Abstract
Background
The N-methyl-D-aspartate receptor (NMDAR) is a major molecular target of alcohol action in the central nervous system, yet many aspects of the manner in which alcohol modulates the activity of this ion channel remain unclear. We and others have shown that ethanol inhibition of NMDAR involves alterations in gating, especially reduction of mean open time. A full description of ethanol’s effects on NMDAR kinetics, however, including fitting to a kinetic model, has not been reported.
Methods
To determine ethanol’s effects on NMDAR kinetics, we used steady-state single-channel recording in outside-out patches from HEK-293 cells transfected with recombinant GluN1/GluN2A or GluN1/GluN2B NMDAR subunits. Very low glutamate concentrations were used in order to isolate individual activations of the receptor.
Results
In both subunit types, ethanol, at approximate whole-cell IC50 values (156 mM, GluN2A; 150 mM, GluN2B), reduced open probability (Po) by approximately 50%, and decreased mean open time without changing frequency of opening. Open and shut time distributions exhibited two and five components, respectively; ethanol selectively decreased the time constant and relative proportion of the longer open time component. In the GluN2A subunit, ethanol increased the time constants of all but the longest shut time components, whereas in the GluN2B subunit, shut times were unchanged by ethanol. Fitting of bursts of openings (representing individual activations of the receptor) to the gating portion of a kinetic model revealed that ethanol altered two rates: the rate associated with activation of the GluN2A or GluN2B subunit, and the rate associated with closing of the longer of the two open states.
Conclusions
These results demonstrate that ethanol selectively alters individual kinetic rates, and thus appears to selectively affect distinct conformational transitions involved in NMDAR gating.
Introduction
Alcohol use disorders present as alterations in behavior, including impairment of motor function, cognition and judgment, and disrupted social interactions during intoxication, but these complex behaviors ultimately arise from changes in synaptic transmission in the brain, due to ethanol-induced alterations in physiological processes including neurotransmitter release, kinase and phosphatase activity, and neuronal ion channel function (Abrahao et al., 2017, McCool, 2011, Ron and Wang, 2009). A large body of evidence supports an important role for the N-methyl-D-aspartate (NMDA) receptor-ion channel in mediating alcohol action in the brain, both in acute intoxication, as well as in phenomena including alcohol craving, tolerance, dependence, withdrawal, and relapse (Chandrasekar, 2013, Holmes et al., 2013, Krystal et al., 2003a, Krystal et al., 2003b, Ron and Wang, 2009, Vengeliene et al., 2008). Although alcohol can modulate NMDA receptor sensitivity and signaling via multiple mechanisms (Ron, 2004), its direct action on NMDA receptors involves altering ion channel gating (Lima-Landman and Albuquerque, 1989, Wright et al., 1996) via an interaction with specific modulatory sites (Honse et al., 2004, Ren et al., 2003, Ren et al., 2007, Ren et al., 2012, Ronald et al., 2001, Smothers and Woodward, 2006).
Previous studies have shown that ethanol inhibits NMDA receptor gating by decreasing both mean open time and frequency of opening, without appreciably changing closed times (Lima-Landman and Albuquerque, 1989, Wright et al., 1996). These studies established that ethanol inhibition involves modulation of gating, but were performed prior to the development of accurate models of ion channel gating, so the precise effects of ethanol on the transitions among the different kinetic states that constitute NMDAR gating behavior have not been determined. In addition, the studies of Lima-Landman and Albuquerque (1989) and Wright et al. (1996) were performed in native CNS neurons in culture. While the conditions used in these studies should closely approximate NMDA receptor modulation by ethanol in vivo, the subunit composition of the receptors tested in these studies is unknown, and most likely was a varying mixture of GluN1/GluN2A, GluN1/GluN2B, and GluN1/GluN2A/GluN2B heterotrimeric NMDA receptors (Al-Hallaq et al., 2007, Rauner and Kohr, 2011). In the present study, we used single-channel recording in recombinant GluN1/GluN2A or GluN1/GluN2B subunit-containing NMDA receptors expressed in the HEK-293 cell line. Using low agonist concentrations to allow separation of groups of opening events into bursts representing individual activations of the receptor, we fit the data to the gating portion of a cyclic model to determine the kinetic effects of ethanol on NMDA receptor gating. Under these conditions, the effect of ethanol was primarily attributable to alteration of two specific ion channel gating rate constants in the kinetic model in both subunit types.
MATERIALS AND METHODS
Materials.
Ethanol (95%, prepared from grain) was obtained from Aaper Alcohol & Chemical Co. (Shelbyville, KY, USA), Cs BAPTA was obtained from Invitrogen ThermoFisher (Waltham, MA), and all other drugs and chemicals were obtained from MilliporeSigma (St. Louis, MO, USA).
Cell culture and transfection.
Human embryonic kidney (HEK) 293 cells obtained from the American Type Culture Collection (Manassas, VA) were cultured as previously described (Ren et al., 2017). Cells were allowed to grow to 75 – 90% confluence before transient transfection with plasmids containing rat GluN1–1a (pRC) and GluN2A (pcDNA1) or GluN2B (pDP3) subunits and green fluorescent protein (Addgene) at a ratio of 2:2:1 using a calcium phosphate transfection kit or Lipofectamine 3000 (Invitrogen ThermoFisher). During and after the transfection, 200 μM D,L-2-amino-5-phosphonovaleric acid (APV) and either 100 μM ketamine or 100 μM memantine were added to the culture media to protect cells from glutamate excitotoxicity. Cells were used in experiments 18 to 72 hr after transfection.
Electrophysiological recording.
Patch-clamp recording was performed at room temperature using an Axopatch 1D or Axopatch 200B (Molecular Devices) amplifier. Patch-pipets were coated with R6101 elastomer (Dow-Corning), and had tip resistances of 8 – 15 MΩ following heat polishing. Outside-out patches were voltage-clamped at −50 mV and superfused in Mg2+-free external recording solution containing (in mM) 150 NaCl, 5 KCl, 0.2 CaCl2, 10 HEPES, 10 μM EDTA, 10 glucose, and 10 sucrose. Solution pH was adjusted to 7.4 by adding a calculated ratio of HEPES free acid to HEPES sodium salt (Buffer Calculator, available at https://www.liverpool.ac.uk/pfg/Tools/BuffferCalc/Buffer.html). Ultrapure salts and chemicals were used to minimize contamination by other cations. The intracellular recording solution contained (in mM) 140 CsCl, 2 Mg4ATP, 10 BAPTA, and 10 HEPES; pH was adjusted to 7.2 with CsOH. Solutions of agonists and ethanol were prepared fresh daily in extracellular solution and applied to cells using a stepper motor-driven rapid solution exchange apparatus (Fast-step, Warner Instrument Co.) and 600 μm id square glass tubing. The ethanol concentrations used were 156 mM (GluN2A) and 150 mM (GluN2B), which we have found in preliminary experiments and previous results using whole-cell patch-clamp recording to be the approximate IC50 values; we have previously reported similar values (Ren et al., 2003, Ren et al., 2012, Zhao et al., 2015), although other laboratories have reported greater differences in sensitivity between the GluN2A and GluN2B subunits (Smothers et al., 2001). Recordings alternated between control and ethanol exposure at one or two minute intervals in order to minimize the influence of any slow changes in ion channel activity. Low concentrations of glutamate in the presence of a saturating concentration of glycine were used to obtain widely separated individual receptor activations (Gibb and Colquhoun, 1991, Gibb and Colquhoun, 1992, Wyllie et al., 1998). Each patch was exposed to an initial concentration of 100 nM glutamate; in some cases a concentration of 1 μM glutamate was used in patches exhibiting very low levels of activity.
Data Analysis.
Data from single-channel recordings were acquired at 50 kHz, digitally filtered at 5 kHz (8-pole Bessel), and idealized using the segmentation K-means algorithm in the QUB software suite (Qin, 2004). Open and shut dwell time histograms were fitted with multiple exponential components using Channelab (Synaptosoft) after imposing a 50–100 μs dead time, and mean open times were obtained from the proportionally-weighted averages of the individual components. Frequency of opening was calculated by dividing the number of openings by the recording time. Data were obtained from 7 patches for each subunit combination. Patches used for each subunit combination had one to three levels of opening, and were obtained on multiple experimental days over the course of several weeks. Burst analysis was performed in steady-state single-channel records by using values of the critical time interval (τcrit) from shut time histograms that minimized the total number of misclassified events. Bursts with more than one level of opening were excluded from analysis. The values for τcrit were calculated to be between the third and fourth components of the shut time in GluN2A and between the fourth and fifth components of the shut time in GluN2B in order to isolate burst events corresponding to individual receptor activations.
Kinetic modeling.
Opening and closing events within bursts were fitted to a kinetic model using the maximum interval likelihood (MIL) function of the QUB program in order to obtain values for the rate constants for subunit activation and channel opening (Qin and Li, 2004). All parameters were allowed to vary freely. We chose to use a simple cyclic gating model of Traynelis and colleagues (Erreger et al., 2005b), which is able to fit both single-channel and macroscopic response data for the GluN2A, GluN2B, and GluN2C subunits (Banke and Traynelis, 2003, Erreger et al., 2005a, Erreger et al., 2005b, Erreger et al., 2007), and which has kinetic states that appear to correspond to the main conformational states of the receptor-ion channel protein (Banke and Traynelis, 2003).
Statistical analysis.
Effects of ethanol on single-channel kinetic measures and rate constants obtained from model fitting were compared in the same patches using paired t-tests or repeated-measures ANOVA.
RESULTS
Effect of Ethanol on NMDA Receptor Single-Channel Conductance.
In outside-out patches from cells expressing GluN1/GluN2A or GluN1/GluN2B NMDA receptor subunits, low concentrations of glutamate (0.1 – 1 μM) in the presence of a saturating concentration of glycine (50 μM) elicited a low level of NMDA receptor activity, with groups of openings widely separated by long closings (Fig. 1.). This pattern of activity was observed in both the absence and the presence of ethanol (156 mM, GluN2A; 150 mM, GluN2B). Ethanol did not alter the single-channel chord conductance determined from all-points histograms (Fig. 2; GluN2A: 54.6 ± 1.63 vs. 56.7 ± 2.59 pS in the absence and presence of ethanol, respectively; paired t-test, P > 0.05, N = 7 patches; GluN2B: 54.2 ± 1.23 vs. 52.5 ± 1.75 pS in the absence and presence of ethanol, respectively; paired t-test, P > 0.05, N = 7 patches), and did not produce any evident alteration in the appearance of the single-channel records, such as flickering block (Fig 3A,C; Fig 4 A, C).
Figure 1.
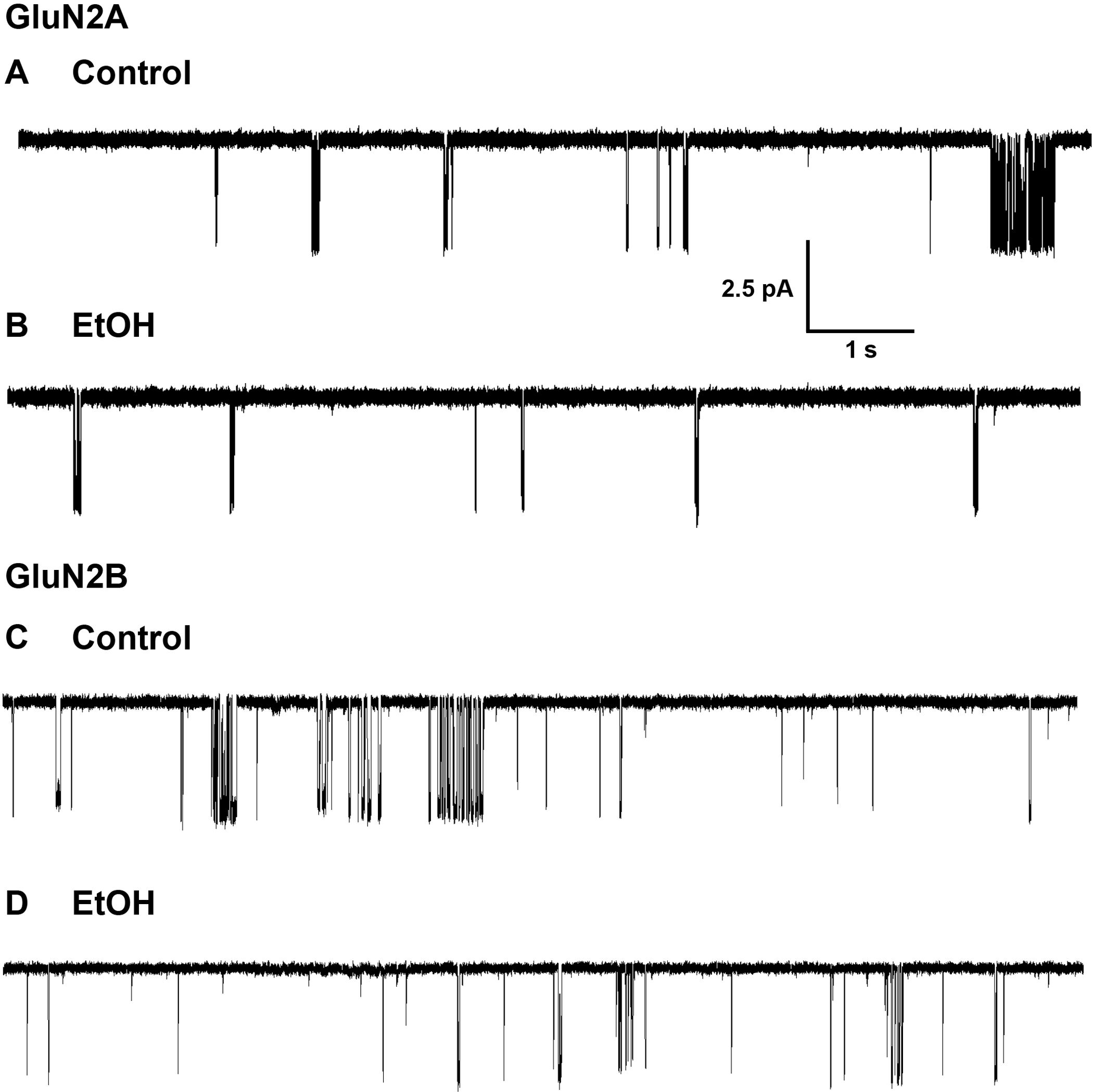
Single-channel currents activated by 0.1 μM glutamate and 50 μM glycine in outside-out patches from cells expressing GluN1/GluN2A (A,B) or GluN1/GluN2B (C,D) NMDA receptor subunits in the absence (A,C) and the presence (B,D) of ethanol (EtOH), 156 mM (B) or 150 mM (D). Channel openings are downward. Scale bars in A apply to all records.
Figure 2.
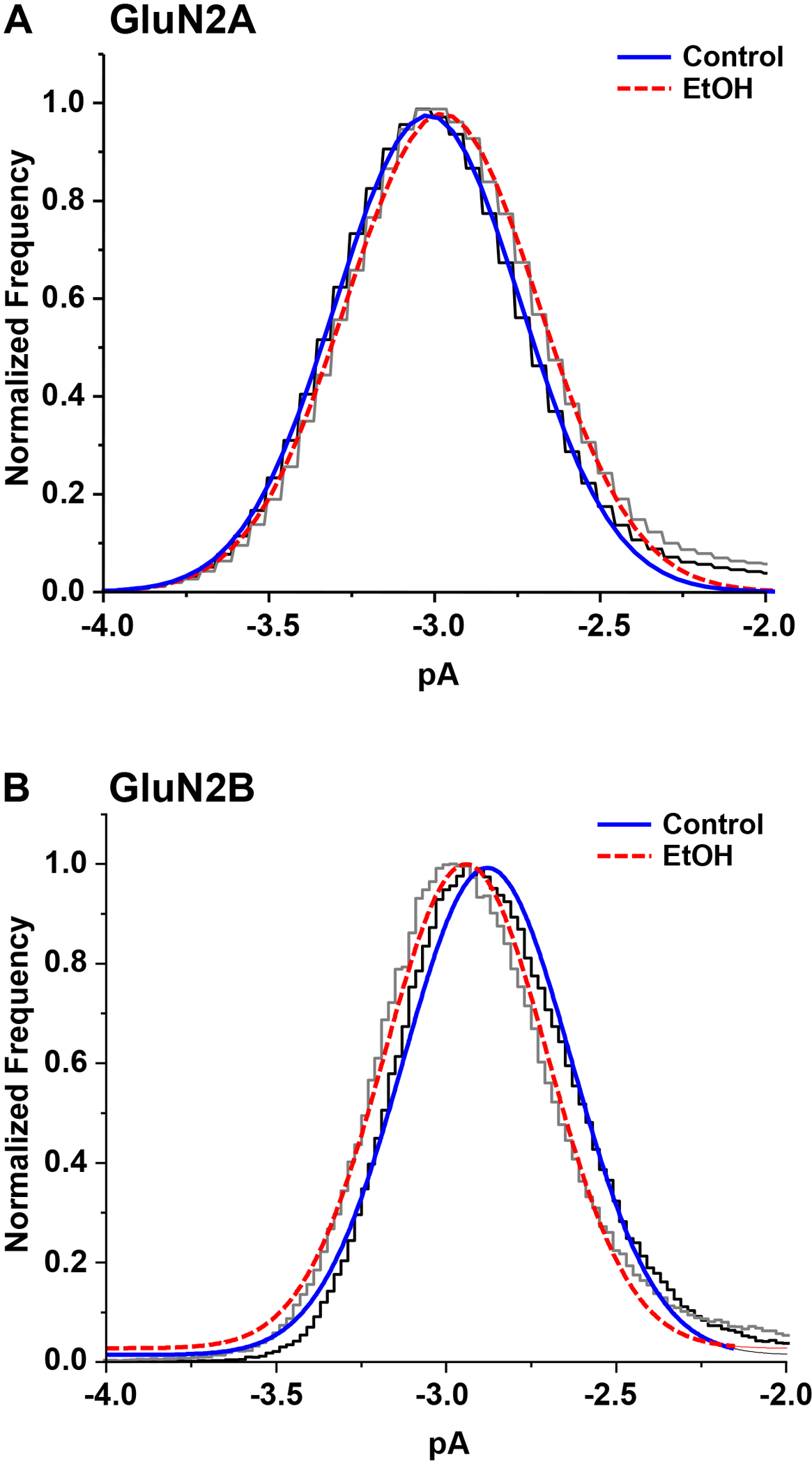
Open-point histograms of glutamate-activated single-channel current amplitudes in outside-out patches from cells expressing GluN1/GluN2A (A) or GluN1/GluN2B (B) subunits in the absence (black) and the presence (gray) of ethanol (EtOH), 156 mM (A) or 150 mM (B). Curves shown are least-squares fits of a Gaussian function to the data in the absence (solid blue) and presence (dashed red) of ethanol.
Figure 3.
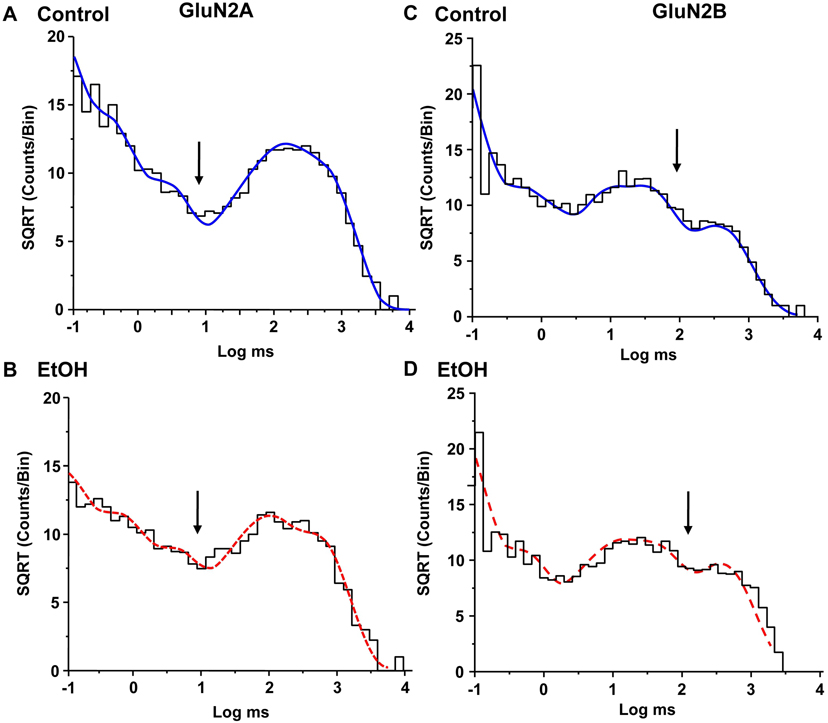
(A,C) Single-channel currents activated by 0.1 μM glutamate and 50 μM glycine in an outside-out patch from a cell expressing GluN1/GluN2A subunits in the absence (A) and the presence (C) of 156 mM ethanol (EtOH). Patches were alternately exposed to control and ethanol solutions at two minute intervals. Channel openings are downward. (B, D) Open time histograms of data from a typical outside-out patch in the absence (B) and presence (D) of ethanol, 156 mM. Curves shown are maximum likelihood multiple exponential fits to the data. Histograms were well fitted by two components. Arrows denote the peaks of the longer components; the dotted line in D indicates the position of the peak in the absence of ethanol.
Figure 4.
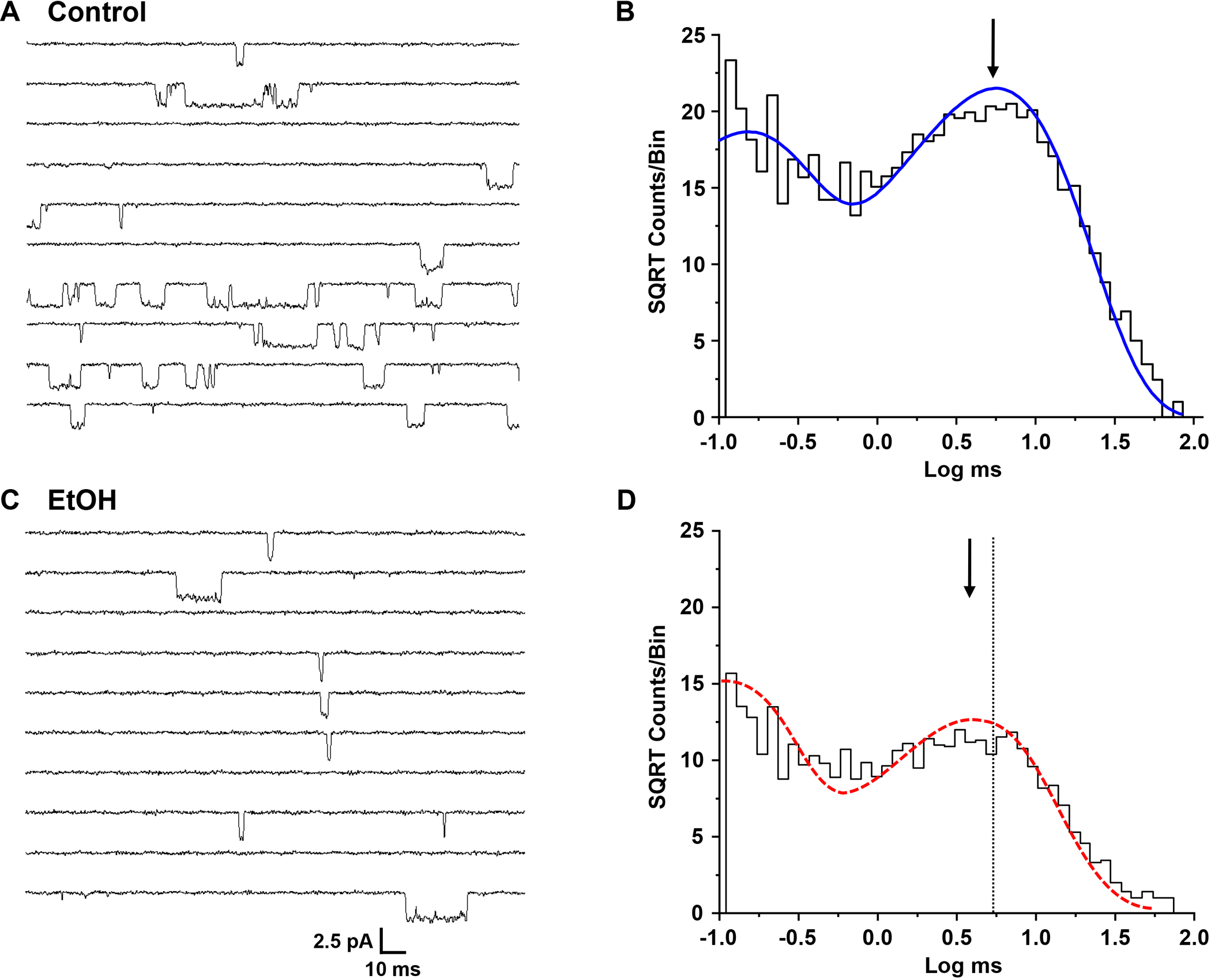
(A,C) Single-channel currents activated by 0.1 μM glutamate and 50 μM glycine in an outside-out patch from a cell expressing GluN1/GluN2B subunits in the absence (A) and the presence (C) of 150 mM ethanol (EtOH). Patches were alternately exposed to control and ethanol solutions at one minute intervals. Channel openings are downward. (B, D) Open time histograms of data from a typical outside-out patch in the absence (B) and presence (D) of ethanol, 150 mM. Curves shown are maximum likelihood multiple exponential fits to the data. Histograms were well fitted by two components. Arrows denote the peaks of the longer components; the dotted line in D indicates the position of the peak in the absence of ethanol.
Effects of Ethanol on Open Times.
Open time distributions for glutamate-activated current in GluN1/GluN2A or GluN1/GluN2B NMDA receptors could be adequately fitted by two components (GluN2A: 62.6 ± 6.24 μs and 4.01 ± 0.441 ms; GluN2B: 130 ± 16.9 μs and 5.39 ± 0.234 ms) with similar proportions of openings (GluN2A: 48.3 ± 2.38 % and 51.8 ± 2.39 %, and GluN2B: 49.7 ± 3.81 % and 50.3 ± 3.81 % for the fast and slow components, respectively, Fig. 3B, 4B). The mean open times were 3.21 ± 0.341 ms (GluN2A) and 3.51 ± 0.216 ms (GluN2B). In the presence of ethanol mean open time was decreased in GluN2A by 33% to 2.17 ± 0.243 ms (Fig 3D; P < 0.001, paired t-test, N = 7 patches), and in GluN2B by 42% to 2.05 ± 0.376 ms (Fig 4D; P < 0.01, paired t-test, N = 7 patches). Open time distributions in the presence of ethanol were also well fitted by two components; the time constant of the fast component was not significantly changed compared to the control condition (GluN2A: 50.3 ± 3.97 μs; paired t-test, P > 0.05, N = 7 patches; GluN2B: 138 ± 42.1 μs; paired t-test, P > 0.05, N = 7 patches), but that of the slow component was significantly decreased (GluN2A: 3.07 ± 0.345 ms; paired t-test, P < 0.001, N = 7 patches; GluN2B: 3.59 ± 0.269 ms; paired t-test, P < 0.01, N = 7 patches). In the GluN2A subunit, but not the GluN2B subunit, the areas of both components were significantly changed, such that there were relatively more fast openings in the presence of ethanol (62.0 ± 4.11 % and 38.0 ± 4.12 % for the fast and slow components, respectively; paired t-test, P < 0.05, N = 7 patches). In contrast, overall frequency of opening was not changed by ethanol in either subunit type (GluN2A: 7.91 ± 1.96 vs. 7.34 ± 1.30 for control and ethanol-treated patches, respectively; paired t-test, P > 0.05, N = 7 patches; GluN2B: 15.8 ± 6.52 vs. 13.6 ± 5.99 for control and ethanol-treated patches, respectively; paired t-test, P > 0.05, N = 7 patches).
Effects of Ethanol on Shut Times.
For both subunit types, shut time distributions for patches in the absence of ethanol could be fitted with five exponential components ranging from approximately 50 μs to 400 ms (Table 1 and Fig 4). Similar distributions were also obtained in the same patches exposed to ethanol. In contrast to its effects on open time, ethanol did not alter any of the shut time constants (repeated measures ANOVA, P > 0.05, N = 7 patches) or their relative areas (repeated measures ANOVA, P > 0.05, N = 7 patches). As would be expected from the observation above that ethanol did not produce flickering block, ethanol did not introduce any additional shut times.
Table 1 -.
Effects of Ethanol on Shut Times
| τ1 (μs) | τ2 | τ3 | τ4 | τ5 | |
|---|---|---|---|---|---|
|
| |||||
| GluN2A | |||||
| Control | 47.6 ± 2.24 (38.2) | 0.267 ± 0.0125 (19.9) | 3.02 ± 0.382 (16.3) | 52.2 ± 12.2 (10.0) | 419 ± 37.3 (15.1) |
| EtOH | 51.3 ± 3.87 (40.0) | 0.319 ± 0.0284 (16.0) | 2.74 ± 0.272 (15.2) | 41.7 ± 11.3 (7.5) | 395 ± 46.1 (18.2) |
| GluN2B | |||||
| Control | 51.0 ± 11.8 (56.0) | 0.435 ± 0.0712 (14.5) | 7.08 ± 2.22 (10.0) | 24.1 ± 3.03 (13.6) | 262 ± 56.6 (6.4) |
| EtOH | 54.0 ± 15.4 (51.5) | 0.424 ± 0.0846 (16.7) | 10.4 ± 2.62 (10.9) | 53.7 ± 22.5 (11.9) | 524 ± 84.3 (9.3) |
GluN1/GluN2A and GluN1/GluN2B NMDA receptor shut times from multiple exponential fits to shut time histograms from outside-out patches under control conditions and in the presence of ethanol (EtOH), 156 mM (GluN2A) or 150 mM (GluN2B). Time constant (τ) values are in ms, except where indicated, and are expressed as the means ± S.E. obtained from N = 7 patches. Values in parentheses are the percentage of the total shut time for each component.
Fitting of Burst Data to a Kinetic Model.
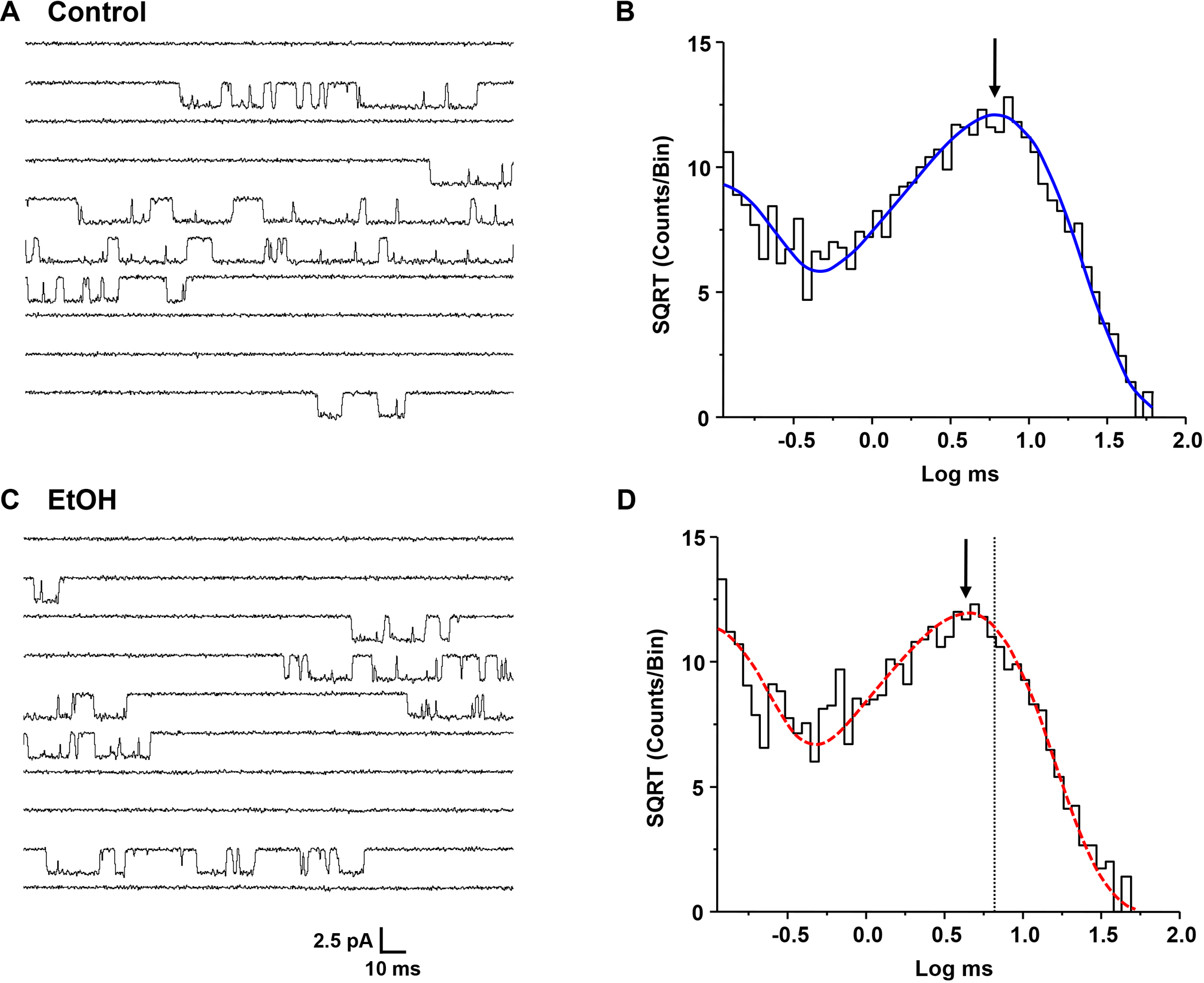
A distinct separation was evident in the shut time distributions between the third and fourth components in GluN2A subunit-containing receptors and between the fourth and fifth components in GluN2B subunit-containing receptors. In the GluN2A subunit, the shortest three shut times are considered to occur within an individual activation of the receptor-ion channel (Gibb and Colquhoun, 1991; Erreger et al, 2005a). Of the longest two shut times, one represents a long-lived desensitized state (within an activation), and one the agonist-unbound state (between activations). Although there is evidence from some studies that ethanol may influence NMDAR desensitization, the absence of a change in the closed time corresponding to the desensitized state in the presence of ethanol is consistent with a negligible effect under the conditions used in this study. In the GluN2B subunit, the longest two shut times are considered to differentiate individual activations of the receptor-ion channel (Erreger et al, 2005a). To determine the effects of ethanol on gating transitions in the fully-liganded, non-desensitized receptor, we grouped opening events into bursts that should correspond to individual activations of the receptor by choosing values of τcrit between the third and fourth (GluN2A) or fourth and fifth (GluN2B) shut time components (Fig 5, arrows). Values of τcrit determined for patches in the absence and presence of ethanol were 10.7 and 8.9 ms, respectively for GluN2A and 83.3 and 151 ms, respectively for GluN2B. We extracted bursts from the idealized data record such that closed times longer than the τcrit value were not included in the analysis. Maximal interval likelihood fitting of bursts to scheme 1 of Erreger et al. (2005b), a cyclic model that does not incorporate agonist binding and unbinding steps or desensitized states (Fig. 6), yielded values for the rate constants that in general were similar to those previously determined (Table 2) (Erreger et al., 2005b). In both GluN2A and GluN2B subunits, ethanol selectively altered two of the kinetic transitions: it decreased the rate constant for the slow forward rate, ks+, by 28.4 % (paired t-test, P < 0.0005) in GluN2A and by 30.2 % (paired t-test, P < 0.001) in GluN2B, and increased the reverse rate between open states, o−, by 49.8 % (t-test, P < 0.001) in GluN2A and 31 % (t-test, P < 0.05) in GluN2B. The remaining kinetic values were not appreciably altered by ethanol (mean change: 8.0 percent; t-tests, P > 0.05). To test whether the two changed rate constants could account for the observed decreases in mean open time and open probability due to ethanol, we substituted these rates in the model and generated simulated current traces using the QUB program, leaving all other rates unchanged from their control values. Changing the slow forward rate, ks+, and the open state reverse rate, o−, to their values in the presence of ethanol accounted for 91% and 90% of the inhibitory effect of ethanol on open probability in the GluN2A and GluN2B subunits, respectively.
Figure 5.
Shut time histograms of data from individual outside-out patches from cells expressing GluN1/GluN2A (A,B) or GluN1/GluN2B (C,D) subunits in the absence (A,C) and presence (B,D) of ethanol (EtOH). Curves shown are maximum likelihood multiple exponential fits; five components were required to adequately fit the data. Arrows indicate the critical time interval (τcrit) for each shut time histogram calculated to minimize the total number of misclassified events.
Figure 6.
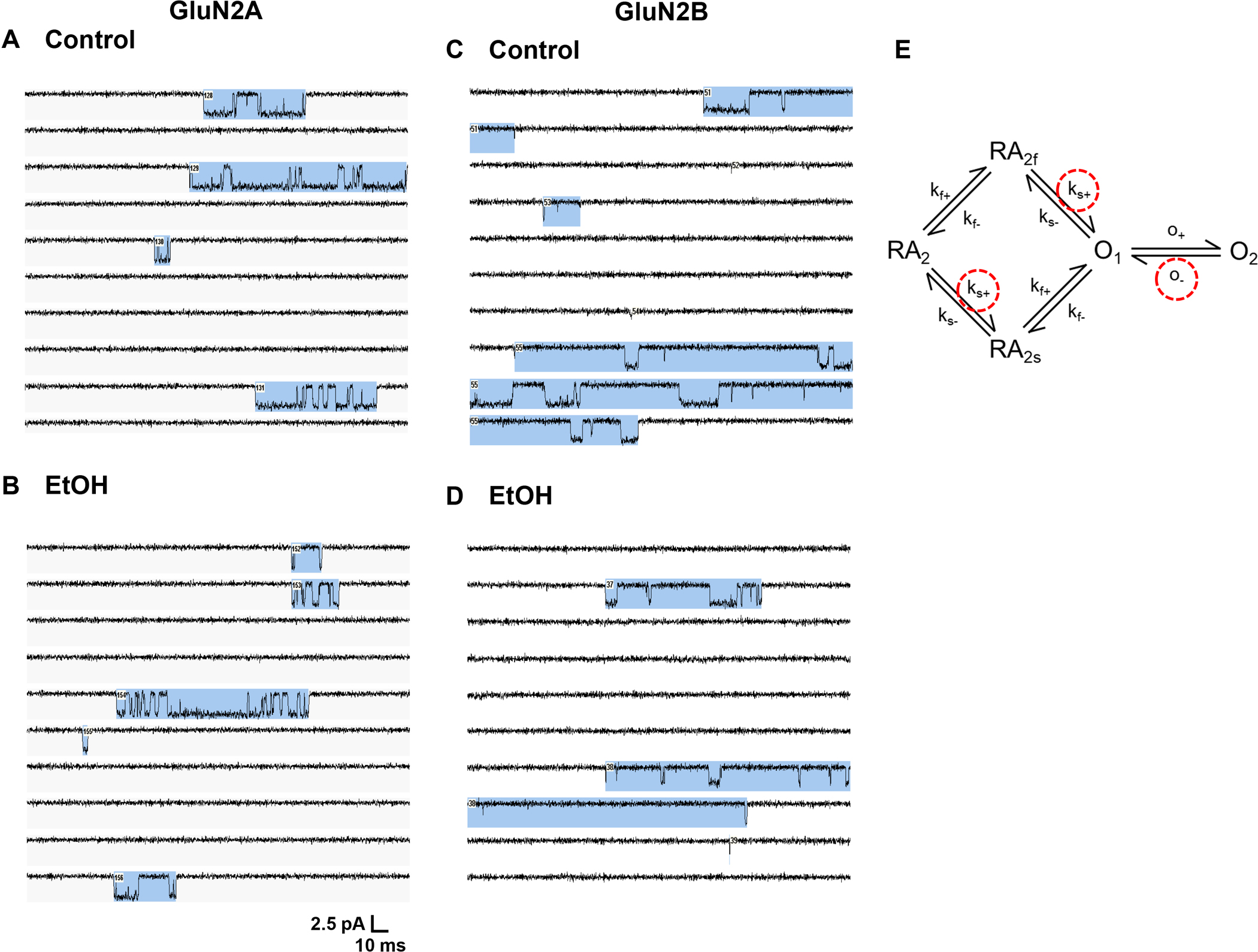
Fitting individual NMDA receptor activations (bursts) to a cyclic kinetic model. (A,B) Single-channel currents activated by 0.1 μM glutamate and 50 μM glycine in an outside-out patch from a cell expressing GluN1/GluN2A subunits in the absence (A) and the presence (B) of 156 mM ethanol (EtOH). (C,D) Single-channel currents activated by 0.1 μM glutamate and 50 μM glycine in an outside-out patch from a cell expressing GluN1/GluN2B subunits in the absence (C) and the presence (D) of 150 mM ethanol (EtOH). Channel openings are downward. Blue shading indicates groups of openings (“bursts”) defined by a critical time interval and considered to represent individual receptor activations. (E) A cyclic kinetic model of the gating of fully-bound GluN1/GluN2A NMDA receptors (Erreger et al., 2005b). Opening and closing events within bursts were fitted to the model using the maximum interval likelihood (MIL) function of the QUB program (Qin and Li, 2004) in order to obtain values for the rate constants for subunit activation and channel opening. Kinetic rates altered by ethanol are indicated by red dashed circles. Abbreviations: A, agonist, R, receptor, O, open, f, fast, s, slow, +, forward, −, reverse.
Table 2 –
Effects of Ethanol on Gating Rate Constants
| kf+ | kf− | ks+ | ks− | o+ | o− | |
|---|---|---|---|---|---|---|
|
| ||||||
| GluN2A | ||||||
| Control | 4110 ± 207 | 2860 ± 379 | 423 ± 21.3 | 956 ± 126 | 4880 ± 407 | 657 ± 67.8 |
| EtOH | 3870 ± 272 | 2600 ± 275 | 303 ± 14.3*** | 829 ± 88.3 | 5063 ± 498 | 984 ± 104*** |
| GluN2B | ||||||
| Control | 3540 ± 436 | 2120 ± 310 | 56 ± 3.1 | 1040 ± 98.5 | 3020 ± 361 | 379 ± 14.2 |
| EtOH | 3200 ± 495 | 2830 ± 870 | 39 ± 3.01*** | 993 ± 172 | 2540 ± 512 | 497 ± 40.6* |
Rate constants obtained from fitting open and shut events within bursts to a kinetic model (Fig. 5C). Values (in s−1) are the means ± S.E. from N = 7 patches. Asterisks denote significant differences from control (paired t-tests; *** P < 0.001, * P < 0.05)
DISCUSSION
In the present study, we used recombinant rat GluN1/GluN2A and GluN1/GluN2B NMDAR to demonstrate that inhibition by ethanol at its approximate IC50 values (GluN2A: 156 mM; GluN2B: 150 mM in this study) involved a decrease in mean open time without appreciable changes in frequency of opening, single-channel conductance, or shut time distribution, and that the effect of ethanol was attributable to selective modulation of two specific ion channel gating rate constants in the kinetic model used.
Following the initial demonstrations that ethanol inhibits NMDA receptor activity (Hoffman et al., 1989, Lima-Landman and Albuquerque, 1989, Lovinger et al., 1989), a few studies used single-channel recording to examine the alterations in receptor kinetics responsible for the inhibitory effect. In the earliest such study, Lima-Landman and Albuquerque (1989) reported that ethanol (86.5 and 174 mM) decreased NMDAR mean open time in outside-out patches from rat hippocampal pyramidal neurons without altering single-channel conductance. These investigators observed a decrease in open probability of approximately 50%, but did not report values for opening frequency. Wright et al. (1996) subsequently showed that in outside-out patches from mouse cerebral cortical neurons, ethanol (200 mM) decreased both mean open time and frequency of opening without altering single-channel conductance or shut times, and without introducing fast flickering behavior. The reduction in mean open time was greater than that in frequency of opening, but both were required to account for the observed reduction in whole-cell current by ethanol. In the present study, at a concentration similar to those used previously, but in recombinant rat GluN1/GluN2A or GluN1/GluN2B NMDA receptors expressed in a cultured cell line rather than in native CNS neurons, we observed similar effects of ethanol: the main effect was to decrease mean open time of the ion channel without altering single channel conductance. There was also no difference in closed time distributions, including no introduction of very short closed times, or fast flickering behavior. Because either an increase in very short closed times or reduced single-channel conductance could indicate an open-channel block mechanism (Hille, 2001), the absence of either finding has been interpreted as evidence that ethanol does not act by occluding the ion channel (Wright et al., 1996). The observation that ethanol primarily shortens mean open time is consistent with a mechanism of inhibition involving an increase in the rate of ion channel closing.
Previous studies have reported that magnesium ions, which normally block NMDA receptors at strongly negative potentials under physiological conditions, can enhance ethanol inhibition of NMDA receptors (Chandler et al., 1994, Chu et al., 1995, Martin et al., 1991, Michaelis and Michaelis, 1994, Morrisett et al., 1991), although this has not been observed in all studies (Bhave et al., 1996, Chu et al., 1995, Peoples et al., 1997). In the present study, experiments were performed in the absence of extracellular magnesium to avoid block at negative membrane potentials. It is thus possible that this resulted in a lesser degree of ethanol inhibition than would be observed at physiological concentrations of magnesium. In addition, because magnesium alters gating kinetics (Kampa et al., 2004), it is also possible that magnesium, if it had been present, could have altered the effects of ethanol on the kinetic model. Many early studies also demonstrated glycine modulation of ethanol inhibition of NMDA receptors and proposed glycine antagonism as a mechanism of inhibition (Buller et al., 1995, Dildy-Mayfield and Leslie, 1991, Rabe and Tabakoff, 1990, Woodward and Gonzales, 1990); however, the binding of glycine or glutamate is not altered by ethanol (Snell et al., 1993), nor is ethanol inhibition competitive with respect to glycine or glutamate (Cebers et al., 1996, Gonzales and Woodward, 1990, Peoples and Weight, 1992, Peoples et al., 1997, Woodward, 1994). In the present study we therefore excluded transitions involved in agonist or coagonist binding by fitting events occurring during bursts, which represent single activations of the ion channel (Gibb and Colquhoun, 1991, Gibb and Colquhoun, 1992), and are separated by one or two long shut times attributable to agonist dissociation and (in the GluN2A subunit) desensitization. Thus we attempted to model the effect of ethanol on gating of the fully-liganded, non-desensitized receptor. Fitting of events during bursts in the absence and presence of ethanol to a simple cyclic model using the maximum interval likelihood (MIL) function of the QUB program revealed that two rate constants in the gating section of the model were appreciably affected by ethanol in both GluN1/GluN2A and GluN1/GluN2B receptors: the slow forward rate, ks+, in the cyclic portion of the model was decreased, and the main open state reverse rate, o−, was increased. The slow forward rate, along with the slow reverse rate, ks−, has been attributed to conformational changes in the GluN2 subunit (Banke and Traynelis, 2003). Thus this result is consistent with ethanol acting via the GluN2 subunit, and the lack of effect of ethanol on the fast forward rate indicates that the corresponding transition in the GluN1 subunit is insensitive to ethanol, which could be due to structural differences between the subunit types underlying the much faster transitions in the GluN1 subunit. The main open state reverse rate affects the longer of the two open times, such that increasing this rate decreases the duration of the longer component. The longer open state accounts for the majority of charge transfer mediated by the NMDAR, so preferentially shortening it has a relative effect greater than would be caused by a similar change to the shorter component. Our simulations indicated that the two states that were measurably altered by ethanol accounted for the great majority of its inhibitory effect.
Although ethanol can modulate the activity of NMDA receptors via multiple actions, such as by altering phosphorylation and interacting proteins (Ron, 2004), in this study we have chosen to focus on its direct action on the NMDA receptor-channel, which can be observed under conditions in which intracellular mechanisms of modulation are minimized or eliminated (Lovinger et al., 1989, Peoples et al., 1997), and which occurs at intoxicating concentrations (Hoffman et al., 1989, Lima-Landman and Albuquerque, 1989, Lovinger et al., 1989). A consideration in the present study is that interacting mechanisms present in CNS neurons may be absent in the expression system used. It should also be noted that the concentrations used in the present study are above the normal range for intoxication in humans (~20 – 50 mM), although even greater concentrations than those used here may be reached in highly-tolerant individuals (Abrahao et al., 2017). Concentrations at approximate IC50 values were used in the present study to ensure that changes in individual microscopic kinetic rates, which act in concert to determine NMDA receptor gating, would be detectable. Results of many studies from this and other laboratories showing sigmoidal ethanol concentration-response curves with slopes of approximately unity are consistent with the view that lower concentrations of ethanol would have qualitatively similar effects (Honse et al., 2004; Jin et al., 2006; Peoples et al., 1997; Ren et al., 2012; Zhao et al., 2015; Smothers and Woodward, 2006). Available evidence supports the view that the NMDA receptor is responsible for some of the effects of intoxicating ethanol concentrations in humans (Holmes et al., 2013, Jury et al., 2018, Krishnan-Sarin et al., 2015, Krystal et al., 2003a, Radke et al., 2017). We and others have identified clusters of adjacent residues in the third and fourth membrane-associated domains of the GluN1 (Jin and Woodward, 2006, Ren et al., 2012, Ronald et al., 2001, Smothers and Woodward, 2006) and GluN2 (Honse et al., 2004, Ren et al., 2003, Ren et al., 2008, Ren et al., 2007, Ren et al., 2012, Wu et al., 2019, Zhao et al., 2015) subunits of the NMDA receptor that strongly regulate ethanol sensitivity, and which appear to form the sites by which ethanol inhibits the receptor under the conditions used in this study. While significant advances have been made in understanding the conformational changes that underlie ion channel gating (Glasgow et al., 2015, Hansen et al., 2021, Karakas and Furukawa, 2014, Kazi et al., 2013, Lee et al., 2014), and available evidence is consistent with movements of the alcohol-sensitive side chains relative to each other during gating transitions, further studies will be required to determine how the ethanol molecule interacts with these domains to alter the kinetics of the conformational changes that are responsible for the rate constants that are regulated by ethanol.
Acknowledgments
This study was supported by grants R01 AA015203–01A1, R01 AA015203–06A1, and R03 AA028903 from the NIAAA, National Institutes of Health, and by a Way Klingler Fellowship Award from Marquette University to RWP. The authors have no conflicts of interest to disclose.
REFERENCES
- Abrahao KP, Salinas AG, Lovinger DM (2017) Alcohol and the Brain: Neuronal Molecular Targets, Synapses, and Circuits. Neuron 96:1223–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Hallaq RA, Conrads TP, Veenstra TD, Wenthold RJ (2007) NMDA Di-Heteromeric Receptor Populations and Associated Proteins in Rat Hippocampus. Journal of Neuroscience 27:8334–8343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banke TG, Traynelis SF (2003) Activation of NR1/NR2B NMDA receptors. Nat Neurosci 6:144–152. [DOI] [PubMed] [Google Scholar]
- Bhave SV, Snell LD, Tabakoff B, Hoffman PL (1996) Mechanism of ethanol inhibition of NMDA receptor function in primary cultures of cerebral cortical cells. Alcohol Clin Exp Res 20:934–941. [DOI] [PubMed] [Google Scholar]
- Buller AL, Larson HC, Morrisett RA, Monaghan DT (1995) Glycine modulates ethanol inhibition of heteromeric N-methyl-D-aspartate receptors expressed in Xenopus oocytes. Mol Pharmacol 48:717–723. [PubMed] [Google Scholar]
- Cebers G, Cebere A, Zharkovsky A, Liljequist S (1996) Glycine does not reverse the inhibitory actions of ethanol on NMDA receptor functions in cerebellar granule cells. Naunyn-Schmiedeberg’s Arch Pharmacol 354:736–745. [DOI] [PubMed] [Google Scholar]
- Chandler LJ, Guzman NJ, Sumners C, Crews FT (1994) Magnesium and zinc potentiate ethanol inhibition of N- methyl-D-aspartate-stimulated nitric oxide synthase in cortical neurons. J Pharmacol Exp Ther 271:67–75. [PubMed] [Google Scholar]
- Chandrasekar R (2013) Alcohol and NMDA receptor: current research and future direction. Front Mol Neurosci 6:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu B, Anantharam V, Treistman SN (1995) Ethanol inhibition of recombinant heteromeric NMDA channels in the presence and absence of modulators. J Neurochem 65:140–148. [DOI] [PubMed] [Google Scholar]
- Dildy-Mayfield JE, Leslie SW (1991) Mechanism of inhibition of N-methyl-D-aspartate-stimulated increases in free intracellular Ca2+ concentration by ethanol. J Neurochem 56:1536–1543. [DOI] [PubMed] [Google Scholar]
- Erreger K, Dravid SM, Banke TG, Wyllie DJA, Traynelis SF (2005a) Subunit-specific gating controls rat NR1/NR2A and NR1/NR2B NMDA channel kinetics and synaptic signalling profiles. J Physiol 563:345–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erreger K, Geballe MT, Dravid SM, Snyder JP, Wyllie DJA, Traynelis SF (2005b) Mechanism of partial agonism at NMDA receptors for a conformationally restricted glutamate analog. J Neurosci 25:7858–7866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erreger K, Geballe MT, Kristensen A, Chen PE, Hansen KB, Lee CJ, Yuan H, Le P, Lyuboslavsky PN, Micale N, Jorgensen L, Clausen RP, Wyllie DJA, Snyder JP, Traynelis SF (2007) Subunit-Specific Agonist Activity at NR2A-, NR2B-, NR2C-, and NR2D-Containing N-Methyl-D-aspartate Glutamate Receptors. Molecular Pharmacology 72:907–920. [DOI] [PubMed] [Google Scholar]
- Gibb AJ, Colquhoun D (1991) Glutamate activation of a single NMDA receptor-channel produces a cluster of channel openings. Proc R Soc Lond [Biol ] 243:39–45. [DOI] [PubMed] [Google Scholar]
- Gibb AJ, Colquhoun D (1992) Activation of N-methyl-D-aspartate receptors by L-glutamate in cells dissociated from adult rat hippocampus. J Physiol 456:143–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasgow NG, Siegler Retchless B, Johnson JW (2015) Molecular bases of NMDA receptor subtype-dependent properties. The Journal of Physiology 593:83–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzales RA, Woodward JJ (1990) Ethanol inhibits N-methyl-D-aspartate-stimulated [3H]norepinephrine release from rat cortical slices. J Pharmacol Exp Ther 253:1138–1144. [PubMed] [Google Scholar]
- Hansen KB, Wollmuth LP, Bowie D, Furukawa H, Menniti FS, Sobolevsky AI, Swanson GT, Swanger SA, Greger IH, Nakagawa T, McBain CJ, Jayaraman V, Low CM, Dell’Acqua ML, Diamond JS, Camp CR, Perszyk RE, Yuan H, Traynelis SF (2021) Structure, Function, and Pharmacology of Glutamate Receptor Ion Channels. Pharmacol Rev 73:298–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille B (2001) Ionic channels of excitable membranes. 3rd ed. Sinauer Associates, Inc., Sunderland, MA. [Google Scholar]
- Hoffman PL, Rabe CS, Moses F, Tabakoff B (1989) N-methyl-D-aspartate receptors and ethanol: inhibition of calcium flux and cyclic GMP production. J Neurochem 52:1937–1940. [DOI] [PubMed] [Google Scholar]
- Holmes A, Spanagel R, Krystal JH (2013) Glutamatergic targets for new alcohol medications. Psychopharmacology (Berl) 229:539–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honse Y, Ren H, Lipsky RH, Peoples RW (2004) Sites in the fourth membrane-associated domain regulate alcohol sensitivity of the NMDA receptor. Neuropharmacol 46:647–654. [DOI] [PubMed] [Google Scholar]
- Jin C, Woodward JJ (2006) Effects of 8 different NR1 splice variants on the ethanol inhibition of recombinant NMDA receptors. Alcohol Clin Exp Res 30:673–679. [DOI] [PubMed] [Google Scholar]
- Jury NJ, Radke AK, Pati D, Kocharian A, Mishina M, Kash TL, Holmes A (2018) NMDA receptor GluN2A subunit deletion protects against dependence-like ethanol drinking. Behav Brain Res 353:124–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karakas E, Furukawa H (2014) Crystal structure of a heterotetrameric NMDA receptor ion channel. Science 344:992–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazi R, Gan Q, Talukder I, Markowitz M, Salussolia CL, Wollmuth LP (2013) Asynchronous Movements Prior to Pore Opening in NMDA Receptors. The Journal of Neuroscience 33:12052–12066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan-Sarin S, O’Malley SS, Franco N, Cavallo DA, Morean M, Shi J, Pittman B, Krystal JH (2015) N-Methyl-d-Aspartate Receptor Antagonism has Differential Effects on Alcohol Craving and Drinking in Heavy Drinkers. Alcohol Clin Exp Res 39:300–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krystal JH, Petrakis IL, Krupitsky E, Schutz C, Trevisan L, D’Souza DC (2003a) NMDA receptor antagonism and the ethanol intoxication signal: from alcoholism risk to pharmacotherapy. Ann N Y Acad Sci 1003:176–184. [DOI] [PubMed] [Google Scholar]
- Krystal JH, Petrakis IL, Mason G, Trevisan L, D’Souza DC (2003b) N-methyl-D-aspartate glutamate receptors and alcoholism: reward, dependence, treatment, and vulnerability. Pharmacology & Therapeutics 99:79–94. [DOI] [PubMed] [Google Scholar]
- Lee CH, Lu W, Michel JC, Goehring A, Du J, Song X, Gouaux E (2014) NMDA receptor structures reveal subunit arrangement and pore architecture. Nature 511:191–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima-Landman MTR, Albuquerque EX (1989) Ethanol potentiates and blocks NMDA-activated single-channel currents in rat hippocampal pyramidal cells. FEBS Lett 247:61–67. [DOI] [PubMed] [Google Scholar]
- Lovinger DM, White G, Weight FF (1989) Ethanol inhibits NMDA-activated ion current in hippocampal neurons. Science 243:1721–1724. [DOI] [PubMed] [Google Scholar]
- Martin D, Morrisett RA, Bian XP, Wilson WA, Swartzwelder HS (1991) Ethanol inhibition of NMDA mediated depolarizations is increased in the presence of Mg2+. Brain Res 546:227–234. [DOI] [PubMed] [Google Scholar]
- McCool BA (2011) Ethanol modulation of synaptic plasticity. Neuropharmacology 61:1097–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaelis ML, Michaelis EK (1994) Effects of ethanol on NMDA receptors in brain: Possibilities for Mg2+-ethanol interactions. Alcoholism 18:1069–1075. [DOI] [PubMed] [Google Scholar]
- Morrisett RA, Martin D, Oetting TA, Lewis DV, Wilson WA, Swartzwelder HS (1991) Ethanol and magnesium ions inhibit N-methyl-D-aspartate-mediated synaptic potentials in an interactive manner. Neuropharmacol 30:1173–1178. [DOI] [PubMed] [Google Scholar]
- Peoples RW, Weight FF (1992) Ethanol inhibition of N-methyl-D-aspartate-activated ion current in rat hippocampal neurons is not competitive with glycine. Brain Res 571:342–344. [DOI] [PubMed] [Google Scholar]
- Peoples RW, White G, Lovinger DM, Weight FF (1997) Ethanol inhibition of N-methyl-D-aspartate-activated current in mouse hippocampal neurones: whole-cell patch-clamp analysis. Br J Pharmacol 122:1035–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin F (2004) Restoration of single-channel currents using the segmental k-means method based on hidden Markov modeling. Biophys J 86:1488–1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin F, Li L (2004) Model-based fitting of single-channel dwell-time distributions. Biophys J 87:1657–1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabe CS, Tabakoff B (1990) Glycine site-directed agonists reverse the actions of ethanol at the N-methyl-D-aspartate receptor. Mol Pharmacol 38:753–757. [PubMed] [Google Scholar]
- Radke AK, Jury NJ, Delpire E, Nakazawa K, Holmes A (2017) Reduced ethanol drinking following selective cortical interneuron deletion of the GluN2B NMDA receptors subunit. Alcohol 58:47–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauner C, Kohr G (2011) Triheteromeric NR1/NR2A/NR2B receptors constitute the major N-methyl-D-aspartate receptor population in adult hippocampal synapses. J Biol Chem 286:7558–7566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren H, Honse Y, Peoples RW (2003) A site of alcohol action in the fourth membrane-associated domain of the NMDA receptor. J Biol Chem 278:48815–48820. [DOI] [PubMed] [Google Scholar]
- Ren H, Salous AK, Paul JM, Lamb KA, Dwyer DS, Peoples RW (2008) Functional interactions of alcohol-sensitive sites in the N-methyl-D-aspartate receptor M3 and M4 domains. J Biol Chem 283:8250–8257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren H, Salous AK, Paul JM, Lipsky RH, Peoples RW (2007) Mutations at F637 in the NMDA receptor NR2A subunit M3 domain influence agonist potency, ion channel gating and alcohol action. Br J Pharmacol 151:749–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren H, Zhao Y, Dwyer DS, Peoples RW (2012) Interactions among positions in the third and fourth membrane-associated domains at the intersubunit interface of the N-methyl-D-aspartate receptor forming sites of alcohol action. J Biol Chem 287:27302–27312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren H, Zhao Y, Wu M, Dwyer DS, Peoples RW (2017) Two adjacent phenylalanines in the NMDA receptor GluN2A subunit M3 domain interactively regulate alcohol sensitivity and ion channel gating. Neuropharmacology 114:20–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ron D (2004) Signaling cascades regulating NMDA receptor sensitivity to ethanol. Neuroscientist 10:325–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ron D, Wang J (2009) The NMDA Receptor and Alcohol Addiction, in Frontiers in Neuroscience, Biology of the NMDA Receptor; (Van Dongen AM ed., Boca Raton (FL). [PubMed] [Google Scholar]
- Ronald KM, Mirshahi T, Woodward JJ (2001) Ethanol inhibition of N-methyl-D-aspartate receptors is reduced by site- directed mutagenesis of a transmembrane domain phenylalanine residue. J Biol Chem 276:44729–44735. [DOI] [PubMed] [Google Scholar]
- Smothers CT, Clayton R, Blevins T, Woodward JJ (2001) Ethanol sensitivity of recombinant human N-methyl-D-aspartate receptors. Neurochem Int 38:333–340. [DOI] [PubMed] [Google Scholar]
- Smothers CT, Woodward JJ (2006) Effects of amino acid substitutions in transmembrane domains of the NR1 subunit on the ethanol inhibition of recombinant N-methyl-D-aspartate receptors. Alcohol Clin Exp Res 30:523–530. [DOI] [PubMed] [Google Scholar]
- Snell LD, Tabakoff B, Hoffman PL (1993) Radioligand binding to the N-methyl-D-aspartate receptor/ionophore complex: Alterations by ethanol in vitro and by chronic in vivo ethanol ingestion. Brain Res 602:91–98. [DOI] [PubMed] [Google Scholar]
- Vengeliene V, Bilbao A, Molander A, Spanagel R (2008) Neuropharmacology of alcohol addiction. Br J Pharmacol 154:299–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward JJ (1994) A comparison of the effects of ethanol and the competitive glycine antagonist 7-chlorokynurenic acid on N-methyl-D-aspartic acid-induced neurotransmitter release from rat hippocampal slices. J Neurochem 62:987–991. [DOI] [PubMed] [Google Scholar]
- Woodward JJ, Gonzales RA (1990) Ethanol inhibition of N-methyl-D-aspartate-stimulated endogenous dopamine release from rat striatal slices: reversal by glycine. J Neurochem 54:712–715. [DOI] [PubMed] [Google Scholar]
- Wright JM, Peoples RW, Weight FF (1996) Single-channel and whole-cell analysis of ethanol inhibition of NMDA-activated currents in cultured mouse cortical and hippocampal neurons. Brain Res 738:249–256. [DOI] [PubMed] [Google Scholar]
- Wu M, Katti P, Zhao Y, Peoples RW (2019) Positions in the N-methyl-D-aspartate Receptor GluN2C Subunit M3 and M4 Domains Regulate Alcohol Sensitivity and Receptor Kinetics. Alcohol Clin Exp Res. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyllie DJA, Behe P, Colquhoun D (1998) Single-channel activations and concentration jumps: comparison of recombinant NR1a/NR2A and NR1a/NR2D NMDA receptors. J Physiol 510:1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Ren H, Dwyer DS, Peoples RW (2015) Different sites of alcohol action in the NMDA receptor GluN2A and GluN2B subunits. Neuropharmacology 97:240–250. [DOI] [PMC free article] [PubMed] [Google Scholar]