Abstract
The streptococcal cysteine protease (SpeB) is one of the major virulence factors produced by group A streptococci (GAS). In this study we investigated if differences exist in SpeB production by clonally related M1T1 clinical isolates derived from patients with invasive infections. Twenty-nine of these isolates were from nonsevere cases and 48 were from severe cases, including streptococcal toxic shock syndrome (STSS) and necrotizing fasciitis (NF) cases. The expression and amount of the 28-kDa SpeB protein produced were determined by quantitative Western blotting, and protease activity was measured by a fluorescent enzymatic assay. A high degree of variation in SpeB expression was seen among the isolates, and this variation seemed to correlate with the severity and/or clinical manifestation of the invasive infection. The mean amount of 28-kDa SpeB protein and cysteine protease activity produced by isolates from nonsevere cases was significantly higher than that from STSS cases (P = 0.001). This difference was partly due to the fact that 41% of STSS isolates produced little or no SpeB compared to only 14% of isolates recovered in nonsevere cases. Moreover, the cysteine protease activity among those isolates that expressed SpeB was significantly lower for STSS isolates than for isolates from nonsevere cases (P = 0.001). Increased SpeB production was also inversely correlated with intact M protein expression, and inhibition of cysteine protease activity blocked the cleavage of the surface M protein. Together, the data support the existence of both an “on-off” and a posttranslational regulatory mechanism(s) controlling SpeB production, and they suggest that isolates with the speB gene in the “off” state are more likely to spare the surface M protein and to be isolated from cases of severe rather than nonsevere invasive infection. These findings may have important implications for the role of SpeB in host-pathogen interactions via regulation of the expression of GAS virulence genes and the severity of invasive disease.
Group A streptococci (GAS) cause a wide variety of human pathological conditions ranging from pharyngitis and impetigo to necrotizing fasciitis (NF) and streptococcal toxic shock syndrome (STSS) (10, 11, 41, 42). GAS produce many virulence factors, including the M protein and several extracellular streptococcal pyrogenic toxins (Spe) or superantigens, such as SpeA, SpeB, SpeC, SpeF, and SSA, which are thought to be involved in the pathogenesis of these infections (1, 15, 19, 20, 28, 30, 39). The major streptococcal cysteine protease, SpeB, is believed to play a major role in GAS pathogenesis. This enzyme is synthesized as a 40-kDa zymogen which is autocleaved to produce a 28-kDa mature form. Although the gene for SpeB is chromosomally located, highly conserved, and found in ≥99% of GAS strains, several studies have documented marked variations in SpeB expression among different strains (9, 43), as well as among clonally related strains (8). The reason for this variation and its impact on diseases caused by GAS remain to be elucidated.
The potential role of SpeB in host-pathogen interactions has been explored in a series of elegant studies performed in vitro, as well as in animal models. This cysteine protease has been shown to process, activate, and alter various host proteins of biological importance. SpeB degrades matrix proteins, generates active interleukin-1β (IL-1β) and kinin from their precursors, and activates human matrix metalloproteases that induce production of tumor necrosis factor alpha (7, 14, 17, 18, 44). The effect of SpeB on host tissue is believed to promote bacterial invasiveness, spread, and growth as well as triggering inflammatory responses. In fact, it has been proposed that the soft-tissue destruction in some patients with NF is partly mediated by the actions of SpeB (7).
In addition to its effect on host tissues and proteins, SpeB also exerts proteolytic activity on several GAS proteins, including the M and M-like proteins, which inhibit complement deposition and confer resistance to phagocytosis. It can also cleave the C5a peptidase, which interferes with neutrophil recruitment at the site of infection (5, 37). Although these posttranslational modifications may be required for certain biological activities of these molecules, overexpression of SpeB would result in nonspecific degradation of key protective virulence proteins and loss of important bacterial defenses (6). This complex interplay between the effects of SpeB on the GAS and its effects on the host requires a highly regulated expression of this protease. Recent studies have shown the existence of several regulatory mechanisms that control expression or posttranslational processing of the protease (12, 13, 22, 23, 27). Furthermore, the surface protein GRAB (protein G-related α2-macroglobulin-binding protein) binds α2-macroglobulin, which is the major protease inhibitor in human plasma, and has been shown to protect the bacterial surface proteins against the proteolytic actions of SpeB (38). The relative contributions of these various regulatory mechanisms to the control of SpeB expression in vivo are not entirely clear. Importantly, the biological effects of variations in SpeB production on GAS pathogenesis in humans need to be investigated.
Studies addressing the role of SpeB in mouse models of infection have generated seemingly conflicting results that might have been due to differences in serotype and/or models of infection used (2, 21, 24–26, 34–36). While some studies suggested that SpeB is essential for virulence (24–26), others showed that low levels of SpeB correlated with increased virulence (2, 36). Studies addressing the role of SpeB in clinical disease have also generated different results. Some studies indicated that increased SpeB production correlated with the more severe form of invasive infections, others found no difference, and still others reported an inverse relation between SpeB production and disease severity (9, 29, 31, 40, 43). The majority of these studies included mixtures of GAS serotypes; therefore, differences in SpeB expression among different serotypes or among different subtypes of the same serotype may have masked significant correlations between SpeB expression or level of SpeB production and disease severity.
The goal of this study was to investigate whether SpeB expression and level of production relate to specific clinical manifestations of invasive GAS infection. To normalize for possible variations among serotypes and between subtypes of the same serotype, we conducted our analysis on a cohort of M1T1 isolates obtained from invasive-infection cases of varying severity, where these isolates were determined to be derived from the same clone by detailed molecular analyses (8). We report considerable variation in SpeB expression among clinical isolates and an apparent correlation between lack of SpeB expression and increased severity of invasive infection. Further, we show that SpeB production is inversely correlated with expression of intact M1 protein, which protects these isolates from phagocytosis. Therefore, it is possible that while SpeB may play a major role in bacterial invasion and inflammation, overproduction of this protease may actually reduce the virulence of the bacteria due to proteolysis of important virulence factors (2, 37).
MATERIALS AND METHODS
Subjects, case definitions, and clinical material.
Patients from whom isolates were obtained were identified through ongoing surveillance for all invasive GAS infections in Ontario, Canada, and were enrolled during 1994 to 1998. All invasive GAS infections were classified according to the scheme proposed by the Working Group on Streptococcal Infections (45). Patients with invasive-infection cases included those with STSS (n = 17), NF (n = 20), and STSS plus NF (n = 11), as well as those with invasive nonsevere infections, i.e., patients with bacteremia, cellulitis, or erysipelas (n = 29).
Characterization of bacterial isolates.
Clinical isolates were identified as Streptococcus pyogenes by standard methodology (11), and each was designated by patient number. M and T serotyping was performed by capillary precipitin and agglutination reactions, respectively, at the National Reference Center for the Streptococcus, Edmonton, Canada. Only clonally related M1T1 strains (8) derived from patients with invasive infections were included in this study (n = 77). The clonality of these emm 1.1-positive isolates was determined as previously described (8). All M1T1 isolates studied here had the speA, speB, speG, smez, and speF genes but not the speC, speH, or ssa genes (28, 32, 33). Forty of the 77 isolates studied were sent encoded.
Preparation of bacterial culture supernatant.
Bacterial isolates were streaked on blood agar plates, and the isolated colonies were cultured overnight in 12.5 ml of Todd-Hewitt broth (THB) supplemented with 1.5% yeast extract (both from Difco, Detroit, Mich.). The bacteria were grown under microaerophilic conditions without shaking at 37°C, although in some experiments, selected isolates were also grown in the presence of 10% CO2 (using Campypacks) or with shaking at 37°C. These different growth conditions had no effect on relative SpeB expression among the isolates tested. The overnight culture supernatants were filter sterilized, aliquoted, and stored at −80°C. Only fresh cultures were used for repeated testing, in order to minimize artifacts that may arise from frequent passages in vitro.
Quantitation of SpeB by use of standardized immunoblots.
The amount of SpeB produced by each isolate was determined by using the quantitative Western immunoblots developed with anti-SpeB antibodies. For quantitation of SpeB, four serial dilutions of standard purified SpeB ranging from 12.5 to 50 ng per lane were run along with appropriate dilutions of the test supernatants on the same blot. Immunodetection was performed by chemiluminescence using monoclonal anti-SpeB antibodies at a dilution of 1:25,000, horseradish peroxidase-conjugated anti-mouse immunoglobulin at a dilution of 1:25,000, and the ECL detection reagents (Amersham Life Sciences Ltd., Little Chalfont, Buckinghamshire, England). The images from the immunoblots were captured using the Fluor-S multi-imager system (Bio-Rad, Hercules, Calif.), and the band corresponding to the active SpeB protease migrating at 28 kDa was scanned separately from the precursor 40-kDa form. A standard curve was generated for each blot using the arbitrary scanning values of the standard 28-kDa SpeB. The concentration of the 28-kDa SpeB protein in each supernatant was deduced from the standard curve, and the dilution factor was taken into consideration for calculating the final concentration.
Measurement of SpeB proteolytic activity.
The proteolytic activity of SpeB was detected using the EnzChek protease assay kit (Molecular Probes Inc., Eugene, Oreg.). This method detects the hydrolysis of a casein derivative heavily labeled with a red fluorescent dye, BODIPY TR-X. Protease-catalyzed hydrolysis of the substrate releases highly fluorescent peptides. The accompanying increase in fluorescence was detected with a microplate fluorometer (Fluoromark; Bio-Rad). The samples were diluted 1:1 in Tris buffer, pH 7.4, and 100 μl was added to the microplate wells, in duplicate. Wells containing N-[N-(l-3-trans-carboxyoxirane-2-carbonyl)-l-leucyl]-agmatine (E-64), a cysteine protease-specific inhibitor that was added at a final concentration of 28 μM, served as a control for specificity. The fluorescent substrate was added, and the plates were incubated, according to the manufacturer's instructions, at room temperature for 24 h in the dark and were then read at excitation and emission wavelengths of 590 and 640 nm, respectively. The protease activity was determined by subtracting the amount of fluorescence obtained in the presence of E-64 from the total fluorescence obtained without the inhibitor. The specific activity was calculated by dividing the fluorescent units (FU) by the amount of 28-kDa SpeB calculated from the Western blots. To rule out the possibility that the differences in protease activity may be affected by the assay temperature, 10 representative isolates were tested in triplicate, both at room temperature and at 37°C in parallel assays. Although the cysteine protease activity was higher at 37°C, the relative differences between isolates remained the same as when the assay was conducted at room temperature.
Expression of protective M protein.
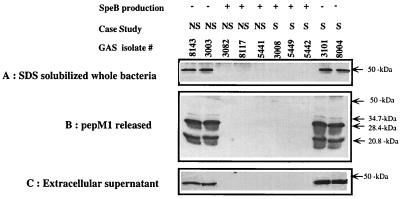
Surface expression of M1 protein was assessed by a Western blot technique from a group of 10 representative GAS isolates (5 from severe cases and 5 from nonsevere cases). These isolates were grown in THB as described above. An aliquot of the bacterial growth was sedimented and directly solubilized in 50 μl of sodium dodecyl sulfate (SDS)-gel loading buffer. PepM fractions were obtained from the remaining cells by digestion with 25 μg of pepsin/ml at a suboptimal pH of 5.8 for 30 min at 37°C in a final volume of 300 μl as detailed elsewhere (4). The pH of the soluble fraction was adjusted to a neutral pH (7.5), and it was dialyzed overnight against water, lyophilized, and solublized in 50 μl of SDS-gel loading buffer. To analyze sheered M1 protein shed in culture supernatants, the extracellular proteins were partially purified by ethanol precipitation as described previously (32). The SDS-solubilized bacterial cells, PepM, or the partially purified proteins from extracellular culture supernatants were transferred to nitrocellulose paper after separation on an SDS–12.5% polyacrylamide gel. The Western blots were probed with anti-M1 specific antibodies kindly provided by James B. Dales. These antibodies were specific to the protective N-terminal region of the M protein.
Statistical analysis.
Statistical differences in the amount and enzyme activity of SpeB among different clinical groups were analyzed by the Student t test (two-tailed). Association between lack of SpeB expression and disease severity was analyzed by Yates' corrected chi-square value, and significance was analyzed by the Fisher exact one-tailed test.
RESULTS
All the GAS isolates included in this study (n = 77) were clonally related M1T1 strains obtained from patients with invasive infections of varying clinical severity, as detailed elsewhere (8). The demographics of patients from whom the isolates were obtained are summarized in Table 1.
TABLE 1.
GAS isolates from patients with severe and nonsevere invasive infections included in the present study
| Isolate source | No. of isolates |
|---|---|
| Total isolates | 77 |
| Severe invasive cases | 48 |
| STSS | 17 |
| NF | 20 |
| STSS plus NF | 11 |
| Nonsevere invasive cases | 29 |
| Soft tissue | 16 |
| Cellulitis | 6 |
| Others | 7 |
Quantitation of 28-kDa SpeB produced by GAS isolates from severe and nonsevere infections.
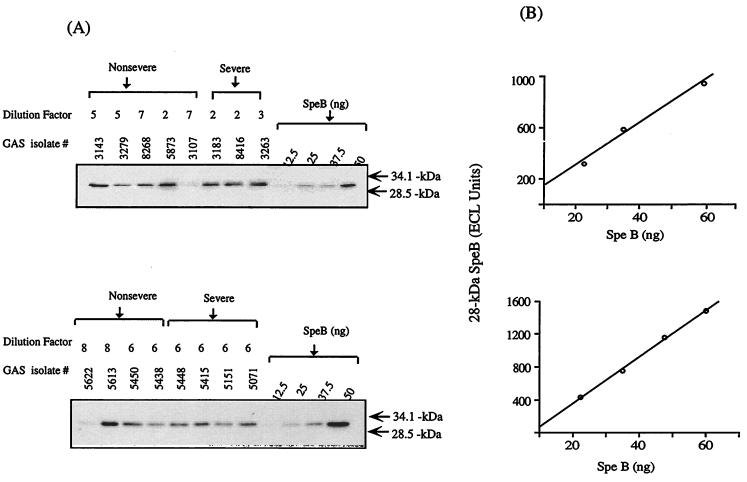
Production of SpeB by the M1T1 isolates was investigated by quantitative Western blotting to allow quantitation of the 28-kDa mature SpeB without interference from the 40-kDa zymogen form. Quantitation by Western blotting and the inclusion of known standards in each blot generated reproducible quantitative data (Fig. 1).
FIG. 1.
Quantitation of SpeB produced by M1T1 isolates by a Western immunoblotting technique. Culture supernatants prepared from overnight cultures were analyzed by Western blotting as detailed in Materials and Methods. (A) Serial dilutions of standard purified SpeB were run along with appropriate dilutions of the test supernatants on the same blot. The blots were developed by use of anti-SpeB antibodies and chemiluminescence detection, and were analyzed by the Fluor-S multi-imager system. (B) A standard curve was generated for each blot using the arbitrary scanning values of the standard 28-kDa SpeB.
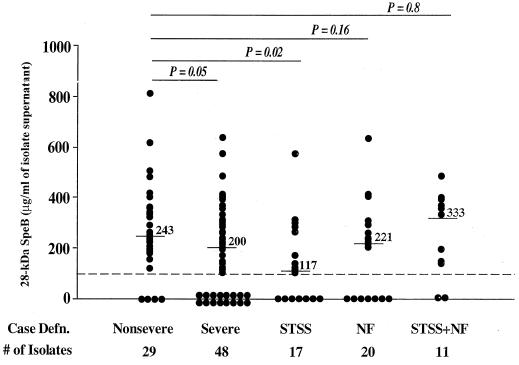
To investigate a possible correlation between SpeB production and outcome of invasive infection, we analyzed the expression and level of production of 28-kDa SpeB in culture supernatants of isolates from nonsevere cases as well as in those from cases of STSS, NF, and STSS plus NF (Fig. 2). The amount of SpeB produced varied considerably among the isolates (Fig. 2). The concentration of the active 28-kDa SpeB produced by isolates from patients with nonsevere infections (n = 29) ranged from 0 to 811 μg/ml, with a mean of 270 ± 183 μg/ml, while the amount produced by isolates from severe cases (n = 48) ranged from 0 to 635 μg/ml, with a mean of 190 ± 171 μg/ml (P = 0.05) (Fig. 2; Table 2). The difference in mean SpeB protein concentration between isolates from nonsevere and STSS cases (n = 17) was more significant (P = 0.02) (Table 2), and there was a significant association between lack of SpeB expression and disease severity (P = 0.049) (Table 3). Although isolates from NF cases (n = 20) produced less SpeB than isolates from nonsevere cases, the difference was not significant (P = 0.16). Differences in SpeB production between isolates from nonsevere cases and isolates from STSS plus NF cases (n = 11) also were not significant, possibly due to small sample size.
FIG. 2.
In vitro production of SpeB by GAS isolates from severe and nonsevere invasive infections. The amount of 28-kDa SpeB protein was determined in culture supernatants prepared from overnight cultures of the isolates using the quantitative method described for Fig. 1. Each data point represents the amount of 28-kDa SpeB produced by one isolate, and the severe-infection group is shown both as a whole and divided into its subgroups STSS, NF, and STSS plus NF. Numbers above lines are median values. Statistical differences between isolates from nonsevere and severe cases were determined by the Student t test. See also Table 2.
TABLE 2.
Amount of 28-kDa SpeB produced by GAS isolated from invasive cases of varying severity
| Isolate source (n) | Amt of 28-kda SpeB (μg/ml)a
|
Pb | |
|---|---|---|---|
| Mean ± SD | Median | ||
| Nonsevere infection (29) | 270 ± 183 | 243 | |
| Severe infection (48) | 190 ± 171 | 200 | 0.05 |
| STSS (17) | 141 ± 162 | 117 | 0.02 |
| NF (20) | 196 ± 176 | 221 | 0.16 |
| STSS + NF (11) | 257 ± 167 | 333 | 0.80 |
The amounts of secreted SpeB in culture supernatants were determined by Western blot analysis as described in Materials and Methods.
Differences between the amounts of SpeB produced by isolates from nonsevere infections and isolates from various subgroups of the severe infections were evaluated by the Student t test.
TABLE 3.
Distribution of SpeB protein activity among GAS isolated from invasive cases of varying severity
| Isolate source (n) | No. (%) of isolates with the following 28-kDa SpeB protein resulta:
|
Statisticsb
|
||||
|---|---|---|---|---|---|---|
| Concn (μg/ml) of:
|
Enzyme activity (101 FU) of:
|
Yates' corrected chi-square value | P | |||
| >100 | <100 | >350 | <350 | |||
| Nonsevere infection (29) | 25 (86) | 4 (14) | 25 (86) | 4 (14) | ||
| Severe infection (48) | 32 (66) | 16 (33) | 32 (66) | 16 (33) | 3.0 | 0.049 |
| STSS (17) | 10 (59) | 7 (41) | 10 (59) | 7 (41) | 3.0 | 0.042 |
| NF (20) | 13 (65) | 7 (35) | 13 (65) | 7 (35) | 2.0 | 0.081 |
| STSS + NF (11) | 9 (82) | 2 (18) | 9 (82) | 2 (18) | 0.02 | 0.531 |
SpeB production was determined by Western blot analysis as well as enzyme assay as described in Materials and Methods.
Association between lack of SpeB expression and disease severity was analyzed by Yates' corrected chi-square value, and significance was analyzed by Fisher's exact one-tailed test.
The difference in SpeB production between isolates from nonsevere cases and isolates from the various subgroups of severe invasive-infection cases was primarily due to the fact that a significantly higher percentage of isolates from severe invasive-infection cases produced little or no SpeB. Almost 41% of STSS isolates produced little or no SpeB, compared to only 14% of isolates from nonsevere cases (P = 0.049) (Table 3). Although the percentage of isolates producing little or no SpeB was also 2.5 times higher in the NF group than in the group of isolates from nonsevere cases, the difference was not significant (Table 3). Therefore, when SpeB production was compared only among isolates that produced detectable levels of the 28-kDa protein, the difference in the amount of SpeB produced by isolates from the various clinical groups was not statistically significant (data not shown).
Enzymatic activity of SpeB produced by GAS isolates from severe and nonsevere invasive infections.
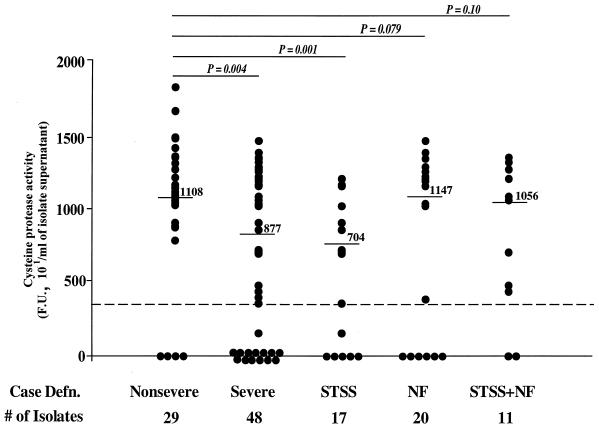
Cysteine protease activities in culture supernatants of isolates from the various clinical-manifestation groups were compared (Fig. 3). Isolates from nonsevere cases produced significantly higher levels of cysteine protease activity than isolates from all severe cases combined (mean activities, in 101 FU/ml, 1,072 ± 437 versus 722 ± 527, respectively [P = 0.004]) (Table 4). The highest significant difference was seen between isolates from nonsevere cases and STSS isolates (P < 0.001); although isolates from NF cases also produced significantly lower amounts of the protease than isolates from nonsevere cases, significance was not reached (P = 0.08). The difference between the cysteine protease activities in culture supernatants of isolates from nonsevere cases and isolates from STSS plus NF cases was also not significant (P = 0.10) (Fig. 3), and this could be related to the small sample size.
FIG. 3.
Enzyme activity of SpeB produced by GAS isolates from severe and nonsevere invasive infections. The cysteine protease activity of SpeB was detected by using a fluorescent enzyme assay as described in Materials and Methods. Each data point represents cysteine protease activity for one isolate, and the severe-infection group is shown both as a whole and divided into its subgroups STSS, NF, and STSS plus NF. Numbers above lines are median values. Statistical differences between isolates from nonsevere cases and isolates from severe cases were determined by the Student t test. See also Table 4.
TABLE 4.
Cysteine protease activities in culture supernatants of GAS isolated from invasive cases of varying severity
| Isolate source (n) | Enzyme activity (101 FU)a
|
Pb | |
|---|---|---|---|
| Mean ± SD | Median | ||
| Nonsevere infection (29) | 1,072 ± 437 | 1,108 | |
| Severe infection (48) | 722 ± 527 | 877 | 0.004 |
| STSS (17) | 564 ± 459 | 704 | 0.001 |
| NF (20) | 810 ± 582 | 1,147 | 0.079 |
| STSS + NF (11) | 804 ± 509 | 1,056 | 0.107 |
The enzyme activity of SpeB was determined by a fluorescent enzyme assay described in Materials and Methods.
Differences between the enzyme activities of SpeB produced by isolates from nonsevere cases and isolates from various subgroups of severe cases were evaluated by the Student t test.
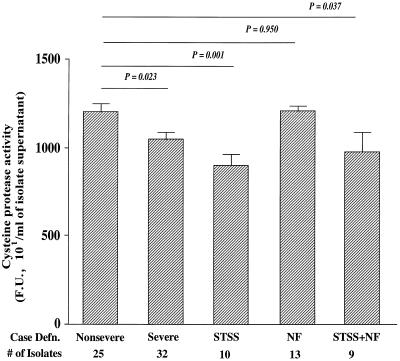
Unlike the analysis of SpeB protein production, differences between protease activities produced by isolates from nonsevere cases and isolates from all severe cases were only partially due to the fact that a higher percentage of isolates from severe cases produced little or no protease activity (Fig. 3). There was a 100% correlation between lack of SpeB protein expression and lack of cysteine protease activity. Again, 33% of isolates from severe cases, and 41% of STSS isolates, did not contain proteolytic activity in their supernatants, compared to only 14% of isolates from nonsevere cases (Table 3). However, a comparison of SpeB enzymatic activity for only those isolates that produced the 28-kDa SpeB protein showed significantly reduced enzymatic activity among STSS isolates (P < 0.001) (Fig. 4; Table 5). Thus, differences in the cysteine protease activity between STSS isolates and isolates from nonsevere infections were not only attributed to the fact that isolates from nonsevere cases are more likely to produce the 28-kDa SpeB protein; it also appears that the protein produced by STSS isolates has diminished cysteine protease activity.
FIG. 4.
Cysteine protease activities produced by SpeB-positive isolates only. SpeB-positive isolates were those showing detectable 28-kDa SpeB protein in Western blots and having cysteine protease activities of ≥350 × 101 FU/ml. The same data are presented in numerical form, together with median values, in Table 5. Statistical differences between isolates from nonsevere cases and isolates from severe cases were determined by the Student t test.
TABLE 5.
Cysteine protease activities in culture supernatants of SpeB-positive GAS isolated from invasive cases of varying severity
| Isolate source (n) | Enzyme activity (101 FU)a
|
Pb | |
|---|---|---|---|
| Mean ± SD | Median | ||
| Nonsevere infection (25) | 1,212 ± 47 | 1,140 | |
| Severe infection (32) | 1,054 ± 46.8 | 1,148 | 0.023 |
| STSS (10) | 908 ± 61.1 | 877 | 0.001 |
| NF (13) | 1,216 ± 34.6 | 1,200 | 0.950 |
| STSS + NF (9) | 982 ± 118.7 | 1,080 | 0.037 |
The enzyme activity of SpeB was determined by a fluorescent enzyme assay described in Materials and Methods.
Differences between the enzyme activities of SpeB produced by isolates from nonsevere cases and isolates from various subgroups of severe cases were evaluated by the Student t test.
Inverse relation between amount of SpeB produced and expression of protective M protein.
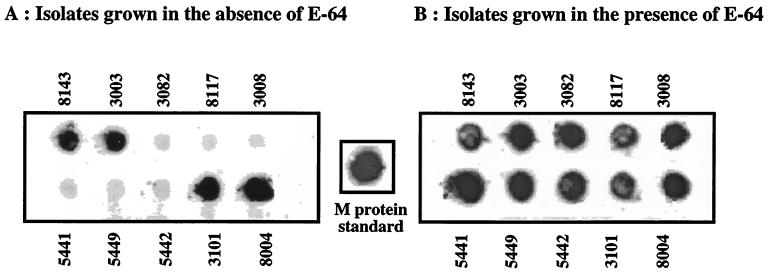
The inverse relation between SpeB production and disease severity led us to hypothesize that overproduction of this protease may lead to degradation of key protective surface proteins, and that this would reduce the virulence of the bacteria in vivo. To test this hypothesis, we analyzed the expression of M1 protein, which is a major virulence factor of GAS that protects the bacteria against phagocytosis, in SpeB-positive and -negative isolates. An antibody directed to the protective N-terminal region of the M1 protein was used to probe SDS-solubilized whole bacteria (Fig. 5A), PepM preparations (Fig. 5B), or extracellular proteins (Fig. 5C). The data show an inverse correlation between SpeB production and intact M1 protein expression. Significant levels of M1 protein could be detected only in isolates that were not producing significant levels of SpeB. In isolates which were producing high levels of SpeB, the protective region of M1 protein was no longer detected. To investigate if this effect is mediated by the proteolytic activity of SpeB, representative isolates were grown in the presence or in the absence of the cysteine protease inhibitor E-64. As shown in Fig. 6, E-64 protected the M1 protein from the proteolytic action of SpeB, inasmuch as the M1 protein was now detected in all isolates grown in the presence of this cysteine protease inhibitor.
FIG. 5.
Inverse relation between SpeB production and expression of intact M1 protein by GAS isolates. M1 protein expression was assessed by Western blotting for a group of 10 representative GAS isolates (5 from severe [S] and 5 from nonsevere [NS] cases). The SDS-solubilized whole bacterial cells (A), the PepM1 preparations (B), and the partially purified proteins from extracellular culture supernatants (C) were transferred to nitrocellulose paper after separation on SDS–12.5% polyacrylamide gels. The Western blots were probed with anti-M1 specific antibodies kindly provided by James B. Dales. These antibodies were specific to the protective N-terminal region of the M1 protein and thus detect intact M1 protein only.
FIG. 6.
Dot blot ELISA with GAS isolates grown in the absence (A) or in the presence (B) of the cysteine protease inhibitor E-64. Overnight bacterial growth from 10 representative GAS isolates was heat killed, blotted onto nitrocellulose paper, and probed with anti-M1 antibodies as detailed in the legend to Fig. 5.
DISCUSSION
The major streptococcal cysteine protease, SpeB, degrades both host and bacterial proteins (5, 16). While the actions of SpeB on host proteins can contribute to the ability of the bacteria to invade tissues and elicit a potent inflammatory response, its ability to hydrolyze M protein, M-like proteins, and C5a peptidase can strip the bacteria of important defenses against phagocytic cells. Thus SpeB can be viewed as a double-edged sword that can work either for or against the bacteria. It is reasonable, therefore, to assume that the effects of SpeB on host versus bacteria in vivo will depend on the balance between the actions of several regulatory systems that control SpeB synthesis, posttranslational modification, and enzymatic activity.
Dynamic regulatory events occurring at the site of infection and surrounding the bacterial surface may determine whether SpeB expression, as well as the level of SpeB production, will increase or reduce the virulence of the organism. The net result of these events may vary for different strains and/or different hosts, as many streptococcal proteins mediate their effects by interacting with host proteins that also exhibit allelic variations. The contribution of these variables may explain the seemingly conflicting reports in the literature concerning the effect of inhibition of SpeB production on increased or decreased virulence of the bacteria in animal models. For example, while some studies have reported that SpeB expression was required for virulence, others found opposite results. Lukomski et al. (24–26) found that insertional inactivation of SpeB reduces the virulence of GAS following intraperitoneal challenge of mice, and Kapur et al. (16) reported that vaccination with the proteinase can provide protection in the same model of infection. In contrast, Ashbaugh et al. (2) found, in a murine model of soft-tissue infection, that there was no difference between the in vivo virulence of a wild-type M3 isolate, recovered from a patient with NF, and that of its isogenic gene replacement mutant deficient in the cysteine protease. These investigators concluded that, in their model, SpeB expression is not critical for the development of tissue necrosis, secondary bacteremia, or lethal infection. Similarly, Raeder et al. (36) examined the role of SpeB expression in an air sac mouse model for skin infection and found that a lack of, or reduction in, SpeB expression correlated both with increased expression of M and M-related proteins and with increased skin-invasive potential of the bacteria.
Similarly, expression of SpeB in clinical isolates has been examined by several groups, and again, variable conclusions were reached (9, 29, 43). In 1993, Talkington et al. (43) found no relation between SpeB production and clinical symptoms of invasive infection caused by either M1 or M3 strains, while SpeB production was inversely related to disease severity among nontypeable OF+ strains. In fact, in that study, 91% of M-nontypeable OF+ isolates from patients with no signs of STSS produced SpeB, compared to only 14% of M-nontypeable OF+ isolates from patients with STSS (P < 0.05) (43). In 1996, Chaussee et al. (9) examined SpeB production by enzyme-linked immunosorbent assay (ELISA) in 117 isolates from 112 patients with a variety of diseases, including NF and STSS, and found no correlation between SpeB production and severity of invasive disease. However, the study included isolates from 14 countries and 18 different serotypes, and therefore it is possible that differences in SpeB production between isolates from severe and nonsevere cases might have been masked by variations in production by different serotypes (9).
In this study, we normalized for possible variations among GAS serotypes by conducting the analysis using a cohort of M1T1 isolates that were determined to be derived from the same clone, by a variety of molecular typing methods (8). This cohort of isolates obtained from invasive-infection cases with varying degrees of severity provided an excellent opportunity to examine if variations in Spe expression and/or the level of Spe production correlate with the severity or clinical manifestation of invasive disease. Recently we reported that expression of SpeA, SpeB, and SpeF varied considerably among these clonal M1T1 isolates (8). However, no correlation was found between the expression of SpeA or SpeF and disease severity. By contrast, there was a trend toward a correlation between SpeB expression and the less severe forms of invasive disease. The data presented here, from a study conducted with a larger sample, clearly show an inverse relation between SpeB expression and increased severity of the invasive infection.
Inasmuch as all our M1T1 isolates were clonal, our data support the existence of an on-off regulatory mechanism(s) controlling SpeB production as well as a posttranslational regulatory mechanism controlling its activity. The finding that the percentage of isolates that do not produce SpeB was threefold higher among STSS isolates than among the clonally related isolates from nonsevere cases, together with the fact that SpeB produced by STSS isolates had significantly less cysteine protease activity than SpeB produced by isolates from nonsevere cases, strongly suggests that events occurring in the host during infection may exert selective pressure or regulatory control over the production and the regulation of the activity of this important virulence factor. We hypothesize that reduced expression or activity of SpeB may be advantageous to the bacteria in cases of STSS, inasmuch as the absence of proteolytic activity would spare important virulence surface proteins. This hypothesis is supported by the studies of Raeder et al. (37) as well as by the data presented here, which showed an inverse relation between SpeB production and expression of intact M1 protein.
Raeder et al. (36) observed that passage of GAS isolates in mice resulted in decreased expression of SpeB accompanied by increased expression of M and M-related proteins, which normally protect the organism against phagocytosis. Similar findings were reported by Ashbaugh et al. (2), who found that the SpeB-negative, large-capsule-producing GAS strains exhibited increased invasiveness in a mouse model of skin infection, whereas SpeB expression was associated with decreased virulence. These investigators concluded that the increased potential of SpeB-negative strains to cause more-invasive infections can be indirectly attributable to the small amount of capsule, loss of M protein, or a combination of both.
Together the data suggest that in the absence of SpeB, the M protein would remain intact and protect the organism against phagocytosis. Although it could be argued that type-specific antibodies directed against the N-terminal region of the M protein would opsonize and eliminate the bacteria, several studies, including our own, have shown that patients with invasive infections caused by M1T1 isolates had significantly lower levels of anti-M1 opsonic antibodies than age- and geographically matched controls (3). Therefore, in the absence of opsonic antibodies, the expression and effects of SpeB on the protective surface proteins would be expected to make a difference in bacterial virulence in vivo, with a higher chance of bacterial survival being associated with low or no SpeB expression. Alternatively, a relatively higher expression of the GRAB protein and inhibition of SpeB by the α2-macroglobulin could protect the organism, but only in the presence of protective opsonic antibodies (38). Clearly, these dynamic interactions may be influenced by the site of infection and may depend on specific host-pathogen interactions that may not be easily discerned. Nonetheless, the above scenario is supported by the results from the present study demonstrating an inverse relation between SpeB expression and invasive-disease severity. Further investigations of the underlying mechanisms controlling speB gene expression in vivo should determine the contribution of host-pathogen interactions in regulating the expression of this and other important streptococcal virulence genes.
ACKNOWLEDGMENTS
This work was supported by grant AI40198 from the National Institute of Allergy and Infectious Diseases (NIAID), National Institutes of Health (NIH) (to M.K.), and by the Research and Development Office, Medical Research Service, Department of Veterans Affairs (merit award to M.K.).
We thank J. Musser, NIAID, NIH, Hamilton, Mont., for providing the mouse monoclonal antibodies to SpeB. We also thank James B. Dale for providing the anti-M1 polyclonal antibody. We are deeply indebted to the physicians and investigators of the Canadian Infectious Disease Society (CIDS) Streptococcal Study Group for help in collecting the clinical material. We also thank H. Courtney, VAMC, Memphis, Tenn., for helpful discussions and suggestions.
REFERENCES
- 1.Alouf J E, Knoll H, Kohler W. The family of mitogenic, shock-inducing and superantigenic toxins from Staphylococci and Streptococci. In: Alouf J E, Freer J H, editors. Sourcebook of bacterial protein toxins. London, United Kingdom: Academic Press; 1991. pp. 367–414. [Google Scholar]
- 2.Ashbaugh C D, Warren H B, Carey V J, Wessels M R. Molecular analysis of the role of the group A streptococcal cysteine protease, hyaluronic acid capsule, and M protein in a murine model of human invasive soft-tissue infection. J Clin Investig. 1998;102:550–560. doi: 10.1172/JCI3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Basma H, Norrby-Teglund A, Guedez Y, McGeer A, Low D E, El-Ahmedy O, Schwartz B, Kotb M. Risk factors in the pathogenesis of invasive group A streptococcal infections: role of protective humoral immunity. Infect Immun. 1999;67:1871–1877. doi: 10.1128/iai.67.4.1871-1877.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beachey E H, Campbell G L, Ofek I. Peptic digestion of streptococcal M protein. II. Extraction of M antigen from group A streptococci with pepsin. Infect Immun. 1974;9:891–896. doi: 10.1128/iai.9.5.891-896.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berge A, Bjorck L. Streptococcal cysteine proteinase releases biologically active fragments of streptococcal surface proteins. J Biol Chem. 1995;270:9862–9867. doi: 10.1074/jbc.270.17.9862. [DOI] [PubMed] [Google Scholar]
- 6.Boyle M D, Raeder R, Flosdorff A, Podbielski A. Role of emm and mrp genes in the virulence of group A streptococcal isolate 64/14 in a mouse model of skin infection. J Infect Dis. 1998;177:991–997. doi: 10.1086/515241. [DOI] [PubMed] [Google Scholar]
- 7.Burns E H, Jr, Marciel A M, Musser J M. Activation of 66-kilodalton human endothelial cell matrix metalloprotease by Streptococcus pyogenes extracellular cysteine protease. Infect Immun. 1996;64:4744–4750. doi: 10.1128/iai.64.11.4744-4750.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chatellier S, Ihendyane N, Kansal R G, Khambaty F, Basma H, Norrby-Teglund A, Low D E, McGeer A, Kotb M. Genetic relatedness and superantigen expression in group A streptococcus serotype M1 isolates from patients with severe and nonsevere invasive diseases. Infect Immun. 2000;68:3523–3534. doi: 10.1128/iai.68.6.3523-3534.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chaussee M S, Liu J, Stevens D L, Ferretti J J. Genetic and phenotypic diversity among isolates of Streptococcus pyogenes from invasive infections. J Infect Dis. 1996;173:901–908. doi: 10.1093/infdis/173.4.901. [DOI] [PubMed] [Google Scholar]
- 10.Davies H D, McGeer A, Schwartz B, Green K, Cann D, Simor A E, Low D E the Ontario Group A Streptococcal Study Group. Invasive group A streptococcal infections in Ontario, Canada. N Engl J Med. 1996;335:547–554. doi: 10.1056/NEJM199608223350803. [DOI] [PubMed] [Google Scholar]
- 11.Demers B, Simor A-E, Vellend H, Schlievert P M, Byrne S, Jamieson F, Valmsley S, Low D E. Severe invasive group A streptococcal infections in Ontario, Canada: 1987–1991. Clin Infect Dis. 1993;16:792–800. doi: 10.1093/clind/16.6.792. [DOI] [PubMed] [Google Scholar]
- 12.Federle M J, McIver K S, Scott J R. A response regulator that represses transcription of several virulence operons in the group A streptococcus. J Bacteriol. 1999;181:3649–3657. doi: 10.1128/jb.181.12.3649-3657.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heath A, DiRita V J, Barg N L, Engleberg N C. A two-component regulatory system, CsrR-CsrS, represses expression of three Streptococcus pyogenes virulence factors, hyaluronic acid capsule, streptolysin S, and pyrogenic exotoxin B. Infect Immun. 1999;67:5298–5305. doi: 10.1128/iai.67.10.5298-5305.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Herwald H, Collin M, Muller-Esterl W, Bjorck L. Streptococcal cysteine proteinase releases kinins: a virulence mechanism. J Exp Med. 1996;184:665–673. doi: 10.1084/jem.184.2.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoge C W, Schwartz B, Talkington D F, Breiman R F, MacNeill E M, Englender S J. The changing epidemiology of invasive group A streptococcal infections and the emergence of streptococcal toxic shock-like syndrome. A retrospective population-based study. JAMA. 1993;269:384–389. [PubMed] [Google Scholar]
- 16.Kapur V, Maffei J T, Greer R S, Li L L, Adams G J, Musser J M. Vaccination with streptococcal extracellular cysteine protease (interleukin-1 beta convertase) protects mice against challenge with heterologous group A streptococci. Microb Pathog. 1994;16:443–450. doi: 10.1006/mpat.1994.1044. [DOI] [PubMed] [Google Scholar]
- 17.Kapur V, Majesky M W, Li L-L, Black R A, Musser J M. Cleavage of interleukin 1β (IL-1β) precursor to produce active IL-1β by a conserved extracellular cysteine protease from Streptococcus pyogenes. Proc Natl Acad Sci USA. 1993;90:7676–7680. doi: 10.1073/pnas.90.16.7676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kapur V, Topouzis S, Majesky M W, Li L L, Hamrick M R, Hamill R J, Patti J M, Musser J M. A conserved Streptococcus pyogenes extracellular cysteine protease cleaves human fibronectin and degrades vitronectin. Microb Pathog. 1993;15:327–346. doi: 10.1006/mpat.1993.1083. [DOI] [PubMed] [Google Scholar]
- 19.Kotb M. Bacterial pyrogenic exotoxins as superantigens. Clin Microbiol Rev. 1995;8:411–426. doi: 10.1128/cmr.8.3.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kotb M. Role of superantigens in the pathogenesis of infectious diseases and their sequelae. Curr Opin Infect Dis. 1992;5:364–374. [Google Scholar]
- 21.Kuo C F, Wu J J, Lin K Y, Tsai P J, Lee S C, Jin Y T, Lei H Y, Lin Y S. Role of streptococcal pyrogenic exotoxin B in the mouse model of group A streptococcal infection. Infect Immun. 1998;66:3931–3935. doi: 10.1128/iai.66.8.3931-3935.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Levin J C, Wessels M R. Identification of csrR/csrS, a genetic locus that regulates hyaluronic acid capsule synthesis in group A Streptococcus. Mol Microbiol. 1998;30:209–219. doi: 10.1046/j.1365-2958.1998.01057.x. [DOI] [PubMed] [Google Scholar]
- 23.Li Z, Sledjeski D D, Kreikemeyer B, Podbielski A, Boyle M D. Identification of pel, a Streptococcus pyogenes locus that affects both surface and secreted proteins. J Bacteriol. 1999;181:6019–6027. doi: 10.1128/jb.181.19.6019-6027.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lukomski S, Burns E H, Jr, Wyde P R, Podbielski A, Rurangirwa J, Moore-Poveda D K, Musser J M. Genetic inactivation of an extracellular cysteine protease (SpeB) expressed by Streptococcus pyogenes decreases resistance to phagocytosis and dissemination to organs. Infect Immun. 1998;66:771–776. doi: 10.1128/iai.66.2.771-776.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lukomski S, Montgomery C A, Rurangirwa J, Geske R S, Barrish J P, Adams G J, Musser J M. Extracellular cysteine protease produced by Streptococcus pyogenes participates in the pathogenesis of invasive skin infection and dissemination in mice. Infect Immun. 1999;67:1779–1788. doi: 10.1128/iai.67.4.1779-1788.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lukomski S, Sreevatsan S, Amberg C, Reichardt W, Woischnik M, Podbielski A, Musser J M. Inactivation of Streptococcus pyogenes extracellular cysteine protease significantly decreases mouse lethality of serotype M3 and M49 strains. J Clin Investig. 1997;99:2574–2580. doi: 10.1172/JCI119445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lyon W R, Gibson C M, Caparon M G. A role for trigger factor and an rgg-like regulator in the transcription, secretion and processing of the cysteine proteinase of Streptococcus pyogenes. EMBO J. 1998;17:6263–6275. doi: 10.1093/emboj/17.21.6263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mollick J, Miller G, Musser J, Cook R, Grossman D, Rich R. A novel superantigen isolated from pathogenic strains of Streptococcus pyogenes with amino-terminal homology to staphylococcal enterotoxins B and C. J Clin Investig. 1993;92:710–719. doi: 10.1172/JCI116641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Muller-Alouf H, Geoffroy C, Geslin P, Bouvet A, Felten A, Gunther E, Ozegowski J H, Alouf J E. Streptococcal pyrogenic exotoxin A, streptolysin O, exoenzymes, serotype and biotype profiles of Streptococcus pyogenes isolates from patients with toxic shock syndrome and other severe infections. Zentbl Bakteriol. 1997;286:421–433. [PubMed] [Google Scholar]
- 30.Musser J M, Kapur V, Kanjilal S, Shah U, Musher D M, Barg N L, Johnston K H, Schlievert P M, Henrichsen J, Gerlach D, et al. Geographic and temporal distribution and molecular characterization of two highly pathogenic clones of Streptococcus pyogenes expressing allelic variants of pyrogenic exotoxin A (Scarlet fever toxin) J Infect Dis. 1993;167:337–346. doi: 10.1093/infdis/167.2.337. [DOI] [PubMed] [Google Scholar]
- 31.Norgren M, Norrby A, Holm S E. Genetic diversity in T1M1 group A streptococci in relation to clinical outcome of infection. J Infect Dis. 1992;166:1014–1020. doi: 10.1093/infdis/166.5.1014. [DOI] [PubMed] [Google Scholar]
- 32.Norrby-Teglund A, Kaul R, Low D E, McGeer A, Newton D W, Andersson J, Andersson U, Kotb M. Plasma from patients with severe invasive group A streptococcal infections treated with normal polyspecific IgG inhibits streptococcal superantigen-induced T cell proliferation and cytokine production. J Immunol. 1996;156:3057–3064. [PubMed] [Google Scholar]
- 33.Proft T, Moffatt S L, Berkahn C J, Fraser J D. Identification and characterization of novel superantigens from Streptococcus pyogenes. J Exp Med. 1999;189:89–102. doi: 10.1084/jem.189.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Raeder R, Boyle M D. Association of type II immunoglobulin G-binding protein expression and survival of group A streptococci in human blood. Infect Immun. 1993;61:3696–3702. doi: 10.1128/iai.61.9.3696-3702.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Raeder R, Boyle M D P. Properties of IgG-binding proteins expressed by Streptococcus pyogenes isolates are predictive of invasive potential. J Infect Dis. 1996;173:888–895. doi: 10.1093/infdis/173.4.888. [DOI] [PubMed] [Google Scholar]
- 36.Raeder R, Harokopakis E, Hollingshead S, Boyle M D. Absence of SpeB production in virulent large capsular forms of group A streptococcal strain 64. Infect Immun. 2000;68:744–751. doi: 10.1128/iai.68.2.744-751.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Raeder R, Woischnik M, Podbielski A, Boyle M D. A secreted streptococcal cysteine protease can cleave a surface-expressed M1 protein and alter the immunoglobulin binding properties. Res Microbiol. 1998;149:539–548. doi: 10.1016/s0923-2508(99)80001-1. [DOI] [PubMed] [Google Scholar]
- 38.Rasmussen M, Muller H P, Bjorck L. Protein GRAB of Streptococcus pyogenes regulates proteolysis at the bacterial surface by binding α2-macroglobulin. J Biol Chem. 1999;274:15336–15344. doi: 10.1074/jbc.274.22.15336. [DOI] [PubMed] [Google Scholar]
- 39.Schlievert P M. Role of superantigens in human diseases. J Infect Dis. 1993;167:997–1002. doi: 10.1093/infdis/167.5.997. [DOI] [PubMed] [Google Scholar]
- 40.Shiseki M, Miwa K, Nemoto Y, Kato H, Suzuki J, Sekiya K, Murai T, Kikuchi T, Yamashita N, Totsuka K, Ooe K, Shimizu Y, Uchiyama T. Comparison of pathogenic factors expressed by group A Streptococci isolated from patients with streptococcal toxic shock syndrome and scarlet fever. Microb Pathog. 1999;27:243–252. doi: 10.1006/mpat.1999.0302. [DOI] [PubMed] [Google Scholar]
- 41.Stevens D L. Invasive group A streptococcus infections. Clin Infect Dis. 1992;14:2–13. doi: 10.1093/clinids/14.1.2. [DOI] [PubMed] [Google Scholar]
- 42.Stevens D L, Tanner M H, Winship J, Swarts R, Ries K M, Schlievert P M, Kaplan E. Severe group A streptococcal infections associated with a toxic shock-like syndrome and scarlet fever toxin A. N Engl J Med. 1989;321:1–7. doi: 10.1056/NEJM198907063210101. [DOI] [PubMed] [Google Scholar]
- 43.Talkington D, Schwartz B, Black C, Todd J, Elliott J, Breiman R, Facklam R. Association of phenotypic and genotypic characteristics of invasive Streptococcus pyogenes isolates with clinical components of streptococcal toxic shock syndrome. Infect Immun. 1993;61:3369–3374. doi: 10.1128/iai.61.8.3369-3374.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wolf B B, Gibson C A, Kapur V, Hussaini I M, Musser J M, Gonias S L. Proteolytically active streptococcal pyrogenic exotoxin B cleaves monocytic cell urokinase receptor and releases an active fragment of the receptor from the cell surface. J Biol Chem. 1994;269:30682–30687. [PubMed] [Google Scholar]
- 45.Working Group on Severe Streptococcal Infections. Defining the group A streptococcal toxic shock syndrome. Rationale and consensus definition. JAMA. 1993;269:390–391. [PubMed] [Google Scholar]