Abstract
Background:
Multiple studies have reported a high burden of hypertension in sub-Saharan Africa (SSA), but none have examined early-stage hypertension. We examined contemporary prevalence of diagnosed, treated, and controlled stage I (130–139/80–89 mmHg) and II (≥140/90 mmHg) hypertension in the general population of SSA.
Methods:
We analyzed World Health Organization STEPwise Approach to Non-Communicable Disease Risk Factor Surveillance surveys from 17 SSA countries including 85,371 respondents representing 85 million individuals from 2010–2017. We extracted demographic variables, blood pressure, self-reported hypertension diagnosis/awareness, and treatment to estimate prevalence of stage I and II hypertension and treatment by country. We examined diagnosis and treatment trends by national sociodemographic index (SDI), a marker of development.
Results:
Stage I hypertension prevalence (regardless of diagnosis/treatment) was ≥25% in 13 of 17 countries, highest in Sudan (35.3% [95% CI 33.7 – 37.0]), and lowest in Eritrea (20.2% [18.8–21.6]). Combined stages I and II hypertension prevalence was >50% in 13 countries; <20% were diagnosed in every country. Treatment among those diagnosed ranged from 26–63%, and control (<140/90 mmHg) from 4–17%. In 8 of 9 countries reporting on behavioral interventions (e.g., salt reduction, weight loss, exercise, smoking cessation), <60% of diagnosed individuals received counseling. Rates of diagnosis, but not treatment, were positively associated with SDI (p = 0.008), though there was substantial variation between countries even at similar levels of development.
Conclusion:
Hypertension is common in SSA, but rates of diagnosis, treatment, and control markedly low. There is a large population with early-stage hypertension that may benefit from behavioral counseling to prevent progression. Our analyses suggest that success in population hypertension care may be achieved independently of socioeconomic development, highlighting a need for policymakers to identify best practices in those countries that outperform similar or more developed countries.
Keywords: Blood pressure, prevalence, hypertension, noncommunicable diseases, developing countries, sub-Saharan Africa, risk factors
Introduction
Hypertension, a major risk factor for cardiovascular disease (CVD), is increasingly prevalent globally, particularly in Sub-Saharan Africa (1). However, contemporary prevalence of early-stage hypertension has not previously been described in Sub-Saharan African countries. This is especially significant given the recommendations for preventative behavioral modifications in people living with stage I hypertension (blood pressure [BP] 130–139/80–89 mmHg), and the role of cost-effective pharmacotherapies for management of stage II hypertension (BP ≥140/90 mmHg) (2). Hypertension interventions in resource-limited settings must account for lack of access to laboratory monitoring that is requisite with certain antihypertensive agents; thus, there is a role for lifestyle-based counseling among those with early-stage hypertension to safely prevent progression.
To date, studies of hypertension in Sub-Saharan Africa have focused on a diagnostic and treatment threshold of ≥140/90 mmHg, despite the documented risk of downstream CVD at levels even lower than stage I (3, 4). Indeed, clinical trials and meta-analyses conducted in mostly high-income countries have demonstrated a significant reduction in cardiovascular events and all-cause mortality among those who were treated to a lower blood pressure target (5, 6). Although pharmacotherapy is not necessarily indicated for stage I hypertension, this represents a population that would benefit from behavioral and lifestyle counseling interventions, which could be delivered at relatively low cost in resource-limited settings. A health systems approach to designing effective population-based hypertension interventions requires knowledge of the burden at various diagnostic thresholds, treatment coverage, and control.
In this study, we examine contemporary survey data collected via the standardized WHO STEPwise Approach to Noncommunicable Disease (NCD) Risk Factor Surveillance (STEPS) survey instrument in multiple countries across Sub-Saharan Africa over the last decade. We apply guideline-directed diagnostic thresholds to describe the burden and severity of hypertension, its treatment, and intensity of treatment across these countries, and compare between-country performance in these domains based upon socioeconomic development indices.
Methods
Study design, data sources, and population
Because of the sensitive nature of the survey data used in this study, requests to access the dataset from qualified researchers trained in human subject confidentiality protocols may be sent to The World Health Organization (WHO)’s STEPwise Approach to Noncommunicable Disease (NCD) Risk Factor Surveillance (STEPS) Microdata Repository (7). The STEPS survey instrument is made freely available by the WHO, but is implemented voluntarily by individual countries and ministries of health for their health surveillance systems. It applies a weighted household survey sampling method to ensure population-representative results and employs a standardized instrument that includes a uniform protocol for measuring blood pressure, among other anthropometric and questionnaire modules. All WHO STEPS surveys are expected to adhere to this standard protocol, as was true for all countries in our sample, unless otherwise stated in the accompanying individual survey documentation (7). Available surveys performed in 2010 or later in sub-Saharan Africa, and data for which could be accessed through the WHO NCD Microdata Repository, were included in our study. Our final survey sample included 85,371 respondents in 17 Sub-Saharan African countries from 2010 to 2017 representing a population of 85 million individuals (characteristics in Table S1, Supplement). Our sample included 9 low-income (Central African Republic, Eritrea, The Gambia, Liberia, Malawi, Rwanda, Sudan, Togo, Uganda), 6 lower-middle-income (Benin, Comoros, Kenya, Lesotho, Eswatini, Tanzania, Zambia), and one upper-middle-income country (Botswana) based on the World Bank classification (8–23). To increase our sample size and the number of represented countries from different African regions, we also included three subnational samples that met our inclusion criteria (Bangui and Ombella M’Poko, Central African Republic; Zanzibar, Tanzania) (24–26). A geographic map of all countries in our sample can be found in Figure S1 (Supplement). We followed the Guidelines for Accurate and Transparent Health Estimates Reporting (GATHER). The University of Washington Institutional Review Board approved the study.
Data extraction and harmonization
Analyses were carried out in RStudio version 1.4.1103 (R Foundation). We extracted variables of interest including age, sex, reported history of hypertension and/or hypertension pharmacotherapy and lifestyle counseling, blood pressure measurements, survey weights and sampling information provided for each country (primary sampling unit, stratum, cluster). Hypertension diagnosis/awareness was determined by whether individuals reported a prior diagnosis, receiving lifestyle advice for blood pressure, taking antihypertensive medications, seeing a traditional healer, or taking traditional remedies for hypertension; we use the terms “diagnosis” and “awareness” interchangeably throughout this paper. Only individuals who reported taking hypertension pharmacotherapy were designated as being treated. For each individual, we calculated systolic (SBP) and diastolic blood pressure (DBP) as the mean of the latter 2 of 3 measurements. Individuals were classified as controlled on treatment if they reported being on pharmacotherapy and had a measured blood pressure lower than one of two chosen thresholds.
Definitions
We defined stage I and II hypertension in accordance with the 2017 ACC/AHA Hypertension Guidelines (2). SBP 130–139 and/or DBP 80–89 mmHg was classified as stage I, while SBP ≥140 or DBP ≥ 90 mmHg constituted stage II hypertension. Isolated diastolic hypertension was designated at two different thresholds as SBP <130 and DBP 80–89 mmHg, or SBP <140 and DBP ≥90 mmHg. We defined controlled hypertension as individuals who were on pharmacotherapy for diagnosed hypertension and had a mean blood pressure below <130/80 (tight threshold) or 130–139/80–89 mmHg (liberal threshold). To determine the extent to which countries were diagnosing and delivering antihypertensive treatment, we assigned all individuals with hypertension to one of five mutually exclusive categories: undiagnosed, diagnosed but untreated, treated but uncontrolled, treated/controlled to a liberal threshold (BP 130–139/80–89), and treated/controlled to a tight threshold (BP <130/80).
Economic and development indices
To examine relationships between population-level hypertension screening and treatment and markers of development, we used gross domestic product per capita (GDP) in base 2010 US dollars and sociodemographic index (SDI) from the Global Burden of Disease (GBD) study. SDI, developed by GBD researchers, was constructed as a composite indicator of development status, and includes total fertility rate under age 25, mean education for those 15 and older, and lag distributed income per capita. SDI, measured on a scale of 0 to 1 with higher values indicating higher development, has been shown to be correlated with positive health outcomes (27). We used values of these indices corresponding to the country-year of each respective STEPS survey.
Statistical analyses
Using the survey and srvyr packages in RStudio version 3.6.0 (R Foundation), we estimated the prevalence of undiagnosed, diagnosed/aware, treated, and controlled hypertension for each country and subnational location in our sample, applying sampling weights included with each country’s dataset. We estimated both overall and age-sex-specific prevalence, as well as 95% confidence intervals using the likelihood ratio method. Missing blood pressure measurements represented <5% of the sample in all countries and were omitted for the relevant analyses. For a subset of the countries in our sample, individuals were asked whether they had received lifestyle/behavioral counseling for hypertension (salt reduction, weight loss, exercise, smoking cessation); in these countries, we calculated the proportion of those diagnosed who had received this counseling. To examine trends in country-level prevalence across levels of development indices, we fit univariable linear regression models with diagnosed, treated, and controlled hypertension as outcomes and GDP or SDI as the independent variable and generated Wald p-values; p <0.05 was considered significant.
Results
Hypertension prevalence.
Hypertension prevalence based on measured blood pressure (regardless of diagnosis or treatment status) is summarized in Table 1. Stage I hypertension was highly prevalent in all countries in our sample, ranging from 20.2% (95% CI 18.8 – 21.6) in Eritrea to 35.3% (33.7 – 37) in Sudan. Stage II hypertension was more variable, with the lowest prevalence again in Eritrea at 13.5% (12.3 – 14.8) and highest in the Central African Republic’s Bangui subnational sample at 33% (30.2 – 35.8). The prevalence of isolated diastolic hypertension at a lower threshold (DBP 80–89 mmHg) was extremely variable, ranging from 7% (5.7 – 8.3) in Zanzibar (Tanzania subnational sample) to 22.9% (21.5 – 24.2) in Sudan. By contrast, the prevalence of isolated diastolic hypertension at a higher threshold (DBP ≥90 mmHg) was over 10% for only three countries (Central African Republic – Bangui, Lesotho, Sudan) and was as low as 2.5% (2 – 3.1) in Eritrea.
Table 1.
Population-level hypertension prevalence, regardless of diagnosis/treatment status, at various thresholds in 17 Sub-Saharan African countries, including three subnational locations, from 2010 – 2017.
| Country | Mean age of survey respondents Years (95% CI) |
Prevalence % (95% CI) |
|||
|---|---|---|---|---|---|
| Stage I hypertension (SBP 130–139 and/or DBP 80–89 mmHg) |
Stage II hypertension (SBP ≥140 or DBP ≥90 mmHg) |
Isolated diastolic hypertension (SBP <130, DBP 80–89 mmHg) |
Isolated diastolic hypertension (SBP <140, DBP ≥90 mmHg) |
||
| Eritrea | 44.3 (43.9–44.6) | 20.2 (18.8–21.6) | 13.5 (12.3–14.8) | 12.1 (10.8–13.4) | 2.5 (2–3.1) |
| Tanzania – Zanzibar | 40.7 (40.3–41.1) | 21.7 (19.2–24.2) | 30.4 (26.6–34.2) | 7 (5.7–8.3) | 2.4 (1.1–3.8) |
| Malawi | 38.1 (37.6–38.5) | 23.1 (19.1–27) | 15 (12.4–17.6) | 11 (9.4–12.6) | 4 (2.9–5.1) |
| Togo | 34 (33.6–34.4) | 23.1 (21.3–24.9) | 18.5 (16.7–20.3) | 12.1 (10.8–13.3) | 5.2 (4.3–6) |
| Liberia | 38.4 (37.9–38.8) | 24.3 (22.5–26) | 29.9 (26.9–32.9) | 13.8 (11.9–15.7) | 6 (4.3–7.7) |
| Central African Republic – Bangui | 40.3 (40–40.7) | 25 (22.9–27.1) | 33 (30.2–35.8) | 14.9 (13.2–16.6) | 10.9 (9.1–12.8) |
| Comoros | 40.1 (39.8–40.4) | 25.4 (23.9–27) | 23.9 (22.2–25.7) | 11 (10–12.1) | 3.8 (3.1–4.4) |
| Zambia | 36.6 (36.2–37) | 26 (24.2–27.8) | 17.7 (16.2–19.2) | 11.5 (10.3–12.8) | 3.8 (3.1–4.6) |
| Central African Republic – Bangui and Ombella M’Poko | N/A* | 26.3 (24.5–28.1) | 27.4 (22.4–32.4) | 17.3 (15.2–19.4) | 7.3 (6.5–8.1) |
| Botswana | 36.4 (36–36.9) | 27 (24.7–29.3) | 26.5 (24.3–28.6) | 15.4 (13.6–17.2) | 6.6 (5.3–7.9) |
| Eswatini | 36.3 (35.8–36.8) | 28.2 (26.3–30.2) | 22.5 (20.4–24.5) | 18.4 (16.7–20) | 7.6 (6.4–8.7) |
| Tanzania | 41.4 (41.1–41.7) | 28.8 (26.9–30.7) | 25.1 (22.8–27.5) | 14.7 (13.5–15.9) | 4.9 (4.1–5.7) |
| Lesotho | 42.9 (42.4–43.4) | 28.9 (26–31.9) | 29.6 (26.6–32.5) | 20.8 (18.2–23.4) | 10.2 (8.2–12.2) |
| Rwanda | 35.3 (35–35.6) | 29.2 (27.7–30.6) | 15.4 (14.1–16.6) | 21.3 (20–22.6) | 5.9 (5.2–6.7) |
| Benin | 37.3 (37–37.7) | 30 (28.2–31.8) | 28.3 (26.2–30.4) | 21.7 (20–23.4) | 9.1 (7.9–10.3) |
| Uganda | 35.4 (35–35.8) | 30.4 (28.3–32.5) | 23.8 (21.7–25.8) | 21.3 (19.5–23.1) | 8.4 (7.3–9.6) |
| Kenya | 37.5 (37.1–37.9) | 32.9 (30.2–35.6) | 22.9 (20.6–25.3) | 22.1 (19.9–24.3) | 8.7 (7.4–9.9) |
| The Gambia | 37.8 (37.5–38.2) | 33.7 (30.7–36.7) | 25.7 (23.2–28.3) | 18.8 (15.7–21.9) | 3.5 (2.7–4.3) |
| Sudan | 38 (37.7–38.3) | 35.3 (33.7–37) | 30 (28.3–31.6) | 22.9 (21.5–24.2) | 11 (9.9–12) |
Central African Republic – Bangui and Ombella M’Poko only reported aggregate age groups (not individual ages) in their survey.
Hypertension diagnosis, treatment, and control.
Figure 1 summarizes population-level prevalence of undiagnosed (stage I or higher), diagnosed/aware (based on self-report), treated and controlled hypertension. In every country in our sample, less than 40% of hypertension was diagnosed, and overall, one half or less of diagnosed hypertension was treated. The prevalence of controlled hypertension was even lower; less than 10% of diagnosed hypertension was treated to BP <140/90 in every country. However, among those with diagnosed hypertension on treatment, a higher proportion of individuals had their BP controlled to a tighter threshold (BP <130/80) than to a liberal threshold (BP 130–139/80–89) in nearly every country. Sudan had the highest prevalence of undiagnosed stage I or higher hypertension (58.5% [56.7 – 60.3]), while Eritrea had the lowest (28.7% [26.8 – 30.7]). Values can be found in Table S2 (Supplement).
Figure 1.
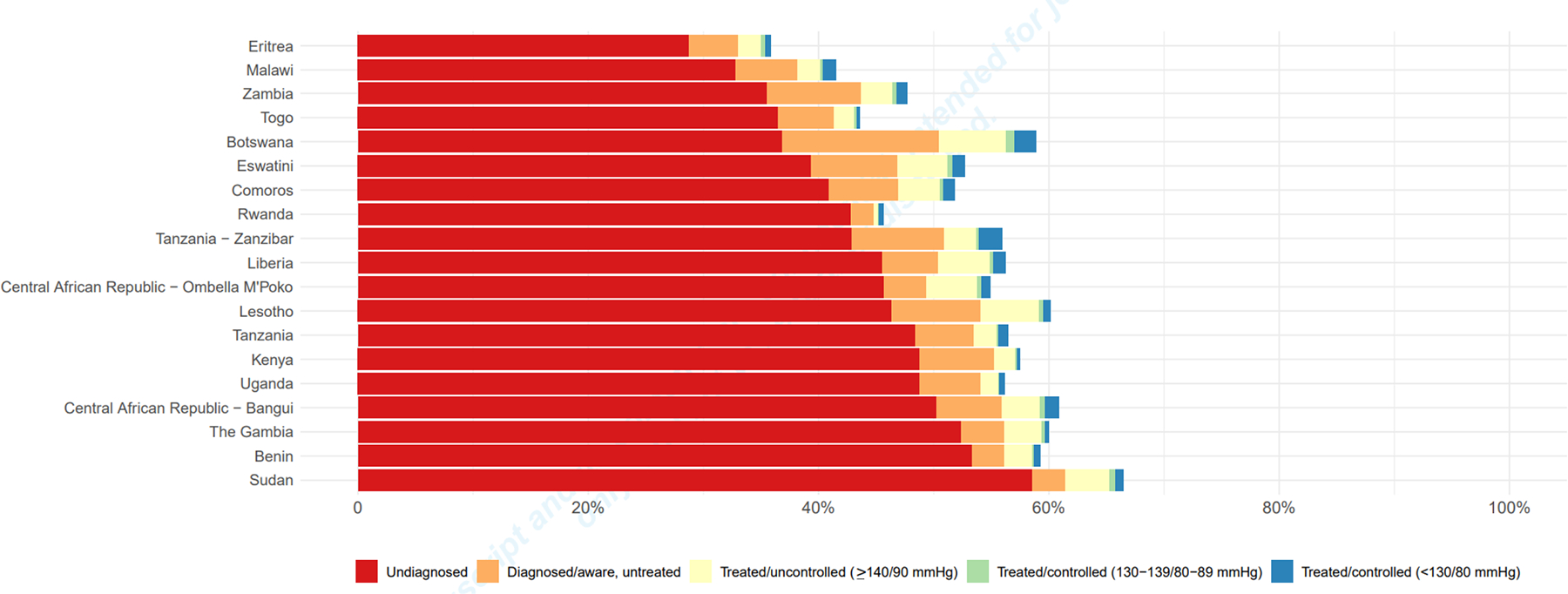
Population-level prevalence of hypertension in 17 Sub-Saharan African countries, including three subnational samples, from 2010–2017. Length of entire bar corresponds to overall prevalence of diagnosed and undiagnosed hypertension; countries are ordered by prevalence of undiagnosed hypertension.
Age- and sex-specific patterns.
In every country, overall hypertension prevalence was higher at older ages, as was diagnosis/awareness in most countries (Figure 2). However, overall hypertension (both diagnosed based on self-report and undiagnosed stage I or II based on measured blood pressure) was more prevalent among younger men compared with younger women in several countries; prevalence was similar by sex among older age groups. In many countries, including Eswatini, Zambia, Botswana, Tanzania, Kenya, and the Central African Republic, hypertension diagnosis/awareness was higher among females compared with males across all age groups.
Figure 2.
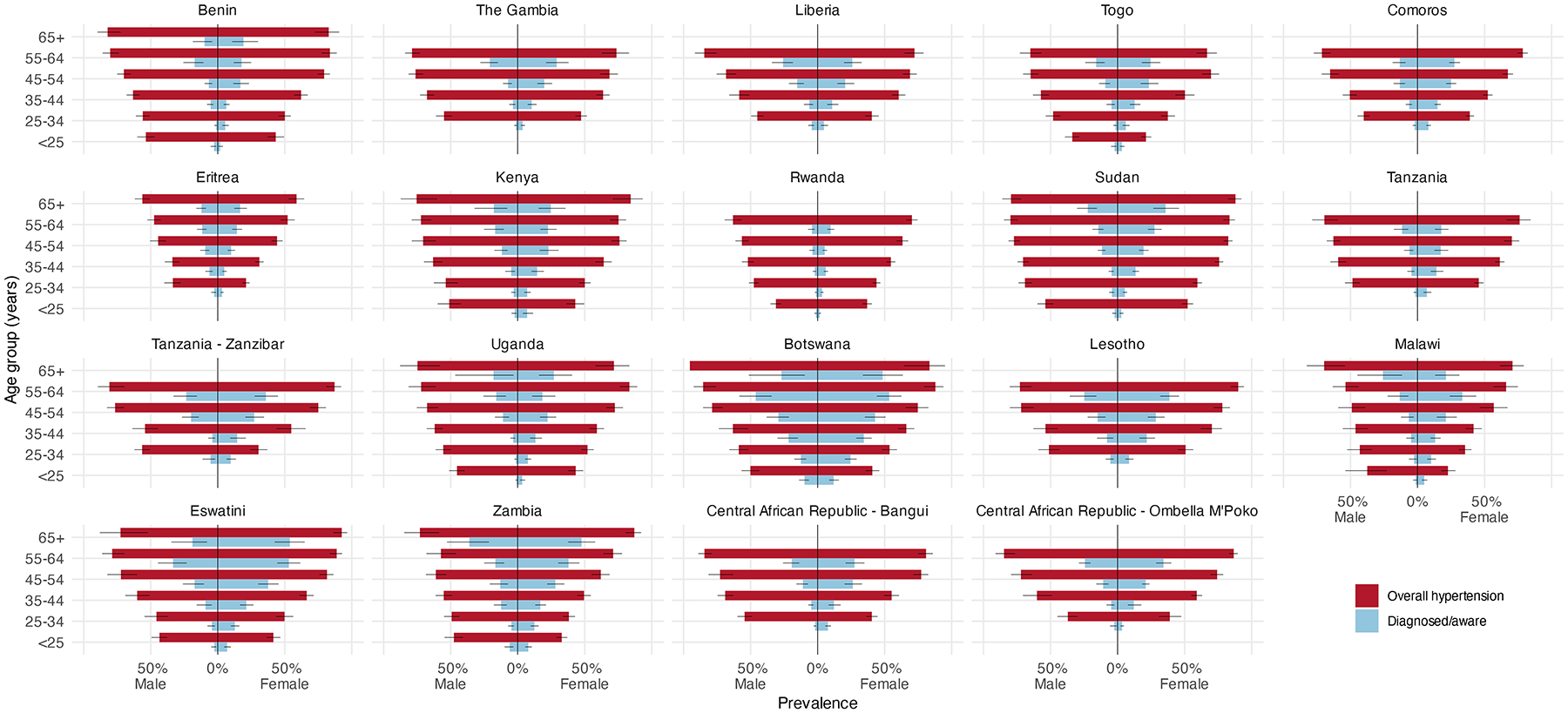
Age- and sex-specific population prevalence of overall hypertension (both diagnosed based on self-report and undiagnosed stage I or II based on measured blood pressure) and diagnosed hypertension in 17 Sub-Saharan African countries, including three subnational samples, from 2010–2017. Countries are arranged by geographic region in Africa (i.e., West Africa, East Africa, Southern Africa, and Central Africa). Horizontal lines represent 95% confidence intervals.
Behavioral/lifestyle intervention counseling.
Table 2 summarizes the prevalence of hypertension diagnosis/awareness, as well as the proportion of those aware who reported receiving lifestyle counseling in a subset of countries that reported this data. Among the behavioral interventions, salt reduction was advised to over half of those with diagnosed hypertension in all but four countries. In most countries, both weight loss and exercise were advised to over 20% of those with hypertension, while the proportion of those advised to quit smoking was comparatively low. The subnational sample in Central African Republic – Ombella M’Poko had the highest rates of lifestyle and behavioral counseling, with 78.2% (73.5 – 82.9) advised on salt reduction, 58.1% (44.1 – 72.1) on weight loss, 55.5% (45.3 – 65.7) on exercise, and 47.5% (37.1 – 57.9) on smoking cessation. Of note, these estimates were markedly higher than the other subnational sample from the Central African Republic (Bangui), despite both locations having comparable prevalence of the other hypertension outcomes.
Table 2.
Behavioral interventions advised among those with diagnosed hypertension (regardless of treatment status) in 9 countries in which this data was collected.
| Country | Prevalence of diagnosed hypertension % (95% CI) |
Proportion of those diagnosed/aware advised on behavioral interventions % (95% CI) |
|||
|---|---|---|---|---|---|
| Salt reduction | Weight loss | Exercise | Smoking cessation | ||
| Lesotho | 13.9 (11.9–15.8) | 50.1 (42.7–57.5) | 27 (21–33.1) | 37.4 (30.1–44.6) | 24 (17.7–30.2) |
| Tanzania - Zanzibar | 13.2 (10.2–16.1) | 52.7 (45.1–60.3) | 23.8 (17.5–30) | 33.1 (26.6–39.5) | 11.8 (7–16.5) |
| Comoros | 11 (9.9–12.1) | 55.8 (51.4–60.2) | 23.5 (19.6–27.4) | 24.5 (20.4–28.6) | 14.6 (11.2–18.1) |
| Liberia | 11 (8.8–13.2) | 53.5 (46.7–60.2) | 27 (21.9–32.1) | 28.4 (21.8–35) | 17.6 (12.7–22.5) |
| Central African Republic - Bangui | 10.7 (8.9–12.5) | 38.3 (31.5–45.2) | 24 (18.4–29.7) | 15.7 (10.5–20.9) | 11.8 (7.4–16.3) |
| Central African Republic - Ombella M’Poko | 9.4 (7.1–11.6) | 78.2 (73.5–82.9) | 58.1 (44.1–72.1) | 55.5 (45.3–65.7) | 47.5 (37.1–57.9) |
| Tanzania | 8.1 (6.7–9.5) | 38.6 (31.6–45.6) | 20.3 (14.4–26.2) | 25.8 (20–31.6) | 15.4 (10.2–20.6) |
| The Gambia | 7.7 (6.4–8.9) | 58.6 (50.5–66.6) | 26 (19.1–33) | 43.1 (34.7–51.6) | 9.1 (4.8–13.5) |
| Eritrea | 7.2 (6.2–8.2) | 70.1 (65.2–75) | 38.1 (31.9–44.3) | 55.2 (48.8–61.6) | 9.2 (4.9–13.4) |
| Togo | 7.2 (6.1–8.3) | 34.2 (27–41.4) | 17.4 (12–22.8) | 14.7 (10.6–18.9) | 4.7 (2.1–7.2) |
| Rwanda | 2.8 (2.4–3.2) | 49.8 (42.3–57.3) | 21 (15.4–26.6) | 20.5 (14.7–26.3) | 9.2 (4.9–13.4) |
Trends by developmental indices.
Figure 3 summarizes proportion of diagnosed, treated, and controlled hypertension by SDI. In our analysis, increasing SDI was associated only with higher levels of hypertension diagnosis/awareness (p = 0.008), but not with the other hypertension outcomes (treatment, control). Among outlier countries, we noted that Rwanda was underperforming in hypertension diagnosis compared to developmental peers but was markedly overperforming in terms of control on treatment. Botswana, which had the highest SDI, had the highest levels of hypertension diagnosis, but performed similarly to the other countries in treatment and control. We also examined trends by GDP per capita, but the interpretation of these results was limited by the relative income homogeneity of our sample; the trends appeared to be primarily driven by Eswatini and Botswana, both of which had much higher GDP than the remaining countries (Figure S2).
Figure 3.
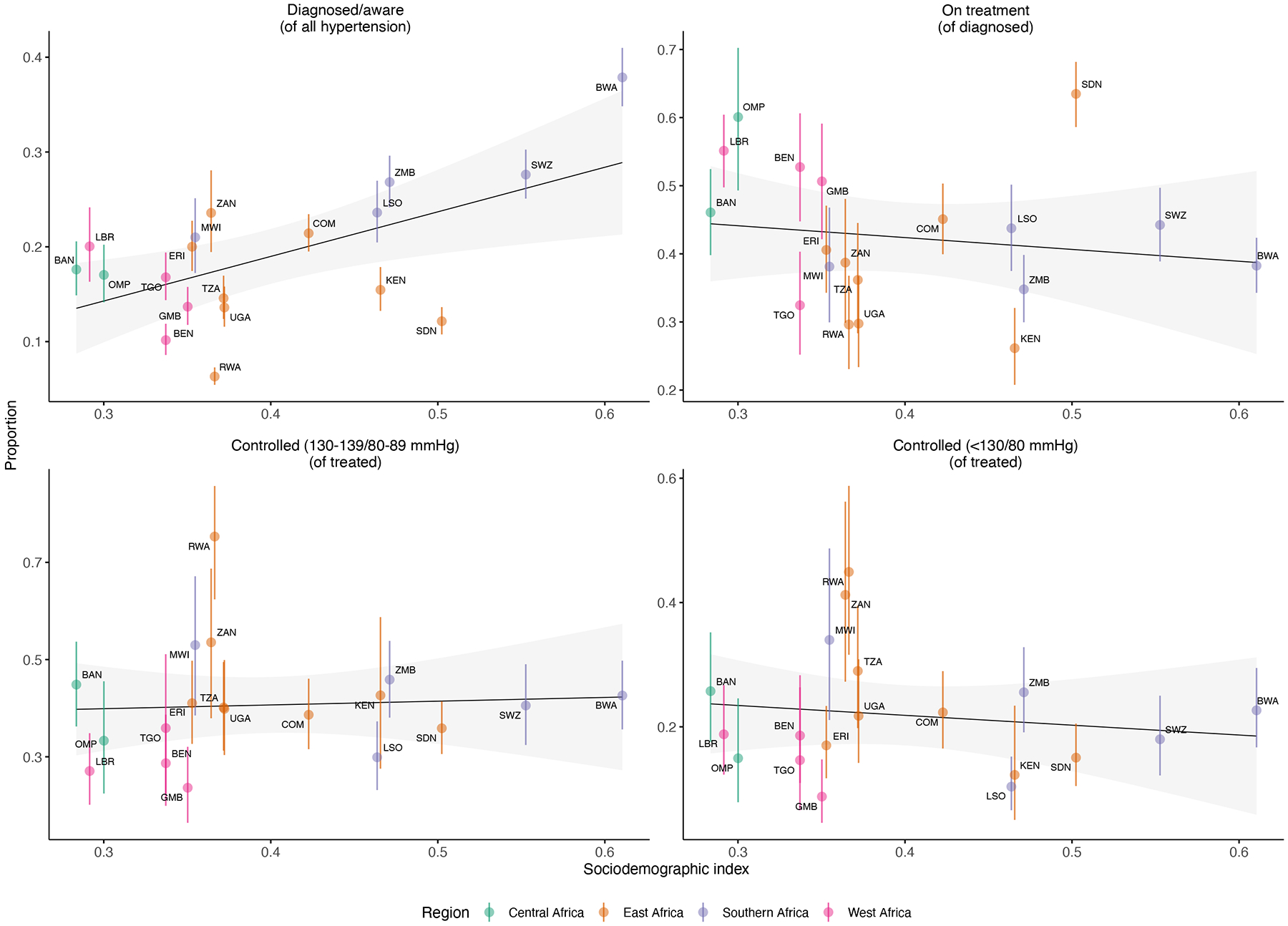
Proportion of diagnosed (stage I or higher), treated, and treated and controlled hypertension vs. sociodemographic index (SDI) in the country-year of survey collection. The gray shaded region indicates the 95% confidence interval (CI) for the linear regression, while vertical bars represent 95% CI for point estimates. A significant relationship was noted only between diagnosis and SDI (p = 0.008). Countries/subnationals are labeled with three-letter codes: BEN = Benin, ERI = Eritrea, MWI = Malawi, TGO = Togo, LBR = Liberia, BAN = Central African Republic (Bangui), OMP = Central African Republic (Bangui & Ombella M’Poko), COM = Comoros, ZMB = Zambia, BWA = Botswana, SWZ = Eswatini, TZA = Tanzania, ZAN = Tanzania (Zanzibar), LSO = Lesotho, RWA = Rwanda, UGA = Uganda, KEN = Kenya, GMB = The Gambia, SDN = Sudan.
Discussion
In our study, we noted that high levels of systolic and diastolic blood pressure are common across Sub-Saharan Africa, but diagnosis and treatment rates remain relatively low, even among countries at higher levels of socioeconomic development. Additionally, our analysis of elements in the population-level hypertension care cascade (diagnosis, treatment, control) demonstrated that higher national development is associated with high levels of diagnosis, but not treatment. This contrasts with other studies that include middle-income countries, which demonstrate greater success in all steps of hypertension care in locations with higher GDP per capita (28). However, such trends in the latter stages of the hypertension care cascade may have been difficult to distinguish in our sample given the relative income homogeneity (Figure S2). More broadly, that development did not predict success in all stages of the hypertension care cascade merits careful consideration. The unique pattern of outliers in our study – that is, countries that underperformed in diagnosis but excelled at control, and vice versa – suggest that existing measures of development may not capture different effects on individual components of the hypertension care cascade. Rwanda, for example, despite having among the lowest SDI, underperformed compared to peer countries in hypertension diagnosis, but markedly overperformed in control. This suggests that population-based screening interventions are failing to identify hypertensive individuals, but that those who are diagnosed are retained in follow-up care. A 2017 study of 6 sites in 4 African countries that did not include Rwanda also noted significant variation in awareness and control of hypertension across sites, especially in South Africa, where some sites were more likely to have higher levels of control, suggesting better retention in care in those settings (29). Rwanda’s health system has a community-based insurance program that subsidizes medication costs for patients, and also allocates newly graduated physicians to rural district hospitals that may offset rural-urban disparities in care (30). These features of the Rwandan health system may explain the relative overperformance in hypertension control compared to peer countries. As another example, the Central African Republic (CAF) subnational locations had among the lowest levels of development. However, CAF – Ombella M’Poko outperformed Botswana, which had the highest SDI, in treatment, and performed similarly in terms of control. Ombella M’Poko also had the highest levels of lifestyle counseling delivered, a low-cost, effective intervention when delivered widely among those diagnosed, which may partially explain the ability to “catch up” to countries at higher levels of development. Although these findings must be interpreted with caution given the potential for systematic bias in survey implementation across different locations, they may suggest that success in the latter stages of the hypertension care cascade can be achieved independently of socioeconomic development or robust health infrastructure. Put differently, as Sub-Saharan Africa develops, there is a possibility that success in comprehensive hypertension management may lag in many countries. This highlights a need for new research to identify, using both quantitative and qualitative methods, those best practices in lower-development countries that allow them to outperform countries with similar or even higher development.
Multiple studies of individual countries and meta-analyses in Sub-Saharan Africa have estimated a high prevalence of stage II hypertension and low levels of treatment and control (31–36). However, ours is the first analysis in the region that also demonstrates prevalence of stage I hypertension in many countries that equals or exceeds that of stage II hypertension, the latter of which is currently the threshold for pharmacotherapy recommended by the International Society of Hypertension 2020 Global Guidelines (37). While most of the surveys in our sample were conducted before widespread uptake of the lower-threshold definition of stage I hypertension, and therefore those who had stage I hypertension may not have been diagnosed based on country guidelines, we newly describe the high burden of hypertension in Africa at lower thresholds that have been correlated with poor cardiovascular outcomes (3, 4). This represents a population of individuals who should receive targeted screening and counseling interventions to prevent progression to stage II hypertension, and some of whom may be at enough cardiovascular risk to warrant adjunctive pharmacotherapy based on the 2017 ACC/AHA guidelines (2). This is particularly relevant in Sub-Saharan Africa, as studies across racial and ethnic groups have found that hypertension among individuals of African descent can be associated with more florid complications and may be more difficult to treat (38). The cornerstone of management for stage I hypertension is behavioral and lifestyle modification to prevent progression (2). Indeed, in a prospective study of normotensive (BP ≤120/70 mmHg) individuals in South Africa, nearly a quarter developed stage II hypertension, and behavioral and lifestyle risks predicted progression (39). In Sudan, which had the highest prevalence of overall and isolated diastolic hypertension in our study, a separate analysis of this same STEPS survey noted that sugar consumption was a key risk factor for hypertension in Sudanese individuals (40). Sugar intake has been associated with significant increases of >5 mmHg in both systolic and diastolic blood pressure (41). Sudan is home to the Kenana Sugar Company, the world’s largest producer of white sugar (42); in addition, the Food and Agriculture Organization of the United Nations estimated that sugar was the second highest source of dietary energy consumption in the country in 2009 (43). While the high prevalence of hypertension in Sudan is likely multifactorial, the literature suggests that dietary and behavioral risks may play a key role. Despite this, in nearly all countries in our study, one half or less of those with diagnosed hypertension reported having received lifestyle counseling. Smoking cessation was the least-delivered counseling intervention, though this may potentially be due to relatively low smoking prevalence in those populations. Given that intensive pharmacologic treatment leads to more complications, in settings with limited access to monitoring (e.g., renal function, potassium), behavioral counseling implemented at earlier diagnostic thresholds may prove a safe adjunct to prevent hypertension progression (44).
In contrast to our findings in Sudan, Eritrea was the country in our sample with the lowest overall prevalence of hypertension and lowest population mean values for systolic and diastolic blood pressure. As few large comparative studies have examined the relatively better performance of Eritrea compared with other African countries, few objective data are available on potential drivers of these differences. However, a qualitative study in Eritrea identified individual knowledge about hypertension, family support, dedication of healthcare professionals, and free supply of antihypertensive medication through initiatives by the Eritrean Ministry of Health as facilitators of successful personal hypertension management (45). While Eritrea’s comparatively low population-level blood pressure may reflect both biological factors and health system measures, further mixed-methods studies are needed to identify opportunities for other countries to adapt successful management models.
The high prevalence of undiagnosed stage I and II hypertension indicates a critical need to integrate screening strategies into the current non-communicable disease (NCD) service framework; unfortunately, a 2020 analysis of 47 countries in the WHO African Region indicate that many fall short of the recommended targets for incorporating NCD management into existing primary health care systems (46). This highlights a need to transition from vertical disease- or condition-oriented health delivery programs (e.g., aimed at HIV or prenatal care) to horizontal programs that holistically address individual and population health needs by leveraging existing infrastructure (47). Specifically, examples of integrated care for the HIV population that also address cardiovascular and metabolic risk factors could be adapted for the general population (48), while other populations that are more likely to seek regular care, such as pregnant women, could be screened for hypertension during their routine visits . The latter is particularly relevant, as a recent meta-analysis suggests that hypertensive disorders of pregnancy, which are associated with significant peripartum and long-term complications, are present in as much as 10% of women in Africa (49).
In line with prior studies, we also observed that hypertension was markedly underdiagnosed in younger individuals, and particularly among young men, in many Sub-Saharan African countries, but that older individuals and women were more likely to be diagnosed/aware (50). This likely reflects demographic differences in health care utilization in many low- and middle-income countries. In a South African study, younger individuals were less likely to seek routine preventive care, and females were significantly more likely to seek health care compared with males; younger females are also more likely to interface with the health care system via antenatal care, during which most women undergo blood pressure screening (51). Although diagnosed and undiagnosed stage I or II hypertension was more prevalent among older age groups in our study, prevalence among the youngest still exceeded 25% for nearly every country in our sample for both sexes. This emphasizes the need to also develop population-based screening protocols tailored toward younger age groups.
The prevalence of isolated diastolic hypertension has not been previously described in Sub-Saharan Africa. Although we noted a relatively high prevalence of mild isolated diastolic hypertension (80–89 mmHg) in some countries, a 2017 study in the US did not find this degree of diastolic hypertension to be associated with an increased risk of cardiovascular or kidney disease (52). However, a much longer longitudinal study of younger individuals noted that even mild isolated diastolic hypertension (80–89 mmHg) was associated with a significantly increased risk of incident cardiovascular events (53). Isolated diastolic hypertension at the higher threshold (≥90 mmHg), which has been associated with incident CVD events, had a relatively low prevalence across most countries in our study. Further prospective studies are needed to understand the implications of isolated diastolic hypertension at various thresholds and for different settings.
Strengths and limitations.
This represents a novel up-to-date multi-country analysis of early-stage hypertension from Sub-Saharan Africa, incorporating different diagnostic thresholds from both the ACC/AHA and the World Hypertension Guidelines (2, 37). Our results are in line with previously published estimates of stage II hypertension prevalence in the region, and we newly report on stage I hypertension (31–36). The household survey methodology and the sample weights applied to estimate prevalence allow for between-country comparisons and mitigate selection bias in populations that could account for trends in the age- and sex-specific analyses.
Despite the standardized survey instrument and collection of biometric data, there may have been differences in how questions were phrased or translated into local languages and, in particular, how blood pressure was measured, including variation in equipment across locations. Although the standard STEPS methodology advises measuring blood pressure after the respondent has sat quietly for 15 minutes with legs uncrossed and an empty bladder, consistently implementing all these steps is often challenging even in highly resourced settings. Fluctuations in blood pressure due to such non-sampling error may have led to artificially elevated measurements, and thus overestimated the burden of undiagnosed hypertension; if these effects were not uniform across locations, they could also account for the variation in our estimates between countries.
Additionally, surveys may have been systematically biased in some locations due to variation in the demographic composition of certain samples with respect to variables not used to explicitly define the sampling strata. The WHO STEPS instruments generally adjust for age and sex in both population and nonresponse weights, but sampling bias could be introduced due to nonrepresentative levels of income, health care access (e.g., urban vs. rural location), or education, all of which may be associated with hypertension outcomes. These issues raise important questions about appraising the validity and improving the robustness of population-based surveillance instruments. Nevertheless, NCD survey data and cross-country comparisons remain an important tool for health policymakers in data-sparse, resource-limited settings.
In addition to potential sources of bias in the survey instrument, our analysis does not account for the year in which the surveys were conducted, which could introduce an additional source of variation in results across countries. Of note, some countries have adopted national hypertension guidelines since the year the last survey was conducted (e.g., Zambia, which established their first national hypertension treatment guideline in 2019;) (54, 55). Given that we did not have access to repeated surveys in the same country in this sample, however, we are not able to definitively identify what effect this may have had on the results. Future work should focus on comparing within-country changes in hypertension care over time across sub-Saharan Africa.
Conclusions
In this household survey analysis of 17 African countries, we found that early-stage hypertension is common, with overall low rates of diagnosis and treatment on a population level regardless of the socioeconomic development of the countries in our sample. In much of Sub-Saharan Africa, where hypertension is highly prevalent but access to treatment and monitoring is limited, behavioral counseling interventions for the large population with early-stage hypertension may prove cost-effective, safe, and scalable strategies to prevent progression. Despite hypertension diagnosis improving with level of national development on average, we observed higher rates of diagnosis and treatment in some very low resource settings, suggesting opportunities to identify best practices from these higher-performing regions. Although health system infrastructure is limited in many Sub-Saharan African countries, our analysis suggests success in the population-level hypertension care cascade may be achieved regardless of socioeconomic development. Such analyses could provide important guidance for policymakers in resource-constrained settings who are allocating limited funds toward reducing hypertension-related cardiovascular disease in their countries.
Supplementary Material
What is known.
Sub-Saharan Africa faces a high burden of hypertension, a primary risk factor for cardiovascular disease.
Early-stage hypertension is known to be associated with poor cardiovascular outcomes; however, most guidelines in low- and middle-income countries (LMICs) do not designate this as a formal diagnosis of hypertension, despite evidence that behavioral/lifestyle modifications at this stage can reduce progression.
What this study adds.
In much of Sub-Saharan Africa, where hypertension is highly prevalent but access to treatment and monitoring is limited, behavioral counseling interventions for the large population with early-stage hypertension may prove cost-effective, safe, and scalable strategies to prevent progression.
Although health system infrastructure is limited in many Sub-Saharan African countries, our analysis suggests that success in the population-level hypertension care cascade may be achieved regardless of socioeconomic development.
Such comparative analyses across LMICs could provide important guidance for policymakers in resource-constrained settings who are allocating limited funds toward reducing hypertension-related cardiovascular disease in their countries.
Acknowledgments
This paper uses data from the following STEPS surveys: Benin 2015, Central African Republic - Bangui 2010, Central African Republic - Bangui & Ombella M’Poko 2017, Comoros 2011, Eritrea 2010, The Gambia 2010, Kenya 2015, Lesotho 2012, Liberia 2012, Sudan 2016, Eswatini 2014, Tanzania 2011, Tanzania - Zanzibar 2011, Togo 2010, Uganda 2014, Zambia 2017; implemented by following agencies/countries: the Ministry of Health (Benin), Central African Republic, Comoros, Eritrea, The Gambia, Ministry of Health (Kenya), Kenya National Bureau of Statistics, Ministry of Health and Social Welfare (Lesotho), Liberia, Malawi, Ministry of Health (Rwanda), National Institute of Statistics of Rwanda, Rwanda Biomedical Center, Centers for Disease Control and Prevention, Federal Ministry of Health (Sudan), Ministry of Health (Swaziland), National Institute for Medical Research (Tanzania), Ministry of Health (Zanzibar), Ministry of Health (Togo), West African Health Organization, Ministry of Health (Uganda), and Zambia, with the support of the World Health Organization. We thank Laura Dwyer-Lindgren at the Institute for Health Metrics and Evaluation, University of Washington, Seattle, USA.
Sources of Funding
Dr Shakil is supported by a training grant (NIH T32 HL007828).
Disclosures:
CTL has received research funding from Medtronic Foundation and Gilead Sciences and has served on an advisory board for Esperion Pharmaceuticals. The other authors report no disclosures.
Non-standard abbreviations and acronyms:
- BP
Blood pressure
- CAF
Central African Republic
- CVD
Cardiovascular disease
- DBP
Diastolic blood pressure
- GBD
Global Burden of Disease
- GDP
Gross domestic product per capita
- NCD
Noncommunicable disease
- SBP
Systolic blood pressure
- SDI
Sociodemographic index
- STEPS
STEPwise Approach to Noncommunicable Disease Risk Factor Surveillance
- WHO
World Health Organization
Footnotes
References
- 1.Zhou B, Bentham J, Cesare MD, Bixby H, Danaei G, Cowan MJ, Paciorek CJ, Singh G, Hajifathalian K, Bennett JE, et al. Worldwide trends in blood pressure from 1975 to 2015: a pooled analysis of 1479 population-based measurement studies with 19·1 million participants. The Lancet. 2017; 389:37–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Whelton PK, Carey RM, Aronow WS, Casey DE, Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2018; 71:e13–e115. [DOI] [PubMed] [Google Scholar]
- 3.Guo X, Zhang X, Guo L, Li Z, Zheng L, Yu S, Yang H, Zhou X, Zhang X, Sun Z, et al. Association between pre-hypertension and cardiovascular outcomes: a systematic review and meta-analysis of prospective studies. Curr Hypertens Rep. 2013; 15:703–716. [DOI] [PubMed] [Google Scholar]
- 4.Huang Y, Wang S, Cai X, Mai W, Hu Y, Tang H, Xu D. Prehypertension and incidence of cardiovascular disease: a meta-analysis. BMC Med. 2013; 11:177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bundy JD, Li C, Stuchlik P, Bu X, Kelly TN, Mills KT, He H, Chen J, Whelton PK, He J. Systolic Blood Pressure Reduction and Risk of Cardiovascular Disease and Mortality: A Systematic Review and Network Meta-analysis. JAMA Cardiology. 2017; 2:775–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.The SPRINT Research Group. A Randomized Trial of Intensive versus Standard Blood-Pressure Control. New England Journal of Medicine. 2015; 373:2103–2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.World Health Organization. STEPwise approach to surveillance (STEPS). Geneva: World Health Organization; 2021. Available from: https://www.who.int/teams/noncommunicable-diseases/surveillance/systems-tools/steps [accessed March 1, 2021]. [Google Scholar]
- 8.World Health Organization (WHO). Eritrea STEPS Noncommunicable Disease Risk Factors Survey 2010.
- 9.World Health Organization (WHO). Gambia STEPS Noncommunicable Disease Risk Factors Survey 2010.
- 10.World Health Organization (WHO). Liberia STEPS Noncommunicable Disease Risk Factors Survey 2011.
- 11.World Health Organization (WHO). Malawi STEPS Noncommunicable Disease Risk Factors Survey 2017.
- 12.Centers for Disease Control and Prevention (CDC), Ministry of Health (Rwanda), National Institute of Statistics of Rwanda, Rwanda Biomedical Center (RBC), World Health Organization (WHO). Rwanda STEPS Noncommunicable Disease Risk Factor Survey 2012–2013.
- 13.Federal Ministry of Health (Sudan), World Health Organization (WHO). Sudan STEPS Noncommunicable Disease Risk Factors Survey 2016. Geneva, Switzerland: World Health Organization (WHO), 2019. [Google Scholar]
- 14.Ministry of Health (Togo), West African Health Organization, World Health Organization (WHO). Togo STEPS Noncommunicable Disease Risk Factors Survey 2010–2011.
- 15.Ministry of Health (Uganda), World Health Organization (WHO). Uganda STEPS Noncommunicable Disease Risk Factors Survey 2014. Geneva, Switzerland: World Health Organization (WHO). [Google Scholar]
- 16.Ministry of Health (Benin), World Health Organization (WHO). Benin STEPS Noncommunicable Disease Risk Factors Survey 2015.
- 17.World Health Organization (WHO). Comoros STEPS Noncommunicable Disease Risk Factors Survey 2011.
- 18.Kenya National Bureau of Statistics, Ministry of Health (Kenya), World Health Organization (WHO). Kenya STEPS Noncommunicable Disease Risk Factors Survey 2015.
- 19.Ministry of Health and Social Welfare (Lesotho), World Health Organization (WHO). Lesotho STEPS Noncommunicable Disease Risk Factors Survey 2012.
- 20.Ministry of Health (Swaziland), World Health Organization (WHO). Swaziland STEPS Noncommunicable Disease Risk Factors Survey 2014.
- 21.National Institute for Medical Research (Tanzania), World Health Organization (WHO). Tanzania STEPS Noncommunicable Disease Risk Factors Survey 2012.
- 22.World Health Organization (WHO). Zambia STEPS Noncommunicable Disease Risk Factors Survey 2017. Geneva, Switzerland: World Health Organization (WHO). [Google Scholar]
- 23.The World Bank. World Bank Country and Lending Groups. https://datahelpdesk.worldbank.org/knowledgebase/articles/906519-world-bank-country-and-lending-groups [accessed August 15, 2021].
- 24.World Health Organization (WHO). Central African Republic - Bangui STEPS Noncommunicable Disease Risk Factors Survey 2010.
- 25.World Health Organization (WHO). Central African Republic - Bangui and Ombella M’Poko STEPS Noncommunicable Disease Risk Factors Survey 2017.
- 26.Ministry of Health (Zanzibar), World Health Organization (WHO). Tanzania - Zanzibar STEPS Noncommunicable Disease Risk Factors Survey 2011.
- 27.Global Burden of Disease Collaborative Network. Global Burden of Disease Study 2019 (GBD 2019) Socio-Demographic Index (SDI) 1950–2019. Seattle, United States of America: Institute for Health Metrics and Evaluation (IHME), 2020. Available from: https://ghdx.healthdata.org/record/ihme-data/gbd-2019-socio-demographic-index-sdi-1950-2019 [accessed August 15, 2021]. [Google Scholar]
- 28.Geldsetzer P, Manne-Goehler J, Marcus ME, Ebert C, Zhumadilov Z, Wesseh CS, Tsabedze L, Supiyev A, Sturua L, Bahendeka SK, et al. The state of hypertension care in 44 low-income and middle-income countries: a cross-sectional study of nationally representative individual-level data from 1·1 million adults. The Lancet. 2019; 394:652–662. [DOI] [PubMed] [Google Scholar]
- 29.Gómez-Olivé FX, Ali SA, Made F, Kyobutungi C, Nonterah E, Micklesfield L, Alberts M, Boua R, Hazelhurst S, Debpuur C, et al. Regional and Sex Differences in the Prevalence and Awareness of Hypertension: An H3Africa AWI-Gen Study Across 6 Sites in Sub-Saharan Africa. Global Heart. 2017; 12:81–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sibomana JP, McNamara RL, Walker TD. Patient, clinician and logistic barriers to blood pressure control among adult hypertensives in rural district hospitals in Rwanda: a cross-sectional study. BMC Cardiovasc Disord. 2019; 19:231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bosu WK, Reilly ST, Aheto JMK, Zucchelli E. Hypertension in older adults in Africa: A systematic review and meta-analysis. PLOS ONE. 2019; 14:e0214934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Houle B, Gaziano T, Farrell M, Gómez-Olivé FX, Kobayashi LC, Crowther NJ, Wade AN, Montana L, Wagner RG, Berkman L, et al. Cognitive function and cardiometabolic disease risk factors in rural South Africa: baseline evidence from the HAALSI study. BMC Public Health. 2019; 19:1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Amare F, Hagos B, Sisay M, Molla B. Uncontrolled hypertension in Ethiopia: a systematic review and meta-analysis of institution-based observational studies. BMC Cardiovasc Disord. 2020; 20:129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ataklte F, Erqou S, Kaptoge S, Taye B, Echouffo-Tcheugui JB, Kengne AP. Burden of Undiagnosed Hypertension in Sub-Saharan Africa. Hypertension. 2015; 65:291–298. [DOI] [PubMed] [Google Scholar]
- 35.Duboz P, Boëtsch G, Gueye L, Macia E. Hypertension in the Ferlo (Northern Senegal): prevalence, awareness, treatment and control. Pan Afr Med J. 2016;25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kuate Defo B, Mbanya JC, Kingue S, Tardif JC, Choukem SP, Perreault S, Fournier P, Ekundayo O, Potvin L, D’Antono B, et al. Blood pressure and burden of hypertension in Cameroon, a microcosm of Africa: a systematic review and meta-analysis of population-based studies. J Hypertens. 2019; 37:2190–2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Unger T, Borghi C, Charchar F, Khan NA, Poulter NR, Prabhakaran D, Ramirez A, Schlaich M, Stergiou GS, Tomaszewski M, et al. 2020 International Society of Hypertension Global Hypertension Practice Guidelines. Hypertension. 2020; 75:1334–1357. [DOI] [PubMed] [Google Scholar]
- 38.Khan JM, Beevers DG. Management of hypertension in ethnic minorities. Heart. 2005;91:1105–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schutte AE, Schutte R, Huisman HW, van Rooyen JM, Fourie CM, Malan NT, Malan L, Mels CM, Smith W, Moss SJ, et al. Are behavioural risk factors to be blamed for the conversion from optimal blood pressure to hypertensive status in Black South Africans? A 5-year prospective study. International Journal of Epidemiology. 2012; 41:1114–1123. [DOI] [PubMed] [Google Scholar]
- 40.Awadalla H, Elmak NE, El-Sayed EF, Almobarak AO, Elmadhoun WM, Osman M, Noor SK, Ahmed MH. Hypertension in Sudanese individuals and associated risk factors: the critical intersection between salt and sugar intake. Cardiovascular Diagnosis and Therapy. 2018; 8:43238–43438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Te Morenga LA, Howatson AJ, Jones RM, Mann J. Dietary sugars and cardiometabolic risk: systematic review and meta-analyses of randomized controlled trials of the effects on blood pressure and lipids. Am J Clin Nutr. 2014; 100:65–79. [DOI] [PubMed] [Google Scholar]
- 42.Institute of Developing Economies-Japan External Trade Organization. Kenana Sugar Company. Sudan/Food, Beverages, Tobacco, IDE-JTO. Available from: https://www.ide.go.jp/English/Data/Africa_file/Company/sudan04.html [accessed May 29, 2022].
- 43.Sudan Integrated Food Security Information for Action (SIFSIA). Food and Nutrition Security Assessment in Sudan 2009. Central Bureau of Statistics, Southern Sudan Commission for Census Statistics and Evaluation. Available from: https://www.fao.org/fileadmin/user_upload/sifsia/docs/SudanFoodInsecurityAssessment_NBHS_July10.pdf [accessed May 29, 2022]. [Google Scholar]
- 44.The SPRINT Research Group. Final Report of a Trial of Intensive versus Standard Blood-Pressure Control. New England Journal of Medicine. 2021; 384:1921–1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gebrezgi MT, Trepka MJ, Kidane EA. Barriers to and facilitators of hypertension management in Asmara, Eritrea: patients’ perspectives. J Health Popul Nutr. 2017; 36:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tesema AG, Ajisegiri WS, Abimbola S, Balane C, Kengne AP, Shiferaw F, Dangou JM, Narasimhan P, Joshi R, Peiris D. How well are non-communicable disease services being integrated into primary health care in Africa: A review of progress against World Health Organization’s African regional targets. PLoS One. 2020; 15:e0240984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mercer T, Chang AC, Fischer L, Gardner A, Kerubo I, Tran DN, Laktabai J, Pastakia S. Mitigating The Burden Of Diabetes In Sub-Saharan Africa Through An Integrated Diagonal Health Systems Approach. Diabetes Metab Syndr Obes. 2019; 12:2261–2272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Birungi J, Kivuyo S, Garrib A, Mugenyi L, Mutungi G, Namakoola I, Mghamba J, Ramaiya K, Wang D, Maongezi S, et al. Integrating health services for HIV infection, diabetes and hypertension in sub-Saharan Africa: a cohort study. BMJ Open. 2021; 11:e053412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Noubiap JJ, Bigna JJ, Nyaga UF, Jingi AM, Kaze AD, Nansseu JR, Fokom Domgue J. The burden of hypertensive disorders of pregnancy in Africa: A systematic review and meta-analysis. J Clin Hypertens (Greenwich). 2019; 21:479–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kayima J, Wanyenze RK, Katamba A, Leontsini E, Nuwaha F. Hypertension awareness, treatment and control in Africa: a systematic review. BMC Cardiovasc Disord. 2013; 13:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Abera Abaerei A, Ncayiyana J, Levin J. Health-care utilization and associated factors in Gauteng province, South Africa. Glob Health Action. 2017; 10:1305765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McEvoy JW, Daya N, Rahman F, Hoogeveen RC, Blumenthal RS, Shah AM, Ballantyne CM, Coresh J, Selvin E. Association of Isolated Diastolic Hypertension as Defined by the 2017 ACC/AHA Blood Pressure Guideline With Incident Cardiovascular Outcomes. JAMA. 2020; 323:329–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lee H, Yano Y, Cho SMJ, Park JH, Park S, Lloyd-Jones DM, Kim HC. Cardiovascular Risk of Isolated Systolic or Diastolic Hypertension in Young Adults. Circulation. 2020; 141:1778–1786. [DOI] [PubMed] [Google Scholar]
- 54.Cadila Pharmaceuticals Limited. “Zambia Hypertension Treatment Guidelines ‘Global to Local’ in association with Cadila & Zambia Heart and Stroke Foundation.” Available from: https://www.cadilapharma.com/blog/innovation/zambia-hypertension-treatment-guidelines-goma/ [accessed May 29, 2022].
- 55.Ministry of Health, Zambia National Formulary Committee (2020). Standard Treatment Guidelines, Essential Medicines List, Essential Laboratory Supplies for Zambia, 5th edition. Zambia Ministry of Health, Lusaka. Available from: https://www.moh.gov.zm/?wpfb_dl=32 [accessed May 29, 2022]. [Google Scholar]
- 56.The World Bank. Population Data. World Development Indicators, The World Bank Group. Availabe from: https://data.worldbank.org/indicator/SP.POP.TOTL [accessed May 29, 2022]. [Google Scholar]
- 57.United Nations Populations Division. World Population Prospects 2019. Department of Economic and Social Affairs, United Nations. Available from: https://population.un.org/wpp/ [accessed May 29, 2022]. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


