Abstract
Background:
Acute kidney injury (AKI) is common in deceased organ donors and is associated with high rates of kidney discard by transplant centers. High discard rates may consequently drive nonprocurement of these kidneys by organ procurement organizations. We aimed to study the relationship between donor AKI and kidney nonprocurement.
Methods:
Using U.S. registry data, we identified donors with at least one organ recovered from 2008–2018. We compared characteristics of donors with no kidneys procured across AKI stages, and used multivariable logistic regression to evaluate the relationship between AKI severity and kidney nonprocurement.
Results:
Overall 14,543 kidneys from 7,620 donors were not procured, among which 93% were from donors with AKI. For 6,945 donors with no kidneys procured but an extrarenal organ recovered, most had stage 3 (51%), followed by stage 1 (27%) and stage 2 AKI (15%). Nonprocured stage 3 donors were the youngest and had the lowest Kidney Donor Risk Index of all nonprocured donors. Adjusted odds of kidney nonprocurement were 1.14 (95%CI 1.02–1.27) for stage 1, 1.25 (95%CI 1.12–1.41) for stage 2, and 10.37 (95%CI 9.30–11.56) for stage 3 donors, compared to non-AKI donors. Among donors with minimum creatinine <1.5 mg/dL, stage 2 and 3 AKI were still associated with significantly higher odds of nonprocurement.
Conclusions:
AKI severity is a strong risk factor for kidney nonprocurement. Efforts to address the organ shortage should focus on encouraging procurement and utilization of kidneys from deceased donors with severe AKI, given the large and rising prevalence of donor AKI and excellent transplant outcomes with these kidneys.
Keywords: Acute Kidney Injury, Creatinine, Deceased Donor, Donor Selection, Kidney Transplantation, Organ Donation, Organ Procurement
1. Introduction
The growing organ shortage has led to innovative strategies for expanding the pool of kidneys for transplantation, including use of kidneys from deceased donors with acute kidney injury (AKI). Multiple studies have demonstrated that kidneys from donors with AKI provide similar short- and long-term outcomes compared to kidneys from donors without AKI, independent of AKI severity.1–9 Despite excellent transplant outcomes, approximately 27% of such kidneys are discarded in the United States.2,4,8 Low utilization of AKI donor kidneys by transplant centers potentially negatively impacts procurement of these kidneys by organ procurement organizations (OPOs). While our prior analysis demonstrated that an elevated terminal creatinine is commonly associated with failure to procure kidneys from deceased donors from whom other solid organs were recovered, the analysis did not include data that would have allowed differentiation of individuals with AKI from those with evidence of chronic kidney disease (CKD).10
In this study, we use changes in creatinine during hospitalization to identify and stage donor AKI, and evaluate the association between AKI severity and kidney nonprocurement. Given evidence that AKI is an independent risk factor for kidney discard,4,8,11 we hypothesize that AKI also adversely impacts kidney procurement.
2. Methods
2.1. Study Design and Participants
We performed a retrospective cohort study on deceased donors with ≥1 solid organ recovered between 1/1/2008 and 12/31/2018 using United Network for Organ Sharing (UNOS) Standard Transplant Analysis and Research (STAR) files from the Organ Procurement and Transportation Network (OPTN) database. Serial creatinine measurements during hospitalization prior to donation were obtained via a UNOS data request and linked to data in the STAR file using encrypted UNOS donor identification numbers. The analysis was based on STAR data as of 3/15/2019 and DonorNet data as of 1/24/2020.
We identified 97,145 deceased donors in the United States with one or more solid organs recovered, i.e. kidney(s), liver, heart, lung(s), intestine, and pancreas (Figure S1). We excluded donors with one or more missing DonorNet creatinine value(s) (n=25) or implausible creatinine value(s) (<0.1 or >40 mg/dL) (n=368), donors without kidney donation consent (n=898), donors with kidneys recovered for reasons other than transplant (n=834), procurements restricted by the medical examiner (n=57), donors with only a pancreas or intestine recovered (n=1), and donors with end-stage kidney disease (ESKD) reported by the organ procurement organization (n=11), for a final cohort of 94,951 donors.
2.2. Acute Kidney Injury, Nonprocurement, and Organ Quality
Donor AKI was the primary exposure variable. We used the lowest and highest serum creatinine (SCr) values from DonorNet to identify donor AKI based on Kidney Disease: Improving Global Outcomes (KDIGO) criteria. Donor AKI was defined as an increase in SCr by ≥0.3mg/dL or ≥1.5 times the lowest value.12 Donor AKI was staged for severity based on KDIGO criteria as stage 1 (≥0.3mg/dL or 1.5–1.9 fold increase), stage 2 (2.0–2.9 fold increase), or stage 3 (≥3 fold increase or a maximum value ≥4.0mg/dL).12 Urine volume and initiation of renal replacement therapy (RRT) were not available.
The primary outcome was deceased donor kidney nonprocurement. Nonprocurement, i.e., failure to recover a kidney from the deceased donor, is distinct from kidney discard, in which a recovered organ is not transplanted. Donors were classified as having no kidneys procured (n=6,945), a single kidney procured (n=675), or all available kidneys procured (n=87,331), which included cases where only 1 kidney was procured due to an absent partner kidney at the time of death (n=258). The number of procured and nonprocured kidneys was counted within each AKI category (Figure S1).
Organ quality was estimated using the Kidney Donor Risk Index (KDRI).13,14 KDRI was mapped onto a cumulative percentage scale using the 2017 scaling factor to generate the kidney donor profile index (KDPI).15,16
2.3. Statistical Analyses
We compared characteristics between donors with no kidneys procured versus all kidneys procured and between donors without AKI versus with AKI by analyzing the absolute standardized mean difference (SMD). The SMD reports the magnitude of difference between groups, and is independent of the sample size. SMD>0.1 (10%) was interpreted as a meaningful effect size.17,18 We compared donor characteristics across AKI stages using Pearson’s chi-squared test for categorical variables and Cuzick’s test based on ranks for continuous variables.
We used bivariable and multivariable logistic regression models to assess the relationship between donor AKI and kidney nonprocurement (versus procurement). Donors with only 1 of 2 available kidneys recovered were excluded from the analysis due to uncertainty over the degree to which the nonprocured kidneys differed from their procured counterparts and the possibility that nonprocurements secondary to unilateral abnormalities were appropriate following visual inspection of the organ. Donor AKI was operationalized as a dichotomous exposure (any AKI versus no AKI) and as a multilevel exposure (stage 1 versus 2 versus 3 versus no AKI), using no AKI as the reference group. In the multivariable model, we adjusted for donor proteinuria (reported as a dichotomous variable by the OPO) and individual components of the KDRI except for terminal creatinine, which was already considered in the donor AKI covariate. To account for potential cases of underlying CKD, we also stratified donors by their lowest SCr during hospitalization (<1.5 mg/dL or ≥1.5 mg/dL). To evaluate changes in procurement decision-making over the study period, we stratified donors by donation period (2008–2011, 2012–2015, and 2016–2018). Statistical analyses were performed using Stata 15.1 (StataCorp, College Station, TX). Statistical significance was set at a two-sided alpha<0.05.
3. Results
3.1. Donor characteristics by kidney procurement status and AKI stage
From 2008 to 2018, we identified 94,951 deceased organ donors meeting inclusion criteria, among which 81,753 (86%) had AKI (Figure S1). Most donors with AKI had stage 1 (53%, n=43,350), followed by stage 2 (30%, n=24,152) and stage 3 (17%, n=14,251) AKI. A total of 14,543 deceased donor kidneys, averaging 1,314 per year, were not procured over the 11-year study period, among which 93% were from donors with AKI. The proportion of kidneys not procured was twice as high for donors with any AKI (8%, n=13,497 out of 163,271) and over six times as high for donors with stage 3 AKI (25%, n=7,204 out of 28,464) compared to donors without AKI (4%, n=1,046 out of 26,373). Rates of kidney nonprocurement varied widely across OPTN regions by AKI stage (Figure S2). Region 6 had the lowest rates of kidney nonprocurement for all donors, with or without AKI (1.3% in donors without AKI, 2.2% in stage 1 donors, 1.8% in stage 2 donors, and 14.1% in stage 3 donors)), whereas Region 3 had the highest rates of kidney nonprocurement for donors with AKI (7.0% in stage 1 donors, 8.0% in stage 2 donors, and 34.2% in stage 3 donors).
Donor characteristics were stratified by kidney procurement status (Table S1). Compared to donors with all kidneys procured, those with no kidneys procured were older (SMD 53%) and more likely to be black (SMD 30%), obese (SMD 11%), hypertensive (SMD 68%), diabetic (SMD 57%), or HCV-positive (SMD 43%). They were more likely to have died from a cerebrovascular accident (SMD 21%) or donated after cardiac death (SMD 26%), and had higher nadir creatinine, terminal creatinine, and KDRI than donors with all kidneys procured. They were more likely to have a liver procured (SMD 44%), and less likely to have a lung (SMD 37%) or heart procured (SMD 47%), than donors with all kidneys procured.
Donor characteristics were also stratified by AKI status (Table S2). Compared to donors without AKI, those with any AKI were younger (SMD 11%) and more likely to be male (SMD 15%), black (SMD 13%), or obese (SMD 12%). They were less likely to have died from a cerebrovascular accident (SMD 24%) and more likely to have donated after cardiac death (SMD 24%). Although donors with AKI had higher nadir and terminal creatinine levels than donors without AKI (nadir creatinine 0.91±0.79 versus 0.76±0.36 mg/dL and terminal creatinine 1.53±1.55 versus 0.84±0.37 mg/dL, respectively), they also had better-quality kidneys as measured by the KDRI (median KDRI 1.26 (IQR 0.98–1.65) versus 1.31 (IQR 0.99–1.68), respectively) and were more likely to have a liver (SMD 12%) or heart (SMD 10%) procured. Stage 2 AKI donors were the youngest; least likely to be hypertensive, obese, or diabetic; and had the lowest nadir creatinine of all AKI donors. Kidney quality was highest for stage 2 AKI kidneys (median KDRI 1.23 (IQR 0.96–1.59)), followed by stage 1 (1.25 (IQR 0.97–1.65)), no AKI (1.31 (IQR 0.99–1.69)), and stage 3 (1.34 (IQR 1.05–1.75)) AKI kidneys.
3.2. Kidney nonprocurement trends
Overall, the rate of kidney nonprocurement declined from 8.6% in 2008 to 6.9% in 2018 (Figure 1). This decline was most pronounced for stage 2 AKI donors, from 6.2% in 2008 to 3.3% 2018 (average annual percentage change (AAPC) −5.1%), followed by stage 3 AKI donors (34.1% in 2008 to 21.2% in 2018 (AAPC −4.5%)), donors without AKI 5.3% in 2018 to 3.2% in 2018 (AAPC −3.5%)), and stage 1 AKI donors (5.3% in 2008 to 3.2% in 2018 (AAPC −3.1%)).
Figure 1:
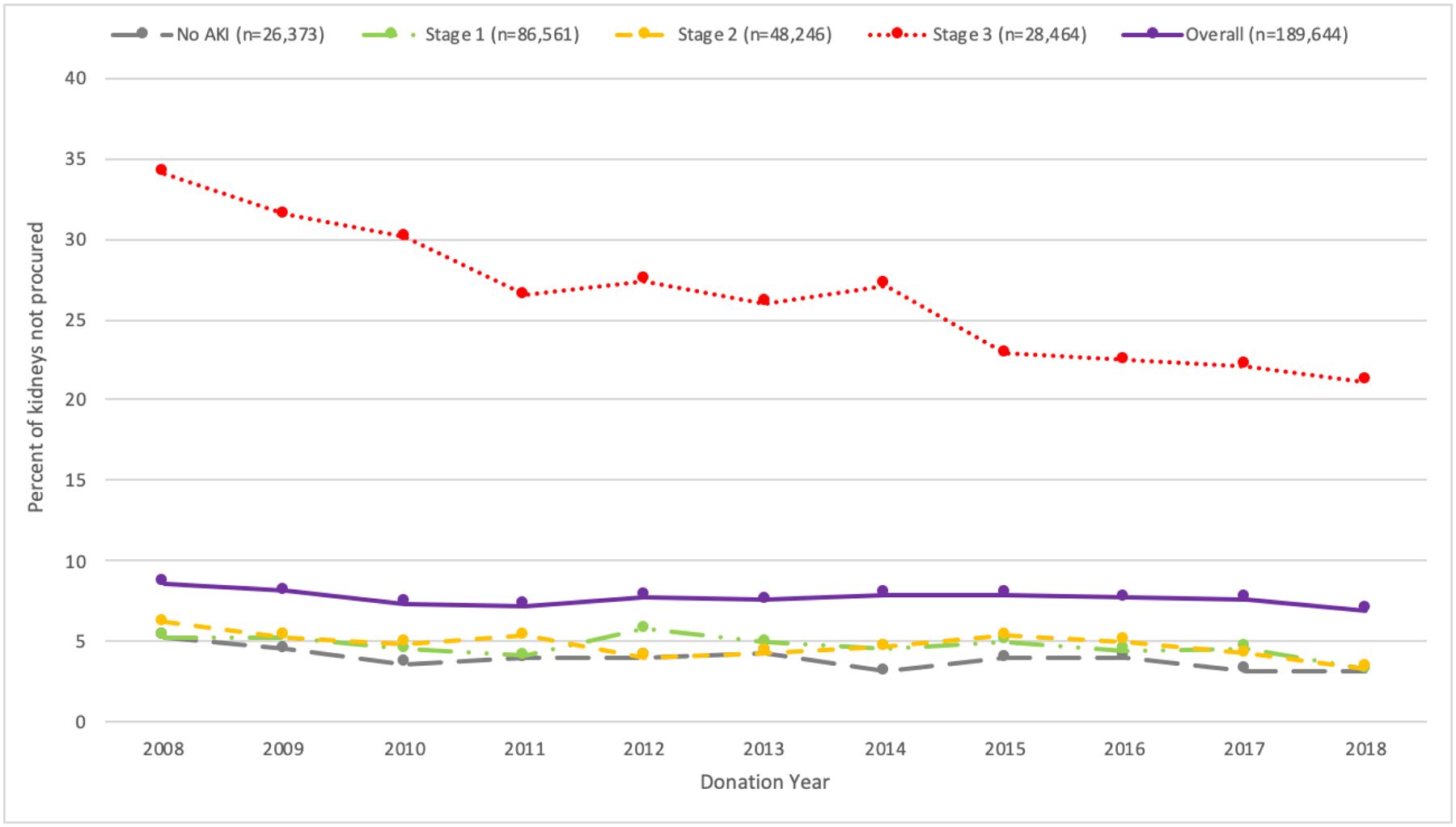
Percent of kidneys not procured by AKI stage and donation year. The average annual percentage change in kidney nonprocurement rate was −2.0% overall, including −3.5% for non-AKI kidneys, −3.3% for stage 1 AKI kidneys, −5.1% for stage 2 AKI kidneys, and −4.5% for stage 3 AKI kidneys.
3.3. Kidney nonprocurement according to donor AKI
Among donors with only extrarenal organs recovered (n=6,945), the most common combinations of procurements were liver only (80%), liver and heart (8%), liver and lung (6%), and heart only (3%) (Figure 2). The majority of donors had stage 3 AKI (51%; n=3,573), followed by stage 1 (27%; n=1,861), stage 2 (15%; n=1,042), and no AKI (7%; n=469) (Table 1). Donors with stage 3 AKI were the youngest, least likely to be HCV-positive, least likely to have died from a cerebrovascular accident, and most likely to have donated after cardiac death (all P<0.001). The median KDRI for nonprocured kidneys decreased with greater AKI severity as follows: 2.20 (IQR 1.63–2.71) for no AKI, 2.10 (IQR 1.56–2.68) for stage 1, 1.90 (IQR 1.46–2.50) for stage 2, and 1.78 (IQR 1.39–2.21) for stage 3 AKI.
Figure 2:
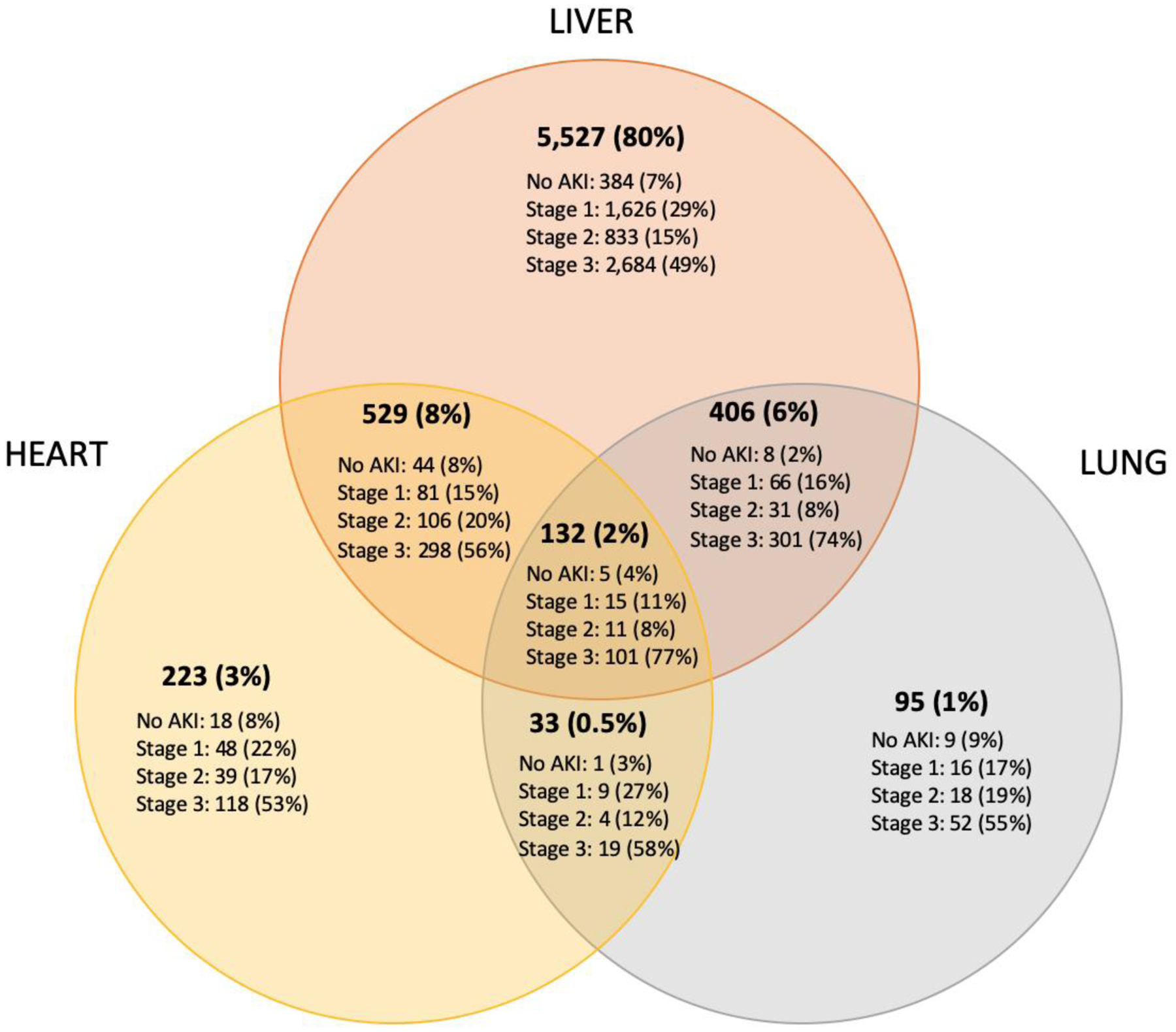
Distribution of AKI severity in donors with no kidney procurements, by non-renal organs procured (n=6,945)
Table 1:
Donor characteristics by AKI stage for donors with no kidneys procured (n=6,945)
| N (%) or mean ± SD | No AKI (7%) (n=469) | Stage 1 (27%) (n=1,861) | Stage 2 (15%) (n=1,042) | Stage 3 (51%) (n=3,573) |
|---|---|---|---|---|
| Age (yr) | 52.7±26.9 | 54.5±18.9 | 47.2±23.5 | 46.0±16.0 |
| Age >50 | 306 (65%) | 1,194 (64%) | 547 (53%) | 1,526 (43%) |
| Female | 249 (53%) | 783 (42%) | 477 (46%) | 1,304 (37%) |
| Black race | 75 (16%) | 409 (22%) | 234 (22%) | 1,170 (33%) |
| BMI (kg/m2) | 25.2±6.7 | 28.3±7.3 | 27.3±7.9 | 29.2±7.7 |
| Obese (BMI>30) | 87 (19%) | 626 (34%) | 328 (32%) | 1,401 (39%) |
| Hypertension | 296 (63%) | 1,258 (68%) | 555 (53%) | 2,335 (65%) |
| Diabetes | 105 (22%) | 614 (33%) | 238 (23%) | 1,274 (36%) |
| Death from CVA | 302 (64%) | 947 (51%) | 425 (41%) | 1,303 (36%) |
| DCD | 29 (6%) | 368 (20%) | 207 (20%) | 1,143 (32%) |
| HCV-positive | 91 (19%) | 458 (25%) | 304 (29%) | 381 (11%) |
| Proteinuria | 217 (46%) | 1,089 (59%) | 586 (56%) | 2,592 (73%) |
| Median KDRI (IQR)† | 2.20 (1.63–2.71) | 2.10 (1.56–2.68) | 1.90 (1.46–2.50) | 1.78 (1.39–2.21) |
| Median KDPI (%) (IQR) | 95 (79–100) | 94 (75–99) | 89 (69–99) | 86 (65–96) |
| KDPI>85% | 317 (68%) | 1,207 (65%) | 580 (56%) | 1,794 (50%) |
| Terminal Cr (mg/dL) | 1.18±1.15 | 1.81±0.89 | 1.83±1.07 | 5.84±2.99 |
| Terminal Cr>2 | 43 (9%) | 662 (36%) | 448 (43%) | 3,343 (94%) |
| Nadir Cr (mg/dL) | 1.09±1.15 | 1.35±0.62 | 0.92±0.43 | 2.89±2.55 |
| Nadir Cr ≥1.5 | 72 (15%) | 714 (38%) | 117 (11%) | 2,310 (65%) |
| Organ(s) procured‡ | ||||
| Liver | 441 (94%) | 1,788 (96%) | 981 (94%) | 3,384 (95%) |
| Lung | 23 (5%) | 106 (6%) | 64 (6%) | 473 (13%) |
| Heart | 68 (14%) | 153 (8%) | 150 (14%) | 536 (15%) |
Note: P <0.001 for all comparisons across AKI groups
Excluding 3 donors who were missing ≥1 donor characteristic used to calculate KDRI
Sum of percentages exceeds 100% because many donors had procurement of ≥2 non-renal organs
After controlling for donor proteinuria and KDRI components, kidneys from donors with any AKI faced over 2-fold higher odds of nonprocurement compared to kidneys from donors without AKI (adjusted OR 2.14 (95%CI 1.94–2.37)) (Table 2). This trend was primarily driven by nonprocurement of stage 3 AKI kidneys; stage 1 AKI donors faced a 1.14-fold higher odds of nonprocurement (95%CI 1.02–1.27), which increased to 1.25-fold higher odds of nonprocurement for stage 2 AKI donors (95%CI 1.12–1.41), and 10.37-fold higher odds of nonprocurement for stage 3 AKI donors (95%CI 9.30–11.56).
Table 2:
Unadjusted and adjusted odds of kidney nonprocurement by AKI stage†
| AKI Status | Number of donors | Nonprocurements (rate) | Unadjusted Odds Ratio (95% CI) | Adjusted Odds Ratio2 (95% CI) |
|---|---|---|---|---|
| No AKI | 13,088 | 469 (4%) | 1.0 (reference) | 1.0 (reference) |
| Any AKI | 81,188 (86%) | 6,476 (8%) | 2.33 (2.12–2.57) | 2.14 (1.94–2.37) |
| No AKI | 13,088 (14%) | 469 (4%) | 1.0 (reference) | 1.0 (reference) |
| Stage 1 | 43,031 (46%) | 1,861 (4%) | 1.22 (1.10–1.35) | 1.14 (1.02–1.27) |
| Stage 2 | 23,973 (25%) | 1,042 (4%) | 1.22 (1.09–1.37) | 1.25 (1.12–1.41) |
| Stage 3 | 14,184 (15%) | 3,573 (25%) | 9.06 (8.20–10.01) | 10.37 (9.30–11.56) |
Excluding donors with nonprocurement of 1 out of 2 available kidneys (n=675)
Adjusted for proteinuria and KDRI components except terminal creatinine, i.e. age, BMI, Black race, death from cerebrovascular accident, donation after cardiac death, hypertension, diabetes, hepatitis C positive
Statification of donors by the lowest SCr during hospitalization revealed similar nonprocurement odds between stage 1 AKI donors and donors without AKI, irrespective of nadir creatinine category (Table S3). For Stage 2 and 3 AKI donors, odds of kidney nonprocurement were significantly increased, even for donors with a nadir creatinine <1.5 mg/dL. Given concerns for double ischemic injury, we tested for interaction terms and identified significant interaction between DCD and AKI severity (p<0.001) (Table S4); however, non-DCD donors with stage III AKI still faced 7.5-fold higher odds of kidney nonprocurement compared to non-DCD donors without AKI, suggesting that factors beyond ischemic injury are driving nonprocurement.
Analysis of procurement decisions by donation period revealed a statistically significant increase in nonprocurement odds for stage 1 AKI donors from 2008–2011 to 2012–2015, and a significant decrease in nonprocurement odds for stage 3 AKI donors from 2008–2011 or 2012–2015 to 2016–2018 (Table S5).
3.4. Sensitivity analysis
Given similarities in nonprocurement rates between stage 1 and 2 AKI donors, we performed sensitivity analysis to evaluate changes in odds of kidney nonprocurement for stage 3 AKI donors, compared to a pooled group of stage 1 and 2 AKI donors (Table S6), and found that stage 1/2 (adjusted OR 1.18, 95%CI 1.06–1.30) and stage 3 AKI (adjusted OR 10.35, 95%CI 9.29–11.53) were significantly associated with nonprocurement. We also evaluated changes in odds of kidney nonprocurement for stage 3 or stage 2 AKI donors, compared to a pooled group of stage 1 and non-AKI donors (Table S7), and found persistent association between stage 2 (aOR 1.13, 95%CI 1.05–1.23) or stage 3 (aOR 9.36, 95%CI 8.78–9.98) AKI and kidney nonprocurement. Given previously identified discrepancies in terminal creatinine values in the OPTN registry,19 we also assessed the impact of using Deceased Donor Registration form (DDR)-based STAR file versus DonorNet terminal creatinine values. We examined differences in mean terminal creatinine, median KDRI, and median KDPI between the two datasets (Table S8), and crossover of donors between terminal creatinine categories and across AKI stages (Table S9), and found that the overall trends across donor kidney procurement status and AKI stages were similar to the primary analysis.
4. Discussion
From 2008 to 2018, the proportion of deceased organ donors with AKI has increased, consistent with rising rates of AKI among hospitalized patients in the United States.20,21 While prior work by our group demonstrated a strong association between elevated terminal creatinine and odds of nonprocurement, in this analysis we used patient creatinine levels during hospitalization to precisely classify donors based on acute kidney injury severity.10 Most donors with AKI had stage 1, followed by stage 2 and stage 3 AKI. Kidney nonprocurement rates decreased for all AKI stages over the study period. The overall nonprocurement rate was similar among stage 1, stage 2, and non-AKI donors; however, stage 3 AKI donors faced kidney nonprocurement rates that were six times higher than donors without AKI. Increasing donor AKI severity corresponded to higher odds of kidney nonprocurement, even after adjusting for donor characteristics and isolating donors with low nadir creatinine. As the transplant community increasingly faces decisions surrounding deceased donor AKI and the discard of these organs,22,23 there is also increasing need for scrutiny of current kidney procurement practices in these donors.
More than nine in ten kidney nonprocurements occurred in the setting of donor AKI. While concurrent CKD would be a recognizable contraindication to kidney procurement, AKI, even at levels severe enough to require transient dialysis, has been shown to provide short- and long-term graft outcomes comparable to non-AKI kidneys.1–9,24 Deceased donors with AKI had high rates of extrarenal organ procurement, with the rate of liver procurement being highest in stage 3 AKI donors (84%), rate of lung recovery being highest in stage 1 AKI donors (24%), and rate of heart recovery being highest in stage 1 and 2 AKI donors (32%). Among stage 3 AKI donors, three-quarters had a nadir creatinine less than 1.5 mg/dL, one-in-five had a lung procurement, and three-in-ten had a heart procurement. The majority of these deceased donors likely had AKI with kidneys potentially suitable for transplantation, given that negative consequences of CKD would likely have also discouraged procurement of these extrarenal organs.25
Previous studies have identified a conservative, risk-averse strategy among transplant centers that has resulted in frequent discard of deceased donor kidneys with AKI.26–29 A recent analysis of trends in kidney utilization found that most transplant centers used few stage 3 AKI kidneys and a quarter of centers did not use any stage 3 AKI kidneys in 2018.22 Although a small number of recovered but unused kidneys may be inevitable to encourage recovery of all usable organs, our paper focuses on the upstream decision of organ procurement. Our findings suggest potentially usable kidneys from donors with severe AKI are not being procured. Transplant center aversion to using stage 3 AKI kidneys, and the associated difficulties faced by OPOs in placing these procured kidneys, may negatively influence OPO willingness to procure these kidneys. Among nonprocured kidneys in our study, greater AKI severity corresponded to a more favorable kidney risk profile. Nonprocured stage 3 AKI kidneys came from the youngest donors (mean age 44.1±18.2 years) who had the lowest rates of HCV-positive status. Kidney quality, as measured by the KDRI, was best in nonprocured kidneys with the greatest AKI severity and worst in nonprocured kidneys without AKI, suggesting that many viable AKI kidneys are not being procured from relatively young and healthy organ donors – underscoring not only the challenges of interpreting large amplitude creatinine fluctuations in younger patients with greater lean body mass, but also the misleading impact of elevated terminal creatinine from AKI on the KDRI.30 Failure to procure these organs contributes to an underestimation of the true potential for transplant centers to appropriately use deceased donor kidneys, since current estimates of kidney discard do not account for the many AKI kidneys that are being left behind by OPOs.
In addition to the strong association between AKI severity and kidney nonprocurement, even for donors with nadir creatinine values <1.5mg/dL, our analysis revealed small changes in the effect size of AKI over the study period. Although odds of nonprocurement decreased for stage 3 donors in 2016–2018, these donors were still at over nine-fold higher odds of nonprocurement compared to donors without AKI. Furthermore, kidney discard rates in the U.S. have continued to rise, with over 21% of procured kidneys being discarded in 2020.31 Liu et al. recently identified increasing discard rates for stage 3 AKI kidneys, despite increasing procurement rates for these organs. The diverging trends suggest that utilization by transplant centers is not keeping up with the supply of available organs.22 The persistent failure to appropriately procure and utilize kidneys from donors with severe AKI, despite successful outcomes for recipients of these organs, follows a trend where organ acceptance practices are not in line with the best available evidence, as has previously been observed in the low procurement and utilization of kidneys from diabetic or HCV-positive donors.4,5,10,32–34
Our findings underscore the need for improved recognition within the transplant community of the benefits of transplanting AKI kidneys to achieve successful outcomes for patients. More detailed reporting systems that include information about renal function trajectories would allow for better evaluation of available donor kidneys. A single-center retrospective study in Korea demonstrated that deceased donor AKI trend (i.e. improving or worsening) was predictive of graft outcomes, and that deceased donor kidneys with improving AKI may be suitable for transplantation regardless of AKI severity.3 Another potential opportunity would be to phenotype the AKI; prerenal AKI resulting from acute hemodynamic changes prior to death is often reversible, and is not necessarily associated with intrinsic tissue injury.26,35 Inclusion of non-creatinine damage and functional biomarkers would allow for more precise assesssment of AKI severity and help identify organs with poor prognoses.36
There are several limitations to our study. The OPTN registry does not consistently capture information on donor urine output or renal replacement therapy, which are part of KDIGO criteria for defining and staging AKI. Our model is unable to account for additional factors associated with nonprocurement that are not reported by OPOs to the data registry, such as logistical challenges relating to procurement. As previously discussed, the database does not distinguish the type of donor AKI. We also assume that the minimum creatinine during hospitalization is similar to the donor’s baseline creatinine, but this value could be different from the actual baseline, particularly if a patient is receiving RRT. While these limitations may lead to misclassification of donor AKI, multiple prior studies have relied on changes in SCr from baseline, independent of time between creatinine measurements (e.g. greater or less than 48 hours per KDIGO criteria), when grading AKI and assessing its impact on organ utilization and transplant outcomes.1,2,11,37,38 Our group’s previous findings of geographic variation in kidney nonprocurements and discards, along with the positive correlation between kidney discard by transplant centers and kidney nonprocurement by the corresponding OPO, suggest that OPO- and transplant center-specific factors may be relevant confounders.8,10 Notably, recent implementation of broader sharing strategies, including KAS250, may help mitigate these regional effects. Our estimates of kidney nonprocurement rates depend on extrarenal organ recovery to identify the baseline number of solid organ donors. Potential donors with no organs procured are thus absent from the analysis. Given that kidney-only donors represented 15% of the study population (n=14,451), the analysis likely underestimates the rate of kidney nonprocurement and the true number of kidneys potentially available for transplantation. Finally, observational studies on transplant outcomes face selection bias in only reporting outcomes of organs that are ultimately procured and transplanted – which may not be generalizable to all organs from deceased donors with AKI. To mitigate this “healthy user” bias, we defined the study population as donors who had at least one solid organ procured – presumably these individuals had already passed an initial health screen by OPOs – and matched donors by kidney quality when comparing nonprocurement between donors with and without AKI.
In conclusion, the majority of kidney nonprocurements from deceased donors in the United States occurs in the setting of donor AKI. Among nonprocured kidneys, more severe AKI is associated with higher-quality kidneys. AKI severity appears to be a strong risk factor for kidney nonprocurement, which may be driven in part by discard of these kidneys by transplant centers. Given the large and rising prevalence of AKI among deceased donors, both individual-level changes to enhance clinician awareness of excellent graft outcomes with AKI kidneys, as well as systems-level changes to incentivize pursuit of these organs, will be crucial to optimize use of deceased donor kidneys.
Supplementary Material
Figure S1: Flowsheet of study cohort distributed among AKI categories (n=94,951)
Figure S2: Percent of kidneys not procured by AKI stage and OPTN region
Table S1: Donor characteristics by kidney procurement status (n=94,276)
Table S2: Donor characteristics by AKI stage (n=94,951)
Table S3: Unadjusted and adjusted odds of kidney nonprocurement by AKI stage, stratified by nadir creatinine
Table S4: Unadjusted odds of kidney nonprocurement by AKI stage, stratified by DCD status.
Table S5: Adjusted odds of kidney nonprocurement, stratified by donation period
Table S6: Sensitivity analysis combining stage 1 and stage 2 AKI donors into one group to evaluate unadjusted and adjusted odds of kidney nonprocurement by AKI stage
Table S7: Sensitivity analysis combining no AKI and stage 1 AKI donors into one group to evaluate unadjusted and adjusted odds of kidney nonprocurement by AKI stage
Table S8: Sensitivity analysis using DonorNet and DDR/STAR terminal creatinine values to compare donor terminal creatinine, KDRI, and KDPI by (a) kidney procurement status (n=94,276), (b) AKI stage (n=94,951), and (c) AKI stage for donors with no kidney procurements (n=6,945)
Table S9: Sensitivity analysis using DonorNet and DDR/STAR terminal creatinine values to assess donor crossover between (a) terminal Cr ≤ and >2 mg/dL (n=94,951) and (b) no AKI, stage 1, stage 2, and stage 3 AKI (n=94,951)
Acknowledgements and Funding
SAH is supported by the National Center for Advancing Translational Sciences (KL2TR001874). CRP is supported by the National Heart, Lung and Blood Institute (R01HL085757) and the National Institute of Diabetes and Digestive and Kidney Diseases (UH3DK114866, U01DK106962, and R01DK093770). SM is supported by National Institute of Diabetes and Digestive and Kidney Diseases (DK114893, DK126739, DK130058, and DK116066) and the National Institute on Minority Health and Health Disparities (MD014161).
This work was supported in part by Health Resources and Services Administration contract 234-2005-370011C. The content is the responsibility of the authors alone and does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
The funders of the study did not have any role in the study design; collection, analysis, and interpretation of data; writing the report; and the decision to submit the report for publication.
Disclosures
C. Parikh is a member of the advisory board of and owns equity in RenalytixAI. He also serves as a consultant for Genfit and Novartis.
S. Mohan received grant funding from Angion Biomedica and personal fees from Kidney International Reports and Health Services Advisory Group outside of the submitted work.
Abbreviations:
- AKI
Acute Kidney Injury
- BMI
Body Mass Index
- CKD
Chronic Kidney Disease
- CVA
Cerebrovascular Accident
- DCD
Deceased after Cardiac Death
- DDR
Deceased Donor Registration form
- DSA
Donor Service Area
- ESKD
End-Stage Kidney Disease
- HCV+
Hepatitis C Positive
- KDIGO
Kidney Disease: Improving Global Outcomes
- KDPI
Kidney Donor Profile Index
- KDRI
Kidney Donor Risk Index
- OPO
Organ Procurement Organization
- OPTN
Organ Procurement and Transplantation Network
- RRT
Renal Replacement Therapy
- SMD
Standardized Mean Difference
- STAR
Standard Transplant Analysis and Research
- UNOS
United Network for Organ Sharing
Data Availability Statement
The data that support the findings of this study are available upon request to the United Network for Organ Sharing (UNOS).
References
- 1.Heilman RL, Smith ML, Smith BH, et al. Long-term Outcomes Following Kidney Transplantation From Donors With Acute Kidney Injury. Transplantation. 2019;103(9). [DOI] [PubMed] [Google Scholar]
- 2.Liu C, Hall IE, Mansour S, Thiessen Philbrook HR, Jia Y, Parikh CR. Association of Deceased Donor Acute Kidney Injury With Recipient Graft Survival. JAMA Network Open. 2020;3(1):e1918634–e1918634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yu M-y, Yu BC, Kim YC, et al. Trend, not severity, of acute kidney injury affects graft outcome in deceased donor kidney transplantation. Clinical Transplantation. 2018;32(12):e13431. [DOI] [PubMed] [Google Scholar]
- 4.Hall IE, Schroppel B, Doshi MD, et al. Associations of deceased donor kidney injury with kidney discard and function after transplantation. Am J Transplant. 2015;15(6):1623–1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hall IE, Akalin E, Bromberg JS, et al. Deceased-donor acute kidney injury is not associated with kidney allograft failure. Kidney Int. 2019;95(1):199–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heilman RL, Smith ML, Kurian SM, et al. Transplanting Kidneys from Deceased Donors With Severe Acute Kidney Injury. Am J Transplant. 2015;15(8):2143–2151. [DOI] [PubMed] [Google Scholar]
- 7.Domagala P, Gorski L, Wszola M, et al. Successful transplantation of kidneys from deceased donors with terminal acute kidney injury. Renal Failure. 2019;41(1):167–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mohan S, Chiles MC, Patzer RE, et al. Factors leading to the discard of deceased donor kidneys in the United States. Kidney Int. 2018;94(1):187–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sanders JM, Opdam HI, Furniss H, Hughes PD, Kanellis J, Jones D. Frequency and outcomes of kidney donation from intensive care patients with acute renal failure requiring renal replacement therapy. Nephrology (Carlton). 2019;24(12):1296–1303. [DOI] [PubMed] [Google Scholar]
- 10.Yu K, King K, Husain SA, et al. Kidney nonprocurement in solid organ donors in the United States. American Journal of Transplantation. 2020;20(12):3413–3425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kayler LK, Garzon P, Magliocca J, et al. Outcomes and utilization of kidneys from deceased donors with acute kidney injury. Am J Transplant. 2009;9(2):367–373. [DOI] [PubMed] [Google Scholar]
- 12.Khwaja A. KDIGO Clinical Practice Guidelines for Acute Kidney Injury. Nephron Clinical Practice. 2012;120(4):c179–c184. [DOI] [PubMed] [Google Scholar]
- 13.Rao PS, Schaubel DE, Guidinger MK, et al. A comprehensive risk quantification score for deceased donor kidneys: the kidney donor risk index. Transplantation. 2009;88(2):231–236. [DOI] [PubMed] [Google Scholar]
- 14.Zhong Y, Schaubel DE, Kalbfleisch JD, Ashby VB, Rao PS, Sung RS. Reevaluation of the Kidney Donor Risk Index (KDRI). Transplantation. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.The Organ Procurement and Transplantation Network UN, for Organ Sharing. A Guide to Calculating and Interpreting the Kidney Donor Profle Index (KDPI) https://optn.transplant.hrsa.gov/media/1512/guide_to_calculating_interpreting_kdpi.pdf.
- 16.The Organ Procurement and Transplantation Network UN, for Organ Sharing. KDRI to KDPI Mapping Table. https://optn.transplant.hrsa.gov/media/2150/kdpi_mapping_table.pdf. Accessed July 22, 2019.
- 17.Gøtzsche PC, Hróbjartsson A, Marić K, Tendal B. Data Extraction Errors in Meta-analyses That Use Standardized Mean Differences. JAMA. 2007;298(4):430–437. [DOI] [PubMed] [Google Scholar]
- 18.Markoulidakis A, Taiyari K, Holmans P, et al. A tutorial comparing different covariate balancing methods with an application evaluating the causal effects of substance use treatment programs for adolescents. Health Services and Outcomes Research Methodology. 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yu K, King K, Husain SA, Mohan S. Variations in Deceased Donor Terminal Creatinine Values Reported in the OPTN Data Registry. Clinical Journal of the American Society of Nephrology. 2022:CJN.15511121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pavkov ME, Harding JL, Burrows NR. Trends in Hospitalizations for Acute Kidney Injury - United States, 2000–2014. MMWR Morb Mortal Wkly Rep. 2018;67(10):289–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sawhney S, Fraser SD. Epidemiology of AKI: Utilizing Large Databases to Determine the Burden of AKI. Advances in chronic kidney disease. 2017;24(4):194–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu C, Alasfar S, Reese PP, et al. Trends in the procurement and discard of kidneys from deceased donors with acute kidney injury. American Journal of Transplantation. 2021;n/a(n/a). [DOI] [PubMed] [Google Scholar]
- 23.Heilman RL, Mathur A, Smith ML, Kaplan B, Reddy KS. Increasing the Use of Kidneys From Unconventional and High-Risk Deceased Donors. American Journal of Transplantation. 2016;16(11):3086–3092. [DOI] [PubMed] [Google Scholar]
- 24.Budhiraja P, Heilman RL, Jadlowiec CC, et al. Successful outcomes with transplanting kidneys from deceased donors with acute kidney injuryon temporary renal replacement therapy. Clinical Transplantation. 2021;35(12):e14465. [DOI] [PubMed] [Google Scholar]
- 25.Israni AK, Zaun D, Hadley N, et al. OPTN/SRTR 2018 Annual Data Report: Deceased Organ Donation. Am J Transplant. 2020;20 Suppl s1:509–541. [DOI] [PubMed] [Google Scholar]
- 26.Koyawala N, Parikh CR. A Review of Donor Acute Kidney Injury and Posttransplant Outcomes. (1534–6080 (Electronic)). [DOI] [PubMed]
- 27.Husain SA, King KL, Pastan S, et al. Association Between Declined Offers of Deceased Donor Kidney Allograft and Outcomes in Kidney Transplant Candidates. JAMA Netw Open. 2019;2(8):e1910312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huml AM, Albert JM, Thornton JD, Sehgal AR. Outcomes of Deceased Donor Kidney Offers to Patients at the Top of the Waiting List. Clin J Am Soc Nephrol. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.King KL, Husain SA, Cohen DJ, Mohan S. Deceased Donor Kidneys Are Harder to Place on the Weekend. Clin J Am Soc Nephrol. 2019;14(6):904–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chiles MC, Husain SA, Skillen W, et al. Predictive Value of Using Initial Versus Terminal Deceased Donor Creatinine to Calculate the Kidney Donor Risk Index. American Journal of Kidney Diseases. 2017;70(1):153–154. [DOI] [PubMed] [Google Scholar]
- 31.Li MT, King KL, Husain SA, Schold JD, Mohan S. Deceased Donor Kidneys Utilization and Discard Rates During COVID-19 Pandemic in the United States. Kidney Int Rep. 2021;6(9):2463–2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mohan S, Tanriover B, Ali N, et al. Availability, utilization and outcomes of deceased diabetic donor kidneys; analysis based on the UNOS registry. Am J Transplant. 2012;12(8):2098–2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.King KL, Husain SA, Mohan S. Trends in Transplantation Center Use of Kidneys From Deceased Donors With Positive Hepatitis C Virus Nucleic Acid Testing. Am J Kidney Dis. 2020;76(5):743–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brennan C, Husain SA, King KL, et al. A Donor Utilization Index to Assess the Utilization and Discard of Deceased Donor Kidneys Perceived as High Risk. Clinical Journal of the American Society of Nephrology. 2019;14(11):1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dupont M, Shrestha K, Singh D, et al. Lack of significant renal tubular injury despite acute kidney injury in acute decompensated heart failure. European Journal of Heart Failure. 2012;14(6):597–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ostermann M, Zarbock A, Goldstein S, et al. Recommendations on Acute Kidney Injury Biomarkers From the Acute Disease Quality Initiative Consensus Conference: A Consensus Statement. JAMA Network Open. 2020;3(10):e2019209–e2019209. [DOI] [PubMed] [Google Scholar]
- 37.Sonnenberg EM, Hsu JY, Cohen JB, et al. Acute Kidney Injury in Deceased Organ Donors and Kidney Transplant Outcomes: A National Cohort Study using a Novel Data Source. Ann Surg. 2020. [DOI] [PubMed] [Google Scholar]
- 38.Hall IE, Akalin E, Bromberg JS, et al. Deceased-donor acute kidney injury is not associated with kidney allograft failure. Kidney International. 2019;95(1):199–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1: Flowsheet of study cohort distributed among AKI categories (n=94,951)
Figure S2: Percent of kidneys not procured by AKI stage and OPTN region
Table S1: Donor characteristics by kidney procurement status (n=94,276)
Table S2: Donor characteristics by AKI stage (n=94,951)
Table S3: Unadjusted and adjusted odds of kidney nonprocurement by AKI stage, stratified by nadir creatinine
Table S4: Unadjusted odds of kidney nonprocurement by AKI stage, stratified by DCD status.
Table S5: Adjusted odds of kidney nonprocurement, stratified by donation period
Table S6: Sensitivity analysis combining stage 1 and stage 2 AKI donors into one group to evaluate unadjusted and adjusted odds of kidney nonprocurement by AKI stage
Table S7: Sensitivity analysis combining no AKI and stage 1 AKI donors into one group to evaluate unadjusted and adjusted odds of kidney nonprocurement by AKI stage
Table S8: Sensitivity analysis using DonorNet and DDR/STAR terminal creatinine values to compare donor terminal creatinine, KDRI, and KDPI by (a) kidney procurement status (n=94,276), (b) AKI stage (n=94,951), and (c) AKI stage for donors with no kidney procurements (n=6,945)
Table S9: Sensitivity analysis using DonorNet and DDR/STAR terminal creatinine values to assess donor crossover between (a) terminal Cr ≤ and >2 mg/dL (n=94,951) and (b) no AKI, stage 1, stage 2, and stage 3 AKI (n=94,951)
Data Availability Statement
The data that support the findings of this study are available upon request to the United Network for Organ Sharing (UNOS).


