Abstract
Background:
Although patient participation in treatment decisions is important for preference-concordant care delivery, it is largely unknown how cognitive impairment influences treatment preferences. We investigated whether treatment preferences for the care of serious illness differ between adults with and without cognitive impairment in hypothetical clinical scenarios.
Methods:
Data from the 2018 Health and Retirement Study were used. The sample included 1,291 self-respondents (201 respondents with cognitive impairment, and 1,090 with normal cognition). We examined treatment preferences for life-extending, limited, and comfort care options in two hypothetical clinical scenarios where the respondent imagines a patient with: (1) good physical health with severe cognitive impairment consistent with dementia; and (2) with physical impairment due to a heart attack, but normal cognition. Respondents specified whether they were unsure, or if they would want or not want each treatment option. Linear probability models were used to compare treatment preferences by cognitive status.
Results:
Respondents with cognitive impairment were more likely to report that they were unsure about treatment options across both clinical scenarios compared to those with normal cognition. For the limited treatment option, cognitive impairment was associated with a lower rate of expressing a treatment preference by 7.3 (p=0.070) and 8.5 (p=0.035) percentage points for the dementia and heart attack scenarios, respectively. Among those who articulated preferences, cognitive impairment was associated with a higher rate of preference for life-extending treatment in both the dementia (30.1% vs 20.0%, p=0.044) and heart attack scenarios (30.0% vs 20.2%, p=0.033).
Conclusions:
Compared to those with normal cognition, cognitive impairment was associated with greater uncertainty about treatment preferences and higher rates of aggressive care preferences among those who specified preferences. Further research should assess whether preferences for aggressive care become more common as cognition declines in order to improve preference-concordant care delivery for patients with cognitive impairment.
Keywords: Cognitive Impairment, treatment preference, dementia, aging, observational study
INTRODUCTION
Patient participation in treatment decisions is important for aligning care with patients’ values, preferences, and goals. However, a common consequence of cognitive impairment is reduced decision-making capacity, which frequently results in a need for surrogate decision-making for healthcare treatment decisions.1 Experts suggest that advance care planning, such as preparation of a living will and durable power of attorney for health care, and discussions surrounding future treatment decisions, values, and care goals, should be completed at early signs of cognitive impairment or before onset to ensure patient preferences are clearly identified and followed.2–5 It is unclear, however, whether treatment preferences expressed prior to the onset of cognitive impairment are similar to those expressed after onset. If preferences, risk tolerance, and perception change as cognition deteriorates, surrogates relying on documents prepared prior to cognitive decline may lack accurate information about current preferences.
Assessing treatment preferences in individuals with early signs of impairment is useful to study because they are still able to participate in research studies. Evidence is conflicting, however, as to whether individuals with early cognitive decline are able to preserve treatment decision capacity. Several studies have found that individuals with mild cognitive impairment (MCI), a clinical diagnosis of an intermediate cognitive state between normal cognition and dementia, are at risk for decline in medical decision-making capacity.6–8 A recent meta-analysis of seven studies found reduced capacity to consent to medical treatment and research participation in persons with MCI.8 Evidence suggests that those with MCI are generally deficient on important consent to treat capacity measures including appreciation, reasoning, and understanding.6, 9, 10 One study found that around 40% of participants with MCI were considered incapable to consent.10 Those with MCI are more likely to exhibit poor decision-making such as susceptibility to scams11 and financial decision-making.12–14 Adverse financial events such as missed credit card payments are more prevalent prior to a diagnosis of dementia.14
However, other studies suggest that most people with MCI are able to retain decisional capacity.15, 16 Karlawish (2008) found that those with mild to moderate Alzheimer’s disease were able to participate in treatment decisions.15 Similarly, Horton-Deutsch and colleagues (2007) found that most participants with mild to moderate dementia were able to explain their rationale for their treatment decisions.16 Other studies suggest that individuals with MCI are able to make daily living decisions such as selecting surrogates,17 making financial decisions,18 and managing medications.19 A National Institute on Aging and Alzheimer’s Association workgroup stated that individuals with MCI, “may take more time, be less efficient, and make more errors at performing such activities than in the past. Nevertheless, they generally maintain their independence of function in daily life, with minimal aids or assistance.”20 Moreover, those with cognitive impairment typically indicate a desire to be the primary decision-maker and participate in treatment decisions.21 Caregivers agree that loved ones with cognitive decline should remain involved in treatment decisions.20, 22 Although cognitive impairment impacts decision-making, providers should involve patients in treatment decisions to the extent possible.
This study assesses whether cognitive impairment is associated with different treatment preferences for serious illness care among older adults who can still participate in survey research. How treatment preferences vary by cognitive status is largely unknown. A recent study found that individuals with MCI express no difference in life-extending treatment preferences compared to those with normal cognition; however, the study included only 66 participants with MCI that were recruited from two academic medical centers.23 We build on this work using new, nationally-representative survey data from the Health and Retirement Study.
METHODS
Data & Sample
We used 2018 data from the Health and Retirement Study (HRS). The HRS is a large, longitudinal, nationally-representative survey that collects detailed health and retirement information on respondents aged 51 years and older and their spouses every two years.24 The HRS provides information on patient demographics, socioeconomic indicators, and health status. In 2018, a 10% random subsample of respondents who did not require assistance from a proxy informant to complete the survey were administered a set of questions about their treatment preferences (n=1,745). There were 341 respondents who declined participation in the module. We excluded 9 respondents due to incomplete treatment preference responses and 61 respondents without cognitive measures. An additional 43 respondents below age 50 were excluded. Our final sample comprised 1,291 respondents, including 201 respondents with cognitive impairment, and 1,090 with normal cognition.
End-of-Life Treatment Preference Measurements
The HRS treatment preference module asked respondents how they would prefer health care decisions to be made under two hypothetical serious illness scenarios:
Dementia Scenario: “Imagine that you have brain damage or some brain disease like dementia which cannot be cured. The condition makes you unable to recognize people and speak understandably. You are physically healthy and could live in this condition for a long time.”
Heart Attack Scenario: “Imagine that you are unconscious due to a sudden, severe disease such as a heart attack. Your doctors believe that treatments can extend your life, but would leave you unable to get out of bed or move around without assistance for the rest of your life. You would not have memory or speech problems.”
For each scenario, respondents were asked about three treatment options–life-extending, limited, and comfort treatments. The HRS describes the life-extending treatment option as, “treatments that extend length of life but may be invasive or painful such as placing a feeding tube into your stomach for liquid food if you cannot eat on your own, or chest compressions to restart your heart if your heart stops beating, or major surgery like open heart surgery.” The limited treatment option was described as, “treatments with few side effects, like providing fluids through your veins to give you water if you cannot drink enough on your own or antibiotics if you develop an infection.” The comfort care treatment option was depicted as, “efforts to keep you as comfortable as possible, including pain medications.” For all three treatment options, respondents were asked whether they would ‘want this treatment’, ‘not want this treatment’, or were ‘unsure about this treatment’.
We created two primary measures of treatment preferences. First, we used a dichotomous indicator for whether the respondent specified a treatment preference. The variable equaled one if the respondent indicated that they either wanted or did not want the respective treatment. The reference group included respondents who indicated that they were unsure of their treatment preference. Second, we used a dichotomous indicator for if the respondent would want the treatment option.
Cognitive Measures
We examined treatment preferences by cognitive functioning. All members of our sample completed a Telephone Interview for Cognitive Status (TICS) as part of the HRS. TICS scores were crosswalked to cognition categories using the Langa-Weir approach. Langa-Weir classifies respondents as persons with normal cognition, mild cognitive impairment, or Dementia based on a subset of HRS cognitive functioning questions. Classification is based on a 27-point scale that assesses immediate and delayed word recall, ability to complete simple subtraction, and backwards counting. Respondents with a cognitive assessment score between 0 and 11 were considered cognitively impaired. Because the HRS only asks treatment preference questions to respondents who are able to complete the survey themselves rather than a proxy respondent, our sample excludes respondents with severe cognitive issues. The sample contained <2% of respondents classified as ‘persons with dementia’. The full methodology to classify cognitive functioning has been described elsewhere.25
The Langa-Weir approach was previously validated from the Aging, Demographics and Memory Study (ADAMS).26–28 The ADAMS study subsampled HRS respondents aged 70 and above. Respondents were given cognitive assessment questions and received a neuropsychiatric assessment as well as detailed physical and cognitive exams. The prevalence of MCI found by the Langa-Weir classification was similar to the neuropsychiatric assessments.
Control Variables
We adjusted for respondent characteristics that have been associated with treatment preferences.29, 30 We used self-reported demographic covariates including age, sex, race (black, white, and another race), Hispanic ethnicity, and marriage status (married or divorced, separated, widowed, or never married). Another race included Alaskan Native, Asian, Native Hawaiian, and Pacific Islander. Socioeconomic variables were included, such as education (less than high school, high school or some college, and college or above), and an indicator for above-median household financial wealth (>$122,000 dollars). We controlled for health status using an indicator for fair/poor self-rated health (versus good or better), one or more comorbidities (diabetes, cancer, lung disease, heart condition, stroke, psychiatric disorder), a hospitalization in the past two years, and a heart condition. We also included an indicator for whether the respondent reported a parent or in-law with dementia since familiarity with parents’ end-of-life care may influence preferences. Religion and spirituality may also be important correlates of treatment preference.31, 32 We measured religiosity as a binary indicator for if the respondent attended a religious event at least monthly.
Statistical Analysis
For both clinical scenarios, we estimated linear probability models to compare preferences of those with and without cognitive impairment. We used linear probability models instead of logistic regression to aid in interpretation of results. Models controlled for demographic, health, and socioeconomic characteristics listed in Table 1. For each treatment option (i.e., life-extending, limited, and comfort care), we first estimated the probability of specifying a treatment preference compared to those who reported they were unsure if they would want or would forgo a treatment. For those who articulated a treatment preference, we then estimated the probability of wanting each treatment option. The reference group for the second model was respondents who ‘did not want’ treatment. All models used robust standard errors. We also conducted sensitivity analyses using logistic regression models and changing the cognitive impairment threshold to 0–10 for minority respondents because ethno-racial differences in performance on cognitive assessments may overestimate the prevalence of cognitive impairment for minority populations.33, 34 All analyses were performed using Stata v.16. This study was determined not to be human subjects research by the Colorado Multiple Institutional Review Board.
Table 1.
Characteristics of HRS respondents by cognitive status, 2018
| Full Sample (n=1,291) | Cognitively Normal (n=1,090) | Cognitive Impairment (n=201) | P-Value | |
|---|---|---|---|---|
| Respondent Characteristics | ||||
| Age (mean) | 66.9 | 66.4 | 69.7 | <0.001 |
| Female | 56.9 | 57.7 | 52.7 | 0.191 |
| Married | 63.2 | 63.6 | 61.2 | 0.520 |
| Black | 20.6 | 19.4 | 26.9 | 0.017 |
| White | 66.5 | 69.7 | 48.8 | <0.001 |
| Other | 12.9 | 10.8 | 24.4 | <0.001 |
| Hispanic | 14.7 | 12.2 | 28.4 | <0.001 |
| Less than High school | 12.9 | 8.8 | 34.8 | <0.001 |
| High school or some college | 60.0 | 60.6 | 56.2 | 0.240 |
| College | 27.2 | 30.6 | 9.0 | <0.001 |
| Above median wealth | 50.1 | 52.8 | 35.8 | <0.001 |
| Children | 90.2 | 89.7 | 92.5 | 0.219 |
| Recent Hospitalization | 22.4 | 22.3 | 22.9 | 0.853 |
| Self-rated Fair or poor health | 26.2 | 23.6 | 40.3 | <0.001 |
| Any comorbidities | 63.3 | 62.8 | 66.2 | 0.356 |
| Heart condition | 24.8 | 23.9 | 29.4 | 0.103 |
| Parent/In-law with dementia | 13.0 | 12.6 | 15.4 | 0.269 |
| Missing/unsure parent dementia | 50.0 | 49.1 | 55.2 | 0.110 |
| Attend religious event monthly | 50.1 | 49.7 | 52.2 | 0.512 |
Notes: Numbers are reported as percentages except for age. P-values are determined from chi-square tests and t-tests for association by cognitive status. A recent hospitalization includes a hospitalization in the past two years. ‘Any comorbidities’ represents respondents with diabetes, cancer, lung disease, a heart condition, past stroke, or a psychiatric disorder.
RESULTS
Respondent characteristics are presented in Table 1. Overall, 15.6% of respondents were classified as cognitively impaired (n=201). The study population had a median age of 65 and ranged from age 50–94. 57% of the sample was female, 63% were married, and 66.5% were white. The majority had at least a high school degree (87%), and around a quarter considered themselves to be in fair or poor health. Respondents whose cognitive function was considered normal were more likely to be younger (66.4 versus 69.7, p<0.001), white (69.7 versus 48.8, p<0.001), non-Hispanic (12.2 versus 28.4, p<0.001), college educated (30.6 versus 9.0, p<0.001), and were less likely to report fair or poor health (23.6 vs 40.3, p<0.001).
Table 2 displays treatment preferences reported in the hypothetical dementia and heart scenarios. We report the number and percent of respondents who selected each response possibility (i.e., unsure, wanted, or did not want treatment) for each treatment option. Overall, respondents were more likely to report wanting comfort or limited treatment options compared to life-extending treatments. Only 12.9% and 14.1% wanted life-extending treatment for the dementia and heart attack scenarios, respectively. More respondents selected unsure about life-extending treatments compared to limited or comfort treatments. Respondents with cognitive impairment were more likely to select unsure relative to respondents with normal cognition across scenarios and treatment options. For the life-extending treatment option in the dementia scenario, 47.3% of respondents with cognitive impairment selected unsure compared to 37.9% who were cognitively normal (p=0.012). For the limited treatment option in both the heart attack and dementia scenarios, around 43% of respondents with cognitive impairment selected unsure, compared to over 28% of those who were cognitively normal (p<0.001). Similarly, around 36% of respondents with cognitive impairment selected they were unsure for the comfort treatment option relative to only a quarter of those with normal cognition (p<0.01).
Table 2.
Treatment preferences of HRS respondents by cognitive status, 2018
| Full Sample (n=1,291) | Cognitively Normal (n=1,090) | Cognitively Impaired (n=201) | P-value | |
|---|---|---|---|---|
| Dementia Scenario | ||||
| Life-Extending Treatment | ||||
| Unsure | 508 (39.3%) | 413 (37.9%) | 95 (47.3%) | 0.012 |
| Wants Treatment | 167 (12.9%) | 131 (12.0%) | 36 (17.9%) | 0.022 |
| Does not want Treatment | 616 (47.7%) | 546 (50.1%) | 70 (34.8%) | <0.001 |
| Limited Treatment | ||||
| Unsure | 390 (30.2%) | 303 (27.8%) | 87 (43.2%) | <0.001 |
| Wants Treatment | 658 (51.0%) | 572 (52.5%) | 86 (42.8%) | 0.012 |
| Does not want Treatment | 243 (18.8%) | 215 (19.7%) | 28 (13.9%) | 0.053 |
| Comfort Treatment | ||||
| Unsure | 358 (27.7%) | 283 (26.0%) | 75 (37.3%) | <0.001 |
| Wants Treatment | 843 (65.3%) | 734 (67.3%) | 109 (54.3%) | <0.001 |
| Does not want Treatment | 90 (7.0%) | 73 (6.7%) | 17 (8.5%) | 0.368 |
| Heart attack scenario | ||||
| Life-Extending Treatment | ||||
| Unsure | 450 (34.9%) | 375 (34.4%) | 75 (37.3%) | 0.426 |
| Wants Treatment | 182 (14.1%) | 143 (13.1%) | 39 (19.4%) | 0.019 |
| Does not want Treatment | 659 (51.0%) | 572 (52.5%) | 87 (43.4%) | 0.017 |
| Limited Treatment | ||||
| Unsure | 397 (30.8%) | 309 (28.4%) | 88 (43.9%) | <0.001 |
| Wants Treatment | 655 (50.7%) | 579 (53.1%) | 76 (37.8%) | <0.001 |
| Does not want Treatment | 239 (18.5%) | 202 (18.5%) | 37 (18.4%) | 0.967 |
| Comfort Treatment | ||||
| Unsure | 352 (27.3%) | 279 (25.6%) | 73 (36.3%) | 0.002 |
| Wants Treatment | 828 (64.1%) | 728 (66.8%) | 100 (49.8%) | <0.001 |
| Does not want Treatment | 111 (8.6%) | 83 (7.6%) | 28 (13.9%) | 0.003 |
Notes: P-values are determined from chi-square tests for association by cognitive status.
There were also notable differences in whether respondents would want or not want each treatment option. For the dementia scenario, an estimated 17.9% of respondents with cognitive impairment preferred life-extending treatments compared to 12.0% of respondents who were cognitively normal (p=0.022). Similarly, respondents with normal cognition were more likely to select that they would not want life-extending treatment (50.1% versus 34.8%, p<0.001). Those with normal cognition were more likely to report wanting limited (52.5% versus 42.8%, p=0.012) and comfort care (67.3% versus 54.3%, p<0.001) treatments compared to those with cognitive impairment in the dementia scenario. For the heart attack scenario, very similar patterns emerged for respondents with normal cognition. However, respondents with cognitive impairment were more likely to specify a preference for life-extending treatment in the heart attack scenario compared to the dementia scenario (63% compared to 53%). Fewer respondents with cognitive impairment also reported that they would want comfort care in the heart attack scenario (50% versus 54%).
Coefficient plots from the linear probability models are displayed in Figures 1 and 2. Results for whether respondents specified a treatment preference are displayed in Figure 1. Cognitive impairment was associated with a decreased probability of expressing a preference across all treatment types and scenarios, although not statistically significantly in all models. For the limited treatment option, those with cognitive impairment were less likely to express a treatment preference by 7.3 (p=0.070) and 8.5 (p=0.035) percentage points in the dementia and heart attack scenarios, respectively. Increasing age was negatively associated with specifying a preference for the limited and comfort treatment options (p<0.01), but not statistically significant for life-extending treatment. In the dementia scenario, the indicator for female was marginally associated with specifying a preference for limited (0.046, p=0.080) and comfort (0.052, p=0.044) treatments. Race and ethnicity were also associated with specifying a preference. Black and Hispanic was associated with a lower likelihood of expressing a treatment preference in both scenarios compared to their white counterparts. Black race was also associated with a 20.5 (p<0.001) and 10.1 (p=0.007) percentage point lower probability of specifying a preference for life-extending treatment in the dementia and heart attack scenario, respectively. A college degree was associated with an increased probability of specifying a preference for life-extending treatment by 9.0 (p=0.004) percentage points in the dementia scenario. A parent or in-law with dementia was associated with a decrease in the probability of specifying a treatment preference for limited (–.076, p=0.067) and comfort care (–.096, p=0.019) in the dementia scenario. Attending religious events at least monthly was also marginally associated with a lower likelihood of specifying a treatment preference.
Figure 1.
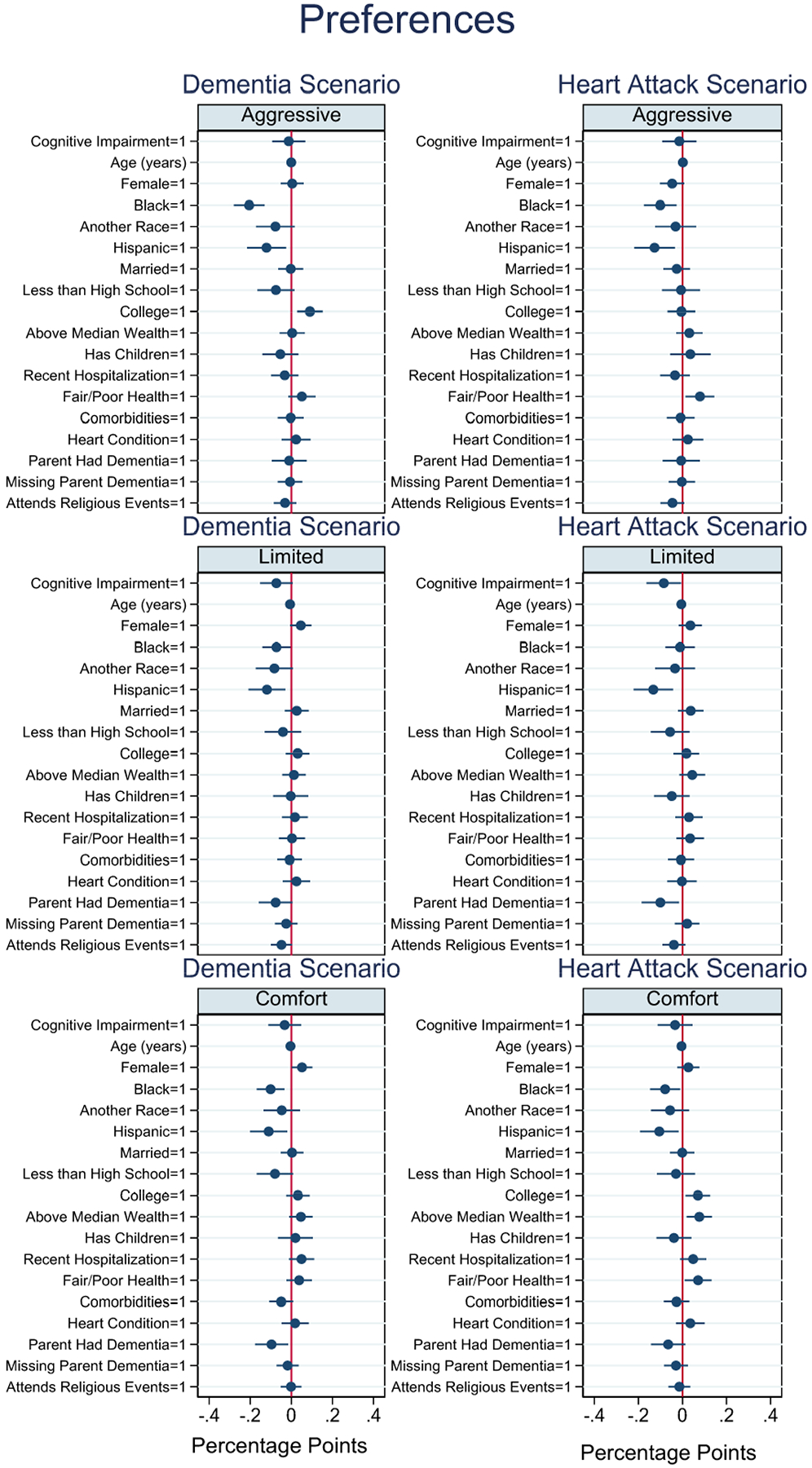
Coefficient plots of specifying a treatment preference
Figure 2.
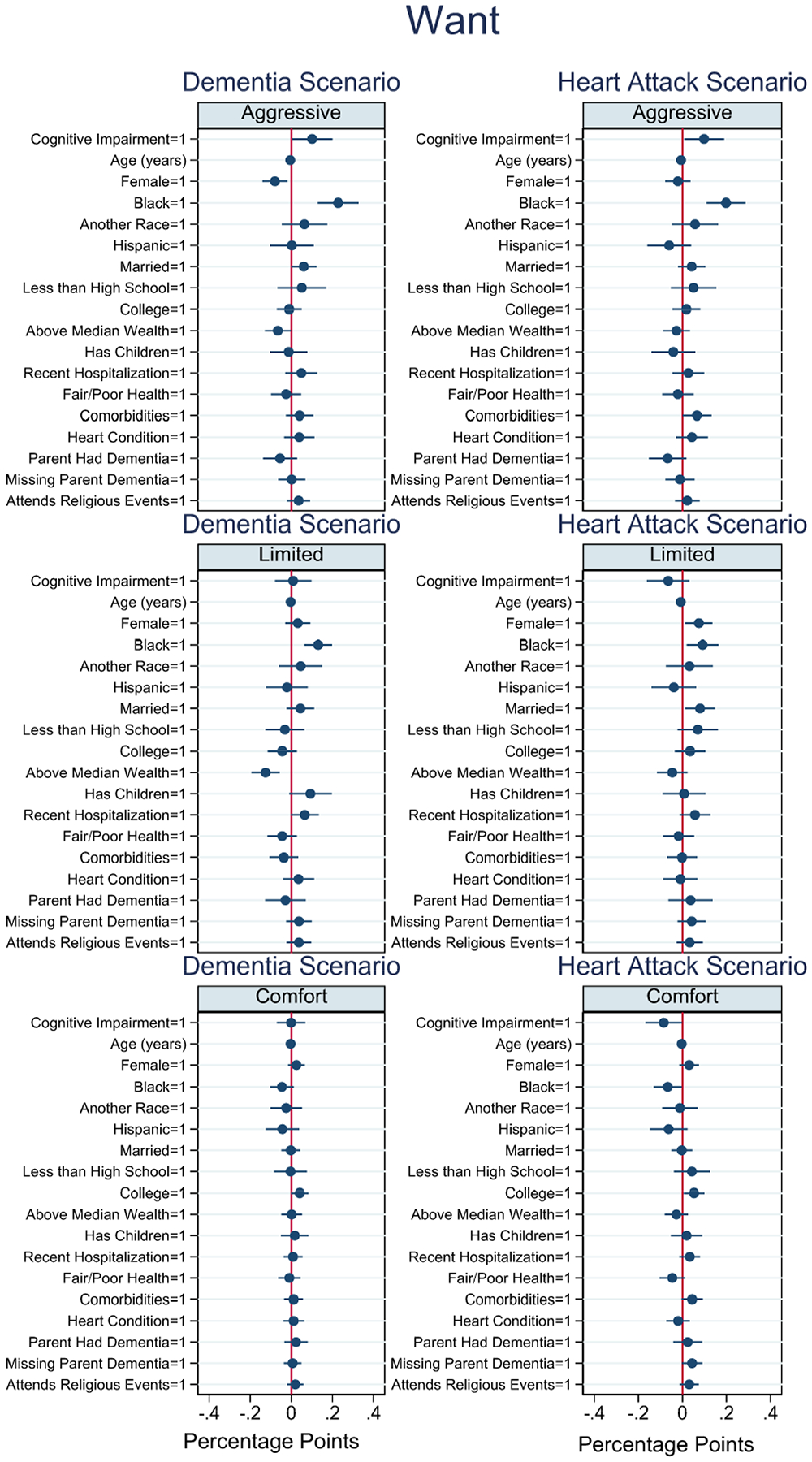
Coefficient plots of wanting a treatment preference
Figure 2 displays whether respondents prefer a given treatment option conditional on specifying a treatment preference. Cognitive impairment was associated with an increased rate of preferring life-extending treatment by 10.1 (p=0.044) and 9.8 (p=0.033) percentage points in the dementia and heart attack scenarios, respectively. Cognitive impairment was not associated with wanting limited or comfort treatments in the dementia scenario. In the heart attack scenario, cognitive impairment was associated with an 8.5 percentage point lower probability of wanting comfort treatment (p=0.044). Age was negatively associated with wanting treatment across all treatment options and scenarios (p<0.05). Female sex was also associated with a reduced probability of preferring life-extending treatment by 8.0 (p=0.009) percentage points in the dementia scenario. Black respondents were more likely to want life-extending and limited treatments relative to white respondents in both scenarios (p<0.05). Respondents above median household wealth were less likely to want life-extending and limited treatments in the dementia scenario (p<0.05). Fair/poor health also reduced the probability of wanting all treatments, however, the findings were not statistically significant.
Full model results and sensitivity analyses can be found in the Supplemental Materials. The average marginal effects computed from the logistic regression models were very similar to the results using linear probability models (Tables S1–S12). Sensitivity analyses using a more conservative measure of cognitive impairment for minority populations were also generally consistent with our main results (Tables S13–S16). Cognitive impairment still decreased the likelihood of specifying a treatment preference for the limited treatment options, however, these results were no longer significant (Tables S13–S14). Cognitive impairment continued to be significantly associated with an increase in the likelihood of wanting life-extending care in both the dementia and heart attack scenarios (Tables S15–S16).
DISCUSSION
We compared serious illness treatment preferences in hypothetical clinical scenarios by cognitive status using a large, nationally-representative survey. We found that even when controlling for a number of health, socioeconomic, and demographic variables, cognitive impairment was associated with multiple dimensions of treatment preferences. Compared to those with normal cognition, cognitive impairment was associated with greater uncertainty across all scenarios and treatment options, although only statistically significantly for the limited treatment option. For respondents who articulated a treatment preference, cognitive impairment was associated with higher rates of preferring life-extending treatments in both the dementia and heart attack scenarios.
Respondents with cognitive impairment possibly had greater difficulty determining their treatment preferences. This finding supports prior research that cognitive impairment negatively impacts decisional capacity.6–11 Greater uncertainty of treatment decisions for individuals with cognitive impairment warrants earlier discussions with all patients on end-of-life treatment preferences. Earlier discussions could promote preference-concordant care given the possibility of compromised decision-making post-cognitive impairment. Moreover, although poor health is generally associated with a preference for forgoing invasive treatments,29 cognitive impairment was associated with increased rates of preferring life-extending care across both dementia and heart attack scenarios. Conversely, cognitive impairment was associated with a lower probability of wanting limited and comfort treatment options in the heart attack scenario. It is possible that compromised decision-making among individuals with cognitive impairment increases the likelihood of preferring life-prolonging or invasive treatment options. Future research should examine to what extent differences in preference due to cognitive impairment is because of deteriorating decision-making and risk assessment capabilities.
Providers may be less likely to recommend guideline-concordant, life-prolonging care as cognition deteriorates.35 Studies have found that patients with preexisting MCI have a lower probability of receiving evidence-based treatments following acute myocardial infarction or acute ischemic stroke.36, 37 Some providers may assume that patients with cognitive impairment prefer less treatment due to perceived lower life expectancy or worsened quality of life.35 However, our findings are consistent with previous studies suggesting that those with cognitive impairment do not prefer fewer life-extending treatments.23 Education efforts may be necessary to improve providers’ understanding of cognitive impairment prognosis and treatment preferences associated with cognitive impairment.
Limitations
Our study should be interpreted in the context of a number of limitations. First, this study is a cross-sectional, observational study design, and we are unable to establish a causal relationship between cognitive status and treatment preferences. We included a number of variables that have been shown to be associated with treatment preferences; nonetheless, there may be unmeasured factors that bias our findings. Treatment decisions are not made in isolation and are influenced by a person’s life experience, values, relationships, and culture, which are unaccounted for in the study. Future studies should assess treatment preferences for those with cognitive impairment longitudinally to understand whether treatment preferences are stable over time.
The HRS contains self-reported data; however, the key measure for cognitive impairment has been externally validated elsewhere.26–28 Although we only observe individuals that are capable of completing the HRS survey independently, we expect that most individuals with cognitive impairment were able to complete the survey. While we did not have clinical diagnoses for sample members, performance on the Telephone Interview for Cognitive Status for those with cognitive impairment was generally consistent with MCI. However, respondents may not have been clinically diagnosed with MCI and we cannot assess whether the knowledge of a MCI diagnosis influences treatment preferences. Nonetheless, it is important to study this population considering studies have demonstrated impacts of cognitive impairment on decision-making before a clinical diagnosis.11, 14, 38–40 In addition, while the 10-percentage point absolute difference in the preference for life-extending care suggests a clinically significant difference in care between those with and without cognitive impairment, the HRS questions are hypothetical clinical scenarios and it is unclear if preferences would differ in real-life clinical circumstances. The analysis also used multiple outcomes and significant findings may be a result of a Type I error, or a false positive result.
Lastly, sensitivity analyses found that the association between cognitive impairment and treatment preferences was attenuated with a more conservative measurement for minority individuals. Experts continue to debate if and how cognitive assessments should adjust for race and ethnicity.41 Changing the cognitive impairment threshold decreased the prevalence of cognitive impairment from 20% to 15% for Blacks and 30% to 23% for Hispanics. A recent study using neurological assessments found the prevalence of MCI was 20% for Hispanics, 25% for Blacks, and 12% for Whites.42 Therefore, the cognitive impairment measure used in the primary analyses may be most consistent with MCI, especially for Black populations.
Conclusions
Cognitive impairment is an important factor associated with treatment preferences in clinical hypothetical scenarios. Cognitive impairment was associated with greater uncertainty in treatment preferences and higher rates of life-extending care preferences. Providers should be aware that cognitive impairment impacts treatment decisions and incorporate discussions of end-of-life treatment preferences early on in the disease process. Future research should assess whether preferences become more aggressive as cognition declines to improve preference-concordant care for patients with cognitive impairment.
Supplementary Material
Key points:
Respondents with cognitive impairment were more likely to report that they were unsure about treatment options and had a higher rate of preference for life-extending treatment compared to those with normal cognition.
Why does this matter? Providers should be aware that cognitive impairment may impact treatment decisions. Treatment preferences are complex and should be discussed earlier in the disease process and reassessed as cognition deteriorates to help caregivers and members of the care team make decisions reflecting patients’ treatment preferences.
ACKNOWLEDGMENTS
Sponsors’ role:
This study was funded by the National Institutes of Aging grant number R01AG059205. The sponsors had no role in the design, analysis, or preparation of the paper.
Footnotes
Supporting Information: Sensitivity analyses.
Tables S1–S12. Sensitivity analyses using logistic regression models
Tables S13–16. Sensitivity analyses with a more restrictive cognitive impairment measure for minority respondents.
Conflict of interest: Authors have no conflicts of interest to disclose.
REFERENCES
- [1].Kim SY, Karlawish JH, Caine ED. Current state of research on decision-making competence of cognitively impaired elderly persons. Am J Geriatr Psychiatry. 2002;10: 151–165. [PubMed] [Google Scholar]
- [2].deLima Thomas J, Sanchez‐Reilly S, Bernacki R, et al. Advance care planning in cognitively impaired older adults. J Am Geriatr Soc. 2018;66: 1469–1474. [DOI] [PubMed] [Google Scholar]
- [3].Garand L, Dew MA, Lingler JH, DeKosky ST. Incidence and predictors of advance care planning among persons with cognitive impairment. Am J Geriatr Psychiatry. 2011;19: 712–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Lyketsos CG, Colenda CC, Beck C, et al. Position statement of the American Association for Geriatric Psychiatry regarding principles of care for patients with dementia resulting from Alzheimer disease. Am J Geriatr Psychiatry. 2006;14: 561–573. [DOI] [PubMed] [Google Scholar]
- [5].Shanley C, Fetherstonhaugh D, McAuliffe L, Bauer M, Beattie E. Providing support to surrogate decision‐makers for people living with dementia: Healthcare professional, organisational and community responsibilities. Health Soc Care Community. 2017;25: 1563–1570. [DOI] [PubMed] [Google Scholar]
- [6].Okonkwo OC, Griffith HR, Copeland JN, et al. Medical decision-making capacity in mild cognitive impairment: a 3-year longitudinal study. Neurology. 2008;71: 1474–1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Han SD, Boyle PA, James BD, Yu L, Bennett DA. Mild cognitive impairment is associated with poorer decision‐making in community‐based older persons. J Am Geriatr Soc. 2015;63: 676–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Parmigiani G, Del Casale A, Mandarelli G, et al. Decisional capacity to consent to treatment and research in patients affected by Mild Cognitive Impairment. A systematic review and meta-analysis. Int Psychogeriatr. 2021: 1–14. [DOI] [PubMed] [Google Scholar]
- [9].Okonkwo O, Griffith H, Belue K, et al. Cognitive models of medical decision-making capacity in patients with mild cognitive impairment. J Int Neuropsychol Soc. 2008;14: 297–308. [DOI] [PubMed] [Google Scholar]
- [10].Jefferson AL, Lambe S, Moser DJ, Byerly LK, Ozonoff A, Karlawish JH. Decisional capacity for research participation in individuals with mild cognitive impairment. J Am Geriatr Soc. 2008;56: 1236–1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Boyle PA, Yu L, Wilson RS, Gamble K, Buchman AS, Bennett DA. Poor decision making is a consequence of cognitive decline among older persons without Alzheimer’s disease or mild cognitive impairment. PLoS One. 2012;7: e43647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Niccolai LM, Triebel KL, Gerstenecker A, et al. Neurocognitive predictors of declining financial capacity in persons with mild cognitive impairment. Clin Gerontol. 2017;40: 14–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Martin RC, Gerstenecker A, Triebel KL, et al. Declining financial capacity in mild cognitive impairment: A six-year longitudinal study. Arch Clin Neuropsychol. 2019;34: 152–161. [DOI] [PubMed] [Google Scholar]
- [14].Nicholas LH, Langa KM, Bynum JP, Hsu JW. Financial presentation of Alzheimer disease and related dementias. JAMA internal medicine. 2021;181: 220–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Karlawish J Measuring decision-making capacity in cognitively impaired individuals. Neurosignals. 2008;16: 91–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Horton-Deutsch S, Twigg P, Evans R. Health care decision-making of persons with dementia. Dementia. 2007;6: 105–120. [Google Scholar]
- [17].Feinberg LF, Whitlatch J. Decision-making for persons with cognitive impairment and their family caregivers. Am J Alzheimers Dis Other Demen. 2002;17: 237–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Lui VW, Lam LC, Chau RC, et al. Structured assessment of mental capacity to make financial decisions in Chinese older persons with mild cognitive impairment and mild Alzheimer disease. J Geriatr Psychiatry Neurol. 2013;26: 69–77. [DOI] [PubMed] [Google Scholar]
- [19].Lui VW-C, Lam LC-W, Chau RC-M, et al. Capacity to make decisions on medication management in Chinese older persons with mild cognitive impairment and mild Alzheimer’s disease. Int Psychogeriatr. 2012;24: 1103–1111. [DOI] [PubMed] [Google Scholar]
- [20].Albert MS, DeKosky ST, Dickson D, et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Focus (Madison). 2013;11: 96–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Miller LM, Whitlatch CJ, Lyons KS. Shared decision-making in dementia: a review of patient and family carer involvement. Dementia. 2016;15: 1141–1157. [DOI] [PubMed] [Google Scholar]
- [22].Hirschman KB, Joyce CM, James BD, Xie SX, Casarett DJ, Karlawish JH. Would caregivers of Alzheimer disease patients involve their relative in a decision to use an AD-slowing medication? Am J Geriatr Psychiatry. 2005;13: 1014–1021. [DOI] [PubMed] [Google Scholar]
- [23].Levine DA, Galecki AT, Plassman BL, et al. The Association Between Mild Cognitive Impairment Diagnosis and Patient Treatment Preferences: a Survey of Older Adults. J Gen Intern Med. 2021: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Sonnega A, Faul JD, Ofstedal MB, Langa KM, Phillips JW, Weir DR. Cohort Profile: the Health and Retirement Study (HRS). Int J Epidemiol. 2014;43: 576–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Langa KM, David R. Weir, Mohammed Kabeto, and Amanda Sonnega. Langa-Weird Classification of Cognitive Function (Onward). Survey Research Center, Institute for Social Research, University of Michigan, 2020. [Google Scholar]
- [26].Crimmins EM, Kim JK, Langa KM, Weir DR. Assessment of cognition using surveys and neuropsychological assessment: the Health and Retirement Study and the Aging, Demographics, and Memory Study. J Gerontol B Psychol Sci Soc Sci. 2011;66: i162–i171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Langa KM, Plassman BL, Wallace RB, et al. The Aging, Demographics, and Memory Study: study design and methods. Neuroepidemiology. 2005;25: 181–191. [DOI] [PubMed] [Google Scholar]
- [28].Plassman BL, Langa KM, Fisher GG, et al. Prevalence of cognitive impairment without dementia in the United States. Ann Intern Med. 2008;148: 427–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Auriemma CL, Nguyen CA, Bronheim R, et al. Stability of end-of-life preferences: a systematic review of the evidence. JAMA internal medicine. 2014;174: 1085–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Quill CM, Ratcliffe SJ, Harhay MO, Halpern SD. Variation in decisions to forgo life-sustaining therapies in US ICUs. Chest. 2014;146: 573–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Balboni TA, Vanderwerker LC, Block SD, et al. Religiousness and spiritual support among advanced cancer patients and associations with end-of-life treatment preferences and quality of life. J Clin Oncol. 2007;25: 555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Carr D, Moorman SM. End‐of‐Life Treatment Preferences Among Older Adults: An Assessment of Psychosocial Influences 1. Sociol Forum, Volume 24: Wiley Online Library, 2009, pp. 754–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Briceño EM, Mehdipanah R, Gonzales XF, et al. Neuropsychological assessment of mild cognitive impairment in Latinx adults: A scoping review. Neuropsychology. 2020;34: 493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Weuve J, Barnes LL, de Leon CFM, et al. Cognitive aging in black and white Americans: cognition, cognitive decline, and incidence of Alzheimer disease dementia. Epidemiology (Cambridge, Mass). 2018;29: 151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Levine DA, Langa KM, Fagerlin A, et al. Physician decision-making and recommendations for stroke and myocardial infarction treatments in older adults with mild cognitive impairment. PLoS One. 2020;15: e0230446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Levine DA, Langa KM, Galecki A, et al. Mild cognitive impairment and receipt of treatments for acute myocardial infarction in older adults. J Gen Intern Med. 2020;35: 28–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Levine DA, Galecki AT, Morgenstern LB, et al. Preexisting Mild Cognitive Impairment, Dementia, and Receipt of Treatments for Acute Ischemic Stroke. Stroke. 2021;52: 2134–2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Boyle PA, Wilson RS, Yu L, et al. Much of late life cognitive decline is not due to common neurodegenerative pathologies. Ann Neurol. 2013;74: 478–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].James BD, Boyle PA, Bennett DA. Correlates of susceptibility to scams in older adults without dementia. Journal of elder abuse & neglect. 2014;26: 107–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Tucker-Drob EM. Neurocognitive functions and everyday functions change together in old age. Neuropsychology. 2011;25: 368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Possin KL, Tsoy E, Windon CC. Perils of race-based norms in cognitive testing: The case of former NFL players. JAMA neurology. 2021;78: 377–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Wright CB, DeRosa JT, Moon MP, et al. Race/ethnic disparities in mild cognitive impairment and dementia: the Northern Manhattan Study. J Alzheimers Dis. 2021;80: 1129–1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


