Abstract
Frailty and Chronic Kidney Disease (CKD) both increase with age and are prevalent in older adults. However, studies in older adults examining the relationship between frailty and milder impairments of kidney function are relatively sparse.
We examined the cross- sectional association of baseline estimated glomerular filtration rate (eGFR), albuminuria, and CKD ((eGFR < 60 ml/min/1.73m2) and/or albuminuria (> 3.0 mg/mmol)) with prefrailty and frailty in the ASPirin in Reducing Events in the Elderly (ASPREE) trial cohort of healthy older participants. Univariate logistic regression models measured the unadjusted odds ratios (OR) and 95% confidence intervals (CI) for prevalent combined prefrailty and frailty (respectively defined as presence of 1-2 or 3+ of 5 modified Fried criteria) for the association between CKD, eGFR, albuminuria, and other potential risk factors.
Multivariable models calculated OR for prefrailty-frailty adjusted for potential confounders and either CKD (i), eGFR and albuminuria measured as either continuous variables (ii) or categorical variables (iii). Of 17,759 eligible participants, 6,934 were classified as prefrail, 389 were frail.
CKD, eGFR and albuminuria were all associated with combined prefrailty-frailty on univariate analysis. In the multivariable modelling, neither CKD (reduced eGFR and/or albuminuria), nor eGFR (either continuous or categorical variables) were associated with prefrailty-frailty. However, albuminuria, either as a continuous variable (OR (95% CI) 1.07 (1.04 – 1.10); p <0.001), or categorical variable (OR 1.21 (1.08 – 1.36); p =0.001) was consistently associated with prefrailty-frailty.
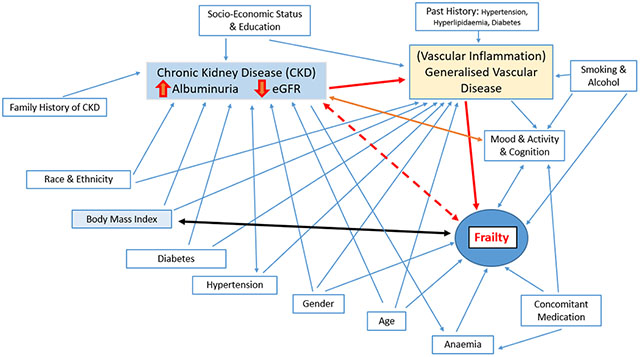
The complex relationship between albuminuria (which may be a biomarker for vascular inflammation), ageing, progressive CKD and frailty requires further investigation.
Keywords: albuminuria, chronic kidney disease, estimated glomerular filtration rate, frail, prefrail, prevalence, older adults
Summary at a Glance
Albuminuria, but not estimated Glomerular Filtration Rated (eGFR), was consistently associated with prevalent prefrailty-frailty, after covariate adjustment in a well population of older adults with Chronic Kidney Disease (CKD). The complex relationship between albuminuria (a possible biomarker for vascular inflammation), ageing, progressive CKD and development of frailty requires further investigation.
Introduction
Frailty and prefrailty increase with age, are more common in women and African Americans, and are exacerbated by other important conditions in older people, such as social isolation, depression, and cognitive impairment (1).
Because chronic kidney disease (CKD) also increases with age (2), there is accruing interest in the relationship between CKD and frailty (1, 3, 4), as CKD is associated with sarcopaenia, poor physical function and cognitive impairment, and CKD and frailty have many shared clinical symptoms, signs, and outcomes (5).
The frailty phenotype incorporates disturbances across five interrelated domains; shrinking (or lean mass reduction (sarcopaenia)), weakness, sub-optimal endurance and energy, slowness, and reduced physical activity. Developed originally in the general older adult population (6), frailty helps describe the accumulation of worsening physiological and organ function and increased vulnerability to ‘stressors’ (1, 6) that lead to increased adverse outcomes (disability, falls, institutionalisation, hospitalisation, and premature death).
High levels of frailty have been described in CKD populations but mostly in dialysis dependent (Stage 5D CKD) subjects (7). The prevalence of frailty in non-dialysis CKD populations varies considerably (7 – 48%) (4, 8). However, similar to dialysis patients (7), from review studies in older predialysis CKD populations, CKD and frailty appear to be (4, 8, 9) independent risk factors for mortality, adverse cardiovascular events, hospitalisations, and other key adverse outcomes.
The ASPREE (10) study’s well characterised generally healthy older participants on enrolment (without diagnosed cardiac events, stroke, dementia, or physical disability), provide an opportunity to assess the relationship between mild to moderate CKD (reduced GFR and/or albuminuria) and frailty (11). Our goal for this analysis was therefore to determine the association between CKD, estimated glomerular filtration rate (eGFR) and albuminuria and the presence of prefrailty or frailty, as measured in the ASPirin in Reducing Events in the Elderly (ASPREE) study adjusted for demographic, comorbidity, and laboratory measures.
Methods
Study Design and Population
The ASPREE study design and main results have previously been published (12) In brief, eligible participants were ≥ 70 years of age, or for US African American or Hispanic participants, ≥ 65 years, and generally healthy. A baseline assessment of kidney function (serum creatinine) and a measure of urinary albumin/creatinine ratio (UACR) was an inclusion requirement for this analysis. ASPREE was approved by multiple Institutional Review Boards in Australia and the US, registered with International Standard Randomized Controlled Trial Number Register (ISRCTN83772183) and clinicaltrials.gov (NCT01038583) and conducted in accordance with the Declaration of Helsinki. Secondary analyses, which includes this project were approved by the Monash University Human Research Ethics Committee (Project ID 24743: ASPREE Secondary Analyses: Factors associated with healthy ageing).
Study Variables and Measures
As assessed at study entry, and as described for ASPREE (10, 12), demographic variables (age, gender, country-ethnicity-race), social determinants of health, (living situation and education status) and other key health measures (smoking and alcohol status, body mass index (BMI), heart rate (HR), blood pressure (systolic blood pressure (SDP); diastolic blood pressure (DBP), hypertension (with and without treatment), diabetes, haemoglobin, dyslipidaemia (with and without treatment), SF-12 (Short-Form 12) quality of life scores (MCS (mental component score) & PCS (physical component score)), depression and cognition measures (3MS (Modified Mini-Mental State examination); global cognition) and CESD10 (Center for Epidemiological Studies scale; depression, 10 questions)), individual nephrotoxic medications, and polypharmacy (≥ 5 prescription medications), as well as family history of kidney disease were used in this study (Table 1).
Table 1.
Comparison of CKD, Demographic and Other Clinical and Health Measure Variables by PreFrail-Frail and Non-Frail status of participants
| Non-Frail (n=10,436 (59%)) | Prefrail-Frail (n=7,323 (41%))† | Total (n = 17,759 (100%)) | Unadjusted Odds Ratio (OR) for Prefrail-Frail |
||||
|---|---|---|---|---|---|---|---|
| OR | (95% CI) | p value | |||||
| Chronic Kidney Disease (CKD) Characteristics, n (%) | |||||||
| Family History of Kidney Disease | 689 (7) | 556 (8) | 1,245 (7) | 1.16 | (1.04 - 1.31) | 0.011 | |
| CKD (KDIGO definition) | 2,467 (24) | 2,270 (31) | 4,737 (27) | 1.45 | (1.36 - 1.55) | <0.001 | |
| eGFR (CKD-EPI)13, ml/min/1.73 m2, mean (SD) | 73.4 (13.1) | 72.2 (15.0) | 72.9 (13.9) | 0.99 | (0.99 - 1.00) | <0.001 | |
| eGFR (Stages of CKD), n(%) | |||||||
| eGFR >= 60 ml/min | 8,729 (84) | 5,778 (79) | 14,507 (82) | 1.00 | |||
| eGFR 45 - 60 ml/min | 1,468 (14) | 1,209 (16) | 2,677 (15) | 1.24 | (1.15 - 1.35) | <0.001 | |
| eGFR < 45 ml/min | 239 (2) | 336 (5) | 575 (3) | 2.12 | (1.79 - 2.52) | <0.001 | |
| Albuminuria (UACR), median, (IQR) | 0.8 (0.5-1.7) | 0.9 (0.4 - 1.3) | 0.8 (0.5-1.4) | 1.02 | (1.01 -1.02) | <0.001 | |
| Albuminuria (log UACR), median, (IQR) | −0.22(−0.92 - 0.26) | −0.11(−0.69 - 0.53) | −0.22(−0.69 - 0.34) | 1.18 | (1.14 - 1.21) | <0.001 | |
| Albuminuria Severity (UACR range), n(%) | |||||||
| No albuminuria, UACR <= 3.0 mg/mmol | 9,567 (92) | 6,395 (87) | 15,962 (90) | 1.00 | |||
| Albuminuria, UACR > 3.0 mg/mmol | 869 (8) | 928 (123 | 1,797 (10) | 1.60 | (1.45 - 1.76) | <0.001 | |
|
| |||||||
| Key Demographic Characteristics | |||||||
| Age at Randomisation, years, mean (SD) | 74.4 (3.9) | 76.1 (5.2) | 75.1 (4.6) | 1.09 | (1.08 – 1.09) | <0.001 | |
| Gender [Female], n(%) | 5,754 (55) | 4,020 (58) | 10,022 (56) | 1.14 | (1.07-1.21) | <0.001 | |
| Country-Ethnicity-Race n(%) | |||||||
| White Caucasian (AUS) | 9,339 (89) | 5,497 (79) | 15,115 (85) | 1.00 | |||
| White Caucasian (US) | 497 (5) | 519 (7) | 1,056 (6) | 1.82 | (1.60 - 2.06) | <0.001 | |
| Black/African American | 271 (3) | 558 (8) | 873 (5) | 3.59 | (3.10 - 4.16) | <0.001 | |
| Hispanic or Latino | 201 (2) | 242 (4) | 463 (3) | 2.11 | (1.75 - 2.54) | <0.001 | |
| Other/Undetermined | 128 (1) | 118 (2) | 252 (1) | 1.57 | (1.22 - 2.01) | <0.001 | |
| Health Behaviours and Social Determinants of Health | |||||||
| Living Situation, n (%) | |||||||
| Home with Family, Friends, Spouse | 7,318 (70) | 4,344 (62) | 11,869 (67) | 1.00 | |||
| Home Alone or Residential Home | 3,118 (30) | 2,772 (38) | 5,890 (33) | 1.43 | (1.34-1.52) | <0.001 | |
| Years of Education, n (%) | |||||||
| < =12 | 5,816 (56) | 4,315 (59) | 10,131 (57) | 1.00 | |||
| > 12 | 4,620 (44) | 3,008 (41) | 7,628 (43) | 0.88 | (0.83 - 0.93) | <0.001 | |
| Smoking, n (%) | |||||||
| Never | 5,831 (56) | 3961 (54) | 9,792 (55) | 1.00 | |||
| Current | 335 (3) | 357 (5) | 692 (4) | 1.57 | (1.34 - 1.83) | <0.001 | |
| Former | 4,270 (41) | 3005 (41) | 7,275 (41) | 1.04 | (0.97 - 1.10) | 0.261 | |
| Alcohol, n (%) | |||||||
| Never | 1,603 (15) | 1448 (20) | 3,091 (17) | 1.00 | |||
| Current | 8,321 (80) | 5278 (72) | 13,599 (77) | 1.46 | (1.35 - 1.58) | <0.001 | |
| Former | 512 (5) | 557 (8) | 1,069 (6) | 1.72 | (1.51 - 1.94) | <0.001 | |
| Clinical and Laboratory Measures | |||||||
| Systolic Blood Pressure, mmHg, mean (SD) | 139,4 (16.1) | 138.9 (17.0) | 139.2 (16.5) | 1.00 | (1.00 – 1.00) | 0.038 | |
| Diastolic Blood Pressure, mmHg, mean (SD) | 77.5 (9.8) | 77.0 (10.2) | 77.3 (10.0) | 1.00 | (0.99 – 1.00) | 0.004 | |
| Heart Rate, beats/min, mean (SD) | 70.5 (10.5) | 71.0 (11.0) | 70.7 (10.7) | 1.01 | (1.00 – 1.01) | <0.001 | |
| Haemoglogbin, g/dL, mean (SD) | 14.3 (1.2) | 14.0 (1.3) | 14.2 (1.2) | 0.86 | (0.84 - 0.88) | <0.001 | |
| Nephrotoxic and Other Medications, n (%) | |||||||
| Proton Pump Inhibitors (PPIs) | 2,425 (23) | 1,925 (26) | 4,350 (24) | 1.10 | (1.03 - 1.17) | 0.003 | |
| Non-Steroidal Anti-inflammatory Agents (NSAIDs) | 1,355 (13) | 1,195 (16) | 2,550 (14) | 1.18 | (1.10 - 1.26) | <0.001 | |
| Polypharmacy | 2,240 (21) | 2,510 (34) | 4,750 (27) | 1.91 | (1.78 - 2.04) | <0.001 | |
| Chronic Conditions | |||||||
| Hypertension & Treatments | |||||||
| Normotensive | 2,796 (27) | 1,753 (24) | 4,549 (26) | 1.00 | |||
| Hypertensive on no Rx | 2,657 (25) | 1,543 (21) | 4,200 (24) | 0.92 | (0.85 – 1.01) | 0.830 | |
| Hypertensive on Rx (not Diuretics and not ACE/ARB) | 461 (4) | 405 (6) | 866 (5) | 1.40 | (1.21 – 1.62) | <0.001 | |
| Hypertensive on Rx (ACE/ARB but not Diuretics | 2,836 (27) | 1,994 (27) | 4,830 (27) | 1.12 | (1.03 – 1.22) | 0.007 | |
| Hypertension on Rx (Diuretics but not ACE/ARB) | 347 (3) | 395 (5) | 742 (4) | 1.82 | (1.55 – 2.12) | <0.001 | |
| Hypertension on Rx (ACE/ARB + Diuretics) | 1,339 (13) | 1,223 (17) | 2,572 (14) | 1.47 | (1.33 – 1.62) | <0.001 | |
| Dyslipidaemia & Treatments, n (%) | |||||||
| Normal lipids and not receiving LLAs | 3,457 (33) | 2,688 (37) | 6,145 (35) | 1.00 | |||
| Abnormal Lipids and not receiving LLAs | 3,530 (34) | 2,058 (28) | 5,588 (31) | 0.98 | (0.91 - 1.06) | 0.590 | |
| Normal lipids and receiving LLAs | 2,837 (27) | 2,161 (30) | 4,979 (28) | 0.75 | (0.69 - 0.81) | <0.001 | |
| Abnormal Lipids and receiving LLAs | 612 (6) | 416 (6) | 1,047 (6) | 0.87 | (0.76 - 1.00) | 0.050 | |
| Diabetes, n (%) | 937 (9) | 977 (13) | 1,914 (11) | 1.56 | (1.42 - 1.72) | <0.001 | |
| Mood, Cognition & Quality of Life | |||||||
| Mental-Component t-score, mean (SD) | 56.2 (6.4) | 55.0 (8.1) | 55.7 (7.1) | 0.98 | (0.97 - 0.98) | <0.001 | |
| Physical Component t-score, mean (SD) | 50.3 (7.4) | 45.4 (9.7) | 48.3 (8.8) | 0.94 | (0.93 - 0.94) | <0.001 | |
| 3MS, mean (SD) | 93.9 (4.4) | 92.8 (4.9) | 93.4 (4.6) | 0,95 | (0.95 - 0.96) | <0.001 | |
| CESD10, mean (SD) | 2.6 (2.6) | 4.1 (3.9) | 3.2 (3.3) | 1.15 | (1.14 - 1.17) | <0.001 | |
| Anthropometrics | |||||||
| Body Mass Index (BMI), kg/m2, mean (SD) | 27.8 (4.1) | 28.6 (5.4) | 28.1 (4.7) | 1.04 | (1.03 −1.04) | <0.001 | |
| BMI Range, n (%) | |||||||
| <20 kg/m2 | 10 (<1) | 324 (4) | 334 (2) | 55.57 | (29.6 - 104.4) | <0.001 | |
| 20 - 30 kg/m2 | 7,682 (74) | 4,470 (61) | 12,161 (69) | 1.00 | |||
| >30 kg/m2 | 2,706 (26) | 2,513 (34) | 5,219 (29) | 1.59 | (1.49 - 1.70) | <0.001 | |
Prefrail (n = 6,934; Frail (n = 389): Total Prefrail-Frail (n = 7,323);
LLAs = Lipid Lowering Agents. ACE= Angiotensin Converting Enzyme Inhibitors, ARB = Angiotensin Receptor Blockers. SD = Standard Deviation; IQR = Inter Quartile Range; Rx = Treatment; UACR = Urinary Albumin/Creatinine Ratio; CI = Confidence Interval.
SDP, DBP and HR measures were the mean of 3 baseline readings (10). Diabetes mellitus and dyslipidaemia were defined as previously published (12) and hypertension defined by the receipt of treatment for high blood pressure or a blood pressure of more than 140/90 mmHg. Treatment for hypertension was sub-categorised (Table 1) to specifically address potential confounding on measurements of eGFR (diuretic induced volume depletion) and attenuation of albuminuria (Angiotensin Receptor Blockers, (ARB) or (Angiotensin Converting Enzyme Inhibitors, (ACE).
Albuminuria (urinary albumin/creatinine ratios) and eGFR (CKD-EPI estimating equation derived from serum creatinine) were obtained from laboratory measures and CKD was defined using the KDIGO (13) criteria; eGFR < 60 ml/min/1.73m2) and/or albuminuria: UACR > 3.0 mg/mmol.
The measures prefrail (1 or 2 domains) and frail (3, 4 or 5 domains) were defined from 5 measures adapted from the Fried (6) frailty domains (body weight, strength, exhaustion, walking speed and physical activity) as described previously (11). A relatively small number of individuals were observed to have frailty (3+ domains) and analyses were conducted of the dichotomous prefrailty-frailty (1+ domains) versus non-frail (fit and robust; 0 domains).
Statistical Analysis
Multivariable logistic regression models were employed relating prefrailty-frailty to each of (i) CKD, (13) (ii) continuous measures of eGFR and UACR, and (iii) categorised eGFR and UACR ranges (Table 2), while adjusting for key demographic and other likely confounders. We identified likely confounders as potential risk factors for frailty or prefrailty from prior studies (summarised in (8)) and plausible shared risk factors for CKD and frailty.
Table 2:
Adjusted odds ratios (OR) for associations between CKD Characteristics/Measures and Prefrailty-Frailty
| Chronic Kidney Disease (CKD) Characteristics | Multivariable Model |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Unadjusted ORs (See Table 1) | (i) CKD(13) | (ii) eGFR / albuminuria (continuous variables) | (iii) eGFR / albuminuria (categorical variables) | |||||||||
|
| ||||||||||||
| OR | (95% CI) | p value | OR | (95% CI) | p value | OR | (95% CI) | p value | ||||
| Family History of Kidney Disease | 1.16 | 1.02 | (0.89 - 1.16) | 0.797 | 1.03 | (0.90 - 1.18) | 0.682 | 1.02 | (0.89 - 1.16) | 0.789 | ||
| CKD (KDIGO Definition13) | 1.45 | 1.06 | (0.98 - 1.14) | 0.152 | ||||||||
| eGFR, ml/min/1.73 m2 | 0.99 | 1.00 | (1.00 - 1.00) | 0.097 | ||||||||
| eGFR Stage | ||||||||||||
| >= 60 ml/min/1.73 m2 | 1.00 | 1.00 | ||||||||||
| 45 - < 60 ml/min/1.73 m2 | 1.24 | 0.94 | (0.86 - 1.04) | 0.217 | ||||||||
| < 45 ml/min /1.73 m2 | 2.13 | 1.16 | (0.96 - 1.41) | 0.126 | ||||||||
| Albuminuria (log UACR, mg/mmol) | 1.18 | 1.07 | (1.04 - 1.10) | <0.001 | ||||||||
| Albuminuria Stage [UACR range, mg/mmol] | ||||||||||||
| no albuminuria (<= 3.0 mg/mmol) | 1.00 | 1.00 | ||||||||||
| albuminuria (> 3.0 mg/mmol) | 1.60 | 1.21 | (1.08 - 1.36) | 0.001 | ||||||||
UACR = Urinary Albumin Creatinine Ratio;
A two-sided P value < 0.05 was considered statistically significant. All analyses were conducted using Stata MP 17.1 (StataCorp, College Station, Texas, USA).
Results
Study Population
Of 19,114 (11) participants, 17,759 satisfied the inclusion criteria. Based on eGFR, only 2,677 (15%) participants had Stage 3a CKD (45-60 ml/min/ 1.73 m2) and 575 (3%) had Stage 3b or Stage 4 CKD (<45 ml/min/1.73m2) and only 1,797 (10%) participants had UACR > 3.0 mg/mmol (Table 1 of which only 120 (< 0.7% of total cohort) had UACR > 30 mg/mmol (macroalbuminuria)).
Prefrailty and Frailty Prevalence
Table 1 shows that participants in the non-frail (n=10,436) and prefrail-frail (n=7323) groups differed on many risk factors of interest.
All CKD related variables were identified as having an association with prefrailty-frailty in univariate analyses (Table 1).
In the multivariable regression model (Table 2 (i)), the adjusted OR for KDIGO-defined CKD (13), was attenuated compared to univariate analysis and lost statistical significance.
Further, neither continuous measures (Table 2 (ii)) nor categorical measures (Table 2 (iii)) of eGFR were found to have associations with prefrailty-frailty after adjustment. However, after the same adjustment, albuminuria did retain an association with prefrailty-frailty whether considered as a continuous variable (OR (95% CI) 1.07 (1.04 – 1.10), p< 0.001; Table 2 (ii)), or categorical variable (OR 1.21 (1.08 – 1.36), p= 0.001; Table 2 (iii)).
Because the adapted definition of prefrailty-frailty used low BMI as a surrogate for weight loss, a separate multivariable analysis, which did not include BMI in the model (data not shown) was carried out and did not influence the conclusions drawn from the results in Table 2.
Discussion
In our study of relatively healthy older individuals, the relationships between either CKD (13) or reduced eGFR and prefrailty-frailty observed on univariate analyses were not apparent when adjusted for other factors known to be associated with frailty syndromes. By contrast albuminuria remained strongly associated with prefrailty-frailty on multivariable analysis.
Prior studies have also noted that albuminuria is more strongly associated than eGFR with cardiovascular and geriatric outcomes such as cognitive impairment and rate of cognitive decline (14, 15). In a systematic review of the relation of UACR with cognitive impairment, in populations with less advanced CKD (eGFR > 45 ml/min/1.73m2), UACR was usually more strongly associated with cognitive impairment than eGFR, whereas in those with more severe CKD (eGFR < 45 ml/min/1.73m2), eGFR was the stronger risk factor (15, 16). One potential explanation is that albuminuria itself is associated with increased risk of decline in eGFR (17) and that this decline has not yet occurred in most participants in this ASPREE population (with mean eGFR of ~ 73 ml/min/1.73m2) and only 3% of subjects having an eGFR < 45 ml/min/1.73m2). Furthermore, as albuminuria is considered a marker of widespread vascular disease and inflammation, it may better reflect the multisystem changes related to inflammation including immune dysregulation and cellular senescence as seen in both frailty and CKD (18, 19) than eGFR.
An important limitation of this study is its cross-sectional nature. The findings are, however, consistent with the recent study (20), documenting a relationship between decline in GFR, but not baseline GFR, and incident frailty. A future examination of longitudinal data from ASPREE to ascertain the relationship between incident and worsening frailty and progressive albuminuria will be valuable. Given that frailty has a relationship to muscle mass another limitation is the lack of another measure of GFR in the ASPREE dataset such as Cystatin C. Notably however, in the multivariable model the adjustment or non-adjustment for BMI, had no impact on the conclusions.
In conclusion, in older adults, albuminuria may be more important to understanding the development of frailty than eGFR. However, the complex relationship between albuminuria (which may be a biomarker for vascular inflammation), ageing, progressive CKD and frailty requires additional study.
Acknowledgements
The work presented here was supported by the National Institute on Aging and the National Cancer Institute at the National Institutes of Health (grant number U01AG029824); the National Health and Medical Research Council of Australia (grant numbers 334047, 1127060); Monash University (Australia); and the Victorian Cancer Agency (Australia). The authors have no conflict of interest to declare in relation to this study.
Footnotes
DISCLOSURE We have no conflict of interest to report.
Address where Project was Carried out
School of Public Health & Preventive Medicine, Monash University, Alfred Campus, Melbourne 3004
References
- 1.Musso CG, Jauregui JR, Macias Nunez JF. Frailty phenotype and chronic kidney disease: a review of the literature. Int Urol Nephrol. 2015;47(11):1801–7. [DOI] [PubMed] [Google Scholar]
- 2.Glassock RJ, Warnock DG, Delanaye P. The global burden of chronic kidney disease: estimates, variability and pitfalls. Nat Rev Nephrol. 2017;13(2):104–14. [DOI] [PubMed] [Google Scholar]
- 3.Bohm C, Storsley L, Tangri N. The assessment of frailty in older people with chronic kidney disease. Curr Opin Nephrol Hypertens. 2015;24(6):498–504. [DOI] [PubMed] [Google Scholar]
- 4.Roshanravan B, Khatri M, Robinson-Cohen C, Levin G, Patel KV, de Boer IH, et al. A prospective study of frailty in nephrology-referred patients with CKD. Am J Kidney Dis. 2012;60(6):912–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weiner DE, Seliger SL. Cognitive and physical function in chronic kidney disease. Curr Opin Nephrol Hypertens. 2014;23(3):291–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3):M146–56. [DOI] [PubMed] [Google Scholar]
- 7.Sy J, Johansen KL. The impact of frailty on outcomes in dialysis. Curr Opin Nephrol Hypertens. 2017;26(6):537–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mei F, Gao Q, Chen F, Zhao L, Shang Y, Hu K, et al. Frailty as a Predictor of Negative Health Outcomes in Chronic Kidney Disease: A Systematic Review and Meta-Analysis. J Am Med Dir Assoc. 2021;22(3):535–43 e7. [DOI] [PubMed] [Google Scholar]
- 9.Chowdhury R, Peel NM, Krosch M, Hubbard RE. Frailty and chronic kidney disease: A systematic review. Arch Gerontol Geriatr. 2017;68:135–42. [DOI] [PubMed] [Google Scholar]
- 10.Aspree Investigator Group. Study design of ASPirin in Reducing Events in the Elderly (ASPREE): a randomized, controlled trial. Contemp Clin Trials. 2013;36(2):555–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Espinoza SE, Woods RL, Ekram A, Ernst ME, Polekhina G, Wolfe R, et al. The effect of low-dose aspirin on frailty phenotype and frailty index in community-dwelling older adults in the ASPirin in Reducing Events in the Elderly study. J Gerontol A Biol Sci Med Sci. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McNeil JJ, Woods RL, Nelson MR, Reid CM, Kirpach B, Wolfe R, et al. Effect of Aspirin on Disability-free Survival in the Healthy Elderly. N Engl J Med. 2018;379(16):1499–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.KDIGO. Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int Suppl. 2013;3((1)):1–150. [Google Scholar]
- 14.Sajjad I, Grodstein F, Kang JH, Curhan GC, Lin J. Kidney dysfunction and cognitive decline in women. Clin J Am Soc Nephrol. 2012;7(3):437–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Murray A, Thao L, Ryan J, Wolfe R, Wetmore J, Woods R, et al. Chronic kidney disease biomarkers, cognitive impairment and incident dementia in an older healthy cohort,. Kidney 360. 2022;3(3):435–445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Georgakis MK, Dimitriou NG, Karalexi MA, Mihas C, Nasothimiou EG, Tousoulis D, et al. Albuminuria in Association with Cognitive Function and Dementia: A Systematic Review and Meta-Analysis. J Am Geriatr Soc. 2017;65(6):1190–8. [DOI] [PubMed] [Google Scholar]
- 17.Oh SW, Kim S, Na KY, Kim KW, Chae DW, Chin HJ. Glomerular filtration rate and proteinuria: association with mortality and renal progression in a prospective cohort of a community-based elderly population. PLoS One. 2014;9(4):e94120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hillege HL, Fidler V, Diercks GF, van Gilst WH, de Zeeuw D, van Veldhuisen DJ, et al. Urinary albumin excretion predicts cardiovascular and noncardiovascular mortality in general population. Circulation. 2002;106(14):1777–82. [DOI] [PubMed] [Google Scholar]
- 19.Thillainadesan J, Scott IA, Le Couteur DG. Frailty, a multisystem ageing syndrome. Age Ageing. 2020;49(5):758–63. [DOI] [PubMed] [Google Scholar]
- 20.Guerville F, de Souto Barreto P, Taton B, Bourdel-Marchasson I, Rolland Y, Vellas B, et al. Estimated Glomerular Filtration Rate Decline and Incident Frailty in Older Adults. Clin J Am Soc Nephrol. 2019;14(11):1597–604. [DOI] [PMC free article] [PubMed] [Google Scholar]


