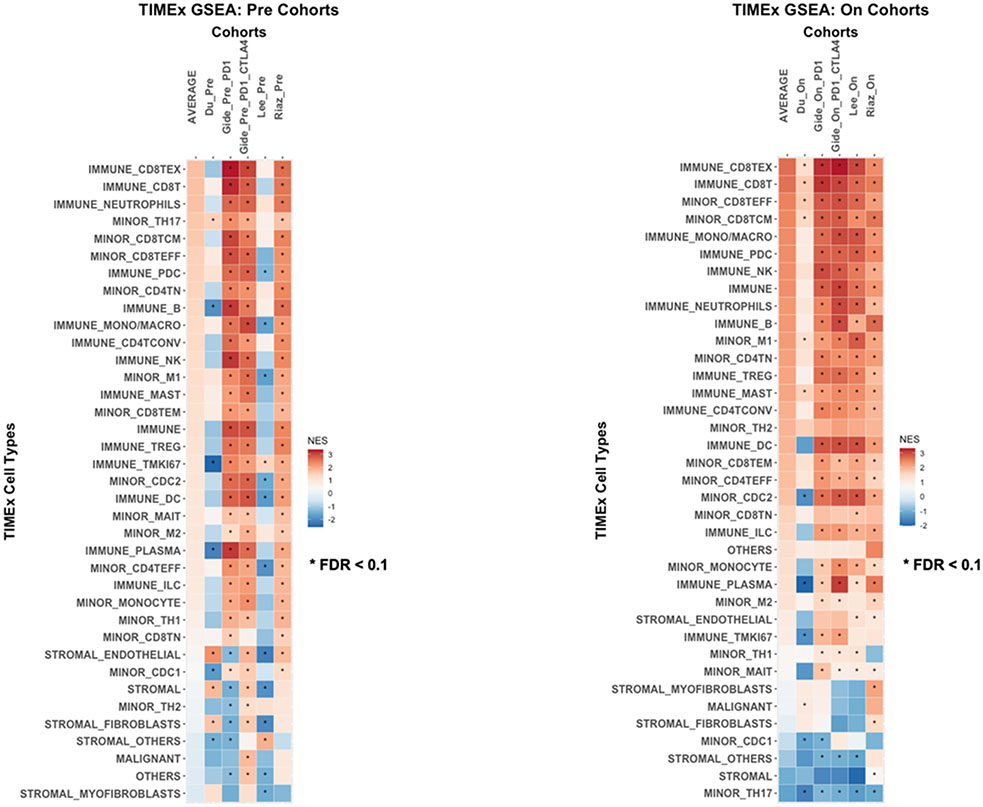
Responders to ICIs were enriched with infiltrated Immune cell types
Finally, we used the TIMEx cell type signatures to deconvolute the tumor microenvironments of the melanoma samples from responders as compared to non-responders to ICIs. As illustrated in Figure 6, in both pre-treatment and on-treatment cohorts, the responders were enriched with infiltrating immune cell types in the bulk transcriptomics, especially CD8 T cells, B cells, monocytes, macrophages, dendritic cells and natural killer cells. Notably, exhausted CD8 T cells (CD8TEx), CD8 T cells, effector CD8 T cells (CD8TEFF) and central memory CD8 T cells (CD8TCM) were significantly enriched (FDR < 0.1) in all on-treatment data sets of the responders to ICIs. The enrichment of immune cell types in the responders are corroborating with the immune active gene signatures are more predictive to ICIs responses. Conversely, the non-responders were enriched with stromal related cell types such as fibroblasts and myofibroblasts. Interestingly, we observed T helper 17 (TH17) cell type was significantly enriched (FDR < 0.1) in non-responders to ICIs across all cohorts.
Figure 6: TIMEx cell types analysis.
GSEA of the pre-treatment and post-treatment cohorts based on the TIMEx cell types. Asterisk (*) indicates gene set with FDR < 0.1. The Average column represents the average enrichment scores across the cohorts.
DISCUSSION
In this study, we evaluated 28 previously published predictive gene expression biomarker signatures for ICI benefits in 15 cohorts across 8 melanoma studies. Using AUROC as the performance metric, we found that 18 predictive gene signatures achieved AUROC > 0.6 in the tested patient cohorts. We noticed that these 18 gene signatures were enriched with genes related to immune activation including pro-inflammatory chemokines and immune cell receptors, cytotoxic T cells, IFN-γ-responsive genes and MHC I and II genes. Conversely, the other 10 gene signatures that had lower AUROC in this evaluation were enriched with tumor cell intrinsic signaling pathways such as glycolysis, MYC, RAS and WNT pathways. In addition, stroma related gene signatures also performed poorly in this study, which may suggest that these signatures are predicting the “immune suppressive” elements in the melanoma samples. For example, there is a close interaction between angiogenesis and immune suppression40,41. Pro-angiogenic factors such as VEGF, NRP-1, PlGF are immune suppressive and negatively impact antigen presentation and effector T cell function through a variety of mechanisms including the stimulation and recruitment of regulatory T cells (Treg), myeloid-derived suppressor cells (MDSC) and tumor-associated macrophages (TAM). These suppressive immune cells can in turn drive angiogenesis and create a vicious cycle of immune deactivation40,41. In fact, combinations of ICI and anti-angiogenic agents are being evaluated in clinical trials42, including in melanoma.
Upon closer examination of the genes involved in the 18 predictive gene signatures with AUROC > 0.6, we found that the “inflammatory” chemokines such as CXCL9, CXCL10, CXCL11, CCL2, CCL3, CCL4, CCL5 were common genes in these signatures. These pro-inflammatory gene expressions have been determined to play an important role in the activation and recruitment of CD8+ cytotoxic T cells in melanoma43. The presence of lymphocytes was previously demonstrated to correlate with defined chemokine gene expressions, and a subset of six chemokines (CCL2, CCL3, CCL4, CCL5, CXCL9, and CXCL10) was previously validated by protein array or quantitative reverse transcription-PCR to be preferentially expressed in tumors that contained T cells, supporting the value of monitoring chemokines as potential predictors of ICI benefit43. In addition, other important players in leukocyte activation and recruitment such as PRF1 were also found to be common among the predictive gene signatures. IFNγ, GZMB and CD8 reflecting cytotoxic T cell activation were also commonly present among the predictive gene signatures. Taken together, the common genes shared among the 18 predictive gene signatures were related to inflammatory chemokines, T-cell activation and interferon-γ signaling.
Due to the complexity and the dynamic nature of the TME, static biomarkers at baseline may not be sufficient to accurately predict response to ICIs. Several studies have indicated that specific immune-related changes in the TME following treatment initiation could provide an early indication of outcomes with immunotherapy8,38. For instance, Tarhini et al. reported that ipilimumab induces massive CD8+ T cell infiltration in the TME early-on following systemic therapy. Furthermore, an immune related gene expression signature was associated with clinical benefits when tested in pre-treatment and early on-treatment specimens and across the clinical endpoints evaluated8,38. To investigate this further, we compared the 18 immune active gene signatures in the five patient cohorts where the pre-treatment and on-treatment gene expression data were available. Overall, we observed that the predictive gene signatures have more predictive power (based on AUROC) in the on-treatment cohorts as compared to the pre-treatment cohorts. This suggests that ICIs induce immune cellular activation and recruitment to the TME and result in dynamic immune changes that could be captured and prove to be more predictive of ICI benefits than gene expression profiling at baseline. Testing early-on treatment samples could be relevant to identify patients who may have primary resistance to ICI therapy and could be saved the toxicities and costs of continued systemic therapy. Along the same lines, a recent study derived predictive pathway-based signatures in pre-treatment and on-treatment melanoma samples based on transcriptomic data and clinical information. They demonstrated that the on-treatment pathway-based signatures were more predictive than the pre-treatment pathway-based signatures in predicting response to ICIs in melanoma31. Similarly, there is an ongoing interest in the development of on-treatment prognostic biomarkers such as the monitoring of circulating tumor cells utilizing liquid biopsies for response to treatment and early detection of disease progression44,45.
To further dissect the molecular pathways and tumor microenvironment in the responders and non-responders to ICIs, we performed gene set enrichment analyses in these cohorts. Similar to the predictive gene signatures results, we observed that the tumors of ICI responders were enriched with genes related to interferon-γ, inflammation and immune activation. In contrast, tumors of non-responders were enriched with genes related to MYC, oxidative phosphorylation, myogenesis and cell cycle regulation. In the immune cell type deconvolution analysis, we observed that CD8+ T cells, B cells, monocytes, macrophages, dendritic cells and natural killer cells were enriched in the tumors of responders. Among the top enriched immune cell types, exhausted CD8+ T cells (CD8TEx), CD8+ T cells, effector CD8+ T cells (CD8TEFF) and central memory CD8+ T cells (CD8TCM) were significantly enriched in all on-treatment data sets of the tumors of responders. This may suggest that ICIs may be reversing effector T cell dysfunction and exhaustion in on-treatment cohorts, thereby enhancing their anti-tumor activities in the responders. On the other hand, the tumors of non-responders were enriched with stroma related cell types such as fibroblasts and myofibroblasts, which have been previously indicated to be associated with resistance in various immunotherapies. Notably, we observed that T-helper 17 (TH17) cell type was significantly enriched in non-responders to ICIs across all on-treatment cohorts. TH17 cells are a subset of helper T cells whose dysregulation has been implicated in the pathogenesis of autoimmune inflammation. In addition, TH17 cells have been shown to trans-differentiate into a more immunosuppressive phenotype that plays a role in tumor immune evasion46. TH17 cells have recently been demonstrated to contribute to resistance to the combination of MEK inhibitor and anti-PD-L1 in pre-clinical lung cancer models47. Furthermore, increasing TH17 associated genes (RORγt, IL17RA, TGFB1, and CCR5) were correlated with poor overall survival and predictive of non-response to ICIs in melanoma patients47. This observation proposes that targeting TH17 could improve responses to ICIs and warrant further investigation in melanoma.
CONCLUSIONS
The goal of developing a reliable and robust gene signature to predict response to ICIs remains a challenging task. Various gene expression signatures have been published, but the reproducibility and predictive power of these signatures have not been independently evaluated. In this study, we evaluated 28 predictive gene signatures across 15 cohorts of patients with melanoma treated with ICIs. We found that predictive gene expression signatures related to IFN-γ signaling, chemokines and immune cell infiltration and activation have higher predictive power for ICI response in this patient population. We also observed that on-treatment gene expression profiles have better predictive value thus capturing the dynamic changes in the TME induced by ICIs. Future biomarker development in the field of ICIs could benefit from integrating multi-omics data and has the potential to move treatment toward a more personalized therapeutic approach for melanoma patients.
Supplementary Material
Supplementary Table 1: Link to public repositories for the data.
Supplementary Table 2: Detailed gene expression signatures evaluated in this study.
Supplementary Table 3: GSEA results using KEGG gene sets in pre- and on-treatment cohorts.
Supplementary Table 4: GSEA results using REACTOME gene sets in pre- and on-treatment cohorts.
Supplementary Figure 1: Correlation of the number of genes in the predictive gene expression signatures and the average AUROC. No correlation was observed between the number of genes in the predictive gene expression signatures and the average AUROC evaluated in this study (p > 0.05).
Figure 5: Gene sets enriched in ICI responders.
Gene set enrichment results of the pre-treatment and post-treatment cohorts based on the Hallmark gene sets. Asterisk (*) indicates gene set with FDR < 0.1. The Average column represents the average enrichment scores across the cohorts.
ACKNOWLEDGMENTS
This work was partly supported by the National Institutes of Health (NIH) under Award Numbers P30CA076292, R01DE030508 and the Oncology Research Information Exchange Network (ORIEN) NOVA Grant (21PRJNOVA009MCC_Tarhini IO NOVA). The content is solely the responsibility of the authors and does not necessarily represent the official views of the funders.
Footnotes
CONFLICT OF INTEREST
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. Unrelated to this work, Dr. Tarhini reports grants from Bristol Myers Squib, Genentech-Roche, Regeneron, Sanofi-Genzyme, Nektar, Clinigen, Merck, Acrotech, Pfizer, Checkmate, OncoSec. Consulting fees from Bristol Myers Squibb, Merck, Easai, Instil Bio, Clinigin, Regeneron, Sanofi-Genzyme, Novartis, Partner Therapeutics, Genentech/Roche, BioNTech.
REFERENCES
- 1.Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin 2022;72:7–33. [DOI] [PubMed] [Google Scholar]
- 2.Hodi FS, O'Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010;363:711–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Robert C, Ribas A, Wolchok JD, et al. Anti-programmed-death-receptor-1 treatment with pembrolizumab in ipilimumab-refractory advanced melanoma: a randomised dose-comparison cohort of a phase 1 trial. Lancet 2014;384:1109–17. [DOI] [PubMed] [Google Scholar]
- 4.Weber JS, D'Angelo SP, Minor D, et al. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol 2015;16:375–84. [DOI] [PubMed] [Google Scholar]
- 5.Postow MA, Chesney J, Pavlick AC, et al. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N Engl J Med 2015;372:2006–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tawbi HA, Schadendorf D, Lipson EJ, et al. Relatlimab and Nivolumab versus Nivolumab in Untreated Advanced Melanoma. N Engl J Med 2022;386:24–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wolchok JD, Chiarion-Sileni V, Gonzalez R, et al. Long-Term Outcomes With Nivolumab Plus Ipilimumab or Nivolumab Alone Versus Ipilimumab in Patients With Advanced Melanoma. J Clin Oncol 2022;40:127–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tarhini A, Kudchadkar RR. Predictive and on-treatment monitoring biomarkers in advanced melanoma: Moving toward personalized medicine. Cancer Treat Rev 2018;71:8–18. [DOI] [PubMed] [Google Scholar]
- 9.Yarchoan M, Hopkins A, Jaffee EM. Tumor Mutational Burden and Response Rate to PD-1 Inhibition. N Engl J Med 2017;377:2500–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McGranahan N, Furness AJ, Rosenthal R, et al. Clonal neoantigens elicit T cell immunoreactivity and sensitivity to immune checkpoint blockade. Science 2016;351:1463–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Iafolla MAJ, Yang C, Chandran V, et al. Predicting Toxicity and Response to Pembrolizumab Through Germline Genomic HLA Class 1 Analysis. JNCI Cancer Spectr 2021;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Postow MA, Manuel M, Wong P, et al. Peripheral T cell receptor diversity is associated with clinical outcomes following ipilimumab treatment in metastatic melanoma. J Immunother Cancer 2015;3:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davoli T, Uno H, Wooten EC, Elledge SJ. Tumor aneuploidy correlates with markers of immune evasion and with reduced response to immunotherapy. Science 2017;355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sayaman RW, Saad M, Thorsson V, et al. Germline genetic contribution to the immune landscape of cancer. Immunity 2021;54:367–86 e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Casey SC, Baylot V, Felsher DW. The MYC oncogene is a global regulator of the immune response. Blood 2018;131:2007–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Spranger S, Bao R, Gajewski TF. Melanoma-intrinsic beta-catenin signalling prevents anti-tumour immunity. Nature 2015;523:231–5. [DOI] [PubMed] [Google Scholar]
- 17.Luke JJ, Bao R, Sweis RF, Spranger S, Gajewski TF. WNT/beta-catenin Pathway Activation Correlates with Immune Exclusion across Human Cancers. Clin Cancer Res 2019;25:3074–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Coelho MA, de Carne Trecesson S, Rana S, et al. Oncogenic RAS Signaling Promotes Tumor Immunoresistance by Stabilizing PD-L1 mRNA. Immunity 2017;47:1083–99 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ayers M, Lunceford J, Nebozhyn M, et al. IFN-gamma-related mRNA profile predicts clinical response to PD-1 blockade. J Clin Invest 2017;127:2930–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Coppola D, Nebozhyn M, Khalil F, et al. Unique ectopic lymph node-like structures present in human primary colorectal carcinoma are identified by immune gene array profiling. Am J Pathol 2011;179:37–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Messina JL, Fenstermacher DA, Eschrich S, et al. 12-Chemokine gene signature identifies lymph node-like structures in melanoma: potential for patient selection for immunotherapy? Sci Rep 2012;2:765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu D, Lin JR, Robitschek EJ, et al. Evolution of delayed resistance to immunotherapy in a melanoma responder. Nat Med 2021;27:985–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fehrenbacher L, Spira A, Ballinger M, et al. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial. Lancet 2016;387:1837–46. [DOI] [PubMed] [Google Scholar]
- 24.Bolen CR, McCord R, Huet S, et al. Mutation load and an effector T-cell gene signature may distinguish immunologically distinct and clinically relevant lymphoma subsets. Blood Adv 2017;1:1884–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gide TN, Quek C, Menzies AM, et al. Distinct Immune Cell Populations Define Response to Anti-PD-1 Monotherapy and Anti-PD-1/Anti-CTLA-4 Combined Therapy. Cancer Cell 2019;35:238–55 e6. [DOI] [PubMed] [Google Scholar]
- 26.Hugo W, Zaretsky JM, Sun L, et al. Genomic and Transcriptomic Features of Response to Anti-PD-1 Therapy in Metastatic Melanoma. Cell 2016;165:35–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee JH, Shklovskaya E, Lim SY, et al. Transcriptional downregulation of MHC class I and melanoma de- differentiation in resistance to PD-1 inhibition. Nat Commun 2020;11:1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu D, Schilling B, Liu D, et al. Integrative molecular and clinical modeling of clinical outcomes to PD1 blockade in patients with metastatic melanoma. Nat Med 2019;25:1916–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Riaz N, Havel JJ, Makarov V, et al. Tumor and Microenvironment Evolution during Immunotherapy with Nivolumab. Cell 2017;171:934–49 e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Van Allen EM, Miao D, Schilling B, et al. Genomic correlates of response to CTLA-4 blockade in metastatic melanoma. Science 2015;350:207–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Du K, Wei S, Wei Z, et al. Pathway signatures derived from on-treatment tumor specimens predict response to anti-PD1 blockade in metastatic melanoma. Nat Commun 2021;12:6023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lauss M, Donia M, Harbst K, et al. Mutational and putative neoantigen load predict clinical benefit of adoptive T cell therapy in melanoma. Nat Commun 2017;8:1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tarhini AA, Lee SJ, Tan AC, et al. Improved prognosis and evidence of enhanced immunogenicity in tumor and circulation of high-risk melanoma patients with unknown primary. J Immunother Cancer 2022;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cristescu R, Nebozhyn M, Zhang C, et al. Transcriptomic Determinants of Response to Pembrolizumab Monotherapy across Solid Tumor Types. Clin Cancer Res 2022;28:1680–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Subramanian A, Tamayo P, Mootha VK, et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci U S A 2005;102:15545–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liberzon A, Subramanian A, Pinchback R, Thorvaldsdottir H, Tamayo P, Mesirov JP. Molecular signatures database (MSigDB) 3.0. Bioinformatics 2011;27:1739–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xie M, Lee K, Lockhart JH, et al. TIMEx: tumor-immune microenvironment deconvolution web-portal for bulk transcriptomics using pan-cancer scRNA-seq signatures. Bioinformatics 2021;37:3681–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tarhini AA, Lin Y, Lin HM, et al. Expression profiles of immune-related genes are associated with neoadjuvant ipilimumab clinical benefit. Oncoimmunology 2017;6:e1231291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chaurio RA, Anadon CM, Lee Costich T, et al. TGF-beta-mediated silencing of genomic organizer SATB1 promotes Tfh cell differentiation and formation of intra-tumoral tertiary lymphoid structures. Immunity 2022;55:115–28 e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rahma OE, Hodi FS. The Intersection between Tumor Angiogenesis and Immune Suppression. Clin Cancer Res 2019;25:5449–57. [DOI] [PubMed] [Google Scholar]
- 41.Khan KA, Kerbel RS. Improving immunotherapy outcomes with anti-angiogenic treatments and vice versa. Nat Rev Clin Oncol 2018;15:310–24. [DOI] [PubMed] [Google Scholar]
- 42.Lee WS, Yang H, Chon HJ, Kim C. Combination of anti-angiogenic therapy and immune checkpoint blockade normalizes vascular-immune crosstalk to potentiate cancer immunity. Exp Mol Med 2020;52:1475–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Harlin H, Meng Y, Peterson AC, et al. Chemokine expression in melanoma metastases associated with CD8+ T-cell recruitment. Cancer Res 2009;69:3077–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tarhini AA, Edington H, Butterfield LH, et al. Immune monitoring of the circulation and the tumor microenvironment in patients with regionally advanced melanoma receiving neoadjuvant ipilimumab. PLoS One 2014;9:e87705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Khunger A, Sarikonda G, Tsau J, et al. Multimarker scores of Th1 and Th2 immune cellular profiles in peripheral blood predict response and immune related toxicity with CTLA4 blockade and IFNalpha in melanoma. Transl Oncol 2021;14:101014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Guery L, Hugues S. Th17 Cell Plasticity and Functions in Cancer Immunity. Biomed Res Int 2015;2015:314620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Peng DH, Rodriguez BL, Diao L, et al. Th17 cells contribute to combination MEK inhibitor and anti-PD-L1 therapy resistance in KRAS/p53 mutant lung cancers. Nat Commun 2021;12:2606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Auslander N, Zhang G, Lee JS, et al. Robust prediction of response to immune checkpoint blockade therapy in metastatic melanoma. Nat Med 2018;24:1545–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Huang AC, Orlowski RJ, Xu X, et al. A single dose of neoadjuvant PD-1 blockade predicts clinical outcomes in resectable melanoma. Nat Med 2019;25:454–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ock CY, Hwang JE, Keam B, et al. Genomic landscape associated with potential response to anti-CTLA-4 treatment in cancers. Nat Commun 2017;8:1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pan YR, Wu CE, Wang YC, et al. Establishment of a novel gene panel as a biomarker of immune checkpoint inhibitor response. Clin Transl Immunology 2020;9:e1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Roh W, Chen PL, Reuben A, et al. Integrated molecular analysis of tumor biopsies on sequential CTLA-4 and PD-1 blockade reveals markers of response and resistance. Sci Transl Med 2017;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rooney MS, Shukla SA, Wu CJ, Getz G, Hacohen N. Molecular and genetic properties of tumors associated with local immune cytolytic activity. Cell 2015;160:48–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang H, Li S, Wang Q, et al. Tumor immunological phenotype signature-based high-throughput screening for the discovery of combination immunotherapy compounds. Sci Adv 2021;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cabrita R, Lauss M, Sanna A, et al. Tertiary lymphoid structures improve immunotherapy and survival in melanoma. Nature 2020;577:561–5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1: Link to public repositories for the data.
Supplementary Table 2: Detailed gene expression signatures evaluated in this study.
Supplementary Table 3: GSEA results using KEGG gene sets in pre- and on-treatment cohorts.
Supplementary Table 4: GSEA results using REACTOME gene sets in pre- and on-treatment cohorts.
Supplementary Figure 1: Correlation of the number of genes in the predictive gene expression signatures and the average AUROC. No correlation was observed between the number of genes in the predictive gene expression signatures and the average AUROC evaluated in this study (p > 0.05).