Abstract
Growth and differentiation factor 15 (GDF15) is a stress-responsive cytokine, and its expression increases during inflammation, hyperoxia, and senescence. Significantly, GDF15 is secreted by the placenta, and maternal levels increase throughout pregnancy. Serum GDF15 level is a promising biomarker for many lung diseases like pulmonary hypertension and pulmonary fibrosis. However, circulating GDF15 levels in preterm infants and their role as a predictor of respiratory outcomes have not been studied. We hypothesized that GDF15 levels would increase with gestational age at birth, and that postnatal GDF15 will be correlated with adverse respiratory outcomes in preterm infants.
Scavenged blood samples were retrieved from 57 preterm infants at five time points, from birth until 36-weeks postmenstrual age (PMA). GDF15 levels were measured using ELISA in 114 samples. We performed two-sample t-test, correlation and linear regression, logistic regression, and mixed-effects linear models for statistical analysis, and significance was identified when p<0.05.
Contrary to our hypothesis, for every one-week increase in gestational age at birth, the predicted GDF15 level decreased by 475.0pg/mL (p<0.001). Greater PMA was significantly associated with lower serum GDF15 levels (p<0.001). Interestingly, higher GDF15 levels were associated with a longer need for mechanical ventilation (p=0.034), prolonged respiratory support need (p<0.001), and length of hospital stay (p=0.006).
In conclusion, in preterm infants, GDF15 levels show an inverse correlation with gestational age at birth, with higher levels in more preterm babies, and levels trend down postnatally. Furthermore, longitudinal GDF15 levels through 36 weeks PMA predict adverse respiratory outcomes in preterm infants.
Keywords: Prematurity, respiratory support, biomarker, bronchopulmonary dysplasia, Growth differentiation factor 15
Introduction
Respiratory morbidity is a significant sequela of prematurity in the neonatal population (1). Despite advances in perinatal care and a decrease in mortality, respiratory morbidity including bronchopulmonary dysplasia (BPD) remain high among these vulnerable patients. Long-term complications (2)(3)(4), including impaired pulmonary function and re-hospitalization, are also prevalent in this patient population. Serum biomarkers that could predict the development of these respiratory morbidities would be beneficial in predicting outcomes and directing therapies to babies most likely to develop long-term respiratory morbidities.
Growth differentiation factor (GDF15) is a divergent member of the transforming growth factor-B (TGF-beta) cytokine superfamily (5). Under physiological conditions, GDF15 expression is low, except during pregnancy (6). In pregnant women, the circulating GDF15 levels continue to increase throughout gestation, with evidence of fetal exposure (7)(8). GDF15 expression increases during pathological states, including hypoxia, inflammation, oxidative stress, cancer, aging, and smoking (9)(10)(11). Increased GDF15 expression may indicate ongoing cellular injury or protective response to biological stress depending on the stress signal and the organ or tissue.
Serum GDF15 levels and trends are associated with many cardiopulmonary disorders in adults, like chronic obstructive pulmonary disease (12), idiopathic pulmonary fibrosis (13), and pulmonary arterial hypertension (14). However, GDF15 levels have been studied in limited pediatric conditions, including mitochondrial diseases (15), pulmonary arterial hypertension (16), and postnatal metabolic status and growth (17). Pre-clinical studies have shown increased GDF15 expression in pulmonary epithelial and endothelial cells upon exposure to hyperoxia (18). GDF15 levels in term newborns are known to be elevated 5- to 10-fold compared to adults and show a rapid decline postnatally (17). However, the circulating GDF15 levels in preterm infants and its longitudinal trends have not been studied, and the association between GDF15 and respiratory outcomes in preterm infants has yet to be identified.
In this study, we tested the hypothesis that there would be increasing GDF15 levels with gestational age and tested its correlation with respiratory outcomes in preterm neonates. We measured baseline GDF15 levels in premature infants and their longitudinal changes postnatally. This study is the first to report GDF15 levels in premature infants born at different gestational ages and its association with respiratory outcomes in this patient population.
Materials and Methods:
Study Population:
A total of 57 infants born at the Pavilion for Women at Texas Children’s Hospital between October 2020 to January 2022 were enrolled in the study. The study was reviewed and approved by the institutional review board at Baylor College of Medicine, and written parental consent was obtained upon infant enrollment. Preterm infants born between 23 weeks and 0 days to 36 weeks and six days gestation admitted to the NICU were enrolled. Patients with major congenital anomalies were excluded from the study.
Specimens and data collection:
Serum samples were collected using scavenged specimens from the aliquots collected for routine laboratory tests. Samples were collected during the NICU stay at five-time points, when available, to measure the longitudinal changes in GDF15 levels postnatally. A total of 114 serum samples were retrieved from 57 patients, and two patients had no samples due to the inadequate volume of the scavenged specimens. The first sample was collected on the day of birth (N=33). The subsequent samples were collected on postnatal days 7, 14, and 28 and at the postmenstrual age of 36 weeks. After sample retrieval, the samples were de-identified and stored at −80°C until the analysis. Baseline characteristics and clinical data of each infant were gathered from birth until discharge or death. BPD was diagnosed in infants who required supplemental oxygen for at least the first 28 days postnatally (19).
GDF15 analysis:
Serum GDF-15 concentrations were quantified using the Quantikine ELISA kit (#DGD150, R&D Systems, Minneapolis, MN) according to the manufacturer’s instructions. The serum samples were diluted 1:10, and the absorbance was read at 450 nm. The concentration of each sample was calculated using a standard curve.
Statistical methods:
Mixed-effects linear models with a first-order autoregressive covariance structure were used to analyze associations with longitudinal GDF15 measurements, while accommodating the correlation among repeated measures from the same patient over time. Linear regression and correlation analysis was used to assess the association of GDF15 level in the first week of life with quantitative outcomes, and logistic regression was used for binary outcomes. GDF15 level in the first week of life was compared between groups using the two-sample t-test. Data were analyzed using SAS version 9.4 (SAS Institute Inc., Cary, North Carolina). A two-sided 5% significance level was used for all hypothesis tests. Power analysis indicated that 46 study patients would provide 80% statistical power to detect a true correlation of r = 0.4 between GDF15 level in the first week of life and gestational age at birth with a two-sided 5% significance level. Due to our approach of utilizing scavenged samples, we had planned to enroll additional subjects as we expected specimen availability might be compromised. Therefore, we continued the patient enrollment process until a sample size of 57 was reached.
Results
Study participants:
The characteristics of the study cohort are shown in Table 1. The average gestational age of our subjects was 30 ± 3.2 weeks (18% of the enrolled neonates were born at 23–27 weeks, 51% between 28–32 weeks, and 31% between 33–36 weeks), and their average birth weight was 1643 ± 635 g. Female infants represented 44% of the studied population. In our study population, among the preterm infants with birth weight <1500g, the frequency of BPD was 38.5%, and sepsis was 23% (Supplemental Table 1). The average length of hospital stay of our cohort was 52 days.
Table 1:
The baseline characteristics and outcomes of the study cohort. A total number of 55 preterm infants are shown in the table.
| Study Cohort (n = 55) a | ||
|---|---|---|
| Infants Baseline Characteristics | ||
| Gestational age – n (%) | ||
| 23 – 27 weeks | 10 (18) | |
| 28 – 32 weeks | 28 (51) | |
| 33 – 36 weeks | 17 (31) | |
| Birth weight (g) – mean ± standard deviation | 1643 ± 635 | |
| Female – n (%) | 24 (44) | |
| Exposure to antenatal steroids – n (%) | 41 (75) | |
| Delivered via Cesarean Section – n (%) | 47 (85) | |
| APGAR <7 at 1 minute – no.(%) | 18 (33) | |
| Pregnancy – n (%) | ||
| Singleton Multiparity |
31 (56) 24 (44) |
|
| Race or ethnic groups, n (%) | ||
| White Black Hispanic Others |
20 (36) 17 (31) 17 (31) 1 (2) |
|
| Hypertensive disorders of pregnancy, n (%) | 20 (36) | |
| Chorioamnionitis, n (%) | 5 (9) | |
| Infants Outcomes | ||
| Bronchopulmonary dysplasia, n (%) | 10 (18) | |
| Duration of respiratory support (days) – mean ± standard deviation | 27 (42) | |
| Patent ductus arteriosus treatment, n (%) | 6 (11) | |
| Necrotizing enterocolitis, n (%) | 4 (7) | |
| Sepsis, n (%) | 6 (11) | |
| Retinopathy of prematurity, n (%) | 11 (20) | |
| Intraventricular hemorrhage, n (%) | 9 (16) | |
| Mortality, n (%) | 1 (2) | |
| Length of stay (days) – mean ± standard deviation | 52 ± 45 | |
Two subjects out of the enrolled 57 were excluded, due to inability to retrieve scavenged specimens.
GDF15 levels show a significant inverse correlation with gestational age, with higher levels in more preterm babies:
Previous studies had reported increasing maternal GDF15 levels with advancing gestation leading us to hypothesize that GDF15 levels would be higher with greater gestational age at birth. However, contrary to our hypothesis, in preterm infants, GDF15 levels within the first week of life were 7201 ± 3880 pg/mL (in infants <= 30 weeks) vs. 3718 ± 2336 pg/mL (in infants > 30 weeks), (p<0.001, Supplemental Table 2). For every one-week increase in gestational age at birth, the predicted GDF15 level in the first week of life decreased by 475.0 pg/ml (r2 = 0.209, p<0.001, Figure 1). The average serum GDF15 level within the first week of life in the entire cohort was 5062 ± 3620 pg/mL.
Figure 1:
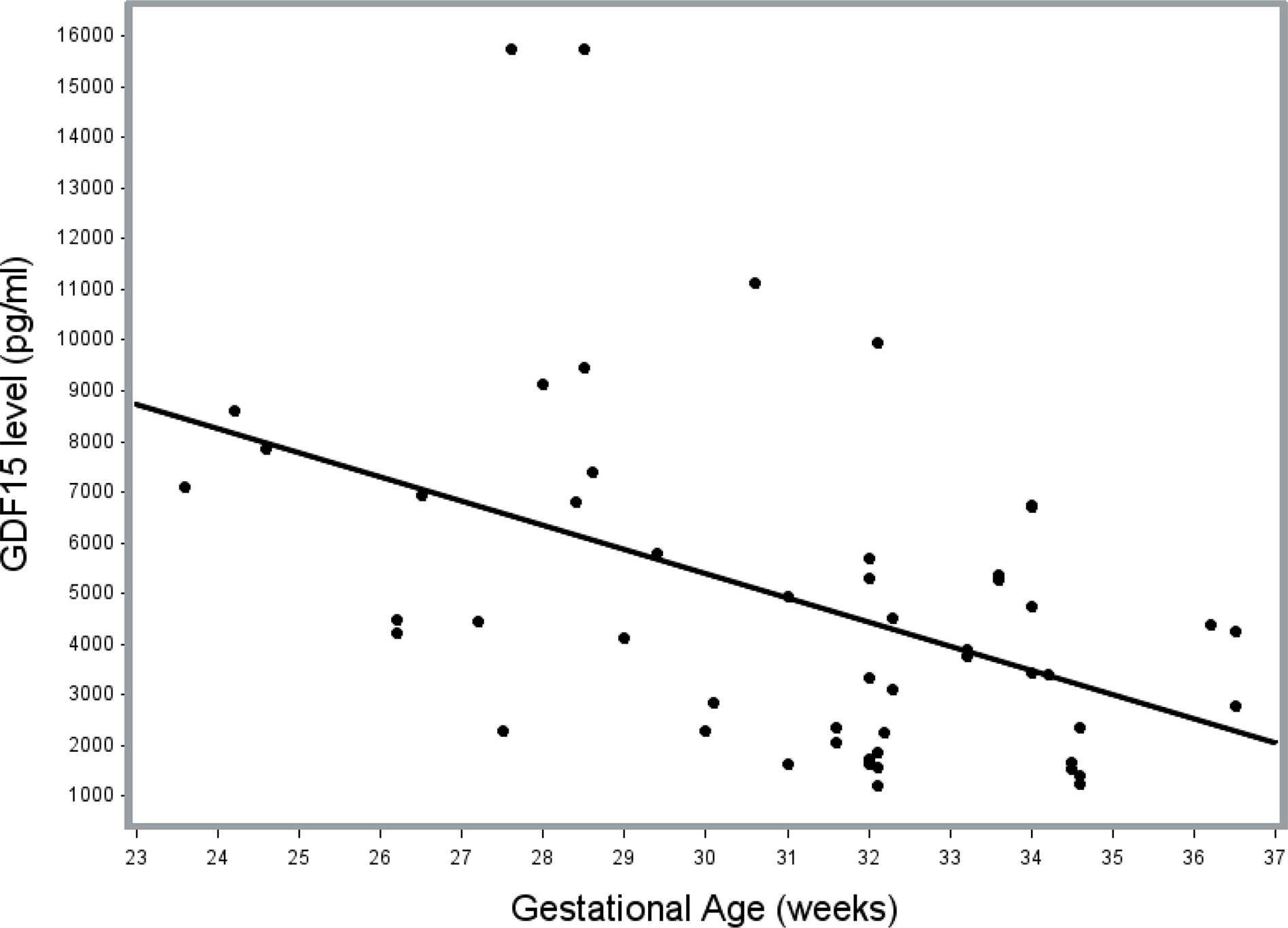
Scatterplot of GDF15 serum levels within the first week of life vs. Gestational age, with superimposed linear regression line. Linear regression analysis showed that for every one-week increase in gestational age at birth, the predicted GDF15 level in the first week of life decreased by 475.0 pg/ml (p<0.001).
Serum GDF15 changes decline with postnatal age in preterm infants:
After controlling for gestational age at birth, for each additional day of life, the predicted GDF15 level decreased by 118.7 pg/ml (p<0.001). Greater postmenstrual age in the preterm infants was significantly correlated with lower serum GDF15 levels (r = −0.618, p<0.001) (Figure 2). The serum GDF15 levels reached their lowest levels at the postmenstrual age of 36 weeks, with an average of 2033±1052 pg/mL (Supplemental Table 3).
Figure 2:
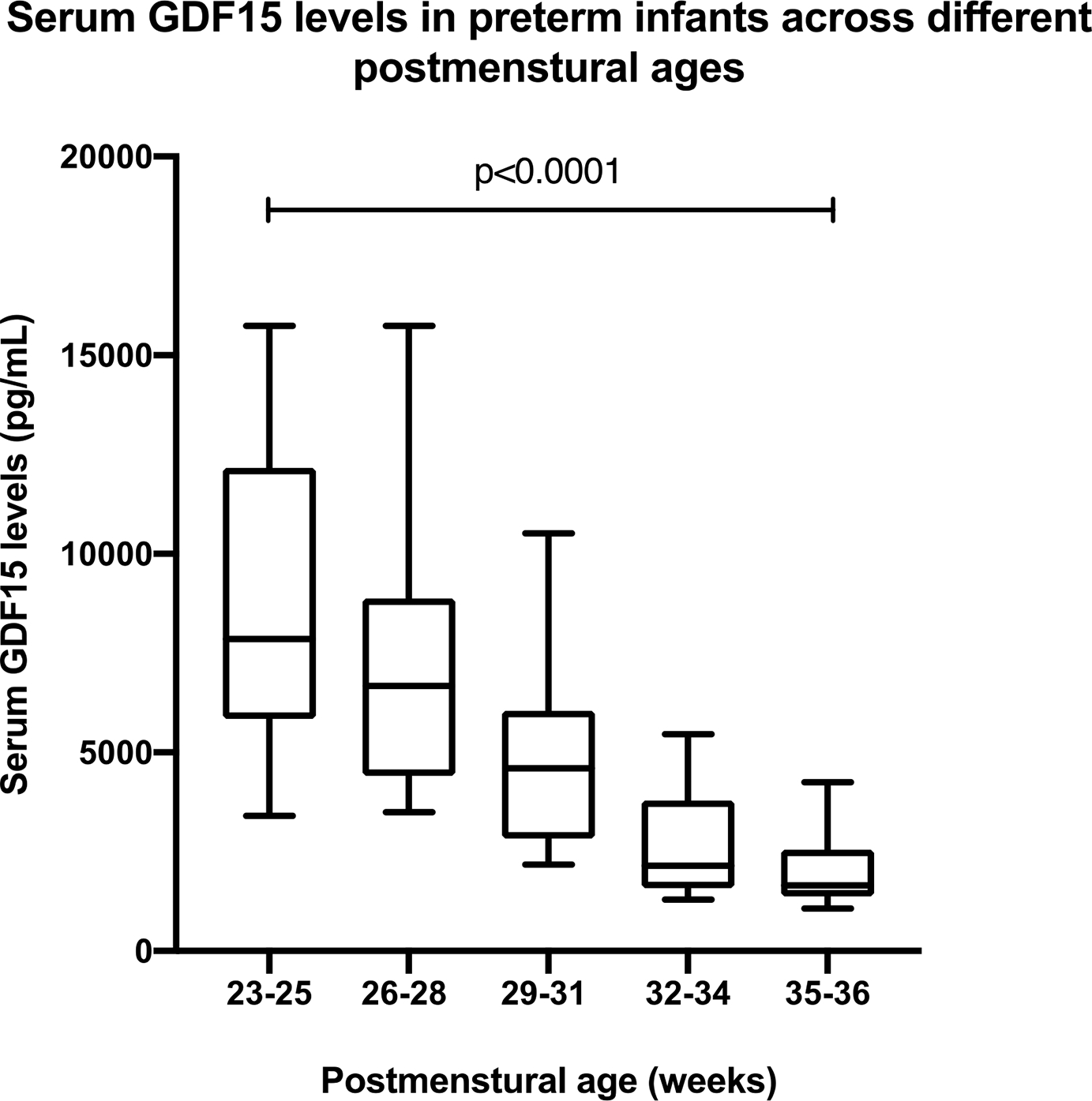
A box and whisker plot showing the serum GDF15 levels in infants at different postmenstrual ages. The data are represented as boxes for the medians and the interquartile ranges, and the whiskers represent the 10th percentile. Pearson’s correlation showed a significant inverse relationship between PMA and GDF15 level. (r = −0.618, p<0.0001).
Longitudinal GDF15 levels are associated with adverse respiratory outcomes in preterm neonates:
A mixed-affect linear model was fit for the respiratory outcome with GDF15 levels, using a first-order autoregressive covariance structure to accommodate the correlation among repeated GDF15 measurements from the same patient over time and after controlling for gestation age at birth and day of life (since both variables were shown to be significantly associated with the longitudinal GDF15 levels). After controlling for gestational age, the longitudinal GDF15 levels were significantly higher in preterm infants who required longer invasive mechanical ventilator support (p = 0.034), more extended period of respiratory support (p<0.001), and increasing length of hospital stay (p=0.006) (Table 2). However, longitudinal GDF15 levels were not significantly different for patients with versus without BPD (p=0.173).
Table 2.
After controlling for gestational age and day of life, the associations of longitudinal GDF15 levels with respiratory outcomes and length of stay in preterm infants
| Slope Coefficient (Standard Error) | p-value | |
|---|---|---|
| Respiratory Outcomes | ||
| Duration of mechanical ventilation (days) | 26.0 (11.4) | 0.034 |
| Duration of any respiratory support (days) | 37.4 (8.8) | <0.001 |
| Oxygen requirement at PMA 36 week | 290.3 | 0.044 |
| Bronchopulmonary dysplasia | 1191.2 (842.5) | 0.173 |
| Length of stay (days) | 27.7 (8.9) | 0.006 |
GDF15 levels within the first week of life predict severe IVH in premature neonates:
We investigated the association between the longitudinal GDF15 levels in preterm infants and prematurity-related outcomes, including intraventricular hemorrhage, retinopathy of prematurity, and necrotizing enterocolitis. After adjusting for the gestational age, and the postnatal day of life, there were no statistically significant associations between the trend in GDF15 levels over time in preterm infants and intraventricular hemorrhage, retinopathy of prematurity, and necrotizing enterocolitis (Table 3). Additionally, no associations were detected with the baseline characteristics of the study subjects, such as birth weight, gender, or exposure to prenatal steroids (Table 3). Since many outcomes in preterm neonates are predicted by their early postnatal clinical course, we assessed the relationship between the GDF15 levels within the first week of life and prematurity-related outcomes. Interestingly, higher initial GDF15 levels were associated with higher grades of intraventricular hemorrhage (p=0.015), even though longitudinal levels were not (p=0.538).
Table 3.
After controlling for gestational age and day of life, the associations between the longitudinal GDF15 levels, cohort baseline characteristics, and other outcomes related to prematurity in preterm infants.
| Slope Coefficient (Standard Error) | p-value | |
|---|---|---|
| Baseline Characteristics | ||
| Birth weight – g | −1.35 (0.65) | 0.051 |
| Birth Length – cm | −40.7 (56.0) | 0.476 |
| Gender – female | −218.0 (363.7) | 0.556 |
| Mode of Delivery – Cesarean Section | −345.5 (704.7) | 0.629 |
| Prolonged rupture of membrane | −730.6 (687.7) | 0.301 |
| Exposure to antenatal steroid | −1309.3 (709.9) | 0.080 |
| Chorioamnionitis | 853.9 (934.4) | 0.372 |
| Hypertensive disorders of pregnancy | 127.0 (569.2) | 0.826 |
| Outcomes related to prematurity | ||
| Intraventricular hemorrhage | 215.9 (326.6) | 0.538 |
| Retinopathy of prematurity | 742.2 (906.9) | 0.423 |
| Necrotizing enterocolitis | 747.8 (453.4) | 0.116 |
Discussion:
Using a prudent approach of scavenged blood specimens from preterm neonates, we report the baseline GDF15 levels in premature infants born at different gestational ages and the association of longitudinal GDF15 levels with respiratory outcomes in preterm neonates. We show that the initial GDF15 level shows an inverse correlation with gestational age in preterm infants, with higher levels in more premature infants. Postnatal GDF15 levels decrease after birth, with their lowest level at 36 weeks PMA. This result was contrary to our hypothesis, based on the rising GDF15 during pregnancy in previous studies, with maternal GDF15 levels peaking at term (6). The higher GDF15 levels in preterm infants may be related to the stress from to preterm birth, as GDF15 is a stress-responsive cytokine. Higher initial GDF15 levels in more preterm neonate may be related to higher induced maternal levels or greater placental production, and the subsequent transfer to the preterm neonates. Increased production in the fetus during an impending preterm birth may be another factor leading to higher GDF15 levels in preterm neonates.
In a human study, circulating GDF-15 was increased in women preceding the development of preeclampsia and in women with diagnosed preeclampsia compared to controls. Maternal levels were higher in women with preterm deliveries due to preeclampsia (20). Other studies, however, have not replicated this association (21). Based on our analysis, there was no association between the initial levels or the trend of GDF15 in preterm infants born to a mother who had a diagnosis of hypertensive disorders of pregnancy.
Analysis of GDF15 levels in amniotic fluid and fetal membranes in preterm labor and premature rupture of membranes did not reveal any differences in levels compared to term deliveries (22). Sugulle et al. reported higher circulating and placental GDF15 levels in mothers with preeclampsia and diabetes mellitus. In addition, GDF-15 was elevated in the amniotic fluid and fetal circulation in pregnancies complicated by preeclampsia and superimposed preeclampsia in diabetes mellitus, as compared with controls (23). Based on the above, maternal and placental GDF15 levels may be higher in conditions that increase the risk of preterm birth.
GDF15 levels in preterm infants under the postmenstrual age of 31 weeks were higher than those observed in term infants (17). The reported GDF15 levels by Díaz et al. for term infants were comparable to the GDF15 levels for preterm infants at a postmenstrual age of 32 to 36 weeks in our study. Similar to our findings, in a study with 18 neonates (mean gestational age of 37.2 weeks), Kinoshita et al. showed that GDF15 levels were negatively correlated with postnatal period and z-score of birth weight. Interestingly, they also noticed a positive correlation of GDF-15 levels with N-terminal pro-brain natriuretic peptide and lactate levels, speculating the association of GDF-15 levels with mitochondrial function (24).
We also demonstrate in this study cohort, that preterm infants who required longer invasive mechanical ventilator support and longer period of any respiratory support had higher GDF15 levels. Higher production of GDF15 in the lung or from other tissues as a response to systemic illness in these neonates, could underlie the higher serum levels. Circulating GDF15 levels are increased in various lung diseases (25), pulmonary hypertension (16), chronic obstructive pulmonary disease (COPD) (26), and idiopathic pulmonary fibrosis (13). GDF15 levels were elevated in those with interstitial lung abnormalities in two large adult cohorts (Farmingham heart study and the COPDGene study) (27). In a mouse model of bleomycin-induced lung injury, lung epithelial cells were the source of GDF15 production and associated with cellular senescence (13). GDF15 expression is also induced in pulmonary endothelial and epithelial cells upon exposure to hyperoxia in vitro, increased cellular viability, and decreased cellular oxidative stress (18). GDF15 expression in lung tissue is linked to cellular senescence, as it expression is increased when epithelial cells are exposed to bleomycin (13). Whether the role of GDF15 overexpression is protective to increase the cellular viability, as shown by Tiwari et al, or a marker of cellular that needs to be discerned. The only known specific receptor identified for GDF15 is the glial-cell-line-derived neurotrophic factor family (GFRAL) receptor, located in the hindbrain (28)(29)(30). Whether all effects of GDF15 are mediated through GFRAL or other GDF15 receptors in different organs are involved is unknown. The binding of GDF15 to GFRAL activates ERK, AKT and PLC-gamma downstream signaling (31). Moreover, GDF15 was found to activate SMAD-mediated signaling in other tissues like airway epithelial cells (32) and cardiomyocytes (33). The metabolic, anti-obesity, and cachectic effects of GDF15 are mediated through its interaction with GFRAL (31). However, this receptor has not been identified in the human lung (13). Díaz et al. reported a more significant decline in GDF-15 levels in small for gestational age (SGA) infants at four months compared to appropriate for gestational age (AGA) infants. They speculated that this might be needed to allow for the catch-up growth in SGA infants (17). In the NICU, extrauterine growth retardation increases morbidity in preterm neonates (34). Higher GDF15 levels could contribute to this phenomenon and thus indirectly modulate other morbidities such as adverse respiratory outcomes.
In neonatology, biomarkers have been studied in various clinical conditions, including chronic lung disease (35), necrotizing enterocolitis, sepsis (36), and patent ductus arteriosus (37). Biochemical markers identify high-risk populations, detect responses to interventions, and predict outcomes. This information can guide clinicians to utilize specifically directed therapies to alleviate or prevent disease and avoid high-risk interventions. The pathophysiology of lung disease in preterm infants is highly complex and mediated by genetic and environmental interactions. Previous studies have highlighted biomarkers that have shown an association with chronic lung disease, like interleukin-6 (IL-6), IL-10, and vascular endothelial growth factor (VEGF) (38). A recent study focusing on the identification of biomarkers for persistence of patent ductus arteriosus in extremely premature (22–27 weeks) infants identified high levels of GDF-15 as one of the candidates among B-type natriuretic peptide, interleukin-6, -8, -10 and -12 (39). A persistent hemodynamically significant PDA is associated with adverse respiratory outcomes in the preterm population. The association in our study between high GDF15 levels and adverse respiratory outcomes could be direct or indirect because of another pathological factor such as a persistent PDA.
Our study highlighted the association of higher initial GDF15 levels in preterm infants with severe IVH. This may reflect the systemic inflammatory stress in these neonates. However, GDF15 might play a causative role, as GDF15 exhibits an inhibitory role on platelet integrins (40) and higher GDF15 levels have been associated with higher incidence of atrial fibrillation-related bleeding in patient on anti-platelet medications (41). GDF15 is also known as nonsteroidal anti-inflammatory drug-activated gene (NAG-1) and is induced by the administration of NSAIDs, including indomethacin (42)and ibuprofen (43). Many preterm neonates are exposed to NSAIDs to prevent IVH or treat a hemodynamically significant PDA. However, there are no long-term benefits of these drugs despite the closure of PDA (44). The indirect effects of these drugs with the possible induction in GDF15 levels in this patient population need to be further explored.
The limitation of our study is the small size of the cohort and the absence of follow-up beyond the NICU discharge regarding the GDF15 levels and long-term respiratory and clinical outcomes. Also, maternal and cord blood GDF15 levels were not measured and were not compared to the initial GDF15 levels in neonates. The strength of our study is that utilizing scavenged specimens avoided any additional blood loss or painful procedures in this vulnerable population, and samples were collected longitudinally from the same patient at different time points, enabling the correlation of GDF15 levels with the clinical course.
In summary, in this pilot study, we show for the first time that GDF15 levels in preterm infants display a significant inverse correlation with gestational age, with higher levels in more preterm babies and postnatally declining levels. We highlight the potential of using GDF15 as a biomarker for predicting adverse respiratory outcomes in preterm infants. Studies in animal models with loss-of-function and gain-of-function of GDF15 will be needed to establish causality for the disease phenotype and further elucidate the cell-autonomous or non-cell-autonomous role of GDF15 will be required. Further studies in larger cohorts will be needed to correlate the GDF15 levels in maternal, placental, and cord blood with its postnatal levels and the role of GDF15 levels in predicting BPD and associated morbidities such as BPD- associated pulmonary hypertension.
Supplementary Material
Acknowledgements
We would like to thank Dr.Rosas for supporting Dr.Almudares with the T32 grant for this scholarly project. We would like to acknowledge the Baylor College of Medicine Clinical Scientist Training Program.
Funding
This word was supported in part by Evangelina “Evie” Whitlock Fellowship Research Award in Neonatology to Faeq Almudares. R01HL144775, R01HL146395 and R21100862 to Krithika Lingappan, T32007747 to Ivan Rosas, and IP42 ES0327725 to Bhagavatula Moorthy.
Footnotes
Conflict of Interest
The authors have no conflict of interest to disclose.
Ethical Statement:
The study protocol was approved by the institutional review board of the Baylor College of Medicine. Written informed consent was obtained before enrolling the infants in the study.
Contributor Information
Faeq Almudares, Department of Pediatrics, Baylor College of Medicine, Houston, USA. Address: 6621 Fannin St, MC: A5590, Houston, Texas, 77030. Phone number: 832-826-1380, Fax number: 832-825-2799.
Joseph Hagan, Department of Pediatrics, Baylor College of Medicine, Houston, USA..
Xinpu Chen, Department of Pathology and Immunology, Baylor College of Medicine, Houston, USA.
Sridevi Devaraj, Department of Pathology and Immunology, Baylor College of Medicine, Houston, USA..
Bhagavatula Moorthy, Department of Pediatrics, Baylor College of Medicine, Houston, USA.
Krithika Lingappan, Department of Pediatrics, Children’s Hospital of Philadelphia, Philadelphia, USA.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References:
- 1.Pramana I, Latzin P, Schlapbach L, Hafen G, Kuehni C, Nelle M, Riedel T, Frey U. Respiratory symptoms in Preterm Infants: Burden of Disease in The First Year of Life. Eur J Med Res (2011)223–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Natarajan G, Pappas A, Shankaran S, Kendrick D, Das A, Higgins R, Laptook A, Bell E, Stoll B, Newman N, et al. Outcomes of extremely low birth weight infants with bronchopulmonary dysplasia: impact of the physiologic definition. Early Hum Dev (2012) 88:509–515. doi: 10.1016/j.earlhumdev.2011.12.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roberts D, Dalziel S. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth (Review). (2006) doi: 10.1002/14651858.CD004454.pub2.www.cochranelibrary.com [DOI] [PubMed]
- 4.Fanaroff AA, Stoll BJ, Wright LL, Carlo WA, Ehrenkranz RA, Stark AR, Bauer CR, Donovan EF, Korones SB, Laptook AR, et al. Trends in neonatal morbidity and mortality for very low birthweight infants. Am J Obstet Gynecol (2007) 196:147.e1–147.e8. doi: 10.1016/j.ajog.2006.09.014 [DOI] [PubMed] [Google Scholar]
- 5.Bootcov M, Bauskin A, Valenzuela S, Moore A, Bansal M, He X, Zhang H, Donnellan M, Mahler S, Pryor K, et al. MIC-1, a novel macrophage inhibitory cytokine, is a divergent member of the TGF-beta superfamily. Proc Natl Acad Sci U S A (1997) 94:11514–11519. doi: 10.1073/pnas.94.21.11514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marjono A, Brown D, Horton K, Wallace E, Breit S, Manuelpillai U. Macrophage inhibitory cytokine-1 in gestational tissues and maternal serum in normal and pre-eclamptic pregnancy. Placenta (2003) 24:100–106. doi: 10.1053/plac.2002.0881 [DOI] [PubMed] [Google Scholar]
- 7.Bresson E, Seaborn T, Côté M, Cormier G, Provost P, Piedboeuf B, Tremblay Y. Gene expression profile of androgen modulated genes in the murine fetal developing lung. Reprod Biol Endocrinol (2010) 8: doi: 10.1186/1477-7827-8-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moore AG, Brown DA, Fairlie WD, Bauskin AR, Brown PK, Munier MLC, Russell PK, Salamonsen LA, Wallace EM, Breit SN. The transforming growth factor-β superfamily cytokine macrophage inhibitory cytokine-1 is present in high concentrations in the serum of pregnant women. J Clin Endocrinol Metab (2000) 85:4781–4788. doi: 10.1210/jc.85.12.4781 [DOI] [PubMed] [Google Scholar]
- 9.Kempf T, Horn-Wichmann R, Brabant G, Peter T, Allhoff T, Klein G, Drexler H, Johnston N, Wallentin L, Wollert K. Circulating concentrations of growth-differentiation factor 15 in apparently healthy elderly individuals and patients with chronic heart failure as assessed by a new immunoradiometric sandwich assay. Clin Chem (2007) 53:284–291. doi: 10.1373/clinchem.2006.076828 [DOI] [PubMed] [Google Scholar]
- 10.Takenouchi Y, Kitakaze K, Tsuboi K, Okamoto Y. Growth differentiation factor 15 facilitates lung fibrosis by activating macrophages and fibroblasts. Exp Cell Res (2020) 391: doi: 10.1016/j.yexcr.2020.112010 [DOI] [PubMed] [Google Scholar]
- 11.Albertoni M, Shaw P, Nozaki M, Godard S, Tenan M, Hamou M, Fairlie D, Breit S, Paralkar V, Tribolet N, et al. Anoxia induces macrophage inhibitory cytokine-1 (MIC-1) in glioblastoma cells independently of p53 and HIF-1. Oncogene (2002) 21:4212–4219. doi: 10.1038/sj.onc.1205610 [DOI] [PubMed] [Google Scholar]
- 12.Verhamme F, Seys L, De Smet E, Provoost S, Janssens W, Elewaut D, Joos G, Brusselle G, Bracke K. Elevated GDF-15 contributes to pulmonary inflammation upon cigarette smoke exposure. Mucosal Immunol (2017) 10:1400–1411. doi: 10.1038/mi.2017.3 [DOI] [PubMed] [Google Scholar]
- 13.Zhang Y, Jiang M, Nouraie M, Roth MG, Tabib T, Winters S, Chen X, Sembrat J, Chu Y, Cardenes N, et al. GDF15 is an epithelial-derived biomarker of idiopathic pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol (2019) 317:L510–L521. doi: 10.1152/ajplung.00062.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Geenen L, Baggen V, Kauling R, Koudstaal T, Boomars K, Boersma E, Roos-Hesselink J, van den Bosch A. Growth differentiation factor-15 as candidate predictor for mortality in adults with pulmonary hypertension. Heart (2020) 106:467–473. doi: 10.1136/heartjnl-2019-315111 [DOI] [PubMed] [Google Scholar]
- 15.Montero R, Yubero D, Villarroya J, Henares D, Jou C, Rodríguez MA, Ramos F, Nascimento A, Ortez CI, Campistol J, et al. GDF-15 is elevated in children with mitochondrial diseases and is induced by mitochondrial dysfunction. PLoS One (2016) 11:1–15. doi: 10.1371/journal.pone.0148709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li G, Li Y, Tan X, Jia P, Zhao J, Liu D, Wang T, Liu B. Plasma Growth Differentiation Factor-15 is a Potential Biomarker for Pediatric Pulmonary Arterial Hypertension Associated with Congenital Heart Disease. Pediatr Cardiol (2017) 38:1620–1626. doi: 10.1007/s00246-017-1705-7 [DOI] [PubMed] [Google Scholar]
- 17.Díaz M, Campderrós L, Guimaraes M, López-Bermejo A, de Zegher F, Villarroya F, Ibáñez L. Circulating growth-and-differentiation factor-15 in early life: relation to prenatal and postnatal growth and adiposity measurements. Pediatr Res (2019) 87:897–902. doi: 10.1038/s41390-019-0633-z [DOI] [PubMed] [Google Scholar]
- 18.Tiwari K, Moorthy B, Lingappan K. Role of GDF15 (growth and differentiation factor 15) in pulmonary oxygen toxicity. Toxicol Vitr (2015) 29:1369–1376. doi: 10.1016/j.tiv.2015.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jobe AH, Bancalari E. NICHD / NHLBI / ORD Workshop Summary. Am J Respir Crit Care Med (2001) 163:1723–1729. [DOI] [PubMed] [Google Scholar]
- 20.Cruickshank T, MacDonald TM, Walker SP, Keenan E, Dane K, Middleton A, Kyritsis V, Myers J, Cluver C, Hastie R, et al. Circulating growth differentiation factor 15 is increased preceding preeclampsia diagnosis: Implications as a disease biomarker. J Am Heart Assoc (2021) 10: doi: 10.1161/JAHA.120.020302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wertaschnigg D, Rolnik DL, Nie G, Teoh SSY, Syngelaki A, da Silva Costa F, Nicolaides KH. Second- and third-trimester serum levels of growth-differentiation factor-15 in prediction of pre-eclampsia. Ultrasound Obstet Gynecol (2020) 56:879–884. doi: 10.1002/uog.22070 [DOI] [PubMed] [Google Scholar]
- 22.Keelan JA, Wang K, Chaiworapongsa T, Romero R, Mitchell MD, Sato TA, Brown DA, Fairlie WD, Breit SN. Macrophage inhibitory cytokine 1 in fetal membranes and amniotic fluid from pregnancies with and without preterm labour and premature rupture of membranes. Mol Hum Reprod (2003) 9:535–540. doi: 10.1093/molehr/gag068 [DOI] [PubMed] [Google Scholar]
- 23.Sugulle M, Dechend R, Herse F, Weedon-Fekjaer MS, Johnsen GM, Brosnihan KB, Anton L, Luft FC, Wollert KC, Kempf T, et al. Circulating and placental growth-differentiation factor 15 in preeclampsia and in pregnancy complicated by diabetes mellitus. Hypertension (2009) 54:106–112. doi: 10.1161/HYPERTENSIONAHA.109.130583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kinoshita M, Yatsuga S, Iwata O, Okamura H, Morisaki T, Iwata S, Hara N, Shindo R, Saikusa M, Harada E, et al. Temporal changes and control variables of growth differentiation factor 15 levels during the first week of life in hospitalised newborn infants. Mitochondrion (2021) 61:25–30. doi: 10.1016/j.mito.2021.09.002 [DOI] [PubMed] [Google Scholar]
- 25.Al-Mudares F, Reddick S, Ren J, Venkatesh A, Zhao C, Lingappan K. Role of Growth Differentiation Factor 15 in Lung Disease and Senescence: Potential Role Across the Lifespan. Front Med (2020) 7:1–8. doi: 10.3389/fmed.2020.594137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mueller T, Leitner I, Egger M, Haltmayer M, Dieplinger B. Association of the biomarkers soluble ST2, galectin-3 and growth-differentiation factor-15 with heart failure and other non-cardiac diseases. Clin Chim Acta (2015) 445:155–160. doi: 10.1016/j.cca.2015.03.033 [DOI] [PubMed] [Google Scholar]
- 27.Sanders JL, Putman RK, Dupuis J, Xu H, Murabito JM, Araki T, Nishino M, Benjamin EJ, Levy DL, Ramachandran VS, et al. The association of aging biomarkers, interstitial lung abnormalities, and mortality. Am J Respir Crit Care Med (2021) 203:1149–1157. doi: 10.1164/rccm.202007-2993OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang L, Chang C, Sun Z, Madsen D, Zhu H, Padkjær S, Wu X, Huang T, Hultman K, Paulsen S, et al. GFRAL is the receptor for GDF15 and is required for the anti-obesity effects of the ligand. Nat Med (2017) 23:1158–1166. doi: 10.1038/nm.4394 [DOI] [PubMed] [Google Scholar]
- 29.Hsu J, Crawley S, Chen M, Ayupova D, Lindhout D, Higbee J, Kutach A, Joo W, Gao Z, Fu D, et al. Non-homeostatic body weight regulation through a brainstem-restricted receptor for GDF15. Nature (2017) 550:255–259. doi: 10.1038/nature24042 [DOI] [PubMed] [Google Scholar]
- 30.Emmerso P, Wang F, Du Y, Liu Q, Pickard R, Gonciarz M, Coskun T, Hamang M, Sindelar D, Ballman K, et al. The metabolic effects of GDF15 are mediated by the orphan receptor GFRAL. Nat Med (2017) 23:1215–1219. doi: 10.1038/nm.4393 [DOI] [PubMed] [Google Scholar]
- 31.Mullican S, Lin-Schmidt X, Chin C, Chavez J, Furman J, Armstrong A, Beck S, South V, Dinh T, Cash-Mason T, et al. GFRAL is the receptor for GDF15 and the ligand promotes weight loss in mice and nonhuman primates. Nat Med (2017) 23:1150–1157. doi: 10.1038/nm.4392 [DOI] [PubMed] [Google Scholar]
- 32.Wu Q, Jiang D, Chu H. Cigarette smoke induces growth differentiation factor 15 production in human lung epithelial cells: implication in mucin over-expression. Innate Immun (2012) 18:617–626. doi: 10.1177/1753425911429837 [DOI] [PubMed] [Google Scholar]
- 33.Xu J, Kimball T, Lorenz J, Brown D, Bauskin A, Klevitsky R, Hewett T, Breit S, Molkentin J. GDF15/MIC-1 functions as a protective and antihypertrophic factor released from the myocardium in association with SMAD protein activation. Circ Res (2006) 98:342–350. doi: 10.1161/01.RES.0000202804.84885.d0 [DOI] [PubMed] [Google Scholar]
- 34.Ofek Shlomai N, Reichman B, Zaslavsky-Paltiel I, Lerner-Geva L, Eventov-Friedman S. Neonatal morbidities and postnatal growth failure in very low birth weight, very preterm infants. Acta Paediatr Int J Paediatr (2022) 111:1536–1545. doi: 10.1111/apa.16380 [DOI] [PubMed] [Google Scholar]
- 35.Bhandari A, Bhandari V. Biomarkers in bronchopulmonary dysplasia. Paediatr Respir Rev (2013) 14:173–179. doi: 10.1016/j.prrv.2013.02.008 [DOI] [PubMed] [Google Scholar]
- 36.Gilfillan M, Bhandari V. Biomarkers for the diagnosis of neonatal sepsis and necrotizing enterocolitis: Clinical practice guidelines. Early Hum Dev (2017) 105:25–33. doi: 10.1016/j.earlhumdev.2016.12.002 [DOI] [PubMed] [Google Scholar]
- 37.Weisz DE, McNamara PJ, El-Khuffash A. Cardiac biomarkers and haemodynamically significant patent ductus arteriosus in preterm infants. Early Hum Dev (2017) 105:41–47. doi: 10.1016/j.earlhumdev.2016.12.007 [DOI] [PubMed] [Google Scholar]
- 38.Hasan J, Beharry KD, Valencia AM, Strauss A, Modanlou HD. Soluble vascular endothelial growth factor receptor 1 in tracheal aspirate fluid of preterm neonates at birth may be predictive of bronchopulmonary dysplasia/chronic lung disease. Pediatrics (2009) 123:1541–1547. doi: 10.1542/peds.2008-1670 [DOI] [PubMed] [Google Scholar]
- 39.Olsson KW, Larsson A, Jonzon A, Sindelar R. Exploration of potential biochemical markers for persistence of patent ductus arteriosus in preterm infants at 22–27 weeks’ gestation. Pediatr Res (2018) doi: 10.1038/s41390-018-0182-x [DOI] [PubMed] [Google Scholar]
- 40.Rossaint J, Vestweber D, Zarbock A. GDF-15 prevents platelet integrin activation and thrombus formation. J Thromb Haemost (2013) 11:335–344. doi: 10.1111/jth.12100 [DOI] [PubMed] [Google Scholar]
- 41.Zhou J, Kang Z, Liu L, Guo Y, Chen S. Predicting value of growth differentiation factor 15 and its correlations with atrial fibrillation. Heart Surg Forum (2020) 23:E452–E460. doi: 10.1532/hsf.2355 [DOI] [PubMed] [Google Scholar]
- 42.Baek SJ, Wilson LC, Lee CH, Eling TE. Dual function of nonsteroidal anti-inflammatory drugs (NSAIDs): Inhibition of cyclooxygenase and induction of NSAID-activated gene. J Pharmacol Exp Ther (2002) 301:1126–1131. doi: 10.1124/jpet.301.3.1126 [DOI] [PubMed] [Google Scholar]
- 43.Kulesza A, Zielniok K, Hawryluk J, Paczek L, Burdzinska A. Ibuprofen in Therapeutic Concentrations Affects the Secretion of Human Bone Marrow Mesenchymal Stromal Cells, but Not Their Proliferative and Migratory Capacity. Biomolecules (2022) 12: doi: 10.3390/biom12020287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Benitz WE. Hey, doctor, leave the PDA alone. Pediatrics (2017) 140:1–2. doi: 10.1542/peds.2017-0566 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


