Abstract
Salvage chemotherapy followed by high-dose chemotherapy (HDC) and autologous stem cell transplantation (ASCT) is a potentially curative treatment for patients with relapsed or refractory large B-cell lymphoma (rrLBCL) with chemosensitive disease. A 18Fluoro-deoxyglucose positron emission tomography (PET) scan after salvage chemotherapy is used to assess response and eligibility for ASCT, but metrics for chemosensitivity in patients with residual disease are not well defined. We performed a single-center retrospective analysis of 92 patients with a partial response (PR) or stable disease (SD) after salvage chemotherapy for rrLBCL who received ASCT to investigate PET-derived parameters and their prognostic utility. The Deauville five-point score (5PS), maximum standardized uptake value (SUVmax), total metabolic tumor volume (TMTV), and total lesion glycolysis (TLG) were calculated from the post-salvage/pre-ASCT PET scan. Five-year progression free survival (PFS) and overall survival (OS) rates were 40% and 54%. 5PS of 5 (p=0.0082, HR 2.09), high SUVmax (p=0.0015, HR 2.48), TMTV (p=0.035, HR 1.83), and TLG (p=0.0036, HR 2.27) were associated with inferior PFS. 5PS of 5 (p=0.030, HR 1.98) and high SUVmax (p=0.0025, HR 2.55) were associated with inferior OS. PET-derived parameters may help prognosticate outcomes after ASCT in patients with rrLBCL with residual disease after salvage chemotherapy.
Keywords: PET scan, residual disease, large B-cell lymphoma, autologous stem cell transplant, Deauville five-point score, quantitative
Introduction
Historically, salvage chemotherapy followed by high dose chemotherapy and autologous stem cell transplant (ASCT) rescue for chemosensitive disease was the only potentially curative treatment option for relapsed or refractory large B-cell lymphoma (rrLBCL).1,2 Chemosensitivity is not well defined, though generally a 18F-flurodeoxyglucose (FDG) positron emission tomography (PET)/computed tomography scan is performed after salvage chemotherapy to assess treatment response and eligibility for ASCT.3–5 The advent of CD19-directed chimeric antigen receptor T-cell therapy (CART19) as another potentially curative treatment modality for rrLBCL after two or more prior lines of therapy6–8 has dampened enthusiasm for ASCT in patients not achieving a complete response (CR) after salvage chemotherapy. While multiple retrospective studies have demonstrated that patients with a partial response (PR) on pre-ASCT PET imaging can experience durable remissions in 30–50% of cases,9–11 there are many publications correlating improved outcomes with documentation of a PET-negative CR prior to transplant.12–16 A recent registry-based analysis of patients who received CART19 or ASCT after a documented PR found that consolidative ASCT after salvage therapy led to similar outcomes as CART19, with a potential lower risk of progression and longer overall survival (OS).17
Quantitative PET parameters include the maximum standardized uptake value (SUVmax), which is measurement of peak tumor glucose metabolism. Total metabolic tumor volume (TMTV) and total lesion glycolysis (TLG) are SUV-based metrics meant to capture overall metabolic tumor burden; TMTV (and TLG) from baseline PET scans before frontline DLBCL therapy have been shown to reliably risk stratify patients from prospective18,19 and retrospective cohorts,20,21 where patients with higher tumor bulk experienced inferior outcomes. TMTV before CART19 was also similarly prognostic.22,23 Prior studies have not comprehensively analyzed PET-derived metrics from rrLBCL patients before ASCT.9–11,17
We hypothesized that the pre-ASCT PET scan could be used to prognosticate outcomes for patients with rrLBCL and residual disease after salvage chemotherapy. We performed a single-center retrospective analysis of patients with a disease response of PR or stable disease (SD) on PET scan after salvage chemotherapy who subsequently received ASCT consolidation, with a focus on qualitative and quantitative PET-derived metrics.
Methods
Patients
We retrospectively identified patients aged ≥18 years with rrLBCL (including diffuse large B-cell lymphoma [DLBCL], primary mediastinal B-cell lymphoma [PMBCL], and transformed indolent B-cell non-Hodgkin lymphoma [TiNHL]) with a disease response of PR or SD on PET scan after salvage chemotherapy who received high dose conditioning chemotherapy and ASCT at our institution between 2010 and 2020. Patients with a CR (5PS 1–3) or progressive disease on PET were excluded. Conditioning regimens were classified as standard (carmustine, etoposide, cytarabine, and melphalan [BEAM]), reduced-dose BEAM, or intensive (containing gemcitabine/busulfan/melphalan. Disease was classified as early-relapsing if refractory to frontline therapy or relapsing within 12 months of frontline therapy and late-relapsing if relapsing more than 12 months from frontline therapy.
Imaging analysis
All patients had an FDG PET scan performed for response assessment at our institution using standardized techniques after at least 2 cycles of salvage chemotherapy. Response status and visual assessment with the five-point scale (5PS) were recorded in accordance with Lugano classification,4 including retrospectively in PET scans originally performed before 2014. Patients were allowed to have received one additional cycle of immunochemotherapy after PET scan for chemomobilization purposes. An expert nuclear radiologist (GX) analyzed all pre-ASCT scans using MIM Encore (MIM Software, Cleveland, OH) with the semiautomated approach with 41% maximum standardized uptake threshold (SUVmax).24,25 In brief, voxels with an SUV value greater than 41% of SUVmax were automatically identified as the regions of interest followed by visual assessment to manually remove areas of physiologic (non-pathologic) uptake. Total metabolic tumor volume (TMTV), average SUV (SUVmean), and total lesion glycolysis (TLG = TMTV * SUVmean) were then automatically calculated. High SUVmax, TMTV, and TLG were defined as values above the 75th percentile (upper quartile) of these values’ distributions. Response to ASCT was determined by PET scan performed on approximately day 30 after ASCT.
Statistical analysis
Patient demographics, disease characteristics, and clinical outcomes were summarized through descriptive statistics. The Chi-square test or Fisher’s exact test was used to evaluate the association between two categorical variables. Wilcoxon rank sum test was used to evaluate the difference in a continuous variable between patient groups. Logarithmic transformation was performed on TLG and TMTV to transform skewed data to approximately conform to normality for the purposes of associating continuous data with outcomes. Receipt of consolidative radiation therapy after ASCT was not analyzed for association with outcomes because inherent selection bias in this post-ASCT treatment decision.
Co-primary endpoints were post-ASCT CR rate and PFS. PFS was defined as time from ASCT to disease progression/relapse or death from any cause, whichever happened first. OS was defined as time from ASCT to death from any cause. Kaplan-Meier method was used for time-to-event analysis. The Log-rank test was used to evaluate the difference in time-to-event endpoints between patient groups. Cox proportional hazards models were used for multivariable analysis. The Schoenfeld residual was used to check the proportional hazards assumption. The variables which had p-value less than 0.2 for PFS or OS from univariate analysis were included in the initial full multivariable model. A backward selection method was used and a significance level of 0.05 was set as the criterion for a variable to stay in the model. Collinearity diagnostics were performed and indicated no collinearity problem. TLG was excluded from multivariate analysis because TMTV is used in its calculation. Multivariable analysis was not performed for subset analysis of only patients with late-relapsing disease because of low numbers of events. Statistical software used included SAS 9.4 (SAS, Cary, NC), S-Plus 8.2 (TIBCO Software Inc., Palo Alto, CA) and R 4.1.2 (R Core Team, Vienna, Austria).
Results
Ninety-two patients with a diagnosis of rrLBCL with either PR or SD after salvage therapy who received ASCT from 4/2010 to 6/2020 were analyzed. An additional 489 patients without PR or SD received ASCT for rrLBCL over the same period at our institution. Patient demographics and disease characteristics (at time of ASCT, when applicable) are summarized in Table 1. Median age at ASCT was 59 years (range 26–77), 65 (71%) of patients were male, 31 (34%) of patients had stage III/IV disease, and 17 (19%) an international prognostic index (IPI) > 2. A total of 26 (28%) of patients experienced late first relapse; 35 (38%), 48 (52%), and 9 (10%) received intensive, standard BEAM, and reduced intensity BEAM conditioning, respectively. Planned consolidative radiation after ASCT was delivered to 11 patients (12%).
Table 1.
Patient, disease, and treatment characteristics (N=92)
| Patient and disease characteristics a | N (%) |
|---|---|
|
| |
| Median age [range] | 58.5 [26–77] |
| Age > 60 | 36 (39) |
|
| |
| Male sex | 65 (71) |
|
| |
| Ann Arbor Stage | |
| I | 37 (40) |
| II | 24 (26) |
| III | 15 (16) |
| IV | 16 (17) |
|
| |
| Lactate dehydrogenase > ULN | 51 (55) |
|
| |
| Extranodal sites > 1 | 7 (8) |
|
| |
| ECOG performance status | |
| 0 | 34 (37) |
| 1 | 52 (56) |
| 2 | 6 (7) |
|
| |
| Karnofsky performance status (N=77) | |
| 100% | 24 (31) |
| 90% | 26 (34) |
| 80% | 20 (26) |
| 70% | 7 (9) |
|
| |
| International prognostic index | |
| 0–1 | 55 (60) |
| 2 | 20 (22) |
| 3 | 17 (18) |
|
| |
| Tumor bulk > 5 cm (N=70) | 47 (67) |
|
| |
| Cell of origin | |
| GCB | 40 (44) |
| Non-GCB | 17 (19) |
| NA | 35 (38) |
|
| |
| Histology | |
| DLBCL | 51 (55) |
| PMBCL | 8 (9) |
| TiNHL | 33 (36) |
|
| |
| Treatment characteristics | |
|
| |
| Frontline therapy | |
| R-CHOP-like | 64 (70) |
| Intensiveb | 20 (22) |
| Other | 8 (9) |
|
| |
| Frontline response | |
| Early relapse/refractory | 66 (72) |
| Late relapse | 26 (28) |
|
| |
| Prior radiation therapy | 17 (19) |
|
| |
| Prior systemic lines of therapy | |
| 2 | 50 (54) |
| 3 | 28 (30) |
| 4 | 12 (13) |
| 5 | 2 (2) |
|
| |
| Conditioning type | |
| Standard BEAM | 48 (52) |
| Reduced intensity BEAMc | 9 (10) |
| Intensive | 35 (38) |
|
| |
| Transplant year | |
| 2010–2011 | 23 (25) |
| 2012–2013 | 28 (30) |
| 2014–2015 | 16 (17) |
| 2016–2017 | 8 (9) |
| 2018–2020 | 17 (19) |
|
| |
| Consolidative radiation therapy | 11 (12) |
N, number; GCB, germinal center B-cell; DLBCL, diffuse large B-cell lymphoma; PMBCL; primary mediastinal B-cell lymphoma; TiNHL, transformed indolent non-Hodgkin lymphoma; ULN, upper limit of normal; ECOG, eastern cooperative oncology group, R-CHOP; rituximab, cyclophosphamide, doxorubicin, vincristine, prednisone; BEAM, carmustine, etoposide, cytarabine, melphalan
Characteristics were recorded at time of transplant (when applicable
Intensive frontline therapy includes R-EPOCH and R-hyperCVAD based regimens
Reduced intensity BEAM dosage was as follows: Carmustine 300 mg/ m2 IV over 1 hour on day −6, cytarabine 100 mg/m2 IV twice a day on days −5 through −2 (total 8 doses), etoposide 100 mg/m2 IV twice a day on days −5 through −2 (total 8 doses), and melphalan 140 mg/ m2 IV on day −1
On the pre-ASCT PET scan, 23 (25%) patients had SD and 23 (25%) a 5PS of 5 (Table 2). Pre-ASCT PET response and 5PS were not significantly associated (p=0.095, odds ratio [OR] of patient with SD having a 5PS of 5 was 2.52 95% CI 0.91–7.02). Median SUVmax was 6.1 (range 2.8–27, interquartile range [IQR] 4.3–11.0), median TMTV was 9.1 cc (range 0.1–1189, IQR 3.9–29.5), and median TLG was 33.5 (range 0.54–3945, IQR 13.0–110.5) (Table 3). Distribution of SUVmax, TMTV, and TLG values are depicted in Figure 1A–C. High SUVmax, TMTV, and TLG were defined as values greater than the 75th percentile: 11.0, 29.5 cc, and 110.5, respectively. 5PS was associated with type of conditioning regimen (p=0.005); 6/48 (13%), 12/35 (34%), and 5/9 (56%) of patients who received standard BEAM, intensive, and reduced intensity BEAM conditioning respectively had a 5PS of 5. Elevated LDH was associated TLG (p=0.050) but not SUVmax and TMTV as continuous variables, nor 5PS (4 vs. 5) as a categorical variable.
Table 2.
Distribution of patients stratified by pre-ASCT PET response and five-point score
| PET 5-point score = 4 | PET 5-point score = 5 | Total (%) | |
|---|---|---|---|
|
| |||
| PET response = PR | 55 | 14 | 69 (75) |
|
|
|||
| PET response = SD | 14 | 9 | 23 (25) |
|
| |||
| Total (%) | 69 (75) | 23 (25) | 92 (100) |
|
|
|||
PET, positron emission tomography; PR, partial response; SD, stable disease
Table 3.
PET-derived metrics from patients before transplant
| Characteristics | Median | Range | Interquartile range |
|---|---|---|---|
| SUVmax | 6.1 | 2.8–27.0 | 4.3–11.0 |
| SUVmean | 3.4 | 1.7–17.0 | 2.7–5.6 |
| TMTV, cc | 9.1 | 0.1–1189.0 | 3.9–29.5 |
| TLG | 33.5 | 0.5–3945.0 | 13.0–110.5 |
PET, positron emission tomography; SUV, standardized uptake value; TMTV; total metabolic tumor volume; cc, cubic centimeters; log, logarithmic transformation; N, number; PR, partial response; SD, stable disease
Figure 1.
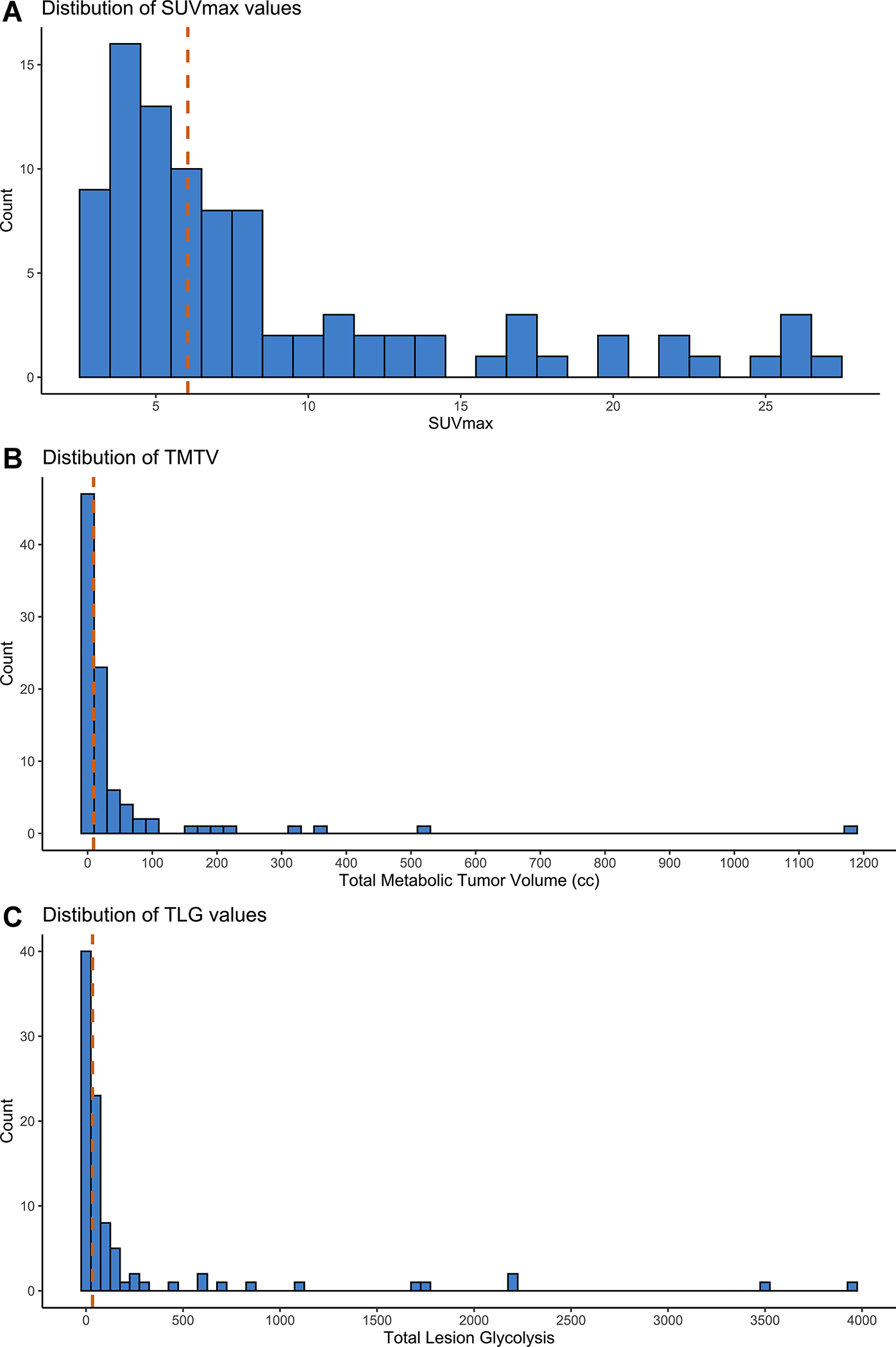
Distribution of quantitative PET-derived parameters. Histograms of maximum standardized uptake (A), total metabolic tumor volume (B), and total lesion glycolysis (C) for the entire cohort. Vertical dashed lines delineate median values.
A total of 90 patients were evaluable for post-ASCT response by PET scan (2 died before evaluation from transplant-related complications). The CR rate was 72.2% (95% CI 61.8–81.1%) and the overall response rate (ORR) was 84.4% (95% CI 75.3–91.2%). Characteristics associated with lower rate of post-ASCT CR were early relapse after frontline therapy (p=0.009, OR 0.16 95% CI 0.03–0.73) and high SUVmax (p=0.004, OR 0.23 95% CI 0.08–0.65). Other characteristics as well as 5PS of 5, High TMTV and high TLG were not significantly associated with lower CR rate (Table S1). SUVmax was higher (median 10 vs. 5.4, p=0.0001) in patients who did not achieve CR post-ASCT compared to those who did.
With a median follow-up of 6.6 years (95% CI 5.9–8.0), 56 patients experienced a PFS event and 45 patients died. Median PFS and OS were 1.0 (95% CI 0.5–5.2) and 7.7 (95% CI 2.4-NA) years, respectively. Five-year PFS and OS rates were 40% (95% CI 30–51%) and 54% (95% CI 44–65%), respectively (Figure 2A–B).
Figure 2.
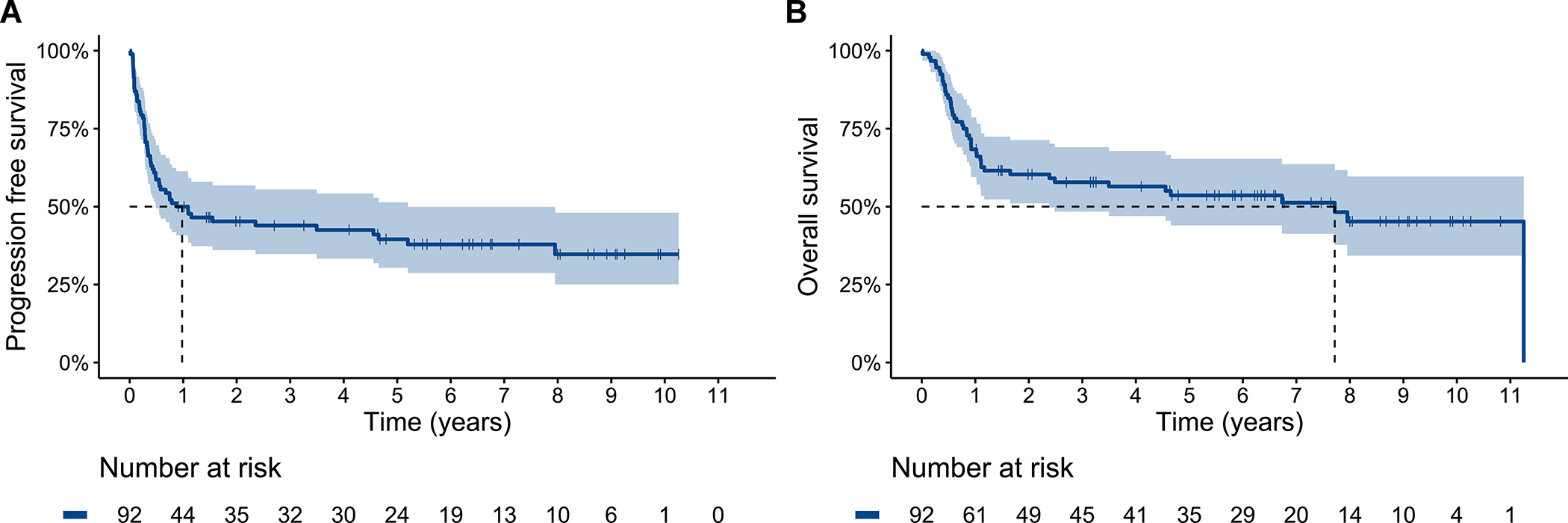
Progression free (A) and overall (B) survival for the entire cohort. Dashed line delineates median survival time.
By univariate analysis for PFS, male sex (p=0.0036, HR 2.67 95% CI 1.34–5.30), elevated Eastern Cooperative Oncology Group (ECOG) performance status (p=0.0038, 1 vs. 0 HR 2.35 95% CI 1.27–4.36 | 2 vs. 0 HR 2.13 95% CI 1.26–3.60), and IPI >2 (p=0.032, HR 1.96 95% CI 1.05–3.66) were associated with shorter PFS. The association between conditioning regimen type and PFS was significant (p=0.019, reduced intensity vs. standard HR 2.84 95% CI 1.27–6.36 | intensive vs. standard HR 1.72 95% CI 0.98–3.05) (Figure S1A).
By pre-ASCT PET metrics, 5PS of 5 (p=0.0082, HR 2.09 95% CI 1.20–3.65, Figure 3A), high SUVmax (p=0.0015, HR 2.48 95% CI 1.39–4.41, Figure 3B), high TMTV (p=0.035, HR 1.83 95% CI 1.03–3.24, Figure 3C), and high TLG (p=0.0036, HR 2.27 95% CI 1.29–3.99, Figure 3D) were significantly associated with shorter PFS but response of SD (p=0.70, HR 0.89 95% CI 0.48–1.63, Figure S2) was not. PFS by SUVmax, TMTV, and TLG quartiles are depicted in Figure S3A–C; PFS was not significantly different between quartiles 1–3 for each of these parameters. As a continuous variable, increasing SUVmax (p=0.0006, HR 1.07, 95% CI 1.03–1.12) and increasing logTLG (p=0.017, HR 1.22 95% CI 1.04–1.43) were associated with shorter PFS.
Figure 3.
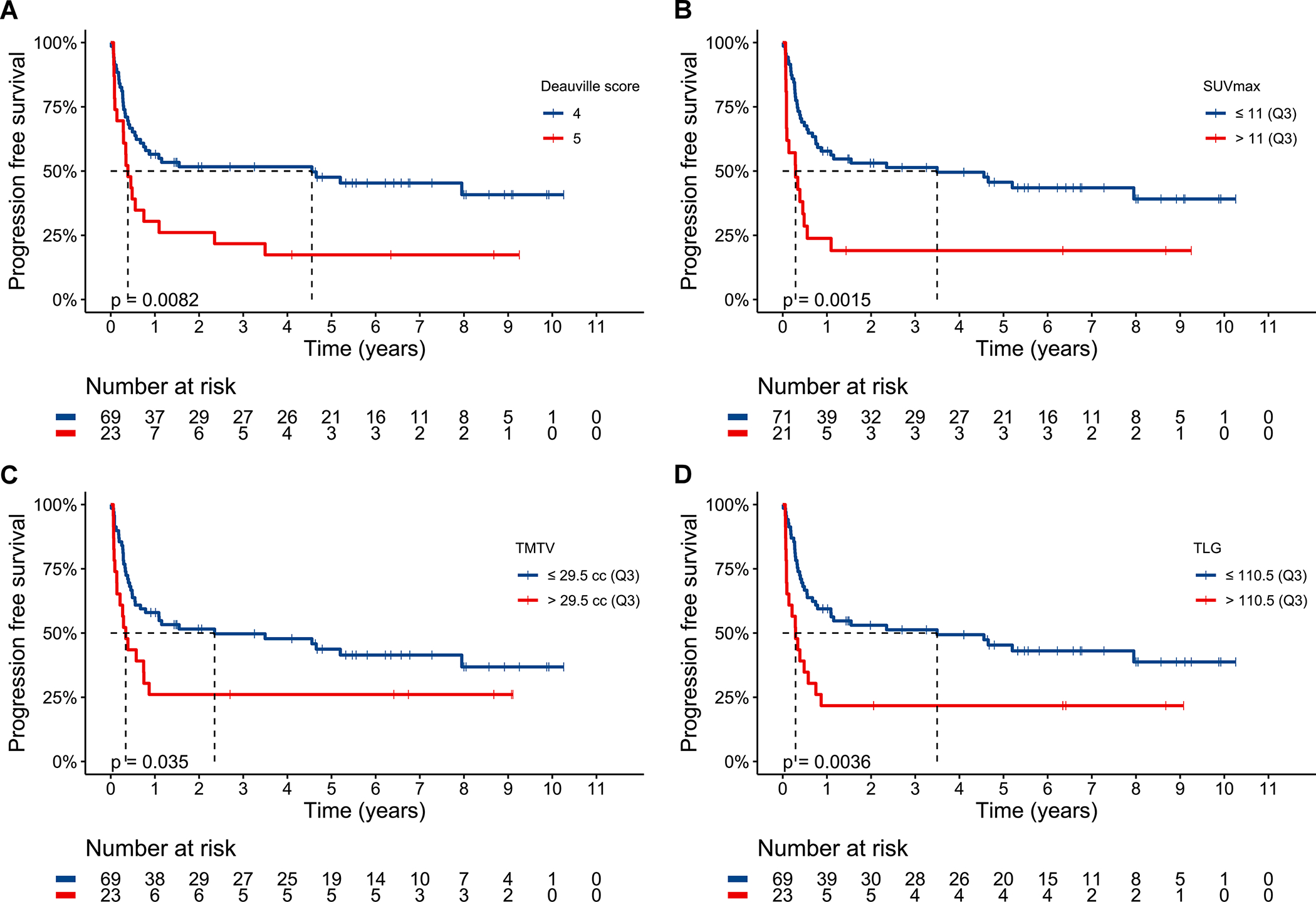
Progression free survival for the entire cohort stratified by Deauville 5-point score (A), 75th percentile maximum standardized uptake value (B), 75th percentile total metabolic tumor volume (C), and 75th percentile total lesion glycolysis (D). Dashed line delineates median survival time. Median PFS for 5PS of 5 was 0.4 years (95% CI 0.3–2.4) and for 5PS of 4 was 4.6 years (95% CI 0.8-NA). Median PFS for high SUVmax was 0.3 years (95% CI 0.09–1.1) and for low SUVmax was 3.5 years (95% CI 0.8-NA). Median PFS for high TMTV was 0.3 years (95% CI 0.1-NA) and for low TMTV was 2.4 years (95% CI 0.7-NA). Median PFS for high TLG was 0.3 years (95% CI 0.1–0.9 years) and for low TLG was 3.5 years (95% CI 0.8-NA).
By multivariate analysis for PFS, the final model selected included the covariates of sex (male vs. female, p=0.0047), IPI (>2 vs. ≤2, p=0.0036), and high SUVmax (>11 vs. ≤11, p=0.019). Association between PFS and covariates of note by univariate and multivariate analysis is summarized in Table S2.
By univariate analysis for OS, early relapse after frontline therapy (p=0.048, HR 2.13 95% Ci 0.99–4.59), receipt of > 2 prior lines of therapy (p=0.018, HR 2.03 95% CI 1.12–3.69), Karnofsky performance status of < 80% (p=0.0023, HR 3.66 95% CI 1.51–8.90), elevated ECOG PS (p=0.0010, 1 vs. 0 HR 1.79 95% CI 0.90–3.53 | 2 vs. 0 HR 2.22 95% CI 1.30–3.79), stage III/IV disease (p=0.011, HR 2.13 95% CI 1.17–3.86), and IPI > 2 (p=0.0021, HR 2.76 95% CI 1.41–5.40) were associated with shorter OS. The association between conditioning regimen and OS was significant (p=0.017, reduced intensity vs. standard HR 2.98 95% CI 1.17–7.58 | intensive vs. standard HR 2.21 95% CI 1.12–4.03) (Figure S1B).
By pre-ASCT PET metrics, 5PS of 5 (p=0.030, HR 1.98 95% CI 1.06–3.70, Figure 4A) and high SUVmax (p=0.0025, HR 2.55 95% CI 1.36–4.79, Figure 4B) were significantly associated with shorter OS but high TMTV (p=0.076, HR 1.76 95% CI 0.93–3.33, Figure S4A), high TLG (p=0.051, HR 1.87 95% CI 0.99–3.53, Figure S4B), and response of SD (p=0.72, HR 0.88 95% CI 0.45–1.75, Figure S4C) were not. As a continuous variable, increasing SUVmax (p=0.0025, HR 1.07 95% CI 1.02–1.11) was associated with shorter OS.
Figure 4.
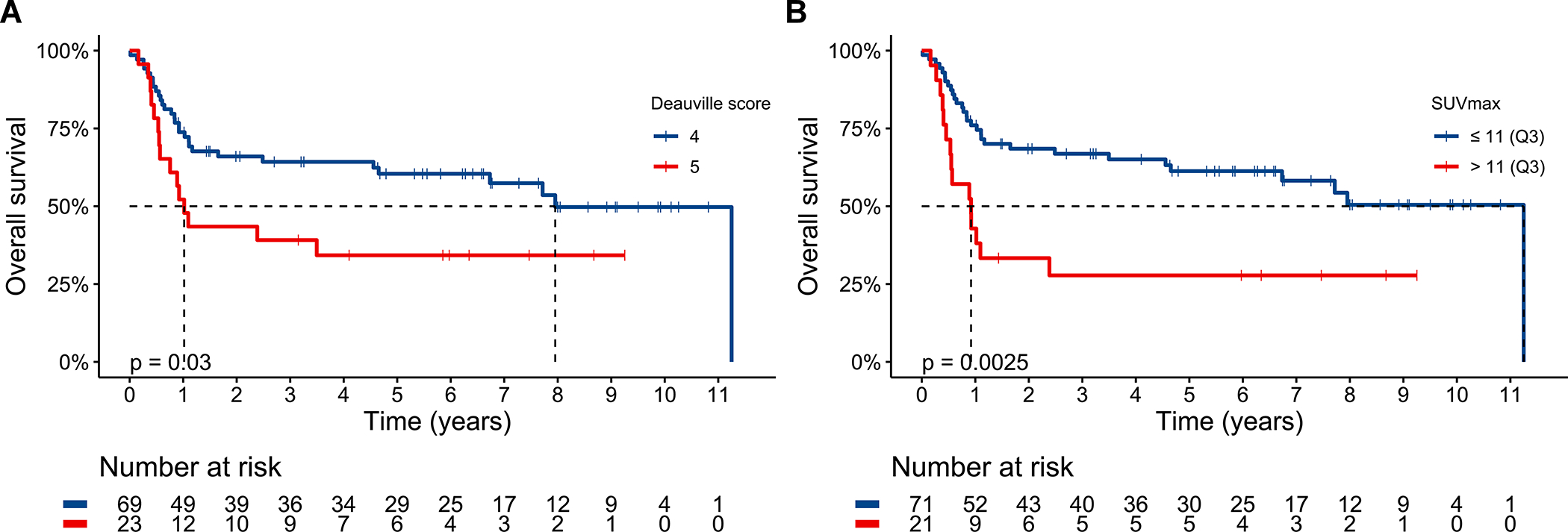
Overall survival for the entire cohort stratified by Deauville 5-point score (A) and 75th percentile maximum standardized uptake value (B). Dashed line delineates median survival time. Median OS for 5PS of 5 was 1.0 years (95% CI 0.6-NA) and 5PS of 4 was 8.0 years (95% CI 4.7-NA). Median OS for high SUVmax was 0.9 years (95% CI 0.5-NA) and for low SUVmax was 11.3 years (95% CI 6.7-NA).
By multivariate analysis for OS, the final model selected included the covariates of ECOG performance status (p=0.0079), stage (III/IV vs. I/II, p=0.0072), and high SUVmax (>11 vs. ≤11, p=0.0096). Association between OS and covariates of note by univariate and multivariate analysis is summarized in Table S3.
A comparison between the 66 (72%) of patients with early first relapse and the 26 (29%) with late first relapse is described in Table S4. Patients with late first relapse were more likely to have received standard BEAM conditioning (p=0.0086) and less likely to have high SUVmax (p=0.031). Restricting analysis to only the patients with late first relapse, SUVmax was higher (median 11.2 vs. 5.1, p=0.040) for patients who did not achieve CR after ASCT compared to those who did. The only covariates associated with shorter PFS were 5PS of 5 (p=0.018, HR 4.69 95% CI 1.15–19.07, Figure S5A) and high SUVmax (p=0.021, HR 5.65 95% CI 1.08–29.51, Figure S5B).
PFS and OS for all patients and for those with late first relapse stratified by both pre-ASCT 5PS and PET response is displayed in Figure S6A–D. Though a response of SD to salvage chemotherapy was not associated with inferior post-ASCT outcomes, a 5PS of 5 signifying metabolically active disease was a negative prognostic marker regardless of response category or time to first relapse.
Discussion
In this single-center retrospective analysis, we analyzed a cohort of 92 patients with rrLBCL and residual disease (PR or SD) on PET scan after salvage therapy who subsequently received high dose chemotherapy and ASCT rescue. We observed durable remission rates in treated patients with 5-year PFS and OS rates of 40% and 54%, respectively. PET-derived parameters collected from the pre-ASCT scan included Deauville 5PS, SUVmax, TMTV, and TLG. High SUVmax was associated with lower rate of CR after ASCT. A 5PS of 5, high SUVmax, high TMTV, and high TLG were associated with shorter PFS, and 5PS of 5 and high SUVmax were associated with shorter OS; high SUVmax remained associated with PFS/OS when adjusting for other covariates. Elevated quantitative PET-metrics were defined as those values above the 75th percentile, thus identifying a high-risk quarter of the cohort with significantly worse outcomes compared to the remaining majority of ASCT recipients.
A response of SD to salvage therapy was not significantly associated with shorter survival compared to a response of PR, suggesting that there may not be a significant difference in chemosensitivity between these two response categories. When limiting analysis only to patients with late relapse after frontline therapy, 5PS and SUVmax were the only factors associated with PFS.
For this cohort, it was clear that patients with significant FDG-uptake on PET scan by visual assessment (Deauville 5PS of 5) and the semiquantitative SUVmax reflective of metabolically active disease experienced poor outcomes irrespective of time to first relapse. The quantitative metric of TMTV was not as prognostic as SUVmax. This finding could be explained by bias inherent in patient selection; with few exceptions, only patients with low volume residual disease would be considered for ASCT, and the distribution of TMTV (and TLG) values in this cohort reflects this bias. Without enough variation in volume of disease between patients, TMTV may be less useful for risk stratification. Studies demonstrating the utility of TMTV analyzed PET scans performed before initiation of anti-lymphoma therapy,18–21 but our study focused on the PET scan performed after salvage therapy for rrLBCL. In comparison, SUVmax values were more evenly distributed. Thus, in the setting of most patients harboring low volume disease, metabolic activity on PET may be a better discriminatory parameter compared to measuring tumor volume. An analysis performed by Brown and colleagues26 found that TMTV was associated with PFS and OS but included patients in a CR before ASCT.
Other limitations of our study stem from its single-institution retrospective nature; there was heterogeneity in patient management surrounding conditioning regimens which was influenced by clinician perception of individual patient risk. Conditioning type was not significantly associated with survival by multivariate analysis; it appears that the worse outcomes experienced by patients receiving intensive conditioning was driven by high-risk disease characteristics such as high SUVmax. Though year of transplant was not associated with survival outcomes, there may have been shifts in patient selection for ASCT starting in 2017 after the approval of CART19 for rrLBCL. A cohort size of 92 patients precluded using statistical methods to identify and validate optimal cutoffs in SUVmax, TMTV, or TLG. Our chosen threshold of 75th percentile identified a high-risk subset of patients, but the lower 3 quartiles experienced similar outcomes. Without validation with a larger independent cohort, use of specific PET-derived metric cutoffs for clinical decision-making is not supported. There were only 26 patients with late first relapse included in that subset analysis, so outcomes analyses lacked statistical power. Though the semiautomated approach using a cutoff of 41% SUVmax to identify regions of interest is widely used in the research setting,27–29 calculation of TMTV and TLG is not standardized nor widely available in routine clinical practice as standard of care.
Reports from 3 randomized studies represent the first time the platform of salvage chemotherapy plus ASCT has been compared to CART19 in the prospective second-line setting,30–32 though in all cases, patients randomized to the control arm only received ASCT if they were responsive to salvage therapy. The ZUMA-7 trial of axicabtagene ciloleucel (axi-cel) and TRANSFORM trial of lisocabtagene maraleucel (liso-cel) versus salvage chemotherapy and ASCT has led to the recent Food and Drug Administration approval of axi-cel and liso-cel in the second-line setting for patients with disease that is refractory to or relapsing within one year of initial therapy; optimal therapy for transplant-eligible patients with more chemosensitive disease and late first relapse is still unclear.
With the advent of CART19, patients with rrLBCL after frontline therapy now have multiple treatment options that can lead to cure. Several novel agents have also been approved in the last several years,33–35 adding to the chemotherapy-free options for patients. Gaining an understanding of which patients should be prioritized for ASCT versus non-chemotherapy treatments is critical; this is particularly true for patients with late first relapse ineligible for axi-cel or liso-cel in the second line setting who may be attractive candidates for salvage chemotherapy given their prior chemosensitivity. Furthermore, despite the approval of CART19 in the second line, in routine practice many patients could still receive at least one cycle of salvage chemotherapy to “bridge” them while being referred to a cellular therapy center. If those patients demonstrate a significant response on restaging, then ASCT should still be considered as a consolidative treatment option.
The strong association between Deauville 5PS and SUVmax in our cohort with PFS (including for late relapsers) and ease of interpretation suggests they are useful parameters for determining suitability for ASCT in patients with rrLBCL and residual disease. There was a high degree of overlap between 5PS of 5 and high SUVmax within this cohort, so 5PS may serve a similar purpose to SUVmax in risk-stratifying patients particularly if the latter is unavailable. Patients with a 5PS of 5 and/or high SUVmax after salvage therapy are likely better served with non-chemotherapy treatments such as CART19. Conversely, there are clearly some patients who still benefit from ASCT consolidation if they have a 5PS of 4 after salvage, even if their disease response is only SD. Without a prospective study randomizing patients who have responded to salvage chemotherapy to CART19 or ASCT, the optimal approach for said patient population is unclear. Thankfully, CART19 remains an effective treatment option for patients who relapse after ASCT.
In summary, a significant proportion of patients with rrLBCL with residual disease after salvage chemotherapy can still experience durable remissions after ASCT consolidation. From the pre-ASCT PET scan, qualitative visual assessment with the Deauville 5PS criteria and the quantitative variable of SUVmax identified a high-risk subset of patients with inferior outcomes after ASCT. Further research is still needed to develop and validate functional imaging as a prognostic tool after salvage chemotherapy in rrLBCL to guide treatment decision making.
Supplementary Material
Acknowledgments
This research was funded by the MD Anderson NIH/NCI Cancer Support Grant under award number P30 CA016672. PS salary is supported by a Lymphoma Research Foundation Career Development Award and an NIH R21.
Footnotes
Conflicts of interest
RS has received research funding from BMS, Seagen, GSK and Rafale pharmaceuticals. PS has received research funding from Astrazeneca Acerta, Sobi, ALX Oncology, ADC Therapeutics and note advisory board participation/consultancy for Hutchinson Medipharma, ADC Therapeutics, TG Therapeutics, Incyte Morphosis, Roche Genentech. CF reports consultancy for AstraZeneca, Bayer, BeiGene, BioAscend, Bristol Myers Squibb, Celgene, Curio Sciences, Denovo Biopharma, Epizyme/Incyte, Foresight Diagnostics, Genentech/Roche, Genmab, MEI Pharmaceuticals, MorphoSys AG, Pharmacyclics/Janssen, SeaGen, and research funding from 4D, Abbvie, Acerta, Adaptimmune, Allogene, Amgen, Bayer, Celgene, Cellectis, EMD, Gilead, Genentech/Roche, Guardant, Iovance, Janssen Pharmaceutical, Kite, Morphosys, Nektar, Novartis, Pfizer, Pharmacyclics, Sanofi, Takeda, TGTherapeutics, Xencor, Ziopharm, Burroughs Wellcome Fund, Eastern Cooperative Oncology Group, National Cancer Institute, V Foundation, Cancer Prevention and Research Institute of Texas: CPRIT Scholar in Cancer Research. MW reports consultancy for AbbVie, AstraZeneca, BeiGene, BioInvent, CSTone, Deciphera, DTRM Biopharma (Cayman) Limited, Epizyme, Genentech, InnoCare, Janssen, Juno Therapeutics, Kite Pharma, Lilly, Loxo Oncology, Miltenyi Biomedicine GmbH, Oncternal, Pepromene Bio, Pharmacyclics, VelosBio, research support from Acerta Pharma, AstraZeneca, BeiGene, BioInvent, Celgene, Genmab, Genentech, Innocare, Janssen, Juno Therapeutics, Kite Pharma, Lilly, Loxo Oncology, Molecular Templates, Oncternal, Pharmacyclics, VelosBio, Vincerx, and honoraria from Acerta Pharma, Anticancer Association, AstraZeneca, BeiGene, BGICS, BioInvent, CAHON, Clinical Care Options, Dava Oncology, Eastern Virginia Medical School, Epizyme, Hebei Cancer Prevention Federation, Imedex, Janssen, Kite Pharma, Leukemia & Lymphoma Society, LLC TS Oncology, Medscape, Meeting Minds Experts, Miltenyi Biomedicine GmbH, First Hospital Zhejiang University, Moffit Cancer Center, Mumbai Hematology Group, OMI, OncLive, Pharmacyclics, Physicians Education Resources (PER), Practice Point Communications (PPC). The other authors have no potential conflicts of interest to declare. PK has received research funding from Ziopharm, Amgen and notes advisory board participation for Kite, Pfizer, and Jazz Pharmaceuticals. SA reports research funding from Seattle Genetics, Merck, Xencor, and Tessa Therapeutics and data safety monitoring board/advisory board participation for Tessa Therapeutics, Myeloid, Servier, and Chimagen. Authors not listed have no disclosures.
Ethics Committee Approval
This study was approved by the Institutional Review Board of MD Anderson Cancer Center under protocol 2021–0242.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- 1.Philip T, Guglielmi C, Hagenbeek A, et al. Autologous Bone Marrow Transplantation as Compared with Salvage Chemotherapy in Relapses of Chemotherapy-Sensitive Non-Hodgkin’s Lymphoma. New England Journal of Medicine. 1995;333(23):1540–1545. doi: 10.1056/NEJM199512073332305 [DOI] [PubMed] [Google Scholar]
- 2.Gisselbrecht C, Glass B, Mounier N, et al. Salvage regimens with autologous transplantation for relapsed large B-cell lymphoma in the rituximab era. Journal of Clinical Oncology. 2010;28(27):4184–4190. doi: 10.1200/JCO.2010.28.1618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Terasawa T, Dahabreh IJ, Nihashi T. Fluorine-18-Fluorodeoxyglucose Positron Emission Tomography in Response Assessment Before High-Dose Chemotherapy for Lymphoma: A Systematic Review and Meta-Analysis. The Oncologist. 2010;15(7):750–759. doi: 10.1634/theoncologist.2010-0054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cheson BD, Fisher RI, Barrington SF, et al. Recommendations for initial evaluation, staging, and response assessment of hodgkin and non-hodgkin lymphoma: The lugano classification. Journal of Clinical Oncology. 2014;32(27):3059–3067. doi: 10.1200/JCO.2013.54.8800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cherng HJJ, Chuang HH, Steiner R, et al. A prospective study on early PET/CT scans during the first cycle of salvage chemotherapy for relapsed or refractory diffuse large B-cell lymphoma. Leukemia and Lymphoma. 2022;63(1):74–83. doi: 10.1080/10428194.2021.1971223/SUPPL_FILE/ILAL_A_1971223_SM0897.DOCX [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Neelapu SS, Locke FL, Bartlett NL, et al. Axicabtagene Ciloleucel CAR T-Cell Therapy in Refractory Large B-Cell Lymphoma. New England Journal of Medicine. 2017;377(26):2531–2544. doi: 10.1056/NEJMoa1707447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schuster SJ, Bishop MR, Tam CS, et al. Tisagenlecleucel in adult relapsed or refractory diffuse large B-cell lymphoma. New England Journal of Medicine. 2019;380(1):45–56. doi: 10.1056/NEJMoa1804980 [DOI] [PubMed] [Google Scholar]
- 8.Abramson JS, Palomba ML, Gordon LI, et al. Lisocabtagene maraleucel for patients with relapsed or refractory large B-cell lymphomas (TRANSCEND NHL 001): a multicentre seamless design study. The Lancet. 2020;396(10254):839–852. doi: 10.1016/S0140-6736(20)31366-0 [DOI] [PubMed] [Google Scholar]
- 9.Armand P, Welch S, Kim HT, et al. Prognostic factors for patients with diffuse large B cell lymphoma and transformed indolent lymphoma undergoing autologous stem cell transplantation in the positron emission tomography era. British Journal of Haematology. 2013;160(5):608–617. doi: 10.1111/bjh.12176 [DOI] [PubMed] [Google Scholar]
- 10.Sauter CS, Matasar MJ, Meikle J, et al. Prognostic value of FDG-PET prior to autologous stem cell transplantation for relapsed and refractory diffuse large B-cell lymphoma. Blood. 2015;125(16):2579–2581. doi: 10.1182/blood-2014-10-606939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shah NN, Ahn KW, Litovich CA, et al. Is Autologous Transplant in Relapsed DLBCL Patients Achieving Only a PET+ PR Appropriate in the CAR-T cell Era? Blood. 2020;137(10):1416–1423. doi: 10.1182/blood.2020007939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johnston PB, Wiseman GA, Micallef INM. Positron emission tomography using F-18 fluorodeoxyglucose pre- and post-autologous stem cell transplant in non-Hodgkin’s lymphoma. Bone Marrow Transplantation 2008 41:11. 2008;41(11):919–925. doi: 10.1038/bmt.2008.82 [DOI] [PubMed] [Google Scholar]
- 13.Filmont JE, Gisselbrecht C, Cuenca X, et al. The impact of pre- and post-transplantation positron emission tomography using 18-fluorodeoxyglucose on poor-prognosis lymphoma patients undergoing autologous stem cell transplantation. Cancer. 2007;110(6):1361–1369. doi: 10.1002/CNCR.22911 [DOI] [PubMed] [Google Scholar]
- 14.Svoboda J, Andreadis C, Elstrom R, et al. Prognostic value of FDG-PET scan imaging in lymphoma patients undergoing autologous stem cell transplantation. Bone Marrow Transplant. 2006;38(3):211–216. doi: 10.1038/SJ.BMT.1705416 [DOI] [PubMed] [Google Scholar]
- 15.Spaepen K, Stroobants S, Dupont P, et al. Prognostic value of pretransplantation positron emission tomography using fluorine 18-fluorodeoxyglucose in patients with aggressive lymphoma treated with high-dose chemotherapy and stem cell transplantation. Blood. 2003;102(1):53–59. doi: 10.1182/BLOOD-2002-12-3842 [DOI] [PubMed] [Google Scholar]
- 16.Alousi AM, Saliba RM, Okoroji GJ, et al. The Influence of PET/Gallium (PET/Gal) Status and High-Dose Rituximab (HDR) in Patients with Aggressive, Large, B-Cell Lymphoma (LBCL) Receiving Autologous Stem Cell Transplants (ASCT). Blood. 2006;108(11):3058. doi: 10.1182/BLOOD.V108.11.3058.3058 [DOI] [Google Scholar]
- 17.Shadman M, Pasquini M, Ahn KW, et al. Autologous transplant vs chimeric antigen receptor T-cell therapy for relapsed DLBCL in partial remission. Blood. 2022;139(9):1330–1339. doi: 10.1182/BLOOD.2021013289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vercellino L, Cottereau AS, Casasnovas O, et al. High total metabolic tumor volume at baseline predicts survival independent of response to therapy. Blood. 2020;135(16):1396–1405. doi: 10.1182/BLOOD.2019003526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ceriani L, Gritti G, Cascione L, et al. SAKK38/07 study: integration of baseline metabolic heterogeneity and metabolic tumor volume in DLBCL prognostic model. Blood Advances. 2020;4(6):1082–1092. doi: 10.1182/BLOODADVANCES.2019001201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mikhaeel NG, Smith D, Dunn JT, et al. Combination of baseline metabolic tumour volume and early response on PET/CT improves progression-free survival prediction in DLBCL. Eur J Nucl Med Mol Imaging. 2016;43(7):1209–1219. doi: 10.1007/S00259-016-3315-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cottereau AS, Lanic H, Mareschal S, et al. Molecular profile and FDG-PET/CT Total metabolic tumor volume improve risk classification at diagnosis for patients with diffuse large B-Cell lymphoma. Clinical Cancer Research. 2016;22(15):3801–3809. doi: 10.1158/1078-0432.CCR-15-2825/274367/AM/MOLECULAR-PROFILE-AND-FDG-PET-CT-TOTAL-METABOLIC [DOI] [PubMed] [Google Scholar]
- 22.Dean EA, Mhaskar RS, Lu H, et al. High metabolic tumor volume is associated with decreased efficacy of axicabtagene ciloleucel in large B-cell lymphoma. Blood Advances. 2020;4(14):3268–3276. doi: 10.1182/bloodadvances.2020001900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vercellino L, di Blasi R, Kanoun S, et al. Predictive factors of early progression after CAR T-cell therapy in relapsed/refractory diffuse large B-cell lymphoma. Blood Adv. 2020;4(22):5607–5615. doi: 10.1182/bloodadvances.2020003001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Meignan M, Sasanelli M, Casasnovas RO, et al. Metabolic tumour volumes measured at staging in lymphoma: methodological evaluation on phantom experiments and patients. Eur J Nucl Med Mol Imaging. 2014;41(6):1113–1122. doi: 10.1007/S00259-014-2705-Y [DOI] [PubMed] [Google Scholar]
- 25.Boellaard R, Delgado-Bolton R, Oyen WJG, et al. FDG PET/CT: EANM procedure guidelines for tumour imaging: version 2.0. Eur J Nucl Med Mol Imaging. 2015;42(2):328–354. doi: 10.1007/S00259-014-2961-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brown R, Lambertini A, Hofman MS, et al. Evaluating the PET Parameters SUVmax and TMTV in the Setting of Autologous Stem Cell Transplantation for DLBCL. Blood. 2020;136(Supplement 1):38–38. doi: 10.1182/BLOOD-2020-135920 [DOI] [Google Scholar]
- 27.Nanni C, Cottereau AS, Lopci E, et al. Report of the 6th International Workshop on PET in lymphoma. Leuk Lymphoma. 2017;58(10):2298–2303. doi: 10.1080/10428194.2017.1298752 [DOI] [PubMed] [Google Scholar]
- 28.Cottereau AS, Buvat I, Kanoun S, et al. Is there an optimal method for measuring baseline metabolic tumor volume in diffuse large B cell lymphoma? Eur J Nucl Med Mol Imaging. 2018;45(8):1463–1464. doi: 10.1007/S00259-018-4005-4 [DOI] [PubMed] [Google Scholar]
- 29.Barrington SF, Meignan M. Time to Prepare for Risk Adaptation in Lymphoma by Standardizing Measurement of Metabolic Tumor Burden. J Nucl Med. 2019;60(8):1096–1102. doi: 10.2967/JNUMED.119.227249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bishop MR, Dickinson M, Purtill D, et al. Second-Line Tisagenlecleucel or Standard Care in Aggressive B-Cell Lymphoma. New England Journal of Medicine. 2022;386(7):629–639. doi: 10.1056/NEJMOA2116596/SUPPL_FILE/NEJMOA2116596_DATA-SHARING.PDF [DOI] [PubMed] [Google Scholar]
- 31.Locke FL, Miklos DB, Jacobson CA, et al. Axicabtagene Ciloleucel as Second-Line Therapy for Large B-Cell Lymphoma. New England Journal of Medicine. 2022;386(7):640–654. doi: 10.1056/NEJMOA2116133/SUPPL_FILE/NEJMOA2116133_DATA-SHARING.PDF [DOI] [PubMed] [Google Scholar]
- 32.Kamdar M, Solomon SR, Arnason J, et al. Lisocabtagene maraleucel versus standard of care with salvage chemotherapy followed by autologous stem cell transplantation as second-line treatment in patients with relapsed or refractory large B-cell lymphoma (TRANSFORM): results from an interim analysis of an open-label, randomised, phase 3 trial. The Lancet. 2022;399(10343):2294–2308. doi: 10.1016/S0140-6736(22)00662-6 [DOI] [PubMed] [Google Scholar]
- 33.Sehn LH, Kamdar M, Herrera AF, et al. Randomized phase 2 trial of polatuzumab vedotin (pola) with bendamustine and rituximab (BR) in relapsed/refractory (r/r) FL and DLBCL. Journal of Clinical Oncology. 2018;36(15_suppl):7507–7507. doi: 10.1200/jco.2018.36.15_suppl.7507 [DOI] [Google Scholar]
- 34.Salles G, Duell J, González Barca E, et al. Tafasitamab plus lenalidomide in relapsed or refractory diffuse large B-cell lymphoma (L-MIND): a multicentre, prospective, single-arm, phase 2 study. Lancet Oncol. Published online June 5, 2020. doi: 10.1016/S1470-2045(20)30225-4 [DOI] [PubMed] [Google Scholar]
- 35.Caimi PF, Ai W, Alderuccio JP, et al. Loncastuximab tesirine in relapsed or refractory diffuse large B-cell lymphoma (LOTIS-2): a multicentre, open-label, single-arm, phase 2 trial. The Lancet Oncology. 2021;0(0). doi: 10.1016/S1470-2045(21)00139-X [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


