Abstract
Objectives:
To compare the incidence, epidemiology, testing patterns, treatment and outcomes of Clostridioides difficile infection (CDI) among hospitalized pediatric patients from 2013–2019.
Study Design:
The Pediatric Health Information System® (PHIS) database was queried for patient admissions (age 0–17 years) with International Classification of Disease (ICD) 9 and 10 codes for diagnoses of CDI with a billing code for a CDI-related antibiotic treatment.
Results:
17,142 pediatric patients were identified, representing 23,052 admissions with CDI. Adjusted annual CDI incidence decreased over the study period from 7.09 cases per 10,000 patient days (95% CI: 6.15 – 8.18) in 2013 to 4.89 cases per 10,000 patient days (95% CI: 4.03 – 5.93) in 2019 (p<0.001). C. difficile specific testing also decreased during the study period (p<0.001). Chronic gastrointestinal conditions (36%) and malignancy (32%) were the most common comorbidities in CDI encounters. Oral metronidazole use decreased during the study period (p<0.01) and oral vancomycin use increased (p<0.001).
Conclusions:
Our study demonstrates a decrease in CDI incidence in hospitalized pediatric patients, a notable change from prior studies, although this may have been influenced by altered testing patterns. We found a high incidence of CDI in patients with cancer and gastrointestinal conditions: groups that warrant targeted evaluation of CDI prevention and treatment.
Keywords: C.difficile, pathogen panel, children, incidence
Introduction
In the last two decades, C. difficile infection (CDI) has emerged as a significant public health threat, and an increase in the incidence of CDI was reported across nearly all geographic regions in both adults and children.1–3 From 2001 to 2006, the rates of CDI nearly doubled in pediatric inpatients4 and a population-based study demonstrated a 12.5-fold increase in CDI in children from 1991 through 2009.5
The last decade has also brought significant changes in the diagnosis and treatment of CDI. Testing algorithms have moved from toxin-based assays, to PCR-based testing, and finally, to multi-step algorithms; all with the goal of improving sensitivity of CDI detection while decreasing detection of C. difficile colonization.3 Treatment recommendations in adults have shifted from metronidazole to vancomycin3,6 with emerging evidence supportive of a similar approach in children.7 To better understand the current incidence, diagnostic testing, comorbidity profiles, and treatment of pediatric CDI, in line with recent changes in guidance, we queried a large pediatric admissions database of hospital encounters from 2013 to 2019.
Methods
The Pediatric Health Information System® (PHIS) is an administrative database that contains inpatient data from 49 pediatric tertiary care centers in the United States. The PHIS database contains demographic information and diagnoses and compiles billing codes for procedures, medications, and laboratory tests performed during hospital admissions. Quality controls ensure accurate data collection across participating hospitals. Given the use of deidentified data, this study was determined to be non-human subjects research by the institutional review board.
For this study, a CDI encounter required an International Classification of Disease (ICD) version 9 or 10 diagnosis code for CDI and a billing code for a CDI-specific antibiotic therapy. Through a prior study involving a small cohort of hospitals from the PHIS databse, use of ICD 9 codes has been independently validated to have high specificity (99.89%) and sensitivity (80.73%) for CDI case identification.8 To further improve case specificity in our study using a larger population of PHIS hospitals, a billing code for a CDI-specific antibiotic therapy was required. Use of ICD-9 with treatment charge has not been independently validated, but Shaklee et al found that including ICD-9 code with billing code for testing and treatment did result in a slight decrease in sensitivity to 76% but improvement in positive predictive value from 74% to 83%.8 CDI-specific antibiotics included metronidazole (oral or intravenous), rifaximin, fidaxomicin, and vancomycin (oral). A diagnostic test was not required to be billed during the encounter and, due to PHIS database limitations, results of diagnostic testing were not available. In addition, testing for CDI prior to hospital admission could not be obtained through the PHIS database, as only inpatient records are available.
The PHIS database was queried for pediatric patient admissions (age <17 years) with a CDI encounter from 2013–2019. Data post-2019 was not collected as the goal of study was to evaluate CDI trends independent of the COVID-19 pandemic. Seven hospitals that did not report over the entire study period were excluded, leaving 42 hospitals with complete data for this retrospective study. Patient demographics, diagnosis codes, and encounter information were compiled for each hospital admission with CDI.
Comorbid conditions were identified by ICD 9 and ICD 10 diagnosis codes using the pediatric complex chronic conditions (CCC) classification system.9 CCCs are defined as medical conditions expected to last at least 12 months, involve several organ systems or one organ system severely enough to require specialty pediatric care, and have a high probability of hospitalization.10 This previously described and validated method for characterizing ICD-9/10–based pediatric complex chronic conditions is represented by 9 categories: neurologic and neuromuscular, cardiovascular, respiratory, renal and urologic, gastrointestinal, hematology and immunodeficiency, metabolic, malignancy, and neonatal. Complications were assessed at the patient-level vs. the encounter-level, and procedural codes (ie. colectomy) were billed during the same encounter as the CDI episode. Related to reliance on billing codes, timing of complications in relation to CDI could not be reliably determined.
Continuous variables were summarized with medians and interquartile ranges (IQR). Categorical variables were expressed as proportions. For regression analysis, incidence rates (cases /10,000 patient-days) were calculated overall and within each age group (<2, 2–5, and 6–17 years) for each year, and correlation was assessed using Spearman rank-order statistics. Adjusted rates were calculated using Poisson generalized linear models, controlling for hospital clustering and allowing for the presence of correlated data within hospitals, nonconstant variability between hospitals, and responses that are not normally distributed. Independent encounter-level variables included sex, race, ethnicity (Hispanic or non-Hispanic), and intensive care unit (ICU) admission.
To categorize testing trends during the study period, testing for CDI across all PHIS hospital encounters (not just those with a CDI encounter) was determined using billing data which included both single target approaches for C. difficile and multipathogen panels (ie. gastrointestinal pathogen panels) and calculated per 10,000 patient days. Each encounter was characterized as having or not having a billing charge for a C. difficile specific test or a multipathogen panel. The inclusion of C. difficile as a target on multipathogen panels could not be determined through the PHIS database. Statistical significance was set at p<0.05. All statistical analyses were performed with SAS version 9.4 (SAS Institute, Cary, North Carolina).
Results
There were 26,592 encounters with an ICD-9 or 10 diagnosis of CDI which were further limited to those that included a billing code for CDI-specific antibiotic treatment (N=23,052). The 23,052 encounters represented 17,142 individual patients admitted to 42 children’s hospitals from 2013–2019. CDI cases had a median age of 7 years (IQR 2–13), and 10,623 (46.1%) patients were female (Table I). Adjusted annual CDI incidence decreased over the study period from 7.09 cases per 10,000 patient days (95% CI: 6.15 – 8.18) in 2013 to 4.89 cases per 10,000 patient days (95% CI: 4.03 – 5.93) in 2019 (p<0.001). Excluding children 0–1 years old, the adjusted annual CDI incidence decreased over the study period from 20.87 cases per 10,000 patient days (95% CI: 18.13 – 24.02) in 2013 to 15.93 cases per 10,000 patient days (95% CI: 13.16 – 19.27) in 2019 (p<0.001). When evaluated by age, adjusted CDI incidence declined in all groupings, including children 0–1 year, 2–5 years, and 6–17 years (all p<0.001) during the study period (Figure 1a).
Table 1.
Demographic summary of inpatient pediatric CDI encounters from 2013–2019 at 42 children’s hospitals in the United States.
| Age | |
| Median (IQR) | 7 (2–13) |
| Ages 0 – 1 | 3,948 (17.1%) |
| Ages 2 – 5 | 6,438 (27.9%) |
| Ages 6 – 17 | 12,666 (55%) |
| Gender | |
| Female | 10,623 (46.1%) |
| Male | 12,429 (53.9%) |
| Race | |
| African American | 2,650 (11.5%) |
| American Indian and Alaskan Native | 137 (0.6%) |
| Asian and Pacific Islander | 680 (2.9%) |
| Other | 3,291 (14.3%) |
| Two or more races | 1,393 (5.6%) |
| White | 14,901 (64.6%) |
| Ethnicity | |
| Hispanic | 5,155 (22.4%) |
| Median Days of Hospitalization Before Testing (IQR) | 1 (0–6) |
| Median Length of Stay (IQR) | 7 (3–18) |
| All-cause Mortality | 429 (1.9%) |
| Gastrointestinal perforation | 91 (0.4%) |
| Colectomy | 74 (0.3%) |
| Colectomy in patients with IBD (percentage of total colectomies) | 55 (74.3%) |
| Toxic Megacolon | 29 (0.1%) |
Figure 1).
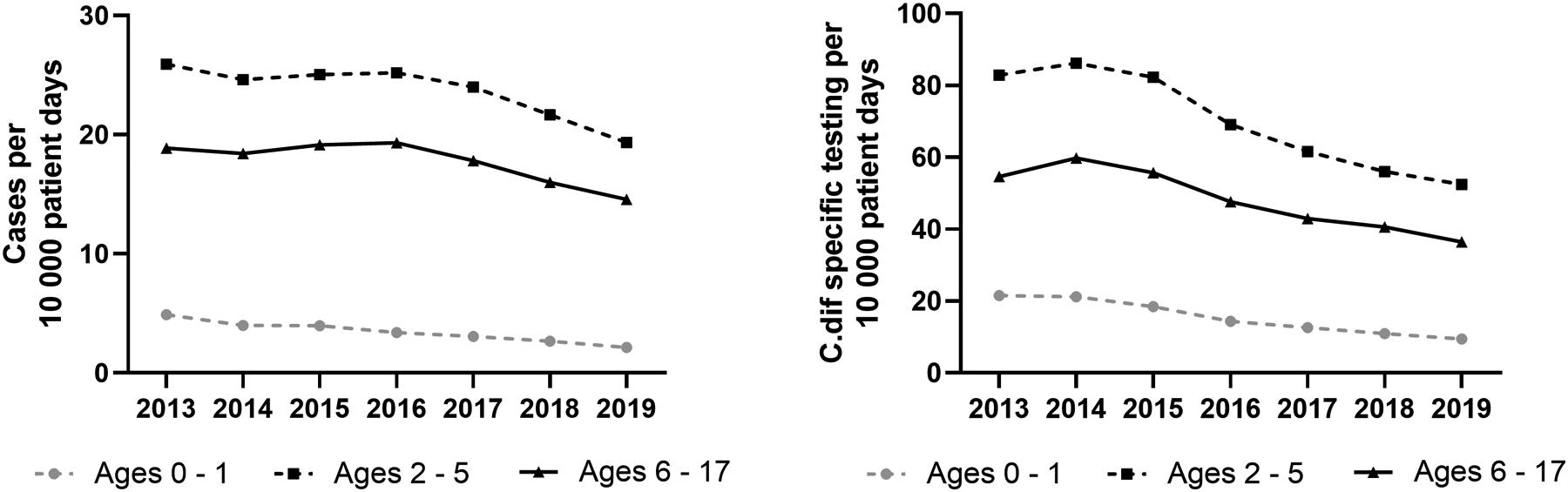
A. The yearly incidence of hospital admissions with a diagnosis of CDI from 2013–2019 in 42 pediatric hospitals (per 10,000 patient-days) were calculated using Poisson generalized linear models, adjusted for patient characteristics and co-morbid conditions. B. Frequency of C. difficile-specific testing per 10,000 patient days.
Among the 23,052 encounters with CDI, 17,247 (74.8%) had C.difficile-specific testing performed during their hospital admission and 3,636 (15.8%) had gastrointestinal panels performed. The most common type of C. difficile specific testing during the study period was nucleic acid amplification (e.g., polymerase chain reaction) testing (NAAT), which occurred in 14,673 encounters (63.7%). In those patients with an NAAT, there were 2,223 (15.2%) that had an additional C. difficile-specific test ordered during the encounter.
Due to the influence of testing rates on CDI incidence, testing per 10,000 patient days was evaluated throughout the study period in all PHIS encounters. The annual overall C. difficile-specific testing per 10,000 patient days decreased during the study period from 52.67 tests (95% CI: 44.67 – 62.11) in 2013 to 33.7 tests (95% CI: 29.48 – 38.52) in 2019 (p<0.001). When evaluated by age, the hospital-based rates of C. difficile specific testing decreased in children ages 0–1 year, 2–5 years, and 6–17 years (all p<0.001) (Figure 1b). When comparing changes in rates between C. difficile specific testing and CDI incidence using Spearmans Correlation, rates were highly correlated in those 0–1 year old (Corr=0.99, p<0.001), and not correlated in those 2–5 years old (corr=0.75, p=0.052) or 6–17 years old (Corr=0.68 (p=0.094). However, panel-based testing (which may or may not include C. difficile) increased during the study period (Figure 2; available at www.jpeds.com).
Figure 2).
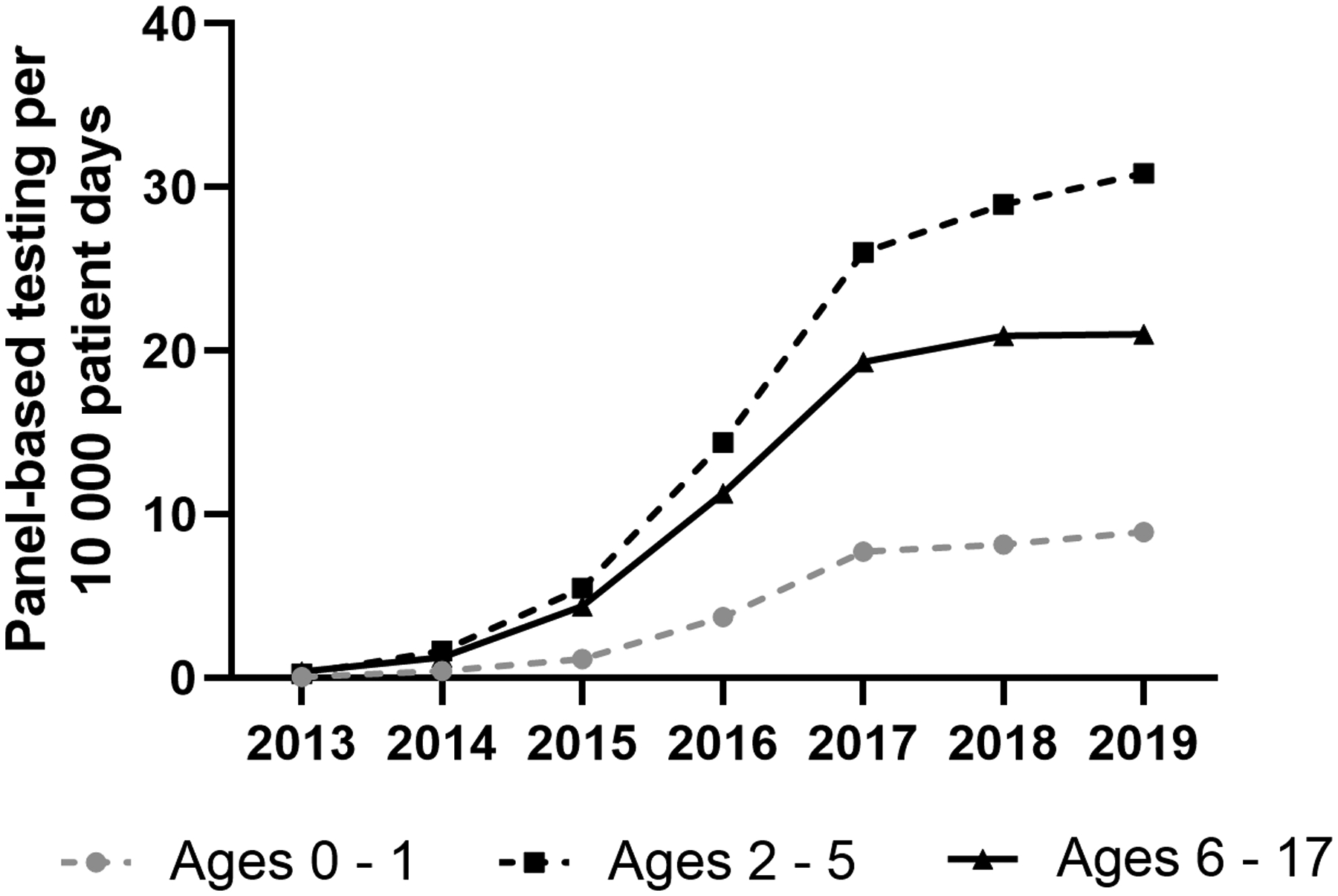
Panel based testing rates per 10,000 patient days in hospitalized children.
Based on CCC classifications, 19,355 (84%) of encounters were in children with a chronic condition. Chronic gastrointestinal conditions (9,332, 41%), malignancy (8,202, 36%), and immunodeficiency (5,520, 24%) were the conditions most frequently associated with CDI encounters (Table II). Nearly a quarter of patients with CDI patient encounters (5,629, 24%) had a gastrointestinal feeding device.
Table 2:
International Classification of Disease 9 and 10 codes of patients with CDI encounter grouped using Feudtner’s Complex Chronic Conditions.
| Patient encounters with Condition within Organ System (percentage of total admissions) | |
|---|---|
| Any Chronic Condition | 19,355 (84%) |
| Cardiovascular | 3,349 (14.5%) |
| Neurologic and Neuromuscular | 3,955 (17.2%) |
| Respiratory | 2,248 (9.8%) |
| Renal and Urologic | 2,914 (12.6%) |
| Gastrointestinal | 9,332 (40.5% ) |
| Inflammatory Bowel Disease | 1,749 (8%) |
| Gastrointestinal Devices (feeding tube or ostomy) |
5,629 (24%) |
| Transplantation (Liver, Small Intestine, or Pancreas) |
572 (2%) |
| Hematologic and/or Immunodeficiency | 5,520 (23.9%) |
| Metabolic | 4,514 (19.6%) |
| Malignancy | 8,202 (35.6%) |
| Neoplasms | 7,045 (31%) |
| Stem Cell Transplantation | 883 (5%) |
| Neonatal (arising in perinatal period) | 473 (2.1%) |
Oral metronidazole was used most frequently (mean annual use: 68.5%) throughout the study period. A decrease in oral metronidazole use was noted in 2018 and 2019 (trend: p<0.010), coincident with an increase in the use of oral vancomycin (trend: p<0.001) (Figure 3), which was used on average in 36.1% of encounters in 2019. Fidaxomicin use was low (mean annual use: 0.5%) during the study period.
Figure 3).
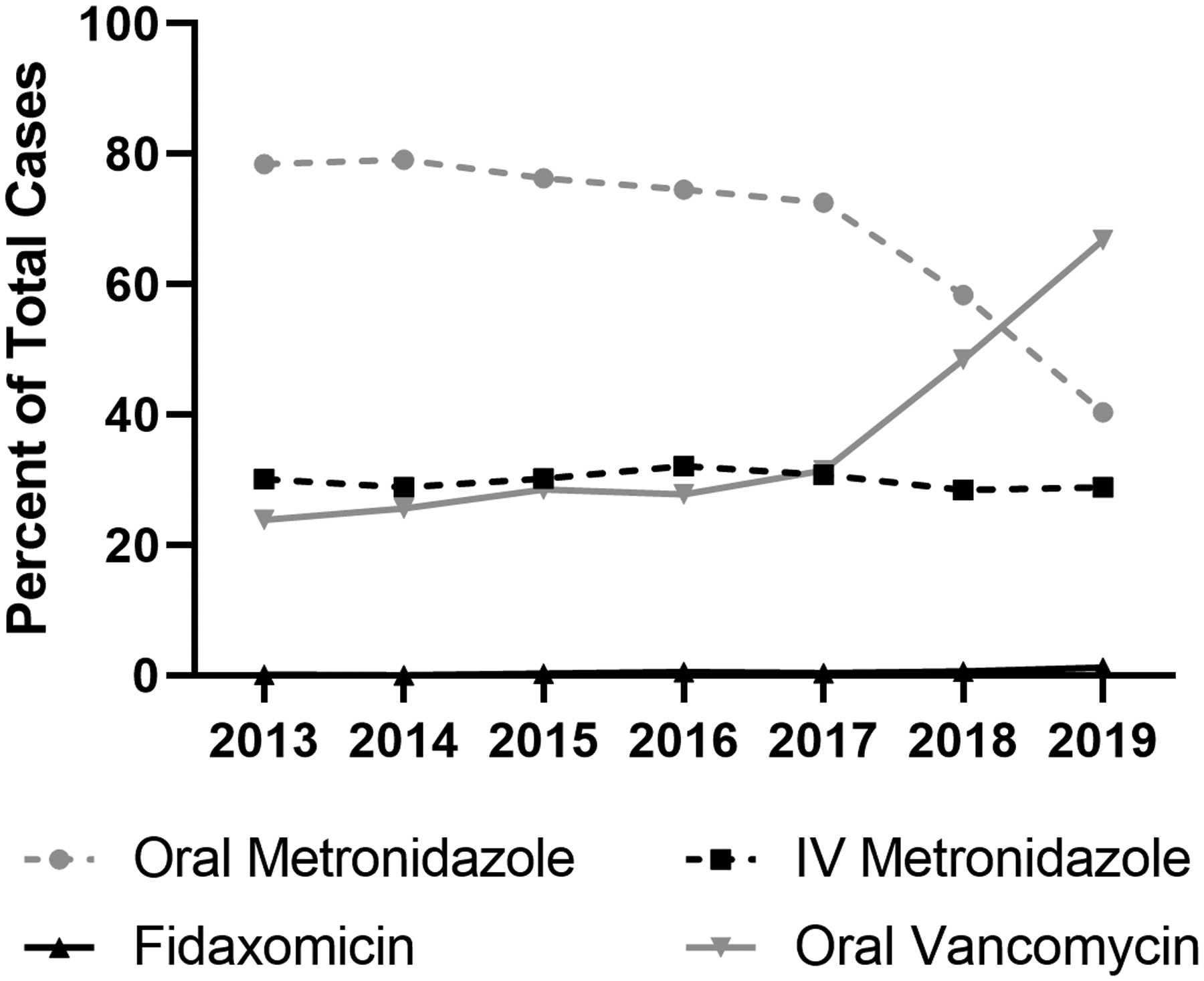
Antibiotic use for the treatment of C. difficile infection in hospitalized children from 2013 to 2019.
The median length of stay for patients with CDI was 7 days (IQR 3–18). Severe outcomes, such as colectomy (74 patients, 0.3%), toxic megacolon (29 patients, 0.1%), and gastrointestinal perforation (91 patients, 0.4%), were uncommon; 55 (74%) of patients who underwent a colectomy also had a diagnosis of inflammatory bowel disease (IBD). There were 429 (1.9%) patients who died of any cause during their hospitalization in the study period but CDI-related causality could not be determined.
Discussion
In 2019, the Centers for Disease Control and Prevention included CDI as one of only five infections in the United States deemed an urgent threat, the highest level of concern to human health.11 Concurrently, recent years have brought enhancements in antibiotic stewardship programs, infection control measures, CDI testing algorithms, and treatment approaches, all directly impacting the incidence and care of children with CDI. These factors illustrate the need for a contemporary assessment of pediatric CDI.
Despite previous studies demonstrating a significant increase in CDI incidence rates prior to 2013, there are limited recent data on CDI incidence, testing and treatment in hospitalized pediatric patients. Our very large cohort including 23,052 pediatric encounters with CDI from 42 tertiary care children’s hospitals across the United States identified a decrease in the adjusted rates of CDI in hospitalized children from 7.09 cases per 10,000 patient days (95% CI: 6.15 – 8.18) in 2013 to 4.89 cases per 10,000 patient days (95% CI: 4.03 – 5.93) in 2019 (p<0.001). Several different initiatives may have contributed to this declining trend in incidence including increased utilization of Antibiotic Stewardship Programs (ASPs), better targeted cleaning protocols, and additional hospital contact precaution policies and procedures. Recent pediatric studies have shown decreases in antibiotic prescribing in both inpatient and outpatient settings,12–14 an important factor when considering CDI risk and incidence in children.
Current data suggest that decreases in CDI incidence are most pronounced in those with healthcare-associated disease, defined as positive C. difficile testing collected > 3 days after admission to the facility.3 Guh et al demonstrated that a national decline in the burden of CDI in the United States from 2011 to 2017 was primarily related to a decline in healthcare-associated CDI with no significant change in community-associated CDI.15 Notably, although once considered primarily a hospital-acquired infection, community-associated (CA)-CDI has been demonstrated to represent 32–75% of CDI cases in population-based cohort studies.16–18 A recent pediatric case-control study of CA-CDI demonstrated a substantial increase from 9.6 per 100,000 children in 2012 to a peak of 16.9 per 100,000 children in 2015.19 As the PHIS database collects inpatient data only, we are unable to evaluate larger trends in CDI incidence in non-hospitalized children, a cohort that requires additional attention and may not be reflected in the trends reported through our study. It is possible that ASPs, which historically have been primarily hospital-based, may have contributed to the differences in CDI incidence in the hospital versus community settings,20,21 although many centers are now focusing on the outpatient antibiotic stewardship with increasing success.22
Of note, changes in incidence in our study may also be related to alterations in testing. C. difficile-specific testing decreased during the study period in all PHIS encounters but panel-based testing, interrogating for multiple enteric pathogens, increased during the study. The presence of C. difficile as a target on panel-based testing cannot be reliably determined through the PHIS database, nor can the precise results of laboratory testing, which are limitations of this study. Those use of alternative pediatric databases with laboratory results would be better suited to evaluate the type and frequency of C. difficile testing and its influence on current incidence rates, particularly considering changes in diagnostic algorithms in the last 10 years with movement from toxin-based testing, to nucleic-acid amplification-based testing (NAAT), and finally, to the use of multi-step algorithms.3,6 However, a recent study mostly in adults but including some children demonstrated that, even when adjusting for the increased sensitivity of NAAT-based testing, the overall incidence of CDI decreased by 24% (95% CI, 6 to 36) from 2011 to 2017.15
Notably, the incidence of CDI in our study area is also likely influenced by issues of C. difficile colonization, loosely defined as the presence of C. difficile in the intestinal microbiota without accompanied clinical symptoms. Colonization with C. difficile occurs more frequently in children <2 years of age and in children with recent hospitalizations or additional comorbidities.23 To date, current testing algorithms have demonstrated difficulty in the differentiation of C. difficile colonization versus infection24 and a careful analysis of clinical symptoms and pre-test probability is warranted for best CDI diagnostics. Clinical symptoms could not be identified through the PHIS database and some children were likely diagnosed and treated for CDI outside of the established CDI clinical definitions.3 It is also possible that the decreased incidence of CDI during the study period was influenced by increased recognition of C. difficile colonization, particularly in those under one year old, and more judicious testing and treatment. Our study identified a significant association of C. difficile specific testing rates and incidence in children 0–1 years old through the study period (Corr=0.99, p<0.001) which likely indicates the high rates of colonization, and therefore inter-connection of testing and incidence, in young children and infants.
Our study identified overall low rates of complications, similar to previous studies of CDI in children.4,25 Complications occurred during the admission with CDI, but timing of the complication in relation to CDI or CDI-related causality could not be fully evaluated using the PHIS database. Colectomy was documented in only 0.3% of the patients in our cohort, which is consistent with previous pediatric data ranging from 0.1% to 1.2% and adult data reporting 0.3% to 1.9%.2,4,26–28 However, most pediatric patients who had a colectomy in our cohort also had a diagnosis of, a known independent risk factor for colectomy.29 It remains unclear if CDI causes worsening of IBD or if patients with severe IBD are at increased risk of acquiring CDI, an area that requires additional study. The 382 (1.8%) inpatient deaths noted during the study period is consistent with prior reported rates of all-cause mortality in pediatric patients with CDI of 2–4%,1,4,30 which remains significantly lower than all-cause mortality related to CDI in adults (13.7%).25 Based on PHIS database limitations, we are unable to determine the cause of death in these patients or if CDI was a contributing factor; however, previous population-based pediatric studies have shown that CDI is an independent predictor of increased hospital stay and all-cause mortality, even when comorbidities are matched.1 Rates of complications should be interpreted with caution, as using ICD codes to identify complications has not been independently validated.
Although data vary between studies, antibiotic exposure, proton pump inhibitor use, healthcare exposure, and the presence of underlying comorbidities have all been associated with increased risk of CDI.3,31 Patients with gastrointestinal conditions, feeding tubes, and immunosuppressed status were highly represented in our study, and although a retrospective study without a matched cohort of patients without CDI cannot assess causality, these findings have been supported by other studies.32–35 The identification of these high risk pediatric populations is critically important, both as a reminder to minimize additional risk factors such as unnecessary antibiotics in these highly vulnerable children and for focus of microbiota-targeting prophylactic treatments. Conversely, 16% of encounters in our study were in children without a chronic condition. Therefore, CDI can and does occur in otherwise healthy children and an appropriate index of suspicious is warranted in any child with a fitting clinical history.
In the 2017 The Infectious Diseases Society of America and The Society for Healthcare Epidemiology of America Guidelines, recommendations were made for the use of oral vancomycin over metronidazole in adult patients for severe and non-severe episodes of CDI, although metronidazole use was still acceptable in the setting of non-severe pediatric CDI due to its high efficacy, especially in CA-CDI.3,19,35These recommendations may have influenced the increasing use of vancomycin noted in our study (Figure 3). Notably, since the release of these guidelines, additional pediatric data have demonstrated earlier symptom resolution associated with vancomycin use over metronidazole use in non-severe CDI,7 although larger studies are needed to guide practice patterns. In one cohort, transition from metronidazole to vancomycin was associated with lower rates of colectomy in adults with IBD, which also may inform prescribing practices in that subset of patients36. Fidaxomicin was infrequently used during our study period, but we anticipate that use will likely increase with a multicenter randomized control trial demonstrating higher rates of global cure for fidaxomicin versus vancomycin in children with CDI and recent Food and Drug Administration approval for pediatric use in 2020.37–39
Our study represents a large pediatric cohort, but reliance on billing, procedure codes, and the retrospective design are notable limitations in a study of this design. C. difficile testing could not be fully evaluated due to the initiation of multi-pathogen panels during the study period and differentiating colonization from CDI cannot be performed through the PHIS database. Finally, identifying complications, comorbidities, and outcomes from a retrospective database relies on accurate documentation of diagnosis codes during the hospital encounter, and sensitivity and specificity for CDI-related complication codes have not been established.
Although rare, severe CDI complications do occur in pediatric patients, and prospective studies will be important to risk-stratify underlying conditions and to test preventive measures for high-risk groups. Continued reduction in the incidence and improved outcomes associated with pediatric CDI will need collaborative efforts to reduce unnecessary antibiotic exposures, identify high-risk groups, tailor infection prevention strategies, evaluate testing practices, and improve prophylaxis and treatment.
Funding:
This work was supported by a National Institute of Health T32 (No. DK007664 to PTE), and an National Institute of Allergy and Infectious Diseases K23 award (No.1K23AI156132–01) to MRN.
Appreviations and Acronyms:
- CDI
C. difficile infection
- PCR
polymerase chain reaction
- COVID-19
- PHIS
Pediatric Health Information System
- ICU
intensive care unit
- ICD
International Classification of Disease
- CCC
complex chronic conditions
- IQR
interquartile range
- IBD
inflammatory bowel disease
- ASPs
antibiotic stewardship programs
- CA-CDI
community-associated CDI
- NAAT
nucleic-acid amplification-based testing
- HCST
hematopoietic stem cell transplantation
Footnotes
Conflict of interest disclosures: The authors declare no conflicts of interest.
Previous Presentations: Portions of this study were presented as a virtual poster (#665) during the North American Society of Pediatric Gastroenterology, Hepatology, and Nutrition (NASPGHAN) national meeting, 2020, virtual.
Data Sharing Statement:
Data are available upon request.
References
- 1.Sammons JS, Localio R, Xiao R, Coffin SE, Zaoutis T. Clostridium difficile infection is associated with increased risk of death and prolonged hospitalization in children. Clin Infect Dis 2013;57(1):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wendt JM, Cohen JA, Mu Y, et al. Clostridium difficile infection among children across diverse US geographic locations. Pediatrics 2014;133(4):651–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McDonald LC, Gerding DN, Johnson S, et al. Clinical Practice Guidelines for Clostridium difficile Infection in Adults and Children: 2017 Update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim J, Smathers SA, Prasad P, Leckerman KH, Coffin S, Zaoutis T. Epidemiological features of Clostridium difficile-associated disease among inpatients at children’s hospitals in the United States, 2001–2006. Pediatrics 2008;122(6):1266–1270. [DOI] [PubMed] [Google Scholar]
- 5.Khanna S, Baddour LM, Huskins WC, et al. The epidemiology of Clostridium difficile infection in children: a population-based study. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2013;56(10):1401–1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kelly CR, Fischer M, Allegretti JR, et al. ACG Clinical Guidelines: Prevention, Diagnosis, and Treatment of Clostridioides difficile Infections. The American journal of gastroenterology. 2021;116(6):1124–1147. [DOI] [PubMed] [Google Scholar]
- 7.Yin J, Kociolek LK, Same RG, Hsu AJ, Amoah J, Tamma PD. Oral Vancomycin May Be Associated With Earlier Symptom Resolution Than Metronidazole for Hospitalized Children With Nonsevere Clostridiodes difficile Infections. Open forum infectious diseases 2019;6(12):ofz492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shaklee J, Zerr DM, Elward A, et al. Improving surveillance for pediatric Clostridium difficile infection: derivation and validation of an accurate case-finding tool. The Pediatric infectious disease journal 2011;30(3):e38–40. [DOI] [PubMed] [Google Scholar]
- 9.Feudtner C, Feinstein JA, Zhong W, Hall M, Dai D. Pediatric complex chronic conditions classification system version 2: updated for ICD-10 and complex medical technology dependence and transplantation. BMC Pediatr 2014;14:199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feudtner C, Christakis DA, Connell FA. Pediatric deaths attributable to complex chronic conditions: a population-based study of Washington State, 1980–1997. Pediatrics 2000;106(1 Pt 2):205–209. [PubMed] [Google Scholar]
- 11.Prevention CfDCa. Antibiotic / Antimicrobial Resistance (AR / AMR); 2019 AR Threats Report. 2019; https://www.cdc.gov/drugresistance/biggest-threats.html#cdiff. [Google Scholar]
- 12.Finkelstein JA, Raebel MA, Nordin JD, Lakoma M, Young JG. Trends in Outpatient Antibiotic Use in 3 Health Plans. Pediatrics 2019;143(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Suda KJ, Hicks LA, Roberts RM, Hunkler RJ, Matusiak LM, Schumock GT. Antibiotic Expenditures by Medication, Class, and Healthcare Setting in the United States, 2010–2015. Clin Infect Dis 2018;66(2):185–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hales CM, Kit BK, Gu Q, Ogden CL. Trends in Prescription Medication Use Among Children and Adolescents-United States, 1999–2014. JAMA 2018;319(19):2009–2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guh AY, Mu Y, Winston LG, et al. Trends in U.S. Burden of Clostridioides difficile Infection and Outcomes. The New England journal of medicine. 2020;382(14):1320–1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khanna S, Pardi DS, Aronson SL, et al. The epidemiology of community-acquired Clostridium difficile infection: a population-based study. The American journal of gastroenterology. 2012;107(1):89–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lessa FC, Mu Y, Winston LG, et al. Determinants of Clostridium difficile Infection Incidence Across Diverse United States Geographic Locations. Open Forum Infect Dis 2014;1(2):ofu048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Benson L, Song X, Campos J, Singh N. Changing epidemiology of Clostridium difficile-associated disease in children. Infection control and hospital epidemiology : the official journal of the Society of Hospital Epidemiologists of America. 2007;28(11):1233–1235. [DOI] [PubMed] [Google Scholar]
- 19.Miranda-Katz M, Parmar D, Dang R, Alabaster A, Greenhow TL. Epidemiology and Risk Factors for Community Associated Clostridioides difficile in Children. J Pediatr 2020;221:99–106. [DOI] [PubMed] [Google Scholar]
- 20.Louh IK, Greendyke WG, Hermann EA, et al. Clostridium Difficile Infection in Acute Care Hospitals: Systematic Review and Best Practices for Prevention. Infect Control Hosp Epidemiol 2017;38(4):476–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Al-Omari A, Al Mutair A, Alhumaid S, et al. The impact of antimicrobial stewardship program implementation at four tertiary private hospitals: results of a five-years pre-post analysis. Antimicrob Resist Infect Control. 2020;9(1):95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Katz SE, Spencer P, Cates J, Harnack L, Xu M, Banerjee R. Improvements in appropriate ambulatory antibiotic prescribing using a bundled antibiotic stewardship intervention in general pediatrics practices. Infection control and hospital epidemiology : the official journal of the Society of Hospital Epidemiologists of America. 2022:1–7. [DOI] [PubMed] [Google Scholar]
- 23.Furuya-Kanamori L, Marquess J, Yakob L, et al. Asymptomatic Clostridium difficile colonization: epidemiology and clinical implications. BMC infectious diseases. 2015;15(1):516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Parnell JM, Fazili I, Bloch SC, et al. Two-step Testing for Clostridioides Difficile is Inadequate in Differentiating Infection From Colonization in Children. Journal of pediatric gastroenterology and nutrition. 2021;72(3):378–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van Dorp SM, Smajlovic E, Knetsch CW, Notermans DW, de Greeff SC, Kuijper EJ. Clinical and Microbiological Characteristics of Clostridium difficile Infection Among Hospitalized Children in the Netherlands. Clin Infect Dis. 2017;64(2):192–198. [DOI] [PubMed] [Google Scholar]
- 26.Nylund CM, Goudie A, Garza JM, Fairbrother G, Cohen MB. Clostridium difficile infection in hospitalized children in the United States. Arch Pediatr Adolesc Med 2011;165(5):451–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Muto CA, Pokrywka M, Shutt K, et al. A large outbreak of Clostridium difficile-associated disease with an unexpected proportion of deaths and colectomies at a teaching hospital following increased fluoroquinolone use. Infect Control Hosp Epidemiol 2005;26(3):273–280. [DOI] [PubMed] [Google Scholar]
- 28.Hensgens MP, Goorhuis A, Dekkers OM, van Benthem BH, Kuijper EJ. All-cause and disease-specific mortality in hospitalized patients with Clostridium difficile infection: a multicenter cohort study. Clin Infect Dis 2013;56(8):1108–1116. [DOI] [PubMed] [Google Scholar]
- 29.Rinawi F, Assa A, Eliakim R, et al. Risk of Colectomy in Patients With Pediatric-onset Ulcerative Colitis. J Pediatr Gastroenterol Nutr 2017;65(4):410–415. [DOI] [PubMed] [Google Scholar]
- 30.Kim J, Shaklee JF, Smathers S, et al. Risk factors and outcomes associated with severe clostridium difficile infection in children. The Pediatric infectious disease journal. 2012;31(2):134–138. [DOI] [PubMed] [Google Scholar]
- 31.Anjewierden S, Han Z, Foster CB, Pant C, Deshpande A. Risk factors for Clostridium difficile infection in pediatric inpatients: A meta-analysis and systematic review. Infect Control Hosp Epidemiol. 2019;40(4):420–426. [DOI] [PubMed] [Google Scholar]
- 32.Ochfeld E, Balmert LC, Patel SJ, Muller WJ, Kociolek LK. Risk factors for Clostridioides (Clostridium) difficile infection following solid organ transplantation in children. Transplant infectious disease : an official journal of the Transplantation Society. 2019;21(5):e13149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chandrakumar A, Zohni H, El-Matary W. Clostridioides difficile Infection in Children With Inflammatory Bowel Disease. Inflamm Bowel Dis 2019. [DOI] [PubMed] [Google Scholar]
- 34.Weng MK, Adkins SH, Bamberg W, et al. Risk factors for community-associated Clostridioides difficile infection in young children. Epidemiol Infect 2019;147:e172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Parmar D, Dang R, Miranda-Katz M, Alabaster A, Greenhow TL. Risk Factors for Recurrent Community-associated Clostridiodes Difficile Infection in Children. Pediatr Infect Dis J 2019;38(11):1073–1078. [DOI] [PubMed] [Google Scholar]
- 36.Issa M, Vijayapal A, Graham MB, et al. Impact of Clostridium difficile on inflammatory bowel disease. Clin Gastroenterol Hepatol 2007;5(3):345–351. [DOI] [PubMed] [Google Scholar]
- 37.Nelson RL, Suda KJ, Evans CT. Antibiotic treatment for Clostridium difficile-associated diarrhoea in adults. Cochrane Database Syst Rev 2017;3:CD004610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Louie TJ, Miller MA, Mullane KM, et al. Fidaxomicin versus vancomycin for Clostridium difficile infection. N Engl J Med 2011;364(5):422–431. [DOI] [PubMed] [Google Scholar]
- 39.Wolf J, Kalocsai K, Fortuny C, et al. Safety and Efficacy of Fidaxomicin and Vancomycin in Children and Adolescents with Clostridioides (Clostridium) difficile Infection: A Phase 3, Multicenter, Randomized, Single-blind Clinical Trial (SUNSHINE). Clin Infect Dis 2020;71(10):2581–2588. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available upon request.


