Abstract
To streamline drug development, the FDA can consider the extrapolation of adult efficacy data to children when the disease and drug effects are sufficiently similar. This study explored whether the relationship between drug exposure and response for selected drugs in systemic lupus erythematosus (SLE) was sufficiently similar to support consideration of extrapolation of adult efficacy data to children ≥ 5 years. An exposure-response analysis of drugs used to treat SLE was conducted using published exposure vs. response and efficacy vs. time data. Statistical analyses included non-compartmental analysis of a drug’s area under the effect curve and direct Imax pharmacodynamic (PD) modeling. Six drugs were included: belimumab, mycophenolate/mycophenolic acid, rituximab, hydroxychloroquine, azathioprine, and cyclophosphamide. For belimumab, the net change in responders at week 52 (primary endpoint) was nearly identical between one adult trial and the pediatric trial. For mycophenolate, PD modeling suggested no significant differences in exposure and SLE disease activity between adults and children. For rituximab, hydroxychloroquine, azathioprine, and cyclophosphamide, data was not sufficient to quantitatively characterize the exposure-response relationship, but the clinical or pharmacologic response between children and adults was similar overall. Adult SLE data should be leveraged to guide pediatric drug development programs and identify areas with residual uncertainty of a drug’s effectiveness or safety in children. The degree to which efficacy extrapolation can reduce clinical trial requirements in pediatric SLE should be individualized for each new drug product, depending in part on the drug’s mechanism of action and the similarity in disease manifestations between children and adults.
Keywords: Pediatrics, pharmacokinetics and drug metabolism, pharmacodynamics, regulatory/scientific affairs, rheumatology
Introduction
To ensure the safety of children in clinical trials, therapeutic agents are often first investigated in adults to provide preliminary evidence of efficacy and identify potential safety concerns and support the prospect of direct benefit to pediatric subjects, prior to initiating clinical trials in children.1 As a result, drug development in children often significantly lags behind drug development in adults, particularly for rare diseases for which enrollment into clinical trials is often difficult. For example, belimumab, a B-cell survival factor (BLyS)-specific inhibitor, was approved for use in adults with systemic lupus erythematosus (SLE) in 2011, yet it took greater than 8 years before being approved for use in pediatric lupus.2 Active SLE can result in permanent organ damage or death,3 so it is imperative to make safe and effective therapeutics available to children with SLE expeditiously in order to improve outcomes.
In drug development programs where the adult and pediatric diseases are considered sufficiently similar, the FDA can consider the extrapolation of efficacy from adult populations to support the efficacy in pediatric populations. By extrapolating efficacy, the amount of efficacy data required from traditional phase 3 trials in children is greatly reduced, and in some cases, eliminated entirely which may result in more timely access to effective treatments.1,4,5 Historically, FDA pediatric extrapolation of efficacy was initially described as full, partial or no extrapolation, with partial extrapolation (e.g., when the exposure-response relationship is not defined or there is residual uncertainty regarding similarity of the disease between adults and pediatric patients) as the principle approach.1 Partial extrapolation has traditionally required dosing studies, and may also include abbreviated efficacy studies. The abbreviated studies can range from a single efficacy trial to a pharmacokinetic/pharmacodynamic (PK/PD) study.1,4,5 Recently, however, regulatory authorities began moving away from strict, discrete categories of full, partial, or no efficacy extrapolation, favoring a more continuous view where the degree of efficacy extrapolation exists on a spectrum based on the abundance and strength of existing data.6 For example, IV belimumab was studied in a randomized, double-blind, placebo-controlled trial in 93 children with SLE. The study was underpowered to demonstrate efficacy in children, but the PK and safety observed were similar to those observed in adults which supported approval.7 Additional support for the efficacy findings of this pediatric study was obtained from a post-hoc Bayesian analysis which borrowed information from two adult belimumab SLE trials to support the efficacy assessment in the pediatric study.7
To support the extrapolation of efficacy in children from adults in SLE, it should be “reasonable to assume” that adults and children have: 1) similar disease progression and response to treatment; and 2) a similar exposure-response relationship.5 Although childhood SLE may be more severe compared to adults,8 SLE is believed to be the same disease in both populations, characterized by the same immunological pathophysiology,9,10 clinical manifestations,8 classification criteria,11 disease activity measurements,12 and treatment armamentarium.13 Disease progression is also similar, though children may accrue damage faster.3,12,13 Nevertheless, no studies have systematically compared the response to treatment between adults and children with SLE, and the exposure-response relationship across several classes of therapeutics is unknown. Therefore, a quantitative and descriptive exposure-response analysis of common therapeutics in SLE was conducted to determine whether use of adult efficacy data could be considered to support efficacy in children.
Methods
The study was considered exempt by the Duke Institutional Review Board (Pro00108606), and consent was not required because the study used only publicly available, de-identified data.
Study Eligibility and Selection
A non-systematic literature search using PubMed was conducted to identify studies evaluating small molecule/synthetic or biologic therapeutics in children or adults with SLE, with and without lupus nephritis. Utilized articles were not restricted based on publication date, and both on-label and off-label drugs were included. Studies were included if: 1) there was a shared outcome measure used in both the adult and pediatric studies; 2) the published manuscript characterized treatment response or an exposure-response relationship; and 3) the data in the published manuscript had tables/graphs/figures suitable for digital data extraction. Based on the literature search, six drugs were identified with studies meeting inclusion criteria (Table S1): 1) belimumab; 2) mycophenolate; 3) hydroxychloroquine; 4) cyclophosphamide; 5) azathioprine; and 6) rituximab. Because lupus nephritis is a separate labelling indication with unique endpoints, when possible, data were presented separately for SLE without nephritis, and SLE with nephritis. Assessment of drug response in the publication was analyzed by a practicing rheumatologist board-certified in both adult and pediatric rheumatology (SJB).
Drug Approval and Regulatory Context for Included Drugs
Belimumab
Belimumab is a monoclonal antibody inhibiting BLyS, reducing several B-cell subsets, and is FDA approved for the treatment of SLE in children ≥5 years of age using an intravenous dosage regimen of 10 mg/kg at 2‑week intervals for the first 3 doses and at 4‑week intervals thereafter.2 Belimumab is also FDA approved in adults for the treatment of SLE and lupus nephritis either intravenously (same weight-based dosage administered in children for SLE) or subcutaneously.
Mycophenolate
The active metabolite of mycophenolate (mycophenolic acid) is a selective, uncompetitive, and reversible inhibitor of inosine monophosphate dehydrogenase (IMPDH), resulting in inhibition of guanosine nucleotide synthesis, and T-lymphocyte and B-lymphocyte proliferation.14 Mycophenolate is used extensively off-label to treat both adult and pediatric SLE with and without lupus nephritis. In this manuscript, ‘mycophenolate’ is used broadly to refer to either mycophenolate mofetil or mycophenolic acid drug products, with the specific drug listed in Table S1.
Hydroxychloroquine
Hydroxychloroquine is an antimalarial with multiple proposed mechanisms of action in SLE, including inhibiting Toll-like receptor signaling and autoantigen presentation, and reducing B-cell and T-cell activation.15 Hydroxychloroquine is FDA approved for the treatment of SLE in adults and is used extensively off-label in children with SLE.
Cyclophosphamide
Cyclosphamide is an oral or intravenous pro-drug that is metabolized to alkylating metabolites, resulting in a cytotoxic effect to lymphocytes.16 Cyclosphamide is used off-label in both adult and pediatric SLE, particularly for lupus nephritis.
Azathioprine
Azathioprine is a derivative of 6-mercaptopurine that undergoes metabolism to multiple metabolites, some of which are incorporated into DNA, stop lymphocyte proliferation, and reduce purine synthesis.17 Azathioprine is used off-label in both adult and pediatric SLE.
Rituximab
Rituximab is a chimeric monoclonal antibody that binds to CD20 on pre-B-lymphoctes and mature B-lymphocytes, resulting in B-cell lysis.18 Rituximab is used off-label in both adult and pediatric SLE, with and without lupus nephritis.
Data Extraction
For the included studies, concentration vs. time profiles, response vs. exposure profiles, and effect (clinical outcome or pharmacologic response) vs. time profiles were extracted from published studies and converted to raw data (comma separated values) using WebPlotDigitizer digitizing software (V4.4, Pacifica, California). In addition, raw data was also extracted directly from tables and figures where applicable in the published manuscripts.
Data Analysis
All statistical analyses were conducted in R version 4.1.1 (R Foundation for Statistical Computing, Vienna, Austria) and RStudio version 1.4.1717 (RStudio, Inc., Boston, MA). All hypothesis testing was conducted using a significance level of 0.05 unless otherwise stated. Graphical analysis was conducted using Phoenix NLME (V8.2, Certara, Raleigh, NC), or R package ggplot2. Specific analyses were dependent on the type of publicly available data and is categorized by each drug below.
Belimumab
The percentage of SRI-4 responders at each time point was digitally extracted from three belimumab studies (Table S1). The SRI-4 is a composite measure for which patients are dichotomized as “responders” or “non-responders”.2 A noncompartmental analysis (NCA) using a linear trapezoidal, linear interpolation method was conducted to compute the area under the time-effect curve (AUEC) from 4 weeks to 52 weeks for 1) the change in SRI responders (%), defined as the difference between belimumab 10 mg/kg and placebo, and 2) the overall effect (% responders) from belimumab 10 mg/kg. A baseline effect of 0 was assumed for both scenarios.
Mycophenolate
Population PD Modeling
The overall modeling approach is depicted in Figure S1. The area under the time-concentration curve (AUC) of mycophenolic acid concentrations and Systemic Lupus Erythematosus Disease Activity Index (SLEDAI) scores for individual patients were extracted from two publications (Table S1, Figure S1). The SLEDAI is a composite disease activity index that is treated on a continuous scale and has a maximum score of 105.12 In addition, mean or median AUC and response (inactive vs. active SLE, renal response) was extracted from four publications (Table S1). To analyze the individual AUC and SLEDAI scores, we conducted a graphical and exposure-response analysis with Phoenix NLME using the Quasi-random Parametric Expectation Maximization algorithm; this algorithm has been shown to produce reliable estimates with sparse data.19 To develop a population PD model to characterize the effect of mycophenolic acid AUC on SLEDAI, we used a direct Imax model as shown in Equation 1.
| Equation 1: |
Where E0 is the baseline SLEDAI, AUC50 is the AUC at which 50% of the maximum inhibitory effect on SLEDAI is observed, and Imax is the maximum inhibitory effect.
Additive (total effect= estimated effect + error term) and proportional (total effect= estimated effect *[1 + error term)] residual error models were explored. Modeling was conducted using three different approaches: 1) fixing both Imax and E0 to 105 based on the maximum SLEDAI score possible; 2) estimating Imax, AUC50, and E0; and 3) fixing Imax and E0 to the maximum individual E0 value observed in the dataset, assuming the maximum possible response is to reduce the baseline SLEDAI to 0. Although fixing Imax and E0 to 105 incorrectly assumes all patients have the highest initial disease possible, this approached was used in the published pediatric model (Chen et al, Table S1) and can facilitate model fitting by reducing the overall number of parameters that need to be estimated. Initially, inter-individual variability (IIV) was estimated on all parameters. However, IIV was removed on E0 and Imax when shrinkage was >40%. IIV was retained on AUC50 regardless of shrinkage to allow for comparison of drug potency with published models and evaluate the impact of covariates on reduction in IIV. Once a final structural model was identified, population PD model parameters were estimated separately for the adult and the pediatric study datasets. Next, the adult and pediatric datasets were combined to estimate a single set of population PD parameters and evaluated the effect of age (adult vs. pediatric) as a covariate. Each final model (e.g., adult, pediatric, and combined adult/pediatric) was selected based on plausibility and precision of parameter estimates, diagnostic plots, successful minimization, and Akaike Information Criterion.
The 95% confidence interval for each final PD model’s parameters was evaluated using 1,000 replicates of non-parametric bootstrapping. Visual predictive checks were performed using the final models and 1000 Monte Carlo simulation replicates per time point of mycophenolic acid AUC. Simulated and observed results were compared by plotting the 5th, 50th, and 95th percentile of each observed vs simulated concentration.
To evaluate the effect of population (adult vs. pediatric) as a binary covariate on PD parameters, a forward-inclusion and backward elimination approach was conducted using a very conservative significance value of p=0.1 and p=0.05, respectively. The P-values correspond to a change in the model objective function value by 2.71 and 3.84 with 1 degree of freedom. Population as a categorical covariate was explored on parameters where IIV was estimated using an exponential function as described in Equation 2.
| Equation 2: |
Where PARn,x denotes the estimate of parameter x in the nth individual; θPop,x is the population value for parameter x; θcov is a parameter which represents the covariate effect, and POPULATION is a dichotomous categorical variable that can take on the value of zero for adults or one for pediatric.
Clinical Outcomes
The average mycophenolic acid AUC over 12 hours (AUC0–12) was compared between adults and children with and without active SLE as defined in the publications using Welch’s t-test. Normality in the average AUC was assumed but could not be verified in the absence of the original dataset. The relationship between mean or median AUC0–12 and renal response was descriptively compared as defined in the original publication (Table S1).
Hydroxychloroquine
Since the number of children with active vs. inactive SLE was not provided in the original pediatric publication,20 no formal statistical comparison was conducted between adults and children. Accordingly, the mean whole blood hydroxychloroquine concentration was descriptively compared between adults and children with and without active SLE as defined in the original publication.
Rituximab
For several of the retrospective pediatric studies, B-cell phenotyping was either not done on all participants, or was done on an inconsistent schedule, potentially leading to bias. After reviewing the available data, differences in study designs and rituximab dosing in the pediatric studies prohibited a statistical comparison of B-cell depletion with adult studies. Accordingly, B-cell depletion over time was visually depicted after receipt of rituximab and changes in SLE biomarkers were descriptively compared between adults and children. For the published Nwobi study, the timing of B-cell depletion was reported over several time intervals (e.g., 0–1 month, 1–3 months, 4–6 months); the midpoint in each time interval in this pediatric study was therefore used to compare to the adult studies.
Results
Belimumab
Included Studies
Brunner et al. conducted a randomized, double-blind, controlled trial of belimumab 10 mg/kg IV vs placebo in 93 children with active SLE aged 5–17 years.21 The primary endpoint was the composite SLE Responder Index-4 (SRI-4) at week 52. Furie et al.22 and Navarra et al.23 conducted independent, phase 3, randomized, double-blind, placebo-controlled trials of belimumab 1 mg/kg IV, 10 mg/kg IV, and placebo in adults with active SLE. The primary outcome, SRI-4 at week 52, was the same in the adult and pediatric trials. The overall study populations were similar in terms of disease and treatment characteristics. In the adult trials, approximately 6.2% in the belimumab 10 mg/kg group withdrew due to lack of efficacy compared to 1.9% in the pediatric trial.
Exposure-Response
SLE without nephritis:
The maximum proportion of children achieving SRI-4 response at any time point was greater than that observed in adults, with overall similar AUC of the time-effect curve (Figure 1, Table 1, and Table S2). However, pediatric participants also had a higher placebo response compared to adults, resulting in periods during the study where the placebo effect was greater than the observed drug effect (negative change in % SRI-4 responders). Specifically, pediatric participants experienced a net positive drug effect for approximately 65.4% of the study duration. Nevertheless, the overall time that the change in SRI-4 AUEC was above 0 (net AUEC) was similar between the pediatric trial and one of two adult trials (Furie et al.), suggesting the overall drug effect was similar over 52 weeks (Figure 1). In addition, the effect of belimumab on several lupus biomarkers were similar in both children and adults (Table 2).
Figure 1. SRI-4 Response in Adult vs. Pediatric Belimumab Studies.
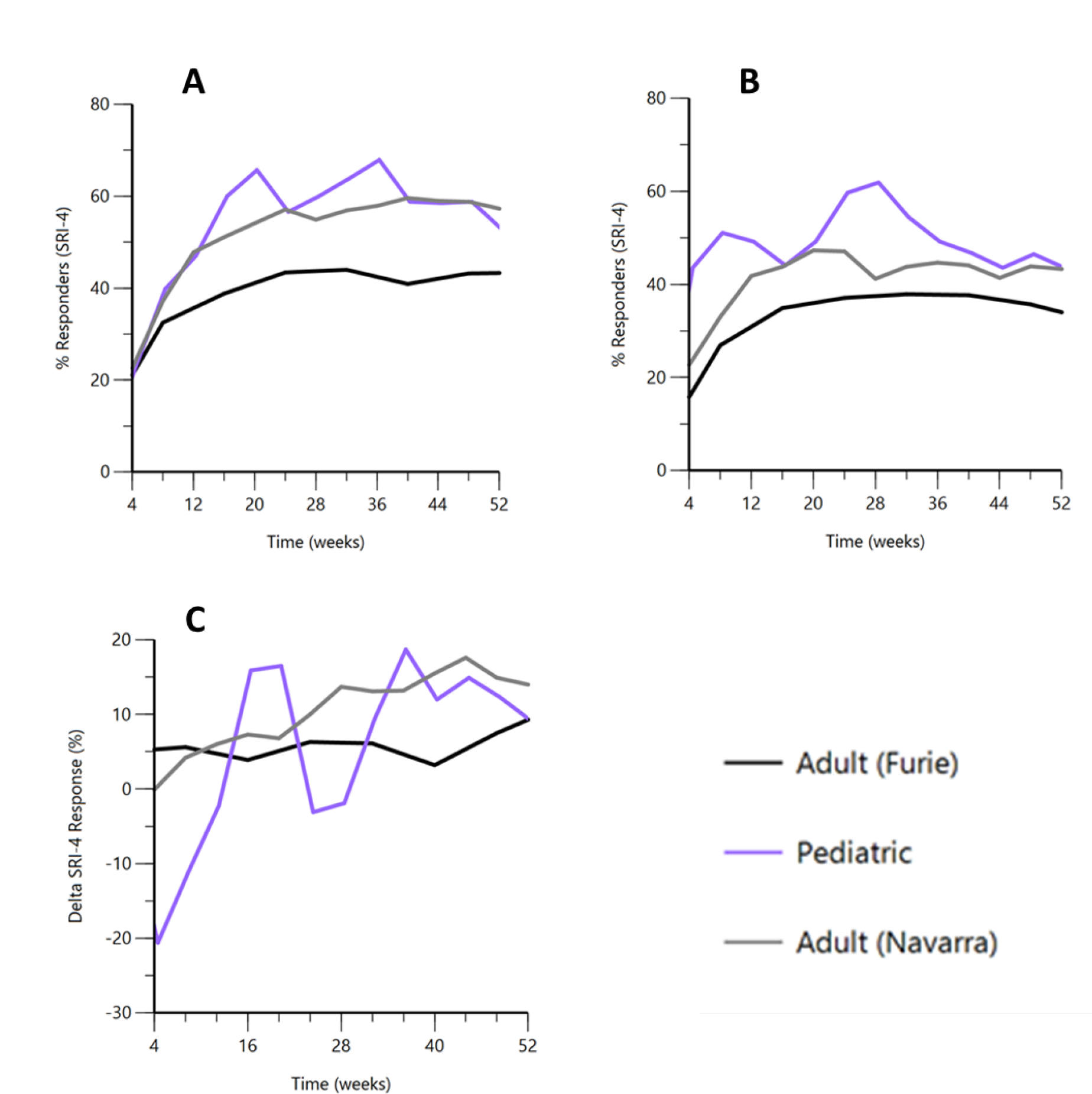
A: Overall SRI-4 Response for Belimumab 10 mg/kg IV; B: Overall SRI-4 Response for Placebo; C: Difference in SRI-4 for Belimumab 10 mg/kg vs Placebo.
SRI-4, Systemic Lupus Erythematosus Responder Index-4
Table 1.
Area Under the Time-Effect Curve for Change in SRI-4 (%), Belimumab 10 mg/kg IV
| Maximum Change in SRI-4 | Minimum Change in SRI-4 | AUEC Above Baselinea | AUEC Below Baselinea | Net AUECb | Time above Baselinea (%) | |
|---|---|---|---|---|---|---|
| Adult22 (Furie) | 9.3 | 3.2 | 274.4 | 0 | 274.4 | 100% |
| Adult23 (Navarra) | 17.6 | −0.1 | 517.0 | 0.2 | 516.8 | 92.1% |
| Pediatric21 (Brunner) | 18.7 | −20.6 | 404.6 | 146.4 | 258.2 | 65.4% |
Baseline represents the SRI-4 response prior to treatment; the AUEC is the area under the SRI effect curve for which the change in SRI-4 response is above or below baseline, respectively.
Net effect is the AUEC above baseline minus AUEC below baseline.
AUEC, area under the time-effect curve; IV, intravenous; SRI-4, Systemic Lupus Erythematosus Responder Index-4
Table 2.
Median Percent Change from Baseline SLE Biomarkers, Belimumab 10 mg/kg IV
| DNA Antibody | Complement C4 | CD20+ | |
|---|---|---|---|
| Adult22 (Furie)a | −49.5 | +51.9 | −54.8 |
| Adult23 (Navarra) | –37.6 | +30.4 | -- |
| Pediatric21 (Brunner) | −44.9 | +50 | −65.8 |
Biomarkers assessed at week 76, all others at week 52
IV, intravenous; SLE, systemic lupus erythematosus
The FDA clinical pharmacology review of pediatric belimumab noted that pediatric exposures at 10 mg/kg were overall similar to adults (average concentration at steady-state 92–112 ug/mL in children vs. 100 ug/mL in adults).24 Additionally, there was no clear dose-response relationship for belimumab in adults, and the PD response in children was similar to adults.24
In summary, clinical trial evidence supports the similarity of exposure and response to belimumab 10 mg/kg in adults and children ≥5 years with SLE.
Mycophenolate
Included Studies
For SLE with or without nephritis where generalized lupus disease activity was the primary outcome, Chen et al. conducted a retrospective cohort study in 67 children with SLE aged 4–18 years. The proportion of children with a history of lupus nephritis was not provided, but there was no significant difference in creatinine clearance between those with SLE Disease Activity Index (SLEDAI) scores <6 and ≥6.25 Zahr et al. conducted a prospective study in 71 adult patients with SLE, 61 of whom had a history of lupus nephritis.26 In both studies, disease activity was measured using the same instrument (SLEDAI) at a single, cross-sectional time point and a cutoff of ≥6 was selected to indicate active disease. The mycophenolate dosing in children was 20–40 mg/kg/day twice daily with maximum 1.5 grams/day; in adults, dosing varied from 0.5 grams to 1.5 grams twice daily (Table S1). However, there were notable differences in the assessment of exposure between the studies; mycophenolic acid was measured using different assays and although both studies used a Bayesian estimator to determine the 12-hour AUC (area under the curve) for drug exposure, sampling times differed slightly.
For studies specifically in lupus nephritis, Sundel et al.27 and Dall’Era et al.28 analyzed data from a single trial (Aspreva Lupus Management Study [ALMS; NCT00377637]) and evaluated renal response at week 24 for mycopenolate mofetil in 10 adolescents compared to 175 adults. Renal response was defined as decrease in the urine protein:creatinine ratio to <3 (if nephrotic at baseline) or by ≥50% (if not nephrotic); and stabilization (+/−25%) or improvement in serum creatinine. The study did not include exposure information but reported the effect of dosage (median 2.6 g/day in adults and 2.3 to 3.0 g/day in children during induction) on renal response (see clinical outcomes below). Godron-Dubrasquet et al.29 and Lertdumrongluk et al.30 conducted studies of children or adults (respectively) with lupus nephritis and compared mycophenolic acid AUC and renal response at 6 months. There were slight differences in the included population, and although the definition used for renal outcome in each study was similar, there were differences in the requirements for inactive urinary sediment and degree of proteinuria (Table S1). Additionally, there were differences in the assays and sampling times, and minor differences in the subtypes of lupus nephritis.
PD Model Development (SLE with or without nephritis)
In general, simultaneously estimating E0, Imax, and AUC50 for the pediatric (Chen) and adult (Zahr) models was difficult due in part to high shrinkage on multiple PD parameters. Model performance for the adult data was greatly improved by fixing Imax and E0 to 105, the maximum possible SLEDAI score, consistent with the approach by Chen et al.25 However, unlike previous models,25 the data was better characterized using an additive error model. When using an additive error model, the pediatric models performed similarly across each tested approach (see methods). Accordingly, to facilitate comparisons across each population, the final PD model was chosen as an Imax model with additive error, fixing Imax and E0 to 105, and estimating between subject variability on AUC50.
As a quality check on our data extraction, we used the published model from Chen et al. and attempted to reproduce their model estimates using the data we extracted from their publication. When refitting the data, similar estimates were observed: AUC50 2.2 vs. 1.97 ug*hr/mL, relative standard error 10.9 vs 14.3%, proportional error 67 vs. 76.7%, and 46.7 vs. 31.6% between subject variability on AUC50.
Results from the PD Model
There was high similarity in the exposure-response curves for mycophenolic acid AUC and SLEDAI between adults and children (Figure 2). Table S3 lists the final parameters for the adult, pediatric, and combined PD models. A very high inter-individual variability for AUC50 in both adults and children (93.8% and 79.4% relative standard error, respectively) was estimated. In general, the estimates for AUC50 were numerically less for adults compared to children, suggesting mycophenolate may be slightly more potent for adults. Alternatively, differences in drug potency may reflect potential differences in the proportion of patients with lupus nephritis. However, the difference in AUC50 estimates were small compared to the 95% confidence intervals for AUC50.
Figure 2. Mycophenolate Exposure and Lupus Disease Activity.
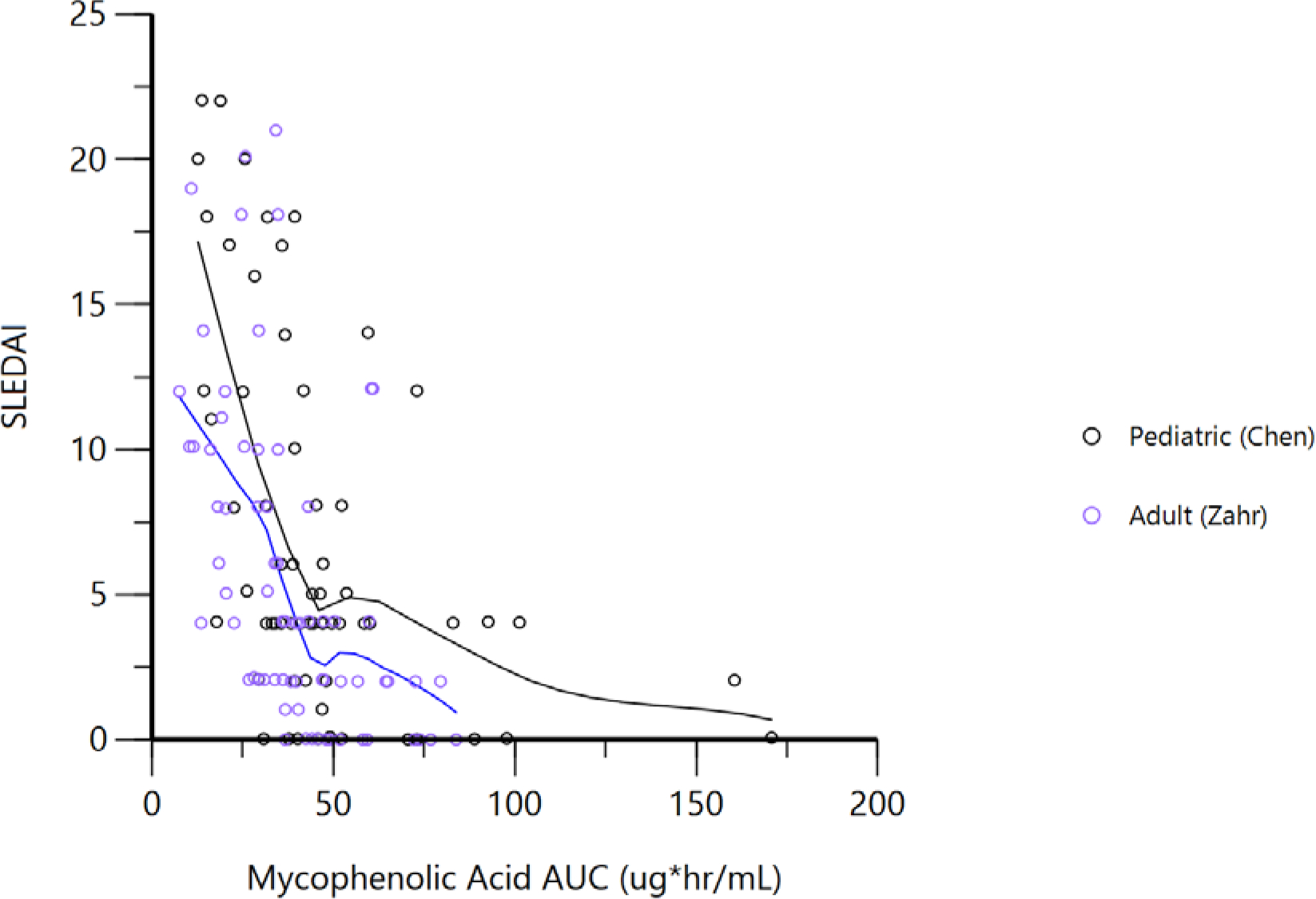
Solid black and blue lines represent a Locally Weighted Scatterplot Smoothing (LOESS) fit.
AUC, area under the curve; SLEDAI, Systemic Lupus Erythematosus Disease Activity Index. AUC represents the 12-hour AUC.
When adding age group (e.g., adult vs pediatric) as a covariate on AUC50, the objective function value was reduced by 6.1 points, which is significant at a p=0.05, but not p=0.01. Using the full model (combined adult and pediatric data with a covariate effect for children as an age group on AUC50), the estimated AUC50 was 1.23 ug*hr/mL for adults and 1.88 ug*hr/mL for children. However, including the population as a covariate only explained 2.5% of the between-patient variability in the AUC50, suggesting that the vast majority of variability in drug potency is not related to the population being adult or pediatric. Visual predictive checks for the final model are shown in Figure S2; additional diagnostic plots are provided in Figure S3.
Clinical Outcomes
SLE with and without nephritis:
Mycophenolic acid exposure is higher in patients with inactive SLE when measured cross-sectionally (Figure 3, Table S4). However, there were no significant differences in mycophenolic acid exposure between adults and children with and without active SLE, although the proportion of patients who may have had concomitant, active lupus nephritis was unclear from the available literature.
Figure 3. Relationship between Mycophenolic Acid Exposure and SLE Disease Activity.
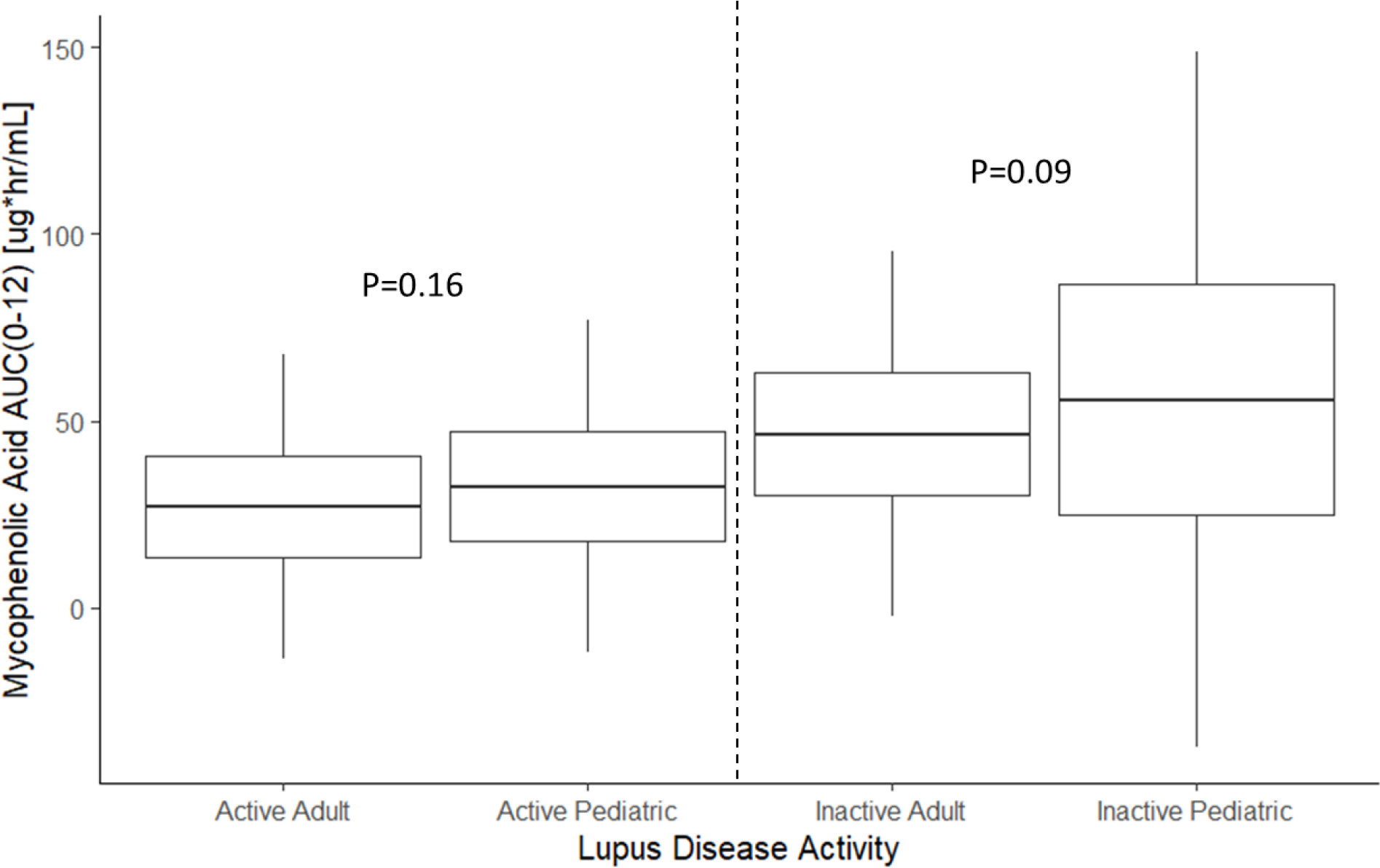
Boxes are mean +/− 1 standard deviation (SD). Whiskers are mean +/− 3x the SD. Active SLE defined a SLEDAI ≥6; Inactive SLE defined as SLEDAI <6.
AUC, area under the curve; SLE, systemic lupus erythematosus; SLEDAI, Systemic Lupus Erythematosus Disease Activity Index
Lupus nephritis:
In a study of mycophenolate where both adolescents and adults with lupus nephritis were enrolled (ALMS), the number of responders was numerically higher for adolescents (7/10, 70%) than adults (97/175, 55.4%).27 In addition, age ≤20 years was not a significant predictor of renal response at 6 months.28 Studies demonstrated that mycophenolic acid exposure is higher in patients with renal response to treatment (Table S5); however, there was overlapping mycophenolic acid exposures between adults and children with and without renal responses.
Cyclophosphamide
Included Studies
For lupus nephritis, in the ALMS study, Sundel evaluted renal response at week 24 in lupus nephritis for IV cyclophosphamide in 14 adolescents and 171 adults using the same treatment protocol and outcome definition for both populations.27 Exposure data was not available and only the dose-response relationship was analyzed. Cyclophosphamide was administered at a dosage of 0.5–1.0 gram/m2 IV monthly.
Clinical Outcomes
Lupus nephritis:
In a study where both adolescents and adults with lupus nephritis were enrolled, the renal response to intravenous cyclophosphamide was similar between adolescents and adults; 8/14 (57.1%) vs. 90/171 (52.6%), respectively.
Azathioprine
Included studies
For lupus nephritis, in the ALMS study, Sundel compared the maintenance of renal remission in lupus nephritis for azathioprine in 8 adolescents and 103 adults using the same treatment protocol and outcome definition for both populations.27 Treatment failure was defined as time to death, end-stage renal disease, doubling of serum creatinine, renal flare, the need for rescue treatment or other LN exacerbation. 27 Exposure data was not available and only the dose-response relationship was analyzed.
Clinical Outcomes
Lupus nephritis:
In a study where both adolescents and adults with lupus nephritis were enrolled, the percentage maintaining renal response to azathioprine was numerically lower for adolescents compared to adults, with 5/8 (62.5%) vs. 30/103 (29.1%) patients experiencing treatment failure, respectively.
Hydroxychloroquine
Included Studies
A 2021 systematic review and meta-analysis identified 16 studies that compared hydroxychloroquine drug concentrations with response,31 yet we only found 1 peer-reviewed, pediatric exposure-response study. The retrospective pediatric study (Zahr et al.)20 compared whole blood concentrations in 55 children with a mean (standard deviation) age of 15 (2) years with and without active SLE as measured by the SLEDAI at several time points per patient. The timing of sample and outcome assessment and handling of multiple SLEDAI scores per patient was not apparent. Correspondingly, we identified one adult study (Costedoat-Chalumeau)32 which also reported whole blood concentrations of hydroxychloroquine and the same outcome categorization of active disease as the pediatric study with a SLEDAI score of ≥6. The study populations were overall similar, but with slightly higher baseline disease activity in children, and more frequent concomitant immunomodulatory use in children. Additionally, a small number of adult patients (10/143) in the adult study had lupus nephritis at baseline.
Exposure-response
SLE with and without nephritis:
There was a wide range in hydroxychloroquine concentrations observed in both the adult and pediatric studies. In both populations, higher hydroxychloroquine concentrations were observed in patients without active SLE, consistent with studies suggesting higher drug concentrations are predictors of lower disease activity. 20,32 In addition, there was significant overlap in whole blood concentrations in adults and children with active SLE, and significant overlap in concentrations between adults and children without active SLE (Figure 4, Table S6), although formal hypothesis testing could not be conducted, due to the lack of reported sample size. Nevertheless, the exposure-response relationship appeared similar in both adults and children.
Figure 4. Hydroxychloroquine Exposure and SLE Disease Activity.
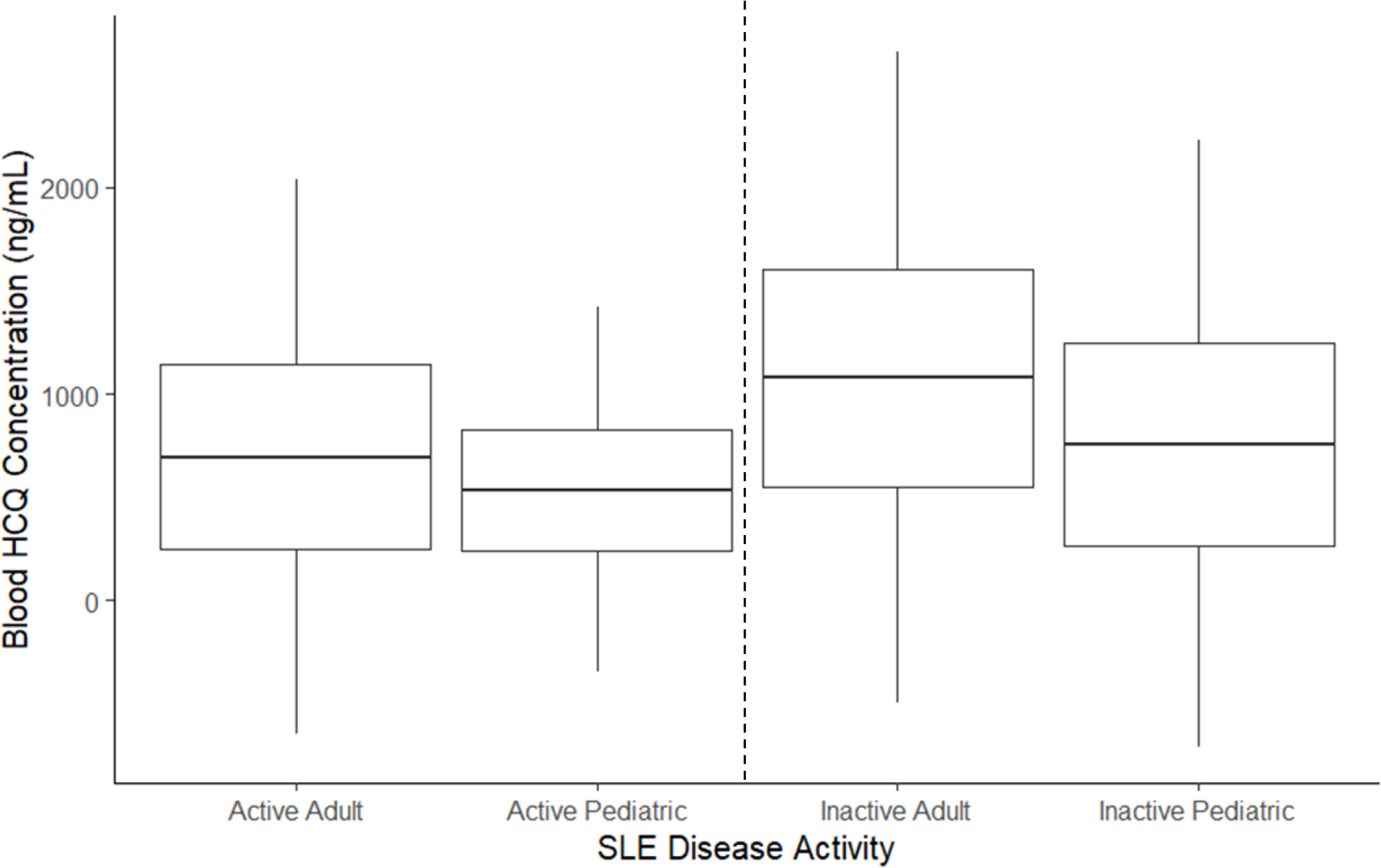
Hypothesis testing not conducted due to lack of sample size for the pediatric study.
Boxes are mean +/− 1 standard deviation (SD). Whiskers are mean +/− 3x the SD. Active SLE defined a SLEDAI ≥6; Inactive SLE defined as SLEDAI <6.
SLE, systemic lupus erythematosus; SLEDAI, Systemic Lupus Erythematosus Disease Activity Index
Rituximab
Included Studies
SLE with and without nephritis:
Mahmoud et al. published a systematic review of rituximab in pediatric SLE in 2017 and identified 12 studies.33 Not including two abstracts, only 1/10 studies were prospective. Unfortunately, there was great heterogeneity across pediatric studies, including: 1) rituximab dosing; 2) outcome measures; and 3) baseline characteristics such as concomitant medications and lupus manifestations. In addition, several studies reported multiple courses of rituximab and the effect of a single rituximab course could not always be determined. Of the available pediatric studies, we only identified three studies where the available outcome measures, populations, and rituximab dosing allowed broad comparison to the two largest adult trials (Table S1).
Exposure-Response
SLE with or without nephritis:
Of the 6 studies analyzed, all but 1 included patients with lupus nephritis (Table 3), although the percentage of patients with nephritis in each study varied. A graphical analysis of B-cell depletion over time from 1 study without lupus nephritis (adult) and 2 studies including lupus nephritis (1 adult, 1 pediatric) suggest that B-cell depletion in children was not as pronounced as in the adult trials (Figure S4); however, this finding could be due to the comparatively lower dosing that children received. Specifically, children in the Nwobi study received rituximab weekly for two to four doses at an initial dosage of 188 mg/m2 and subsequent doses of 375 mg/m2. Adults in the EXPLORER and LUNAR trials initially received 1,000 mg of rituximab two weeks apart; and assuming a range of adult body surface areas (BSA) between 1.73–2.04,40 this equates to an average of 490–578 mg/m2/dose.
Table 3.
Change in Systemic Lupus Erythematosus Biomarkers after Rituximab
| Lupus Nephritis Included | Time Biomarker Assessed: (Months) | Rituximab Dosing | DNA Antibody (Ratio endpoint:baseline) | Complement C3 (mg/dL) (Mean or Median Change from Baseline) | Complement C4 (mg/dL) (Mean or Median Change from Baseline) | |
|---|---|---|---|---|---|---|
| Adult34 (Merrill) | No | 12 | 1,000 mg on days 1, 15, 168, and 182 | 0.24 | - | - |
| Adult35 (Rovin) | Yes | 12 | 1,000 mg on days 1, 15, 168, and 182. | 0.31 | +37.5 | +9.9 |
| Pediatric36 (Watson) | Yes | 2.5 (IQR 1.6–4.3) | 750mg/m2 × 2 doses, approximately 14 days apart; 73% received IV cyclophosphamide with 1st course; 30% of cohort received >1 course of rituximab | 0.32 | +6 | +6 |
| Pediatric37 (Nwobi) | Yes | 2, 4, 6 | Weekly for 2–4 doses. Initial dosage 188 mg/m2, subsequent doses 375 mg/m2 | 0.18 | +24.6 | +3.4 |
| Pediatric38 (Podolskaya) | Yes | 12 | Most 2 infusions of 750 mg/m2, 2 weeks apart | 0.39 | +57.1 | +10.3 |
| Pediatric39 (Tambralli) | Yes | 12 | Typically 750mg/m2 × 2 doses (maximum 1g), approximately 14 days apart | 0.1 | +44.6 | +14 |
IQR, interquartile range; IV, intravenous
The change in laboratory biomarkers of lupus disease activity in adults and children after receiving rituximab is noted in Table 3. In general, the decrease in DNA antibody, and increases in C3 and C4, were similar across adult and pediatric studies that included patients with lupus nephritis. For example, the ratio of DNA antibody (12 month:baseline) ranged from 0.1–0.39 in children and was 0.31 in adults. Similarly, the mean or median change at 12 month compared to baseline for C3 increased 44.6–57.1 for children and 37.5 for adults; for C4 there was an increase of 10.3–14 for children and 9.9 for adults. However, there were important differences in treatment regimens and study populations (Supplemental Table S1). For the one adult study without lupus nephritis, there was no comparable pediatric study. Nevertheless, the change in DNA antibody was similar in adults without lupus nephritis compared to the studies including lupus nephritis in both adults and children.
Discussion
A quantitative and descriptive exposure-response analysis of frequently used therapeutics for SLE was conducted to determine whether there was similarity in response to therapy to support the extrapolation of adult efficacy data to children. Overall, both children ≥ 5 years and adults with SLE responded similarly to treatment across several different biologic and non-biologic drug classes. For products where there is limited data available, there may be additional considerations to partial extrapolation or other borrowing approaches, based on the specific drug and available information.
The extrapolation of efficacy from adults to pediatric patients was described in the final rule on pediatric labeling of 1994 as a means of providing more complete information about the use of a drug in the pediatric population in FDA labels when the course of disease and drug effects are sufficiently similar in both populations.41 Since that time, the ICH E11 guidance on Investigation of Medicinal Products in the Pediatric Population42 was revised to describe the extensive analysis needed to show similarity of disease and response to therapy between the pediatric and the reference population.43 While histopathologic, pathobiologic, and disease progression criteria are critical to show disease similarity, the exposure-response analysis can provide evidence of similar drug effects and response to therapy.44 When there is strong evidence in favor of disease similarity and response to treatment, exposure matching may support efficacy extrapolation.45 Conversely, when there is weak or uncertain evidence supporting similarity, additional pediatric efficacy trials may be necessary.
Demonstrating drug effectiveness usually requires two adequate and well-controlled clinical trials, although there are some situations where substantial evidence can be provided by one adequate and well controlled trial along with confirmatory evidence.1 Extrapolating efficacy from adults to children can reduce the number and complexity of required pediatric clinical trials,1 increasing study efficiency, and potentially limiting the exposure to placebo for pediatric subjects. This is particularly relevant to the rheumatology community, where a survey found that more than 70% of pediatric rheumatologists preferred an open-label PK/PD study design over a traditional phase 3 efficacy study in children with SLE.12 The high preference for open-label PK/PD studies reflect the importance of limiting placebo use or ineffective standard of care treatment in chronic, systemic illnesses like SLE where permanent organ damage can occur with active disease. Additionally, traditional phase 3 efficacy studies in children with SLE are extremely challenging to conduct due to disease heterogeneity, small sample sizes, ethical considerations, and other feasibility concerns.
For belimumab, several key findings were observed. First, children experienced a higher cumulative placebo response compared to adults, with a net positive drug effect for only two-thirds of the 52-week study. A high placebo response in children has been observed with treatment for other rheumatic diseases in children,46 and may be one reason for clinical trials failing to show a positive drug effect. However, FDA analyses of pediatric placebo-controlled trials across different therapeutic areas suggests differences in the placebo response rate between adults and children are most often associated with differences in study designs and endpoints.47 Second, despite a higher placebo response, the treatment effect of belimumab was similar between adults and children, with the net change in SRI-4 responders at week 52 (primary endpoint) being nearly identical between the pediatric trial and one of two adult trials. Additionally, adults and children experienced similar changes in disease activity biomarkers. Although the maximum percentage of SRI-4 responders to belimumab was higher in children compared to both adult studies, this could be due to inherent variability in the SRI-4 measurement and does not imply a difference in treatment response as the overall time that the change in SRI-4 AUEC was above 0 (net AUEC) was similar between the pediatric trial and one of two adult trials. Accordingly, the results from our analysis are consistent with the FDA’s decision to approve belimumab for pediatric lupus in part on a Bayesian analysis where efficacy data was borrowed from adults.7
For mycophenolate, a PD model was developed to quantify the exposure-response relationship between adults and children. Although different estimates in the potency for mycophenolic acid on lupus disease activity (AUC50) was observed, these differences were small and likely not clinically relevant. The differences in AUC50 may also be explained by different assays used to measure drug concentration, differences in sampling times and the calculation of AUC, and the underlying population. Due possibly to sparse data, the best PD model performance was obtained by fixing E0 and Imax to the maximum possible SLEDAI score (105); this assumption could result in biased AUC50 estimates- but would not obscure differences between children and adults. Furthermore, due to lack of baseline SLEDAI data, the analysis could not evaluate the change in SLEDAI score with treatment, and the number of patients who may have had active lupus nephritis was unclear. Because the published studies did not have many patients with very severe SLE, it is possible that the true drug potency is underestimated. Conversely, there was no statistically significant difference in mycophenolic acid AUC between adults and children with and without active SLE. In addition, similar treatment response to mycophenolate was observed in a clinical trial in which both adolescents and adults with lupus nephritis were enrolled and treated under the same protocol, although sample sizes in children were small.27,28 In summary, limited observational data supports a similar exposure-response relationship between mycophenolate and generalized SLE disease activity in adults and children, whereas clinical trial data with small sample sizes suggests a similar exposure-response relationship specifically for lupus nephritis.
For the other drugs analyzed (rituximab, hydroxychloroquine, azathioprine, and cyclophosphamide), there was not sufficient publicly available data to quantitatively evaluate the exposure-response relationship between adults and children. Nevertheless, some general observations can be made: 1) the change in SLE disease activity biomarkers were similar across adults and children receiving rituximab, most of whom had lupus nephritis; 2) whole blood concentrations of hydroxychloroquine overlapped in adults and children both with and without active SLE; and 3) the renal response to cyclophosphamide in lupus nephritis was numerically higher in adolescents compared to adults. Conversely, the only drug where there was a notable difference in response was the maintenance of renal remission with azathioprine in lupus nephritis, for which the small sample size (n=8) limits definitive conclusions.
There are several limitations to our study. The quality of much of the available data for many of the drugs was low, with few efficacy trials of similar design in both children and adults to support comparison. While prospective clinical trials in SLE are usually conducted separately in patients with and without lupus nephritis due to different endpoints, our study found that most observational studies included both patients with and without active nephritis, making it difficult to compare the exposure-response relationship across studies. The majority of pediatric studies enrolled older children and adolescents, potentially limiting conclusions about similarity of exposure-response in children <5 and perhaps up to 10 years of age. Drug/metabolite concentration may (e.g., mycophenolate) or may not (e.g., belimumab) have obvious exposure-response relationship for efficacy, particularly if there are limited dose levels and a narrow range of exposures. For belimumab, the high dosages administered produces exposures already at the upper end of the exposure-response curve. 48 Additionally, the methods were limited to including studies with sufficient publicly available data to compare exposures and outcomes between adults and children. Also, data was extracted from published manuscripts without access to the original datasets. Data extraction is increasingly used in the scientific literature for meta-analyses,49,50 but error can be introduced depending on the type and quality of published figures and tables. For example, only 63 out of 67 data points could be extracted from the pediatric PK/PD study of mycophenolate, due to overlapping data points.25 Despite this potential limitation, the extraction software used in this study has been shown to have very high inter-coder reliability (r=0.997) and validity compared to known values (r=0.989), with up to 10% of values only having a small degree of discrepancy.51 In addition, a sensitivity analysis was conducted whereby the PD model in the pediatric mycophenolate study was reproduced and obtained AUC50 estimates very similar to the original publication (2.2 vs. 1.97 ug*hr/mL).25 Therefore, the study conclusions are likely not impacted by using publicly available, digitized data. Several studies also evaluated the exposure-response relationship at a single time point (e.g., cross-sectional), and the relationship is likely better characterized using a change in outcome (e.g., SLEDAI) as a function of drug exposure. Finally, SLE is a heterogeneous disease and some studies included patients with and without lupus nephritis or other severe disease manifestations, making evaluation of the exposure-response relationship difficult.
Conclusions
Out of the six drugs assessed in this study, quality evidence suggests that the response to belimumab is similar between adults and children with SLE, and the response to mycophenolate is similar between adult and children with lupus nephritis. While the available data was too limited to quantitatively assess the exposure-response relationship for other therapeutics, the data analyzed herein suggests that adults and children with SLE and lupus nephritis may respond similarly to treatment across several different drug classes. Accordingly, adult SLE data should be leveraged to guide the pediatric drug development program and identify areas with residual uncertainty of a drug’s effectiveness or safety in children. The degree to which efficacy extrapolation can reduce clinical trial requirements for a new drug product in pediatric SLE should be individualized, depending in part on the drug’s mechanism of action and the similarity in disease manifestations between children and adults.
Supplementary Material
Acknowledgments:
We would like to thank Erin Campbell, MS, for manuscript review. Ms. Campbell did not receive compensation for her contributions, apart from her employment at Duke University.
Footnotes
Conflict of interest disclosures: SJB receives support from the National Institutes of Health, the US Food and Drug Administration, the Patient-Centered Outcomes Research Institute, the Rheumatology Research Foundation’s Scientist Development Award, the Childhood Arthritis and Rheumatology Research Alliance, Purdue Pharma, and consulting for UCB. LES receives support from CARRA and has research grants from PCORI, BMS and consults for UCB, Sanofi. DG receives research support from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (5R01HD096435-04 and 1R01HD102949-01A1) and from Nabriva Therapeutics through a contract with the University of North Carolina at Chapel Hill. In addition, D.G. serves as a consultant for Tellus Therapeutics, focusing on neonatal drug development. CPH receives salary support for research from National Institute for Child Health and Human Development (NICHD) (R13HD102136; RL1HD107784; R01HD106588), the National Heart Lung and Blood Institute (NHLBI) (R61/R33HL147833), the US Food and Drug Administration (R01-FD006099, PI Laughon; and U18-FD006298), the U.S. government for his work in pediatric clinical pharmacology (Government Contract HHSN275201800003I, PI: Benjamin under the Best Pharmaceuticals for Children Act), the non-profit Burrhoughs Wellcome Fund, and other sponsors for drug development in adults and children (https://dcri.org/about-us/conflict-of-interest/
Disclaimer: The opinions expressed in this article are those of the authors and should not be interpreted as the position of the U.S. Food and Drug Administration.
Data Accessibility:
All data used in this study were extracted from the literature and are available at the respective references.
References
- 1.Dunne J, Rodriguez WJ, Murphy MD, et al. Extrapolation of adult data and other data in pediatric drug-development programs. Pediatrics. 2011;128(5):e1242–1249. [DOI] [PubMed] [Google Scholar]
- 2.Product Label. Belimumab injection. GlaxoSmithKline LP, PA. March 11, 2021. [Google Scholar]
- 3.Livingston B, Bonner A, Pope J. Differences in clinical manifestations between childhood-onset lupus and adult-onset lupus: a meta-analysis. Lupus. 2011;20(13):1345–1355. [DOI] [PubMed] [Google Scholar]
- 4.United States Food and Drug Administration (FDA). Pediatric science and research activities. FDA web site. https://www.fda.gov/science-research/pediatrics/pediatric-science-and-research-activities. Updated October 15, 2021. Accessed October 25, 2021. [Google Scholar]
- 5.United States Food and Drug Administration (FDA). Guidance document: general clinical pharmacology considerations for pediatric studies for drugs and biological products. December 2014. FDA web site. https://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM425885.pdf. Accessed October 25, 2021. [Google Scholar]
- 6.European Medicines Agency (EMA). Reflection paper on the use of extrapolation in the development of medicines for paediatrics. EMA web site. https://www.ema.europa.eu/en/documents/scientific-guideline/adopted-reflection-paper-use-extrapolation-development-medicines-paediatrics-revision-1_en.pdf. Updated October 7, 2018. Accessed April 19, 2022. [Google Scholar]
- 7.Pottackal G TJ, Neuner R, Rothwell R, Levin G, Nie L, Niu J, Marathe A, Nikolov N. Application of Bayesian statistics to support approval of intravenous belimumab in children with systemic lupus erythematosus in the United States [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/application-of-bayesian-statistics-to-support-approval-of-intravenous-belimumab-in-children-with-systemic-lupus-erythematosus-in-the-united-states/. Accessed October 25, 2021. [Google Scholar]
- 8.Bundhun PK, Kumari A, Huang F. Differences in clinical features observed between childhood-onset versus adult-onset systemic lupus erythematosus: a systematic review and meta-analysis. Medicine (Baltimore). 2017;96(37):e8086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tsokos GC, Lo MS, Reis PC, Sullivan KE. New insights into the immunopathogenesis of systemic lupus erythematosus. Nat Rev Rheumatol. 2016;12(12):716–730. [DOI] [PubMed] [Google Scholar]
- 10.Wright TB, Punaro M. Paediatric systemic lupus erythematosus: insights from translational research. Rheumatology (Oxford). 2017;56(suppl_1):i24–i31. [DOI] [PubMed] [Google Scholar]
- 11.Tao JJ, Hiraki LT, Levy DM, Silverman ED. Comparison of sensitivities of American College of Rheumatology and Systemic Lupus International Collaborating Clinics classification criteria in childhood-onset systemic lupus erythematosus. J Rheumatol. 2019;46(7):731–738. [DOI] [PubMed] [Google Scholar]
- 12.Brunner HI, Martini A, Lovell DJ, Ruperto N. Clinical trials in children and adolescents with systemic lupus erythematosus: methodological aspects, regulatory landscape and future opportunities. Ann Rheum Dis. 2019;78(2):162–170. [DOI] [PubMed] [Google Scholar]
- 13.Mina R, Brunner HI. Update on differences between childhood-onset and adult-onset systemic lupus erythematosus. Arthritis Res Ther. 2013;15(4):218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Product Label. Mycophenolate Mofetil. West-Ward Pharmaceuticals Corporation. Eatontown NJ, 2021. [Google Scholar]
- 15.Udupa A, Leverenz D, Balevic SJ, Sadun RE, Tarrant TK, Rogers JL. Hydroxychloroquine and COVID-19: a rheumatologist’s take on the lessons learned. Curr Allergy Asthma Rep. 2021;21(1):5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Product Label. Cyclophosphamide capsule. Cipla USA IW, NJ. March 11, 2021. [Google Scholar]
- 17.Product Label. Azathioprine tablet. Salix Pharmaceuticals. Bridgewater NJ. July 12, 2021. [Google Scholar]
- 18.Product Label. Rituximab injection. Biogen and Genentech, Inc. June 30, 2021. [Google Scholar]
- 19.Certara. QRPEM: A New Standard of Accuracy, Precision and Efficiency in NLME Population PK/PD Methods. Available at: https://www.certara.com/app/uploads/2020/06/WP_QRPEMPhoenixNLMEAlgorithm.pdf. Accessed June 29 2022.
- 20.Zahr N, Urien S, Funck-Brentano C, et al. Evaluation of hydroxychloroquine blood concentrations and effects in childhood-onset systemic lupus erythematosus. Pharmaceuticals (Basel). 2021;14(3):273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brunner HI, Abud-Mendoza C, Viola DO, et al. Safety and efficacy of intravenous belimumab in children with systemic lupus erythematosus: results from a randomised, placebo-controlled trial. Ann Rheum Dis. 2020;79(10):1340–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Furie R, Petri M, Zamani O, et al. A phase III, randomized, placebo-controlled study of belimumab, a monoclonal antibody that inhibits B lymphocyte stimulator, in patients with systemic lupus erythematosus. Arthritis Rheum. 2011;63(12):3918–3930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Navarra SV, Guzman RM, Gallacher AE, et al. Efficacy and safety of belimumab in patients with active systemic lupus erythematosus: a randomised, placebo-controlled, phase 3 trial. Lancet. 2011;377(9767):721–731. [DOI] [PubMed] [Google Scholar]
- 24.United States Food and Drug Administration (FDA). BLA 125370/s-064 and BLA 761043/s-007 Multi-disciplinary review and evaluation: Benlysta® (belimumab) for intravenous infusion in children 5 to 17 years of age with SLE. FDA web site. https://www.fda.gov/media/127912/download. Version October 12, 2018. Accessed October 25, 2021. [Google Scholar]
- 25.Chen Y, Sun L, Xu H, et al. PK/PD study of mycophenolate mofetil in children with systemic lupus erythematosus to inform model-based precision dosing. Front Pharmacol. 2020;11:605060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zahr N, Arnaud L, Marquet P, et al. Mycophenolic acid area under the curve correlates with disease activity in lupus patients treated with mycophenolate mofetil. Arthritis Rheum. 2010;62(7):2047–2054. [DOI] [PubMed] [Google Scholar]
- 27.Sundel R, Solomons N, Lisk L, Aspreva Lupus Management Study (ALMS) Group. Efficacy of mycophenolate mofetil in adolescent patients with lupus nephritis: evidence from a two-phase, prospective randomized trial. Lupus. 2012;21(13):1433–1443. [DOI] [PubMed] [Google Scholar]
- 28.Dall’Era M, Stone D, Levesque V, Cisternas M, Wofsy D. Identification of biomarkers that predict response to treatment of lupus nephritis with mycophenolate mofetil or pulse cyclophosphamide. Arthritis Care Res (Hoboken). 2011;63(3):351–357. [DOI] [PubMed] [Google Scholar]
- 29.Godron-Dubrasquet A, Woillard JB, Decramer S, et al. Mycophenolic acid area under the concentration-time curve is associated with therapeutic response in childhood-onset lupus nephritis. Pediatr Nephrol. 2021;36(2):341–347. [DOI] [PubMed] [Google Scholar]
- 30.Lertdumrongluk P, Somparn P, Kittanamongkolchai W, Traitanon O, Vadcharavivad S, Avihingsanon Y. Pharmacokinetics of mycophenolic acid in severe lupus nephritis. Kidney Int. 2010;78(4):389–395. [DOI] [PubMed] [Google Scholar]
- 31.Garg S, Unnithan R, Hansen KE, Costedoat-Chalumeau N, Bartels CM. Clinical significance of monitoring hydroxychloroquine levels in patients with systemic lupus erythematosus: a systematic review and meta-analysis. Arthritis Care Res (Hoboken). 2021;73(5):707–716. [DOI] [PubMed] [Google Scholar]
- 32.Costedoat-Chalumeau N, Amoura Z, Hulot JS, et al. Low blood concentration of hydroxychloroquine is a marker for and predictor of disease exacerbations in patients with systemic lupus erythematosus. Arthritis Rheum. 2006;54(10):3284–3290. [DOI] [PubMed] [Google Scholar]
- 33.Mahmoud I, Jellouli M, Boukhris I, et al. Efficacy and safety of rituximab in the management of pediatric systemic lupus erythematosus: a systematic review. J Pediatr. 2017;187:213–219.e2. [DOI] [PubMed] [Google Scholar]
- 34.Merrill JT, Neuwelt CM, Wallace DJ, et al. Efficacy and safety of rituximab in moderately-to-severely active systemic lupus erythematosus: the randomized, double-blind, phase II/III systemic lupus erythematosus evaluation of rituximab trial. Arthritis Rheum. 2010;62(1):222–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rovin BH, Furie R, Latinis K, et al. Efficacy and safety of rituximab in patients with active proliferative lupus nephritis: the Lupus Nephritis Assessment with Rituximab study. Arthritis Rheum. 2012;64(4):1215–1226. [DOI] [PubMed] [Google Scholar]
- 36.Watson L, Beresford MW, Maynes C, et al. The indications, efficacy and adverse events of rituximab in a large cohort of patients with juvenile-onset SLE. Lupus. 2015;24(1):10–17. [DOI] [PubMed] [Google Scholar]
- 37.Nwobi O, Abitbol CL, Chandar J, Seeherunvong W, Zilleruelo G. Rituximab therapy for juvenile-onset systemic lupus erythematosus. Pediatr Nephrol. 2008;23(3):413–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Podolskaya A, Stadermann M, Pilkington C, Marks SD, Tullus K. B cell depletion therapy for 19 patients with refractory systemic lupus erythematosus. Arch Dis Child. 2008;93(5):401–406. [DOI] [PubMed] [Google Scholar]
- 39.Tambralli A, Beukelman T, Cron RQ, Stoll ML. Safety and efficacy of rituximab in childhood-onset systemic lupus erythematosus and other rheumatic diseases. J Rheumatol. 2015;42(3):541–546. [DOI] [PubMed] [Google Scholar]
- 40.Verbraecken J, Van de Heyning P, De Backer W, Van Gaal L. Body surface area in normal-weight, overweight, and obese adults. A comparison study. Metabolism. 2006;55(4):515–524. [DOI] [PubMed] [Google Scholar]
- 41.United States Food and Drug Administration. 21 CFR Part 201. Specific requirements on content and format of labeling for human prescription drugs; revision of “Pediatric Use” subsection in the labeling; final rule. Govinfo web site. https://www.govinfo.gov/content/pkg/FR-1994-12-13/html/94-30238.htm. [Google Scholar]
- 42.Center for Drug Evaluation and Research and Center for Biologics Evaluation and Research; United States Food and Drug Administration (FDA). Guidance document: E11 clinical investigation of medicinal products in the pediatric population. FDA web site. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/e11-clinical-investigation-medicinal-products-pediatric-population. Published December 2000. Accessed January 10, 2022.
- 43.European Medicines Agency (EMA). ICH E11(R1) step 5 guideline on clinical investigation of medicinal products in the pediatric population. EMA web site. https://www.ema.europa.eu/en/ich-e11r1-step-5-guideline-clinical-investigation-medicinal-products-pediatric-population. Updated June 10, 2017. Accessed October 25, 2021.
- 44.United States (U.S.) Department of Health and Human Services, Food and Drug Administration (FDA), Center for Drug Evaluation and Research (CDER), Center for Biologics Evaluation and Research (CBER). FDA web site. Guidance for industry: Providing clinical evidence of effectiveness for human drug and biological products. https://www.fda.gov/media/71655/download. Published May 1998. Accessed January 10, 2022.
- 45.Zhang Y, Wang Y, Khurana M, et al. Exposure-response assessment in pediatric drug development studies submitted to the US Food and Drug Administration. Clin Pharmacol Ther. 2020;108:90–98. [DOI] [PubMed] [Google Scholar]
- 46.Demirkaya E, Lanni S, Bovis F, et al. A meta-analysis to estimate the placebo effect in randomized controlled trials in juvenile idiopathic arthritis. Arthritis Rheumatol. 2016;68(6):1540–1550. [DOI] [PubMed] [Google Scholar]
- 47.Park K, Tran N, Momper J, Green, DJ, Burckart GJ. Pediatric and Adult Placebo Response Rates in Placebo-Controlled Clinical Trials Submitted to the US FDA 2012–2020. J Clin Pharmacol. 2022. Feb 4. doi: 10.1002/jcph.2035 [Online ahead of print]. [DOI] [PubMed] [Google Scholar]
- 48.Struemper H, Thapar M, Roth D. Population Pharmacokinetic and Pharmacodynamic Analysis of Belimumab Administered Subcutaneously in Healthy Volunteers and Patients with Systemic Lupus Erythematosus. Clin Pharmacokinet. 2018. Jun;57(6):717–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Burda BU, O’Connor EA, Webber EM, Redmond N, Perdue LA. Estimating data from figures with a web-based program: considerations for a systematic review. Res Synth Methods. 2017;8(3):258–262. [DOI] [PubMed] [Google Scholar]
- 50.Premaratne S, Newman J, Hobbs S, Garnham A, Wall M. Meta-analysis of direct surgical versus endovascular revascularization for aortoiliac occlusive disease. J Vasc Surg. 2020;72(2):726–737. [DOI] [PubMed] [Google Scholar]
- 51.Drevon D, Fursa SR, Malcolm AL. Intercoder reliability and validity of WebPlotDigitizer in extracting graphed data. Behav Modif. 2017;41(2):323–339. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data used in this study were extracted from the literature and are available at the respective references.


