Abstract
Antibodies to the mycobacterial surface lipoglycan lipoarabinomannan (LAM) and its related capsular polysaccharide arabinomannan (AM) are increasingly important for investigations focused on both understanding mechanisms of protection against Mycobacterium tuberculosis and developing next generation point-of-care tuberculosis diagnostics. We provide here an overview of the growing pipeline of monoclonal antibodies (mAbs) to LAM/AM. Old and new methodologies for their generation are reviewed and we outline and discuss their glycan epitope specificity and other features with implications for the tuberculosis field.
Implications of antibodies for tuberculosis control
Active tuberculosis (TB) is, next to COVID-19, the deadliest infectious disease caused by a single pathogen. A quarter of the world’s population is estimated to be infected with Mycobacterium tuberculosis (Mtb) and 5–10% of Mtb infected individuals progress to transmissible disease over their lifetime, with higher proportions in immunocompromised individuals [1]. In 2021, ~10 million people developed TB with 1.5 million associated deaths, a mortality rising for the first time in decades [2]. Projections suggest that TB incidence could increase globally in 2022 and 2023, highlighting the urgency of better control measures. The M. bovis-based bacillus Calmette–Guérin (BCG) vaccine, given at birth in TB endemic countries, is ineffective against initial infection with Mtb, or developing TB later in life and new vaccine candidates are being evaluated [3, 4]. Thus, there is an urgent need for ongoing investigations towards novel effective vaccines and treatment options against TB and simple point-of-care (POC) diagnostic tests to control this major global health problem.
Surface components, including lipoglycans, glycolipids and polysaccharides, play important roles in interactions of Mtb and other mycobacteria with the host and impact immune responses. Increasing evidence for a protective role of antibodies against Mtb has led to investigations of potentially protective Mtb antigens that could inform TB vaccine development (reviewed in [5–8]). Simple POC diagnostics could improve timely TB diagnosis and treatment initiation, reducing Mtb transmission. Such diagnostics should be suitable for use during the initial community health care evaluation in resource-limited settings [9]. Identifying protective antibodies against Mtb and developing POC tests for TB are important research areas for which generating and characterizing monoclonal antibodies (mAbs) is imperative. In particular, mAbs to the lipoglycan lipoarabinomannan (LAM) and its related derivative arabinomannan (AM), have attracted significant interest.
Structure and functions of LAM and AM
The highly immunogenic LAM accounts for about 15% of Mtb’s mass [10] and is located in the inner and outer membranes, and in extracellular membrane vesicles produced by the bacterium [11–13]. LAM contains four structural domains (Box 1 with Figure I, and Figure 1). AM, which constitutes 10–20% of the polysaccharides in the mycobacterial capsule, lacks the lipid anchor of LAM [14] and is an important virulence factor that defines pathogenic species [15, 16]. Slow growing mycobacteria have a higher polysaccharide than protein content, while fast-growing strains have larger protein content [16–18]. LAM/AM varies structurally within and between Mtb complex species and nontuberculous mycobacteria (NTM; Box 1) highlighting the importance of understanding these differences and their impact on host–pathogen interactions, mAb generation, and the performance of LAM detection-based diagnostics.
Box 1: LAM structure.
Our knowledge of the structure of mycobacterial LAM continues to evolve [27, 28], and there are differences across species, but the general features are well understood. This lipoglycan contains four structural domains (Figure 1 in main text and Figure I below) – a mannosylated phosphatidyl inositol (PI) anchor, a mannan core, an arabinan domain and different capping motifs that contribute to species and strain diversity (reviewed in [19, 20, 29]). The mannan core consists of a chain of α-(1→6)-linked mannopyranose (Manp) residues, some of which are modified by the addition of α-(1→2)-linked Manp motifs, usually, but not always, as a single residue. An arabinan, composed solely of arabinofuranose (Araf) residues, is attached to the mannan core. The arabinan is connected primarily through α-(1→5)-linkages with occasional α-(1→3)-linked branching residues, from which additional α-(1→5)-linked chains are present. The arabinan contains terminal β-Araf-(1→2)-α-Araf motifs leading to two structures: Ara4 (β-Araf-(1→2)-α-Araf-(1→5)-α-Araf-(1→5)-α-Araf) and Ara6 (β-Araf-(1→2)-α-Araf-(1→5)-(β-Araf-(1→2)-α-Araf-(1→3))-α-Araf-(1→5)-α-Araf). Both line-bond and symbolic structures for Ara4 and Ara6 are provided below. Capping motifs can be added to these motifs at specific positions (blue shaded ovals) contributing to intra- and inter-species structural variability [30]. For example, while fast growing mycobacterial species predominately produce AraLAM (uncapped LAM, M. abscessus) or PILAM (phosphoinositol capped, M. smegmatis [31]), slow growing mycobacteria like Mtb and M. leprae produce LAM with α-(1→2)-linked Manp capping residues, giving a molecule referred to as ManLAM [32, 33]. Within the Mtb complex group, comprised of Mtb, M. bovis, M. microti, and M. africanum, variations regarding primarily the degree of terminal mannose capping can range between 40–70% [33–36]. However, some fast-growing and/or non-pathogenic mycobacteria also produce ManLAM but differ in the mannose content of the capping motifs. For example, M. avium produces predominately single mannose Manp caps compared to the dominant disaccharide produced in pathogenic Mtb species [34, 37]. In addition to Manp capping, ManLAM from bacteria of the Mtb complex group contains a unique residue – 5-deoxy-5-methylthio-xylofuranose (MTX, below) on the terminal Manp [38–41]. LAM of the NTM M. kansasii also contains MTX but in contrast to the Mtb complex group, it is linked to the mannan core [42, 43]. Further variability comes from acylation of the arabinan (orange shaded ovals), most commonly succinylation, which can be found either in the internal [27] or termini [28] of this domain.
Figure I:
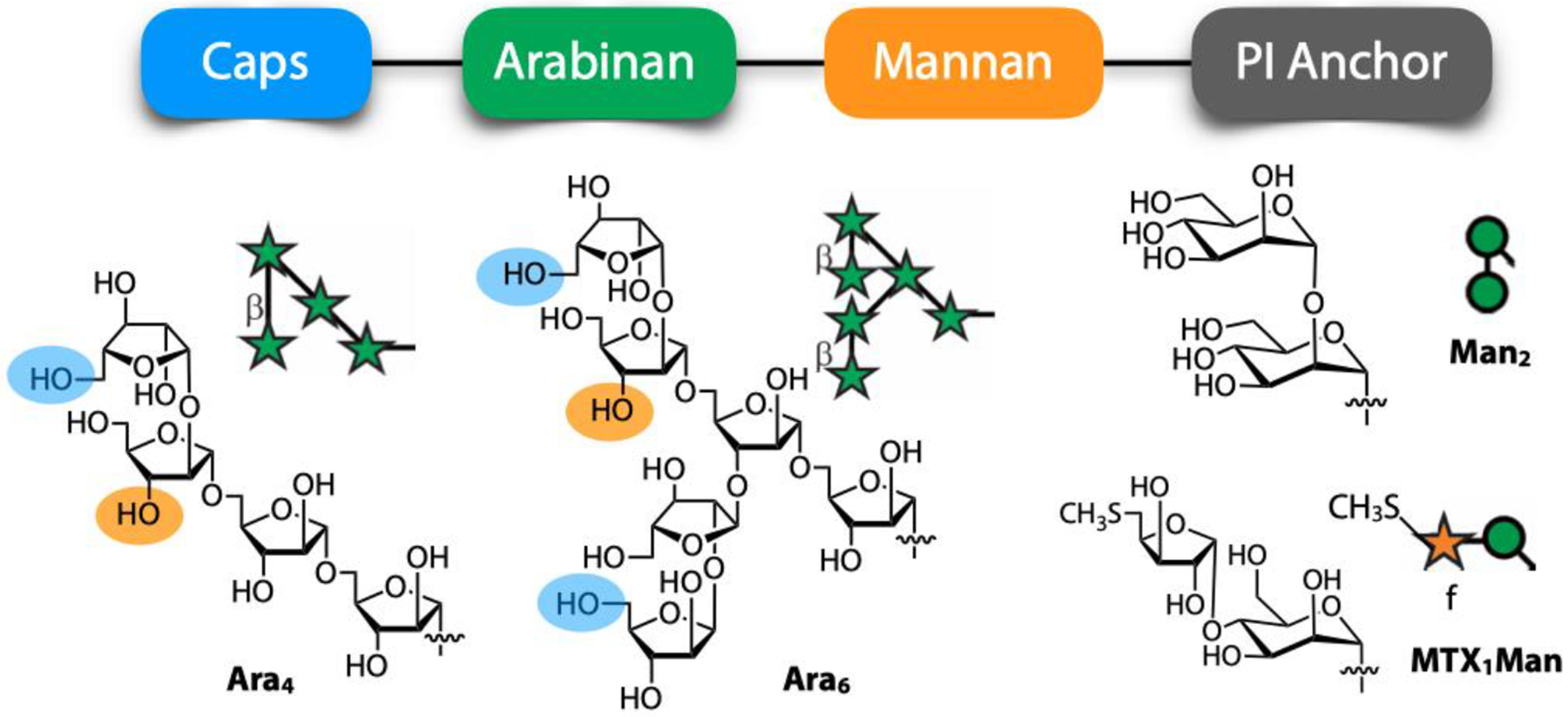
Four structural domains in LAM (top) and representative terminal arabinose motifs (Ara4 and Ara6) and capping motifs (Man2 and MTX1Man) shown in both line-bond structures and symbolic nomenclature (bottom). Capping sites on Ara4/Ara6 are indicated with blue shaded ovals. Sites for the attachment of succinate and other acyl groups are indicated with orange shaded ovals.
Figure 1. Array of synthetic oligosaccharide (OS) fragments corresponding to structural motifs in LAM/AM, organized into seven groups based on their structural/chemical similarities and location.
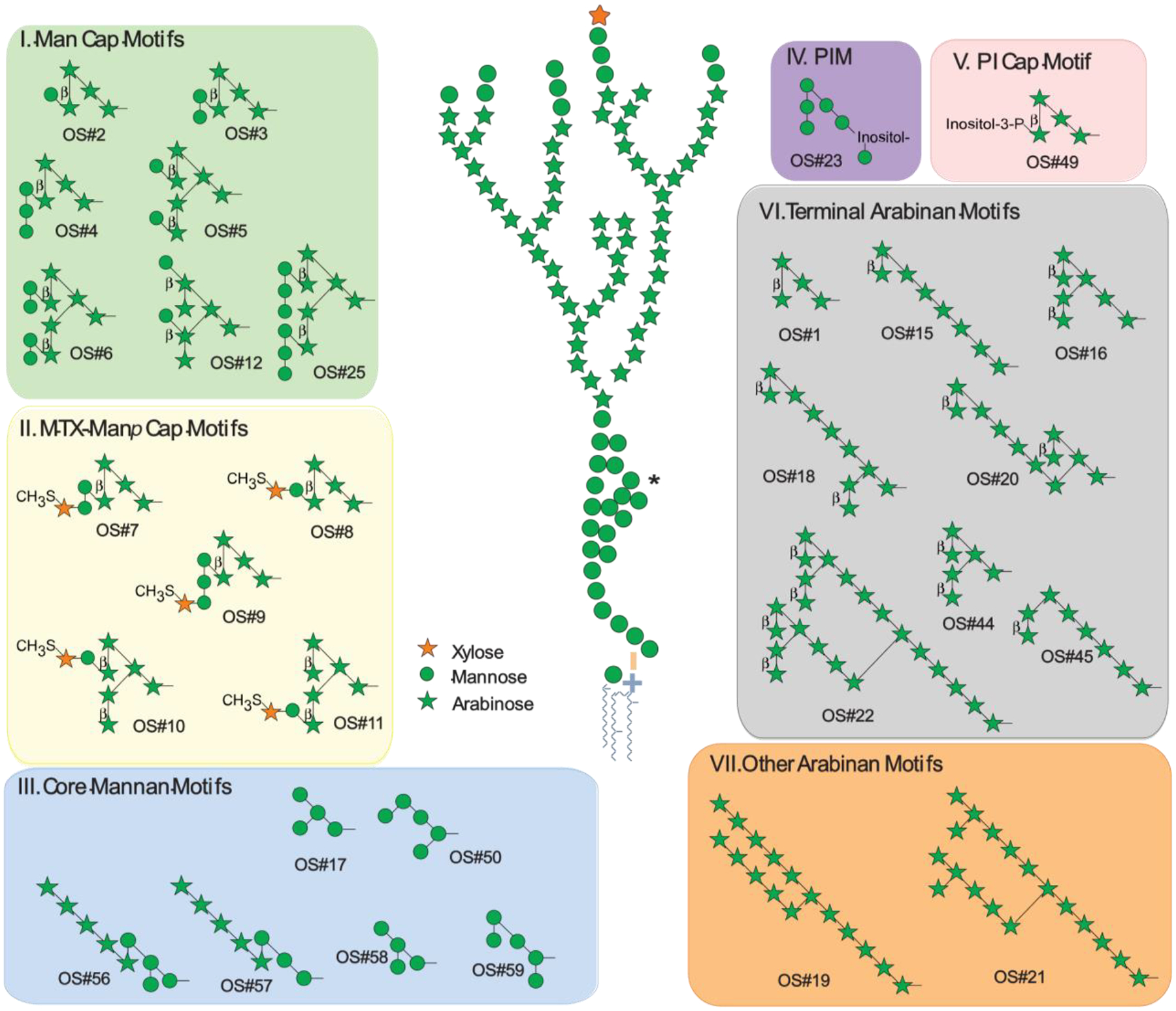
Numbers under each structure refer to positions on the array used for determining binding specificity [26]. LAM structure figure adapted from [27]. All glycosidic linkages are α unless otherwise indicated; all arabinose and xylose residues are in the furanose form, and all mannose residues are in the pyranose ring form. *A secondary mannan side chain of the mannan core, included in the composite LAM structure shown here, was described in 2020, but corresponding OS motifs have not yet been included in the glycan array. OSs by group and location on the glycan array (isomeric structures are differentiated by a numerical suffix (e.g., −1, −2)): Mannose-capped (Man Cap) Motifs (Group I): Man1Ara4 (OS#2), Man2Ara4 (OS#3), Man3Ara4 (OS#4), Man2Ara6-1 (OS#5), Man4Ara6 (OS#6), Man2Ara6-2 (OS#12), Man6Ara6 (OS#25). 5-methylthioxylofuranose (MTX)-Manp Cap Motifs (Group II): MTX1Man2Ara4 (OS#7), MTX1Man1Ara4 (OS#8), MTX1Man3Ara4 (OS#9), MTX1Man1Ara6-1 (OS#10), MTX1Man1Ara6-2 (OS#11). Core Mannan Motifs (Group III): Man4-1 (OS#17), Man5-1 (OS#50), Ara5Man4 (OS#56), Ara5Man3 (OS#57), Man4-2 (OS#58), Man5-2 (OS#59). Phosphatidyl-myo-inositol mannoside (PIM; Group IV): PIM-6 (OS#23). myo-Inositol-phosphate (PI) Cap Motif (Group V): PI1Ara4 (OS#49). Terminal Arabinan (Ara) Motifs (Group VI): Ara4 (OS#1), Ara8-1 (OS#15), Ara7 (OS#16), Ara10 (OS#18), Ara11 (OS#20), Ara22 (OS#22), Ara6 (OS#44), Ara8-2 (OS#45). Other Arabinan Motifs (Group VII): Ara16 (OS#19), Ara18 (OS#21).
Immune responses to LAM/AM are critical because these glycans and their components are integral in TB pathogenesis with multiple functions impacting Mtb infection (Box 2; reviewed in [10, 19–21]). The presentation of LAM and AM to the host varies based on multiple factors. Mtb sheds LAM and AM in cell culture and during infection by various means, such as extracellular vesicles and disassociation of capsular material from the bacterium [17, 22, 23]. Like all glycans, LAM and AM are conformationally more flexible than proteins, thereby presenting the immune system with multiple conformational epitopes [24]. Moreover, enzymes in body fluids and tissues can impact LAM/AM composition. For example, glycosidases in the lung can result in a 30–70% reduction in LAM on the Mtb surface [25]. Knowledge about the variability of LAM in different body fluids and tissues is evolving (below) and is critical for to study antibody responses, functions, and LAM detection-based diagnostics.
Box 2: Functions of LAM and AM.
LAM and AM have multiple functions that facilitate Mtb infection (reviewed in [10, 19–21]). For example, during infection, LAM aids the uptake and survival of Mtb in macrophages and other innate cells by interacting with host receptors, such as the mannose receptor (MR), dectin-2, langerin, dendritic cell-specific intercellular adhesion molecule-grabbing nonintegrin (DC-SIGN), a C-type lectin receptor, and/or the homolog DC-SIGN-related (DC-SIGNR, aka L-SIGN) [19, 26, 44, 45]. Many of these receptors interact primarily with Man caps [26]. To promote survival, LAM activates signaling pathways that inhibit apoptosis of infected macrophages and inhibit cytosolic Ca2+ increases to prevent phagosomal maturation (reviewed in [20]). Through downregulation of MHC class II-mediated antigen presentation, LAM contributes to killing evasion of Mtb-infected cells by T cells [21, 46]. LAM further induces an anti-inflammatory host response via inhibiting IL-12 production by dendritic cells, which promotes Mtb survival [47]. Compared to the exponential Mtb growth phase, it has been theorized that LAM production is upregulated during bacterial stasis with an increase in its arabinose:mannose ratio [21]. This suggests this ratio could also have a role in the establishment of LTBI (reviewed in [21]).
Although AM has been characterized to a lesser degree than LAM, there are known functional similarities between the two. For example, ManAM, like ManLAM, interact swith human DC-SIGN to modulate the host immune response (reviewed in [20, 48]). Because of LAM’s localization in the lipid layers of the cell envelope compared to AM’s localization to the capsule, it has been hypothesized that AM, not LAM, is the ligand that primarily interacts with the mannose-binding receptor [14]. However, the extent to which the localization of LAM and AM impacts their functions remains incompletely understood [20, 49].
A glycan array containing structurally defined LAM/AM oligosaccharides (OSs)
Recent studies have shown structural differences between LAM and AM during mycobacterial infection and bacterial growth in vitro and in vivo [40]. Strain-to-strain variations are also seen (Box 1). Therefore, using native LAM/AM to characterize the specificity of proteins recognizing these molecules is fraught with potential reproducibility issues, including the possibility that LAM produced in vitro may not represent that present during infection. Such specificity studies are further complicated by difficulties in accessing homogenous fragments from native LAM/AM. To circumvent some of these issues, structurally defined OSs corresponding to structural motifs (referred to as OS motifs) in LAM/AM (Figure 1), and other surface glycans, have been chemically synthesized, enabling the development of a mycobacterial glycan array [26]. This array, which currently has 33 LAM/AM fragments, is a unique resource to probe the specificity of proteins that recognize LAM/AM and other mycobacterial glycans. This specificity information is essential for both functional and diagnostic antibody studies.
Roles of antibodies to LAM/AM
Roles of antibodies to LAM/AM in protection against Mtb infection.
LAM has immunomodulatory effects on all immune arms (reviewed in [10]). Decades ago, a lack of serum IgG to LAM/AM in children was associated with disseminated TB [50]; more recently, we showed this association in adults [51]. In passive transfer experiments, the use of anti-LAM/AM mAbs can improve outcomes of Mtb infection in mice (reviewed in [52]), and we and others have shown that immunizing mice with an AM-protein conjugate vaccine leads to enhanced control of Mtb infection [53, 54]. In humans, antibodies to LAM/AM have been implicated in enhancing innate and cell-mediated immune responses to BCG and/or Mtb following BCG vaccination [51, 55–57]. Subsequently, we showed that polyclonal serum IgG to AM from asymptomatic individuals with Mtb exposure or latent infection protects against Mtb in vitro and in vivo [51]. Human IgG responses to LAM and AM from the same Mtb strain also correlate highly and significantly [57, 58], demonstrating, concordant with murine mAb studies [52], that the humoral immune response targets the AM component of LAM. Most recently, serum and lung mucosal IgM to LAM in i.v. BCG-vaccinated Rhesus macaques correlated with protection against Mtb [59]. Collectively, these findings provide compelling evidence that antibodies to LAM/AM are protective against Mtb and suggest roles for various isotypes. Moreover, our studies highlight the tremendous heterogeneity of human antibodies to AM, not just titers and isotypes, but also their specificity to different AM structural motifs (also referred to as OS motifs) [51, 57]. However, we still have an incomplete understanding of immunogenic LAM/AM motifs, what influences their preferential recognition, and how this immune specificity influences antibody functions against Mtb and TB diagnostic test performance.
Value of antibodies to LAM/AM for TB diagnosis.
There is an urgent need for simple non-sputum-based POC tests for TB, suitable for use by community health care providers in resource-limited settings [9]. LAM can be detected in various body fluids of TB patients including sputum, serum, and urine at estimated concentrations of 15 pg/ml – 2 ug/ml, 6 pg/ml – 70 ng/ml, and 12 pg/ml – 70 mg/ml, respectively [60].To date, the most promising simple TB POC test approach is detection of LAM in urine (U-LAM) by anti-LAM antibodies using a lateral flow format (reviewed in [60–62]). The currently available Determine™ TB LAM Ag test (Abbott; former Alere Determine™ TB LAM Ag), however, has low sensitivity (<50% in HIV-associated TB, <20% in non-HIV TB) that is inversely correlated with CD4 cell counts [61]. Thus, the WHO recommends its use only in people living with HIV (PLHIV) with CD4 counts <100 [43]. Its reliance on polyclonal rabbit sera for LAM capture and detection is another limitation and details about the recognition of specific structural motifs in U-LAM remain unknown. Several newer U-LAM detection assays using old and new anti-LAM/AM mAbs have enhanced sensitivity [60, 62]. While these newer tests have high specificity in patients with other respiratory diseases, their specificity for TB compared to NTM disease remains to be determined. The recently developed FujiLAM lateral flow test uses two new anti-LAM mAbs but requires an extra silver amplification step and its sensitivity remains <50% in PLHIV with >200 CD4 cells and ~50% in HIV uninfected TB patients [63, 64]. Therefore, efforts to isolate new anti-LAM mAbs and evaluate them with existing high-affinity mAbs for detecting LAM in urine and other bodily fluids are ongoing.
Generation of mAbs to LAM/AM
Methods for generating mAbs to LAM/AM range from classical hybridoma technology to phage display and, more recently, human B cell culturing and single B cell cloning (Table 1). Thus far, 52 anti-AM/LAM mAbs have been reported (Table 1, Supplementary Table 1), and their number keeps increasing. Of these, 21 have been characterized for their epitope specificity (Table 1). To gain insight into both the glycan specificity of anti-AM/LAM mAbs and how generation methodology might impact this specificity, we focus here on these 21 mAbs.
Table 1.
Anti-AM/LAM mAbs with known reactivity to glycan structural motifs
| Species | mAb | Epitope(s) Recognized | Isotype | Method | Strain/Antigen | References |
|---|---|---|---|---|---|---|
| Human | T1AM09 | Long chain Ara | IgG1 | Single B cell sort | TST+ IGRA- subject sorted w/ Mtb H37Rv AM | [79] |
| L1AM04 | Man3 | IgG1 | TST+ IGRA+ subject sorted w/ Mtb H37Rv AM | [79] | ||
| A194–01 | Ara4/Ara 6 +/− Man1 | IgG1 | B cell culture | TB+ subject cultured w/ Mtb Erdman ManLAM | [70, 76] | |
| P83A8 | Ara4/Ara 6 | IgG1 | ||||
| P30B9 | Man2 | IgM | ||||
| P95C1 | Man only | IgM | ||||
| P65H1 | Man2 cap | IgM | ||||
| My2F12 | Ara4/Ara6 + Man2 | IgG1 | Phage display library | Non-immune screened w/ Mtb AoyamaB ManLAM | [80] | |
| Murine | CS-35 | Ara4/Ara6 +/− caps | IgG3 | Hybridoma | Immunization w/ M. leprae i.p | [65, 68] |
| CS-40 | Ara4/Ara6 + Man1 +/− MTX | IgG3 | Immunization w/ Mtb Erdman ManLAM i.p. | [71, 72] | ||
| 922.5* | Ara4/Ara6 | IgG3 | Immunization w/ M. leprae, boost w/ LAM i.p. | [68] | ||
| 908.1* | Ara4/Ara6 | IgG3 | ||||
| FIND25* | Ara6+/− cap | IgG1 | Immunization w/ hk Mtb H37Rv and LAM i.p. | [69] | ||
| FIND28* | Ara6 + Man cap | IgG1 | ||||
| FIND170* | Ara6 +/− cap | IgG1 | ||||
| 9d8 | Ara6 + Man1/Man2 | IgG3 | Immunization w/ hk Mtb Erdman i.p. | [53, 66] | ||
| Rabbit | 27D2 | Ara4/Ara6 +/− Man1 | IgG | B cell culture | Immunization w/ synthetic LAM OS motifs (S#16, S#44, S#22), boosted on days 7 and 14 | [76] |
| 13H3 | Ara6 +/− Man1 | IgG | ||||
| S4–20+ | Man Cap + MTX | IgG1 | Phage display library | Immunization w/ BCG and hk Mtb H37Ra s.c. screened w/ LAM | [77] | |
| O-TB× | Man Cap +/− MTX | IgG1 | ||||
| Chicken | G3^ | Man Cap − MTX | IgG1 |
Epitope(s) recognized: the AM/LAM structural motifs predominantly recognized by the respective mAb. Method: describes methodology used to isolate mAb; Strain/Antigen: describes immunization and other factors contributing to mAb generation. Mtb.: Mycobacterium tuberculosis; i.p.: intra-peritoneal; s.c.: subcutaneous; hk.: heat-killed. Detailed information on reactivities to AM oligosaccharide motifs is shown in Figure 2.
mAbs clonally related to some others listed, including to those in supplemental Table S1 (see online Supplemental Information Table S1).
Other names for mAbs:
MoAb1,
MoAb3,
MoAb2
Murine mAbs to LAM/AM.
The first mAbs to AM/LAM were generated via hybridoma technology. To elicit a sufficient immune response, immunization was usually done intraperitoneally (i.p.) with very high-dose whole mycobacteria, (e.g., BCG, M. leprae, or heat-inactivated Mtb) with or without boosting with purified LAM (Table 1; [65–69]). Three of the 21 characterized mAbs to AM/LAM are from M. leprae immunized mice (CS-35, 908.1, and 922.5) [65, 68, 70–72]. Of these, CS-35 has, for an anti-glycan mAb, exceptionally high affinity and value as a capture mAb in U-LAM detection assays [73].
When mice and other animals were immunized with virulent Mtb, mycobacteria were heat-killed. However, only ~25% of heat-killed bacilli show intact cell wall architecture, with ~60% less LAM on their surface than live Mtb [74]. Further studies are needed to elucidate the effect of heat-killing on LAM composition. In addition to the differences in the immunizing strains (avirulent and heat-killed virulent laboratory versus clinical Mtb strains), the infecting doses (e.g., up to 9.7 × 109 bacteria [66] versus as little as 10 in humans), the use of in vitro generated LAM that differs in structure from that detected in vivo [40], and the routes of infections (e.g., i.p. versus airway infection), mice differ considerably in their pathogenesis and immune response to Mtb compared to humans [75]. It can therefore be anticipated that anti-AM/LAM mAbs generated from immunized mice might differ in their glycan epitope specificity compared to those obtained from B cells of Mtb infected humans.
MAbs to LAM/AM generated from other immunized animals.
Two recently reported mAbs provide proof-of-concept that synthetic OS fragments of AM, when conjugated to bovine serum albumin (BSA), are sufficiently immunogenic to induce a structure-specific humoral immune response in rabbits [76] (Table 1 & Figure 2). Phage display libraries have also been successfully used to generate mAbs from both rabbits and chickens (S4–20, O-TB, G3) [77]. The singular chicken-derived mAb, G3, represents the only mAb generated using a non-mammalian source. This antibody recognizes a unique set of structural motifs (Table 1 & Figure 2) and offers an opportunity to explore the immunogenicity of AM/LAM in non-mammalian hosts.
Figure 2. Reactivities of mAbs to AM/LAM structural motifs.
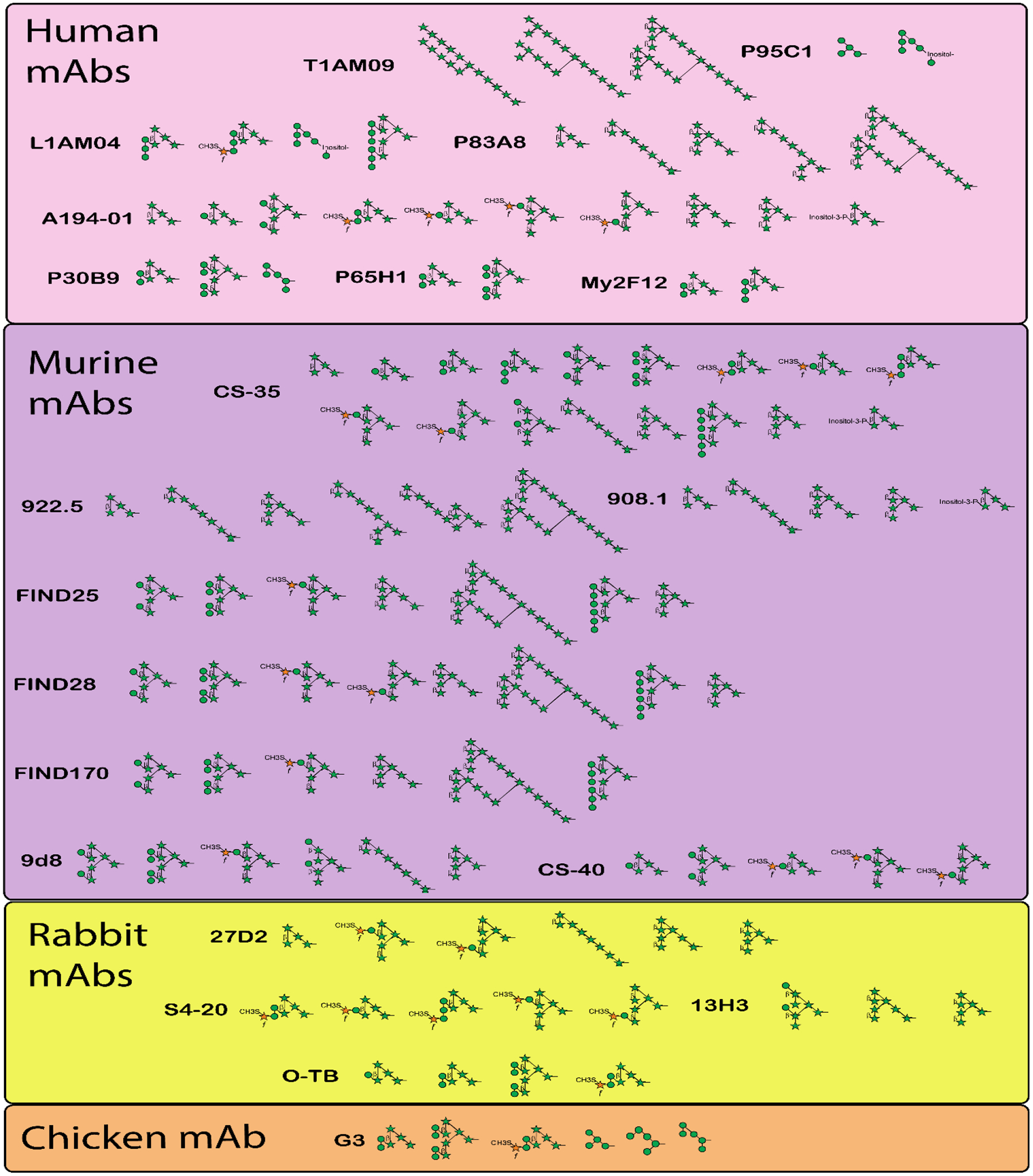
Characterized anti-AM/LAM mAbs (n = 21), organized by species in which they were generated, with the structural OS motifs they recognize as reported by respective authors (see Table 1).
Human mAbs to LAM/AM.
In the past few years, human B cell culturing and targeted single B cell sorting have been used to generate anti-AM/LAM antibodies. This approach circumvents the laborious process of immunizing animals and generating hybridomas (Table 1). Because these approaches isolate mAbs induced during natural human Mtb infection, they increase the likelihood of generating anti-AM/LAM mAbs that recognize clinically relevant epitopes. Five mAbs were generated from TB patients via B cell culturing methods [70, 78], two from asymptomatic latently infected or Mtb exposed humans via single B cell sorting to AM [79], and one from a non-immune human phage display library [80]. Evidence for human mAbs recognizing LAM/AM epitopes distinct from mAbs developed by hybridoma technology (Table 1 & Figure 2), comes from recent studies in which anti-AM mAbs were generated from LTBI individuals [79]. The mAbs generated from these asymptomatic individuals had distinct specificity from human mAbs generated from culturing B cells of TB patients [70]. These data suggest that the state of Mtb infection (i.e., controlled in LTBI versus uncontrolled in TB) can influence the specificity of the resulting mAbs.
Heterogeneity and frequency of mAb reactivity with LAM/AM motifs
Delineating antibody reactivity to individual LAM/AM structural motifs allows uncovering: i) motifs that are more immunogenic, ii) how methodology might influence reactivity, and iii) the relevance in reactivity to certain motifs for antibody functions against Mtb and for U-LAM based POC diagnosis of TB. Anti-LAM mAbs are heterogeneous in their epitope-binding patterns, with arabinan termini and capping motifs being among the most recognized (Figure 3). Some mAbs react with very few, while others bind to more than a dozen motifs. For example, CS35, generated from mice immunized with M. leprae, is one of the most broadly reactive anti-AM/LAM mAbs [70]. However, two other mAbs generated from M. leprae immunized mice (922.5 and 908.1) react with a narrower range of structural motifs. Among human mAbs, those generated from asymptomatic Mtb exposed or latently infected individuals appear to recognize different, and a narrower range of, AM/LAM glycan epitopes (T1AM09 and L1AM04) than those generated from TB patients (e.g., A194; Figure 2) [70, 79]. This finding suggests that infection state impacts the specificity of antibodies generated to LAM/AM.
Figure 3. Frequency of anti-AM/LAM mAbs reacting with individual AM/LAM structural motifs.
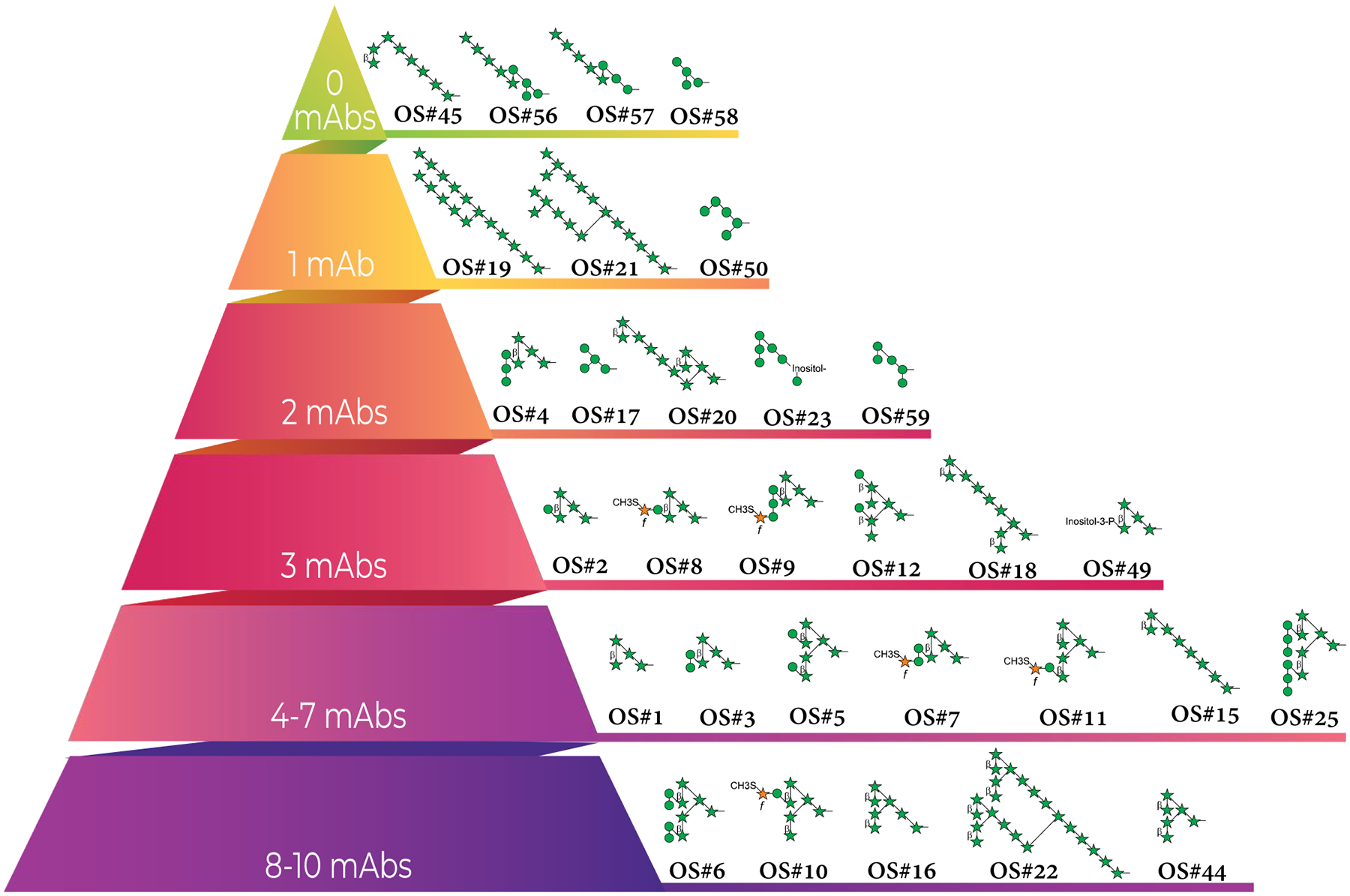
Total mAbs with reported reactivity to AM oligosaccharide motifs (n = 21), regardless of isotype. Motif reactivity was previously defined and reported by respective authors.
The distinct reactivity of the human mAb L1AM04 provides an example [79]. This mAb recognizes the α-Manp-(1→2)-α-Manp-(1→2)-α-Manp motif present in Man3Ara4 (OS#4) and the related branched Man6Ara6 (OS#25). It does not, however, recognize motifs with a single Manp residue (e.g., Man1Ara4, OS#2) nor those with two α-(1→2)-linked Manp residues (e.g., Man2Ara4, OS#3), the latter of which is the motif most frequently occurring in Mtb AM/LAM. On the other hand, some of the mAbs obtained from immunization (e.g., CS-40) bind to the α-Manp-α-(1→2)-Manp motif (Table 1 and Figure 2). Thus, these closely related structural motifs appear not just to be immunogenically distinct, but might also be displayed to the immune system in different ways during uncontrolled (disease) and controlled (latent) infection. Moreover, both T1AM09 and L1AM04, mAbs isolated from latently Mtb-infected individuals, originated from different germlines and were less than 10% mutated at the nucleotide level. To what degree the spectrum of reactivity and affinity to glycan epitopes is influenced by somatic hypermutation, the state of Mtb infection, and/or the infecting strain warrants further investigation.
Ara4 and Ara6 containing motifs are among the most commonly recognized (Table 1 & Figure 3). The most widely recognized structure, by 10/21 mAbs, is Man4Ara6 (OS#6), which contains two α-(1→2)-linked Manp disaccharide residues attached to Ara6. The next most recognized is Man2Ara4 (OS#3), bound by 6/21 mAbs. Their frequent recognition by mAbs is not surprising because these structures are among the most common capping groups [81]. Of the four motifs not recognized by mAbs, Man4Ara5 (OS#56) and Man3Ara5 (OS#57) were strongly recognized by serum IgG from both LTBI and TB patients [51]. Human mAbs recently generated also bind strongly to the Man4Ara5 and Man3Ara5 motifs (unpublished data), suggesting that these structures, consisting of a linear Ara5 structure linked to an underlying tri- or tetra-mannoside, are immunogenic. Overall, the variations in methods used to produce anti-LAM/AM mAbs complicate determining the degree to which the infecting strain and methodology influence glycan specificity and highlight the need to isolate more human mAbs to LAM/AM to better understand these factors.
Importance of antibody reactivity to LAM/AM motifs
Well-known features such as epitope specificity, isotype, Fc glycosylation, avidity and affinity influence the functions and diagnostic value of antibodies. Methods to determine binding of mAbs to LAM/LAM vary broadly and little has been reported about mAb binding kinetics using methods such as Biolayer Interferometry (BLI) or Surface Plasmon Resonance (SPR) [79]. Recent studies, however, suggest that in addition to affinity, both the functions and diagnostic value of antibodies to LAM/AM are influenced by their epitope specificity [51, 57, 76].
Importance of epitope specificity for antibody functions.
A better understanding of the LAM/AM motifs/epitopes that are most protective could inform the development of immunotherapeutic mAbs and TB vaccines. Human polyclonal IgG antibodies are tremendously heterogeneous in their binding to LAM/AM motifs and reactivity with specific motifs correlating with IgG functions in vitro [51, 57]. Specifically, in LTBI individuals, reactivity to OS#7, OS#8, OS#9, OS#49, and OS#3, all containing the Ara4 motif with 0–3 Manp residues, and for OS#7, OS#8 and OS#9 an MTX capping residue (Figure 1 and Figure I in Box 1), correlated significantly with enhancement of Mtb phagocytosis in human macrophages [51]. Similarly, in adults that had undergone primary and secondary BCG vaccination, there was a significant correlation with overlapping motifs, specifically Man2Ara4 (OS#3), Man4Ara6 (OS#6) and Man2Ara6-2 (OS#12), containing Ara4 or Ara6 with 1–2 Manp residues, but no MTX residue [57]. The glycan motifs present in OS#3 and OS#6, both containing the α-Manp-α-(1→2)-Manp motif, have important roles in Mtb virulence. These are the primary mannose residues involved in mannose receptor (MR)-facilitated phagocytosis of Mtb [19]. In addition to the MR, the mannose-rich caps of LAM further bind to dendritic cell-specific intercellular adhesion molecule-grabbing nonintegrin (DC-SIGN), langerin, and dectin-2, facilitating the uptake of mycobacteria and/or inducing intracellular signaling [26, 44, 45] (Box 2). Although data on the role of Man caps in the virulence and persistence of Mtb in host cells is controversial [44, 82, 83], it is conceivable that antibodies targeting this disaccharide motif could interfere with Mtb pathogenesis. For example, such antibodies could reduce the uptake of Mtb via the MR and enhance its uptake of via FcRs, which was shown to lead to enhanced phagolysosomal fusion in macrophages [51, 57]. Some, but not all, anti-AM/LAM mAbs have protective efficacy in Mtb-infected mice [67, 84–86]. The only characterized mAb among these is 9d8, which recognizes Man4Ara6 (OS#6) [57, 85]. Although most of the anti-AM/LAM mAbs used in passive transfer experiments thus far have not been characterized in detail, it is conceivable that their differences in both reactivity to glycan motifs and FcR-binding contribute to their variations in in vivo efficacy against Mtb. Recent studies with nonhuman primates show that airway IgA to Man4Ara5 (OS#56) and Man3Ara5 (OS#57) and serum reactivity to Man2Ara4 (OS#3), Man3Ara4 (OS#4), MTX1Man2Ara4 (OS#7) and/or Man4Ara5 (OS#56) prior to Mtb infection is associated with protection against TB (unpublished data) but little is known about the roles of the Man4Ara5 and Man3Ara5 motifs in TB [26]. Further functional studies to better understand how, and to what degree, reactivity to specific glycan motifs influences antibody functions against Mtb are needed and will be facilitated by using mAbs with varying Fc structures and defined reactivity to diverse AM/LAM motifs.
Importance of mAb reactivity with OS motifs for TB diagnostic LAM detection.
In addition to high affinity, low background, and stability in POC formats, the most useful mAbs for TB diagnostic LAM detection assays should react with a range of clinically relevant LAM motifs. MAbs should also lack of cross-reactivity with other bacteria and, ideally, with NTMs. Binding to distinct LAM motifs will reduce competition between mAbs for LAM capture and detection and epitope specificity is thus important for optimal performance of LAM detection assays [76]. Several mAbs have value as research and diagnostic tools, especially for the detection of U-LAM in TB patients. For example, an improved capture-ELISA for U-LAM detection was developed using the broadly reactive murine mAbs CS35 as a capture together with the recently generated human mAb A194–01 as a detection mAb [73]. In an evaluation of 100 new anti-LAM mAb pair combinations on a multiplexed sandwich immunoassay and electrochemiluminescence platform, the 12 best pairs detecting spiked U-LAM were evaluated with clinical samples [76]. While 7/8 capture mAbs detected U-LAM in HIV-infected TB patients, only three (S4–20, FIND 28, and 13H3) mAbs with A194 for detection were able to detect U-LAM in HIV uninfected TB patients. This finding highlights the implications of structural differences between LAM generated in vitro and present in vivo and suggests U-LAM heterogeneity. Of these, using the rabbit mAb S4–20 as the capture with the human mAb A194–01 for detection had the best sensitivity/specificity profile [76], resulting in a next generation U-LAM detection POC test (FujiLAM) [63, 64]. Whereas CS35 reacts with a broad range of structural motifs, S4–20 recognizes only MTX1ManX motifs (Table 1 and Figures 1 & 2 [70, 76]). These data have led to the belief that reactivity to the Mtb-defining MTX-containing Man caps is critical for LAM capture in urine from TB patients [60, 62]. However, the rabbit mAb 13H3, which worked well as a capture mAb with clinical urine samples [76], does not react with MTX1ManX motifs. Neither does the human mAb L1AM04 (Table 1 and Figure 2), which, in preliminary studies, detected spiked and clinical U-LAM comparable to the capture and detection pair CS35/A194–01 [79].
A194–01 reacts with a several arabinan termini and Man cap motifs (Figure 2; [70, 76]). Although it is an excellent LAM detection mAb, it leads to much lower sensitivities when used as a capture mAb with several other mAbs [73, 76]. Collectively, the data highlight the complexity of optimal mAb pairs for U-LAM detection and suggest that reactivity to Man caps with and without MTX residues as well terminal arabinan motifs is critical. These studies support the heterogeneity of LAM motifs in clinical urine samples and lead to a more nuanced, yet incomplete, understanding of which LAM motifs are more abundantly excreted in urine.
Recent studies suggest that certain AM/LAM motifs, such as Ara4 and a Ara5 motif containing an additional reducing end α-(1→5)-Araf residue are more abundantly excreted in the urine of TB patients than other structures [28]. Data further suggest that AM/LAM components in the urine could be influenced by the infecting strain [28, 87]. Analysis of LAM isolated from three different clinical strains further identified varying degrees of acylation, most notably succinylation in ~45% of LAM motifs from clinical strains, compared to only ~30% of H37Rv [28]. It currently is unclear whether these acylation motifs will play a role in functions and/or the detection of LAM by mAbs [28, 40, 88]. In addition, structural differences between LAM made in vitro and in vivo [40] highlight the intricacy of factors that could influence both the generation of anti-LAM mAbs and their ability to bind to LAM in body fluids. Complicating matters further, mAb pairs used for detection of U-LAM in adult TB are less sensitive in pediatric TB and vice versa, suggesting that the disease state and host also impacts the LAM/AM motifs that are most abundant in urine (unpublished). These data highlight the need for further studies to delineate the heterogeneity and specificity of anti-LAM/AM mAbs critical for U-LAM detection.
Second generation U-LAM detection tests (e.g., FujiLAM) still detect only around half of PLHIV with >200 CD4 cells and HIV uninfected TB patients [63, 64]. In addition to investigating novel high-affinity anti-LAM mAbs, efforts to increase sensitivity through various LAM concentration methods and different diagnostic platforms are ongoing [60]. Pre-treatment of urine with enzymes can make certain LAM structures more accessible and enhance LAM detection in body fluids. For example, delipidation by pre-treatment of urine containing spiked LAM with chloroform, or removal of the Man caps by treatment with α-mannosidase, led to a 10-fold improvement in LAM detection with the Determine™ TB LAM Ag test [87]. Interestingly, whereas pre-treatment of LAM-spiked urine samples with other enzymes, including Proteinase-K, did not improve performance, Proteinase-K, possibly through releasing protein-bound LAM, was shown to improve LAM detection in clinical urine samples with next generation capture ELISA assays [89, 90]. Whether these differences in LAM detection after enzymatic pre-treatment were due to diversity in strain-specific LAM, spiked versus clinical LAM, and/or specificities of capture and detection antibodies could provide further insights into the many variables influencing U-LAM detection.
Detection of LAM in other body fluids has not been investigated as extensively as in urine and we do not know if LAM structures in these differ from those in urine. Both the presence of LAM degrading hydrolases in the lung mucosa [25] and structural differences of the non-reducing arabinan termini between LAM isolated from the lungs of Mtb-infected mice and LAM isolated from the urine of a TB patient [40], suggest that this might be the case. However, investigations to explore this further are needed. Despite higher LAM concentrations in sputum [60], attempts to detect LAM have been more challenging with low sensitivity and specificity, likely due to sputum viscosity and interfering oral flora [91, 92]. The amount of LAM in sputum correlated with bacterial load and TB treatment response in a small number of TB patients employing capture by mAbs S4–20 combined with G3 and detection with mAb O-TB. However, the sensitivity was lower than GeneXpert [77]. Serum, compared to urine, has the advantage of being less impacted by hydration status but immune complexes and low LAM concentrations in humans limit sensitivity [60]. While these findings show that LAM can be detected in other body fluids, we first need to better understand which of the likely diverse LAM structures are most abundant in the various organs/body fluids, determine optimal detection methods, including best mAbs pairs, and compare these to U-LAM detection.
Concluding Remarks
Antibodies to the mycobacterial surface glycans LAM and AM have protective functions against Mtb and are critical reagents for POC TB diagnosis. Recent efforts have increased the pipeline of anti-LAM/AM mAbs and have led to improved U-LAM detection assays. These, together with a new glycan array containing chemically defined AM structural motifs, have enhanced our knowledge of the breadth of mAb reactivity and importance of AM epitope recognition. While studies suggest the relevance of antibody reactivity to specific AM motifs for functions against Mtb, mAbs with varying Fc structures and defined reactivity to diverse AM/LAM motifs are needed to dissect the impact of FcR-mediated effects and epitope specificity.
As for TB diagnostics, a growing body of data suggest that LAM structures are diverse among and within various body fluids and are likely influenced by both pathogen and host factors. However, knowledge of the detailed LAM structures most abundantly present in urine and other body fluids and their extent of heterogeneity remains incomplete. Moreover, recently discovered structural differences between LAM from Mtb grown in vitro and U-LAM from TB patients infected with various clinical strains highlights further challenges that need to be considered when generating mAbs by immunizing animals and when assessing assays for LAM detection. Second generation POC tests based on U-LAM detection still fall short of WHO target profiles with desired sensitivities of 80–90% for all TB patients. One of the major challenges is the detection of low U-LAM concentrations in paucibacillary TB. Overcoming this challenge will likely require a combination of LAM concentration and urine pre-treatment on an ideal POC platform, possibly with a variety of and/or bi-specific mAbs.
While our understanding of the importance of glycan motif recognition for antibody functions, diagnostic LAM detection, and research applications at large has progressed, major knowledge gaps remain. Continued generation of high-affinity mAbs to diverse and clinically relevant LAM/AM motifs combined with studies on how their epitope specificity and other antibody features influence functions and diagnostic performance will be essential to both determine their role in the protection against TB and achieve the high bar of a universal simple TB POC test.
Supplementary Material
Outstanding Questions:
Which specific glycan epitopes influence antibody functions against Mtb?
What is the structural basis of the recognition of LAM/AM by anti-LAM antibodies?
How is LAM degraded in the host and how does that impact LAM structure and ability to be detected in bodily fluids?
What LAM motifs are most critical for highly sensitive U-LAM detection assays?
Are antibody reactivities to succinylation and other acylation motifs of LAM relevant for function and ability to detect LAM in body fluids.
Do LAM structures differ in various body fluids and tissues?
Can additional anti-LAM/AM mAbs improve sensitivity of next generation U-LAM detection assays?
Can the detection of LAM in other body fluids be complementary or superior to U-LAM detection?
To which degree is the abundance of LAM motifs in clinical samples influenced by the infecting Mtb strain, the state of the disease, and the host?
How does the method used for generating anti-LAM/AM mAbs impact their function, and sensitivity and specificity for LAM detection?
Highlights:
The surface glycans LAM and AM play important roles in interactions between M. tuberculosis and other mycobacteria and the host. These conformationally flexible glycans are shed in vitro and in vivo.
The pipeline of mAbs to LAM/AM has grown as antibodies to LAM and AM are important for studies both to understand mechanisms of protection against Mtb and to develop next generation simple point-of-care tuberculosis diagnostics based on LAM detection in body fluids.
The synthesis of oligosaccharides corresponding to structurally defined LAM/AM motifs, and their display in an array format, has enabled convenient characterization of anti-LAM/AM antibody reactivity to glycan epitopes.
To date, the epitope specificity of 21 mAbs to LAM/AM has been determined. These data enhance our understanding of LAM/AM motif recognition and its relevance for antibody functions and diagnostic LAM detection.
Acknowledgements and funding:
This work was supported in part by funds from the National Institute of Health (NIH)/National Institute of Allergy and Infectious Diseases (NIAID) to J.M.A. (AI146329, AI127173, and AI117927) and D.C. (R01 AI132680). E.I. was supported by NIH Institutional Clinical and Translational Science Award (U54) grant (5TL1TR001072-06) and NIH T32 Fellowship in Geographic Medicine and Emerging Infectious Diseases (2T32AI070117-14). T.L.L. is thankful for intramural support from Academia Sinica.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Boom WH, et al. (2021) The knowns and unknowns of latent Mycobacterium tuberculosis infection. J Clin Invest 131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Global Tuberculosis Report 2021. Geneva: World Health Organization; 2021, License: CC BY-NC-SA 3.0 IGO [Google Scholar]
- 3.Schrager LK, et al. (2020) The status of tuberculosis vaccine development. Lancet Infect Dis 20, e28–e37 [DOI] [PubMed] [Google Scholar]
- 4.Mangtani P, et al. (2014) Protection by BCG vaccine against tuberculosis: a systematic review of randomized controlled trials. Clin Infect Dis 58, 470–480 [DOI] [PubMed] [Google Scholar]
- 5.Achkar JM and Prados-Rosales R (2018) Updates on antibody functions in Mycobacterium tuberculosis infection and their relevance for developing a vaccine against tuberculosis. Curr Opin Immunol 53, 30–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kawahara JY, et al. (2019) A Case for Antibodies as Mechanistic Correlates of Immunity in Tuberculosis. Front Immunol 10, 996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li H and Javid B (2018) Antibodies and tuberculosis: finally coming of age? Nat Rev Immunol 18, 591–596 [DOI] [PubMed] [Google Scholar]
- 8.Rijnink WF, et al. (2021) B-Cells and Antibodies as Contributors to Effector Immune Responses in Tuberculosis. Front Immunol 12, 640168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Denkinger CM, et al. (2015) Defining the needs for next generation assays for tuberculosis. J Infect Dis 211 Suppl 2, S29–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Correia-Neves M, et al. (2019) Lipoarabinomannan in Active and Passive Protection Against Tuberculosis. Front Immunol 10, 1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Palacios A, et al. (2019) Mycobacterium tuberculosis extracellular vesicle-associated lipoprotein LpqH as a potential biomarker to distinguish paratuberculosis infection or vaccination from tuberculosis infection. BMC Vet Res 15, 188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Prados-Rosales R, et al. (2011) Mycobacteria release active membrane vesicles that modulate immune responses in a TLR2-dependent manner in mice. J Clin Invest 121, 1471–1483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Layre E (2020) Trafficking of Mycobacterium tuberculosis Envelope Components and Release Within Extracellular Vesicles: Host-Pathogen Interactions Beyond the Wall. Front Immunol 11, 1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kalscheuer R, et al. (2019) The Mycobacterium tuberculosis capsule: a cell structure with key implications in pathogenesis. Biochem J 476, 1995–2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ortalo-Magne A, et al. (1995) Molecular composition of the outermost capsular material of the tubercle bacillus. Microbiology (Reading) 141 (Pt 7), 1609–1620 [DOI] [PubMed] [Google Scholar]
- 16.Daffe M and Marrakchi H (2019) Unraveling the Structure of the Mycobacterial Envelope. Microbiol Spectr 7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Daffe M and Etienne G (1999) The capsule of Mycobacterium tuberculosis and its implications for pathogenicity. Tuber Lung Dis 79, 153–169 [DOI] [PubMed] [Google Scholar]
- 18.Daffé M, et al. (2014) Genetics of Capsular Polysaccharides and Cell Envelope (Glyco)lipids. Microbiology Spectrum 2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Turner J and Torrelles JB (2018) Mannose-capped lipoarabinomannan in Mycobacterium tuberculosis pathogenesis. Pathog Dis 76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mishra AK, et al. (2011) Lipoarabinomannan and related glycoconjugates: structure, biogenesis and role in Mycobacterium tuberculosis physiology and host-pathogen interaction. FEMS Microbiol Rev 35, 1126–1157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dulberger CL, et al. (2020) The mycobacterial cell envelope - a moving target. Nat Rev Microbiol 18, 47–59 [DOI] [PubMed] [Google Scholar]
- 22.Lemassu A, et al. (1996) Extracellular and surface-exposed polysaccharides of non-tuberculous mycobacteria. Microbiology (Reading) 142 (Pt 6), 1513–1520 [DOI] [PubMed] [Google Scholar]
- 23.Schwebach JR, et al. (2001) Expression of a Mycobacterium tuberculosis arabinomannan antigen in vitro and in vivo. Infect Immun 69, 5671–5678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Haji-Ghassemi O, et al. (2015) Antibody recognition of carbohydrate epitopes. Glycobiology 25, 920–952 [DOI] [PubMed] [Google Scholar]
- 25.Arcos J, et al. (2011) Human lung hydrolases delineate Mycobacterium tuberculosis-macrophage interactions and the capacity to control infection. J Immunol 187, 372–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zheng RB, et al. (2017) Insights into Interactions of Mycobacteria with the Host Innate Immune System from a Novel Array of Synthetic Mycobacterial Glycans. ACS Chem Biol 12, 2990–3002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Angala SK, et al. (2020) Secondary Extended Mannan Side Chains and Attachment of the Arabinan in Mycobacterial Lipoarabinomannan. Commun Chem 3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.De P, et al. (2021) Structural implications of lipoarabinomannan glycans from global clinical isolates in diagnosis of Mycobacterium tuberculosis infection. J Biol Chem 297, 101265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nigou J, et al. (2003) Lipoarabinomannans: from structure to biosynthesis. Biochimie 85, 153–166 [DOI] [PubMed] [Google Scholar]
- 30.Chatterjee D and Brennan PJ (2010) Glycosylated components of the mycobacterial cell wall. In Microbial Glycobiology, pp. 147–167, Elsevier [Google Scholar]
- 31.Palcekova Z, et al. (2020) Polysaccharide Succinylation Enhances the Intracellular Survival of Mycobacterium abscessus. ACS Infect Dis 6, 2235–2248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chatterjee D and Khoo KH (1998) Mycobacterial lipoarabinomannan: an extraordinary lipoheteroglycan with profound physiological effects. Glycobiology 8, 113–120 [DOI] [PubMed] [Google Scholar]
- 33.Khoo KH, et al. (1995) Inositol phosphate capping of the nonreducing termini of lipoarabinomannan from rapidly growing strains of Mycobacterium. J Biol Chem 270, 12380–12389 [DOI] [PubMed] [Google Scholar]
- 34.Khoo KH, et al. (2001) Variation in mannose-capped terminal arabinan motifs of lipoarabinomannans from clinical isolates of Mycobacterium tuberculosis and Mycobacterium avium complex. J Biol Chem 276, 3863–3871 [DOI] [PubMed] [Google Scholar]
- 35.Chatterjee D, et al. (1992) Structural basis of capacity of lipoarabinomannan to induce secretion of tumor necrosis factor. Infect Immun 60, 1249–1253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chatterjee D, et al. (1991) Structural features of the arabinan component of the lipoarabinomannan of Mycobacterium tuberculosis. J Biol Chem 266, 9652–9660 [PubMed] [Google Scholar]
- 37.Souza C, et al. (2013) Mannosylated lipoarabinomannans from Mycobacterium avium subsp. paratuberculosis alters the inflammatory response by bovine macrophages and suppresses killing of Mycobacterium avium subsp. avium organisms. PLoS One 8, e75924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Treumann A, et al. (2002) 5-Methylthiopentose: a new substituent on lipoarabinomannan in Mycobacterium tuberculosis. J Mol Biol 316, 89–100 [DOI] [PubMed] [Google Scholar]
- 39.Joe M, et al. (2006) The 5-deoxy-5-methylthio-xylofuranose residue in mycobacterial lipoarabinomannan. absolute stereochemistry, linkage position, conformation, and immunomodulatory activity. J Am Chem Soc 128, 5059–5072 [DOI] [PubMed] [Google Scholar]
- 40.De P, et al. (2020) Comparative Structural Study of Terminal Ends of Lipoarabinomannan from Mice Infected Lung Tissues and Urine of a Tuberculosis Positive Patient. ACS Infect Dis 6, 291–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Angala SK, et al. (2017) Biosynthesis of the Methylthioxylose Capping Motif of Lipoarabinomannan in Mycobacterium tuberculosis. ACS Chem Biol 12, 682–691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Guerardel Y, et al. (2003) Lipomannan and lipoarabinomannan from a clinical isolate of Mycobacterium kansasii: novel structural features and apoptosis-inducing properties. J Biol Chem 278, 36637–36651 [DOI] [PubMed] [Google Scholar]
- 43.World Health Organization (2015) The use of lateral flow urine lipoarabinomannan assay (LF-LAM) for the diagnosis and screening of active tuberculosis in people living with HIV. World Health Organization [Google Scholar]
- 44.Decout A, et al. (2018) Deciphering the molecular basis of mycobacteria and lipoglycan recognition by the C-type lectin Dectin-2. Sci Rep 8, 16840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hoving JC, et al. (2014) Signalling C-type lectin receptors, microbial recognition and immunity. Cell Microbiol 16, 185–194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Harding CV and Boom WH (2010) Regulation of antigen presentation by Mycobacterium tuberculosis: a role for Toll-like receptors. Nat Rev Microbiol 8, 296–307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nigou J, et al. (2002) Mycobacterial lipoarabinomannans: modulators of dendritic cell function and the apoptotic response. Microbes Infect 4, 945–953 [DOI] [PubMed] [Google Scholar]
- 48.van Kooyk Y and Geijtenbeek TB (2003) DC-SIGN: escape mechanism for pathogens. Nat Rev Immunol 3, 697–709 [DOI] [PubMed] [Google Scholar]
- 49.Sani M, et al. (2010) Direct visualization by cryo-EM of the mycobacterial capsular layer: a labile structure containing ESX-1-secreted proteins. PLoS Pathog 6, e1000794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Costello AM, et al. (1992) Does antibody to mycobacterial antigens, including lipoarabinomannan, limit dissemination in childhood tuberculosis? Trans R Soc Trop Med Hyg 86, 686–692 [DOI] [PubMed] [Google Scholar]
- 51.Chen T, et al. (2020) Capsular glycan recognition provides antibody-mediated immunity against tuberculosis. J Clin Invest 130, 1808–1822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Achkar JM and Casadevall A (2013) Antibody-mediated immunity against tuberculosis: implications for vaccine development. Cell Host Microbe 13, 250–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Prados-Rosales R, et al. (2017) Enhanced control of Mycobacterium tuberculosis extrapulmonary dissemination in mice by an arabinomannan-protein conjugate vaccine. PLoS Pathog 13, e1006250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hamasur B, et al. (2003) Mycobacterium tuberculosis arabinomannan-protein conjugates protect against tuberculosis. Vaccine 21, 4081–4093 [DOI] [PubMed] [Google Scholar]
- 55.Poyntz HC, et al. (2014) Non-tuberculous mycobacteria have diverse effects on BCG efficacy against Mycobacterium tuberculosis. Tuberculosis (Edinb) 94, 226–237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.de Valliere S, et al. (2005) Enhancement of innate and cell-mediated immunity by antimycobacterial antibodies. Infect Immun 73, 6711–6720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chen T, et al. (2016) Association of Human Antibodies to Arabinomannan With Enhanced Mycobacterial Opsonophagocytosis and Intracellular Growth Reduction. J Infect Dis 214, 300–310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Younis H, et al. (2019) Combining urine lipoarabinomannan with antibody detection as a simple non-sputum-based screening method for HIV-associated tuberculosis. PLoS One 14, e0218606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Irvine EB, et al. (2021) Robust IgM responses following intravenous vaccination with Bacille Calmette-Guerin associate with prevention of Mycobacterium tuberculosis infection in macaques. Nat Immunol 22, 1515–1523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bulterys MA, et al. (2019) Point-Of-Care Urine LAM Tests for Tuberculosis Diagnosis: A Status Update. J Clin Med 9, 111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shah M, et al. (2016) Lateral flow urine lipoarabinomannan assay for detecting active tuberculosis in HIV-positive adults. Cochrane Database Syst Rev, CD011420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Flores J, et al. (2021) Lipoarabinomannan as a Point-of-Care Assay for Diagnosis of Tuberculosis: How Far Are We to Use It? Front Microbiol 12, 638047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Broger T, et al. (2019) Novel lipoarabinomannan point-of-care tuberculosis test for people with HIV: a diagnostic accuracy study. Lancet Infect Dis 19, 852–861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Broger T, et al. (2020) Diagnostic accuracy of 3 urine lipoarabinomannan tuberculosis assays in HIV-negative outpatients. J Clin Invest 130, 5756–5764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gaylord H, et al. (1987) Most Mycobacterium leprae carbohydrate-reactive monoclonal antibodies are directed to lipoarabinomannan. Infect Immun 55, 2860–2863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Glatman-Freedman A, et al. (1996) Monoclonal antibodies to surface antigens of Mycobacterium tuberculosis and their use in a modified enzyme-linked immunosorbent spot assay for detection of mycobacteria. J Clin Microbiol 34, 2795–2802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hamasur B, et al. (2004) A mycobacterial lipoarabinomannan specific monoclonal antibody and its F(ab’) fragment prolong survival of mice infected with Mycobacterium tuberculosis. Clin Exp Immunol 138, 30–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hunter SW, et al. (1986) Structure and antigenicity of the phosphorylated lipopolysaccharide antigens from the leprosy and tubercle bacilli. J Biol Chem 261, 12345–12351 [PubMed] [Google Scholar]
- 69.Hamasur B, et al. (2015) A sensitive urinary lipoarabinomannan test for tuberculosis. PLoS One 10, e0123457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Choudhary A, et al. (2018) Characterization of the Antigenic Heterogeneity of Lipoarabinomannan, the Major Surface Glycolipid of Mycobacterium tuberculosis, and Complexity of Antibody Specificities toward This Antigen. J Immunol 200, 3053–3066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chatterjee D, et al. (1992) Lipoarabinomannan of Mycobacterium-Tuberculosis - Capping with Mannosyl Residues in Some Strains. Journal of Biological Chemistry 267, 6234–6239 [PubMed] [Google Scholar]
- 72.Prinzis S, et al. (1993) Structure and antigenicity of lipoarabinomannan from Mycobacterium bovis BCG. J Gen Microbiol 139, 2649–2658 [DOI] [PubMed] [Google Scholar]
- 73.Amin AG, et al. (2018) Detection of lipoarabinomannan in urine and serum of HIV-positive and HIV-negative TB suspects using an improved capture-enzyme linked immuno absorbent assay and gas chromatography/mass spectrometry. Tuberculosis (Edinb) 111, 178–187 [DOI] [PubMed] [Google Scholar]
- 74.Kang PB, et al. (2005) The human macrophage mannose receptor directs Mycobacterium tuberculosis lipoarabinomannan-mediated phagosome biogenesis. J Exp Med 202, 987–999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bucsan AN, et al. (2019) The current state of animal models and genomic approaches towards identifying and validating molecular determinants of Mycobacterium tuberculosis infection and tuberculosis disease. Pathog Dis 77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sigal GB, et al. (2018) A Novel Sensitive Immunoassay Targeting the 5-Methylthiod-Xylofuranose-Lipoarabinomannan Epitope Meets the WHO’s Performance Target for Tuberculosis Diagnosis. J Clin Microbiol 56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kawasaki M, et al. (2019) Lipoarabinomannan in sputum to detect bacterial load and treatment response in patients with pulmonary tuberculosis: Analytic validation and evaluation in two cohorts. PLoS Med 16, e1002780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pinter A, et al. (2017) Novel anti-lam and anti-pim6/lam monoclonal antibodies for diagnosis and treatment of mycobacterium tuberculosis infections. World Intellectual Property Organization United States of America, WO2017/139153A139151 [Google Scholar]
- 79.Ishida E, et al. (2021) Monoclonal antibodies from humans with Mycobacterium tuberculosis exposure or latent infection recognize distinct arabinomannan epitopes. Commun Biol 4, 1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chan CE, et al. (2015) The diagnostic targeting of a carbohydrate virulence factor from M.Tuberculosis. Sci Rep 5, 10281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chatterjee D, et al. (1993) Structural definition of the non-reducing termini of mannose-capped LAM from Mycobacterium tuberculosis through selective enzymatic degradation and fast atom bombardment-mass spectrometry. Glycobiology 3, 497–506 [DOI] [PubMed] [Google Scholar]
- 82.Afonso-Barroso A, et al. (2013) Lipoarabinomannan mannose caps do not affect mycobacterial virulence or the induction of protective immunity in experimental animal models of infection and have minimal impact on in vitro inflammatory responses. Cell Microbiol 15, 660–674 [DOI] [PubMed] [Google Scholar]
- 83.Appelmelk BJ, et al. (2008) The mannose cap of mycobacterial lipoarabinomannan does not dominate the Mycobacterium-host interaction. Cell Microbiol 10, 930–944 [DOI] [PubMed] [Google Scholar]
- 84.Glatman-Freedman A, et al. (2000) Clearance and organ distribution of Mycobacterium tuberculosis lipoarabinomannan (LAM) in the presence and absence of LAM-binding immunoglobulin M. Infect Immun 68, 335–341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Teitelbaum R, et al. (1998) A mAb recognizing a surface antigen of Mycobacterium tuberculosis enhances host survival. Proc Natl Acad Sci U S A 95, 15688–15693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Glatman-Freedman A, et al. (2004) Antigenic evidence of prevalence and diversity of Mycobacterium tuberculosis arabinomannan. J Clin Microbiol 42, 3225–3231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Garcia JI, et al. (2019) Improved Alere Determine Lipoarabinomannan Antigen Detection Test for the Diagnosis of Human and Bovine Tuberculosis by Manipulating Urine and Milk. Sci Rep 9, 18012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lowary TL and Achkar JM (2022) Tailor made: New insights into lipoarabinomannan structure may improve TB diagnosis. J Biol Chem 298, 101678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Panraksa Y, et al. (2021) Immobilization of Proteinase K for urine pretreatment to improve diagnostic accuracy of active tuberculosis. PLoS One 16, e0257615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Amin AG, et al. (2021) Urine lipoarabinomannan in HIV uninfected, smear negative, symptomatic TB patients: effective sample pretreatment for a sensitive immunoassay and mass spectrometry. Sci Rep 11, 2922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Dheda K, et al. (2010) Clinical utility of a commercial LAM-ELISA assay for TB diagnosis in HIV-infected patients using urine and sputum samples. PLoS One 5, e9848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Peter JG, et al. (2012) Diagnostic accuracy of induced sputum LAM ELISA for tuberculosis diagnosis in sputum-scarce patients. Int J Tuberc Lung Dis 16, 1108–1112 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


