Abstract
The standardization of variant curation criteria is essential for accurate interpretation of genetic results and clinical care of patients. The variant curation guidelines developed by the American College of Medical Genetics and Genomics (ACMG) and the Association for Molecular Pathology (AMP) in 2015 are widely used but are not gene specific. To address this issue, the Clinical Genome Resource (ClinGen) Variant Curation Expert Panels (VCEP) have been tasked with developing gene-specific variant curation guidelines. The Glaucoma VCEP was created to develop rule specifications for genes associated with primary glaucoma, including myocilin (MYOC), the most common cause of Mendelian glaucoma. Of the 28 ACMG/AMP criteria, the Glaucoma VCEP adapted 15 rules to MYOC and determined 13 rules not applicable. Key specifications included determining minor allele frequency thresholds, developing an approach to counting probands and segregations, and reviewing functional assays. The rules were piloted on 81 variants and led to a change in classification in 40% of those that were classified in ClinVar, with functional evidence influencing the classification of 18 variants. The standardized variant curation guidelines for MYOC provide a framework for the consistent application of the rules between laboratories, to improve MYOC genetic testing in the management of glaucoma.
Keywords: Variant classification, primary open-angle glaucoma, juvenile open-angle glaucoma, variant interpretation, genetic testing, variant curation expert panel, MYOC
INTRODUCTION
Accurately interpreting sequence variants is a key component in genetic diagnosis. In 2015, the American College of Medical Genetics and Genomics (ACMG) and the Association for Molecular Pathology (AMP) published guidelines to standardize the interpretation and classification of sequence variants in genes associated with Mendelian diseases (Richards et al., 2015). The guidelines have since been widely adopted by the genetics community. The ACMG/AMP guidelines were intended to be broadly applicable and were designed to provide flexibility and adaptability. However, recent studies have reported discrepancies and inconsistency in variant interpretation and classification between laboratories using the guidelines (Amendola et al., 2016; Amendola et al., 2020; Harrison et al., 2017). This highlights the need to develop further expert-led guidance specific to the gene or disease.
The Clinical Genome Resource (ClinGen, www.clinicalgenome.org) aims to define the clinical relevance of genes and variants through collaborative international efforts to improve genetic diagnosis. Within ClinGen, the Variant Curation Expert Panels (VCEP) are tasked with developing gene-specific variant curation guidelines (Rivera-Munoz et al., 2018). The Glaucoma VCEP was formed to review the evidence and curate variants in genes associated with primary glaucoma.
Glaucoma refers to a heterogeneous group of disorders characterized by progressive optic neuropathy with associated visual field defects (Casson et al., 2012). Primary open-angle glaucoma (POAG; MIM# 137750) is the most common type of glaucoma and is characterized by an open anterior chamber angle and no developmental defects or other underlying disease (American Academy of Ophthalmology Preferred Practice Pattern Glaucoma Committee, 2020). POAG with an early age of onset is called juvenile open-angle glaucoma (JOAG) and is usually defined as an age at diagnosis <30 to 40 years (but after age 5) (Turalba & Chen, 2008).
Pathogenic variants in the myocilin gene (MYOC, formerly trabecular meshwork-induced glucocorticoid response gene or TIGR; MIM# 601652) are the most common cause of Mendelian JOAG and POAG (Stone et al., 1997). They account for 8–36% of JOAG (Shimizu et al., 2000; Vincent et al., 2002; Wiggs et al., 1998) and 2–4% of POAG (Fingert et al., 1999; Souzeau et al., 2013). In high-risk individuals with a strong family history of glaucoma, cascade genetic screening can allow targeted clinical screening and facilitate early diagnosis and treatment of glaucoma (Souzeau et al., 2017). The MYOC database compiles all published variants and currently contains 358 variants (www.myocilin.com, accessed 7 June 2022) (Hewitt et al., 2008). Glaucoma-causing MYOC variants are transmitted in an autosomal dominant manner, often with an age-related, incomplete penetrance. The most common pathogenic MYOC variant is p.Gln368Ter, accounting for 1.6–2.6% of individuals with POAG (Fingert et al., 1999; Souzeau et al., 2013). This variant is present in as many as 1/300 individuals in reference databases (highest prevalence reported in gnomad.broadinstitute.org, version 2.1.1 accessed 7 June 2022), and previous studies support a common European founder effect (Baird et al., 2003; Fingert et al., 1999).
MYOC contains 3 exons and encodes a 504 amino acid protein. Most reported disease-causing variants are located in the olfactomedin domain coded by exon 3 (amino acid residues 246-502) (Hewitt et al., 2008). MYOC whole gene deletions are not associated with disease in humans (Wiggs & Vollrath, 2001) and absence of disease with heterozygous or homozygous premature termination variants in the first two exons that would be expected to be subject to nonsense-mediated decay in humans (Lam et al., 2000) or mouse models (Kim et al., 2001) strongly argues against haploinsufficiency as a disease mechanism. The mechanism by which MYOC variants cause POAG is not fully understood but current evidence supports a toxic gain-of-function mechanism. Evidence suggests that MYOC variants lead to misfolded protein (Burns et al., 2011; Liu & Vollrath, 2004; Vollrath & Liu, 2006) and that the mutant protein is not secreted extracellularly (Caballero et al., 2000; Gobeil et al., 2004; Izumi et al., 2003; Jacobson et al., 2001; Nakahara & Hulleman, 2022; Vollrath & Liu, 2006; Zadoo et al., 2016). Moreover, mutant MYOC protein is not folded correctly in the endoplasmic reticulum, leading to accumulation of insoluble aggregates inside the trabecular meshwork cells, (Caballero & Borrás, 2001; Liu & Vollrath, 2004) inducing the unfolded protein response and resulting in endoplasmic reticulum stress-induced apoptosis (Yam et al., 2007).
The Glaucoma VCEP was created with the goal of generating specifications to the ACMG/AMP guidelines for MYOC. Herein, we describe the process of developing specified variant classification rules for MYOC, validating the rules on a set of variants, and curating MYOC variants with submission to ClinVar with expert panel review status. ClinVar is a freely accessible, public archive which reports the relationships among human variations and phenotypes, with supporting evidence (Landrum et al., 2018).
MATERIALS AND METHODS
VCEP membership and framework
The Glaucoma VCEP sits within the ClinGen Ocular Clinical Domain Working Group. Members were selected to provide broad expertise including clinical ophthalmology and research, with an emphasis on glaucoma as a sub-specialty, molecular biology, molecular diagnostics and genetic counselling. Representation from multiple countries was also sought, with panel members from Australia, the United States and Germany. Details of composition and membership can be found on the VCEP website (https://clinicalgenome.org/affiliation/50053/).
Project design
The Glaucoma VCEP met regularly through video and telephone conferences to review the ACMG/AMP guidelines (Richards et al., 2015) and discuss changes and specifications relevant to MYOC POAG/JOAG. The abbreviations for each rule and its original specification can be found in Table 1 (and Supp Table S1 for rules deemed not applicable). Individuals or small working groups were convened to gather relevant evidence and information for each rule, and this was presented to the VCEP for discussion. Consensus decisions were reached on the conference call and minutes circulated with the accompanying information to members unable to attend.
Table 1:
MYOC rule specifications.
| PATHOGENIC CRITERIA |
ACMG criteria | STRONG | MODERATE | SUPPORTING | Specification | |
|---|---|---|---|---|---|---|
| Population data | ||||||
| PM2 | Absent in population databases. | Allele frequency ≤ 0.0001 in population databases. | Highest allele frequency in population databases should be used. Only applies to populations with ≥ 10,000 alleles. | |||
| PS4 | The prevalence of the variant in affected individuals is significantly increased compared with the prevalence in controls. | ≥ 15 probands from multiple independent studies | ≥ 6 probands from multiple independent studies | ≥ 2 probands from multiple independent studies | Probands counted should be clinically assessed and have a diagnosis of JOAG or POAG. PM2_Supporting must be met. | |
| Computational and predictive data | ||||||
| PP3 | Multiple lines of computational evidence support a deleterious effect on the gene or gene product. | Applies to missense variants with a REVEL score of ≥ 0.7 | ||||
| PM5 | Novel missense change at an amino acid residue where a different missense change determined to be pathogenic has been seen before. | Same residue as a previously established P variant (assessed independently of PM5) or 2 previously established LP variants (both assessed independently of PM5) | Same residue as a previously established LP variant (assessed independently of PM5) | The novel change must not affect splicing (SpliceAI ≤ 0.2), must meet PP3 and have a Grantham score equal or greater than the previously established P/LP variants. | ||
| PS1 | Same amino acid change as a previously established pathogenic variant regardless of nucleotide change. | Same amino acid change as a previously established P variant (assessed independently of PS1) | Same amino acid change as a previously established LP variant (assessed independently of PS1) | The novel change must not affect splicing (SpliceAI ≤ 0.2). | ||
| PM4 | Protein length changes as a result of in-frame deletions/insertions in a nonrepeat region or stop-loss variants. | In-frame del/ins and truncating variants involving > 10% of the protein | In-frame del/ins and truncating variants involving ≤ 10% of the protein | Variant must be located within the conserved olfactomedin domain (AA 246-502). | ||
| Functional Data | ||||||
| PS3 | Well-established in vitro or in vivo functional studies supportive of a damaging effect on the gene or gene product. | Assays with OddsPath > 18.7 | Assays with OddsPath > 4.3 | Assays with OddsPath > 2.1 | Applies to the following functional studies: Solubility or secretion assays or animal models that replicate the glaucoma phenotype. | |
| Segregation Data | ||||||
| PP1 | Cosegregation with disease in multiple affected family members in a gene definitively known to cause the disease. | ≥7 meioses in >1 family | ≥ 5 meioses | ≥ 3 meioses | BA1 and BS1 must not be met. Only genotype-positive/phenotype-positive and obligate carriers/phenotype-positive individuals should be counted as segregations. Phenotype-positive need to be clinically assessed and either have a diagnosis of glaucoma or suspicious signs of glaucoma. | |
| De novo Data | ||||||
| PS2/PM6 | De novo in a patient with disease and no family history. | ≥ 2 confirmed de novo in JOAG | ≥ 2 confirmed de novo in POAG or 1 confirmed de novo in JOAG or ≥2 assumed de novo in JOAG | 1 confirmed de novo in POAG or ≥ 2 assumed de novo in POAG or 1 assumed de novo in JOAG | Use proposed SVI point recommendations for “phenotype consistent with the gene but not highly specific and with high genetic heterogeneity” for POAG and “phenotype consistent with gene but not highly specific” for JOAG. Both maternity and paternity need to be proven for confirmed de novo variants. Parents need to be clinically assessed and not have a diagnosis of glaucoma. | |
| BENIGN CRITERIA |
ACMG criteria | STAND ALONE | STRONG | MODERATE | SUPPORTING | Specification |
| Population data | ||||||
| BA1/BS1 | Allele frequency is greater than expected for disorder. |
BA1 Allele frequency ≥ 0.01 in population databases |
BS1 Allele frequency ≥ 0.001 in population databases. Does not apply to p.Gln368Ter. |
The highest allele frequency in population databases should be used. Variant must be present in ≥ 5 alleles in any validated general continental population dataset of at least 2,000 observed alleles. | ||
| Computational and predictive data | ||||||
| BP4 | Multiple lines of computational evidence suggest no impact on gene/gene product. | Applies to missense variants with a REVEL score ≤ 0.15 or synonymous or noncoding variants with CADD ≤ 10 AND SpliceAI ≤ 0.2 | ||||
| BP7 | A synonymous (silent) variant for which splicing prediction algorithms predict no impact to the splice consensus sequence nor the creation of a new splice site AND the nucleotide is not highly conserved. | Applies to synonymous variants or noncoding variants with no impact on splicing (SpliceAI ≤ 0.2) AND GERP < 0 for conservation |
||||
| Functional Data | ||||||
| BS3 | Well-established in vitro or in vivo functional studies show no damaging effect on protein function or splicing. | Assays with OddsPath < 0.23 | Assays with OddsPath < 0.48 | Applies to variants showing solubility and secretion in functional assays. | ||
JOAG: juvenile open-angle glaucoma, POAG: primary open-angle glaucoma, B: benign, LB: likely benign; LP: likely pathogenic, P: pathogenic, AA: amino acid, SVI: ClinGen Sequence Variant Interpretation Working Group
Draft rules were applied to a list of 81 pilot variants, which included the majority of variants with previous classifications from ClinVar, to assess how the refined criteria performed against previous classifications. The list covered variants from all 3 exons, different categories of variants (truncating variants [nonsense, frameshift], missense, synonymous, in-frame indels), different variant classifications (benign [B]/likely benign [LB], pathogenic [P]/likely pathogenic [LP], and variants of uncertain significance [VUS]) and all variants with conflicting evidence from ClinVar). Additional variants were included to ensure that all criteria were applied in the pilot. All variants with functional evidence were included to review the level of strength of these studies. Two biocurators applied the draft rules to each pilot variant (P.G. and J.H.), noting issues that arose while doing so. These were discussed by a core pilot working group consisting of a genetic counsellor (E.S.), an ophthalmologist (D.A.M.), a molecular geneticist (K.P.B.), a clinical scientist (A.D.), and the biocurators. This group made recommendations for further modifications and refinements to the rules for discussion by the full VCEP in an iterative process. The final set of rules was disseminated to all VCEP members for comment before submission to the ClinGen Sequence Variant Interpretation (SVI) Working Group, which provides harmonization across Clinical Domain Working Groups.
Variant curations were performed by the biocurators in the ClinGen Variant Curation Interface (https://curation.clinicalgenome.org/) and were reviewed by a core approval member (E.S.) for initial assessment, with additional review by two more core approval members (A.D., K.P.B., F.P., K.W.) for final approval. A summary of approved variant interpretations was then sent to all VCEP members for feedback. All variant classifications, criteria applied and supporting evidence were submitted to ClinVar with expert status (3-star level). Variants were annotated using GenBank reference sequence NM_000261.2 and NP_000252.1 using genome build GRCh37/hg19.
Data sources
Publicly available variant data were obtained between June and November 2021 from ClinVar (https://www.ncbi.nlm.nih.gov/clinvar) and the Myocilin database (www.myocilin.com) (Hewitt et al. 2008), which compiles all variants reported in the literature. Additional published and unpublished case-level data were used for variant curation from research databases of VCEP panel members including J.E.C. (The Australian and New Zealand Registry of Advanced Glaucoma [ANZRAG]) (Souzeau et al., 2013), D.A.M. (The Glaucoma Inheritance Study of Tasmania [GIST]) (Mackey et al., 2019), F.P., and T.Y. All variants are publicly listed in the Myocilin database. We defined JOAG as a diagnosis at ≤40 years old.
Rule specific approaches
Population data (BA1, BS1, PM2)
Inheritance pattern, disease prevalence, allelic contribution and penetrance information were gathered from the literature and used in the Whiffin/Ware calculator (https://cardiodb.org/allelefrequencyapp/) (Whiffin et al., 2017) to establish minor allele frequency thresholds for BA1, BS1 and PM2 specific to MYOC.
Proband counts (PS4)
To set thresholds for the number of probands to indicate a significant increase in prevalence of a variant in affected individuals compared to controls, odds ratios (ORs) were calculated for hypothetical variants with varied numbers of observed probands and minor allele counts in population databases, similar to the approach taken by Kelly et al. (2018) Sample size was set to 3,522 cases, (based on the number of POAG/JOAG probands with MYOC results in the ANZRAG/GIST databases in March 2021) and 125,748 unrelated reference samples (as recorded in gnomAD v2.1.1 at the same date). Given that ANZRAG predominantly contains individuals of European descent, the process was repeated with 3,277 cases and 56,885 unrelated reference samples, representing the Non-Finnish European (NFE) cohort in gnomAD. Proband count for Supporting, Moderate and Strong thresholds were selected as those that gave rise to ORs of 10, 30 and 100, as per Kelly et al. (2018)
Segregation data (PP1)
Thresholds for the number of meioses required to apply PP1 at each level of strength were taken from Kelly et al. (2018) and Jarvik and Browning, (2016) and the classification outcomes were compared. The thresholds giving the most conservative classification were selected.
Computational predictive tools (PP3, BP4, BP7)
Rare Exome Variant Ensemble Learner (REVEL) (Ioannidis et al., 2016), Functional Analysis Through Hidden Markov Models (FATHMM) (Shihab et al., 2013) and Combined Annotation-Dependent Depletion (CADD) (Kircher et al., 2014; Rentzsch et al., 2019) scores were calculated for all non-synonymous and synonymous (where possible) MYOC variants reported in ClinVar (July 2021) and compared to each other and against the classifications reported in ClinVar. HSF (Desmet et al., 2009), MaxEntScan (Yeo & Burge, 2004), NeuralNetwork (Reese et al., 1997), and SpliceAI (Jaganathan et al., 2019), were used to predict effects on splicing for synonymous variants. Tools and threshold selection were made based on the ability of each tool to discriminate variants previously classified in ClinVar as P/LP or B/LB, combined with the recommendations for interpreting output from each tool.
RESULTS
Summary of rule specifications
The final rule specifications adapted from the ACMG/AMP guidelines for MYOC by the Glaucoma VCEP were approved by the SVI Working Group on 12 October 2021. These are summarized in Table 1 and are available online (https://www.clinicalgenome.org/affiliation/50053/). Of the 28 criteria, 11 were determined not applicable to MYOC (PVS1, PM1, PM3, PP2, PP4, BS2, BS4, BP1, BP2, BP3, BP5, Supp Table S1) and 2 were excluded based on previous SVI recommendations (PP5, BP6) (Biesecker & Harrison, 2018). The remaining 15 rules were all modified to be specific to MYOC and/or the associated phenotype. The level of strength was modified for two rules (PM2, BS3) and additional levels added for 8 (PS1, PS2/PM6, PS3, PS4, PM4, PM5, PP1, BS3).
Population data
Allele frequency data (BA1, BS1, PM2_Supporting)
BA1, BS1 and PM2 apply to the frequency of a variant in a control or general population. Allele frequency thresholds for BA1 (allele frequency >5%) and BS1 (allele frequency greater than expected for the disorder) specific to MYOC were calculated with the Whiffin/Ware calculator.
The highest prevalence for POAG is found in African populations (1/24) (Quigley & Broman, 2006; Tham et al., 2014). The maximum proportion of individuals with JOAG or POAG potentially attributable to a single allele corresponds to the MYOC p.Gln368Ter variant, (the most frequently reported variant) with a prevalence of 1.6% to 2.6% depending on the study (Fingert et al., 1999; Siggs et al., 2021; Souzeau et al., 2013). Analysis of the penetrance of p.Gln368Ter showed that it was much lower in a population-based study (UK Biobank [UKB], 7.6%) than in family-based studies (ANZRAG/GIST, 56%) (Han et al., 2019), although both studies have their own biases. The UKB relied in part on self-report for diagnosis in addition to elevated intraocular pressure or ICD-9/ICD-10 coding. It is well established that at least 50% of individuals with glaucoma are undiagnosed (Mitchell et al., 1996; Soh et al., 2021), so reliance on self-report can lead to underestimation of penetrance. More recent analyses indicate the penetrance of the p.Gln368Ter variant in UKB is around 25% (Zebardast et al., 2021). Conversely, the penetrance in family-based studies may be inflated if individuals with glaucoma are more likely to participate than unaffected family members. Using the prevalence of POAG in Africans, a maximum allelic contribution at 2.6% and a conservative estimate for the penetrance at 7.6%, we calculated a maximum credible population allele frequency for BA1 at 0.007, which was rounded up to 0.01 (1%). Using the same prevalence and maximum allelic contribution, and a more realistic estimate of the penetrance at 56%, we calculated an allele frequency threshold for BS1 at 0.001 (0.1%). We adopted the SVI recommendations that the variant be present in ≥5 alleles in any validated general continental population dataset of at least 2,000 observed alleles (Ghosh et al., 2018; Sequence Variant Interpretation Working Group, 2020), to prevent using less well-characterized populations in the assessment of BA1 and BS1.
An exception was applied to the BS1 rule for variant p.Gln368Ter, based on its penetrance and the presence of a common disease haplotype in all carriers (Baird et al., 2003). Its allele frequency was 0.0025 in the UKB (Han et al., 2019) and 0.001588 in Non-Finnish Europeans in gnomAD, with the highest allele frequency at 0.003344 in Finnish Europeans, which were all above the BS1 threshold established. However, MYOC p.Gln368Ter is a definitively established pathogenic variant (Fingert et al., 1999; Jacobson et al., 2001; Zhou & Vollrath, 1999), displaying variable expressivity (Craig et al., 2001) and reduced penetrance (Han et al., 2019).
PM2 was initially defined by the ACMG/AMP guidelines as the absence of the variant from population databases. Although most are absent, MYOC pathogenic variants may be present as POAG is a common complex disease with late onset and age-related penetrance, and glaucoma phenotypes are not actively excluded from gnomAD. We therefore decided to allow the presence of MYOC variants in population databases for PM2. The filtering allele frequency for PM2 was set one order of magnitude lower than BS1 at 0.0001 (0.01%). PM2 only applies to populations of ≥ 10,000 alleles, to prevent the occurrence of a variant by chance in a small population subset. The Glaucoma VCEP endorsed the SVI recommendation to decrease the weight of PM2 to a Supporting level (Sequence Variant Interpretation Working Group, 2020) and recommended using the highest allele frequency in population databases when assessing BA1, BS1 and PM2_Supporting.
Phenotype data (PS4, PS4_Moderate, PS4_Supporting)
PS4 relates to the prevalence of the variant in affected individuals (cases) compared to unaffected controls. Reliable case-control data in individual studies was limited for most MYOC variants, with the size of the control cohort often not large enough for a reliable estimate of frequency. Therefore, we adopted the “proband counting” approach recommended by the ACMG/AMP guidelines, which allows counting of probands across multiple independent studies for a “quasi case-control study”.
All probands counted must be clinically assessed and have had a diagnosis of JOAG or POAG. In order to apply PS4, BA1 and BS1 must not be met to avoid counting the occurrence of common variants in affected individuals. PM2_Supporting must be met to indicate a rare allele. Efforts should be made to ensure that probands counted are from independent cohorts and probands should not be counted toward PS4 if uncertainty remains (e.g., published by same groups or authors).
We calculated proband count thresholds at which statistical significance would be reached when compared to a large reference dataset such as gnomAD. Evaluation of the ANZRAG/GIST cohorts as a basis for calculations revealed that no pathogenic variants from the cohorts were present at more than 6 alleles in gnomAD (excluding p.Gln368Ter). Using the presence of 6 alleles in gnomAD, ORs of 10, 30 and 100, as per Kelly et al.,(2018) were reached for 2, 6 and 17 probands (Supp Table S2). Calculations on cases and controls from a European-only population gave similar thresholds of 2, 6 and 18 probands, when 3 alleles were present in the reduced gnomAD dataset (Supp Table S2). Based on similar results to Kelly et al., the same thresholds of 2, 6, and 15 probands for Supporting, Moderate and Strong were selected for the application of PS4.
Computational and predictive data
Computational predictive tools (PP3, BP4, BP7)
PP3 and BP4 require multiple lines of computation evidence predicting pathogenic or benign characteristics respectively while BP7 relates specifically to non-conserved synonymous variants with no evidence of an effect on splicing. Evaluation of a range of computational predictive tools revealed overlap in the evidence used to make predictions. In order not to overstate the importance of computational evidence, and in line with reports that a lower rate of concordance is achieved when multiple software tools are used (Ghosh et al., 2017), we decided to use a single tool that collates multiple sources of computational evidence, and where necessary, supplement that with additional sources of evidence.
A comparison of REVEL, FATHMM and CADD was undertaken for nonsynonymous variants deposited in ClinVar. FATHMM was optimized towards assessing non-coding variants and was discounted. Comparison of the REVEL (https://sites.google.com/site/revelgenomics/downloads) and CADD (Version 1.6; https://cadd.gs.washington.edu/snv) scores revealed that REVEL was more conservative (Supp Figure S1). Multiple benign variants and VUS had CADD scores >20, but REVEL scores <0.5; however, there was broad agreement between the tools at the pathogenic end. Based on this comparison, and the sensitivity/specificity reported for REVEL when predicting pathogenicity of ClinVar variants (Ioannidis et al., 2016), the Glaucoma VCEP recommended defining PP3 as REVEL ≥0.7 (58% sensitivity, 96% specificity) and BP4 as REVEL ≤0.15 (55% sensitivity, 95% specificity) for missense variants, similar to other VCEPs curating variants for autosomal dominant diseases (Luo et al., 2019; Oza et al., 2018).
Several criteria require specific evaluation of potential splicing effects. REVEL considers splicing effects but is unable to score synonymous variants. We evaluated several splice prediction tools for this purpose, seeking one that was freely available and straightforward to use. SpliceAI (https://spliceailookup.broadinstitute.org/) (Jaganathan et al., 2019) met these criteria and had clear interpretation guides in the reporting literature. We did not find any evidence in the literature of MYOC variants believed to act through altered splicing and all 19 synonymous variants reported in ClinVar at the time of assessment were classified as B/LB, VUS or conflicting interpretations. All of these had SpliceAI scores <0.2 for all splicing measures, consistent with no in silico evidence of effects on splicing. Therefore, we chose a SpliceAI scores ≤ 0.2 for assessing splicing effects of synonymous variants.
BP4 requires multiple lines of computational evidence suggesting no impact on gene product. As CADD scores can be calculated for any type of variant and incorporate multiple lines of evidence, we specified that a CADD score of ≤10 and SpliceAI score ≤0.2 were required to apply BP4 to synonymous variants. For the application of BP7 for synonymous variants, we specified a GERP score <0 (https://genome.ucsc.edu/cgi-bin/hgGateway), indicating the nucleotide is not highly conserved, as well as SpliceAI ≤0.2. BP4 and BP7 extend to noncoding variants; however, based on the disease mechanism and the absence of evidence supporting pathogenicity of intronic/noncoding variants, curation of these variants will be deprioritized.
Protein length changing variants (PM4, PM4_Supporting)
The Glaucoma VCEP decided that PM4 (protein length changes due to in-frame deletions/insertions and stop losses) would be more appropriate for truncating variants in the last exon that are predicted to escape nonsense-mediated decay, instead of PVS1, which is intended for loss-of-function (LoF) variants. We decided to expand PM4 to any protein length-changing variants, including truncating variants, found within the conserved olfactomedin domain, which is located in exon 3 (aa 246-502). Variants occurring after the last 50bp of exon 2, equating to aa227 would be expected to escape nonsense mediated decay. The ACMG/AMP guidelines emphasized that larger deletions, insertions or extensions would be considered stronger evidence toward pathogenicity. Therefore, we decided to apply PM4 at a Moderate level if the protein length-changing variant was involving ≥10% of the protein and at a Supporting level if involving <10% of the protein.
Variants affecting the same amino acid residue (PS1, PS1_Moderate, PM5, PM5_Supporting)
PS1 applies to a novel nucleotide change leading to the same amino acid residue while PM5 applies to a different missense change. There was only one variant listed in the MYOC database that consists of a different nucleotide leading to the same amino acid (p.Asn480Lys caused by c.1440C>A and c.1440C>G). There were 24 variants in the MYOC database with 2 different missense variants at the same residue and 6 with >2 different missense variants at the same residue. The VCEP decided to apply different levels of strength to PS1 and PM5 depending on the pathogenicity of the previously established variant. PS1 applies to the same amino acid change as a previously established P variant while PS1_Moderate applies to the same amino acid change as a previously established LP variant. Similarly, PM5 applies to the same residue as a previously established P variant or to two previously established LP variants while PM5_Supporting applies to the same residue as a previously established LP variant. The previously established P or LP variants need to reach their classification without the use of PS1 or PM5. In line with the ACMG/AMP guidelines, the novel change must not affect splicing for PS1 or PM5 to apply (as assessed by SpliceAI with a score ≤ 0.2). Additionally, to apply PM5, the variant must meet PP3 and have a Grantham score equal or greater than the previously established P or LP variant to ensure the novel amino acid is predicted to impact function.
Functional data (PS3, PS3_Moderate, PS3_Supporting, BS3_Moderate, BS3_Supporting)
PS3 applies to functional evidence from well-established in vitro or in vivo functional studies supportive of a damaging effect on the gene or gene product. The current body of literature supports a role for MYOC variants causing JOAG or POAG via a mechanism by which the mutant protein is not secreted extracellularly (Caballero et al., 2000; Gobeil et al., 2004; Izumi et al., 2003; Jacobson et al., 2001; Vollrath & Liu, 2006), leading to the accumulation of insoluble aggregates in the cells (Caballero & Borrás, 2001; Liu & Vollrath, 2004). The Glaucoma VCEP determined that assays that report on the solubility and secretion of MYOC were suitable, with evidence supporting insolubility and non-secretion of mutant MYOC protein indicating impact on protein function. Transgenic mice expressing the Tyr423His MYOC mutant (corresponding to human MYOC p.Tyr437His) (Senatorov et al., 2006), or the human p.Tyr437His (Zode et al., 2011), develop elevated intraocular pressure, retinal ganglion cell death and axonal degeneration, reproducing the glaucoma phenotype seen in humans. We determined that animal models that replicate the glaucoma phenotype were also suitable.
Following the recommendations from Brnich et al. (2019), PS3 should only be applied if the assay includes negative (e.g., empty vector) and positive (e.g., wild-type) controls and includes technical and/or biological replicates. The Glaucoma VCEP reviewed all variants included in functional assays that reported on the solubility or the secretion of MYOC (Supp Tables S3a and S3b). We decided to apply different levels of strength based on the odds of pathogenicity (OddsPath) (Brnich et al., 2019) with Strong, Moderate or Supporting levels for OddsPath of >18.7, >4.3 and >2.1, respectively. Validation controls (variants classified as P/LP (pathogenic control) or B/LB (benign control) without the use of PS3 or BS3) from studies that reported on the same class of assay and had the same methodology were combined to calculate the OddsPath.
All coding variants curated in the pilot phase as B/LB for which functional evidence was available were secreted and/or soluble. Similarly, among the coding variants showing evidence for solubility and/or secretion, none were classified as P/LP. Therefore, we decided to apply BS3 (no evidence of damaging effects on protein function in well-established assays) to variants showing solubility or secretion in functional assays that meet the OddsPath of <0.48 or <0.23 for a Supporting or Moderate level, respectively (Brnich et al., 2019). Although there is no evidence currently supporting other protein functions leading to the condition, we recommended not applying BS3 at a Strong level, as we could not completely rule out other mechanisms for pathogenicity.
If multiple results from functional assays are available for a single variant, then the evidence from the assay that is best validated should apply as suggested (Brnich et al., 2019). If results from different assays are conflicting for a single variant, then the result from the assay with the highest level of validation and a conclusive result should override the result from the other assay. However, if the results from the study with the highest level of validation are inconclusive (partial secretion or insoluble levels), then PS3/BS3 should not be applied.
Segregation data (PP1_Strong, PP1_Moderate, PP1)
In the ACMG/AMP guidelines, PP1 applies for co-segregation with disease but is not quantitatively defined. Two studies have since provided quantitative guidelines for assessing segregation of a variant. Jarvik and Browning (2016) calculated a probability that the observed variant and affection status occurs by chance rather than due to co-segregation. Kelly et al (2018) adopted different numbers of meioses based on likelihood ratios of 10 (LOD 0.9), 30 (LOD 1.5) and 100 (LOD 2.1) for Supporting, Moderate and Strong evidence. The pilot study compared the thresholds for the number of meioses required to apply each level of evidence using both methods. Of the 81 pilot-phase variants, only two had a different strength of evidence based on the two approaches: p.Ala363Thr and p.Asn480Lys. The p.Ala363Thr variant had 5 meioses in 3 families, meeting PP1_Moderate according to Kelly et al. but PP1_Strong according to Jarvik and Browning, but both approaches resulted in an LP classification. The p.Asn480Lys (c.1440C>G) variant had 4 meioses in 1 family that met PP1 and was classified LP according to Kelly et al. but PP1_Moderate with classification of P according to Jarvik and Browning. The Glaucoma VCEP recommends a conservative approach and applied the recommendations from Kelly et al (2018) with ≥7, ≥5, and ≥3 meioses for Strong, Moderate and Supporting levels. In addition, a Strong level should only be applied if the variant is present in more than one family, to limit the risk of detecting segregation with a second variant in strong linkage disequilibrium with the one under assessment.
Benign variants may segregate in a family by chance or appear to segregate because they are common in the population. The Glaucoma VCEP agreed that PP1 would only apply if BA1 or BS1 were not met. Due to the late age of onset of POAG, the documented reduced penetrance of many variants, and the potential for phenocopies within families, only genotype-positive/phenotype-positive individuals and obligate carriers/phenotype-positive individuals should be counted. Individuals who carry the variant but do not have a diagnosis of JOAG/POAG (genotype-positive/phenotype-negative) should not be included when counting meioses nor should JOAG/POAG patients who do not carry the variant (genotype-negative/phenotype-positive). Phenotype-positive individuals need to have been clinically assessed and either have a diagnosis of glaucoma (POAG or JOAG) or suspicious signs of glaucoma (e.g., ocular hypertension, suspicious discs).
De novo data (PS2/PM6_Strong, PS2_Moderate/PM6, PS2_Supporting/PM6_Supporting)
PS2 and PM6 apply to de novo variants. MYOC de novo variants are rare, with only two reports in the literature: p.Val251Ala (Kuchtey et al., 2013) and p.Pro254Leu (Souzeau et al., 2016), both confirmed as de novo. The Glaucoma VCEP adopted the SVI-proposed point recommendations for PS2 and PM6 (Supp Figure S2) (Sequence Variant Interpretation Working Group, 2021). Under the scheme, the two criteria are equivalent and only one should be applied. We recommended applying the point-based system for a “phenotype consistent with the gene but not highly specific and with high genetic heterogeneity” for POAG and a higher point-based system for JOAG, using a “phenotype consistent with gene but not highly specific”. Paternity and maternity need to be confirmed to demonstrate a variant is de novo, as per the ACMG/AMP guidelines. Both parents need to be clinically assessed and should not have a diagnosis of glaucoma. If a parent has suspicious signs of glaucoma, their age and the severity of the symptoms should be considered before applying these criteria.
Rules removed or determined not applicable
PP5 (reputable source reports as pathogenic) and BP6 (reputable source reports as benign) were removed based on the recommendation from the SVI Working Group to use primary data instead of relying on assertions (Biesecker & Harrison, 2018). The 11 rules determined not applicable in the context of MYOC with POAG/JOAG are detailed in Supp Table S1. Of note, PVS1 (truncating variants) was removed as it refers specifically to null variants where loss of function is a known mechanism. MYOC variants are known to cause disease through gain-of-function mechanisms and pathogenic truncating variants are not null alleles. These types of variants have been included in the specification for PM4 (protein length changes). The other excluded rules also relate to characteristics of the gene or disease that are not relevant to MYOC or POAG/JOAG.
Combining criteria
Tavtigian et al. (2018) showed that the ACMG/AMP guidelines were compatible with a Bayesian framework and that they could be converted into a point-based system (Tavtigian et al., 2020). With the point-based system, variants with conflicting evidence can be classified as pathogenic or benign depending on the number and/or the strength of the criteria applied. Additionally, this approach allows the use of criteria strength combinations not specifically listed in the ACMG/AMP guidelines. The Glaucoma VCEP decided to apply the scaled point system recently developed by Tavtigian et al. (2020) (Tables 2 and 3). We modified the threshold for LB from −1 to −2 to follow the ACMG/AMP guidelines that require ≥2 Benign Supporting criteria for a LB classification.
Table 2:
Point Values for ACMG/AMP strength of evidence categories(Tavtigian et al., 2020)
| Evidence Strength |
Point scale | |
|---|---|---|
| Pathogenic | Benign | |
| Indeterminate | 0 | 0 |
| Supporting | 1 | −1 |
| Moderate | 2 | −2 |
| Strong | 4 | −4 |
| Very Strong | 8 | −8 |
Table 3:
Point-based variant classification categories, modified from Tavtigian et al.(2020)
| Category | Point ranges |
|---|---|
| Pathogenic | ≥10 |
| Likely Pathogenic | 6 to 9 |
| Uncertain | −1 to 5 |
| Likely Benign | −2 to −6 |
| Benign | ≤−7 |
Application of the rules in a pilot study
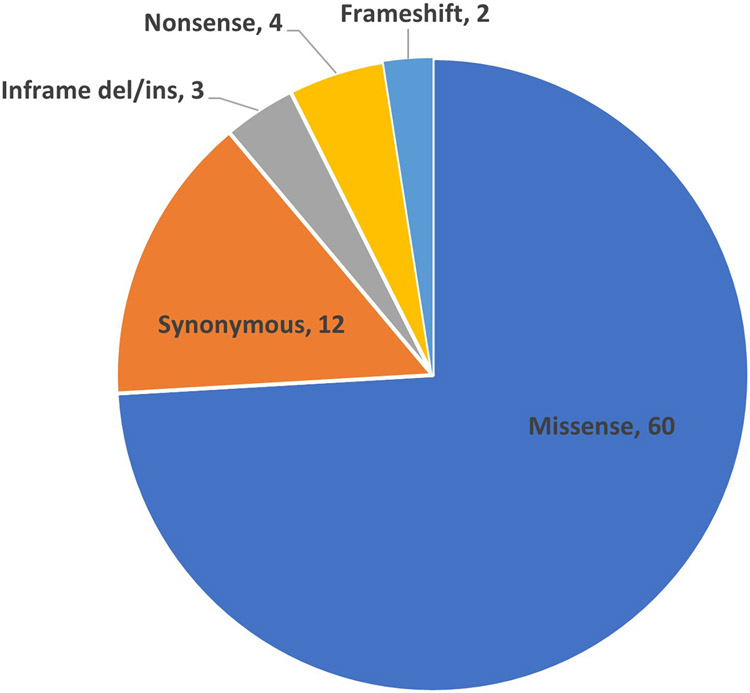
We curated 81 MYOC variants (Supp Table S4) using the specified rules (Table 1). The majority of variants (74%, 60/81) were missense (Figure 1) and were located in exon 3 (78%, 63/81) where the largest numbers of causative variants have been reported.
Figure 1:
Distribution of 81 MYOC variants classified in the pilot study
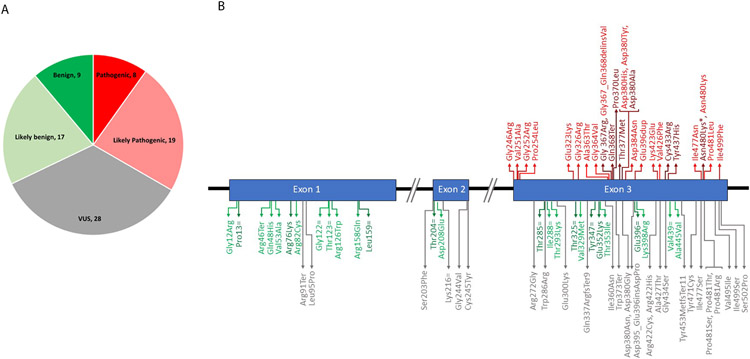
Figure 2 shows the final classification of the pilot variants. All of the LP and P variants were located in exon 3 (Figure 2b). The B, LB and VUS variants were located throughout the gene.
Figure 2:
Final classification of pilot variants by the Glaucoma VCEP using the specified rules. A) number of variants in each classification.
B) position of each variant in the MYOC gene.
Dark red = P; Light red = LP; Grey = VUS; Light green = LB; Dark green = B.
*there are 2 variants encoding p.Asn480Lys, c.1440C>A was classified as P and c.1440C>G as LP.
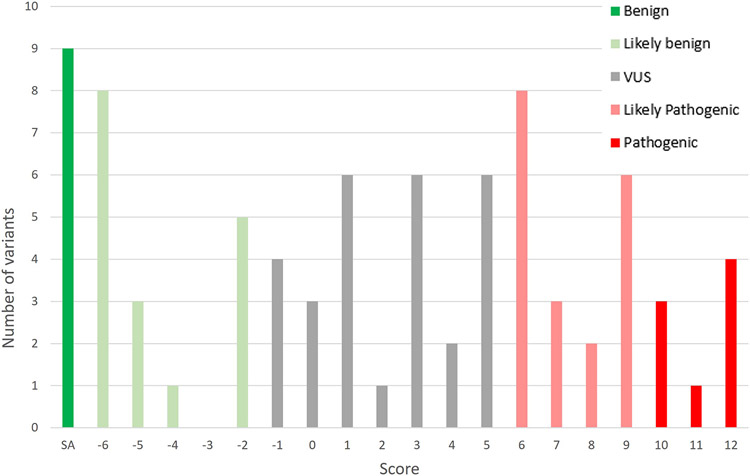
Figure 3 shows the distribution of the classification scores for the variants (Supp Table S4). All the variants classified as B met BA1. All the variants with a score of −6 met BS1 as well as BS3_Moderate or a combination of BP4 and BP7. All of the variants classified on the upper end of LB, with a score of −2, solely met BS3_Moderate.
Figure 3:
Distribution of the final Glaucoma VCEP classification scores
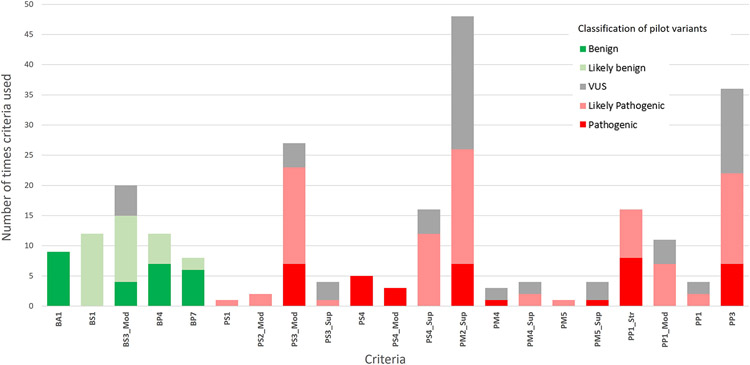
PM2_Supporting was the most applied criterion (Figure 4), used in 59% (48/81) of the classifications including 7 P, 19 LP and 22 VUS. Because no criteria are applied at a Very Strong level with the specified rules, at least one Strong criterion is required to reach a P classification. PP1_Strong was used in the classification of all P variants along with PS4 (Strong in 5/8 and Moderate in 3/8 variants), with PS3_Moderate, PM2_Supporting and PP3 all included in 7 out of the 8 P classifications.
Figure 4:
Criteria applied in the pilot phase with final Glaucoma VCEP classification of variants
BA1 was used to classify 9 variants as B. BS1 and BS3_Moderate were the most used criteria for the variants classified as LB (applied 12 and 11 times respectively). Five variants had conflicting evidence. BS3_Moderate was included in all, as was PM2_Supporting and/or PP3. All of these variants were classified as VUS.
Supp Figure S3 shows that most variants classified as LP or P had PM2_Supporting applied and were not reported in gnomAD, with the exception of the two well-characterized common variants p.Gln368Ter and p.Thr377Met. It is also evident that BS1 plays an important role in discriminating LB variants from VUS, with 81% (21/26) of variants classified as LB/B meeting BS1 or BA1.
PM5 was used once with an unmodified strength and four times as PM5_Supporting. Based on the LP classification of p.Asp380His, PM5_Supporting was applied to p.Asp380Ala, which has a higher Grantham score and was classified as P. This P variant was then used to apply PM5 to p.Asp380Tyr, as it has a higher Grantham score than p.Asp380Ala, increasing its classification to LP. The LP variant p.Asp380His was used to apply PM5_Supporting to p.Asp380Gly, which was classified as VUS. Additionally, PM5_Supporting was applied to p.Pro481Arg and p.Ile499Ser, but these variants also remained as VUS.
The Glaucoma VCEP reviewed evidence from 10 published studies with functional assays, which included 63 variants from the pilot list. BS3_Moderate was applied 20 times in total, with 11 of these involved with LB classifications. For 5 variants, BS3_Moderate was the only criterion applied, which resulted in an LB classification. PS3 was applied 31 times, 27 at the Moderate level and 4 at the Supporting level. Functional evidence influenced the classification for 29% (18/63) of variants, including 10 from VUS to LP, 5 from VUS to LB and 3 from LP to P.
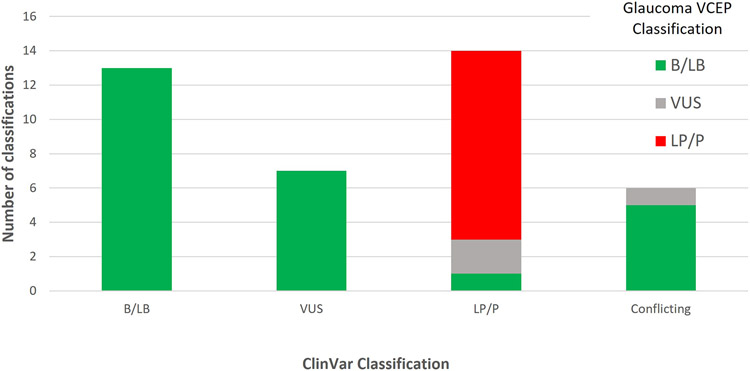
Among the 40 variants that had a ClinVar classification (accessed 10 January 2022), 40% (16/40) had a change of classification after Glaucoma VCEP curation (Figure 5). Variants with a discordant classification from ClinVar included 7 VUS reclassified as B/LB, 3 LP/P reclassified (2 as VUS and 1 as B/LB) and all 6 variants with conflicting interpretation reclassified (5 as B/LB and 1 as VUS). BA1 and BS1 were applied in the curations of 8 of the 13 variants reclassified as B/LB by the VCEP. BS3_Moderate was applied to 10 of these variants. The number of variants in ClinVar classified as VUS or as variants with conflicting interpretations decreased from 13 to 1 after VCEP curation.
Figure 5:
ClinVar vs Glaucoma VCEP classification of MYOC variants from the pilot phase. Colours indicate the Glaucoma VCEP classification
DISCUSSION
The specifications of the ACMG/AMP guidelines for the MYOC gene in relation to POAG/JOAG has resulted in the reclassification of over one-third of the variants reported in ClinVar. The process has led to fewer VUS, with clearer definitions of LB and B, largely related to population frequency and functional evidence. Similarly, for LP and P classifications, population and functional criteria were influential, but in contrast to the benign variants, in silico predictions were frequently applied and influenced the classification.
Challenges were revealed where information from previously classified variants was required to classify a variant of interest. This was evident in the application of PS1, PM5 and PS3/BS3. Curators were first required to identify variants in our pilot list that met the LP or P classifications without the application of these rules before we could apply these rules to other variants. For example, PM5_Supporting or PM5 can only be applied if another variant at the same residue was previously classified as LP or P and the novel variant must have a higher Grantham score than the previously classified variant. This means biocurators were required to seek information about many other variants in order to apply the rule to one variant. Similarly, for PS3 and BS3, it was necessary to count the number of variants assessed by that assay that were classified B/LB or LP/P without functional evidence, and label these as validation controls for that assay to determine the strength at which each rule can be applied. We expanded the pilot variant list to include all variants with functional evaluation in the literature to facilitate this process. Generating functional evidence for additional variants beyond the pilot list will be highly valuable, especially for variants classified as VUS in the absence of functional data.
The addition of carefully assessed functional data to the classification resulted in 15 VUS now meeting the thresholds for LB or LP. We took a conservative approach, carefully evaluating the strength of each assay, guided by the work by Brnich et al. (2019) to quantify the required numbers of validation controls. Even with the conservative evidence-based thresholds, these criteria played an important role in the classification. The VCEP chose to specify the application of BS3 at the Moderate level when the appropriate OddsPath was reached, as described by Brnich et al. This is in contrast to the original ACMG/AMP guidelines, which did not allow for any benign criteria to be applied at the Moderate level. We do not recommend applying BS3 at the Strong level. With uncertainty remaining as to whether the solubility and secretion assays test all the appropriate or possible disease mechanisms, the VCEP took the conservative approach of not overclassifying variants towards benign. As knowledge of POAG molecular mechanisms increases and more thorough assays are developed, the specifications for this criterion may need to be re-assessed. The application of BS3_Moderate allowed variants to be classified as LB with the application of BS3_Moderate alone (score of −2). This decision was taken to reflect a consistent approach in the level of evidence required for both BS3 and PS3 and reflects the level of confidence the Glaucoma VCEP had in the quality and validity of the functional assays described for MYOC.
Despite PP1 in the original guidelines being at the Supporting level, this rule is often applied arbitrarily at higher levels. The work by Kelly et al. (2018) in relation to the MYH7 gene, and Jarvik and Browning (2016) for more general application, provide an evidence base for rational decision-making on when to apply each level of evidence. Both are simple to apply, requiring only the counting of meioses with thresholds for each level of significance correlating to the probabilities of multiple co-segregating transmissions. We chose the slightly more conservative classifications of Kelly et al. (2018) to limit over-interpretation; however, the marginal differences in outcome reflect the similarity in the underlying approaches. PS4 was originally defined for case-control studies, or, when they do not reach statistical significance, for multiple independent affected individuals. Similar to PP1, we have developed an easy-to-use points system that allows counting of independent probands and the application of PS4 at different levels of evidence.
The standard benign criteria for population data are BA1 (allele frequency >0.05) and BS1 (allele frequency greater than expected for the disease). However, it has been recognized that the prevalence and penetrance of the disease as well as the gene contribution should be considered when applying these rules (Whiffin et al., 2017). The Glaucoma VCEP developed allele frequency thresholds for BA1 and BS1 that reflect the architecture of the disease and the MYOC gene. Our pilot data show that 81% of LB/B variants met one of these criteria, validating our approach. Similarly, PM2 was initially defined as an absence of the variant from controls. We set a conservative threshold to account for the possibility of pathogenic variants being present in population databases in the context of incomplete age-related penetrance and undiagnosed glaucoma in the population. Nevertheless, all but two of the variants classified as LP/P from the pilot study were absent from gnomAD, highlighting the rarity of pathogenic MYOC variants in population databases. Although we used gnomAD version 2 for assessing allele frequency in the pilot phase, we encourage the use of other large population datasets that become available or that may be specific to some populations.
The Glaucoma VCEP accessed allele frequency information from gnomAD and recommended applying the most relevant population specific information where available. It should be noted that gnomAD and most other population databases are limited in their content from non-European populations. Our pilot variant list included several variants predominantly reported in non-European probands. Several were classified as B (p.Pro13Pro seen in African/African American) or LB (p.Gly12Arg, p.Arg46Ter, p.Gln48His, p.Asp208Glu, p.Thr353Ile reported in East Asian/South Asian). Others were classified as VUS (p.Trp286Arg in Latino/Admixed American and p.Glu300Lys in East Asian) and two as P (p.Thr377Met and p.Cys433Arg - African/African American). As access to genetic research and testing increases in populations of non-European descent, sourcing appropriate population frequencies will be critically important for correctly interpreting novel variants. New information from other populations should also be considered for variants already classified that may have inadvertently been labelled rare based on European information but are more common in other populations.
Access to high quality data is important for criteria requiring counting of genotype- and phenotype-positive individuals (PS4, PP1). In the pilot study, this was largely achieved through manual curation of published literature, including, where necessary, contacting the corresponding authors to confirm overlapping individuals between publications. Where this information could not be obtained directly, we took the conservative approach of only counting each possibly duplicated patient once. In addition, the Glaucoma VCEP accessed multiple research databases, increasing the number of probands counted for some variants. This highlights the importance of data sharing through individual research groups and accredited genetic-testing laboratories publishing and/or depositing the information for each observed variant in publicly accessible locations. This is especially important for rarely observed variants where every piece of information can have a significant effect on the overall classification.
The thresholds for each criterion are in line with those recommended by other VCEPs for similar autosomal dominant heterogeneous diseases such as MYH7-associated inherited cardiomyopathies (Kelly et al., 2018), genetic hearing loss (Oza et al., 2018) and myeloid malignancy caused by RUNX1 variants (Luo et al., 2019). This consistency in approach provides a framework that can be applied to other diseases and genes that have similar characteristics but have not yet had the benefit of dedicated VCEP review and rule specification. This is important, as the workload for defining specific rules for every disease-causing gene is daunting. The genetic hearing loss VCEP has specified a set of rules to be applied across a range of genetic hearing loss-related genes (Oza et al., 2018), streamlining the process for this group of genes that all have similar characteristics. This approach will be necessary for the efficient specification of rules for other heterogeneous but monogenic diseases.
The Glaucoma VCEP has curated 81 variants using MYOC-specific rules within the framework of the ACMG/AMP guidelines and these classifications are available in ClinVar. The VCEP will curate the remaining reported variants and novel variants as they are reported. We will also review any variants submitted to ClinVar with a different classification to that assigned by the VCEP, as additional evidence may change the classifications. Variants with a medically significant difference (P/LP vs B/LB/VUS) will be reassessed within 3 months of being notified of the discrepant ClinVar classification. Variants classified as LP and VUS will be reviewed every 2 years and LB variants will be reassessed when new large population datasets are released as per ClinGen protocol, to ensure up-to-date information is available for all variants. The VCEP will review its MYOC-specified rules every 2 years or sooner as necessary if new knowledge or recommendations from ClinGen arise.
Supplementary Material
ACKNOWLEDGEMENTS
This work was funded by a National Health and Medical Research Council (NHMRC) of Australia Centre for Research Excellence grant (GNT1116360), Program Grant (GNT1150144), Practitioner Fellowships to J.E.C, D.A.M and A.W.H., The Hospital Research Foundation Early Career Fellowship to E.S, A Core Grant for Vision Research from the National Eye Institute/National Institutes of Health to the University of Wisconsin-Madison (P30EY016665) and an Unrestricted Grant from Research to Prevent Blindness, Inc. to the UW-Madison Department of Ophthalmology and Visual Sciences to T.L.Y. and K.N.W. The authors would like to acknowledge the support of the ClinGen Sequence Variant Interpretation and Ocular Clinical Domain Working Groups, especially Kristy Lee.
Footnotes
CONFLICTS OF INTEREST
The authors have no conflicts of interest to declare
WEB RESOURCES
Whiffin/Ware calculator: https://cardiodb.org/allelefrequencyapp/
Glaucoma VCEP: https://www.clinicalgenome.org/affiliation/50053/
REVEL score: https://sites.google.com/site/revelgenomics/downloads
CADD score (v1.6): https://cadd.gs.washington.edu/snv
SpliceAI score: https://spliceailookup.broadinstitute.org/
GERP score: https://genome.ucsc.edu/cgi-bin/hgGateway
ClinGen: https://clinicalgenome.org/
ClinGen Variant Curation Interface: https://curation.clinicalgenome.org/
ClinVar: https://www.ncbi.nlm.nih.gov/clinvar/
gnomAD: https://gnomad.broadinstitute.org/
Myocilin database: http://www.myocilin.com
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are openly available in ClinVar at https://www.ncbi.nlm.nih.gov/clinvar/, accession numbers for each variant can be found in Supp Table S4.
References
- Amendola LM, Jarvik GP, Leo MC, McLaughlin HM, Akkari Y, Amaral MD, Berg JS, Biswas S, Bowling KM, Conlin LK, Cooper GM, Dorschner MO, Dulik MC, Ghazani AA, Ghosh R, Green RC, Hart R, Horton C, Johnston JJ, . . . Rehm HL (2016). Performance of ACMG-AMP variant-interpretation guidelines among nine laboratories in the clinical sequencing exploratory research consortium. Am J Hum Genet, 98(6), 1067–1076. 10.1016/j.ajhg.2016.03.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amendola LM, Muenzen K, Biesecker LG, Bowling KM, Cooper GM, Dorschner MO, Driscoll C, Foreman AKM, Golden-Grant K, Greally JM, Hindorff L, Kanavy D, Jobanputra V, Johnston JJ, Kenny EE, McNulty S, Murali P, Ou J, Powell BC, . . . Jarvik GP (2020). Variant Classification Concordance using the ACMG-AMP Variant Interpretation Guidelines across Nine Genomic Implementation Research Studies. Am J Hum Genet, 107(5), 932–941. 10.1016/j.ajhg.2020.09.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Academy of Ophthalmology Preferred Practice Pattern Glaucoma Committee. (2020). Primary Open-Angle Glaucoma Preferred Practice Pattern. American Academy of Ophthalmology. https://www.aao.org/preferred-practice-pattern/primary-open-angle-glaucoma-ppp [Google Scholar]
- Baird PN, Craig JE, Richardson AJ, Ring MA, Sim P, Stanwix S, Foote SJ, & Mackey DA (2003). Analysis of 15 primary open-angle glaucoma families from Australia identifies a founder effect for the Q368STOP mutation of myocilin. Hum Genet, 112(2), 110–116. 10.1007/s00439-002-0865-5 [DOI] [PubMed] [Google Scholar]
- Biesecker LG, & Harrison SM (2018). The ACMG/AMP reputable source criteria for the interpretation of sequence variants. Genet Med, 20(12), 1687–1688. 10.1038/gim.2018.42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brnich SE, Abou Tayoun AN, Couch FJ, Cutting GR, Greenblatt MS, Heinen CD, Kanavy DM, Luo X, McNulty SM, Starita LM, Tavtigian SV, Wright MW, Harrison SM, Biesecker LG, & Berg JS (2019). Recommendations for application of the functional evidence PS3/BS3 criterion using the ACMG/AMP sequence variant interpretation framework. Genome Med, 12(1), 3. 10.1186/s13073-019-0690-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns JN, Turnage KC, Walker CA, & Lieberman RL (2011). The stability of myocilin olfactomedin domain variants provides new insight into glaucoma as a protein misfolding disorder. Biochemistry, 50(26), 5824–5833. 10.1021/bi200231x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caballero M, & Borrás T (2001). Inefficient processing of an olfactomedin-deficient myocilin mutant: potential physiological relevance to glaucoma. Biochem Biophys Res Commun, 282(3), 662–670. 10.1006/bbrc.2001.4624 [DOI] [PubMed] [Google Scholar]
- Caballero M, Rowlette LL, & Borrás T (2000). Altered secretion of a TIGR/MYOC mutant lacking the olfactomedin domain. Biochim Biophys Acta, 1502(3), 447–460. 10.1016/s0925-4439(00)00068-5 [DOI] [PubMed] [Google Scholar]
- Casson RJ, Chidlow G, Wood JP, Crowston JG, & Goldberg I (2012). Definition of glaucoma: clinical and experimental concepts. Clin Experiment Ophthalmol, 40(4), 341–349. 10.1111/j.1442-9071.2012.02773.x [DOI] [PubMed] [Google Scholar]
- Craig JE, Baird PN, Healey DL, McNaught AI, McCartney PJ, Rait JL, Dickinson JL, Roe L, Fingert JH, Stone EM, & Mackey DA (2001). Evidence for genetic heterogeneity within eight glaucoma families, with the GLC1A Gln368STOP mutation being an important phenotypic modifier. Ophthalmology, 108(9), 1607–1620. 10.1016/s0161-6420(01)00654-6 [DOI] [PubMed] [Google Scholar]
- Desmet FO, Hamroun D, Lalande M, Collod-Béroud G, Claustres M, & Béroud C (2009). Human Splicing Finder: an online bioinformatics tool to predict splicing signals. Nucleic Acids Res, 37(9), e67. 10.1093/nar/gkp215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fingert JH, Heon E, Liebmann JM, Yamamoto T, Craig JE, Rait J, Kawase K, Hoh ST, Buys YM, Dickinson J, Hockey RR, Williams-Lyn D, Trope G, Kitazawa Y, Ritch R, Mackey DA, Alward WL, Sheffield VC, & Stone EM (1999). Analysis of myocilin mutations in 1703 glaucoma patients from five different populations. Hum Mol Genet, 8(5), 899–905. 10.1093/hmg/8.5.899 [DOI] [PubMed] [Google Scholar]
- Ghosh R, Harrison SM, Rehm HL, Plon SE, & Biesecker LG (2018). Updated recommendation for the benign stand-alone ACMG/AMP criterion. Hum Mutat, 39(11), 1525–1530. 10.1002/humu.23642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh R, Oak N, & Plon SE (2017). Evaluation of in silico algorithms for use with ACMG/AMP clinical variant interpretation guidelines. Genome Biol, 18(1), 225. 10.1186/s13059-017-1353-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gobeil S, Rodrigue MA, Moisan S, Nguyen TD, Polansky JR, Morissette J, & Raymond V (2004). Intracellular sequestration of hetero-oligomers formed by wild-type and glaucoma-causing myocilin mutants. Invest Ophthalmol Vis Sci, 45(10), 3560–3567. 10.1167/iovs.04-0300 [DOI] [PubMed] [Google Scholar]
- Han X, Souzeau E, Ong JS, An J, Siggs OM, Burdon KP, Best S, Goldberg I, Healey PR, Graham SL, Ruddle JB, Mills RA, Landers J, Galanopoulos A, White AJR, Casson R, Mackey DA, Hewitt AW, Gharahkhani P, . . . MacGregor S (2019). Myocilin gene Gln368Ter variant penetrance and association with glaucoma in population-based and registry-based studies. JAMA Ophthalmol, 137(1), 28–35. 10.1001/jamaophthalmol.2018.4477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison SM, Dolinsky JS, Knight Johnson AE, Pesaran T, Azzariti DR, Bale S, Chao EC, Das S, Vincent L, & Rehm HL (2017). Clinical laboratories collaborate to resolve differences in variant interpretations submitted to ClinVar. Genet Med, 19(10), 1096–1104. 10.1038/gim.2017.14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewitt AW, Mackey DA, & Craig JE (2008). Myocilin allele-specific glaucoma phenotype database. Hum Mutat, 29(2), 207–211. 10.1002/humu.20634 [DOI] [PubMed] [Google Scholar]
- Ioannidis NM, Rothstein JH, Pejaver V, Middha S, McDonnell SK, Baheti S, Musolf A, Li Q, Holzinger E, Karyadi D, Cannon-Albright LA, Teerlink CC, Stanford JL, Isaacs WB, Xu J, Cooney KA, Lange EM, Schleutker J, Carpten JD, . . . Sieh W (2016). REVEL: An Ensemble Method for Predicting the Pathogenicity of Rare Missense Variants. Am J Hum Genet, 99(4), 877–885. 10.1016/j.ajhg.2016.08.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izumi K, Mashima Y, Obazawa M, Ohtake Y, Tanino T, Miyata H, Zhang Q, Oguchi Y, Tanaka Y, & Iwata T (2003). Variants of the myocilin gene in Japanese patients with normal-tension glaucoma. Ophthalmic Res, 35(6), 345–350. 10.1159/000074075 [DOI] [PubMed] [Google Scholar]
- Jacobson N, Andrews M, Shepard AR, Nishimura D, Searby C, Fingert JH, Hageman G, Mullins R, Davidson BL, Kwon YH, Alward WL, Stone EM, Clark AF, & Sheffield VC (2001). Non-secretion of mutant proteins of the glaucoma gene myocilin in cultured trabecular meshwork cells and in aqueous humor. Hum Mol Genet, 10(2), 117–125. 10.1093/hmg/10.2.117 [DOI] [PubMed] [Google Scholar]
- Jaganathan K, Kyriazopoulou Panagiotopoulou S, McRae JF, Darbandi SF, Knowles D, Li YI, Kosmicki JA, Arbelaez J, Cui W, Schwartz GB, Chow ED, Kanterakis E, Gao H, Kia A, Batzoglou S, Sanders SJ, & Farh KK (2019). Predicting Splicing from Primary Sequence with Deep Learning. Cell, 176(3), 535–548.e524. 10.1016/j.cell.2018.12.015 [DOI] [PubMed] [Google Scholar]
- Jarvik GP, & Browning BL (2016). Consideration of Cosegregation in the Pathogenicity Classification of Genomic Variants. Am J Hum Genet, 98(6), 1077–1081. 10.1016/j.ajhg.2016.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly MA, Caleshu C, Morales A, Buchan J, Wolf Z, Harrison SM, Cook S, Dillon MW, Garcia J, Haverfield E, Jongbloed JDH, Macaya D, Manrai A, Orland K, Richard G, Spoonamore K, Thomas M, Thomson K, Vincent LM, . . . Funke B (2018). Adaptation and validation of the ACMG/AMP variant classification framework for MYH7-associated inherited cardiomyopathies: recommendations by ClinGen's Inherited Cardiomyopathy Expert Panel. Genet Med, 20(3), 351–359. 10.1038/gim.2017.218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim BS, Savinova OV, Reedy MV, Martin J, Lun Y, Gan L, Smith RS, Tomarev SI, John SW, & Johnson RL (2001). Targeted Disruption of the Myocilin Gene (Myoc) Suggests that Human Glaucoma-Causing Mutations Are Gain of Function. Mol Cell Biol, 21(22), 7707–7713. 10.1128/mcb.21.22.7707-7713.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kircher M, Witten DM, Jain P, O'Roak BJ, Cooper GM, & Shendure J (2014). A general framework for estimating the relative pathogenicity of human genetic variants. Nat Genet, 46(3), 310–315. 10.1038/ng.2892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuchtey J, Chowdhury UR, Uptegraft CC, Fautsch MP, & Kuchtey RW (2013). A de novo MYOC mutation detected in juvenile open angle glaucoma associated with reduced myocilin protein in aqueous humor. Eur J Med Genet, 56(6), 292–296. 10.1016/j.ejmg.2013.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam DS, Leung YF, Chua JK, Baum L, Fan DS, Choy KW, & Pang CP (2000). Truncations in the TIGR gene in individuals with and without primary open-angle glaucoma. Invest Ophthalmol Vis Sci, 41(6), 1386–1391. https://iovs.arvojournals.org/article.aspx?articleid=2123288 [PubMed] [Google Scholar]
- Landrum MJ, Lee JM, Benson M, Brown GR, Chao C, Chitipiralla S, Gu B, Hart J, Hoffman D, Jang W, Karapetyan K, Katz K, Liu C, Maddipatla Z, Malheiro A, McDaniel K, Ovetsky M, Riley G, Zhou G, . . . Maglott DR (2018). ClinVar: improving access to variant interpretations and supporting evidence. Nucleic Acids Res, 46(D1), D1062–D1067. 10.1093/nar/gkx1153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, & Vollrath D (2004). Reversal of mutant myocilin non-secretion and cell killing: implications for glaucoma. Hum Mol Genet, 13(11), 1193–1204. 10.1093/hmg/ddh128 [DOI] [PubMed] [Google Scholar]
- Luo X, Feurstein S, Mohan S, Porter CC, Jackson SA, Keel S, Chicka M, Brown AL, Kesserwan C, Agarwal A, Luo M, Li Z, Ross JE, Baliakas P, Pineda-Alvarez D, DiNardo CD, Bertuch AA, Mehta N, Vulliamy T, . . . Godley LA (2019). ClinGen Myeloid Malignancy Variant Curation Expert Panel recommendations for germline RUNX1 variants. Blood Adv, 3(20), 2962–2979. 10.1182/bloodadvances.2019000644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackey DA, Craig JE, & Hewitt AW (2019). Seeing the impact of the Glaucoma Inheritance Study in Tasmania after 25 years. Clin Exp Ophthalmol, 47(5), 677–679. 10.1111/ceo.13446 [DOI] [PubMed] [Google Scholar]
- Mitchell P, Smith W, Attebo K, & Healey PR (1996). Prevalence of open-angle glaucoma in Australia. The Blue Mountains Eye Study. Ophthalmology, 103(10), 1661–1669. 10.1016/s0161-6420(96)30449-1 [DOI] [PubMed] [Google Scholar]
- Nakahara E, & Hulleman JD (2022). A simple secretion assay for assessing new and existing myocilin variants. Curr Eye Res, 25, 1–5. 10.1080/02713683.2022.2047205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oza AM, DiStefano MT, Hemphill SE, Cushman BJ, Grant AR, Siegert RK, Shen J, Chapin A, Boczek NJ, Schimmenti LA, Murry JB, Hasadsri L, Nara K, Kenna M, Booth KT, Azaiez H, Griffith A, Avraham KB, Kremer H, . . . Abou Tayoun AN (2018). Expert specification of the ACMG/AMP variant interpretation guidelines for genetic hearing loss. Hum Mutat, 39(11), 1593–1613. 10.1002/humu.23630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quigley HA, & Broman AT (2006). The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol, 90(3), 262–267. 10.1136/bjo.2005.081224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reese MG, Eeckman FH, Kulp D, & Haussler D (1997). Improved splice site detection in Genie. J Comput Biol, 4(3), 311–323. 10.1089/cmb.1997.4.311 [DOI] [PubMed] [Google Scholar]
- Rentzsch P, Witten D, Cooper GM, Shendure J, & Kircher M (2019). CADD: predicting the deleteriousness of variants throughout the human genome. Nucleic Acids Res, 47(D1), D886–d894. 10.1093/nar/gky1016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, Grody WW, Hegde M, Lyon E, Spector E, Voelkerding K, & Rehm HL (2015). Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med, 17(5), 405–424. 10.1038/gim.2015.30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera-Munoz EA, Milko LV, Harrison SM, Azzariti DR, Kurtz CL, Lee K, Mester JL, Weaver MA, Currey E, Craigen W, Eng C, Funke B, Hegde M, Hershberger RE, Mao R, Steiner RD, Vincent LM, Martin CL, Plon SE, . . . Berg JS (2018). ClinGen Variant Curation Expert Panel experiences and standardized processes for disease and gene-level specification of the ACMG/AMP guidelines for sequence variant interpretation. Hum Mutat, 39(11), 1614–1622. 10.1002/humu.23645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senatorov V, Malyukova I, Fariss R, Wawrousek EF, Swaminathan S, Sharan SK, & Tomarev S (2006). Expression of mutated mouse myocilin induces open-angle glaucoma in transgenic mice. J Neurosci, 26(46), 11903–11914. 10.1523/jneurosci.3020-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sequence Variant Interpretation Working Group. (2020). Sequence Variant Interpretation Recommendation for Absence/Rarity (PM2) - Version 1.0. https://clinicalgenome.org/site/assets/files/5182/pm2_-_svi_recommendation_-_approved_sept2020.pdf
- Sequence Variant Interpretation Working Group. (2021). Sequence Variant Interpretation Recommendation for De Novo Criteria (PS2 & PM6) - Version 1.1. https://www.clinicalgenome.org/site/assets/files/3461/svi_proposal_for_de_novo_criteria_v1_1.pdf
- Shihab HA, Gough J, Cooper DN, Stenson PD, Barker GL, Edwards KJ, Day IN, & Gaunt TR (2013). Predicting the functional, molecular, and phenotypic consequences of amino acid substitutions using hidden Markov models. Hum Mutat, 34(1), 57–65. 10.1002/humu.22225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu S, Lichter PR, Johnson AT, Zhou Z, Higashi M, Gottfredsdottir M, Othman M, Moroi SE, Rozsa FW, Schertzer RM, Clarke MS, Schwartz AL, Downs CA, Vollrath D, & Richards JE (2000). Age-dependent prevalence of mutations at the GLC1A locus in primary open-angle glaucoma. Am J Ophthalmol, 130(2), 165–177. 10.1016/s0002-9394(00)00536-5 [DOI] [PubMed] [Google Scholar]
- Siggs OM, Han X, Qassim A, Souzeau E, Kuruvilla S, Marshall HN, Mullany S, Mackey DA, Hewitt AW, Gharahkhani P, MacGregor S, & Craig JE (2021). Association of Monogenic and Polygenic Risk With the Prevalence of Open-Angle Glaucoma. JAMA Ophthalmol, 139(9), 1023–1028. 10.1001/jamaophthalmol.2021.2440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soh Z, Yu M, Betzler BK, Majithia S, Thakur S, Tham YC, Wong TY, Aung T, Friedman DS, & Cheng CY (2021). The global extent of undetected glaucoma in adults: a systematic review and meta-analysis. Ophthalmology, 128(10), 1393–1404. 10.1016/j.ophtha.2021.04.009 [DOI] [PubMed] [Google Scholar]
- Souzeau E, Burdon KP, Dubowsky A, Grist S, Usher B, Fitzgerald JT, Crawford A, Hewitt AW, Goldberg I, Mills RA, Ruddle JB, Landers J, Mackey DA, & Craig JE (2013). Higher prevalence of myocilin mutations in advanced glaucoma in comparison with less advanced disease in an Australasian disease registry. Ophthalmology, 120(6), 1135–1143. [DOI] [PubMed] [Google Scholar]
- Souzeau E, Burdon KP, Ridge B, Dubowsky A, Ruddle JB, & Craig JE (2016). A novel de novo Myocilin variant in a patient with sporadic juvenile open angle glaucoma. BMC Med Genet, 17, 30. 10.1186/s12881-016-0291-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souzeau E, Tram KH, Witney M, Ruddle JB, Graham SL, Healey PR, Goldberg I, Mackey DA, Hewitt AW, Burdon KP, & Craig JE (2017). Myocilin Predictive Genetic Testing for Primary Open-Angle Glaucoma Leads to Early Identification of At-Risk Individuals. Ophthalmology, 124(3), 303–309. 10.1016/j.ophtha.2016.11.011 [DOI] [PubMed] [Google Scholar]
- Stone EM, Fingert JH, Alward WL, Nguyen TD, Polansky JR, Sunden SL, Nishimura D, Clark AF, Nystuen A, Nichols BE, Mackey DA, Ritch R, Kalenak JW, Craven ER, & Sheffield VC (1997). Identification of a gene that causes primary open angle glaucoma. Science, 275(5300), 668–670. 10.1126/science.275.5300.668 [DOI] [PubMed] [Google Scholar]
- Tavtigian SV, Greenblatt MS, Harrison SM, Nussbaum RL, Prabhu SA, Boucher KM, & Biesecker LG (2018). Modeling the ACMG/AMP variant classification guidelines as a Bayesian classification framework. Genet Med, 20(9), 1054–1060. 10.1038/gim.2017.210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavtigian SV, Harrison SM, Boucher KM, & Biesecker LG (2020). Fitting a naturally scaled point system to the ACMG/AMP variant classification guidelines. Hum Mutat, 41(10), 1734–1737. 10.1002/humu.24088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tham YC, Li X, Wong TY, Quigley HA, Aung T, & Cheng CY (2014). Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and meta-analysis. Ophthalmology, 121(11), 2081–2090. 10.1016/j.ophtha.2014.05.013 [DOI] [PubMed] [Google Scholar]
- Turalba AV, & Chen TC (2008). Clinical and genetic characteristics of primary juvenile-onset open-angle glaucoma (JOAG). Semin Ophthalmol, 23(1), 19–25. 10.1080/08820530701745199 [DOI] [PubMed] [Google Scholar]
- Vincent AL, Billingsley G, Buys Y, Levin AV, Priston M, Trope G, Williams-Lyn D, & Heon E (2002). Digenic inheritance of early-onset glaucoma: CYP1B1, a potential modifier gene. Am J Hum Genet, 70(2), 448–460. 10.1086/338709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollrath D, & Liu Y (2006). Temperature sensitive secretion of mutant myocilins. Exp Eye Res, 82(6), 1030–1036. 10.1016/j.exer.2005.10.007 [DOI] [PubMed] [Google Scholar]
- Whiffin N, Minikel E, Walsh R, O'Donnell-Luria AH, Karczewski K, Ing AY, Barton PJR, Funke B, Cook SA, MacArthur D, & Ware JS (2017). Using high-resolution variant frequencies to empower clinical genome interpretation. Genet Med, 19(10), 1151–1158. 10.1038/gim.2017.26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiggs JL, Allingham RR, Vollrath D, Jones KH, De La Paz M, Kern J, Patterson K, Babb VL, Del Bono EA, Broomer BW, Pericak-Vance MA, & Haines JL (1998). Prevalence of mutations in TIGR/Myocilin in patients with adult and juvenile primary open-angle glaucoma. Am J Hum Genet, 63(5), 1549–1552. 10.1086/302098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiggs JL, & Vollrath D (2001). Molecular and clinical evaluation of a patient hemizygous for TIGR/MYOC. Arch Ophthalmol, 119(11), 1674–1678. 10.1001/archopht.119.11.1674 [DOI] [PubMed] [Google Scholar]
- Yam GH, Gaplovska-Kysela K, Zuber C, & Roth J (2007). Aggregated myocilin induces russell bodies and causes apoptosis: implications for the pathogenesis of myocilin-caused primary open-angle glaucoma. Am J Pathol, 170(1), 100–109. 10.2353/ajpath.2007.060806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo G, & Burge CB (2004). Maximum entropy modeling of short sequence motifs with applications to RNA splicing signals. J Comput Biol, 11(2-3), 377–394. 10.1089/1066527041410418 [DOI] [PubMed] [Google Scholar]
- Zadoo S, Nguyen A, Zode G, & Hulleman JD (2016). A Novel Luciferase Assay For Sensitively Monitoring Myocilin Variants in Cell Culture. Invest Ophthalmol Vis Sci, 57(4), 1939–1950. 10.1167/iovs.15-18789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zebardast N, Sekimitsu S, Wang J, Elze T, Gharahkhani P, Cole BS, Lin MM, Segre AV, Wiggs JL, & International Glaucoma Genetics C (2021). Characteristics of p.Gln368Ter Myocilin Variant and Influence of Polygenic Risk on Glaucoma Penetrance in the UK Biobank. Ophthalmology, 128(9), 1300–1311. 10.1016/j.ophtha.2021.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z, & Vollrath D (1999). A cellular assay distinguishes normal and mutant TIGR/myocilin protein. Hum Mol Genet, 8(12), 2221–2228. 10.1093/hmg/8.12.2221 [DOI] [PubMed] [Google Scholar]
- Zode GS, Kuehn MH, Nishimura DY, Searby CC, Mohan K, Grozdanic SD, Bugge K, Anderson MG, Clark AF, Stone EM, & Sheffield VC (2011). Reduction of ER stress via a chemical chaperone prevents disease phenotypes in a mouse model of primary open angle glaucoma. J Clin Invest, 121(9), 3542–3553. 10.1172/jci58183 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are openly available in ClinVar at https://www.ncbi.nlm.nih.gov/clinvar/, accession numbers for each variant can be found in Supp Table S4.