Abstract
Objective
As single pulse electrical stimulation (SPES) is increasingly utilized to help localize the seizure onset zone (SOZ), it is important to understand how stimulation intensity can affect the ability to use cortico-cortical evoked potentials (CCEPs) to delineate epileptogenic regions.
Methods
We studied 15 drug-resistant epilepsy patients undergoing intracranial EEG monitoring and SPES with titrations of stimulation intensity. The N1 amplitude and distribution of CCEPs elicited in the SOZ and non-seizure onset zone (nSOZ) were quantified at each intensity. The separability of the SOZ and nSOZ using N1 amplitudes was compared between models using responses to titrations, responses to one maximal intensity, or both.
Results
At 2 mA and above, the increase in N1 amplitude with current intensity was greater for responses within the SOZ, and SOZ response distribution was maximized by 4-6 mA. Models incorporating titrations achieved better separability of SOZ and nSOZ compared to those using one maximal intensity.
Conclusions
We demonstrated that differences in CCEP amplitude over a range of current intensities can improve discriminability of SOZ regions.
Significance
This study provides insight into the underlying excitability of the SOZ and how differences in current-dependent amplitudes of CCEPs may be used to help localize epileptogenic sites.
Keywords: cortico-cortical evoked potential, single pulse electrical stimulation, intracranial EEG, effective connectivity, epileptogenic network, epilepsy
1. Introduction
Epilepsy is a complex neurological disease characterized by recurrent unprovoked seizures and affects over 70 million people worldwide (Singh and Trevick, 2016). While antiepileptic drug (AED) therapy can be an effective treatment, nearly a third of epilepsy patients continue to have seizures despite trials of multiple AEDs (Kwan and Brodie, 2010). These patients with drug-resistant epilepsy may be candidates for surgical treatments targeting the epileptogenic zone, defined as the brain region for which resection or disconnection results in seizure-freedom. The location of the epileptogenic zone is informed by many clinical factors and diagnostic tools, including the location of the seizure onset zone, the brain region from which clinical seizures are generated (Rosenow and Lüders, 2001). While surgery can be the most effective treatment for drug-resistant focal epilepsy, success rates vary due to the difficulty in accurately localizing the epileptogenic zone (Wiebe et al., 2001; Jeha et al., 2007; González-Martínez et al., 2007; Ramey et al., 2013; Jobst and Cascino, 2015; Malmgren and Edelvik 2017).
Single pulse electrical stimulation (SPES) is a procedure that can be used to measure effective connectivity in the brain by applying repeated trials of single pulses delivered at multiple stimulation sites and recording the responses elicited throughout the brain. This active technique can supplement passive intracranial EEG monitoring in localizing the seizure onset zone in drug-resistant epilepsy patients, as SPES has been increasingly used to differentiate epileptogenic networks ever since early work showed that delayed responses from SPES can be used to identify epileptogenic regions (Valentin et al., 2002, 2005). However, later studies focused on the characteristic responses produced by SPES known as cortico-cortical evoked potentials (CCEPs) that have a distinct early response (Matsumoto et al., 2004). While initial studies investigating accentuated CCEPs in seizure onset zones used graded stimulation intensities to show increased excitability persisting over a range of stimulation currents (Iwasaki et al., 2010, Enatsu et al., 2012a), more recent studies often rely on responses elicited from a single current intensity. While intensity titrations are often initially performed to determine safe parameters that do not produce pain, twitching, or after-discharges, many SPES studies investigating epileptogenic networks primarily report results from one final current intensity per patient or stimulation site (van ‘t Klooster et al., 2011; Enatsu et al., 2012b; Mouthaan et al., 2016; Kobayashi et al., 2017; van Blooijs et al., 2018; Zhang et al., 2018; Hays et al., 2021a). The effect of current intensity on responses to SPES has been studied further but is often not within the context of epileptogenic networks, either with the effect of epileptogenicity not investigated (Trebaul et al., 2018; Crowther et al., 2019; Hays et al., 2021b) or not differentiated at each current intensity (Keller et al., 2014; Donos et al., 2016; Kundu et al., 2020).
The goal of this study was to determine how evoked responses in epileptogenic and non-epileptogenic sites differ across current intensity and whether those differences can improve the localization of the seizure onset zone from SPES data. We conducted SPES with incremental current intensity titrations in 15 epilepsy patients to investigate the effect of current intensity on the resulting CCEPs. We will use the term SPES to refer to the stimulation procedure and the term CCEPs to refer to the responses elicited by SPES. We first quantified how the early response magnitude (N1 potential) and spatial distribution of CCEPs change in response to increasing current intensity, for CCEPs elicited in the seizure onset zone (SOZ) or non-seizure onset zone (nSOZ) based on the location of the response site. Then, we investigated how these differences in the current-dependent response amplitudes may be used to classify and predict sites involved in the SOZ, and how performance is affected by using responses to the titrated currents instead of, or in addition to, the more common practice of using responses to a single maximal current intensity.
2. Materials
2.1. Patients and electrodes
This study expands upon our previously published work, Hays et al., 2021b, and therefore includes similar experimental methods; we will briefly summarize here. Fifteen patients with medically refractory epilepsy who required implantation of intracranial electrodes for seizure localization were included in this study with the Johns Hopkins School of Medicine Institutional Review Board approval. The patients were implanted with stereo-electroencephalography (S-EEG) electrodes or both S-EEG and subdural electrocorticography (ECoG) electrodes in one patient (P2). Electrodes were placed according to target the epileptogenic zone informed by a combination of clinical seizure history, noninvasive neuroimaging and neuropsychology tests, and scalp EEG recordings for each patient. S-EEG electrodes had platinum contacts that were 2.3 mm long with 5 mm spacing between centers, and the ECoG grid for P2 was an array of platinum electrodes with 2.3 mm diameter and 10 mm spacing between centers (Ad-Tech Medical Instrument Corporation, Racine, WI, USA). The BioImage Suite was used to localize implanted electrodes through co-registration of pre-implantation brain MRI with post-implantation CT (Duncan et al., 2004), and FreeSurfer (Fischl, 2012) was used to identify which electrodes were within gray or white matter, confirmed by visual inspection. Clinical labels identifying the seizure onset zone (SOZ), sites of early propagation (EP), and irritative zone (IZ) were determined independently from this study based on comprehensive evaluation of clinical and intracranial EEG data by a board-certified epileptologist.
2.2. Single pulse electrical stimulation
The SPES procedure we used was described previously (Hays et al., 2021b). In brief, ECoG and S-EEG signals were recorded, filtered, and down-sampled to 1 kHz using a NeuroPort amplifier (Blackrock Microsystems, Salt Lake City, UT, USA). A CereStim R96 stimulator (Blackrock Microsystems, Salt Lake City, UT, USA) was used to conduct bipolar stimulation of adjacent electrodes at 0.4 Hz (P1-P4) or 0.5 Hz (P5-P15) using biphasic 0.15 ms/phase pulses. At each stimulation site, titration blocks with increasing current intensity were performed first (10 pulses at each intensity), followed by a full block of 50 (P1-P4) or 40 (P5-P15) pulses at a maximum intensity (4-10 mA, median 6 mA) (Figure 1A, Table 1). The maximum intensity was set per patient at an intensity that could consistently elicit evoked potentials while no after discharges were observed, up to 10 mA. Stimulation locations were chosen to target sites within gray matter.
Figure 1.
Single pulse electrical stimulation (SPES) procedure and analysis. (A) SPES was conducted using bipolar stimulation on adjacent electrodes. At each site, current titration blocks were first applied, consisting of 10 pulses at each incremental intensity. This was followed by a full block, consisting of 40-50 pulses delivered at a maximum intensity. (B) Trial responses of bipolar montage signals were baseline-centered and averaged for each titration and full block. N1 potentials were identified within 10 to 50 ms post-stimulus, and the absolute value of the N1 peak voltage was used as the amplitude of the average response. Responses with full block N1 voltages greater than six standard deviations of the pre-stimulus baseline (−500 to −10 ms) were labeled as significant. (C) Responses were classified as being within the seizure onset zone (SOZ) or non-seizure onset zone (nSOZ), designated here as red or blue, respectively. Whether the response was elicited from stimulation within or outside of SOZ was noted but not used to group responses for most of this study. For two example full blocks stimulating one site inside and one site outside the SOZ, significant responses are shown as red (SOZ) or blue (nSOZ) squares located between the electrode contacts used for each bipolar channel with lines coming from the stimulated contacts highlighted yellow. (D) The responses of four example stimulation-response pairs over the range of intensities used in the current titration blocks are shown for each connection type with respect to SOZ. (E) The N1 voltages of SOZ and nSOZ responses over a range of titrating current intensities were compared across current intensity and across connection type. As an illustrative example, the N1 voltage of each stimulation-response pair shown in D for patient P8 is plotted here for each current intensity.
Table 1:
Patient characteristics.
| Patient Number |
Sex | Age | Implant Type | Number of Electrodes Implanted (Stimulated) |
Titration Block Current Intensity Range (Increments) [mA] |
Full Block Current Intensity [mA] |
Number of Significant Connections (in SOZ) |
|---|---|---|---|---|---|---|---|
| P1 | F | 51 | S-EEG | 88 (16) | 0.5-4.0 (0.5) | 4.0 | 102 (9) |
| P2 | F | 42 | S-EEG, Grid | 214 (18) | 0.5-4.5 (1.0) | 4.5 | 197 (26) |
| P3 | M | 48 | S-EEG | 103 (34) | 0.5-5.5 (1.0) | 5.5 | 190 (3) |
| P4 | F | 23 | S-EEG | 108 (35) | 1.0-6.0 (1.0) | 6.0 | 386 (64) |
| P5 | F | 23 | S-EEG | 168 (20) | 1.0-5.0 (1.0) | 5.0 | 109 (21) |
| P6 | M | 51 | S-EEG | 114 (18) | 1.0-4.0 (1.0) | 5.0 | 108 (9) |
| P7 | M | 45 | S-EEG | 70 (47) | 1.0-5.0 (1.0) | 5.0 | 201 (20) |
| P8 | F | 39 | S-EEG | 112 (68) | 1.0-10.0 (1.0) | 5.0 | 1341 (235) |
| P9 | F | 48 | S-EEG | 54 (34) | 1.0-10.0 (1.0) | 10.0 | 330 (22) |
| P10 | M | 33 | S-EEG | 52 (42) | 1.0-10.0 (1.0) | 10.0 | 336 (20) |
| P11 | M | 24 | S-EEG | 102 (62) | 1.0-10.0 (1.0) | 10.0 | 407 (49) |
| P12 | F | 48 | S-EEG | 111 (69) | 1.0-10.0 (1.0) | 10.0 | 731 (41) |
| P13 | F | 54 | S-EEG | 92 (50) | 1.0-10.0 (1.0) | 10.0 | 793 |
| P14 | F | 19 | S-EEG | 81 (34) | 1.0-10.0 (1.0) | 10.0 | 424 |
| P15 | F | 48 | S-EEG | 114 (63) | 1.0-10.0 (1.0) | 10.0 | 540 |
M, Male; F, Female; S-EEG, Stereo-electroencephalography
Adapted from Hays et al., 2021b
Electrodes determined to have excessive noise under visual inspection were rejected, followed by bipolar montage re-referencing of all remaining channels. To remove stimulus artifacts in response channels while preserving the surrounding signals, data from −5 to 10 ms relative to the stimulus were replaced with reversed, tapered copies of the signals immediately before and after this period (Crowther et al., 2019). Signals were then low pass filtered at 50 Hz and divided into time-locked windows for each stimulation trial, from −500 to 1500 ms relative to the stimulus. Custom Matlab scripts (R2020a, MathWorks, Natick, MA) were used to process and analyze all signals.
2.3. Cortico-cortical evoked potential quantification
Average responses to each titration and full block were obtained by baseline-centering and averaging the stimulus-locked signals from −500 ms to 1500 ms for all 10 trials in titration blocks and 40 or 50 trials in the full blocks. CCEPs can be quantified by the amplitude of the N1 potential, an early negative deflection in voltage that typically occurs 10-50 ms post-stimulus, thought to represent direct connectivity between stimulation and response sites (Matsumoto et al., 2004, 2007, 2012). We chose to focus on this early component of the CCEP waveform for this study because later components, such as N2, may arise from indirect connectivity through polysynaptic and/or subcortical pathways (Matsumoto et al., 2004, 2017), and the N1 potential has been shown to be accentuated with epileptogenicity (Iwasaki et al., 2010, Enatsu et al., 2012a). N1 potentials were identified in each response using peak detection from 10 to 50 ms post-stimulus. The latency of the identified N1 peak was marked, and the absolute value of the N1 voltage with respect to the zero-mean pre-stimulus baseline (−500 to −10 ms) was used to quantify the amplitude of each response (Figure 1B). To determine which responses may represent a significant evoked potential, the N1 voltages of the full blocks were divided by the standard deviation of the pre-stimulus baseline (−500 to −10 ms) to obtain Z-scores, and responses with Z-scores greater than six standard deviations were classified as significant (Keller et al., 2011, 2014). Analysis of responses was performed on all bipolar montage signals, regardless of whether the electrodes were located within gray or white matter.
We quantified how the spatial distribution of responses varied across current intensity by calculating the proportion of channels with a significant response to the full block (Z-score > 6) that also had a significant signal to noise ratio (SNR) in the titration blocks. The SNR was calculated as the ratio of the variance from 10 to 100 ms post-stimulus to the variance of the baseline (−500 to −10 ms pre-stimulus) (Hays et al., 2021b). SNR significance was determined by the probability of observing greater SNR values than the observed SNR, using null distributions generated from 500 repeated SNR calculations using random circular time shifts of each trial response to derive P-values, with P < 0.05 considered significant. SNR was used to determine the significance of average responses in titration blocks rather than Z-score since it is a more general measure of the evoked responses that does not rely on the existence of distinct N1 potentials that may not be elicited in responses to lower stimulating currents.
2.4. Statistical Analysis
2.4.1. N1 voltage in SOZ and nSOZ
We define the term “stimulation-response pair” as the pair of locations that comprise a stimulation site and a response site. Each stimulation-response pair was first classified as being either significantly responsive or non-responsive if its full block Z-score was greater or less than six standard deviations of the pre-stimulus baseline, as described above. Significantly responsive pairs were further separated based on the response site being in the seizure onset zone (SOZ) or non-seizure onset zone (nSOZ) (Figure 1C-E). This resulted in three groups: significantly responsive pair ending in SOZ, significantly responsive pair ending in nSOZ, and non-responsive pair. The numbers of stimulated electrodes and significant stimulation-response pairs for each patient are listed in Table 1.
Kruskal-Wallis tests were conducted to determine whether N1 voltage varied significantly across current intensity within each group (SOZ, nSOZ, and non-responsive) and across group within each current intensity. Post-hoc Dunn’s tests were conducted for pairwise comparisons, and Bonferroni corrected P-values less than 0.05 were significant. Effect sizes of Kruskal-Wallis tests were determined by eta-squared using the H statistic, and effect sizes of Dunn’s tests were determined by Pearson’s correlation r (Tomczak and Tomczak, 2014).
2.4.2. Separability of SOZ and nSOZ using titration and full blocks
We next examined how the separability of SOZ and nSOZ using responses to a range of titrating current intensities compares to that of responses to a single maximal current intensity. Our conjecture was that the response amplitudes over a range of stimulating currents provides additional information to improve discrimination between SOZ and nSOZ. To investigate this, three different logistic regression models were fit for each patient: Titration+Full, using responses to both titration blocks and full blocks; Titration, using only responses to titration blocks; and Full, using only responses to full blocks. The N1 voltage responses to each current intensity were used as predictor variables to classify whether each stimulation-response pair was labeled as SOZ or nSOZ based on the location of the response site. Classification of individual sites was done by averaging the model probability over all connections ending at each site to obtain one probability for each site. The hypothesis was that if a particular site has many connections with high SOZ probability then it is more likely within the SOZ, whereas a site that has mostly low SOZ probability connections is not as likely to be within SOZ. This was based on the idea that SOZ sites are more excitable given any stimulation, whereas sites with strong structural or functional connections may only respond strongly to stimulation at the sites of those connections.
The ability of each model to separate SOZ from nSOZ was quantified by the area under the curve (AUC) of the receiver operating characteristic (ROC) curves, and the three model types per patient were compared using DeLong tests to determine significant differences between AUC, with false-discovery rate (FDR) P values < 0.05 considered significant (Benjamini and Hochberg, 1995). N1 voltages were log scaled prior to fitting the models to normalize the distribution and achieve better fitting models.
2.4.3. SOZ and nSOZ prediction
To assess the ability of responses to titrations and full blocks to predict SOZ for new patients, we tested the accuracy of predicting SOZ sites in several patients individually excluded from multi-patient training models. We adapted the classification models to include multiple patients using a generalized linear mixed effect logistic regression model, with the N1 voltage responses to each current as fixed effects (same as the predictor variables from the single-patient logistic regression) and patient number as a random effect. Only patients P8-P15 who received the same 1-10 mA titration blocks were included in this part so that the fixed effects would be identical with no missing data. Since there were no spontaneous seizures captured for patients P13-P15 for clinicians to determine SOZ labels, these patients were analyzed separately. For the patients with SOZ labels (P8-P12), models were trained on each combination of four of the five patients and tested on the patient left out of the training set. The threshold that maximized Youden’s J statistic in each training model was used to classify the sites of the test patient as SOZ or nSOZ (Youden, 1950). For patients without SOZ labels (P13-P15), we used models trained on P8-P12 to predict SOZ sites in these three patients as examples of how CCEPs may benefit seizure localization in cases when no spontaneous seizures are observed during intracranial EEG monitoring.
Statistical analysis was calculated using custom scripts in R (R Core Team 2019) with rstatix (Kassambara, 2021) and nlme (Pinheiro et al., 2021) packages.
3. Results
Fifteen patients (5 male, 10 female, median age 45) were included in this study (Table 1, adapted from Hays et al., 2021b). Across all patients and current intensities, we observed 208,245 responses (per patient: median 9,226, range 3,984-34,903), with 61,942 classified as responses from significant connections (per patient: median 3,611, range 522-14,751). No spontaneous seizures were observed in P13-P15 during intracranial EEG monitoring, so electrode sites could not be given clinically designated SOZ labels, and these patients were not included in the SOZ separability portions of this study. For the remaining 12 patients (P1-P12) with clinically labeled SOZ, a median of 21.5 (range 3-235) significant connections with response sites in SOZ, and median of 247.5 (range 88-1,106) significant connections with response sites outside of SOZ.
3.1. Effect of current intensity and SOZ on N1 voltage
The N1 voltages of evoked responses varied significantly across current intensity in both the SOZ and nSOZ groups (Kruskal-Walls tests, P < 0.05), with large or moderate effect sizes (eta-squared > 0.14 or 0.06, respectively) in every patient except P3 (Figure 2, Supplementary Figure 1A). For these patients, the effect size was greater within the SOZ group than in the nSOZ group, indicating that the N1 voltages of responses within the SOZ vary more with current intensity than responses outside the SOZ. While statistically significant, the effect sizes in the non-responsive group were small (eta-squared < 0.06). The N1 voltages of SOZ and nSOZ groups were significantly different at lower current intensities (Dunn’s tests, P < 0.05), but once a high enough current was reached, responses were no longer significantly different (Figure 2, Supplementary Figure 1B). This can be observed visually in the current versus N1 voltage plots in Figure 2 where there is a greater slope at low currents and a plateauing at greater currents. While this was seen in both the SOZ and nSOZ responses, the current at which the magnitude of the responses plateaued in the SOZ group was equal or lower than that for the nSOZ group in almost every patient (2-5 mA, median 3.5 mA for SOZ; 2-6 mA, median 4.5 mA for nSOZ).
Figure 2.
N1 voltage responses across current intensity. Stimulation-response pairs were separated based on the location of the response and the significance of the response in the full block: significant response in the seizure onset zone (SOZ), significant response in the non-seizure onset zone (nSOZ), or no significant response. N1 voltage was compared using Kruskal-Wallis tests across current intensities within each group and across groups within each current intensity, followed by post-hoc Dunn’s tests for pairwise comparisons. Error bars represent 95% confidence interval of the median.
Group (significant SOZ, significant nSOZ, and non-responsive) had a significant effect on N1 voltage at 2 mA and above (Kruskal-Wallis, P < 0.05) for each patient, and effect size generally increased with increasing current intensity (Figure 2, Supplementary Figure 2A). As expected, post-hoc pairwise comparisons showed significant differences in N1 voltage of SOZ and nSOZ stimulation response-pairs compared to that of non-responsive stimulation response-pairs. Interestingly, in most patients, while effect size between non-responsive and nSOZ reached greater magnitudes at the highest current intensities, the effect size between non-responsive and SOZ increased at lower currents then kept constant. Median N1 voltages of SOZ responses were significantly greater than those of nSOZ responses across almost the whole current range for P4, P5, P7-P12 (Figure 2, Supplementary Figure 2B). This is also shown by the greatest effect size between nSOZ and SOZ occurring at lower current intensities for most patients that showed a significant difference.
3.2. Epileptogenic network connectivity
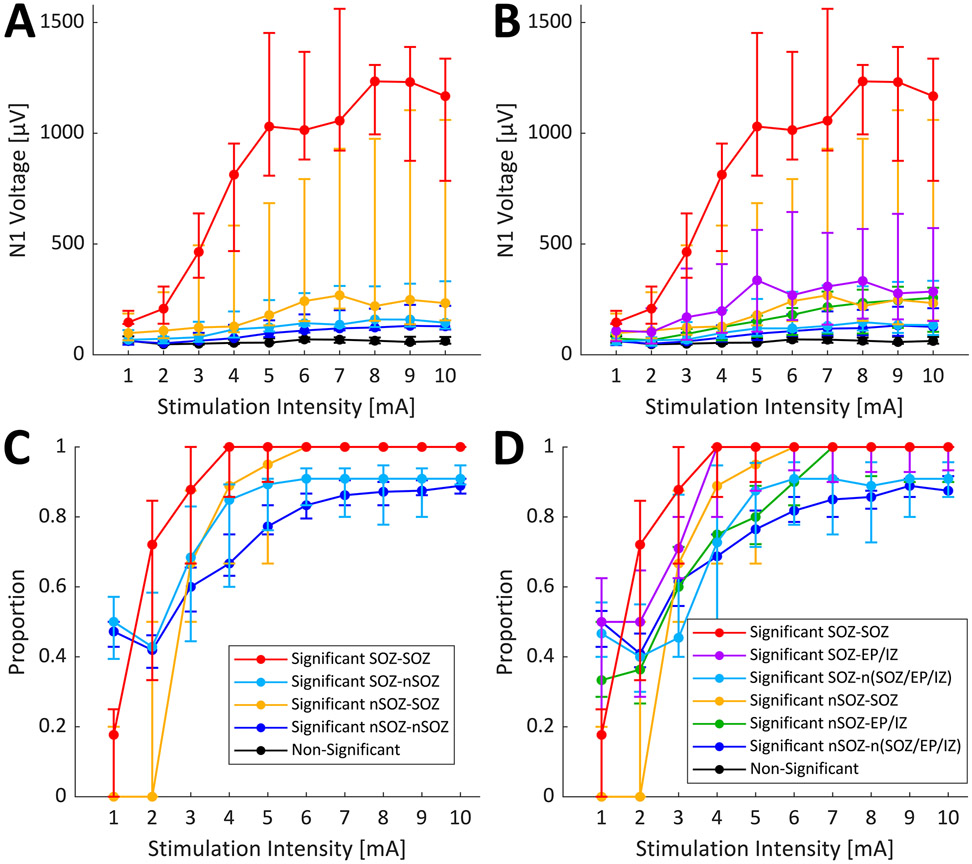
To further investigate the excitability of the epileptogenic network over a range of current intensities, N1 voltage responses were also separated based on location of both the stimulation and response site with respect to SOZ. Additionally, response sites in nSOZ were further split into sites involved in the EP or IZ (EP/IZ) and sites not involved in SOZ, EP, or IZ [n(SOZ/EP/IZ)]. Since further separation of connection type decreased the number of connections within each group, the results for individual patients had large confidence intervals and variation in trends from patient to patient (Supplementary Figure 3). Instead, we grouped the median responses of each connection type from each patient that had the same maximal range of titration parameters (P8-P12). While this grouping still may not have provided considerable statistical power, it did allow us to observe the overall trends across these patients. The median values across the five patients are plotted in Figure 3A and 3B, without and with differentiation of EP/IZ sites respectively. Current intensity had the largest effect size on SOZ-SOZ connection N1 voltage, and connection type had a significant effect on N1 voltage with effect size generally increasing with current intensity (Kruskal-Wallis tests, P < 0.05). Pairwise comparisons did not show many significant differences, although SOZ-SOZ and nSOZ-SOZ were both consistently significantly different from non-responsive pairs (Dunn’s tests, P < 0.05), which followed the visual trends in Figure 3A and 3B. There was a clear separation of the N1 voltage responses of SOZ-SOZ connections compared to the rest of the connection types, and nSOZ-SOZ connections had a slightly greater slope than the remaining connections. Together this may indicate that SOZ sites are more excitable than nSOZ sites, especially when stimulating from another SOZ site. When differentiating EP/IZ sites, it appears that EP/IZ sites may also be more excitable than n(SOZ/EP/IZ), with this effect possibly increased for stimulation within SOZ.
Figure 3.
Epileptogenic network connection response magnitude and distribution over current intensity range. Connections were separated based on location of stimulation and response site with respect to seizure onset zone (SOZ) (A and C) or with respect to SOZ, early propagation, and irritative zone (EP/IZ) (B and D). For stimulations within the SOZ, responses observed in the SOZ or non-seizure onset zone (nSOZ) are labeled as SOZ-SOZ or SOZ-nSOZ, in panels A and C. In panels B and D, responses within the SOZ, within the EP or IZ, or in neither SOZ, EP, or IZ are labeled as SOZ-SOZ, SOZ-EP/IZ, or SOZ-n(SOZ/EP/IZ). Similarly, for stimulations within the nSOZ, responses are labeled as nSOZ-SOZ or nSOZ-nSOZ in panels A and C, and as nSOZ-SOZ, nSOZ-EP/IZ, or nSOZ-n(SOZ/EP/IZ) in panels B and D. N1 voltages are plotted for each connection type over current intensity range (A and B). The proportion of connections with a significant normalized N1 amplitude in the full block that also showed a significant signal to noise ratio in the titration blocks are plotted for each current intensity (C and D). Error bars represent 95% confidence interval of the median across included patients P8-P12.
The proportions of significant connections (Z-score > 6) within each connection type that had significant SNRs in titration blocks are shown in Figure 3C and 3D. Proportions for each stimulation across patients P8-P12 were grouped since they had the same maximal titration current range (1-10 mA). While greater median proportions of connections ending in nSOZ showed significant activity at 1 mA compared to that of connections ending in SOZ, the proportion of significant SOZ connections quickly increased with increasing current intensity, especially those from SOZ stimulation (Figure 3C). The distribution of significant connections ending in SOZ was maximized between 4-6 mA, whereas the median proportions of connections ending in nSOZ instead increased more gradually and leveled off around 6-7 mA. However, by additionally separating nSOZ response sites into EP/IZ or n(SOZ/EP/IZ), we see that significant SOZ to EP/IZ connections showed maximal response proportions by 4-6 mA (Figure 3D).
3.3. Responses over range of stimulation intensities improve SOZ classification
The AUC of the logistic regression models classifying SOZ for each patient are shown in Table 2 with individual ROC curves shown in Supplementary Figure 4. For classification of connections as ending in SOZ or nSOZ, the AUC for Titration+Full or Titration models was greater than the AUC of the Full model in every patient, and the differences were significant in 6 patients (DeLong tests, P < 0.05) (Table 2, Supplementary Figure 4A). This supports our hypothesis that the responses over a range of current intensities can be used to improve SOZ separability. The Titration+Full model was only significantly better than the Titration model in P10. While the AUC varied by patient, good performance was generally achieved with the AUC of the best model for each patient ranging from 0.61-0.92 (median 0.75).
Table 2:
AUC for SOZ Logistic Regression on Single Patients
| Patient | Titration+Full Model Connection- based AUC |
Titration Model Connection- based AUC |
Full Model Connection- based AUC |
Titration+Full Model Site- based AUC |
Titration Model Site- based AUC |
Full Model Site-based AUC |
|---|---|---|---|---|---|---|
| P1 | 0.77 | 0.78 | 0.69 | 0.85 | 0.85 | 0.75 |
| P2 | 0.59 | 0.58 | 0.57 | 0.61 | 0.60 | 0.65 |
| P3 | 0.71 | 0.70 | 0.64 | 0.73* | 0.74* | 0.62 |
| P4 | 0.69 | 0.69 | 0.65 | 0.94 | 0.94 | 0.93 |
| P5 | 0.67 | 0.68 | 0.62 | 0.82 | 0.80 | 0.78 |
| P6 | 0.68 | 0.65 | 0.65 | 0.74 | 0.72 | 0.74 |
| P7 | 0.76*** | 0.75*** | 0.60 | 0.84 | 0.81 | 0.75 |
| P8 | 0.79*** | 0.79*** | 0.73 | 0.88 | 0.87 | 0.88 |
| P9 | 0.83*** | 0.83*** | 0.69 | 0.88 | 0.88 | 0.81 |
| P10 | 0.92*** | 0.89*** | 0.60 | 0.98* | 0.96* | 0.72 |
| P11 | 0.81*** | 0.81*** | 0.72 | 0.87 | 0.87 | 0.83 |
| P12 | 0.74*** | 0.73*** | 0.63 | 0.89 | 0.87 | 0.87 |
SOZ, seizure onset zone; AUC, area under the curve; Titration+Full or Titration AUC being significantly different from Full AUC is represented by:
P < 0.05
P < 0.001
A similar pattern was seen for the models that classified individual response sites as SOZ or nSOZ using averaged probabilities from the connection-based classification. In almost every patient, either both or one of the Titration+Full and Titration models had greater AUC than that of the Full model (Table 2, Supplementary Figure 4B). However, differences were only significant in P3 and P10 (DeLong tests, P < 0.05), and the Full model performed best in P2 and P8 (Table 2, Supplementary Figure 4). The performance of site-based classification was generally better than that of the connection-based classification, with the best AUC for each patient ranging from 0.65-0.98 (median 0.86).
3.4. Predicting SOZ
For P8-P12, additional multi-patient models were trained on each combination of four patients and tested on the excluded patient. For all five patients, the AUC of both the Titration+Full and Titration models for connection-based classification were significantly greater than that of the Full model (Supplementary Table 1). For each patient, the AUC from this model was only slightly lower than the AUC from the model trained on that patient alone, suggesting that there is a consistency across patients in how the voltages responses over a current range can predict SOZ. Using the threshold determined by maximizing Youden’s J statistic for the model trained on four of the five patients, each site in the remaining patient was classified as SOZ or nSOZ. The accuracy, sensitivity, and specificity of these predictions are shown in Table 3. While performance varied, the Titration+Full and Titration model had greater accuracy than Full alone in P9, P10, and P12. In most cases, the predictions had greater specificity than sensitivity, and models including Titrations generally had greater sensitivity than Full alone. Interestingly, a considerable proportion of the false positive sites were sites that were labeled as EP/IZ, indicating that the responses at those sites resemble that of SOZ sites.
Table 3:
SOZ Site Prediction Accuracy
| Patient | Titration+Full Accuracy (Sensitivity, Specificity) |
Titration Accuracy (Sensitivity, Specificity) |
Full Accuracy (Sensitivity, Specificity) |
|---|---|---|---|
| P8 | 0.74 (0.90, 0.70) | 0.75 (0.90, 0.71) | 0.77 (0.78, 0.77) |
| P9 | 0.91 (0.50, 0.97) | 0.91 (0.50, 0.97) | 0.76 (0.17, 0.85) |
| P10 | 0.85 (0.43, 0.94) | 0.83 (0.29, 0.94) | 0.80 (0.14, 0.94) |
| P11 | 0.81 (0.53, 0.88) | 0.81 (0.47, 0.89) | 0.84 (0.20, 0.98) |
| P12 | 0.85 (0.60, 0.86) | 0.84 (0.60, 0.85) | 0.74 (1.00, 0.73) |
SOZ, seizure onset zone
Since P13-P15 did not have spontaneous seizures captured for clinicians to determine SOZ or EP labels, we compared the sites predicted to be SOZ by a P8-P12 model to sites labeled as IZ. The sites that were predicted as SOZ often coincided with these sites, however this performance varied by model type (Table 4).
Table 4:
SOZ Site Predictions in Patients Without SOZ Labels
| Patient | Number of Predicted SOZ Sites (within IZ) For Titration+Full Model |
Number of Predicted SOZ Sites (within IZ) For Titration Model |
Number of Predicted SOZ Sites (within IZ) For Full Model |
|---|---|---|---|
| P13 | 7 (4) | 7 (4) | 25 (7) |
| P14 | 6 (3) | 6 (3) | 6 (2) |
| P15 | 38 (11) | 37 (11) | 7 (3) |
SOZ, seizure onset zone; IZ, irritative zone
4. Discussion
In this study, we investigated how the excitability of effective connections in the epileptogenic network varies in response to SPES over a range of stimulation intensities and determined how differences in current-dependent excitability may be used to discriminate between intracranial recording sites within and outside epileptogenic brain regions. We found that for 2 mA and above, response sites in SOZ tended to have greater magnitude N1 responses compared to that of nSOZ sites, and with increasing intensity, response sites in SOZ had greater increases in voltages for a given increase in current and may plateau at lower currents. This excitability pattern in SOZ sites was more accentuated when stimulation also occurred within the SOZ. The proportion of significant stimulation-response pairs showing significant SNR during titrations was maximized for SOZ sites at 4-6 mA, while that of nSOZ sites gradually increased over the whole intensity range. When classifying SOZ and nSOZ connections and sites with logistic regression models, the models that incorporated responses to the titration blocks were able to better separate SOZ from nSOZ (connection-based median AUC of 0.75 versus 0.64 and site-based median AUC of 0.86 versus 0.77). Predictions in patients excluded from training sets were able to identify SOZ and nSOZ sites with relatively good accuracy (range of 0.74-0.91 across all models), with false positives often being sites that were still involved in the greater epileptogenic network, such as early propagation and irritative zones. Together, our findings provide insight into the current-dependent excitability of epileptogenic and non-epileptogenic regions and how utilizing responses to a range of current intensities may improve the separability of epileptogenic and non-epileptogenic regions compared to relying on responses to a single maximum intensity.
Early studies showed that responses to SPES, particularly abnormal delayed and repetitive responses could be used to identify epileptogenic regions (Valentín et al., 2002, 2005). More recently, early responses have been used to probe the excitability of SOZ regions and measure the connectivity of epileptogenic networks due to the accentuated amplitude of CCEPs observed within SOZ (Iwasaki et al., 2010; Enatsu et al., 2012a). These two studies showed that iCCEPs (equivalent of SOZ-SOZ CCEPs in the present study) generally showed greater response amplitudes over a range of currents compared to nCCEPs (equivalent of nSOZ-nSOZ CCEPs in the present study). Accordingly, we observed that SOZ-SOZ connections stood out as having the greatest amplitude responses to increasing currents, followed by nSOZ-SOZ connections. This elevated excitability of SOZ is consistent with other SPES studies showing that the SOZ has greater amplitude connections with itself (Parker et al., 2018; Van Blooijs et al., 2018; Guo et al., 2020; Hays et al., 2021a). It has also been shown that responses in SOZ are greater regardless of stimulation location (Keller et al., 2014; Parker et al., 2018; Van Blooijs et al., 2018; Zhang et al., 2018) and that stimulating the SOZ produces stronger responses elsewhere (Van Blooijs et al., 2018; Guo et al., 2020; Hays et al., 2021a). These notions of SOZ being both more receptive and more propagative of activity may reflect greater overall excitability in the SOZ, possibly due to imbalanced network dynamics (Kramer and Cash, 2012; Bartolomei et al., 2017; Gupta et al., 2020; Li et al., 2021; Gunnarsdottir et al., 2021).
While we primarily focused on comparing responses in SOZ and nSOZ, we also analyzed different connection types within the greater epileptogenic network by separating nSOZ sites into sites where early propagation of seizures was observed and sites in the irritable zone. Previous studies have also used CCEPs to investigate the connectivity between sites of seizure onset and propagation, finding increased prevalence and amplitude of CCEPs from areas of seizure onset to areas of early spread (Enatsu et al., 2012b; Lega et al., 2015; Parker et al., 2018; Zhang et al., 2018; Guo et al., 2020). We similarly observed that responses in sites classified as early propagation or initiative zones had a trend for greater amplitude responses over the titration current range, especially from stimulation in SOZ, and SOZ to EP/IZ connections showed maximal distributions were achieved at lower currents. Additionally, many false positives when predicting SOZ were EP/IZ sites, indicating that these types of connections do have elevated strength, resembling the amplitude response of SOZ connections. For the three patients without SOZ labels, the predicted sites were often IZ sites.
Why did the titration responses improve separability of SOZ and nSOZ regions rather than being repetitive of the information acquired from responses to a single maximal current? Part of this may be due to the nonlinearity of the voltage response to increasing current. The voltage responses are relatively linear once they start to increase at lower current intensities but begin to plateau at higher current intensities. However, as Iwasaki et al., 2010 and Enatsu et al., 2012a first demonstrated, the voltage response curves for SOZ and nSOZ have different slopes and shifted currents at which they begin to increase and then plateau. So, while the greater slope of the voltage response in SOZ allows for separability from nSOZ at lower titrating currents, some responses in SOZ may start to level off with increasing current while responses in nSOZ continue to increase, potentially decreasing separability at high currents. This was evidenced in our analysis of the pairwise comparisons of N1 voltage responses and the distribution of significant connections. Altogether these results indicate that responses in SOZ and nSOZ may have better or equal separation at lower currents. This may be because greater charge levels increase the probability of observing CCEP connections, leading to larger network activation and revealing more functional connectivity (Trebaul et al., 2018). So, greater currents may maximally excite all connections, whether they are representative of structural and/or functional relationships between stimulation and response sites (Trebaul et al., 2018; Hebbink et al., 2019; Crocker et al., 2021) or due to effects of epileptogenicity (Valentín et al., 2002; Valentín et al., 2005; Iwasaki et al., 2010; Enatsu et al., 2012a). However, it is possible that at lower currents, the increased excitability of SOZ makes those regions more sensitive to stimulation currents that might be sub-threshold for evoking responses that relate to physiological or functional connections.
This study used the amplitude of the N1 potential of CCEPs to quantify the responses of sites to SPES since this is thought to relate to the direct effective connectivity between stimulation and response sites (Matsumoto et al., 2004). However, further research may benefit from quantifying excitability using other robust metrics of effective connectivity using such as the root-mean-squared (RMS) of the early response in CCEPs (Prime et al., 2018) or evoked spectral responses (Crowther et al., 2019). While SPES studies investigating epileptogenic networks often use CCEP responses to quantify connections, research has also shown that SPES can elicit increases high frequency activity in early responses within epileptogenic regions (van ’t Klooster et al., 2011; Mouthaan et al., 2016; Mălîia et al., 2017; Kobayashi et al., 2017). This increased high frequency activity could be further used to differentiate SOZ and nSOZ when measured over a range of current intensities. Given that Kundu et al., 2020 demonstrated that SPES-evoked high gamma responses increase and plateau over a range of increasing current intensity similar to the amplitude of CCEP responses, we may expect similar differentiable excitability pattern for high gamma responses in epileptogenic regions.
This study also has some limitations. One important factor that was not explored in this study was how the excitability of SOZ may vary across different anatomical locations or for different epilepsy types. For example, how do the responses within a mesial temporal SOZ compare to those within a SOZ in cortical regions? There may be expected variation since the effect of epileptogenicity on evoked responses to SPES has been shown to be greater in mesial temporal structures (Kobayashi et al., 2017; Guo et al., 2020; Hays et al., 2021a). The anatomical locations of SOZ for the patients included in this study did vary and did include those with mesial temporal lobe epilepsy. As a result, the effect of anatomical location may have contributed to the observed variability in our results across SOZ sites. Since networks of SPES-evoked responses are known to reflect structural and functional connectivity (Hebbink et al., 2019; Crocker et al., 2021), this contributed to the variability in the evoked responses across nSOZ sites as well. For example, stimulation of eloquent cortices is associated with larger or more robust evoked responses (Matsumoto et al., 2004; Matsumoto et al., 2007; Keller et al., 2011; Connor et al., 2011; Kikuchi et al., 2012; Sonoda et al., 2021). We expect that large nSOZ CCEPs from connections involved in these strong functional networks likely contributed to the nSOZ sites that were misclassified as SOZ sites by the logistic regression models. Future studies with larger sample sizes that enable controlling for location would be needed to account for differences between regions, possibly improving classification performance.
Similarly, tissue type is another potentially confounding factor since the SOZ is often localized to gray matter. While white matter stimulation can elicit more or larger CCEPs (Trebaul et al., 2018; Mitsuhashi et al., 2020), stimulations in this study targeted gray matter contacts to focus on connections between cortex or subcortical structures and avoid stimulating along white matter tracts. However, responses across all electrode contacts were analyzed and included in the reported results. To ensure that differences across tissue type did not confound our results, analysis of N1 voltage across current intensity was repeated using only responses in gray matter, and the results were not qualitatively different (Supplementary Figure 5).
Lastly, while we are using the clinically annotated SOZ as labels for grouping, classifying, and predicting, we recognize that these may not entirely reflect what part of the brain is responsible for generating and propagating seizure activity. The SOZ is only one factor indicating the location of the epileptogenic zone (Rosenow and Lüders, 2001), and observation is always limited to the electrode coverage. However, given the relatively high separability of SOZ and nSOZ across all patients, the concordance between electrophysiological responses to SPES and clinical observation of the SOZ indicates that CCEPs are measuring and identifying some aspect of the underlying excitability of epileptogenic brain tissue.
5. Conclusions
As SPES is increasingly utilized as a tool to delineate epileptogenic networks, it is important to understand how the choice of stimulation parameters can affect the ability to measure differences in excitability of epileptogenic and non-epileptogenic brain regions through evoked responses. By investigating CCEPs elicited from incremental titrations of current intensity, we found that sites within epileptogenic regions showed increasingly greater response amplitude compared to non-epileptogenic sites at 2 mA and above, especially from stimulation at other epileptogenic sites. Additionally, the distribution of significant responses in epileptogenic regions was maximized by 4-6 mA while that of non-epileptogenic regions steadily increased. We found that this excitability pattern over a range of current intensities provided additional information to help improve discriminability between epileptogenic and non-epileptogenic regions compared to relying on responses to a single maximum stimulation intensity. This research provides insight into the underlying excitability of epileptogenic regions and how differences in this current-dependent responses can be used to localize epileptogenic sites.
Supplementary Material
Highlights.
Cortico-cortical evoked potential amplitude increases with current intensity with a greater effect in the seizure onset zone (SOZ)
SOZ response distributions are maximized at lower currents, while those outside the SOZ gradually increase with current intensity
Responses to a range of current intensities provide better classification of the SOZ than responses to only one maximal intensity
Acknowledgments
This work was supported by the NIH NINDS Grant R01 NS115929.
Abbreviations:
- AUC
area under the curve
- CCEP
cortico-cortical evoked potential
- EEG
electroencephalography
- EP
early propagation
- IZ
irritative zone
- nSOZ
non-seizure onset zone
- RMS
root mean square
- ROC
receiver operating characteristic
- S-EEG
stereo-electroencephalography
- SNR
signal to noise ratio
- SOZ
seizure onset zone
- SPES
single pulse electrical stimulation
Footnotes
Conflict of interest statement
None of the authors have potential conflicts of interest to be disclosed.
Data availability statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- Bartolomei F, Lagarde S, Wendling F, McGonigal A, Jirsa V, Guye M. et al. Defining epileptogenic networks: Contribution of SEEG and signal analysis. Epilepsia 2017; 58: 1131–1147. 10.1111/epi.13791 [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J R Stat Soc Ser B Methodol 1995; 57: 289–300. 10.1111/j.2517-6161.1995.tb02031.x [DOI] [Google Scholar]
- Conner CR, Ellmore TM, DiSano MA, Pieters TA, Potter AW, Tandon N. Anatomic and electro-physiologic connectivity of the language system: a combined DTI-CCEP study. Comput Biol Med 2011; 41(12):1100–9. 10.1016/j.compbiomed.2011.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crocker B, Ostrowski L, Williams ZM, Dougherty DD, Eskandar EN, Widge AS, et al. Local and Distant responses to single pulse electrical stimulation reflect different forms of connectivity. NeuroImage 2021; 237:118094. 10.1016/j.neuroimage.2021.118094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowther LJ, Brunner P, Kapeller C, Guger C, Kamada K, Bunch ME, et al. A quantitative method for evaluating cortical responses to electrical stimulation. J Neurosci Methods 2019; 311: 67–75. 10.1016/j.jneumeth.2018.09.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donos C, Mîndruţă I, Ciurea J, Mălîia MD, Barborica A. A comparative study of the effects of pulse parameters for intracranial direct electrical stimulation in epilepsy. Clin Neurophysiol 2016; 127(1): 91–101. 10.1016/j.clinph.2015.02.013 [DOI] [PubMed] [Google Scholar]
- Duncan JS, Papademetris X, Yang J, Jackowski M, Zeng X, Staib LH. Geometric strategies for neuroanatomic analysis from MRI. NeuroImage 2004; 23(1): S34–S45. 10.1016/j.neuroimage.2004.07.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enatsu R, Piao Z, O’Connor T, Horning K, Mosher J, Burgess R, et al. Cortical excitability varies upon ictal onset patterns in neocortical epilepsy: A cortico-cortical evoked potential study. Clin Neurophysiol 2012a; 123: 252–260. 10.1016/j.clinph.2011.06.030 [DOI] [PubMed] [Google Scholar]
- Enatsu R, Jin K, Elwan S, Kubota Y, Piao Z, O’Connor T, et al. Correlations between ictal propagation and response to electrical cortical stimulation: A cortico-cortical evoked potential study. Epilepsy Res 2012b; 101(1–2): 76–87. 10.1016/j.eplepsyres.2012.03.004 [DOI] [PubMed] [Google Scholar]
- Fischl B. FreeSurfer. NeuroImage 2012; 62: 774–781. 10.1016/j.neuroimage.2012.01.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Martínez JA, Srikijvilaikul T, Nair D, Bingaman WE. Long-term Seizure Outcome in Reoperation after Failure of Epilepsy Surgery. Neurosurgery 2007; 60(5): 873–880. 10.1227/01.NEU.0000255438.13871.FA [DOI] [PubMed] [Google Scholar]
- Gunnarsdottir KM, Li A, Smith RJ, Kang JY, Korzeniewska A, Crone NE, et al. Source-sink connectivity: A novel interictal EEG marker for seizure localization. bioRxiv 2021.10.15.464594 10.1101/2021.10.15.464594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Z, Zhao B, Toprani S, Hu W, Zhang C, Wang X, et al. Epileptogenic network of focal epilepsies mapped with cortico-cortical evoked potentials. Clin Neurophysiol 2020; 131(11): 2657–2666. 10.1016/j.clinph.2020.08.012. [DOI] [PubMed] [Google Scholar]
- Gupta K, Grover P, Abel TJ. Current Conceptual Understanding of the Epileptogenic Network From Stereoelectroencephalography-Based Connectivity Inferences. Front Neurol 2020; 11: 1441 10.3389/fneur.2020.569699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hays MA, Coogan C, Crone NE, Kang JY. Graph theoretical analysis of evoked potentials shows network influence of epileptogenic mesial temporal region. Hum Brain Mapp. 2021a; 42: 4173– 4186. 10.1002/hbm.25418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hays MA, Smith RJ, Haridas B, Coogan C, Crone NE, Kang JY. Effects of stimulation intensity on intracranial cortico-cortical evoked potentials: A titration study. Clin Neurophysiol 2021b; 132(11): 2766–2777. 10.1016/j.clinph.2021.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebbink J, van Blooijs D, Huiskamp G, Leijten FSS, van Gils SA, Meijer HGE. A Comparison of Evoked and Non-evoked Functional Networks. Brain Topogr 2019; 32:405–417. 10.1007/s10548-018-0692-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasaki M, Enatsu R, Matsumoto R, Novak E, Thankappen B, Piao Z, et al. Accentuated cortico-cortical evoked potentials in neocortical epilepsy in areas of ictal onset. Epileptic Disord 2010; 12(4): 292–302. 10.1684/epd.2010.0334 [DOI] [PubMed] [Google Scholar]
- Jeha LE, Najm I, Bingaman W, Dinner D, Widdess-Walsh P, Lüders H, Surgical outcome and prognostic factors of frontal lobe epilepsy surgery. Brain 2007; 130(2): 574–584. 10.1093/brain/awl364 [DOI] [PubMed] [Google Scholar]
- Jobst BC, Cascino GD. Resective Epilepsy Surgery for Drug-Resistant Focal Epilepsy: A Review. JAMA 2015; 313(3): 285–293. 10.1001/jama.2014.17426 [DOI] [PubMed] [Google Scholar]
- Kassambara A 2021. rstatix: Pipe-Friendly Framework for Basic Statistical Tests. R package version 0.7.0, https://CRAN.R-project.org/package=rstatix [Google Scholar]
- Keller CJ, Bickel S, Entz L, Ulbert I, Milham MP, Kelly C. Intrinsic functional architecture predicts electrically evoked responses in the human brain. Proc Natl Acad Sci 2011; 108(25): 10308–10313. 10.1073/pnas.1019750108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller CJ, Honey CJ, Entz L, Bickel S, Groppe DM, Toth E, et al. Corticocortical Evoked Potentials Reveal Projectors and Integrators in Human Brain Networks. J Neurosci 2014; 34(27): 9152–9163. 10.1523/jneurosci.4289-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuchi T, Matsumoto R, Mikuni N, Yokoyama Y, Matsumoto A, Ikeda A, et al. Asymmetric bilateral effect of the supplementary motor area proper in the human motor system. Clin Neurophysiol 2012; 123(2): 324–334. 10.1016/j.clinph.2011.06.011 [DOI] [PubMed] [Google Scholar]
- Kobayashi K, Matsumoto R, Matsuhashi M, Usami K, Shimotake A, Kunieda T., et al. High frequency activity overriding cortico-cortical evoked potentials reflects altered excitability in the human epileptic focus. Clin Neurophysiol 2017; 128(9): 1673–1681. 10.1016/j.clinph.2017.06.249. [DOI] [PubMed] [Google Scholar]
- Kramer MA, Cash SS. Epilepsy as a Disorder of Cortical Network Organization. Neuroscientist. 2012; 18(4): 360–372. 10.1177/1073858411422754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kundu B, Davis TS, Philip B, Smith EH, Arain A, Peters A, et al. A systematic exploration of parameters affecting evoked intracranial potentials in patients with epilepsy. Brain Stimul 2020; 13(5): 1232–1244. 10.1016/j.brs.2020.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwan P, Brodie MJ. Definition of refractory epilepsy: defining the indefinable? Lancet Neurol 2010; 9(1): 27–29. 10.1016/S1474-4422(09)70304-7. [DOI] [PubMed] [Google Scholar]
- Lega B, Dionisio S, Flanigan P, Bingaman W, Najm I, Nair D, et al. Cortico-cortical evoked potentials for sites of early versus late seizure spread in stereoelectroencephalography. Epilepsy Res 2015; 115: 17–29. 10.1016/j.eplepsyres.2015.04.009 [DOI] [PubMed] [Google Scholar]
- Li A, Huynh C, Fitzgerald Z, Cajigas I, Brusko D, Jagid J, et al. Neural fragility as an EEG marker of the seizure onset zone. Nat Neurosci 2021; 24: 1465–1474. 10.1038/s41593-021-00901-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mălîia MD, Donos C, Barborica A, Mindruta I, Popa I, Ene M, et al. High frequency spectral changes induced by single-pulse electric stimulation: Comparison between physiologic and pathologic networks. Clin Neurophysiol 2017; 128(6): 1053–1060. 10.1016/j.clinph.2016.12.016 [DOI] [PubMed] [Google Scholar]
- Malmgren K, Edelvik A. Long-term outcomes of surgical treatment for epilepsy in adults with regard to seizures, antiepileptic drug treatment and employment. Seizure 2017; 44: 217–224. 10.1016/j.seizure.2016.10.015 [DOI] [PubMed] [Google Scholar]
- Matsumoto R, Nair DR, LaPresto E, Najm I, Bingaman W, Shibasaki H, et al. Functional connectivity in the human language system: A cortico-cortical evoked potential study. Brain 2004; 127(10): 2316–2330. 10.1093/brain/awh246 [DOI] [PubMed] [Google Scholar]
- Matsumoto R, Nair DR, LaPresto E, Bingaman W, Shibasaki H, Lüders HO. Functional connectivity in human cortical motor system: a cortico-cortical evoked potential study. Brain 2007; 130(1): 181–197. 10.1093/brain/awl257 [DOI] [PubMed] [Google Scholar]
- Matsumoto R, Nair DR, Ikeda A, Fumuro T, LaPresto E, Mikuni N, et al. Parieto-frontal network in humans studied by cortico-cortical evoked potential. Hum. Brain Mapp 2012; 33: 2856–2872. 10.1002/hbm.21407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto R, Kunieda T, Nair D. Single pulse electrical stimulation to probe functional and pathological connectivity in epilepsy. Seizure 2017; 44: 27–36. 10.1016/j.seizure.2016.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsuhashi T, Sonoda M, Iwaki H, Luat AF, Sood S, Asano E. Effects of depth electrode montage and single-pulse electrical stimulation sites on neuronal responses and effective connectivity. Clin Neurophysiol 2020; 131(12): 2781–2792. 10.1016/j.clinph.2020.09.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouthaan BE, van ‘t Klooster MA, Keizer D, Hebbink GJ, Leijten FSS, Ferrier CH, et al. Single Pulse Electrical Stimulation to identify epileptogenic cortex: Clinical information obtained from early evoked responses. Clin Neurophysiol 2016; 127(2): 1088–1098. 10.1016/j.clinph.2015.07.031 [DOI] [PubMed] [Google Scholar]
- Parker CS, Clayden JD, Cardoso MJ, Rodionov R, Duncan JS, Scott C, et al. Structural and effective connectivity in focal epilepsy. NeuroImage Clin 2018; 17: 943–952. 10.1016/j.nicl.2017.12.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinheiro J, Bates D, DebRoy S, Sarkar D, R Core Team (2021). nlme: Linear and Nonlinear Mixed Effects Models. R package version 3.1–153, https://CRAN.R-project.org/package=nlme. [Google Scholar]
- Prime D, Woolfe M, Rowlands D, O’Keefe S, Dionisio S. Comparing connectivity metrics in cortico-cortical evoked potentials using synthetic cortical response patterns. J Neurosci Methods 2020; 334: 108559. 10.1016/j.jneumeth.2019.108559 [DOI] [PubMed] [Google Scholar]
- R Core Team 2019. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/ [Google Scholar]
- Ramey WL, Martirosyan NL, Lieu CM, Hasham HA, Lemole GM, Weinand ME. Current management and surgical outcomes of medically intractable epilepsy. Clin Neurol Neurosurg 2013; 115(12): 2411–2418. 10.1016/j.clineuro.2013.09.035 [DOI] [PubMed] [Google Scholar]
- Rosenow F, Lüders H. Presurgical evaluation of epilepsy. Brain 2001; 124(9): 1683–1700. 10.1093/brain/124.9.1683 [DOI] [PubMed] [Google Scholar]
- Singh A, Trevick S. The Epidemiology of Global Epilepsy. Neurol Clin 2016; 34: 837–847. 10.1016/j.ncl.2016.06.015 [DOI] [PubMed] [Google Scholar]
- Sonoda M, Silverstein BH, Jeong JW, Sugiura A, Nakai Y, Mitsuhashi T, et al. Six-dimensional dynamic tractography atlas of language connectivity in the developing brain. Brain 2021; 144(11): 3340–3354. 10.1093/brain/awab225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomczak M, Tomczak E. The need to report effect size estimates revisited. An overview of some recommended measures of effect size. Trends Sport Sci. 2014; 1(21):19–25 [Google Scholar]
- Trebaul L, Deman P, Tuyisenge V, Jedynak M, Hugues E, Rudrauf D, et al. Probabilistic functional tractography of the human cortex revisited. NeuroImage 2018; 181:414–429. 10.1016/j.neuroimage.2018.07.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentín A, Anderson M, Alarcón G, García Seoane JJ, Selway R, Binnie CD, et al. Responses to single pulse electrical stimulation identify epileptogenesis in the human brain in vivo. Brain 2002; 125(8): 1709–1718. 10.1093/brain/awf187 [DOI] [PubMed] [Google Scholar]
- Valentín A, Alarcón G, Honavar M, García Seoane JJ, Selway RP, Polkey CE, et al. Single pulse electrical stimulation for identification of structural abnormalities and prediction of seizure outcome after epilepsy surgery: A prospective study. Lancet Neurol 2005; 4(11): 718–726. 10.1016/S1474-4422(05)70200-3 [DOI] [PubMed] [Google Scholar]
- van Blooijs D, Leijten FSS, van Rijen PC, Meijer HGE, Huiskamp GJM. Evoked directional network characteristics of epileptogenic tissue derived from single pulse electrical stimulation. Hum Brain Mapp 2018; 39(11): 4611–4622. 10.1002/hbm.24309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van ’t Klooster MA, Zijlmans M, Leijten FSS, Ferrier CH, van Putten MJAM, Huiskamp GJM. Time-frequency analysis of single pulse electrical stimulation to assist delineation of epileptogenic cortex. Brain 2011; 134(10): 2855–2866. 10.1093/brain/awr211 [DOI] [PubMed] [Google Scholar]
- Wiebe S, Blume WT, Girvin JP, Eliasziw M. A Randomized, Controlled Trial of Surgery for Temporal-Lobe Epilepsy. N Engl J Med 2001; 345(5): 311–318 10.1056/NEJM200108023450501 [DOI] [PubMed] [Google Scholar]
- Youden WJ (1950), Index for rating diagnostic tests. Cancer 1950; 3: 32–35. [DOI] [PubMed] [Google Scholar]
- Zhang N, Zhang B, Rajah GB, Geng X, Singh R, Yang Y, et al. The effectiveness of cortico-cortical evoked potential in detecting seizure onset zones. Neurol Res 2018; 40(6): 480–490. 10.1080/01616412.2018.1454092 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.