Abstract
In the present study we investigated the role of platelet-activating factor (PAF) and prostaglandins in experimental Leishmania (Leishmania) amazonensis infection and the relationship between these mediators and nitric oxide (NO) production. Mouse peritoneal macrophages elicited with thioglicolate were infected with leishmania amastigotes, and the infection index determined 48 h later. The course of infection was monitored for 5 weeks in mice infected in the footpad with promastigotes by measuring the footpad swelling and parasite load in regional lymph nodes and spleen. The addition of PAF to C57BL/6 mouse macrophages significantly inhibited parasite growth and induced NO production. Treatment of macrophages with a selective PAF antagonist, WEB2086, increased the infection, indicating that endogenously produced PAF regulates macrophage ability to control leishmania infection. This effect of PAF was abolished by addition of the inhibitor of NO synthesis, L-NAME, to the cultures. The addition of prostaglandin E2 significantly increased the infection and NO production. Treatment with cyclo-oxygenase inhibitor, indomethacin, reduced the infection and PAF-induced release of NO. Thus, the increased NO production induced by PAF seems to be mediated by prostaglandins. The more-selective inhibitors of cyclo-oxygenase 2, nimesulide and NS-398, had no significant effect. Thus, antileishmanial activity correlates better with the presence of PAF or absence of prostaglandins than with NO production. In vivo treatment with PAF antagonists significantly increased leishmania lesions, as well as the parasite load, in regional lymph nodes and spleens. These findings indicate that PAF is essential for the control of leishmania infection.
Leishmania species have a worldwide distribution and can infect humans, causing a spectrum of diseases ranging from small cutaneous lesions to disseminated visceral leishmaniasis (11). Characteristically, Leishmania parasites multiply exclusively in the cells of the mononuclear phagocytic system (5). In murine resident macrophages, Leishmania parasites can survive within the phagolysosome and multiply extensively until lysing these cells (22). However, in activated macrophages, Leishmania parasites are promptly killed (32). Experimental infection with Leishmania parasites inducing cutaneous lesions in susceptible mice results in a disseminated and lethal infection, accompanied by an immune response dominated by CD4+ T helper 2 (Th2) cells secreting interleukin 4 (IL-4), IL-5, and IL-10 (5, 36). In contrast, resistant strains of mouse which exhibit a self-limiting infection develop an immune response dominated by CD4+ Th1 cells secreting gamma interferon (IFN-γ), IL-2, and tumor necrosis factor (TNF) (33). However, there are evidences that immunity to Leishmania is more complex and cannot be explained simply by the Th1-Th2 dichotomy (5, 43). It is well established in murine models that in cytokine-activated macrophages, the increased leishmanicidal activity correlates with increased NO (nitric oxide) production (15, 21, 27). The importance of NO in controlling Leishmania infection has been confirmed also in vivo, since mice treated with an inhibitor of NO synthesis, L-NAME, developed larger lesions with a higher parasite load than did untreated mice (21, 24). Accordingly, resistant mouse strains produce more NO and express higher levels of inducible NO synthase (iNOS) than did susceptible strains (4, 22). Moreover, cytokines that inhibit NO production also inhibit macrophage leishmanicidal activity. For instance, treatment of resident macrophages with IL-4 prior to activation with lipopolysaccharide (LPS) and IFN-γ inhibited NO production and increased parasite multiplication (22). Similar results were observed with IL-10 (9) or with transforming growth factor β (TGF-β) treatments (2). The vast majority of studies on immunity to Leishmania infection have focused on the relationship between cytokines and the production of NO and oxygen intermediates. The involvement of other cell mediators, such as lipids derived from the arachidonic acid metabolism and platelet-activating factor (PAF), in immunity to Leishmania has been largely neglected. There is one report showing that prostaglandins exacerbate the outcome of infection with L. major in BALB/c mice (12), and increased production of prostaglandin E2 (PGE2), PGF2α, LTC4, and PGD2 during murine infection with L. donovani has been described (37, 38, 39). We have shown that prostaglandins, either endogenously produced or added to the macrophage cultures, enhance Leishmania (Leishmania) amazonensis growth in resident murine (BALB/c) macrophages. Moreover, we provided the first evidence that PAF modulates macrophage leishmanicidal activity, causing a marked decrease of the in vitro infection (25). This effect of PAF appeared to be mediated by an NO-dependent mechanism, since the addition of NO inhibitors reverted the protective effect of PAF. However, NO was not detected in these cultures (25). In the present study, we further examined the relationship between lipid mediators, NO production, and the leishmanicidal activity of macrophages. In order to better monitor NO production, these experiments were conducted in thioglycolate (TG)-elicited macrophages obtained from susceptible or resistant mouse strains. NO production and parasite growth were determined in macrophages infected with L. (L.) amazonensis and treated with PGE2 or PAF or with their respective inhibitors or antagonists. We show that the inhibition of parasite growth correlates better with the presence of PAF or absence of PGE2 than with the levels of NO production. More importantly, we show that after treatment of mice with PAF antagonists, the course of infection in a relatively resistant strain (C57BL/6) became similar to that of a more susceptible strain (BALB/c). These findings outline an essential role for PAF in the control of cutaneous leishmaniasis.
MATERIALS AND METHODS
Mice and parasites.
Male BALB/c and C57BL/6 mice, 8 to 10 weeks old, from our own animal facilities were used. The L. (L.) amazonensis strain, MHOM/BR/73/M2269, was kindly provided by J. J. Shaw, Instituto Evandro Chagas, Belém, Pará, Brazil, and maintained as amastigotes by inoculation into the footpads of golden hamsters every 4 to 6 weeks. Amastigote suspensions were prepared as previously described (3). Briefly, the excised lesion were homogenized using a Potter glass homogenizer, the resulting supernatant was centrifuged at 1,400 × g for 10 min, and the pellet was resuspended in RPMI 1640. Promastigotes were isolated from the lymph nodes of infected mice and cultured in medium 199 containing 20% fetal calf serum (FCS), 2 mM l-glutamine, penicillin (100 U/ml), and streptomycin (0.1 mg/ml) at 26°C, they were then used in the stationary phase of growth (day 6 of culture).
Macrophage leishmanicidal activity.
Macrophages were harvested from the peritoneal cavities of BALB/c or C57BL/6 mice by lavage with phosphate-buffered saline (PBS) (resident macrophages) or 4 days after the injection of 1 ml of 3% TG (TG-elicited macrophages). In each group, three different macrophage suspensions were analyzed. Each suspension consisted of a pool of peritoneal cells obtained from at least two animals and was assayed in duplicate. Thus, the values in each group represent the mean of six coverslips examined. About 5 × 105 cells were allowed to attach for 60 min to round, 13-mm-diameter glass coverslips placed in 24-well plates (Costar) containing 0.5 ml of RPMI 1640. The nonadherent cells were removed by three washings in warm medium. The adherent cells were incubated in RPMI 1640 supplemented with 10% FCS, penicillin (100 U/ml), and streptomycin (0.1 mg/ml) for 48 h at 37°C in 5% CO2. The cells were infected with L. (L.) amazonensis amastigotes at a ratio of three amastigotes/macrophage and, at different times after infection, the supernatants were removed for nitrite or eicosanoid determination. The coverslips were washed with PBS, stained with a HEMA 3-Stain set, dried, mounted on glass slides, and examined microscopically. The number of infected macrophages and the average number of parasites per macrophage was determined in 200 cells. The results were expressed as the infection index, which is the percentage of infected macrophages multiplied by the average number of amastigotes per macrophage.
Course of infection.
Mice were infected subcutaneously with 107 promastigotes of L. (L.) amazonensis in 25 μl of PBS in the right footpad and with the same volume of PBS in the left paw. The footpad swelling was monitored weekly with a caliper (Mitutoyo) and was expressed as the thickness of the infected footpad minus that of the uninfected contralateral footpad.
Parasite load in lymph nodes and spleen.
The popliteal lymph nodes draining the infected footpad and the spleen were removed, weighed, and then homogenized with a Potter glass homogenizer in medium 199 supplemented with 20% FCS–2 mM l-glutamine–penicillin (100 U/ml)–streptomycin (0.1 mg/ml) as previously described (7). Briefly, under sterile conditions, serial fourfold dilutions were prepared and distributed in 96-well microtiter plates (Costar) in duplicates. After 5 to 12 days of incubation at 26°C, the wells were examined in an inverted microscope (Nikon, Inc.) at ×100 or ×200 magnification for the presence or the absence of promastigotes. The final titer was the last dilution for which the well contained at least one parasite. The parasite load (number of parasites/gram of tissue) was calculated as follows: the geometric mean of the reciprocal of the positive titers from each duplicate was divided by the weight of the lymph node or spleen. The value obtained was multiplied by the reciprocal fraction of the homogenized organ inoculated into the first well.
Determination of nitrites.
The nitrite concentration was measured by the Griess reagent standard reaction (10). Briefly, 50 μl of culture supernatant was incubated with 50 μl of Griess reagent (1% sulfanilimide–0.1% N-1-naphthylenediamine dihydrochloride–2.5% H3PO4) at room temperature for 10 min. The absorbance at 540 nm with a 620-nm reference filter was detected by a Titertek Multiskan microplate reader. The nitrite concentration was determined from a sodium nitrite standard curve.
Measurement of PGE2.
The concentration of PGE2 in the culture supernatants was determined by a specific enzyme immunoassay (Cayman Chemical Co.) according to the method of Pradelles and Maclouf (35). Briefly, dilutions of the supernatants were incubated with the conjugated eicosanoid-acetylcholinesterase and with the specific antiserum in 96-well plates precoated with anti-rabbit immunoglobulin G antibodies. After overnight incubation at 4°C, the plates were washed and the enzyme substrate (Ellman's reagent) was added for 60 to 120 min at 25°C. The optical density of the samples was determined at 412 nm in a microplate reader, and the concentration of eicosanoids was calculated from standard curve.
TNF bioassay.
TNF activity was measured by a cytotoxicity assay using L929 tumor cells (13). Briefly, 100 μl of the diluted samples was pipetted into 96-well microtiter plates containing target L-929 cells (5 × 104 cell/100 μl) in presence of actinomycin D (2 μg/ml). The cells were incubated with the samples for 20 h at 37°C in 5% CO2. The supernatants were then discarded, and the remaining viable adherent cells were washed with PBS and stained with crystal violet for 15 min. The absorbance of samples was read at 620 nm (Titertek Multiskan). The TNF titer (units/milliliter) was defined as the reciprocal of the dilution that induced 50% of L929 cells lysis.
Drugs and treatments.
PAF (10−6, 10−9, or 10−12 M) and PGE2 (10−5, 10−6, or 10−7 M) were added to macrophage cultures at the moment the infection. L-NAME (10 mM), indomethacin (10 μg/ml), nimesulide (10−5 M), NS-398 (10−6 M), BN52021 (10−5 M), and WEB2170 (10−5 M) were added 1 h before infection. The drugs were maintained throughout the time of the assays. PAF, PGE2, and indomethacin were dissolved in ethanol and further diluted in complete medium. The final concentration of ethanol did not exceed 0.01%. The others drugs were dissolved in PBS and diluted in cultured medium. None of the drugs used affected the macrophage viability, as measured by the trypan blue exclusion test. The viability of macrophages cultured with the drugs for 48 h was always >98%. In a set of experiments, the amastigotes were incubated for 24 h with different concentrations of PAF and indomethacin and then cultured with macrophages. We observed that the infection index was similar to that obtained with untreated amastigotes, indicating that these compounds do not have a direct effect on leishmania. For in vivo treatments, BN52021 and WEB2170 at 5 mg/kg were administered intraperitoneally 1 h before infection, twice daily in the first week, and once a day thereafter till week 4 of infection. Control groups received the vehicle of the drugs on the same schedule.
Reagents and media.
PAF was purchased from Bachem, Inc.; WEB2170 was from Boehringer Ingelheim; BN52021 was from Institut Henri Beaufour; L-NAME, PGE2, indomethacin, RPMI 1640 medium, and supplements were from Sigma Chemical Co., St. Louis, Mo. Compound NS-398 and nimesulide from Cayman Chemical Co. TG medium was from Difco Laboratories, Detroit, Mich.; 199 medium was from Serva Feinbiochemica; the HEMA 3-Stain set from Biochemical Sciences, Inc.
Statistical analysis.
The data were subjected to the Kolmogorov-Smirnov test; those showing normal distribution were submitted to Student's t test when comparing two groups or analysis of variance for more than two groups. The course of infection and parasite load data were analyzed by using the Mann-Whitney U test. All analyses were made using the Instat Program (Graph PAD Software, Inc., San Diego, Calif.). The differences were considered significant at a 5% level.
RESULTS
PAF stimulates macrophage leishmanicidal activity and NO production.
We first compared the leishmanicidal activity of resident peritoneal macrophages from susceptible BALB/c and resistant C57BL/6 mice. It was found that the infection index was roughly similar in both strains: in BALB/c it was 351.21 ± 14.75, and in C57BL/6 it was 348.78 ± 14.41 (n = 3; 48-h cultures). Regarding NO production, resident macrophages from both strains, infected or not with Leishmania and treated or not with PAF, did not release NO.
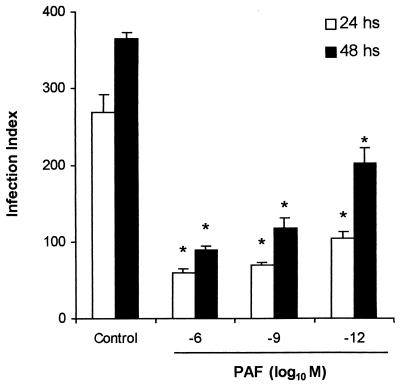
We then focused on C57BL/6 mice and TG-elicited peritoneal macrophages. It was found that the addition of PAF to the infected macrophages induced a dose-dependent inhibition of the infection index (Fig. 1), indicating that PAF inhibits the parasite growth. Pretreatment of macrophages with WEB2170, a selective PAF antagonist, markedly increased the infection index, indicating endogenous production of PAF. The dose of the antagonist used (10−5 M) was able to completely reverse the effect of PAF (Table 1).
FIG. 1.
Effect of exogenous PAF on infection index. TG-elicited peritoneal macrophages from C57BL/6, treated with PAF (10−6, 10−9, or 10−12 M) at the moment of infection with L. (L.) amazonensis amastigotes and the infection index determined 24 and 48 h later. Untreated macrophages were used as a control. In each group, three different macrophage suspensions were analyzed. Each suspension was a pool of peritoneal cells from at least two mice and was assayed in duplicate. The data represent the mean ± the standard error of the mean (SEM) of six coverslips examined. ∗, P < 0.01 (comparing PAF-treated with the untreated group).
TABLE 1.
Effect of PAF antagonist on the infection indexa
| Treatment (M) | Mean infection index ± SEM at 48 h |
|---|---|
| None | 287.53 ± 11.30 |
| PAF (10−6) | 163.24 ± 13.20* |
| WEB2170 | 419.57 ± 57.28* |
| PAF plus WEB2170 | 308.41 ± 4.80# |
TG-elicited, C57BL/6 mouse peritoneal macrophages infected with L. (L.) amazonensis were treated with PAF at the moment of infection. WEB2170 (10−5 M) was added to macrophage cultures 1 h before the addition of PAF to infected cultures. The infection index was determined in 48-h cultures. In each group, three different macrophage suspensions were analyzed. Each suspension consisted of a pool of peritoneal cells obtained from at least two animals and was assayed in duplicate. The data represent the mean ± the SEM of six coverslips examined. ∗, P < 0.05 (compared to vehicle-treated group); #, P < 0.05 (compared to the group treated with PAF).
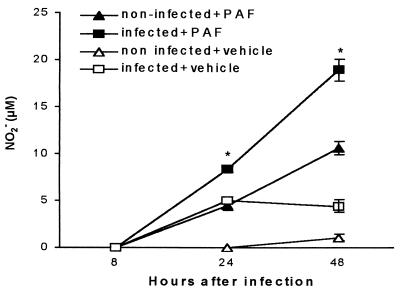
Next, we investigated the effect of PAF on NO production by TG-elicited macrophages infected or not with Leishmania. Figure 2 shows that the addition of PAF to noninfected macrophages significantly increased NO production in the 24- to 48-h time interval compared to macrophages treated with the PAF vehicle. In infected macrophages, the addition of PAF further increased NO production. This effect was significant at 0- to 24-h and at 24- to 48-h time intervals compared to infected and vehicle-treated macrophages.
FIG. 2.
Kinetics of NO release induced by PAF. TG-elicited peritoneal macrophages from C57BL/6, infected or not with L. (L.) amazonensis and treated with PAF (10−6 M) or its vehicle. Nitrite levels were measured in the supernatants from cultures at 8, 24, and 48 h. The data represent the mean ± the SEM of three experiments performed as described in legend to Fig. 1. ∗, P < 0.01 (comparing PAF-treated with vehicle treated group).
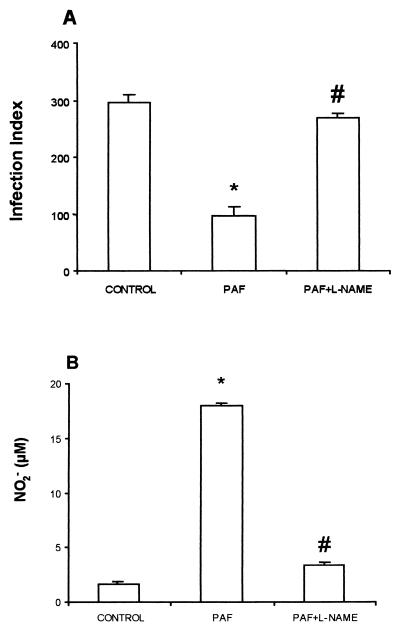
Since PAF induced NO production and increased leishmanicidal activity, the infection index and NO production were measured in the presence of an inhibitor of NO production, L-NAME. It is shown in Fig. 3 that the increased leishmanicidal activity (Fig. 3A) and NO production (Fig. 3B) induced by PAF in infected TG-elicited macrophages were totally abrogated by treatment with L-NAME.
FIG. 3.
Effect of L-NAME on PAF-induced effects. TG-elicited peritoneal macrophages from C57BL/6 mice were infected with L. (L.) amazonensis (control) and treated with 10−6 M PAF at the moment of infection. In the other group, L-NAME was given as a pretreatment 1 h before the PAF addition. The infection index (A) and nitrite levels (B) were determined 48 h after infection. The data represent the mean ± the SEM of three experiments performed as described in the legend to Fig. 1. ∗, P < 0.01 (compared to the control group); #, P < 0.01 (comparing the PAF plus L-NAME group with the PAF-treated group).
PGE2 decreases macrophage leishmanicidal activity.
In a previous study we showed that the addition of PGE2 to resident macrophages obtained from susceptible BALB/c mice induced a significant increase in the infection index (25). Here we confirmed this effect of PGE2 in TG-elicited macrophages of C57BL/6 mice. It can be seen in Table 2 that the addition of PGE2 caused a dose-dependent increase in the infection index compared to the vehicle-treated group. In another set of experiments, we investigated the effect of inhibitors of prostaglandin production—indomethacin, nimesulide, and NS-398—on the leishmanicidal activity. As shown in Table 2, pretreatment of macrophages with indomethacin, a preferential inhibitor of COX-1, diminished significantly the infection index. Interestingly, nimesulide and NS-398, compounds that are more selective for COX-2 inhibition had no significant effect on parasite growth. The data of each treated group were compared to data from the untreated group tested on the same day. Collectively, our results indicate that prostaglandins produced by macrophages, mainly via COX-1 stimulation, are involved in downregulating macrophage leishmanicidal activity.
TABLE 2.
Effect of PGE2 and inhibitors of prostaglandin synthesis on L. (L.) amazonensis infectiona
| Treatment (M) | Mean infection index ± SEM at 48 h |
|---|---|
| None | 230.26 ± 2.56 |
| PGE2 (10−7) | 291.64 ± 6.89 |
| PGE2 (10−6) | 307.57 ± 15.85* |
| PGE2 (10−5) | 337.79 ± 2.42* |
| None | 269.45 ± 3.61 |
| Indomethacin | 107.95 ± 8.68* |
| Nimesulide | 249.93 ± 14.11 |
| NS-398 | 270.51 ± 11.52 |
TG-elicited C57BL/6 peritoneal macrophages were treated with indomethacin (10 μg/ml), nimesulide (10−5 M), or NS-398 (10−6 M) for 1 h before infection with L. (L.) amazonensis. PGE2 was added at the moment of the infection. A untreated group was also run in each assay. The data represent the mean ± the SEM of three experiments performed as described in the legend to Fig. 1. ∗, P < 0.01 (compared to the respective control group).
Modulation of leishmanicidal activity by prostaglandins and PAF does not correlate with the level of NO production.
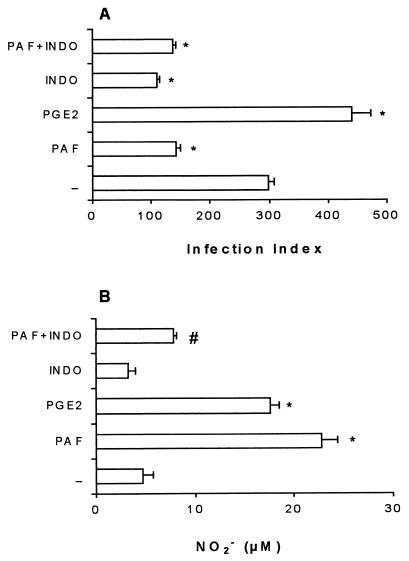
Since PAF and PGE2 exerted opposite effects on leishmanicidal activity, we investigated whether the modulation of leishmanicidal activity by these mediators was associated with NO production. As expected, the addition of PAF decreased, while the addition of PGE2 increased the macrophage infection index (Fig. 4A). Surprisingly, both PAF and PGE2 increased NO production (Fig. 4B). Pretreatment of the macrophages with indomethacin decreased the infection index but did not increase NO production compared with control macrophages (Fig. 4). Treatment with indomethacin plus PAF did not decrease further the infection index (Fig. 4A). Interestingly, indomethacin treatment inhibited the PAF-induced NO production (Fig. 4B). Thus, it appears that the increased NO production induced by PAF is mediated by prostaglandins and that the leishmanicidal activity does not correlate with the level of NO production.
FIG. 4.
Infection index and NO production under different treatments. TG-elicited peritoneal macrophages from C57BL/6 mice infected with L. (L.) amazonensis were treated with PAF (10−6 M) or PGE2 (10−5 M) at the moment of infection. Indomethacin (10 μg/ml) was given as a pretreatment 1 h before infection and maintained throughout the infection. The infection index (A) and the levels of nitrite (B) were determined 48 h after infection. The data represent the mean ± the SEM of three experiments performed as described in the legend to Fig. 1. ∗, P < 0.01 (compared with the untreated group); #, P < 0.01 (comparing PAF plus indomethacin with PAF-alone groups).
Resistance to Leishmania infection is dependent on endogenous PAF production.
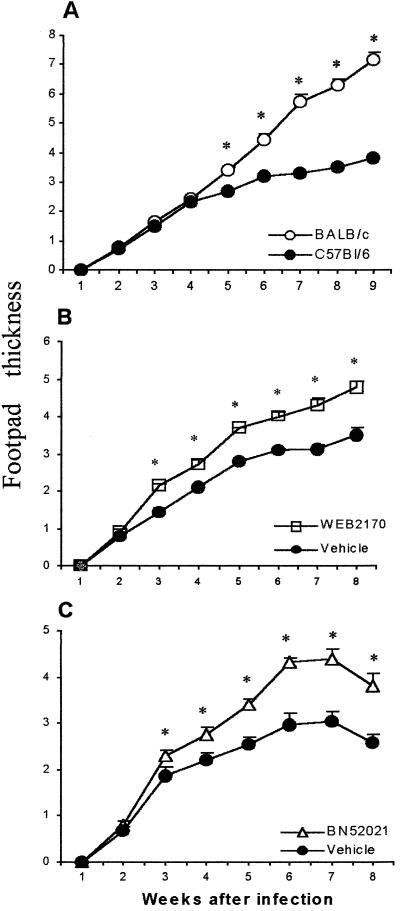
Data from the in vitro studies suggested that during L. (L.) amazonensis infection there is endogenous production of PAF which controls the infection by increasing macrophage leishmanicidal activity. We then investigated whether PAF would affect the outcome of the infection in vivo. We first compared the course of the infection in BALB/c and C57BL/6 mouse strains after infection with promastigote forms of L. (L.) amazonensis. Upon analysis of the evolution of the footpad lesions, during the first 4 weeks of infection, no differences were observed between these strains (Fig. 5A). From 5 weeks onward, the footpad thickness was significantly higher in BALB/c mice compared to C57BL/6 mice. Moreover, BALB/c mice presented a sustained increase in footpad thickness for up to 9 weeks, while C57BL/6 mice showed a modest increase in footpad thickness (Fig. 5A). Thus, BALB/c and C57BL/6 mice differ in their susceptibility to L. (L.) amazonensis infection. Next, we investigated the effect of two selective PAF antagonists, WEB2170 and BN52021, on the course of infection in the resistant C57BL/6 mice. The PAF antagonists were administered before and every day after the infection for four consecutive weeks. As shown in Fig. 5B and C, treatment with PAF antagonists resulted in significantly higher lesions compared to vehicle-treated animals. This indicates that PAF is produced during the infection and that the inhibition of endogenously produced PAF converts a resistant strain into a susceptible one.
FIG. 5.
Time course of L. (L.) amazonensis infection in BALB/c and C57BL/6 mice and the effect of PAF antagonists. (A) BALB/c or C57BL/6 mice were infected with 107 promastigote forms of L. (L.) amazonensis, and the course of infection was monitored by weekly measurements of footpad thickness. (B and C) C57BL/6 mice were treated or not treated with a 5-mg/kg intraperitoneal injection of either WEB2170 or BN52021. The first dose of the antagonists was given 1 h before infection, twice a day during the first week, and once a day thereafter till week 4. Control groups received daily intraperitoneal injections of the vehicles of the drugs. The infected and contralateral footpads were measured weekly. The data represent the mean ± the SEM of the infected contralateral footpad of seven animals. ∗, P < 0.01 compared to the vehicle-treated group.
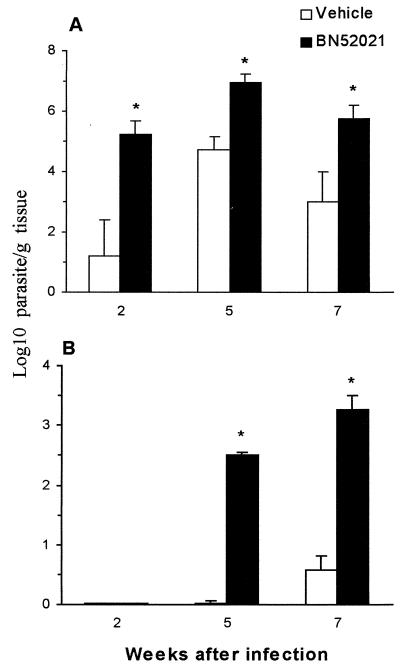
Parasite load of lymph nodes and spleen increase after treatment with PAF antagonist.
The effect of the PAF antagonist (BN52021) on the parasite load of the regional (popliteal) lymph nodes and spleen was then determined. In draining lymph nodes, treatment with PAF antagonist resulted in a significant increase of parasite load compared to vehicle-treated animals at all time points studied (Fig. 6A). Regarding the effect of the PAF antagonist on parasite load in the spleen, it was found that, at 2 weeks postinfection, parasites were not detected in the spleen of control or BN52021-treated animals (Fig. 6B). However, at 5 weeks postinfection a dramatic increase in the number of parasites was observed in BN52021-treated animals, whereas untreated animals presented a very low parasite load (Fig. 6B). At 7 weeks postinfection (3 weeks after discontinuing drug treatment), the number of parasites in the spleen was 54 times higher in the BN52021-treated group than in the vehicle-treated animals (Fig. 6B).
FIG. 6.
Effect of the treatment with PAF antagonist on the parasite load in lymph nodes and spleen. Mice were infected and treated with BN52021 as described in Fig. 4. At 2, 5, and 7 weeks post-infection the animals were killed and the popliteal lymph nodes (A) and spleens (B) were removed for quantification of the parasite load by limiting dilution. The data are expressed as the log10 of the number of parasites/gram of tissue and are shown as the mean ± the SEM of four animals. ∗, P < 0.01 (comparing the drug-treated group with the vehicle-treated group at each time point).
DISCUSSION
Murine models have firmly established that an inappropriate T-cell response can cause severe cutaneous leishmaniasis, while Th1 cells that secrete macrophage-activating cytokines such as IFN-γ lead to resistance (16, 20, 31, 40). Also, the role of different cytokines and NO production on the outcome of disease is well documented (5, 8, 17, 23, 41, 42). However, little is known about the role of lipid mediators in Leishmania infection.
We have previously shown that the addition of PGE2 to resident BALB/c macrophages strongly reduced their leishmanicidal capacity (25). In the present study we confirmed this observation in TG-elicited macrophages obtained from C57BL/6 mice. Prostaglandins are among the major arachidonic acid metabolites produced by macrophages. They are known to exert a negative control of macrophage activation, possibly by increasing cyclic AMP levels (29, 34, 44). In this study, the inhibition of prostaglandin synthesis by indomethacin, significantly increased the leishmanicidal activity. The more-selective inhibitors of COX-2, nimesulide (47) and NS-398 (14, 26), did not have any effect. These results suggest that prostaglandins are produced in this experimental condition mainly by activation of COX-1. However, it is not possible to exclude the participation of COX-2. It is possible to speculate that the amount of prostaglandins produced via COX-1 is high enough to modulate the macrophages and thus any additional prostaglandin produced via COX-2 would have no relevant effect. These results indicate that endogenous prostaglandin production enhances macrophage infection with Leishmania and corroborate previous findings (6, 12, 28, 46).
We also showed in a previous study that PAF suppressed L. (L.) amazonensis growth in resident macrophages and that this suppression appeared to be mediated by NO since L-NAME, an NO inhibitor, blocked the effect of PAF. However, we could not detect NO production in culture supernatants (25). Since in that study we used resident macrophages from a susceptible strain (BALB/c), we next assayed macrophages from a resistant strain (C57BL/6) and found that they are also unable to produce detectable levels of NO, even after stimulation with PAF. However, TG-elicited macrophages released significant amounts of NO after PAF addition to the cultures, and this production was further increased when they were infected with L. (L.) amazonensis. In turn, PAF-stimulated macrophages showed higher antileishmanial activity than control macrophages. The effect of PAF appears to be leishmanicidal because the infection index at 24 to 48 h after PAF addition is lower than that at the beginning of the infection (data not shown). As expected, the PAF antagonist (WEB2170) or the NOS inhibitor (L-NAME) abolished the effect of PAF. There is one report presenting evidence that PAF can induce and augment macrophage tumoricidal activity by an NO-dependent mechanism (18) and another showing that PAF is able to induce NOS expression and NO production after LPS activation (30, 45).
We also found that the infection index of TG-elicited macrophages is lower than that exhibited by resident macrophages, indicating that elicited macrophages are more efficient at restricting the parasite growth than the resident ones (unpublished results). Comparing the present results of leishmanicidal activity exhibited by TG-elicited macrophages which produce substantial amounts of NO upon PAF stimulation with those obtained previously with resident macrophages, which were unable to produce detectable amounts of NO, it is clear that PAF exerts its activity independently of the amount of NO produced. Another evidence for the lack of correlation between leishmanicidal activity and the levels of NO production came from experiments with PGE2. The addition of PGE2 to infected TG-elicited macrophages induced a substantial NO production and, paradoxically, parasite growth was strongly enhanced. In contrast, the addition of indomethacin did not increase NO production and yet increased significantly the macrophage leishmanicidal activity. Finally, indomethacin inhibited PAF-induced NO production but did not affect leishmanicidal activity. The latter result indicates that the PAF-induced NO secretion occurs via a COX-1-dependent mechanism. It is noteworthy that PAF and indomethacin do not appear to act synergistically, because the addition of both compounds did not increase further the macrophage leishmanicidal activity observed when they were added separately. Incubation of Leishmania amastigotes with PAF or indomethacin for 24 h had no effect on the infection index, which indicates that these drugs do not affect the viability of leishmania amastigotes.
Our results are in line with two recent reports describing the protective role of PAF on Candida albicans and Trypanosoma cruzi infections. In C. albicans infection, the protective role of PAF was blocked by anti-TNF treatment (19). Also, anti-TNF antibody inhibited NO production induced by PAF in T. cruzi-infected, TG-elicited macrophages (1). In our experiments, we could not detect TNF production in supernatants collected 48 h after infection and PAF treatment (data not shown). However, it remains to be tested whether anti-TNF antibody will affect PAF-induced leishmanicidal activity.
Having established the role of PAF in controlling L. (L.) amazonensis infection in vitro, we then tested the effect of PAF antagonists on the course of infection in C57BL/6 mice. The in vivo results clearly indicate that PAF is produced endogenously during infection and that its inhibition increased the severity of footpad lesions. In addition, the administration of PAF antagonist increased the parasite load of popliteal lymph nodes and spleen. Also, PAF antagonist accelerated the visceralization of L. (L.) amazonensis, since at 5 weeks postinfection parasites were readily found in the spleen of PAF antagonist-treated animals but not in vehicle-treated animals.
Since the experiments in vivo confirmed the requirement of PAF for the control of parasite growth, it appears that during the infection, macrophages produce PAF that in turn activates them in an autocrine fashion for leishmanicidal activity. Nevertheless, it is also possible that the inhibition of PAF action by the antagonist may interfere with antigen presentation by macrophages resulting in the selective activation of T lymphocytes secreting IL-4, IL-10, and TGF-β, which are known to promote parasite multiplication (2, 9, 22).
The fact that PAF exerts its action on Leishmania infection independently of the level of NO production may be important for studies with human mononuclear phagocytes. It is known that human phagocytes usually produce very low amounts of NO. Therefore, it will be important to determine if PAF can increase the leishmanicidal activity of human mononuclear phagocytes.
Finally, whatever the mechanism of PAF-induced leishmanicidal activity, the major conclusion that can be drawn from our experiments is that PAF is essential for the control of L. (L.) amazonensis infection. Based on this conclusion we speculate whether PAF applied topically might be of therapeutical value in cutaneous leishmaniasis, alone or in combination with cytokines.
ACKNOWLEDGMENTS
This work was supported by grants from the Fundação de Amparo a Pesquisa do Estado de São Paulo (FAPESP) and the Conselho Nacional de Pesquisa e Desenvolvimento (CNPq).
We thank Eliane Aparecida Gomes de Mello and Richardt Gama Landgraf for excellent technical assistance.
REFERENCES
- 1.Aliberti J C S, Machado F S, Gazzinelli R T, Teixeira M M, Silva J S. Platelet-activating factor induces nitric oxide synthesis in Trypanosoma cruzi-infected macrophages and mediates resistance to parasite infection in mice. Infect Immun. 1999;67:2810–2814. doi: 10.1128/iai.67.6.2810-2814.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barral A, Barral-Neto M E C, Yong, Brownell C E, Twardzik D, Reed S G. TGF-β as a virulence mechanism for L. brasiliensis. Proc Natl Acad Sci USA. 1993;90:3442–3446. doi: 10.1073/pnas.90.8.3442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beyrot C G P, Pinto A R, Freymüller E, Barbiéri C L. Characterization of an antigen from Leishmania amazonensis amastigotes able to elicit protective responses in a murine model. Infect Immun. 1997;65:2052–2059. doi: 10.1128/iai.65.6.2052-2059.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blackwell J M, Roach T I A, Atkinson S E, Ajioka J W, Barton C H, Shaw M A. Genetic regulation of macrophage priming activation: the Lsh gene story. Immunol Lett. 1991;30:241–248. doi: 10.1016/0165-2478(91)90032-6. [DOI] [PubMed] [Google Scholar]
- 5.Bogdan C, Gessner A, Solbach W, Rollinghoff M. Invasion, control and persistence of Leishmania parasites. Curr Opin Immunol. 1996;8:517–525. doi: 10.1016/s0952-7915(96)80040-9. [DOI] [PubMed] [Google Scholar]
- 6.Buchmüller-Rouiller Y, Betz-Corradin S, Mauel J. Differential effects of prostaglandins on macrophage activation induced by calcium ionophore A23187 or IFN-γ. J Immunol. 1992;148:1171–1175. [PubMed] [Google Scholar]
- 7.Buffet P A, Sulahian A, Garin Y J F, Nassar N, Derouin F. Culture microtitration: a sensitive method for quantifying Leishmania infantum in tissues of infected mice. Anitimicrob Agents Chemother. 1995;39:2167–2168. doi: 10.1128/aac.39.9.2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chaterlain R, Varkila K, Coffman R L. IL-4 induces a Th2 response in Leishmania major-infected mice. J Immunol. 1992;148:1182–1187. [PubMed] [Google Scholar]
- 9.Cunha F Q, Moncada S, Liew F Y. Interleukin 10 (IL-10) inhibits the induction of nitric oxide synthase by IFN-γ in murine macrophages. Biochem Biophys Res Commun. 1992;182:1155–1159. doi: 10.1016/0006-291x(92)91852-h. [DOI] [PubMed] [Google Scholar]
- 10.Ding A H, Nathan C F, Stuehr D J. Release of reactive nitrogen intermediates and reactive oxygen intermediates from mouse peritoneal macrophages: comparison of activating cytokines and evidence for independent production. J Immunol. 1988;141:2407–2412. [PubMed] [Google Scholar]
- 11.Division of Communicable Disease Program, HPC/HCT, PAHO. Leishmaniasis in the Americas. Epidemiol Bull. 1994;15:8–11. [PubMed] [Google Scholar]
- 12.Farrell J P, Kirkpatrich C E. Experimental cutaneous leishmaniasis. J Immunol. 1987;138:902–907. [PubMed] [Google Scholar]
- 13.Flick D A, Gifford G E. Comparison of “in vitro” cell cytotoxicity assays for tumor necrosis factor. J Immunol. 1984;68:167–175. doi: 10.1016/0022-1759(84)90147-9. [DOI] [PubMed] [Google Scholar]
- 14.Futaki N, Takahashi S, Yokoyama M, Arai I, Higuchi S, Otomo S. NS-398, a new anti-inflammatory agent, selectively inhibits prostaglandin G/H synthase/cyclooxygenase (COX-2) activity ‘in vitro’. Prostaglandins. 1994;47:55–59. doi: 10.1016/0090-6980(94)90074-4. [DOI] [PubMed] [Google Scholar]
- 15.Green S J, Meltzer M S, Hibbs J B, Jr, Nacy C A. Activated macrophages destroy intracellular Leishmania major amastigotes by an l-arginine-dependent killing mechanism. J Immunol. 1990;144:278–283. [PubMed] [Google Scholar]
- 16.Güler M L, Gorham J D, Hsieh C S, Mackey A J, Steen R G, Dietrich W F, Murphy K M. Genetics susceptibility to Leishmania: IL-12 responsiveness in Th1 cell development. Science. 1996;271:984–986. doi: 10.1126/science.271.5251.984. [DOI] [PubMed] [Google Scholar]
- 17.Heinzel F P, Rerko R M, Ahmed F, Pearlman C. Endogenous IL-12 is required for control of Th2 cytokine response capable of exacerbating leishmaniasis in normally resistant mice. J Immunol. 1995;155:730–739. [PubMed] [Google Scholar]
- 18.Howard A D, Erickson K L. The induction and augmentation of macrophage tumoricidal responses by platelet-activating factor. Cell Immunol. 1995;164:105–112. doi: 10.1006/cimm.1995.1148. [DOI] [PubMed] [Google Scholar]
- 19.Im S Y, Choi J H, Ko H M, Han S J, Chun S B, Lee H K, Ha T Y. A protective role of platelet-activating factor in murine candidiasis. Infect Immun. 1997;65:1321–1326. doi: 10.1128/iai.65.4.1321-1326.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kemp M, Jorgen J A L, Bendtzen K, Poulsen L K, Hansen M B, Koech D K, Kharazmi A, Theander T G. Leishmania donovani-reactive Th1- and Th2-like T-cell clones from individuals who have recovered from visceral leishmaniasis. Infect Immun. 1993;61:1069–1073. doi: 10.1128/iai.61.3.1069-1073.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liew F Y, Yun L, Millott S. Tumor necrosis factor α synergizes with IFN-γ in mediating killing of Leishmania major through the induction of nitric oxide. J Immunol. 1990;145:4306–4310. [PubMed] [Google Scholar]
- 22.Liew F Y. Role of cytokines in killing of intracellular pathogens. Immunol Lett. 1991;30:193–198. doi: 10.1016/0165-2478(91)90024-5. [DOI] [PubMed] [Google Scholar]
- 23.Liew F Y, O'Donnell C A. Immunology of leishmaniasis. Adv Parasitol. 1993;32:161–259. doi: 10.1016/s0065-308x(08)60208-0. [DOI] [PubMed] [Google Scholar]
- 24.Liew F Y, Li Y, Moss D, Parkinson C, Roger M V, Moncada S. Resistance to L. major infection correlates with the induction of nitric oxide synthase in murine macrophages. Eur J Immunol. 1991;21:3009–3014. doi: 10.1002/eji.1830211216. [DOI] [PubMed] [Google Scholar]
- 25.Lonardoni M V C, Barbieri C L, Russo M, Jancar S. Modulation of Leishmania (L.) amazonensis growth in cultured mouse macrophages by prostaglandins and platelet-activating factors. Mediators Inflamm. 1994;3:137–141. doi: 10.1155/S0962935194000177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Masferrer J L, Zweifel B S, Manning P T, Hauser S D, Leahy K M, Smith W G, Isakson P, Seibert K. Selective inhibition of inducible cyclooxygenase 2 ‘in vivo’ is antiinflammatory and nonulcerogenic. Proc Natl Acad Sci USA. 1994;91:3228–3232. doi: 10.1073/pnas.91.8.3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mauël J, Ransijn A, Buchmüller-Rouiller Y. Killing of Leishmania parasites in activated murine macrophages is based on a l-arginine-dependent process that produces nitrogen derivatives. J Leukoc Biol. 1991;49:73–82. doi: 10.1002/jlb.49.1.73. [DOI] [PubMed] [Google Scholar]
- 28.Milano S, Arcoleo F, Dieli M, D'Agostino R, Nucci G, D'Agostino P, Cillari E. Ex vivo evidence for PGE2 and LTB4 involvement in cutaneous leishmaniasis: relation with infection status and cytokine production. Parasitology. 1996;112:13–19. doi: 10.1017/s0031182000065033. [DOI] [PubMed] [Google Scholar]
- 29.Minakuchi R, Wacholtz M C, Davis L S, Lipsky P E. Delineation of the mechanism of inhibition of human T cell activation by PGE2 J. Immunol. 1990;145:2616–2625. [PubMed] [Google Scholar]
- 30.Mustafa S B, Howard K M, Olson M S. Platelet-activating factor augments lipopolysaccharide induced nitric oxide formation by rat Kupffer cells. Hepatology. 1996;23:1622–1630. doi: 10.1002/hep.510230645. [DOI] [PubMed] [Google Scholar]
- 31.Nabors G S. Modulating ongoing Th2-cell responses in experimental leishmaniasis. Parasitol Today. 1997;13:76–79. doi: 10.1016/s0169-4758(96)10078-8. [DOI] [PubMed] [Google Scholar]
- 32.Nacy C A, Fortier A H, Meltzer M S, Buchmeier N A, Schreiber R D. Macrophage activation to kill Leishmania major: activation of macrophage for intracellular destruction of amastigotes can be induced by both recombinant interferon-γ and non-interferon lymphokines. J Immunol. 1985;135:3505–3511. [PubMed] [Google Scholar]
- 33.O'Garra A, Murphy K. Role of cytokines in determining T-lymphocyte function. Curr Opin Immunol. 1994;6:458–466. doi: 10.1016/0952-7915(94)90128-7. [DOI] [PubMed] [Google Scholar]
- 34.Phipps R P, Stein S H, Roper R L. A new view of prostaglandin E regulation of the immune response. Immunol Today. 1991;12:349–352. doi: 10.1016/0167-5699(91)90064-Z. [DOI] [PubMed] [Google Scholar]
- 35.Pradelles P J, Maclouf J. Enzyme immunoassays of eicosanoids using acetylcolinesterase as label: an alternative to radioimmunoassay. Anal Chem. 1985;57:1170–1173. doi: 10.1021/ac00284a003. [DOI] [PubMed] [Google Scholar]
- 36.Reed S G, Scott P. T-cell and cytokine responses in leishmaniasis. Curr Opin Immunol. 1993;5:524–531. doi: 10.1016/0952-7915(93)90033-o. [DOI] [PubMed] [Google Scholar]
- 37.Reiner N E, Malemud C J. Arachidonic acid metabolism in murine leishmaniasis (donovani): ex vivo evidence for increased ciclo-oxygenase and 5-lipoxygenase activity in spleen cells. Cell Immunol. 1984;88:501–510. doi: 10.1016/0008-8749(84)90181-3. [DOI] [PubMed] [Google Scholar]
- 38.Reiner N E, Malemud C J. Arachidonic acid metabolism by murine peritoneal macrophages infected with Leishmania donovani: ‘in vitro’ evidence for parasite-induced alterations in cyclooxygenase and lipoxygenase pathways. J Immunol. 1985;134:556–563. [PubMed] [Google Scholar]
- 39.Reiner N E, Schultz L A, Malemud C J. Eicosanoid metabolism by Leishmania donovani-infected macrophages: mouse strain responses in prostanoid synthesis. Am J Trop Med Hyg. 1988;38:59–64. doi: 10.4269/ajtmh.1988.38.59. [DOI] [PubMed] [Google Scholar]
- 40.Rossi-Bergmann B, Müller I, Godinho E B. Th1 and Th2 T-cell subsets are differentially activated by macrophages and B cells in murine leishmaniasis. Infect Immun. 1993;61:2266–2269. doi: 10.1128/iai.61.5.2266-2269.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Scharton-Kersten T, Scott P. The role of the innate immune response in Th1 cell development following Leishmania major infection. J Leukoc Biol. 1995;57:515–522. doi: 10.1002/jlb.57.4.515. [DOI] [PubMed] [Google Scholar]
- 42.Scott P. IFN-γ modulates the early development of Th1 and Th2 responses in a murine model of cutaneous leishmaniasis. J Immunol. 1991;147:3149–3155. [PubMed] [Google Scholar]
- 43.Shankar A H, Titus R G. T cell and non-T cell compartments can independently determine resistance to Leishmania major. J Exp Med. 1995;181:845–855. doi: 10.1084/jem.181.3.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stein S H, Phipps R P. Anti-class II antibodies potentiate IgG2a production by lipopolysaccharide-stimulated B lymphocytes treated with prostaglandin E2 and IFN-gamma. J Immunol. 1992;148:3943–3949. [PubMed] [Google Scholar]
- 45.Szabo A, Wu C C, Mitchel J A, Gross S S, Thiemermann C, Vane J R. Platelet-Activating Factor contributes to the induction of nitric oxide synthase by bacterial lipopolysaccharide. Circ Res. 1993;73:991–999. doi: 10.1161/01.res.73.6.991. [DOI] [PubMed] [Google Scholar]
- 46.Titus R G, Theodos C M, Shankar A, Hall L R. Interactions between Leishmania major and macrophages. In: Zwilling B S, Eisenstein T K, editors. Macrophage-pathogen interactions. New York, N.Y: Marcel Dekker; 1994. pp. 437–459. [PubMed] [Google Scholar]
- 47.Vigdahl R L, Tukey R H. Mechanism of action of novel anti-inflammatory drugs diflumidone and R-805. Biochem Pharmacol. 1977;26:307–311. doi: 10.1016/0006-2952(77)90182-4. [DOI] [PubMed] [Google Scholar]