Abstract
Background.
The clinical outcomes associated with, and risk factors for, carbapenem-resistant Enterobacterales (CRE) bloodstream infections (BSIs) in solid organ transplant (SOT) recipients remain ill-defined.
Methods.
A multicenter retrospective cohort study was performed, including SOT recipients with an Enterobacterales BSI between 2005 and 2018. Exposed subjects were those with a CRE BSI. Unexposed subjects were those with a non-CRE BSI. A multivariable survival analysis was performed to determine the association between CRE BSI and risk of all-cause mortality within 60 d. Multivariable logistic regression analysis was performed to determine independent risk factors for CRE BSI.
Results.
Of 897 cases of Enterobacterales BSI in SOT recipients, 70 (8%) were due to CRE. On multivariable analysis, CRE BSI was associated with a significantly increased hazard of all-cause mortality (adjusted hazard ratio, 2.85; 95% confidence interval [CI], 1.68-4.84; P < 0.001). Independent risk factors for CRE BSI included prior CRE colonization or infection (adjusted odds ratio [aOR] 9.86; 95% CI, 4.88-19.93; P < 0.001), liver transplantation (aOR, 2.64; 95% CI, 1.23-5.65; P = 0.012), lung transplantation (aOR, 3.76; 95% CI, 1.40-10.09; P = 0.009), and exposure to a third-generation cephalosporin (aOR, 2.21; 95% CI, 1.17-4.17; P = 0.015) or carbapenem (aOR, 2.80; 95% CI, 1.54-5.10; P = 0.001) in the prior 6 months.
Conclusions.
CRE BSI is associated with significantly worse outcomes than more antibiotic-susceptible Enterobacterales BSI in SOT recipients.
INTRODUCTION
Carbapenem-resistant Enterobacterales (CRE) infections have been designated an urgent antibiotic resistance threat by the Centers for Disease Control and Prevention.1 Although the prevalence varies by region, CRE are increasingly endemic throughout the United States2 and globally.3–5
CRE infections have disproportionately affected the solid organ transplant (SOT) population. SOT recipients experience rates of CRE infection up to five-times greater than the general population.6–8 This is likely driven by SOT recipients’ multicomorbidity, frequent healthcare and antibiotic exposures, frequent procedures, and chronic indwelling medical devices.9–12 Notably, however, several studies have identified transplant status as an independent risk factor for CRE infection, even after adjusting for other risk factors,7 suggesting there may be issues unique to transplantation that increase the risk for CRE infection.12 Furthermore, prior studies have indicated that outcomes following CRE infection in SOT recipients are poor, with rates of graft failure and death ranging between 12% and 66%, depending on the transplant cohort, the type of CRE infection studied, and the duration of follow-up.6,13–16
Despite the outsized impact of CRE on SOT recipients, there is a limited understanding of the clinical risk factors for CRE infection and the impact of CRE infection on SOT recipient outcomes. Much of the data regarding CRE infections in SOT recipients are derived from small single-center studies, limited to abdominal organ transplant recipients, include heterogeneous infection types (eg, CRE urinary tract infections and bloodstream infections [BSIs]), and have limited durations of patient follow-up.6,17,18
As a result, in this study, we sought to determine (1) the impact of CRE BSI on the risk of all-cause mortality and new-onset graft failure and (2) the risk factors for developing CRE BSI in SOT recipients, using a large multicenter cohort of all organ transplant types. This is the largest and only multicenter cohort of SOT recipients with CRE BSI reported to date.14,17,19–21
MATERIALS AND METHODS
Study Design and Setting
A multicenter retrospective cohort study was performed at three tertiary care transplant centers in the United States: The Hospital of the University of Pennsylvania (HUP) (776 beds), The Johns Hopkins Hospital (JHH) (1154 beds), and the University of Maryland Medical Center (UMMC) (767 beds).
Study Population
The initial source population included all SOT recipients with an Enterobacterales (EB) BSI identified at HUP or UMMC between January 1, 2007, and June 30, 2018, and at JHH between January 1, 2005, and December 31, 2015 (with the varying time periods being due to differences in data availability). Because the microbiology laboratories at each institution process both inpatient and outpatient cultures, the cohort included any SOT recipient with an EB BSI regardless of the geographic location at which the BSI was identified, although all subjects were ultimately hospitalized. For those SOT recipients with multiple EB BSI during the study period, only the first episode was included.
For the determination of the impact of CRE BSI on clinical outcomes, the cohort was divided into exposed and unexposed subjects: Exposed subjects were those with a CRE BSI. CRE BSI was defined by any EB on blood culture that exhibited in vitro nonsusceptibility to any carbapenem, that is, minimum inhibitory concentration [MIC] ≥2 μg/mL for ertapenem or MIC ≥4 μg/mL for meropenem, doripenem, or imipenem before 2011, or MIC ≥1 μg/mL for ertapenem or MIC ≥2 μg/mL for meropenem, doripenem, or imipenem after 2011 per Clinical and Laboratory Standards Institute guidelines.22 EB isolates were not routinely screened for carbapenemase production. Unexposed subjects were those with a non-CRE EB BSI, defined as any EB on blood culture that exhibited in vitro susceptibility to all carbapenems. This control population was selected to focus on the impact of carbapenem resistance, rather than EB BSI, on outcomes.
For the determination of risk factors for CRE BSI, a case-control study design was employed. Case subjects were those with a CRE BSI, and control subjects were those with a non-CRE EB BSI (as defined above). All cases and controls were included in the analysis; no matching was performed.
This study was approved by the Institutional Review Board at each of the participating transplant centers (see Text S1, SDC, http://links.lww.com/TP/C498).
Primary Outcomes
There were two primary outcomes: (1) all-cause mortality within 60 d after the EB BSI, and (2) new-onset graft failure within 60 d after the EB BSI. Graft failure was defined by retransplantation of the same organ, relisting for transplantation of the same organ, return to dialysis for kidney transplant recipients,23 or a cardiac index ≤2 or ejection fraction ≤40% with need for inotropes or persistent mechanical support in heart transplant recipients.24 For the analysis of new-onset graft failure, those SOT recipients with graft failure before the EB BSI onset were excluded from the cohort. We chose not to restrict graft failure or death to those that were thought to be directly attributable to the EB BSI episode because the relatedness of graft failure or death to a single infectious event can be difficult to ascertain retrospectively and can lead to misclassification of outcomes. Subjects were followed for 60 d because this is a timeframe in which subsequent graft failure or death may be attributable, at least in part, to the EB BSI. The follow-up period began immediately after the first positive blood culture was collected.
Data Collection
Data on SOT recipients were abstracted from the electronic medical records at each study site by a combination of electronic data extraction, with validation of variables, and manual chart review. Information was collected on demographics, comorbidities, medications, and details of the EB BSI episode (see Text S2 parts B1 and B2, SDC, http://links.lww.com/TP/C498 for details).
Susceptibility Testing of EB Isolates
All EB isolates identified from study subjects were tested as part of routine care for susceptibility to antibiotics at each of the centers’ clinical microbiology laboratories. At HUP, the semiautomated Vitek 2 identification and susceptibility system (bioMerieux, Inc, Durham, NC) was utilized; at JHH, the BD Phoenix Automated System (BD Diagnostics, Sparks, MD) was used; and at UMMC, disk diffusion was used before 2010 and the Vitek 2 (bioMerieux, Inc, Durham, NC) was used after 2010.
Statistical Analysis
Continuous variables were summarized using median and interquartile range, and categorical variables were summarized using proportions. Continuous variables were compared using the Wilcoxon rank-sum test (because distributions were non-normal), and categorical variables were compared using the χ2 or Fisher exact test.
For the determination of the association between CRE BSI and risk of death, we performed a survival analysis. Time zero was defined as the date of the first blood culture that grew an EB organism, and the time at risk was measured in days. The day on which the SOT recipient first met criteria for the outcome (ie, date of death) following the EB BSI was the failure date, and subjects were censored after 60 d of follow-up. For the unadjusted analyses, a Kaplan Meier curve was plotted to assess the time to the primary outcome (death), stratified by exposure status (CRE versus non-CRE EB BSI), and a log-rank test was performed. For the adjusted analysis, a mixed-effects multivariable frailty model using the Weibull distribution was developed, with a random effect for study site. Bivariate regression was used to examine the relationship between the primary exposure, as well as other baseline factors, and the outcome. Variables from bivariate analyses with P < 0.20 were considered for inclusion in the final multivariable model. Variables were retained in the final multivariable model if they were confounders of the primary association (defined by a 15% or more change in the effect estimate of the association between the primary exposure and outcome) or had a P of <0.05 in the multivariable model. Hazard ratios (HRs) and 95% confidence intervals (CIs) were calculated to evaluate the strength of any associations.
For the determination of the association between CRE BSI and risk of new-onset graft failure, we again performed a survival analysis, but used multivariable competing risks regression, with death modeled as a competing risk. Study site was incorporated as a fixed effect. Measurement of time at risk, duration of follow-up, and variable selection for the multivariable model were performed in the same manner as described above. For this analysis, subhazard ratios (SHRs) and 95% CIs were calculated.
For the determination of risk factors for CRE BSI, we performed mixed-effects multivariable logistic regression analyses. Bivariate mixed-effects logistic regression was used to examine the relationship between each potential risk factor and CRE BSI. Variables from bivariate analyses with P < 0.20 were considered for inclusion in the final multivariable model. Manual forward selection was performed to build the multivariable model. Variables were retained in the final model if they were significantly associated with the outcome (P < 0.05). Odds ratios (ORs) and 95% CIs were calculated to evaluate the strength of any association.
Secondary Analyses
We performed several secondary analyses, including: (1) Stratified analyses by organ transplant type. (2) An analysis of outcomes after further stratifying the exposure into three groups: CRE BSI, extended-spectrum beta-lactamase (ESBL)–producing EB BSI (with ESBL status determined by either confirmatory testing using the double disk method with both cefotaxime and ceftazidime,25 the ESBL ETEST [bioMerieux, Durham, NC], or a ceftriaxone MIC of ≥8 μg/mL26), and susceptible EB BSI. (3) An analysis of outcomes after stratifying the CRE exposure into two mutually exclusive groups: CRE BSI nonsusceptible to all carbapenems and CRE BSI nonsusceptible to ertapenem only.
Mediation Analysis
A mediation analysis was performed in which we evaluated whether time to effective antibiotic therapy (measured in days) was the primary driver of the association between CRE BSI and all-cause mortality and/or new-onset graft failure. Effective antibiotic therapy is defined in Text S2 part B3, SDC, http://links.lww.com/TP/C498.
Risk Factors for Death Following CRE BSI
An exploratory analysis was performed in which we identified the risk factors for death among those with CRE BSI. In this analysis, only those with a CRE BSI were included in the cohort. Cases were those who died within 60 d after the CRE BSI, and controls were those who did not.
The same statistical approach was used for all secondary analyses as is described for the primary analyses. All analyses were performed with STATA/SE 15.1 (StataCorp, College Station, TX).
RESULTS
Study Population
A total of 897 SOT recipients developed an EB BSI during the study period, of which 70 (8%) cases were due to CRE. Among those with a CRE BSI (Table 1), the median age was 56 y old (interquartile range [IQR], 48–63), 24 (34%) were women, 40 (57%) had received a liver transplant, 13 (19%) a kidney, 12 (17%) a lung, 4 (6%) a heart, and 1 (1%) a pancreas transplant. The remainder of the cohort has been described previously.12
TABLE 1.
Baseline characteristics of solid organ transplant recipients with CRE and non-CRE BSI
| Baseline characteristica,b | CRE BSI (N = 70) | Non-CRE EB BSI (N = 827) | P c |
|---|---|---|---|
| Study site | |||
| Site 1 | 24 (34%) | 250 (30%) | 0.057 |
| Site 2 | 12 (17%) | 253 (31%) | |
| Site 3 | 34 (49%) | 324 (39%) | |
| Demographics | |||
| Age, y, median (IQR) | 56 (48–63) | 57 (47–64) | 0.721 |
| Female gender | 24 (34%) | 346 (42%) | 0.218 |
| Black/African American | 7 (10%) | 219 (26%) | 0.002 |
| White/Caucasian | 23 (33%) | 265 (32%) | 0.889 |
| Hispanic ethnicity | 4 (6%) | 18 (2%) | 0.085 |
| Comorbiditiesd | |||
| Diabetes mellitus | 21 (30%) | 398 (48%) | 0.004 |
| Chronic kidney disease (not on dialysis) | 2 (3%) | 92 (11%) | 0.025 |
| End-stage kidney disease requiring dialysis | 19 (27%) | 276 (33%) | 0.287 |
| Cirrhosis | 14 (20%) | 137 (17%) | 0.461 |
| Heart failure | 3 (4%) | 82 (10%) | 0.140 |
| Chronic pulmonary disease | 7 (10%) | 60 (7%) | 0.350 |
| Malignancy requiring chemotherapy in the prior 6 mo | 8 (11%) | 58 (7%) | 0.174 |
| Weight, kg, median (IQR) | 76 (60–86) | 74 (62–88) | 0.500 |
| Organ transplant type | |||
| Kidney | 21 (30%) | 503 (61%) | <0.001 |
| Liver | 40 (57%) | 238 (29%) | <0.001 |
| Heart | 4 (6%) | 65 (8%) | 0.645 |
| Lung | 12 (17%) | 46 (6%) | <0.001 |
| Pancreas | 1 (1%) | 26 (3%) | 0.716 |
| Transplant characteristics | |||
| Time from transplant to BSI, d, median (IQR) | 120 (50–462) | 760 (122–3062) | <0.001 |
| Induction immunosuppression given at time of transplantation | 15 (23%) | 345 (49%) | <0.001 |
| Graft failure before the EB BSI | 26 (38%) | 200 (25%) | 0.018 |
| Primary graft dysfunction after transplant | 18 (28%) | 105 (15%) | 0.005 |
| Reoperation within 4 wk of transplant | 27 (45%) | 210 (31%) | 0.024 |
| Living donor | 9 (13%) | 190 (23%) | 0.050 |
| CMV infection before the EB BSIe | 23 (34%) | 168 (21%) | 0.012 |
| Rejection episode before the EB BSIf | 5 (7%) | 85 (10%) | 0.387 |
| Chronic immunosuppression at the time of the EB BSI | |||
| Azathioprine | 6 (9%) | 31 (4%) | 0.051 |
| Corticosteroids | 57 (81%) | 559 (68%) | 0.017 |
| Cyclosporine | 4 (6%) | 44 (5%) | 0.784 |
| Mycophenolate | 25 (36%) | 500 (60%) | <0.001 |
| Sirolimus | 6 (9%) | 66 (8%) | 0.861 |
| Tacrolimus | 48 (69%) | 593 (72%) | 0.577 |
| Prior healthcare and antibiotic exposuresg | |||
| Hospitalization in prior 6 mo | 54 (77%) | 587 (71%) | 0.273 |
| Antimicrobial exposure in prior 6 mo | 66 (94%) | 614 (74%) | <0.001 |
| Aminoglycoside | 32 (46%) | 63 (8%) | <0.001 |
| Third-generation cephalosporin | 26 (37%) | 139 (17%) | <0.001 |
| Fourth-generation cephalosporin | 31 (44%) | 193 (23%) | <0.001 |
| Fluoroquinolone | 31 (44%) | 233 (28%) | 0.005 |
| Polymyxin | 15 (21%) | 13 (2%) | <0.001 |
| Carbapenem | 39 (56%) | 156 (19%) | <0.001 |
| Piperacillin-tazobactam | 42 (60%) | 312 (38%) | <0.001 |
| Tigecycline | 17 (24%) | 15 (2%) | <0.001 |
| TMP-SMX | 47 (67%) | 271 (33%) | <0.001 |
| IV vancomycin | 55 (79%) | 358 (43%) | <0.001 |
| Prior colonization with EBh | |||
| Prior ESBL-EB colonization/infection | 38 (54%) | 99 (12%) | <0.001 |
| Prior CRE colonization/infection | 29 (41%) | 25 (3%) | <0.001 |
| EB on prior culture | 46 (67%) | 378 (48%) | 0.003 |
| EB on prior respiratory tract culture | 17 (24%) | 76 (9%) | <0.001 |
| EB on prior genitourinary tract culture | 17 (24%) | 255 (31%) | 0.252 |
| EB on prior biliary culture | 2 (3%) | 10 (1%) | 0.239 |
| EB on prior intra-abdominal culture | 12 (17%) | 26 (3%) | <0.001 |
| EB on prior blood culture | 16 (23%) | 67 (8%) | <0.001 |
| Severity of illnessi | |||
| Pitt bacteremia score, median (IQR), points | 3 (1–4) | 1 (0–3) | 0.001 |
| Intensive care unit admission | 40 (61%) | 240 (31%) | <0.001 |
| Neutropenicj | 10 (14%) | 56 (7%) | 0.021 |
| Creatinine, median (IQR), mg/dL among nonkidney transplant recipients | 1.2 (0.8–2.0) | 1.6 (1.0–2.5) | 0.079 |
| Creatinine, median (IQR), mg/dL among kidney transplant recipients | 2.0 (1.0–2.7) | 2.1 (1.4–3.3) | 0.162 |
Data are presented as n(%) except where noted. The percentages represent the proportion of exposed and unexposed subjects with the specified baseline characteristic, except where noted.
Only those variables with a P <0.20 and those of notable biologic importance are included in this table.
Continuous variables were compared using the Wilcoxon rank-sum test (because distributions were non-normal), and categorical variables were compared using the χ2 or Fisher exact test.
Comorbidities assessed at the time of EB BSI.
CMV infection assessed through 6 mo before the EB BSI.
Acute rejection assessed through 3 mo before the EB BSI.
Hospital and antibiotic exposures assessed through 6 mo before the EB BSI.
Microbiological results assessed through 1 y before the EB BSI. No surveillance cultures for ESBL-EB or CRE are performed at any of the included study sites.
Severity of illness measures were assessed on day 1 of the EB BSI (the day that the first positive blood culture was collected).
Neutropenia defined by an absolute neutrophil count <1500 cells/μL.
BSI, bloodstream infection; CMV, cytomegalovirus; CRE, carbapenem-resistant EB; EB, Enterobacterales; ESBL, extended-spectrum beta-lactamase; IQR, interquartile range; IV, intravenous; TMP-SMX, trimethoprim-sulfamethoxazole.
Overview of CRE BSI Microbiology
Among those with a CRE BSI (Table 2), the most common identifiable sources were biliary (19, 27%), genitourinary (13, 19%), lower respiratory tract (13, 19%), and intra-abdominal (12, 17%). The most common CRE organisms were Klebsiella (37, 53%) and Enterobacter (25, 36%) species. The median duration of bacteremia was 1 d (IQR, 1–2). The microbiology of the remainder of the cohort has been described previously.12
TABLE 2.
Overview of CRE and non-CRE BSI microbiology
| Microbiology characteristica | CRE BSI (N = 70) | Non-CRE EB BSI (N = 827) | P |
|---|---|---|---|
| Source of BSIb | |||
| Genitourinary | 13 (19%) | 363 (43%) | <0.001 |
| Central venous catheter | 7 (10%) | 102 (12%) | 0.566 |
| Intra-abdominal | 12 (17%) | 91 (11%) | 0.122 |
| Biliary/hepatic | 19 (27%) | 95 (27%) | <0.001 |
| Lower respiratory tract | 13 (19%) | 44 (5%) | <0.001 |
| Skin or soft tissue | 3 (4%) | 20 (2%) | 0.415 |
| Organism on blood culture | |||
| Klebsiella spp | 37 (53%) | 334 (40%) | 0.042 |
| Escherichia coli | 5 (7%) | 333 (40%) | <0.001 |
| Enterobacter spp | 25 (36%) | 94 (11%) | <0.001 |
| Serratia spp | 1 (1%) | 29 (4%) | 0.724 |
| Proteus spp | 0 (0%) | 22 (3%) | 0.406 |
| Citrobacter spp | 2 (3%) | 12 (1%) | 0.299 |
| In vitro susceptibilities | |||
| Resistant to extended-spectrum cephalosporins | 70 (100%) | 286 (35%) | <0.001 |
| Resistant to piperacillin-tazobactam | 59 (84%) | 92 (11%) | <0.001 |
| Resistant to fluoroquinolones | 47 (67%) | 180 (22%) | <0.001 |
| Resistant to polymyxins | 5 (7%) | 0 (0%) | <0.001 |
| Duration of bacteremia, d, median (IQR) | 1 (1–2) | 1 (1–1) | 0.007 |
Data are presented as n(%) except where noted.
The source of the EB BSI is displayed for those episodes in which it was possible to determine.
The source was determined by an infectious diseases–trained physician at each study site.
BSI, bloodstream infection; CRE, carbapenem-resistant Enterobacterales; EB, Enterobacterales; IQR, interquartile range; spp, species.
Impact of CRE BSI on Risk of All-cause Mortality
Among those SOT recipients with a CRE BSI, 33 (47%) died within 60 d of the BSI, compared to 102 (12%) among those with a non-CRE EB BSI (Figure 1A, log-rank P < 0.001). On multivariable analysis (Table 3A), CRE BSI was associated with a significantly increased hazard of death within 60 d (adjusted hazard ratio [aHR] 2.85; 95% CI, 1.68-4.84; P < 0.001), after adjusting for age, organ type, prior CRE and EB colonization/infection, prior exposure to polymyxins, graft failure before the EB BSI, and recent allograft rejection treated with corticosteroids. Notably, the year of transplant and year of EB BSI were not confounders or significantly associated with the outcome.
FIGURE 1.
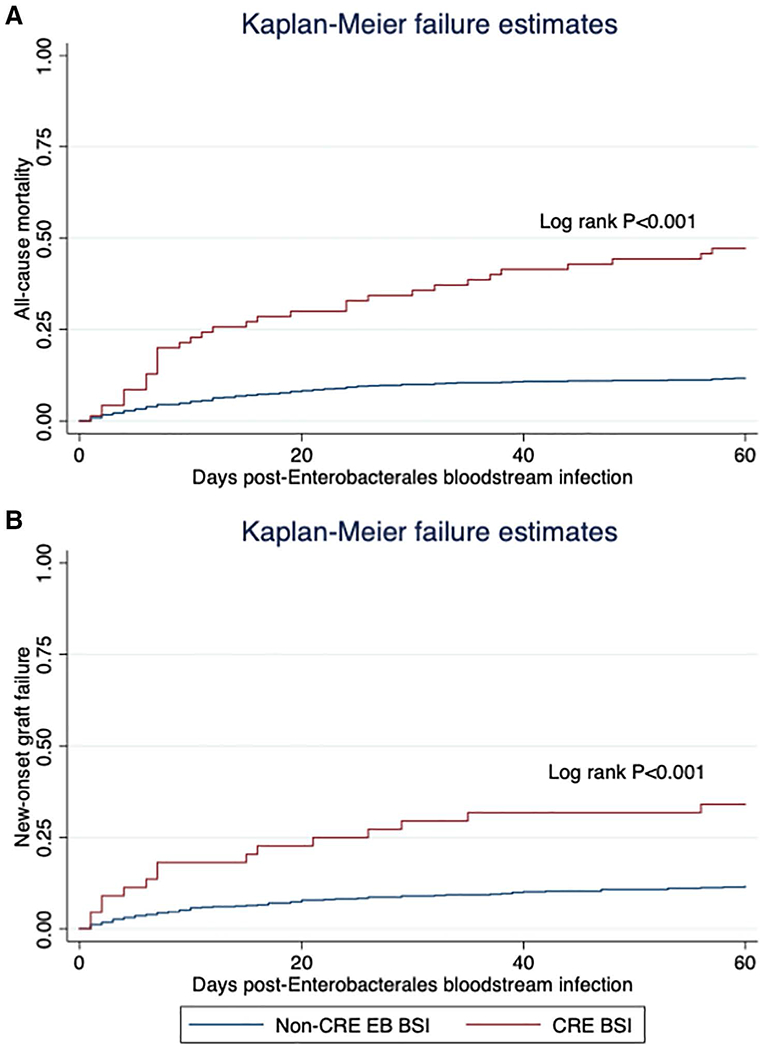
Kaplan Meier curve of (A) all-cause mortality and (B) new-onset graft failure following EB BSI, stratified by carbapenem resistance. BSI, bloodstream infection; CRE, carbapenem-resistant Enterobacterales; EB, Enterobacterales.
TABLE 3.
Multivariable survival analysis of the association between CRE BSI and (A) all-cause mortality, (B) new-onset graft failure
| A. All-cause mortality | |||
|---|---|---|---|
|
| |||
| Characteristic | aHR | 95% CI | P |
| CRE BSI (compared to non-CRE EB BSI) | 2.85 | 1.68-4.84 | <0.001 |
| Prior CRE colonization/infectiona | 0.90 | 0.45-1.77 | 0.749 |
| Age (per y) | 1.03 | 1.01-1.05 | <0.001 |
| Prior EB on respiratory culturea | 3.08 | 2.00-4.74 | <0.001 |
| Prior exposure to a polymyxinb | 2.51 | 1.32-4.79 | 0.005 |
| Rejection before the EB BSI, treated with corticosteroidsc | 1.91 | 1.05-3.47 | 0.034 |
| Graft failure before the EB BSI | 1.90 | 1.30-2.77 | 0.001 |
| Organ transplant type | |||
| Kidney | Ref | ||
| Liver | 2.68 | 1.72-4.19 | <0.001 |
| Heart | 1.22 | 0.54-2.77 | 0.631 |
| Lung | 1.45 | 0.73-2.89 | 0.293 |
| Pancreas | 3.79 | 1.46-9.82 | 0.006 |
| B. New-onset graft failure | |||
|
| |||
| Characteristic | aSHR | 95% CI | P |
|
| |||
| CRE BSI (compared to non-CRE EB BSI) | 2.42 | 1.33-4.39 | 0.004 |
| Rejection before the EB BSIc | 1.86 | 1.03-3.36 | 0.040 |
| Prior EB on genitourinary culturea | 0.43 | 0.23-0.81 | 0.009 |
| Prior EB on respiratory culturea | 2.53 | 1.31-4.87 | 0.006 |
| Prior exposure to metronidazoleb | 1.77 | 1.05-2.96 | 0.030 |
| Prior exposure to an aminoglycosideb | 0.90 | 0.46-1.80 | 0.756 |
| Organ transplant type | |||
| Kidney | Ref | ||
| Liver | 2.00 | 1.10-3.65 | 0.024 |
| Heart | 1.41 | 0.53-3.71 | 0.491 |
| Lung | 1.47 | 0.59-3.64 | 0.408 |
| Pancreas | 3.49 | 1.40-8.69 | 0.007 |
| Study site | |||
| Site 1 | Ref | ||
| Site 2 | 0.59 | 0.35-1.02 | 0.059 |
| Site 3 | 0.47 | 0.28-0.80 | 0.005 |
Organism isolated on any clinical culture from any anatomical site in the 12 mo before the EB BSI. (No surveillance cultures performed at the included study sites.)
Exposure within the 6 mo before the EB BSI.
Rejection within the 3 mo before the EB BSI.
aHR, adjusted hazard ratio; aSHR, adjusted subhazard ratio; BSI, bloodstream infection; CI, confidence interval; CRE, carbapenem-resistant EB; EB, Enterobacterales; ref, reference.
Impact of CRE BSI on Risk of New-onset Graft Failure
There were 663 SOT recipients with a functioning graft at the time of their EB BSI, of which 44 (7%) had a CRE BSI. Among those with a CRE BSI, 15 (34%) developed new-onset graft failure within 60 d of the EB BSI, compared to 72 (12%) in the non-CRE BSI group (Figure 1B, log-rank P < 0.001). On multivariable analysis, in which death was modeled as a competing risk (Table 3B), CRE BSI was associated with a significantly increased hazard of new-onset graft failure within 60 d (aSHR, 2.42; 95% CI, 1.33-4.39; P = 0.004), after adjusting for organ type, study site, prior antibiotic exposures, prior EB colonization/infection, and recent allograft rejection.
Risk Factors for CRE BSI
On multivariable analysis, the independent risk factors for CRE BSI (compared to non-CRE EB BSI) included (Table 4) prior CRE colonization/infection (adjusted odds ratio [aOR], 9.86; 95% CI, 4.88-19.93; P < 0.001), liver transplantation (aOR, 2.64; 95% CI, 1.23-5.65; P = 0.012), lung transplantation (aOR, 3.76; 95% CI, 1.40-10.09; P = 0.009), exposure to a third-generation cephalosporin in the prior 6 mo (aOR, 2.21; 95% CI, 1.17-4.17; P = 0.015), and exposure to a carbapenem in the prior 6 mo (aOR, 2.80; 95% CI, 1.54-5.10; P = 0.001). Conversely, there was a significantly decreased odds of CRE BSI in SOT recipients on mycophenolate immunosuppression (aOR, 0.47; 95% CI, 0.25-0.89; P = 0.021), and a reduction in the odds of CRE BSI with increasing time posttransplant (aOR, 0.99; 95% CI, 0.99-1.00; P = 0.051 per day forward posttransplant).
Table 4.
Mixed-effects multivariable logistic regression model of risk factors for CRE BSI
| Baseline characteristic | aOR | 95% CI | P |
|---|---|---|---|
| Prior exposure to a third-generation cephalosporina | 2.21 | 1.17-4.17 | 0.015 |
| Prior exposure to a carbapenema | 2.80 | 1.54-5.10 | 0.001 |
| Prior CRE colonization/infectionb | 9.86 | 4.88-19.93 | <0.001 |
| Time from transplantation to EB BSI (per d) | 0.99 | 0.99-1.00 | 0.051 |
| Chronic immunosuppression with mycophenolatec | 0.47 | 0.25-0.89 | 0.021 |
| Organ transplant type | |||
| Kidney | Ref | ||
| Liver | 2.64 | 1.23-5.65 | 0.012 |
| Heart | 2.69 | 0.80-9.02 | 0.108 |
| Lung | 3.76 | 1.40-10.09 | 0.009 |
| Pancreas | 0.68 | 0.08-6.57 | 0.740 |
Exposure within the 6 mo before the EB BSI.
CRE organism isolated on any microbiological culture from any anatomical site in the 12 months before the EB BSI. No surveillance cultures were performed at the included study sites.
Immunosuppression assessed at the time of the EB BSI.
aOR, adjusted odds ratio; BSI, bloodstream infection; CI, confidence interval; CRE, carbapenem-resistant Enterobacterales; EB, Enterobacterales; ref, reference.
Secondary Analyses
Among liver transplant recipients, there was not a significant association between CRE BSI and the hazard of all-cause mortality (aHR, 1.60; 95% CI, 0.83-3.05; P = 0.158) or new-onset graft failure (aHR, 0.70; 95% CI, 0.25-1.99; P = 0.506) (Table S1, SDC, http://links.lww.com/TP/C498). In examining risk factors for CRE BSI in liver recipients, there was a significantly decreased odds of CRE BSI among those who received their allograft from a living donor (aOR, 0.18; 95% CI, 0.05-0.72; P = 0.016) (Table S2, SDC, http://links.lww.com/TP/C498).
Among kidney, lung, and pancreas transplant recipients (Table S3A, SDC, http://links.lww.com/TP/C498), CRE BSI was associated with a significantly increased hazard of all-cause mortality on bivariate analysis. Among all non–liver transplant recipients (Table S3B, SDC, http://links.lww.com/TP/C498), CRE BSI was associated with a significant increase in the hazard of new-onset graft failure on bivariate analysis.
After stratifying the exposure into 3 groups based on resistance to carbapenems and extended-spectrum cephalosporins (namely, CRE, ESBL-EB, and susceptible EB BSI) (Figure S1, SDC, http://links.lww.com/TP/C498), an unadjusted analysis showed that CRE BSI was associated with a significant increase in the hazard of all-cause mortality (HR, 5.46; 95% CI, 3.56-8.36; P < 0.001), whereas ESBL-EB BSI was not (HR, 1.22; 95% CI, 0.81-1.84; P = 0.348).
After stratifying the CRE exposure into 2 groups, namely, CRE nonsusceptible to all carbapenems and CRE nonsusceptible to ertapenem only (Figure S2, SDC, http://links.lww.com/TP/C498), an unadjusted analysis showed that both CRE types were associated with a significantly increased hazard of all-cause mortality (CRE BSI nonsusceptible to all carbapenems HR, 6.64; 95% CI, 4.32-10.18; P < 0.001; CRE nonsusceptible to only ertapenem HR, 2.48; 95% CI, 1.09-5.65; P = 0.031).
Mediation Analysis of Time to Effective Antibiotic Therapy
Among those with a CRE BSI, 27 (39%) received effective antibiotic therapy within 24 h of their first positive blood culture, compared to 597 (72%) among those with a non-CRE EB BSI (χ2 P < 0.001) (Table S4, SDC, http://links.lww.com/TP/C498). Among those with a CRE BSI, the median time to effective antibiotics was 3 d (IQR, 0–90) compared to 1 d (IQR, 0–2) among those with a non-CRE EB BSI (Wilcoxon rank-sum P < 0.001). However, among those with a CRE BSI, the median time to effective antibiotics was reduced to 1 d (IQR, 0–4) for those with prior CRE colonization/infection. Furthermore, among those with a CRE BSI, the median time to effective antibiotics was 2 d (IQR, 0–8) in liver recipients, compared to 4 d (IQR, 1–90) in non–liver recipients (Wilcoxon rank-sum P = 0.058). There was no significant multicollinearity observed between prior CRE colonization/infection, liver transplantation, prior polymyxin exposure, and time to effective antibiotics (variance inflation factor values, 1.00–1.29; tolerance values, 0.78–1.14).
When time to effective therapy was incorporated into the analysis of CRE BSI and its impact on all-cause mortality and new-onset graft failure (Table S5, SDC, http://links.lww.com/TP/C498), we found that (1) with increasing time to effective antibiotics, there was a significant increase in the hazard of all-cause mortality (aHR, 1.01; 95% CI, 1.00-1.01; P = 0.034 per day without effective antibiotics) and new-onset graft failure (aSHR, 1.01; 95% CI, 1.00-1.02; P = 0.009); (2) there remained a significant association between CRE BSI and all-cause mortality (aHR, 2.60; 95% CI, 1.54-4.41; P < 0.001) and new-onset graft failure (aSHR, 2.03; 95% CI, 1.09-3.78; P = 0.025).
Risk Factors for Death Following CRE BSI
In the subgroup with a CRE BSI (N = 70), there were 33 (47%) who died within 60 d of the BSI. The independent risk factors for death within 60 d included (Table S6, SDC, http://links.lww.com/TP/C498): polymyxin exposure in the prior 6 mo (aOR, 6.44; 95% CI, 1.01-40.93; P = 0.049), Pitt bacteremia score (aOR, 1.36; 95% CI, 1.00-1.84; P = 0.053 per additional point), and graft failure before the CRE BSI (aOR, 7.66; 95% CI, 1.16-50.33; P = 0.034). Conversely, a genitourinary source of the CRE BSI (eg, urinary tract infection) was associated with a reduced odds of death (aOR, 0.05; 95% CI, 0.002-0.93; P = 0.045). Time to effective antibiotics was not significantly associated with the odds of death (aOR, 1.00; 95% CI, 0.98-1.02; P = 0.966).
DISCUSSION
In this multicenter retrospective cohort study of SOT recipients, we demonstrated a significant reduction in graft and patient survival—with over twice the hazard of all-cause mortality and new-onset graft failure—in the 60 d following a CRE BSI compared to those with a more antibiotic-susceptible EB BSI. After adjusting for time to effective antibiotics, this relationship was slightly attenuated, but remained significant, suggesting that poor outcomes with CRE BSI are not simply due to delays in detection and treatment. We further identified several important risk factors for CRE BSI in SOT recipients, including prior colonization or infection with a CRE organism, a history of liver or lung transplantation (as compared to other transplant types), and recent exposure to third-generation cephalosporins or carbapenems.
Although studies have previously demonstrated poor outcomes in SOT recipients with CRE infections,6,13–16,27,28 our study adds meaningfully to this literature by quantifying the specific impact of carbapenem resistance, even after accounting for antibiotic appropriateness and timing, on the risk of graft failure and death in the largest and only multicenter cohort of SOT recipients with CRE BSI reported to date. The reason for such poor outcomes is likely multifactorial: First, those with CRE BSI are less likely to receive early effective antibiotic therapy; indeed, we found that the time to effective antibiotic therapy was longer for those with CRE BSI than non-CRE BSI, was predictive of poor outcomes, and served as a partial mediator between CRE BSI and patient outcomes. Another possible mechanism is via increased pathogenicity: some CRE have been shown to be more pathogenic than antibiotic-susceptible EB organisms, due to harboring additional virulence factors such as those affecting iron scavenging or adhesion factors.29–31 Klebsiella pneumoniae is the most common organism in the CRE group, and is also among the most pathogenic of EB organisms.32–34 Third, it is likely that a CRE BSI results in a cascade of necessary management decisions, such as reduction in immunosuppression or use of antibiotics with greater potential toxicity (eg, polymyxins, as shown in the secondary analysis of risk factors for death following CRE BSI), that may compromise graft function and subsequently result in poorer outcomes.
In two secondary analyses, we found a stepwise relationship between the degree of EB resistance and clinical outcomes, where ESBL-EB did not significantly affect mortality, CRE with nonsusceptibility to only ertapenem increased the hazard of mortality 2-fold, and CRE with nonsusceptibility to all carbapenems increased the hazard of mortality 6-fold. This stepwise increase in the risk of mortality with increasing degree of antibiotic resistance further underscores the finding that the drug resistance itself contributes to poor outcomes. Taken together, these data suggest that it will be imperative to not only more rapidly detect and treat CRE BSI but also develop novel approaches to preventing CRE infection, to improve SOT recipient outcomes.
In one subgroup analysis of note, we found that CRE BSI did not significantly affect outcomes in liver transplant recipients. We hypothesized that this was likely due to earlier effective antibiotic therapy in this subgroup because liver recipients are known to be at higher risk for CRE infection.35,36 Our analysis of time to effective antibiotic therapy confirmed this hypothesis, showing that liver recipients had a shorter median time to effective antibiotics in the setting of CRE BSI.
In our analysis of risk factors for CRE BSI, the single most predictive factor was colonization or infection with CRE in the prior 12 months, which increased the odds more than nine-fold. CRE colonization has been shown previously to be a risk factor for CRE infection in SOT recipients,17,19,21,37–39 but the magnitude of this risk, particularly for thoracic organ recipients and after adjusting for other risk factors, has not been previously quantified. Although none of the transplant centers included in this study perform routine fecal sample surveillance for CRE colonization, this finding raises the question of whether transplant recipients should be proactively screened for CRE colonization pretransplantation or posttransplantation to guide antibiotic stewardship and infection prevention efforts as well as empiric antibiotic therapy choices.
Interestingly, we found that chronic immunosuppression with mycophenolate was associated with a reduced odds of CRE BSI. Although this finding is perhaps counterintuitive, it is possible that mycophenolate immunosuppression is a marker of those SOT recipients who have had fewer prior infectious complications, and fewer antibiotic exposures, because mycophenolate is often discontinued when infectious complications occur.40,41 We also found that the risk of CRE BSI decreased with increasing time posttransplant, which is consistent with prior studies that have shown that multidrug-resistant Gram-negative infections are most common in the first year posttransplant and decrease thereafter,27 likely due to the reduction in immunosuppression, hospital exposures, and antibiotic exposures with increasing time posttransplant.
There are several limitations to this work: (1) We chose to employ a control group with susceptible EB BSI, rather than including all hospitalized SOT recipients, which can result in an overestimation of the risk of antibiotic exposures.42 (2) None of the included hospitals perform surveillance cultures for CRE colonization, so our analyses of prior CRE colonization/infection are limited to those CRE captured on clinical cultures. (3) Before the adjustment of MIC breakpoints for CRE in 2011, the incidence of CRE may have been underestimated because of limitations in the ability of automated susceptibility platforms to reliably detect carbapenem resistance.43 Furthermore, because of the changes in MIC breakpoints in 2011, there may be some heterogeneity in the CRE exposure distribution based on era. However, the year of BSI was not significantly associated with the primary outcomes and did not significantly alter the association between CRE status and the primary outcomes, suggesting the change in definitions in 2011 did not significantly alter our findings. (4) We were not able to perform molecular analyses to identify the carbapenemases produced by CRE organisms because of the retrospective nature of this study. (5) We chose not to limit the primary outcomes to EB BSI-related graft failure or death because this can be difficult to classify accurately, but, as a result, the proportion of death and graft failure outcomes that were directly due to the EB BSI are unknown. (6) Because this was a retrospective observational study, there may have been residual unmeasured confounders that if measured, might account for the differences in outcomes observed with CRE BSI. (7) Though this is a large multicenter cohort, the results may not be generalizable to other transplant centers in other geographic regions.
In summary, in this multicenter retrospective cohort study, we found that CRE BSI significantly reduced graft and patient survival in SOT recipients, even after accounting for antibiotic appropriateness and timing. More intensive antibiotic stewardship and rapid antimicrobial susceptibility testing are urgently needed in the transplant population to mitigate the burden and impact of CRE infections on SOT recipient outcomes.
Supplementary Material
ACKNOWLEDGMENTS
The authors wish to thank the Antibacterial Resistance Leadership Group and the CDC Prevention Epicenters Program for their support of this study.
This work was supported by the Antibacterial Resistance Leadership Group (grant number 5 UM 1AI104681-05 with a subaward fellowship grant to J.A.A.); the Transplant Foundation’s Innovative Research Grant Program, an affiliate of the Gift of Life Donor Program (Donation and Transplantation Grant to J.A.A.); the National Institutes of Health (NIH; grant numbers K24-AI080942 to E.L., K01-AI137317 to J.A.A.); and a Centers for Disease Control and Prevention (CDC) Cooperative Agreement FOA no. CK16-004-Epicenters for the Prevention of Healthcare-Associated Infections (to E.L.). The content is solely the responsibility of the authors and does not necessarily represent the offfcial views of the NIH.
E.A.B. receives research support from Merck, Takeda, and Hologic; is a member of a Data and Safety Monitoring Board (DSMB) for Amplyx; and is a member of Scientific Advisory Committees for Merck and Takeda. E.L. is a member of a DSMB for Merck and is a member of a scientific advisory committee for Paratek and Shionogi. K.A. serves on a scientific advisory board for Becton Dickinson. J.H.H. was affiliated with the University of Pennsylvania during the conduct of this research and is now employed by, and holds shares in, the GlaxoSmithKline group of companies. None of these conflicts are relevant to this article. The other authors declare no conflicts of interest.
J.A.A. led research design, performance of research, data analysis, and writing of the article. E.L. participated in research design, performance of research, data analysis, and review of the article. K.A.T. participated in performance of research and review of the article. P.D.T. participated in performance of research and review of the article. E.A.B. participated in performance of research and review of the article. K.A. participated in performance of research, analytic tools, and review of the article. W.B.B. participated in data analysis and review of the article. A.W. participated in performance of research. A.A. participated in performance of research and data analysis. P.T., J.O., and L.P. participated in performance of research. J.H.H. participated in research design, performance of research, data analysis, and review of the article.
Footnotes
Supplemental digital content (SDC) is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML text of this article on the journal’s Web site (www.transplantjournal.com).
REFERENCES
- 1.Centers for Disease Control and Prevention. Antibiotic resistance threats in the United States. 2019. Available at https://www.cdc.gov/drugresistance/pdf/threats-report/2019-ar-threats-report-508.pdf. Accessed March 7, 2022.
- 2.Satlin MJ, Chen L, Patel G, et al. Multicenter clinical and molecular epidemiological analysis of bacteremia due to carbapenem-resistant Enterobacteriaceae (CRE) in the CRE Epicenter of the United States. Antimicrob Agents Chemother. 2017;61:e02349–e02316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang Y Wang Q, Yin Y et al. Epidemiology of carbapenem-resistant Enterobacteriaceae infections: report from the China CRE Network. Antimicrob Agents Chemother. 2018;62:e01882–e01817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ferreira J, Couto B, Starling C. Secular trends in nosocomial carbapenem-resistant enterobacteriaceae (CRE): twenty-five years of surveillance in Brazilian hospitals. Infect Control Hosp Epidemiol. 2020;41:S383–S384. [Google Scholar]
- 5.Castanheira M, Deshpande LM, Mendes RE, et al. Variations in the occurrence of resistance phenotypes and carbapenemase genes among Enterobacteriaceae isolates in 20 years of the SENTRY antimicrobial surveillance program. Open Forum Infect Dis. 2019;6:S23–S33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Satlin MJ, Jenkins SG, Walsh TJ. The global challenge of carbapenem-resistant Enterobacteriaceae in transplant recipients and patients with hematologic malignancies. Clin Infect Dis. 2014;58:1274–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rodríguez-Baño J, Picón E, Gijón P et al. Risk factors and prognosis of nosocomial bloodstream infections caused by extended-spectrum-beta-lactamase-producing Escherichia coli. J Clin Microbiol. 2010;48:1726–1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brink AJ. Epidemiology of carbapenem-resistant Gram-negative infections globally. Curr Opin Infect Dis. 2019;32:609–616. [DOI] [PubMed] [Google Scholar]
- 9.Smibert O, Satlin MJ, Nellore A, et al. Carbapenem-resistant Enterobacteriaceae in solid organ transplantation: management principles. Curr Infect Dis Rep. 2019;21:26. [DOI] [PubMed] [Google Scholar]
- 10.Moreno Camacho A, Ruiz Camps I. [Nosocomial infection in patients receiving a solid organ transplant or haematopoietic stem cell transplant]. Enferm Infecc Microbiol Clin. 2014;32:386–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schwaber MJ, Klarfeld-Lidji S, Navon-Venezia S, et al. Predictors of carbapenem-resistant Klebsiella pneumoniae acquisition among hospitalized adults and effect of acquisition on mortality. Antimicrob Agents Chemother. 2008;52:1028–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Anesi JA, Lautenbach E, Tamma PD, et al. Risk factors for extended-spectrum β-lactamase-producing enterobacterales bloodstream infection among solid-organ transplant recipients. Clin Infect Dis. 2021;72:953–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pouch SM, Satlin MJ. Carbapenem-resistant Enterobacteriaceae in special populations: solid organ transplant recipients, stem cell transplant recipients, and patients with hematologic malignancies. Virulence. 2017;8:391–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Taimur S, Pouch SM, Zubizarreta N, et al. Impact of pre-transplant carbapenem-resistant Enterobacterales colonization and/or infection on solid organ transplant outcomes. Clin Transplant. 2021;35:e14239. [DOI] [PubMed] [Google Scholar]
- 15.Hong Nguyen M, Shields RK, Chen L, et al. Molecular epidemiology, natural history, and long-term outcomes of multidrug-resistant enterobacterales colonization and infections among solid organ transplant recipients. Clin Infect Dis. 2022;74:395–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heldman MR, Guo K, Nelson B, et al. Treatment of multidrug-resistant gram-negative bacilli after solid organ transplant: outcomes and complications. Transpl Infect Dis. 2021;23:e13474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mouloudi E, Massa E, Papadopoulos S, et al. Bloodstream infections caused by carbapenemase-producing Klebsiella pneumoniae among intensive care unit patients after orthotopic liver transplantation: risk factors for infection and impact of resistance on outcomes. Transplant Proc. 2014;46:3216–3218. [DOI] [PubMed] [Google Scholar]
- 18.Aguiar EB, Maciel LC, Halpern M, et al. Outcome of bacteremia caused by extended-spectrum β-lactamase-producing Enterobacteriaceae after solid organ transplantation. Transplant Proc. 2014;46:1753–1756. [DOI] [PubMed] [Google Scholar]
- 19.Simkins J, Muggia V Cohen HW, et al. Carbapenem-resistant Klebsiella pneumoniae infections in kidney transplant recipients: a case-control study. Transpl Infect Dis. 2014;16:775–782. [DOI] [PubMed] [Google Scholar]
- 20.Kalpoe JS, Sonnenberg E, Factor SH, et al. Mortality associated with carbapenem-resistant Klebsiella pneumoniae infections in liver transplant recipients. Liver Transpl. 2012;18:468–474. [DOI] [PubMed] [Google Scholar]
- 21.Pereira MR, Scully BF, Pouch SM, et al. Risk factors and outcomes of carbapenem-resistant Klebsiella pneumoniae infections in liver transplant recipients. Liver Transpl. 2015;21:1511–1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Clinical Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing. 31st ed. CLSI; 2021. [Google Scholar]
- 23.Kabore R, Haller MC, Harambat J, et al. Risk prediction models for graft failure in kidney transplantation: a systematic review. Nephrol Dial Transplant. 2017;32(suppl_2):ii68–ii76. [DOI] [PubMed] [Google Scholar]
- 24.Kobashigawa J, Zuckermann A, Macdonald P, et al. ; Consensus Conference participants. Report from a consensus conference on primary graft dysfunction after cardiac transplantation. J Heart Lung Transplant. 2014;33:327–340. [DOI] [PubMed] [Google Scholar]
- 25.Clinical Laboratory Standards Institute. M100: Performance Standards for Antimicrobial Susceptibility Testing. 20th ed. Wayne, PA: Clinical Laboratory Standards Institute; 2010. [Google Scholar]
- 26.Huang Y, Carroll KC, Cosgrove SE, et al. Determining the optimal ceftriaxone MIC for triggering extended-spectrum β-lactamase confirmatory testing. J Clin Microbiol. 2014;52:2228–2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lanini S, Costa AN, Puro V et al. ; Donor-Recipient Infection (DRIn) Collaborative Study Group. Incidence of carbapenem-resistant gram negatives in Italian transplant recipients: a nationwide surveillance study. PLoS One. 2015;10:e0123706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mularoni A, Martucci G, Douradinha B, et al. Epidemiology and successful containment of a carbapenem-resistant Enterobacteriaceae outbreak in a Southern Italian Transplant Institute. Transpl Infect Dis. 2019;21:e13119. [DOI] [PubMed] [Google Scholar]
- 29.Lapp Z, Han JH, Wiens J, et al. Patient and microbial genomic factors associated with carbapenem-resistant klebsiella pneumoniae extraintestinal colonization and infection. Msystems. 2021;6:e00177–e00121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Martin RM, Cao J, Wu W, et al. Identification of pathogenicity-associated loci in klebsiella pneumoniae from hospitalized patients. Msystems. 2018;3:e00015–e00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Biggel M, Xavier BB, Johnson JR, et al. Horizontally acquired papGII-containing pathogenicity islands underlie the emergence of invasive uropathogenic Escherichia coli lineages. Nat Commun. 2020;11:5968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Highsmith AK, Jarvis WR. Klebsiella pneumoniae: selected virulence factors that contribute to pathogenicity. Infect Control. 1985;6:75–77. [DOI] [PubMed] [Google Scholar]
- 33.Podschun R, Ullmann U. Klebsiella spp. as nosocomial pathogens: epidemiology, taxonomy, typing methods, and pathogenicity factors. Clin Microbiol Rev. 1998;11:589–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pomakova DK, Hsiao CB, Beanan JM, et al. Clinical and phenotypic differences between classic and hypervirulent Klebsiella pneumonia: an emerging and under-recognized pathogenic variant. Eur J Clin Microbiol Infect Dis. 2012;31:981–989. [DOI] [PubMed] [Google Scholar]
- 35.Blot S, Depuydt P Vogelaers D, et al. Colonization status and appropriate antibiotic therapy for nosocomial bacteremia caused by antibiotic-resistant gram-negative bacteria in an intensive care unit. Infect Control Hosp Epidemiol. 2005;26:575–579. [DOI] [PubMed] [Google Scholar]
- 36.Pouch SM, Patel G; AST Infectious Diseases Community of Practice. Multidrug-resistant Gram-negative bacterial infections in solid organ transplant recipients-Guidelines from the American Society of Transplantation Infectious Diseases Community of Practice. Clin Transplant. 2019;33:e13594. [DOI] [PubMed] [Google Scholar]
- 37.Freire MP, Carvalho LB, Reusing JO Jr, et al. Carbapenem-resistant Enterobacteriaceae among kidney transplant recipients–insights on the risk of acquisition and CRE infection. Infect Dis (Lond). 2021;53:430–439. [DOI] [PubMed] [Google Scholar]
- 38.Pouch SM, Kubin CJ, Satlin MJ, et al. Epidemiology and outcomes of carbapenem-resistant Klebsiella pneumoniae bacteriuria in kidney transplant recipients. Transpl Infect Dis. 2015;17:800–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Giannella M, Bartoletti M, Morelli MC, et al. Risk factors for infection with carbapenem-resistant Klebsiella pneumoniae after liver transplantation: the importance of pre- and posttransplant colonization. Am J Transplant. 2015;15:1708–1715. [DOI] [PubMed] [Google Scholar]
- 40.Ritter ML, Pirofski L. Mycophenolate mofetil: effects on cellular immune subsets, infectious complications, and antimicrobial activity. Transpl Infect Dis. 2009;11:290–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bernabeu-Wittel M, Naranjo M, Cisneros JM, et al. Infections in renal transplant recipients receiving mycophenolate versus azathioprine-based immunosuppression. Eur J Clin Microbiol Infect Dis. 2002;21:173–180. [DOI] [PubMed] [Google Scholar]
- 42.Harris AD, Karchmer TB, Carmeli Y, et al. Methodological principles of case-control studies that analyzed risk factors for antibiotic resistance: a systematic review. Clin Infect Dis. 2001;32:1055–1061. [DOI] [PubMed] [Google Scholar]
- 43.He Q, Chen W, Huang L, et al. Performance evaluation of three automated identification systems in detecting carbapenem-resistant Enterobacteriaceae. Ann Clin Microbiol Antimicrob. 2016;15:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


