Abstract
The hepatitis B virus (HBV) is a major cause of cirrhosis and hepatocellular carcinoma worldwide. Despite an effective vaccine the prevalence of chronic infection remains high. Current therapy is effective at achieving on-treatment but not off-treatment viral suppression. Loss of hepatitis B surface antigen (HBsAg), the best surrogate marker of off-treatment viral suppression, is associated with improved clinical outcomes. Unfortunately, this endpoint is rarely achieved with current therapy because of their lack of effect on covalently closed circular DNA, the template of viral transcription and genome replication. Major advancements in our understanding of HBV virology along with better understanding of immunopathogenesis have led to the development of a multitude of novel therapeutic approaches with the prospect of achieving functional cure (HBsAg loss) and perhaps complete cure (clearance of cccDNA and integrated HBV DNA). This review will cover current best practice for managing chronic HBV infection and emerging novel therapies for HBV infection and their prospect for cure.
Introduction
The hepatitis B virus (HBV) is a small hepatropic DNA virus that has been infecting humans for millennia. An ancestral strain was likely present in hunter-gatherers during the early Holocene period (~20,000–12,000 years ago).[1]. During human evolution, spread of HBV was likely facilitated by the establishment of agrarian societies in the Neolithic and Bronze Ages.[2] Currently, it is estimated that over 2 billion persons have been exposed to HBV, of whom 296 million (~3.7% of the human population) have chronic infection [3, 4]. Chronic HBV infection is responsible for ~820,000 deaths annually worldwide from complications of cirrhosis and hepatocellular carcinoma (HCC) [5]. Despite the availability of an effective vaccine, ~1.5 million new infections occur annually.
Nevertheless, the HBV vaccine has had a profound impact on the prevalence of chronic HBV infection and complication rate, particularly in high prevalence regions.[6, 7] Given the significant burden on global public health, the World Health Organization (WHO) has set a goal of complete eradication of HBV by 2030, defined as a 65% reduction in mortality and a 90% reduction in incidence compared with the baseline levels obtained in 2015.[8]. Currently, only 12% of countries are on track to meet WHO elimination targets.[9]
HBV lifecycle
The intact virion or Dane particle has an outer lipid envelope that surrounds a viral nucleocapsid containing the viral DNA and polymerase. The genome is partially double-stranded DNA with four overlapping open reading frames that encode for seven viral proteins: polymerase, core, hepatitis B e antigen (HBeAg), large, middle, and small HBsAg and X protein.[10].
The viral lifecycle is illustrated in Figure 1. During replication, double-stranded linear DNA (dslDNA) forms are produced (~5–10%) that may integrate randomly into the host genome by utilizing random sites of host cell DNA breaks.[11] Apart from being a constant source of RNA and viral proteins, integration is also considered to be a contributor to the development of HCC. A substantial proportion of HBsAg may be derived from integrated HBV DNA, particularly among HBeAg negative patients,[12], Figure 1, suggesting that HBsAg loss may ultimately require the elimination of integrated HBV DNA.
Figure 1: HBV lifecycle and targets of drug development.

Viral entry is a multi-step process beginning with viral attachment to the hepatocyte surface via a loose interaction with heparan sulfate proteoglycans[145]. This is followed by stronger interaction between the pre-S1 domain and the hepatocyte bile salt transporter, the sodium taurocholate co-transporting polypeptide (NTCP) which facilitates entry[146]. The NTCP receptor confers species specificity to HBV. Viral entry is thought to occur via endocytosis. Following entry, there is uncoating and release of the partially double-stranded, relaxed circular DNA genome (rcDNA), which is transported to the hepatocyte nucleus where host cellular enzymes repair the rcDNA to form the covalently closed circular DNA (cccDNA). cccDNA serves as the transcriptional template for all mRNAs including the pre-genomic RNA, which also serves as the template for genome replication. Viral transcription and translation are under the control of viral promoters and enhancers. Four viral transcripts, polymerase, core, surface, and X are transported to the cytoplasm where they are translated into 7 viral proteins. In the cytoplasm, core proteins self-assemble and through an encapsidation reaction, the pgRNA and viral polymerase are packaged to form the nucleocapsid. Viral replication occurs within the nucleocapsid through a reverse transcription step. The mature viral capsids containing rcDNA are then enveloped with the small, medium, and large (S, M, L) surface proteins in the endoplasmic reticulum and secreted from the infected cell as intact virions, (Dane particle), or transported back to the nucleus to replenish the cccDNA pool. Several sub-viral filamentous and spherical particles that are devoid of viral DNA are also produced in vast excess of the Dane particle.
1)Targeting viral entry; 2) Targeting covalently closed circular DNA (cccDNA) via elimination or silencing; 3) Targeting viral transcription 4) Targeting the HBV core protein; 5) Targeting the HBV polymerase; and 6) Targeting hepatitis B surface antigen (HBsAg) secretion.
Immunopathogenesis of HBV
The immune response contributes to both HBV clearance and liver injury. HBV does not readily activate the intracellular innate defense mechanisms including type I IFN pathway [13, 14]. However, HBV replication is inhibited by pharmacological activation of type I/III IFNs and intracellular antiviral sensors such as the toll like receptors (TLRs) and retinoid acid inducible gene-I (RIG-I) like receptors as well as exogenous IFN therapy [15]. HBV can also induce type III IFN through the interaction between the HBV pregenomic RNA and RIG-I [16] and with a biphasic interferon stimulated gene (ISG) induction in hepatocytes in-vitro [17]. In addition, natural killer (NK) and NKT cells can be activated early in acutely HBV-infected patients [18]. Thus, once HBV replication is established in hepatocytes, type III IFN, and activation of NK and Kupffer cells may help to modulate viral replication and viral spread during the early stages of infection.
As for the adaptive immune response, T-cells play a key role in HBV clearance and liver disease pathogenesis as shown in experimental animal models [19]. CD8 T-cells directly recognize and kill (or cure) virus-infected hepatocytes that express viral epitopes on class I MHC, whereas CD4 T-cells provide critical T-cell help and orchestrate the overall adaptive immune response. In acutely HBV-infected patients, spontaneous viral clearance and disease resolution is characterized by a broadly specific and durable antiviral CD8 and CD4 T-cell responses as well as HBsAg-specific neutralizing antibody response. Importantly, memory T-cell response to HBV can persist for decades after clinical resolution of acute HBV infection, maintained by trace amounts of virus in-vivo [20] and likely mediating virus control—raising the possibility for their role for sustained virus control of HBV post-therapy. Similarly, a critical role for B-cells in HBV control is suggested by HBV reactivation by immunosuppressive regimens that deplete B cells [21, 22].
Evolution of acute HBV infection to chronic likely involves both host and viral factors [23, 24], although precise mechanisms are not well defined. Established chronic HBV infection is characterized by both HBV-specific and global T- [25, 26] and B-cell dysfunction [27, 28], due to prolonged exposure to viral antigens and inflammatory mediators that leads to immune exhaustion with the induction of regulatory pathways and immune checkpoints including regulatory T-cells, programmed cell death protein 1 (PD1)[29] and cytotoxic T lymphocyte-associated antigen-4 (CTLA-4) [26], in addition to altered gammadelta T-cells[30] and metabolic immune dysregulation through myeloid derived suppressor cells and arginase [31] and antiviral T-cell elimination through activated NK cells and death pathways (e.g., Bim) [31, 32]. Lacking adaptive immune control, non-specific inflammatory infiltrates combining innate and adaptive immune cells accumulate in HBV-infected liver and promote hepatocellular injury and fibrosis without virus suppression [33]. Thus, immune-mediated HBV therapy requires a fine balance between immune control of the virus and hepatocellular injury to avoid adverse clinical consequences.
Preventing infection
HBV vaccination has resulted in a significant reduction in both disease prevalence and complications of HBV including HCC [6, 7]. In the U.S., the Advisory Committee on Immunization practices (ACIP)[34] recommends vaccination of all infants, children, adolescents, and adults through 59 years of age as well as adults >60 years with risk factors. Additional information regarding available infant, child, adolescent and adult vaccines, vaccinee schedules and at-risk populations are provided in Supplementary Tables 1 and 2.
Treatment
Goals of treatment
The primary goal of therapy is to prevent cirrhosis, development of HCC and liver-related mortality. However, these endpoints take decades to develop. Therefore, studies evaluating therapies for chronic HBV infection have relied on surrogate endpoints. These include undetectable HBV DNA using a sensitive PCR-based assay, normalization of serum alanine aminotransferase (ALT), loss of HBeAg, loss of HBsAg and histological improvement. HBsAg loss is considered the best endpoint because it is associated with durable suppression of HBV DNA and improvement in clinical outcomes such as hepatic decompensation, hepatocellular carcinoma and liver-related death.[35, 36] However, as reported in two meta-analyses the rate of spontaneous and treatment-related HBsAg loss is low, approximately 1% annually.[37, 38]
Indications for treatment
Chronic hepatitis B (CHB) is a dynamic disease characterized by frequent fluctuations in disease activity. Historically, HBeAg status, HBV DNA and ALT levels are used to assess disease activity. The three major liver societies, the American Association for the Study of Liver Diseases (AASLD), the European Association for the Study of the Liver (EASL) and the Asian Pacific Association for the Study of the Liver (APASL) have provided guidance on indications for treatment, Table 1. [39–41] Additionally, the World Health Organization (WHO) has developed a more simplified approach to treatment for low and middle income countries that may lack access to viral load testing.[42] All guidelines strongly agree that patients with decompensated liver disease, cirrhosis and those with active disease (defined as those with elevated HBV DNA and ALT levels) should receive treatment. There are minor regional differences in the choice of HBV DNA level (for example HBV DNA level of 20,000 IU/ml (AASLD and APASL) or 2,000 IU/mL (EASL) in an HBeAg positive patient) and ALT cut-offs (either twice the laboratory upper limit of normal (ULN) (APASL and EASL), >ULN if moderate liver necroinflammation or fibrosis is present (EASL), or gender specific ALT cut-offs −35 U/L for males and 25 U/L for females (AASLD)) to initiate therapy. Similarly, there is general agreement that patients whose disease is inactive (HBeAg negative with low HBV DNA (<2,000 IU/mL) and normal ALT levels can be safely observed without the need for treatment. There is some controversy on how patients with elevated HBV DNA but normal (≤1xULN) or mildly elevated ALT levels (>1-<2xULN) should be managed. In these situations, obtaining additional evidence on disease severity either through a liver biopsy or non-invasive assessment of fibrosis is advised to assist in decision making. Among non-invasive tests, transient elastography (TE)[43] or shear wave elastography (SWE)[44] generally have higher diagnostic accuracy over serum biomarkers such as APRI and FIB-4 and are therefore preferred for assessing fibrosis in the absence of a liver biopsy, Supplementary Tables 3a and 3b. Non-invasive tests perform better at excluding than establishing advanced fibrosis/cirrhosis. Additionally, other factors such as age over 40 years, family history of HCC, lengthy disease duration, HBsAg level ≥1,000 IU/mL and a patient’s willingness to receive treatment should be considered in the decision to recommend therapy. [39–41, 45, 46]. Other indications for treatment or prophylaxis are listed in Table 2.
Table 1:
Indications for Treatment by Liver Society Guidelines and World Health Organization.
| Indication | AASLD | EASL | APASL | WHO |
|---|---|---|---|---|
| Cirrhosis (any detectable HBV DNA) | Treat | Treat | Treat | Treat |
| HBeAg positive CHB | Treat if: ^ALT≥ 2 X ULN and HBV DNA> 20,000 IU/mL |
Treat if: HBV DNA> 2,000 IU/mL,#ALT>ULN and/or at least moderate liver necroinflammatio n or fibrosis* |
Treat if: |
Treat all adults above the age of 30 if:
|
| HBeAg negative CHB | Treat if: ALT≥ 2 X ULN and HBV DNA> 2,000 IU/mL |
Treat if: HBV DNA> 2,000 IU/mL, ALT>ULN and/or at least moderate liver necroinflammation or fibrosis* |
Treat if:
|
|
| CHB reactivation | Treat | Treat | Treat | Treat |
| Pregnant women with HBV DNA >200,000 IU/mL on 3rd trimester | Treat | Treat | Treat** | Decision to treat should be based on regular treatment indications. No specific recommendation regarding prevention of vertical transmission |
Normal ALT defined as ≤35 and ≤25 U/L for males and females, respectively.
Normal ALT defined as ≤ laboratory upper limit of normal (~40 U/L)
Based on histologic assessment of liver biopsy including moderate to severe inflammation by either Ishak activity score >3 or METAVIR activity score above A2. Fibrosis by Ishak score > 3 or METAVIR >2, Elastography (Fibroscan©) > 8kPa.
If HBV DNA is above 6–7 log IU/mL
ALT, alanine aminotransferase, AASLD, American Association for the Study of Liver Diseases; EASL, European Association for the Study of the Liver; APASL, Asian-Pacific Association for the Study of Liver Diseases; WHO, World Health Organization.
Table 2.
Indications for treatment and prophylaxis
| Indications for treatment | Indications for prophylaxis (Prevention of HBV transmission/re-activation) |
|---|---|
| Decompensated cirrhosis | Post-liver transplantation |
| Compensated cirrhosis regardless of HBV DNA and ALT levels | Post-liver transplantation from anti-HBc positive donor to HBsAg negative recipient |
| HBV presenting with acute liver failure | HBsAg positive mother during the third trimester with HBV DNA >200,000 IU/mL |
| HBeAg positive immune active (HBV DNA >20,000 IU/mL and ALT >2XULN) | HBsAg positive patients receiving immunosuppression/chemotherapy |
| HBeAg negative immune active (HBV DNA >2,000 IU/mL and ALT >2XULN) | HBsAg negative, anti-HBc positive patients receiving immunosuppression / chemotherapy and at high risk for reactivation |
| HBV/HDV Co-infection with HBV DNA >2,000 IU/mL | |
| HBV / HIV Co-infection | |
| HBV presenting with extrahepatic manifestations | |
| HBsAg positive healthcare worker with HBV DNA>2,000 IU/mL |
An alternate and more simplified approach to treatment being put forth by some experts, but not endorsed by any of the major liver society guidelines, is a “treat all” approach in which any HBsAg positive individual with detectable viremia regardless of ALT level would receive treatment. In the case of HBeAg positive patients with markedly elevated HBV DNA (>108 IU/mL) and normal ALT levels (immunetolerant or HBeAg positive chronic infection with no clear evidence of hepatocellular damage), the recommendation to treat is driven by a desire to limit the risk for HBV-specific T-cell depletion, DNA integrations that drive HCC risk, silent fibrosis progression, and risk of transmission. Indeed, this approach is supported by the Risk Evaluation of Viral Load Elevation and Associated Liver Disease (REVEAL) study[47, 48] which showed a relationship between elevated HBV DNA levels and subsequent development of cirrhosis and HCC and a Korean study reporting that untreated immunetolerant patients have higher risk of HCC and death/transplantation than nucleos(t)ide analogue treated immuneactive-phase.[49] However, as data from the REVEAL study was obtained from an older, predominantly male, HBeAg negative cohort, caution is advised in extrapolating to a younger HBeAg positive cohort. Also, in the latter study from Korea, the HCC risk was lowest among patients with highest HBV DNA levels and normal ALT levels (true immunetolerant patients). Notably in that study, the mean age of the immunetolerant patients was 38 years, thus, many or most would have met the three liver association guidelines for treatment. Furthermore, there is currently no evidence that lowering viral load would necessarily reduce HCC incidence in patients with immunetolerant disease. Moreover, spontaneous HBeAg seroconversion occurs in a majority of patients with low rates of progression to HBeAg negative immuneactive disease, cirrhosis or HCC,[50] achieving undetectable HBV DNA is challenging, integration cannot be prevented, and paired liver biopsy studies showed minimal if any fibrosis progression in patients with immunetolerant disease.[51] However, some studies suggest unacceptably high rates of HCC and other clinical outcomes among indeterminate/grey zone patients (HBeAg negative with elevated HBV DNA and mildly elevated ALT levels) or who do not meet criteria for treatment according to APASL, AASLD and EASL and that treatment may be of benefit to these patients.[52–54] Future studies are needed to address management of these controversial group of patients. Until such results are available, we recommend a “case by case” approach, considering presence of risk factors for disease progression and HCC and patient’s willingness for treatment for cases outside the guideline treatment criteria. Please see supplementary data for more in-depth discussion of management of controversial patients.
Current treatment options
There are seven licensed agents for treatment of chronic HBV infection in the U.S., standard interferon alfa-2b (no longer available in the U.S. and Europe), pegylated interferon alfa-2a, lamivudine (LAM), adefovir, telbivudine, entecavir (ETV), tenofovir disoproxil fumarate (TDF) and tenofovir alafenamide (TAF). Pegylated interferon is preferred over standard interferon due its more favorable pharmacokinetics and dosing schedule (once weekly versus thrice weekly). Among the nucleos(t)ide analogues, entecavir, TDF and TAF are recommended over LAM, adefovir, telbivudine because of their greater potency and lower rates of antiviral resistance. The sustained, on-treatment viral suppression that can be achieved with these agents is associated with less progression to cirrhosis or even reversal of cirrhosis, prevention of decompensation, reduction but not prevention of HCC and lower mortality.
Two treatment strategies are recommended by liver society guidelines, Table 3. One is a finite 48-week treatment course with pegylated interferon alfa-2a and the other is long-term therapy with one of the recommended nucleos(t)ide analogues.
Table 3.
Efficacy of Currently Approved Agents for Therapy of Chronic Hepatitis B
| HBeAg positive | PegIFN (180mcg/week SC) |
Entecavir (0.5mg/day PO) |
Tenofovir disoproxil fumarate (245–300mg/day PO) | Tenofovir alafenamide (25mg/day PO) |
|---|---|---|---|---|
| Anti-HBeAg seroconversion | 32%1 | 21%2 23%3 |
21%2 27%4 |
10%2 |
| HBV DNA < 60–80 lU/mL | 14%1 | 67%2 94%3 |
76%2 98%4 |
64%2 |
| ALT normalization | 41%1 | 68%2 80%3 |
68%2 78%4 |
72%2 |
| HBsAg loss | 3–7%1 | 2%2 1.4%3 |
3%2 5%4 |
1%2 |
| HBeAg negative | PegIFN (180mcg/week) |
Entecavir (0.5mg/day) |
Tenofovir disoproxil fumarate (245–300mg/day) | Tenofovir alafenamide (25mg/day) |
| HBV DNA < 60–80 lU/mL | 19%1 | 90%2 | 93%2 100%4 |
94%2 |
| ALT normalization |
59%1 | 78%2 | 76%2 83%4 |
83%2 |
| HBsAg loss | 4%1 | 0%2 | 0%2 3%4 |
0%2 |
Table adapted from EASL Clinical practice guidelines J Hepatol 2017; 67:370–398 and Terrault N et al Hepatology 2018; 67:1560–1599.
pegIFN, pegylated interferon alfa-2a; HBeAg, hepatitis B e antigen; HBsAg, hepatitis B s antigen; ALT, alanine aminotransferase.
ALT normalization defined by laboratory upper limit of normal
Evaluated 6 months following 48–52 weeks of treatment
Evaluated at 48–96 weeks of continuous therapy
The entecavir long-term cohort consisted of 183 HBeAg positive patients who received ≥1 year of entecavir 0.5 mg in the registration trial (ETV-022) and then entered long-term treatment (ETV-901) with a treatment gap ≤35 days. In ETV-901 the entecavir dose was increased to 1.0 mg daily.[144]
Results based on a sub-set of patients 203/641 (32%) HBeAg‐positive (n=80) and HBeAg-negative (n=118) patients who were initially randomized and treated and who were followed for 10 years.[93]
Pegylated interferon alfa-2a
The mechanism of action of pegylated interferon alfa-2a is not fully understood but the drug has both antiviral and immunomodulatory properties. The recommended dose for both HBeAg positive and negative patients is 180 μg once weekly by subcutaneous (SC) injection for 48 weeks. Pegylated interferon can be discontinued early for futility among HBeAg positive patients, if at week 12 HBsAg levels are ≥20,000 IU/ml for genotypes B and C, or no decline of HBsAg levels are observed for genotypes A and D and at week 24 if HBsAg levels ≥20,000 IU/ml in patients with genotypes A-D. Pegylated interferon can be stopped among HBeAg negative patients with genotype D at week 12 if there is no decrease in HBsAg levels and <2 log10 IU/ml reduction in serum HBV DNA. In addition to the finite dosing schedule, other advantages of pegylated interferon are a relatively high rate of HBeAg loss (>30%) in HBeAg positive patients [55, 56] and the potential to clear HBsAg (2–7% in HBeAg-positive and 4% in HBeAg-negative patients) with a relatively short duration of therapy, especially in patients with HBV genotypes A and B. Moreover, HBeAg loss and HBsAg loss are durable. Disadvantages of pegylated interferon include the need for administration by SC injection as well as the numerous substantial adverse events.[57] Additionally, peginterferon is contraindicated in patients with decompensated cirrhosis and compensated cirrhosis with clinically significant portal hypertension, due to the risk of hepatic decompensation, and during pregnancy. Some studies have shown benefit in extending treatment duration beyond 48 weeks especially among HBeAg negative patients[58, 59] but in practice this is difficult due to poor patient tolerance.
Nucleos(t)ide analogues
Nucleos(t)ide analogues, incorporated into nascent DNA by the HBV reverse transcriptase, inhibit viral replication by functioning as DNA chain terminators.[60] They are more potent inhibitors of viral replication compared to pegylated interferon but recrudescence of viral replication following their withdrawal is common due their lack of activity on covalently closed circular DNA (cccDNA). Recently, it was shown that nucleos(t)ide analogues (tenofovir) may decrease the number of transcriptionally active distinct HBV-host DNA integrations among patients with mild CHB.[61] However, it remains uncertain if nucleos(t)ide analogues can alter clonal expansion of hepatocytes carrying unique viral integrations, the expression level of an integrated sequence or if reducing integrations would result in a lower incidence of HCC. Efficacy of recommended nucleos(t)ide analogues are shown in Table 3. Rates of HBsAg loss after 1 year are 1–3% in HBeAg positive and ≤1% in HBeAg negative patients. In comparison to pegylated interferon, nucleos(t)ide analogues are orally administered, are well tolerated, and have an excellent safety profile. There is a small risk for nephropathy and bone loss, particularly with TDF. They may be used in patients with decompensated cirrhosis and in pregnant women as treatment or for prevention of mother-to-infant transmission. In the absence of co-morbid conditions, selection of one nucleos(t)ide analogue over another is based on patient preference and cost. In patients with renal or bone disease, entecavir or TAF are preferred. For treatment experienced patients or HIV-HBV co-infection, TDF or TAF are the preferred agents due to the high rate of resistance to entecavir in LAM-experienced patients. In pregnant women, TDF is preferred because data on the safety of TAF[62–64] are not yet as extensive as TDF and ETV is contraindicated during pregnancy.
Special populations
Treatment of special populations is beyond the scope of this review and readers are referred to guidelines that cover this topic.[39–41] An abridged overview of management is provided in Supplementary Table 4. Patients scheduled to receive immunosuppressive or cytotoxic therapy are at risk for reactivation of HBV infection. Consequently, all patients should be screened for current or past HBV infection using HBsAg and anti-HBc. Those who test positive for HBV markers should be further risk stratified based on the immunosuppressive regimen. Patients at high risk for HBV reactivation (e.g., use of anti-CD20 agent) should receive prophylactic antiviral therapy. Those at moderate risk (e.g., anti-TNF or low dose steroids) could either receive prophylactic antiviral therapy or be monitored closely with HBV DNA and ALT testing every 3 months. If reactivation occurs, patients should immediately start antiviral therapy. Patients at low risk (short term steroids) do not require monitoring for reactivation. The preferred prophylactic treatment of choice is either TDF, TAF or ETV. Ideally, treatment should be initiated 2–4 weeks before a planned course of immunosuppression and continued for an additional 6 months after immunosuppression is stopped. An exception is for patients receiving B-cell depleting regimens (e.g., anti-CD20) where treatment should continue for 12–18 months after immunosuppression is stopped. [39–41, 65]
Endpoints of treatment
Pegylated interferon is administered for a finite duration of 48 weeks. Among HBeAg positive patients, pegylated interferon-related HBeAg seroconversion is a durable endpoint. Approximately twenty-five percent of patients will have sustained suppression of HBV DNA < 2000 IU/mL in the short-term (6–12 months off-treatment).[66] In contrast, among HBeAg negative patients, only 19% can maintain HBV DNA suppression < 400 copies/mL, off-treatment.[67]
The optimal endpoint during therapy with nucleos(t)ide analogues is HBsAg loss that is confirmed on at least two occasions, with or without development of anti-HBs. Nucleos(t)ide analogues can be discontinued in non-cirrhotic, HBeAg positive patients who achieve HBeAg seroconversion and undetectable HBV DNA and who receive at least 12 months of consolidation therapy. Post-treatment monitoring is advised (every 3 months) for at least a year to detect a return of active disease. For HBeAg negative patients, AASLD recommends indefinite therapy until HBsAg loss occurs. However, APASL and EASL recommend that treatment can be discontinued in selected patients without cirrhosis who achieve undetectable HBV DNA and normal ALT levels for a period of 2–3 years. This recommendation is based on data reporting rates of HBsAg loss of ~20% among Caucasian patients and 2–3% among Asian patients 3 years after withdrawal and 20–30% maintaining a low HBV DNA (<2,000 IU/mL) and normal ALT after withdrawal of nucleos(t)ide analogues.[68] HBsAg cutoffs of <1,000 IU/mL among Caucasians and <100 IU/mL among Asians were associated with the highest rates of HBsAg loss.[69] In practice, virological relapse is almost universal, withdrawal flares are observed in 10–30% of patients within the first 3 months of stopping treatment and almost half of patients require re-initiation of treatment. Thus, the decision to withdraw treatment requires careful deliberation with the patient and the patient must agree to close monitoring after withdrawal of therapy. Patients with cirrhosis should not stop antiviral therapy due to the risk of hepatic decompensation.
Monitoring untreated patients and Screening
Due to the dynamic nature of chronic HBV infection, untreated patients should be monitored, with serial HBV DNA and ALT levels every 3–6 months, for evidence of disease progression until spontaneous HBsAg loss occurs. Among HBeAg positive patients HBeAg status should be checked every 6–12 months. Among HBeAg negative patients with low HBV DNA levels (<2,000 IU/mL and normal ALT level, (inactive carrier/ HBeAg negative chronic infection)) HBV DNA and ALT levels should be checked every 3 months for one year to confirm inactive disease after which they may be monitored every 6–12 months. Testing for HBsAg loss should be performed annually. If available, monitoring quantitative HBsAg levels annually in HBeAg negative patients with HBV DNA levels <2,000 IU/mL may be helpful to allow HCC risk stratification and determining the monitoring schedule. A non-invasive assessment of liver fibrosis should be considered every 2–3 years.
HCC surveillance is considered cost-effective if the annual risk of HCC is ≥0.2%. Consequently, all patients with cirrhosis should be screened with ultrasound with or without alfa fetoprotein (AFP) testing every 6 months. HBsAg-positive adults without cirrhosis but considered at high risk for HCC including Asian or Black men over 40 years and Asian women over 50 years of age, persons with a first-degree relative with a history of HCC, or persons with hepatitis D virus (HDV) should also undergo screening every 6 months. After HBsAg loss, surveillance for HCC should continue for patients with cirrhosis, those who have a first-degree relative with HCC, or a long duration of infection (>40 years for males and >50 years for females).
Limitations of current therapy
Although current therapy is associated with less outcomes, it is not curative and does not eliminate HCC risk. This is due to their limited effect on cccDNA and integrated HBV DNA. Additionally, current treatment does not restore the immune dysfunction that is characteristic of chronic HBV infection. Thus, there is an urgent need for short duration regimens that can achieve high rates of HBsAg loss.
Novel treatments
The development of curative therapy for HCV infection and limitations of current therapy for HBV infection has renewed interest in curing chronic HBV infection. A more comprehensive understanding of the HBV lifecycle and immunopathogenesis of persistent infection coupled with innovations in drug development and delivery have led to multiple new approaches to treat chronic HBV infection. These include innovative means to interrupt viral production, Table 4, Figure 1 and/or to restore or boost the exhausted immune response, Table 4, Figure 2. Along with the development of novel therapy, newer tools to monitor the virological and immunological response such as HBV RNA, HBcrAg and cytokine panels will be needed. An in-depth review of these markers is beyond the scope of this review, but readers are directed to an excellent review on the topic.[70]
Table 4.
Direct and Indirect Antiviral Agents Currently in Development
| Target | Mechanism of Action | Agent in Development | Current Stage of Development |
|---|---|---|---|
| Direct | |||
| Viral Entry | Blockage of the NTCP receptor | Bulevirtide | Phase 3* |
| Hepalatide | Phase 2 | ||
| Monoclonal antibody against the pre-S1 domain | VIR-3434 | Phase 1 | |
| Viral Transcription | mRNA disruption by siRNA | JNJ-3989 | Phase 2 |
| AB-729 | Phase 2 | ||
| RG6346 | Phase 2 | ||
| VIR-2218 | Phase 2 | ||
| ALG-125755 | Phase 1 | ||
| BB-103 | Pre-Clinical | ||
| mRNA disruption by ASO | Bepirovirsen | Phase 2 | |
| IONIS-HBVLRx | Phase 2 | ||
| Core Protein | Capsid Inhibitor | EDP-514 | Phase 2 |
| Morphothiadin | Phase 2 | ||
| RG7907 | Phase 2 | ||
| Vebicorvir | Phase 2 | ||
| JNJ 56136379 | Phase 2 | ||
| ABI-H3733 | Phase 1 | ||
| AB-836 | Phase 1 | ||
| ALG-000184 | Phase 1 | ||
| QL-007 | Phase 1 | ||
| VNRX-9945 | Phase 1 | ||
| ZM-H1505R | Phase 1 | ||
| GLP-26 | Pre-Clinical | ||
| ABI-4334 | Pre-Clinical | ||
| cccDNA | Reducing HBX expression | Pevonedistat | Pre-Clinical |
| Dicoumarol | Pre-Clinical | ||
| HBV polymerase | Prodrugs of nucleotide analogues | Pradefovir | Phase 3 |
| HS-10234 | Phase 3 | ||
| NCO-48 fumarate | Phase 1 | ||
| Non-chain terminating nucleotide analogue | AT-2173 | Phase 2 | |
| HBsAg release | Nucleic Acid Polymers (NAPs) | REP 2139/2165 | Phase 2 |
| S-Antigen Transport-inhibiting Oligonucleotide Polymer (STOPS) | ALG-010133 | Discontinued | |
| Indirect | |||
| Innate immunity | TLR 7 agonist | Vesatolimod | Phase 2 |
| RG7854 | Phase 1 | ||
| TLR 8 agonist | GS-9688 | Phase 2 | |
| SBT 8230 | Preclinical | ||
| Adaptive immunity | Checkpoint inhibitor | Nivolumab | Phase 2 |
| Envafolimbab (ASC22) | Phase 2 | ||
| Immune Mobilizing Monoclonal T-cell Receptors Against Virus (ImmTAV) | IMC-I109V | Phase 1/2 | |
| Therapeutic vaccines | DNA vaccines | GS-4774 | Phase 2 |
| HB-110 | Phase 1 | ||
| INO-1800/9112 | Phase 1 | ||
| JNJ-64300535 | Phase 1 | ||
| MVA-HBV (VTP-300) | Phase 1 | ||
| TG1050 | Phase 1 | ||
| VRON-0200 | Preclinical | ||
| T-cell or B-cell epitope vaccine | εPA-44 | Phase 3 | |
| FP-02.2 (HepTcell) | Phase 2 | ||
| HBV envelope antigen vaccines | NASVAC | Phase 4 | |
| BRII-179 | Phase 2 | ||
| VVX001 | Phase 2 |
NTCP, sodium taurocholate co-transporting polypeptide; HBX, HBV X protein; HBsAg, HBV surface antigen; cccDNA, covalently closed circular DNA; siRNA, small interfering RNAs; ASO, antisense oligonucleotides.
For HBV/HDV co-infection not HBV monotherapy.
Figure 2: Immune subsets involved in HBV pathogenesis and approaches for immune-modulatory therapy.
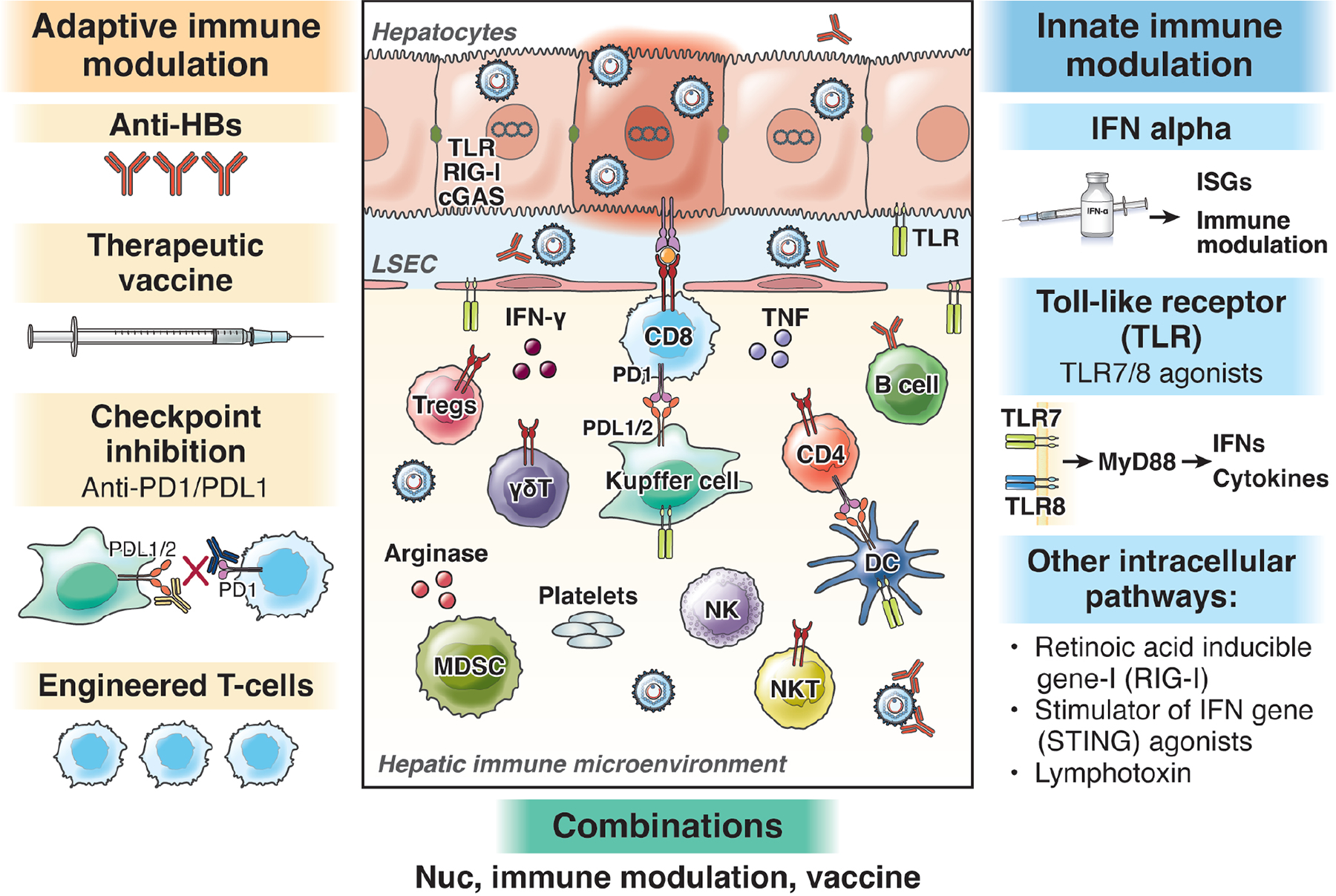
Multiple immune subsets participate in virus control and disease pathogenesis in chronic hepatitis B. Adaptive immune modulatory approaches in exploration include augmentation of antiviral T and B cells by therapeutic vaccination, checkpoint inhibition (e.g. blockade of PD1/PDL1 or CTLA4/CD28 interactions) as well as supplementation by providing engineered T-cells or antibodies. Innate immune modulatory include IFN alpha (already in clinical use with pleiotropic antiviral and immune modulatory effects) in addition to evolving clinical and pre-clinical evaluations for cellular antiviral pathways including agonists for toll like receptors (e.g TLR7/8), RIG-I,* STING agonists and lymphotoxins.
*No longer in clinical development
The focus of novel therapy is to achieve HBsAg loss or “functional cure”. A true cure or “complete cure” would require eradication of the non-integrated cccDNA as well as integrated HBV DNA. Currently, this is not technically feasible. An alternate but less desirable goal is sustained off-treatment inhibition of viral replication or “partial cure”. Although this is associated with improved clinical outcomes, the response is often not durable.
Direct Antiviral agents in development
Agents Targeting Viral Entry
The premise of targeting viral entry is to block new rounds of hepatocyte infection, thereby preventing cccDNA formation and reducing the cccDNA pool. Bulevirtide (previously Myrcludex B), is a synthetic, N-acylated pre-S1 peptide that irreversibly blocks the sodium taurocholate co-transporting polypeptide (NTCP) receptor, thereby preventing viral entry.[71] Bulevirtide is conditionally approved by the European Medicine Agency (EMA) for the treatment of chronic HDV infection.[72] There is limited clinical data in the HBV mono-infected population. The results of a small unpublished study, indicated that bulevirtide administered at different doses for 12 weeks in patients with HBeAg negative CHB led to a ≥0.5 log IU/mL decline in HBsAg level at week 12 in a minority of patients but none lost HBsAg.[73] Asymptomatic bile acid elevation was noted. These findings are perhaps not surprising given the relatively long half-life of cccDNA. It is predicted that treatment may have to be administered long-term to have any meaningful effect on HBsAg loss. Hepalatide is another NTCP receptor blocker currently being evaluated for CHB treatment in combination with pegylated interferon versus pegylated interferon alone in a double blind, placebo-controlled phase 2 study (NCT04426968).
Rather than targeting the receptor, several monoclonal/polyclonal antibody preparations are being developed that bind to the N-terminal region of pre-S1, the site of viral interaction with the NTCP receptor.[74] In addition to blocking viral entry, monoclonal antibodies can lower viremia and the level of subviral particles and may cross-present viral antigens with stimulation of T-cells leading to HBsAg loss. GC1102 is a recombinant hepatitis B immunoglobulin currently in a phase 2 trial. VIR-3434, is a novel monoclonal antibody in a phase 1 study in virally suppressed patients (NCT04423393). Preliminary results suggest a rapid dose dependent decline in HBsAg levels without significant adverse events.[75] Further studies with longer term follow-up regarding safety and efficacy of entry inhibitors and monoclonal antibodies are eagerly awaited.
Agents Targeting Viral Transcripts
Another approach to inhibit viral production is to target the protein encoding mRNAs via RNA interference and antisense oligonucleotides (ASOs). A therapeutic advantage of this approach is that multiple viral transcripts may be silenced by a single siRNA/ASO because all HBV mRNAs share the same terminal 3’ sequence. Potential advantages of an ASOs are its ability to interact with pre-mRNA, which permits targeting of splicing and increases the amount of target RNA sequence for ASO binding, which can also limit off-target effects and there is no requirement for a carrier vehicle. Conversely, GalNAc conjugation improves accumulation of siRNAs in the target organ and facilitates their cellular uptake
Small interfering RNAs (siRNA) are short nucleic acid duplexes that bind to their target mRNA and induce the cellular RNA-induced silencing complex to degrade the targeted viral RNA. Their delivery and uptake can be enhanced using lipid nanoparticles or conjugation with N-acetylgalactosamine. First generation siRNAs, ARC-520 and ARC-521 demonstrated proof-in-principle, durable knockdown of target genes (HBsAg) in virally suppressed HBeAg positive and negative patients. However, development of these compounds was stopped due to toxicity from the delivery vehicle. Subsequently, studies with other siRNAs AB-729, JNJ-3989, RG6346 and VIR-2218 in viremic and virally suppressed patients on NAs demonstrated mean 1.5 to ~2.0 log10 IU/mL decline in HBsAg levels following monthly administration over 2 to 4 months.[76–79] However, with extended dosing up to one year further decline in HBsAg levels was minimal.[79] Plateau in HBsAg decline suggests that long-term siRNA use is probably not a viable approach. Rather the siRNA may be administered as induction therapy for a finite period to lower HBsAg levels and then followed by another agent such as an immune modulator e.g. peginterferon or therapeutic vaccine or alternatively as repeated short courses but the benefit of this latter approach is not proven. Suppression of HBsAg with AB-729 was shown to result in an increase in HBV-specific immune response in some but not all patients, providing another benefit of these agents.[80]
ASOs are single-stranded DNA molecules that can bind to viral mRNA and induce their degradation via RNAseH1 or steric hindrance to prevent translation. Two anti-sense molecules, Bepirovirsen, (previously, IONIS-HBVRx or GSK3228836) and IONIS-HBVLRx (GSK33389404) are currently in phase 2a trials among nucleos(t)ide analogue-naïve and treated patients. Administration for four weeks resulted in greater declines among nucleos(t)ide analogue-treated patients 1.99 log10 IU/ml compared to nucleos(t)ide analogue-naïve patients 1.56 log10 IU/ml.[81] Reductions in HBsAg levels were durable in some patients up to six months off treatment. Recently, it was reported that the addition of bepirovirsen 300 mg for 24 weeks to ongoing nucleos(t)ide analogue therapy, resulted in HBsAg below the lower limit of quantitation in 28% (18/64) of patients at end of treatment.[82]
Agents Targeting Core Protein
The HBV core protein has multiple regulatory roles in the viral lifecycle and the host immune response, making it an attractive target for drug development. Its primary role is to serve as the structural protein of the viral nucleocapsid, the site of reverse transcription and replication of the viral genome. The core protein also regulates subcellular trafficking and release of the HBV genome, RNA metabolism, cccDNA transcription and inhibiting the host innate immune response. Core protein allosteric modulators (CpAMs) are synthetically derived compounds that bind to a small hydrophobic pocket between core protein dimers and augment dimer-dimer interaction to modify nucleocapsid assembly.[83–91] CpAMs inhibit HBV replication through formation of aberrantly assembled nucleocapsids, or morphologically normal capsids devoid of pgRNA, or both. CpAMs are classified based on their mechanism of action. Type 1 CpAMs such as heteroaryldihydropyrimidine (HAP) derivatives lead to the formation of aberrantly assembled nucleocapsids. Type 2 CpAMs for example phenylpropenamides and sulfamoylbenzamides result in the formation of morphologically normal but empty nucleocapsids due to an inability to encapsidate the pre-genomic RNA. Some CpAMs may have additional effects, such as affecting the conversion of rcDNA to cccDNA.
There is great interest in developing CpAMs due to their potent inhibition of viral replication and oral route of administration. At least a dozen CpAMs are in various stages of drug development, Table 4. CpAMs as a class are very effective at inhibiting HBV replication across all HBV genotypes. However, they must be combined with one or more antiviral agents of a different class because of rapid development of resistance when used as monotherapy. When combined with NAs in viremic patients, they generally lead to faster and greater inhibition of viral replication compared to NA alone and deeper suppression of HBV DNA in patients already treated with a NA.[83–85, 90] Thus, they may offer the potential to increase on-treatment response particularly in highly viremic patients. However, minimal changes in HBeAg and HBsAg levels with short term administration coupled with a high rate of virological relapse upon withdrawal of the CpAM and nucleos(t)ide analogue,[92] raises questions whether they could achieve functional cure with finite therapy.
Agents Targeting the HBV Polymerase
Drugs targeting the reverse transcriptase function of the HBV polymerase are the most widely used agents to treat chronic HBV infection. Nucleos(t)ide analogues inhibit viral replication but not viral transcription or translation (i.e., viral antigen).[93] The focus of development of next generation nucleos(t)ide analogues are to improve their efficacy and safety, through novel prodrug approaches.
ATI-2173 is a non-competitive, non-chain terminating, clevudine derivative able to inhibit the HBV polymerase via active site distortion.[94] In phase I studies, 28-day dosing resulted in a mean HBV DNA reduction of 2.8 log10IU/mL without any serious adverse events.[95] Expectedly, no changes were seen in HBsAg levels over the short dosing interval. Phase II studies are in progress (NCT04847440). Other new nucleos(t)ide analogue prodrugs, pradefovir, HS-10234 and NCO-48 fumarate, derived from adefovir and tenofovir respectively are designed to increase antiviral potency and reduce metabolite toxicity.[96–98] Preliminary data suggest similar effectiveness to TDF. Agents targeting the RNase H function of the HBV polymerase are in preclinical development.[99]
Agents Targeting HBsAg Release
As HBsAg loss defines functional cure, there is great interest in developing agents to reduce HBsAg levels and limit viral production. In addition, given that HBsAg circulates in vast quantities as subviral particles in chronic HBV infection, it is hoped that therapeutic HBsAg reduction might restore the immune response. Nucleic acid polymers (NAPs) are short, synthetic oligonucleotides able to interact with HBV subviral particles through a poorly understood mechanism.[100] It was proposed that NAPs may interfere with the assembly/release of HBV subviral particles.[101] In a small 40 patient study two NAPs, REP 2139 or REP 2165, were evaluated in combination with tenofovir and pegylated interferon and compared to tenofovir plus pegylated interferon for 48 weeks. At the end of 48 weeks follow-up the addition of NAPs to the regimen was associated with sustained suppression of HBsAg to below level of detection in 44% all of whom developed antiHBs compared to the tenofovir and pegylated interferon arm in which 37% achieved sustained suppression of HBsAg, of whom two-thirds developed anti-HBs.[102] Grade 3–4 ALT flares were observed in the majority of NAP treated patients, 90%, compared to 20% among non-NAP treated patients. Controlled studies with larger groups of patients are needed to further evaluate the efficacy and safety of NAPs.
A similar approach to disrupting HBsAg secretion involves the use of S-Antigen Transport-inhibiting Oligonucleotide Polymers (STOPS). Like NAPs, STOPS are single-stranded oligonucleotides that sequester cellular proteins necessary for HBsAg production.[103] STOPS were shown to have greater potency than NAPS in-vitro. However, results of a phase 1 study evaluating the STOPS agent ALG-010133 demonstrated no meaningful HBsAg reduction and further development of this compound has been discontinued.[104]
Agents Targeting cccDNA
Elimination of cccDNA within the hepatocyte nucleus is the key to curing chronic HBV infection. Several approaches to eliminate or silence cccDNA are in pre-clinical development. However, the potential for off-target effects is a major safety concern that may limit this exciting approach.
Gene editing technology such as using zinc-finger nucleases (ZFNs), transcription activator-like effector nucleases (TALENs) and clustered regularly interspaced short palindromic repeats associated system 9 (CRISP/Cas-9) proteins are being evaluated to inactivate cccDNA by introducing targeted breaks in double stranded DNA that are then repaired by homologous repair creating mutations at the cleavage site. A study utilizing CRISPR/Cas9 systems from Streptococcus thermophilus on HBV infected cell lines was successful in eradicating 90% of HBV cccDNA[105]. However, several challenges need to be overcome before this approach can be used in the clinic, including target specificity, safe and efficient delivery systems to the hepatocyte nucleus, and increasing editing efficiency to eliminate all cccDNA molecules.
In the nucleus, cccDNA is organized into a chromatin-like structure which makes it amenable to epigenetic manipulation.[106] Several compounds have been shown in-vitro to silence cccDNA transcription. Interferon-α inhibits transcription of genomic and subgenomic RNAs derived from cccDNA, both in HBV-replicating cells in culture and in HBV-infected chimeric uPA/SCID mice repopulated with primary human hepatocytes.[107] Interestingly, the HBV X protein (HBX) which is essential for viral transcription has been shown to act through degradation of the host structural maintenance of chromosomes (Smc) complex, Smc5/6, which selectively blocks extrachromosomal DNA transcription. HBX destroys the Smc5/6 complex removing the brake on transcription and allowing hepatitis B virus gene expression to occur.[108, 109] Thus, targeting the HBX might be a viable approach to silencing cccDNA. Pevonedistat, a neuronal precursor cell-expressed developmentally down-regulated protein 8- (NEDD-8) activating enzyme inhibitor and dicoumarol, an inhibitor of NAD(P)H:quinone oxidoreductase 1 (NQO1) were shown to reduce HBX expression,[110, 111] restore Smc5/6 levels and suppress viral transcription in cultured hepatocytes [111] and in a humanized mouse model.[110] However, the observation that there is reactivation of cccDNA as soon as HBX becomes available again may be a major limitation to this approach.
Finally, an interesting prospect for targeting cccDNA rests in the enhancement of the apolipoprotein B mRNA editing catalytic subunit 3A and 3B deaminases (APOBEC3A/B). Upregulation of APOBEC3A/B by interferon-α and lymphotoxin-b has been shown to lead to non-cytolytic degradation of cccDNA in-vitro, but the degradation of the cccDNA pool is incomplete.[112]
Indirect Antiviral agents
One of the unanswered questions related to therapy is whether cure can be achieved through a purely antiviral approach or if the addition of an immunemodulator will be necessary. Current immunological approaches have been targeting both innate and adaptive immune system to broadly bolster cellular defense and to promote HBV-specific adaptive immune response (Figure 2).
Targeting the Innate Immune system
HBV is poorly sensed by the innate immune system and therefore is considered a stealth virus.[13, 14] However, the observation that certain cytokines (IFN-a, IFN-g, TNF-a, and IL-1a), produced by non-parenchymal liver cells, and LTβR-mediated activation of APOBEC (apolipoprotein B mRNA editing enzyme, catalytic polypeptide–like) or activation of retinoic acid-inducible gene-I (RIG-I) can suppress or even eradicate HBV from infected hepatocytes through a non-cytolytic mechanism [16, 107, 112, 113] provides a rationale for developing exogenous activators of the innate immune response. Several agonists of pathogen recognition sensors, e.g., TLRs, RIG-I, and stimulator of interferon genes (STING) have been shown to induce production of interferon-stimulated genes (ISGs) and proinflammatory cytokines than can cytopathically or non-cytopathically clear virus.
Agonists of innate immunity
TLRs are expressed in many cell subsets (including immune cells) and play an important role in host defense as sensors of viral and bacterial pathogen-associated molecular patterns (PAMPs). Combination treatment with CpG oligodeoxynucleotides (CpG ODN) and entecavir was shown in the woodchuck model to suppress woodchuck hepatitis virus (WHV) viral load.[114] Several oral TLR-7/8 agonists are in clinical trials, including GS-9620, RO7020531, RG7795 (ANA773), RG7854, JNJ-4964 (AL-034/TQ-A3334), and GS-9688.
Vesatolimod (GS-9620), a TLR-7 agonist, can activate intrahepatic dendritic cells among others, triggering the production of type I and II interferons and activating intra-hepatic NK and mucosal-associated invariant T (MAIT) cells. In proof-of-principle studies, vesatolimod reduced viral load and HBsAg antigenemia in chimpanzees and woodchucks but not in humans.[115–117] Vesatolimod in untreated viremic and virally suppressed patients on NAs was well-tolerated but did not substantially reduce HBsAg level.[118, 119] Differences in response observed in animal and human studies may relate to use of sub-therapeutic doses in human compared to animal studies.
GS-9688 (selgantolimod), a TLR-8 agonist, can activate intrahepatic dendritic cells, NK cells and MAIT cells and induce production of cytokines (IL-12/IL-18). In chronically WHV-infected woodchucks, short duration therapy with GS-9688 induced a sustained antiviral response and reduced WHV surface antigen (WHsAg) levels to below the limit of detection in half of the woodchucks.[120] Among virally suppressed patients, modest declines in HBsAg levels were seen. One patient (5%) achieved HBsAg loss and 16% HBeAg loss.[121] Selgantolimod induced dose dependent cytokine responses that did not correlate with HBsAg decline.
Inarigivir (SB9200), an oral dinucleotide RIG-I and nucleotide-binding oligomerization domain-containing protein 2 (NOD2) agonist in combination with TDF demonstrated dose dependent reduction in HBV DNA level with a maximal reduction of 3.26 log10[122], with serum ALT flares in 10% of patients. Further development of this compound was discontinued due to a patient death possibly related to liver injury.
Cyclic GMP-AMP synthetase (cGAS) can recognize HBV DNA and activate its adaptor protein STING, leading to ISG56 expression thereby inhibiting nucleocapsid formation.[123] Additionally, activation of the cGAS STING pathway by dsDNA or cGAMP was shown to markedly inhibit HBV replication in cell and mouse models.[124] However, a recent study suggesting that human hepatocytes do not express STING raises questions whether such an approach will be clinically effective.[125]
Lymphotoxin-b–mediated activation of APOBEC or activation of RIG-I was reported to suppress HBV replication by cytidine deamination, leading to cccDNA degradation.[112] Lymphotoxin-a (LTa), lymphotoxin-b (LTb), and cluster of differentiation (CD) 258 are the natural ligands of lymphotoxin-b receptor (LTbR). The risk of severe side effects with these cytokines limits their therapeutic use. However, activating the receptor using other ligands, tetravalent bispecific (BS1) and bivalent (CBE11) agonistic anti-LTβR antibodies to non-cytolytically degrade cccDNA has been demonstrated as a proof-in-principle for this approach.[112]
Agents stimulating and restoring adaptive immunity
Several strategies have been explored to re-invigorate the weak adaptive immune responses to HBV with a key consideration being to induce a therapeutic response safely without causing severe hepatocellular damage and clinical decompensation.
Checkpoint Blockade
Given the success of treating certain malignancies with checkpoint inhibitors, there is interest in using this approach for chronic HBV infection. However, use of these agents in the clinic in a non-malignant setting has been constrained by concerns for widespread hepatocyte death precipitating acute liver failure coupled with the risk of autoimmunity. In-vitro, incubation of T-cells from patients with chronic HBV infection with anti-PD1 antibodies led to proliferation of CD8+ cells with increased production of IL-2 and interferon gamma [126]. In a small pilot study, the PD-1 inhibitor nivolumab was evaluated with and without the therapeutic vaccine GS-4774 in virally suppressed patients on nucleos(t)ide analogue therapy [127]. Minor declines in HBsAg were noted. One patient achieved HBsAg sero-conversion, that was preceded by grade 3 ALT flare. No serious adverse events were observed. A recent phase 2 study evaluated the PD-1 monoclonal antibody envafolimab (ASC22) in nucleos(t)ide analogue experienced patients. A maximum HBsAg reduction of 1.2 Log10 IU/mL was seen without significant ALT flares.[128] The treatment was well tolerated. Another approach to targeting PD-1 pathway involves degradation of the PD-1 ligand (PD-L1) mRNA via the ribonuclease H (RNH) pathway.[129] Despite the potential usefulness of checkpoint blockade, concerns regarding safety and unpredictable response may limit their use in chronic HBV infection. Future larger studies are awaited.
Genetically engineered T-cells
Success of immunotherapy in cancer and demonstration of viral clearance in chronically HBV-infected recipients of bone marrow from recovered or vaccinated donors paved the way to develop genetically engineered HBV-specific T-cells[130–132], including ongoing efforts utilizing chimeric antigen receptor (CAR) T-cells [133], T-cell receptor (TCR) gene transfer [134] and TCR activation via immune mobilizing monoclonal T-cell receptors against virus (ImmTAV) molecules[135]. The ImmTAV molecule is a fusion protein consisting of an affinity-enhanced T-Cell receptor with an anti-CD3 T-Cell-activating moiety that can activate and redirect T-cells against HBV infected cells in-vitro [135]. Preliminary evidence in an animal model demonstrate reduction of HBsAg and HBV DNA levels without inducing significant liver damage. Proof-of-principle of the safety and efficacy of using genetically engineered T-cells expressing a HBsAg specific T cell receptor or adoptive transfer of autologous T cells expressing HBV-specific TCR, were demonstrated in patients with HBV-related HCC.[136, 137] Results from clinical studies utilizing these novel approaches in non-HCC patients are necessary to better understand their safety profile and effectiveness.
Novel therapeutic vaccines
The premise behind therapeutic vaccination is to break immune tolerance and augment the HBV-specific T-cell response to mediate functional cure (HBsAg loss). Unfortunately, previous attempts using multiple antigens (pre-S1/S2), peptide-based T-cell vaccines, DNA vaccines with novel adjuvants or nucleos(t)ide analogues were unsuccessful. Updated vaccine approaches are employing unique strategies to enhance vaccine efficacy including: 1) inclusion of multiple antigens to broaden the T-cell response (GS-4774, yeast-based vector with multiple viral antigens including HBsAg, HBcAg and HBX); 2) delivery systems (electroporation e.g. INO-1800 vaccine encoding HBsAg and HBcAg, JNJ-64300535 vaccine encoding HBV core and polymerase proteins); 3) novel adjuvants (INO-1800 vaccine plus INO-9112 (a DNA plasmid for IL-12)); 4) combination with checkpoint inhibitors; 5) use of viral vectors (primed non-replicative human adenovirus, chimpanzee adenovirus (ChAd), modified Vaccinia virus Ankara (MVA) and arenavirus). An advantage of viral vectors is they express antigen intracellularly and induce a robust cytotoxic T lymphocyte (CTL) response. [138] [139] A promising approach to generate high levels of memory T-cells is heterologous prime/boost vaccination. In this strategy, different antigen delivery systems are used to sequentially administer vaccine. In pre-clinical studies, a MVA expressing HBV antigens was used to boost protein-prime (HBsAg and HB core antigen) vaccinations in wildtype and HBV-transgenic (HBVtg) mice. Protein-prime/MVA-boost vaccination was able to overcome HBV-specific tolerance in HBVtg mice with low and medium but not with high antigenemia. Using the same model system, knockdown of viral antigenemia using siRNAs followed by therapeutic vaccination led to the development of polyfunctional, HBV-specific CD8+ T-cells, and elimination of HBV.[140] This vaccine is being tested in phase I studies.
New approaches to increase immunogenicity of peptide-based vaccines include novel nasal formulation NASVAC [141], the Sci-B-Vac derivative BRII-179[142] and HepTcell which is composed of nine synthetic HBV-derived peptides formulated with IC31®, a TLR9-based adjuvant.
Despite the many approaches and advances, none have been shown to restore immunity and clear infection in patients. Other strategies will therefore be required such as combination with potent antivirals, agents to lower viral antigen burden and other immunological boosters.
Combination Therapy
Given the success of combination therapy in chronic HCV infection and other infectious diseases, a combination approach will likely be needed to achieve durable HBV suppression, likely including both antiviral and immunomodulatory therapy. Almost every possible combination of agents is being evaluated in pre-clinical and clinical studies and results are eagerly awaited. However, a word of caution is in order. A recent study evaluating the combination of siRNA (JNJ-3989) with or without a CpAM (JNJ-6379) plus a NA, reported a surprising result in which the triple arm regimen (siRNA plus CpAM plus NA) had the lowest rate of response (ALT<3XULN, HBV DNA <LLOQ, HBeAg negative and HBsAg <10 IU/mL at end of treatment) compared to the two siRNA plus NA comparator arms.[143] This raises the possibility of an interaction between the CpAM and siRNA and suggests that not all combinations will result on synergy.
Conclusion
Chronic HBV infection results in a chronic hepatitis that carries a lifetime risk for progression to cirrhosis and HCC. Consequently, lifelong monitoring is necessary to detect disease progression and surveillance is recommended for individuals at increased risk for HCC. Persons at risk for cirrhosis and HCC should be offered antiviral treatment. Although current therapy is associated with improved clinical outcome it is not curative because of lack of effect on cccDNA and integrated HBV DNA. Stopping therapy in the absence of HBsAg loss usually leads to relapse to active disease in most patients and thus treatment must be administered long term.
Given the global burden of disease there is an urgent need for more effective therapy. A better understanding of the HBV lifecycle and immunopathogenesis of persistent infection together with innovations in drug development and delivery have led to multiple new approaches to treat chronic HBV infection. A regimen to achieve functional cure will likely require a combination of agents including an antiviral, an agent to reduce viral antigen burden and an immunemodulator to boost the immune response. Complete cure will require the refinement of gene editing therapy. The burden of disease is greatest in low-middle income countries. Therefore, to achieve WHO elimination goals will require development of a safe, effective, finite duration therapy that is affordable. Although many challenges remain, the sheer breadth of therapeutic approaches in development holds great promise for curing and eliminating chronic HBV infection.
Supplementary Material
Grant support:
This work was supported by the Intramural Research Program of the NIDDK, NIIH
Abbreviations:
- AASLD
American Association for the Study of Liver Diseases
- ALT
alanine aminotransferase
- APASL
Asian Pacific Association for the Study of the Liver
- ASO
antisense oligonucleotide
- CAR
chimeric antigen receptor
- cccDNA
covalently closed circular DNA
- cGAS
cyclic GMP-AMP synthetase
- CpAMs
core protein allosteric modulators
- CTLA-4
cytotoxic T lymphocyte-associated antigen-4
- dslDNA
double-stranded linear DNA
- EASL
European Association for the Study of the Liver
- HBV
hepatitis B virus
- HBeAg
hepatitis B e antigen
- HBsAg
hepatitis B surface antigen
- HBX
HBV X protein
- HCC
hepatocellular carcinoma
- HDV
hepatitis D virus
- ISG
interferon stimulated gene
- LAM
lamivudine
- MAIT
mucosal-associated invariant T
- NAPs
Nucleic acid polymers
- NK
natural killer
- NTCP
sodium taurocholate co-transporting polypeptide
- PD1
programmed cell death protein 1
- rcDNA
relaxed circular DNA
- RIG-I
retinoid acid inducible gene-I
- SC
subcutaneous
- siRNA
small interfering RNA
- Smc
structural maintenance of chromosomes
- STING
stimulator of interferon genes
- STOPS
S-Antigen Transport-inhibiting Oligonucleotide Polymers
- TAF
tenofovir alafenamide
- TCR
T-cell receptor
- TDF
tenofovir disoproxil fumarate
- TLR
toll like receptor
- ULN
upper limit of normal
- WHO
world health organization
- WHV
woodchuck hepatitis virus
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures:
David Yardeni: Nothing to disclose
Kyongi-Mi Chang: Nothing to disclose
Marc G. Ghany: Nothing to disclose.
References:
- 1.Kocher A, Papac L, Barquera R, Key FM, Spyrou MA, Hübler R, Rohrlach AB, Aron F, Stahl R, Wissgott A et al. : Ten millennia of hepatitis B virus evolution. Science 2021, 374(6564):182–188. [DOI] [PubMed] [Google Scholar]
- 2.Locarnini SA, Littlejohn M, Yuen LKW: Origins and Evolution of the Primate Hepatitis B Virus. Front Microbiol 2021, 12:653684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Global prevalence, treatment, and prevention of hepatitis B virus infection in 2016: a modelling study. Lancet Gastroenterol Hepatol 2018, 3(6):383–403. [DOI] [PubMed] [Google Scholar]
- 4.Schweitzer A, Horn J, Mikolajczyk RT, Krause G, Ott JJ: Estimations of worldwide prevalence of chronic hepatitis B virus infection: a systematic review of data published between 1965 and 2013. Lancet 2015, 386(10003):1546–1555. [DOI] [PubMed] [Google Scholar]
- 5.Hepatitis B Fact Sheet [https://www.who.int/news-room/fact-sheets/detail/hepatitis-b]
- 6.Chang MH, Shau WY, Chen CJ, Wu TC, Kong MS, Liang DC, Hsu HM, Chen HL, Hsu HY, Chen DS et al. : Hepatitis B vaccination and hepatocellular carcinoma rates in boys and girls. JAMA 2000, 284(23):3040–3042. [DOI] [PubMed] [Google Scholar]
- 7.Chang MH, You SL, Chen CJ, Liu CJ, Lai MW, Wu TC, Wu SF, Lee CM, Yang SS, Chu HC et al. : Long-term Effects of Hepatitis B Immunization of Infants in Preventing Liver Cancer. Gastroenterology 2016, 151(3):472–480 e471. [DOI] [PubMed] [Google Scholar]
- 8.Cox AL, El-Sayed MH, Kao JH, Lazarus JV, Lemoine M, Lok AS, Zoulim F: Progress towards elimination goals for viral hepatitis. Nat Rev Gastroenterol Hepatol 2020, 17(9):533–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Analysis. POCfD:https://cdafound.org/polaris-public-faq/#sec13 Last accessed February 14, 2022.
- 10.Tsukuda S, Watashi K: Hepatitis B virus biology and life cycle. Antiviral Res 2020, 182:104925. [DOI] [PubMed] [Google Scholar]
- 11.Pollicino T, Caminiti G: HBV-Integration Studies in the Clinic: Role in the Natural History of Infection. Viruses 2021, 13(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wooddell CI, Yuen MF, Chan HL, Gish RG, Locarnini SA, Chavez D, Ferrari C, Given BD, Hamilton J, Kanner SB et al. : RNAi-based treatment of chronically infected patients and chimpanzees reveals that integrated hepatitis B virus DNA is a source of HBsAg. Sci Transl Med 2017, 9(409). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wieland S, Thimme R, Purcell RH, Chisari FV: Genomic analysis of the host response to hepatitis B virus infection. Proc Natl Acad Sci U S A 2004, 101(17):6669–6674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wieland SF, Chisari FV: Stealth and cunning: hepatitis B and hepatitis C viruses. J Virol 2005, 79(15):9369–9380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chang J, Guo JT: Treatment of chronic hepatitis B with pattern recognition receptor agonists: Current status and potential for a cure. Antiviral Res 2015, 121:152–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sato S, Li K, Kameyama T, Hayashi T, Ishida Y, Murakami S, Watanabe T, Iijima S, Sakurai Y, Watashi K et al. : The RNA sensor RIG-I dually functions as an innate sensor and direct antiviral factor for hepatitis B virus. Immunity 2015, 42(1):123–132. [DOI] [PubMed] [Google Scholar]
- 17.Shlomai A, Schwartz RE, Ramanan V, Bhatta A, de Jong YP, Bhatia SN, Rice CM: Modeling host interactions with hepatitis B virus using primary and induced pluripotent stem cell-derived hepatocellular systems. Proceedings of the National Academy of Sciences of the United States of America 2014, 111(33):12193–12198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fisicaro P, Valdatta C, Boni C, Massari M, Mori C, Zerbini A, Orlandini A, Sacchelli L, Missale G, Ferrari C: Early kinetics of innate and adaptive immune responses during hepatitis B virus infection. Gut 2009, 58(7):974–982. [DOI] [PubMed] [Google Scholar]
- 19.Guidotti LG, Chisari FV: Immunobiology and pathogenesis of viral hepatitis. Annu Rev Pathol 2006, 1:23–61. [DOI] [PubMed] [Google Scholar]
- 20.Rehermann B, Ferrari C, Pasquinelli C, Chisari FV: The hepatitis B virus persists for decades after patients’ recovery from acute viral hepatitis despite active maintenance of a cytotoxic T-lymphocyte response. Nat Med 1996, 2(10):1104–1108. [DOI] [PubMed] [Google Scholar]
- 21.Hwang JP, Barbo AG, Perrillo RP: Hepatitis B reactivation during cancer chemotherapy: an international survey of the membership of the American Association for the Study of Liver Diseases. Journal of viral hepatitis 2015, 22(3):346–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Di Bisceglie AM, Lok AS, Martin P, Terrault N, Perrillo RP, Hoofnagle JH: Recent US Food and Drug Administration warnings on hepatitis B reactivation with immune-suppressing and anticancer drugs: just the tip of the iceberg? Hepatology 2015, 61(2):703–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Milich D, Liang TJ: Exploring the biological basis of hepatitis B e antigen in hepatitis B virus infection. Hepatology 2003, 38(5):1075–1086. [DOI] [PubMed] [Google Scholar]
- 24.Asabe S, Wieland SF, Chattopadhyay PK, Roederer M, Engle RE, Purcell RH, Chisari FV: The size of the viral inoculum contributes to the outcome of hepatitis B virus infection. Journal of virology 2009, 83(19):9652–9662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ferrari C, Boni C, Rossi M, Vecchi A, Barili V, Laccabue D, Fisicaro P, Missale G: T cell regulation in HBV-related chronic liver disease. Journal of hepatology 2017, 66(5):1096–1098. [DOI] [PubMed] [Google Scholar]
- 26.Park JJ, Wong DK, Wahed AS, Lee WM, Feld JJ, Terrault N, Khalili M, Sterling RK, Kowdley KV, Bzowej N et al. : Hepatitis B Virus-Specific and Global T-Cell Dysfunction in Chronic Hepatitis B. Gastroenterology 2016, 150(3):684–695 e685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Salimzadeh L, Le Bert N, Dutertre CA, Gill US, Newell EW, Frey C, Hung M, Novikov N, Fletcher S, Kennedy PT et al. : PD-1 blockade partially recovers dysfunctional virus-specific B cells in chronic hepatitis B infection. J Clin Invest 2018, 128(10):4573–4587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Burton AR, Pallett LJ, McCoy LE, Suveizdyte K, Amin OE, Swadling L, Alberts E, Davidson BR, Kennedy PT, Gill US et al. : Circulating and intrahepatic antiviral B cells are defective in hepatitis B. J Clin Invest 2018, 128(10):4588–4603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bengsch B, Martin B, Thimme R: Restoration of HBV-specific CD8+ T cell function by PD-1 blockade in inactive carrier patients is linked to T cell differentiation. J Hepatol 2014, 61(6):1212–1219. [DOI] [PubMed] [Google Scholar]
- 30.Chang KM, Traum D, Park JJ, Ho S, Ojiro K, Wong DK, Wahed AS, Terrault NA, Khalili M, Sterling RK et al. : Distinct phenotype and function of circulating Vdelta1+ and Vdelta2+ gammadeltaT-cells in acute and chronic hepatitis B. PLoS Pathog 2019, 15(4):e1007715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fisicaro P, Barili V, Rossi M, Montali I, Vecchi A, Acerbi G, Laccabue D, Zecca A, Penna A, Missale G et al. : Pathogenetic Mechanisms of T Cell Dysfunction in Chronic HBV Infection and Related Therapeutic Approaches. Front Immunol 2020, 11:849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lopes AR, Kellam P, Das A, Dunn C, Kwan A, Turner J, Peppa D, Gilson RJ, Gehring A, Bertoletti A et al. : Bim-mediated deletion of antigen-specific CD8 T cells in patients unable to control HBV infection. J Clin Invest 2008, 118(5):1835–1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Traum D, Wang YJ, Schwarz KB, Schug J, Wong DK, Janssen H, Terrault NA, Khalili M, Wahed AS, Murray KF et al. : Highly multiplexed 2-dimensional imaging mass cytometry analysis of HBV-infected liver. JCI Insight 2021, 6(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hepatitis B Vaccination: Information for Healthcare Providers [https://www.cdc.gov/vaccines/vpd/hepb/hcp/index.html]
- 35.Lok AS, Zoulim F, Dusheiko G, Ghany MG: Hepatitis B cure: From discovery to regulatory approval. J Hepatol 2017, 67(4):847–861. [DOI] [PubMed] [Google Scholar]
- 36.Cornberg M, Lok AS, Terrault NA, Zoulim F: Guidance for design and endpoints of clinical trials in chronic hepatitis B - Report from the 2019 EASL-AASLD HBV Treatment Endpoints Conference. Hepatology 2019. [DOI] [PubMed] [Google Scholar]
- 37.Yeo YH, Ho HJ, Yang HI, Tseng TC, Hosaka T, Trinh HN, Kwak MS, Park YM, Fung JYY, Buti M et al. : Factors Associated With Rates of HBsAg Seroclearance in Adults With Chronic HBV Infection: A Systematic Review and Meta-analysis. Gastroenterology 2019, 156(3):635–646.e639. [DOI] [PubMed] [Google Scholar]
- 38.Zhou K, Contag C, Whitaker E, Terrault N: Spontaneous loss of surface antigen among adults living with chronic hepatitis B virus infection: a systematic review and pooled meta-analyses. Lancet Gastroenterol Hepatol 2019, 4(3):227–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Terrault NA, Lok ASF, McMahon BJ, Chang KM, Hwang JP, Jonas MM, Brown RS Jr., Bzowej NH, Wong JB: Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology 2018, 67(4):1560–1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J Hepatol 2017, 67(2):370–398. [DOI] [PubMed] [Google Scholar]
- 41.Sarin SK, Kumar M, Lau GK, Abbas Z, Chan HL, Chen CJ, Chen DS, Chen HL, Chen PJ, Chien RN et al. : Asian-Pacific clinical practice guidelines on the management of hepatitis B: a 2015 update. Hepatol Int 2016, 10(1):1–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.WHO: http://apps.who.int/iris/bitstream/handle/10665/154590/9789241549059_eng.pdf. Last accessed 3/10/22 2015.
- 43.Singh S, Muir AJ, Dieterich DT, Falck-Ytter YT: American Gastroenterological Association Institute Technical Review on the Role of Elastography in Chronic Liver Diseases. Gastroenterology 2017, 152(6):1544–1577. [DOI] [PubMed] [Google Scholar]
- 44.Herrmann E, de Ledinghen V, Cassinotto C, Chu WC, Leung VY, Ferraioli G, Filice C, Castera L, Vilgrain V, Ronot M et al. : Assessment of biopsy-proven liver fibrosis by two-dimensional shear wave elastography: An individual patient data-based meta-analysis. Hepatology 2018, 67(1):260–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen CJ, Yang HI, Su J, Jen CL, You SL, Lu SN, Huang GT, Iloeje UH: Risk of hepatocellular carcinoma across a biological gradient of serum hepatitis B virus DNA level. Jama 2006, 295(1):65–73. [DOI] [PubMed] [Google Scholar]
- 46.Chen CF, Lee WC, Yang HI, Chang HC, Jen CL, Iloeje UH, Su J, Hsiao CK, Wang LY, You SL et al. : Changes in serum levels of HBV DNA and alanine aminotransferase determine risk for hepatocellular carcinoma. Gastroenterology 2011, 141(4):1240–1248, 1248.e1241–1242. [DOI] [PubMed] [Google Scholar]
- 47.Chen CJ, Yang HI, Su J, Jen CL, You SL, Lu SN, Huang GT, Iloeje UH, Group R-HS: Risk of hepatocellular carcinoma across a biological gradient of serum hepatitis B virus DNA level. JAMA 2006, 295(1):65–73. [DOI] [PubMed] [Google Scholar]
- 48.Iloeje UH, Yang HI, Su J, Jen CL, You SL, Chen CJ, Risk Evaluation of Viral Load E, Associated Liver Disease/Cancer-In HBVSG: Predicting cirrhosis risk based on the level of circulating hepatitis B viral load. Gastroenterology 2006, 130(3):678–686. [DOI] [PubMed] [Google Scholar]
- 49.Kim GA, Lim YS, Han S, Choi J, Shim JH, Kim KM, Lee HC, Lee YS: High risk of hepatocellular carcinoma and death in patients with immune-tolerant-phase chronic hepatitis B. Gut 2018, 67(5):945–952. [DOI] [PubMed] [Google Scholar]
- 50.Chu CM, Hung SJ, Lin J, Tai DI, Liaw YF: Natural history of hepatitis B e antigen to antibody seroconversion in patients with normal serum aminotransferase levels. Am J Med 2004, 116(12):829–834. [DOI] [PubMed] [Google Scholar]
- 51.Hui CK, Leung N, Yuen ST, Zhang HY, Leung KW, Lu L, Cheung SK, Wong WM, Lau GK, Hong Kong Liver Fibrosis Study G: Natural history and disease progression in Chinese chronic hepatitis B patients in immune-tolerant phase. Hepatology 2007, 46(2):395–401. [DOI] [PubMed] [Google Scholar]
- 52.Sinn DH, Kim SE, Kim BK, Kim JH, Choi MS: The risk of hepatocellular carcinoma among chronic hepatitis B virus-infected patients outside current treatment criteria. J Viral Hepat 2019, 26(12):1465–1472. [DOI] [PubMed] [Google Scholar]
- 53.Choi GH, Kim GA, Choi J, Han S, Lim YS: High risk of clinical events in untreated HBeAg-negative chronic hepatitis B patients with high viral load and no significant ALT elevation. Alimentary Pharmacology and Therapeutics 2019, 50(2):215–226. [DOI] [PubMed] [Google Scholar]
- 54.Hsu YC, Chen CY, Chang IW, Chang CY, Wu CY, Lee TY, Wu MS, Bair MJ, Chen JJ, Chen CC et al. : Once-daily tenofovir disoproxil fumarate in treatment-naive Taiwanese patients with chronic hepatitis B and minimally raised alanine aminotransferase (TORCH-B): a multicentre, double-blind, placebo-controlled, parallel-group, randomised trial. Lancet Infect Dis 2021, 21(6):823–833. [DOI] [PubMed] [Google Scholar]
- 55.Buster EH, Flink HJ, Cakaloglu Y, Simon K, Trojan J, Tabak F, So TM, Feinman SV, Mach T, Akarca US et al. : Sustained HBeAg and HBsAg loss after long-term follow-up of HBeAg-positive patients treated with peginterferon alpha-2b. Gastroenterology 2008, 135(2):459–467. [DOI] [PubMed] [Google Scholar]
- 56.Janssen HL, van Zonneveld M, Senturk H, Zeuzem S, Akarca US, Cakaloglu Y, Simon C, So TM, Gerken G, de Man RA et al. : Pegylated interferon alfa-2b alone or in combination with lamivudine for HBeAg-positive chronic hepatitis B: a randomised trial. Lancet 2005, 365(9454):123–129. [DOI] [PubMed] [Google Scholar]
- 57.Konerman MA, Lok AS: Interferon Treatment for Hepatitis B. Clin Liver Dis 2016, 20(4):645–665. [DOI] [PubMed] [Google Scholar]
- 58.Zhu YY, Wu YL, Chen J, Zheng Q, Dong J, Jiang JJ: [Prolonged duration of the routine pegylated-interferon alfa-2a therapy produces superior virological response in HBeAg-positive chronic hepatitis B patients: a single-center cohort study]. Zhonghua Gan Zang Bing Za Zhi 2012, 20(10):737–741. [DOI] [PubMed] [Google Scholar]
- 59.Cao ZH, Ma LN, Zhang HW, Liu YL, Chen XY: Extended treatment with peginterferon α-2a in combination with lamivudine or adefovir for 96 weeks yields high rates of HBeAg and HBsAg seroconversion. J Dig Dis 2013, 14(8):446–450. [DOI] [PubMed] [Google Scholar]
- 60.Menendez-Arias L, Alvarez M, Pacheco B: Nucleoside/nucleotide analog inhibitors of hepatitis B virus polymerase: mechanism of action and resistance. Curr Opin Virol 2014, 8:1–9. [DOI] [PubMed] [Google Scholar]
- 61.Hsu YC, Suri V, Nguyen MH, Huang YT, Chen CY, Chang IW, Tseng CH, Wu CY, Lin JT, Pan DZ et al. : Inhibition of Viral Replication Reduces Transcriptionally Active Distinct Hepatitis B Virus Integrations with Implications on Host Gene Dysregulation. Gastroenterology 2022. [DOI] [PubMed] [Google Scholar]
- 62.Li B, Liu Z, Liu X, Liu D, Duan M, Gu Y, Liu Q, Ma Q, Wei Y, Wang Y: Efficacy and safety of tenofovir disoproxil fumarate and tenofovir alafenamide fumarate in preventing HBV vertical transmission of high maternal viral load. Hepatol Int 2021, 15(5):1103–1108. [DOI] [PubMed] [Google Scholar]
- 63.Zeng QL, Yu ZJ, Ji F, Li GM, Zhang GF, Xu JH, Chen ZM, Cui GL, Li W, Zhang DW et al. : Tenofovir Alafenamide to Prevent Perinatal Hepatitis B Transmission: A Multicenter, Prospective, Observational Study. Clin Infect Dis 2021, 73(9):e3324–e3332. [DOI] [PubMed] [Google Scholar]
- 64.Ding Y, Cao L, Zhu L, Huang Y, Lin C, Wang Y, Liu Y, Sheng Q, Wang S, Fan J et al. : Efficacy and safety of tenofovir alafenamide fumarate for preventing mother-to-child transmission of hepatitis B virus: a national cohort study. Aliment Pharmacol Ther 2020, 52(8):1377–1386. [DOI] [PubMed] [Google Scholar]
- 65.Loomba R, Liang TJ: Hepatitis B Reactivation Associated With Immune Suppressive and Biological Modifier Therapies: Current Concepts, Management Strategies, and Future Directions. Gastroenterology 2017, 152(6):1297–1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lau GK, Piratvisuth T, Luo KX, Marcellin P, Thongsawat S, Cooksley G, Gane E, Fried MW, Chow WC, Paik SW et al. : Peginterferon Alfa-2a, lamivudine, and the combination for HBeAg-positive chronic hepatitis B. N Engl J Med 2005, 352(26):2682–2695. [DOI] [PubMed] [Google Scholar]
- 67.Marcellin P, Lau GK, Bonino F, Farci P, Hadziyannis S, Jin R, Lu ZM, Piratvisuth T, Germanidis G, Yurdaydin C et al. : Peginterferon alfa-2a alone, lamivudine alone, and the two in combination in patients with HBeAg-negative chronic hepatitis B. N Engl J Med 2004, 351(12):1206–1217. [DOI] [PubMed] [Google Scholar]
- 68.Tout I, Lampertico P, Berg T, Asselah T: Perspectives on stopping nucleos(t)ide analogues therapy in patients with chronic hepatitis B. Antiviral Res 2021, 185:104992. [DOI] [PubMed] [Google Scholar]
- 69.Hirode G, Choi HSJ, Chen CH, Su TH, Seto WK, Van Hees S, Papatheodoridi M, Lens S, Wong G, Brakenhoff SM et al. : Off-Therapy Response After Nucleos(t)ide Analogue Withdrawal in Patients With Chronic Hepatitis B: An International, Multicenter, Multiethnic Cohort (RETRACT-B Study). Gastroenterology 2021. [DOI] [PubMed] [Google Scholar]
- 70.Coffin CS, Zhou K, Terrault NA: New and Old Biomarkers for Diagnosis and Management of Chronic Hepatitis B Virus Infection. Gastroenterology 2019, 156(2):355–368.e353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Blank A, Markert C, Hohmann N, Carls A, Mikus G, Lehr T, Alexandrov A, Haag M, Schwab M, Urban S et al. : First-in-human application of the novel hepatitis B and hepatitis D virus entry inhibitor myrcludex B. J Hepatol 2016, 65(3):483–489. [DOI] [PubMed] [Google Scholar]
- 72.Kang C, Syed YY: Bulevirtide: First Approval. Drugs 2020, 80(15):1601–1605. [DOI] [PubMed] [Google Scholar]
- 73.A Phase 1b/2a Randomized, Open-label Clinical Trial of Daily Myrcludex B vs Entecavir in Patients With HBeAg Negative Chronic Hepatitis B [https://clinicaltrials.gov/ct2/show/study/NCT02881008]
- 74.Tu T, Urban S: Virus entry and its inhibition to prevent and treat hepatitis B and hepatitis D virus infections. Curr Opin Virol 2018, 30:68–79. [DOI] [PubMed] [Google Scholar]
- 75.Agarwal KYM, Wedemeyer H, Arizpe A, Shen L, Cloutier D, Tay C, Gupta S, Fanget M, Dholakiya S et al. : Rapid HBsAg reduction in chronic hepatitis B virus infection: preliminary re-sults from a phase 1 study evaluating a single dose of VIR-3434, a novel neutralizing, vaccinal monoclonal antibody. Hepatology 2021, 74:514A, Abstract 839. [Google Scholar]
- 76.Yuen MF LT KW, Tongkijvonich P, Yoon JH, Sievert W et al. : HBV RNAi INHIBITOR RG6346 IN PHASE 1b-2a TRIAL WAS SAFE, WELL-TOLERATED, AND RESULTED IN SUBSTANTIAL AND DURABLE REDUCTIONS IN SERUM HBsAg LEVELS. . Hepatology 2020, 72:LO9. [Google Scholar]
- 77.Gane ELY, Cloutier D, et: Safety and antiviral activity of VIR-2218, an X-targeting RNAi therapeutic, in participants with chronic hepatitis B infection: week 48 follow-up results. . Journal of Hepatolgy 2021, 75:S287:OS244;. [Google Scholar]
- 78.Gane ELS, Lim TH, Strasser S, Sievert W, Cheng W, et al. : Short interfering RNA JNJ-3989 combination therapy in chronic hepatitis B shows potent reduction of all viral markers but no correlate was identified for HBsAg reduction and baseline factors. Journal of Hepatology 2021, 75:S289:(Abstract OS-1430). [Google Scholar]
- 79.Yuen MF BE, Kim YJ, Holmes JA, Lim Y-S, Strasser SI et al. : Safety and pharmacodynamics of the GalNAc-siRNA AB-729 in subjects with chronic hepatitis B infection. Hepatology 2020, 72:62A:Abstract 83. [Google Scholar]
- 80.Paratala BPJ-J, Ganchua SC, Gane E, Yuen M-F et al. : Inhibition of HBsAg in patients with CHB by RNA interference therapeutic AB-729 is accompanied by upregulation of HBVspecific T cell activation markers. J Hepatology 2021, 75(2):S294–S803. [Google Scholar]
- 81.Yuen MF, Heo J, Jang JW, Yoon JH, Kweon YO, Park SJ, Tami Y, You S, Yates P, Tao Y et al. : Safety, tolerability and antiviral activity of the antisense oligonucleotide bepirovirsen in patients with chronic hepatitis B: a phase 2 randomized controlled trial. Nat Med 2021, 27(10):1725–1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yuen M-FPR, Lim SG, Tsuji K, Diaconescu G, Gadano A, et al. : Efficacy and safety of bepirovirsen in patients with chronic hepatitis B virus infection on stable nucleos (t)ide analogue therapy: interim results from the randomised phase 2b B-Clear study. Journal of Hepatology 2022, 77(S1);S13:LB004B. [Google Scholar]
- 83.Yuen MF, Gane EJ, Kim DJ, Weilert F, Yuen Chan HL, Lalezari J, Hwang SG, Nguyen T, Flores O, Hartman G et al. : Antiviral Activity, Safety, and Pharmacokinetics of Capsid Assembly Modulator NVR 3–778 in Patients with Chronic HBV Infection. Gastroenterology 2019, 156(5):1392–1403.e1397. [DOI] [PubMed] [Google Scholar]
- 84.Yuen MF, Agarwal K, Gane EJ, Schwabe C, Ahn SH, Kim DJ, Lim YS, Cheng W, Sievert W, Visvanathan K et al. : Safety, pharmacokinetics, and antiviral effects of ABI-H0731, a hepatitis B virus core inhibitor: a randomised, placebo-controlled phase 1 trial. Lancet Gastroenterol Hepatol 2020, 5(2):152–166. [DOI] [PubMed] [Google Scholar]
- 85.Zoulim F, Lenz O, Vandenbossche JJ, Talloen W, Verbinnen T, Moscalu I, Streinu-Cercel A, Bourgeois S, Buti M, Crespo J et al. : JNJ-56136379, an HBV Capsid Assembly Modulator, Is Well-Tolerated and Has Antiviral Activity in a Phase 1 Study of Patients With Chronic Infection. Gastroenterology 2020, 159(2):521–533.e529. [DOI] [PubMed] [Google Scholar]
- 86.Jacobson IM MX, Nguyen T, Schiff ER, Yuen MF, Hann H-WL et al. : Analysis of the longer-term safety profile of the hepatitis B virus core inhibitor ABI-H0731 in an open-label extension study. . Hepatology 2020, 72:501A:Abstract 820. [Google Scholar]
- 87.Gane EJ YM, Jucov A, Le K, Westland C, Schwabe C, Agarwal K et al. : Safety, Pharmacokinetics (PK), and Antiviral Activity of the Capsid Assembly Modulator (CAM) ALG-000184 in Subjects with Chronic Hepatitis B (CHB). Heptology 2021, 74. [Google Scholar]
- 88.Yuen M-FCW-L, Peng C-Y, Jeng W-J, Su W-W, Chang T-T, et al. : EDP-514, a Novel Pangenotypic Class II Hepatitis B Virus Core Inhibitor Demonstrates Significant HBV DNA and HBV RNA Reductions in a Phase 1b Study in Viremic, Chronic Hepatitis B Infected Patients Hepatology 2021, 74:505A:Abstract 823. [Google Scholar]
- 89.Feld JJLE, Nguyen T, Lalezari JP, Hassanein T, Martin P, et al. : EDP-514, A NOVEL PANGENOTYPIC CLASS II HEPATITIS B VIRUS CORE INHIBITOR: PRELIMINARY RESULTS OF A 28-DAY PHASE 1b STUDY IN NUC-SUPPRESSED CHB PATIENTS. Hepatology 2021, 74:504A:Abstract 822. [Google Scholar]
- 90.Yuen MF, Zhou X, Gane E, Schwabe C, Tanwandee T, Feng S, Jin Y, Triyatni M, Lemenuel-Diot A, Cosson V et al. : Safety, pharmacokinetics, and antiviral activity of RO7049389, a core protein allosteric modulator, in patients with chronic hepatitis B virus infection: a multicentre, randomised, placebo-controlled, phase 1 trial. Lancet Gastroenterol Hepatol 2021, 6(9):723–732. [DOI] [PubMed] [Google Scholar]
- 91.https://investor.arbutusbio.com/news-releases/news-release-details/preliminary-data-shows-arbutus-capsid-inhibitor-ab-836-generally. Last accessed January 4th.
- 92.Yuen MF MX, Hassanein TI, Kwo PY, Ma J, Li L, et al. : HBV PGRNA AND DNA BOTH REBOUND IMMEDIATELY FOLLOWING DISCONTINUATION OF THE CORE INHIBITOR VEBICORVIR DESPITE CONTINUED NRTI TREATMENT IN PATIENTS WITH HBEAG POSITIVE CHRONIC HEPATITIS B VIRUS INFECTION: FINDINGS FROM A PHASE 2 OPEN LABEL STUDY. Hepatology 2021, 74:65A Abstract 96. [Google Scholar]
- 93.Marcellin P, Wong DK, Sievert W, Buggisch P, Petersen J, Flisiak R, Manns M, Kaita K, Krastev Z, Lee SS et al. : Ten-year efficacy and safety of tenofovir disoproxil fumarate treatment for chronic hepatitis B virus infection. Liver Int 2019, 39(10):1868–1875. [DOI] [PubMed] [Google Scholar]
- 94.Squires KE, Mayers DL, Bluemling GR, Kolykhalov AA, Guthrie DB, Reddy P, Mitchell DG, Saindane MT, Sticher ZM, Edpuganti V et al. : ATI-2173, a Novel Liver-Targeted Non-Chain-Terminating Nucleotide for Hepatitis B Virus Cure Regimens. Antimicrob Agents Chemother 2020, 64(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Mayers DJA, Anastasiy I, Ghicavii N, Ogilvie L, Squires K et al. : Phase 1 Results for ATI-2173, a novel active site polymerase inhibitor nucleotide (ASPIN), in HBV- infected subjects. . Journal of Hepatology 2021, 75(2):S743:Abstract PO- 1251. [Google Scholar]
- 96.Gao Y, Kong F, Song X, Shang J, Yao L, Xia J, Peng Y, Liu W, Gong H, Mu M et al. : Pradefovir Treatment in Patients with Chronic Hepatitis B: Week 24 Results from a Multicenter, Double-blind, Randomized, Noninferiority, Phase 2 Trial. Clin Infect Dis 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zhang H, Hu Y, Wu M, Liu J, Zhu X, Li X, Chen H, Li C, Liu C, Niu J et al. : Randomised clinical trial: safety, efficacy and pharmacokinetics of HS-10234 versus tenofovir for the treatment of chronic hepatitis B infection. Aliment Pharmacol Ther 2021, 53(2):243–252. [DOI] [PubMed] [Google Scholar]
- 98.Zhi LVE, Vrishabhendra L, Hu D, Lu T-S and Marschke KB: Single-dose Pharmacokinetics and Safety of NCO-48 Fumarate, a Novel Liver-Targeted Prodrug (LTP) of Tenofovir (TFV). Hepatology 2021, 74:519A: Abstract 847.33226658 [Google Scholar]
- 99.Pierra Rouviere C, Dousson CB, Tavis JE: HBV replication inhibitors. Antiviral Res 2020, 179:104815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Al-Mahtab M, Bazinet M, Vaillant A: Safety and Efficacy of Nucleic Acid Polymers in Monotherapy and Combined with Immunotherapy in Treatment-Naive Bangladeshi Patients with HBeAg+ Chronic Hepatitis B Infection. PLoS One 2016, 11(6):e0156667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Vaillant A: Nucleic acid polymers: Broad spectrum antiviral activity, antiviral mechanisms and optimization for the treatment of hepatitis B and hepatitis D infection. Antiviral Res 2016, 133:32–40. [DOI] [PubMed] [Google Scholar]
- 102.Bazinet M, Pântea V, Placinta G, Moscalu I, Cebotarescu V, Cojuhari L, Jimbei P, Iarovoi L, Smesnoi V, Musteata T et al. : Safety and Efficacy of 48 Weeks REP 2139 or REP 2165, Tenofovir Disoproxil, and Pegylated Interferon Alfa-2a in Patients With Chronic HBV Infection Naïve to Nucleos(t)ide Therapy. Gastroenterology 2020, 158(8):2180–2194. [DOI] [PubMed] [Google Scholar]
- 103.Kao CC, Nie Y, Ren S, De Costa N, Pandey RK, Hong J, Smith DB, Symons JA, Beigelman L, Blatt LM: Mechanism of action of hepatitis B virus S antigen transport-inhibiting oligonucleotide polymer, STOPS, molecules. Mol Ther Nucleic Acids 2022, 27:335–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Aligos: Aligos Halting Further Development of STOPS™ Drug Candidate, ALG-010133. In.; 2022.
- 105.Kostyushev D, Brezgin S, Kostyusheva A, Zarifyan D, Goptar I, Chulanov V: Orthologous CRISPR/Cas9 systems for specific and efficient degradation of covalently closed circular DNA of hepatitis B virus. Cell Mol Life Sci 2019, 76(9):1779–1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Tropberger P, Mercier A, Robinson M, Zhong W, Ganem DE, Holdorf M: Mapping of histone modifications in episomal HBV cccDNA uncovers an unusual chromatin organization amenable to epigenetic manipulation. Proc Natl Acad Sci U S A 2015, 112(42):E5715–5724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Belloni L, Allweiss L, Guerrieri F, Pediconi N, Volz T, Pollicino T, Petersen J, Raimondo G, Dandri M, Levrero M: IFN-alpha inhibits HBV transcription and replication in cell culture and in humanized mice by targeting the epigenetic regulation of the nuclear cccDNA minichromosome. J Clin Invest 2012, 122(2):529–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Xu Z, Yen TS, Wu L, Madden CR, Tan W, Slagle BL, Ou JH: Enhancement of hepatitis B virus replication by its X protein in transgenic mice. J Virol 2002, 76(5):2579–2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Decorsière A, Mueller H, van Breugel PC, Abdul F, Gerossier L, Beran RK, Livingston CM, Niu C, Fletcher SP, Hantz O et al. : Hepatitis B virus X protein identifies the Smc5/6 complex as a host restriction factor. Nature 2016, 531(7594):386–389. [DOI] [PubMed] [Google Scholar]
- 110.Cheng ST, Hu JL, Ren JH, Yu HB, Zhong S, Wai Wong VK, Kwan Law BY, Chen WX, Xu HM, Zhang ZZ et al. : Dicoumarol, an NQO1 inhibitor, blocks cccDNA transcription by promoting degradation of HBx. J Hepatol 2021, 74(3):522–534. [DOI] [PubMed] [Google Scholar]
- 111.Sekiba K, Otsuka M, Ohno M, Yamagami M, Kishikawa T, Seimiya T, Suzuki T, Tanaka E, Ishibashi R, Funato K et al. : Pevonedistat, a Neuronal Precursor Cell-Expressed Developmentally Down-Regulated Protein 8-Activating Enzyme Inhibitor, Is a Potent Inhibitor of Hepatitis B Virus. Hepatology 2019, 69(5):1903–1915. [DOI] [PubMed] [Google Scholar]
- 112.Lucifora J, Xia Y, Reisinger F, Zhang K, Stadler D, Cheng X, Sprinzl MF, Koppensteiner H, Makowska Z, Volz T et al. : Specific and nonhepatotoxic degradation of nuclear hepatitis B virus cccDNA. Science 2014, 343(6176):1221–1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Maini MK, Gehring AJ: The role of innate immunity in the immunopathology and treatment of HBV infection. J Hepatol 2016, 64(1 Suppl):S60–S70. [DOI] [PubMed] [Google Scholar]
- 114.Meng Z, Zhang X, Pei R, Zhang E, Kemper T, Vollmer J, Davis HL, Glebe D, Gerlich W, Roggendorf M et al. : Combination therapy including CpG oligodeoxynucleotides and entecavir induces early viral response and enhanced inhibition of viral replication in a woodchuck model of chronic hepadnaviral infection. Antiviral Res 2016, 125:14–24. [DOI] [PubMed] [Google Scholar]
- 115.Lanford RE, Guerra B, Chavez D, Giavedoni L, Hodara VL, Brasky KM, Fosdick A, Frey CR, Zheng J, Wolfgang G et al. : GS-9620, an oral agonist of Toll-like receptor-7, induces prolonged suppression of hepatitis B virus in chronically infected chimpanzees. Gastroenterology 2013, 144(7):1508–1517, 1517 e1501–1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Menne S, Tumas DB, Liu KH, Thampi L, AlDeghaither D, Baldwin BH, Bellezza CA, Cote PJ, Zheng J, Halcomb R et al. : Sustained efficacy and seroconversion with the Toll-like receptor 7 agonist GS-9620 in the Woodchuck model of chronic hepatitis B. J Hepatol 2015, 62(6):1237–1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Gane EJ, Lim YS, Gordon SC, Visvanathan K, Sicard E, Fedorak RN, Roberts S, Massetto B, Ye Z, Pflanz S et al. : The oral toll-like receptor-7 agonist GS-9620 in patients with chronic hepatitis B virus infection. J Hepatol 2015, 63(2):320–328. [DOI] [PubMed] [Google Scholar]
- 118.Janssen HLA, Brunetto MR, Kim YJ, Ferrari C, Massetto B, Nguyen AH, Joshi A, Woo J, Lau AH, Gaggar A et al. : Safety, efficacy and pharmacodynamics of vesatolimod (GS-9620) in virally suppressed patients with chronic hepatitis B. J Hepatol 2018, 68(3):431–440. [DOI] [PubMed] [Google Scholar]
- 119.Agarwal K, Ahn SH, Elkhashab M, Lau AH, Gaggar A, Bulusu A, Tian X, Cathcart AL, Woo J, Subramanian GM et al. : Safety and efficacy of vesatolimod (GS-9620) in patients with chronic hepatitis B who are not currently on antiviral treatment. J Viral Hepat 2018, 25(11):1331–1340. [DOI] [PubMed] [Google Scholar]
- 120.Daffis S, Balsitis S, Chamberlain J, Zheng J, Santos R, Rowe W, Ramakrishnan D, Pattabiraman D, Spurlock S, Chu R et al. : Toll-Like Receptor 8 Agonist GS-9688 Induces Sustained Efficacy in the Woodchuck Model of Chronic Hepatitis B. Hepatology 2021, 73(1):53–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Gane E DP BA, Zhao Y, Tan SK et al. : Efficacy and Safety of 24 Weeks Treatment with Oral TLR8 Agonist Selgantolimod (GS-9688, SLGN) in Virally Suppressed Adult Patients with Chronic Hepatitis B: A Phase 2 Study. Journal of Hepatology 2020, 73 S52:Abstract ASO71. [Google Scholar]
- 122.Yuen MC CC, Liu CY et al. : Ascending dose cohort study of inarigivir-A novel RIG I agonist in chronic HBV patients: Final results of the ACHIEVE trial. J Hepatology 2019, 70:e45–79. [Google Scholar]
- 123.Dansako H, Ueda Y, Okumura N, Satoh S, Sugiyama M, Mizokami M, Ikeda M, Kato N: The cyclic GMP-AMP synthetase-STING signaling pathway is required for both the innate immune response against HBV and the suppression of HBV assembly. FEBS J 2016, 283(1):144–156. [DOI] [PubMed] [Google Scholar]
- 124.He J, Hao R, Liu D, Liu X, Wu S, Guo S, Wang Y, Tien P, Guo D: Inhibition of hepatitis B virus replication by activation of the cGAS-STING pathway. J Gen Virol 2016, 97(12):3368–3378. [DOI] [PubMed] [Google Scholar]
- 125.Thomsen MK, Nandakumar R, Stadler D, Malo A, Valls RM, Wang F, Reinert LS, Dagnaes-Hansen F, Hollensen AK, Mikkelsen JG et al. : Lack of immunological DNA sensing in hepatocytes facilitates hepatitis B virus infection. Hepatology 2016, 64(3):746–759. [DOI] [PubMed] [Google Scholar]
- 126.Fisicaro P, Valdatta C, Massari M, Loggi E, Biasini E, Sacchelli L, Cavallo MC, Silini EM, Andreone P, Missale G et al. : Antiviral intrahepatic T-cell responses can be restored by blocking programmed death-1 pathway in chronic hepatitis B. Gastroenterology 2010, 138(2):682–693, 693.e681–684. [DOI] [PubMed] [Google Scholar]
- 127.Gane E, Verdon DJ, Brooks AE, Gaggar A, Nguyen AH, Subramanian GM, Schwabe C, Dunbar PR: Anti-PD-1 blockade with nivolumab with and without therapeutic vaccination for virally suppressed chronic hepatitis B: A pilot study. J Hepatol 2019, 71(5):900–907. [DOI] [PubMed] [Google Scholar]
- 128.Wang GQJ, Cui Y, Yan Y, He H, Wu J et al. : A Phase IIa trial of Subcutaneously Administered PD-L1 Antibody ASC22 (Envafolimab) in Patients with Chronic Hepatitis B. Hepatology 2021, 74:19: Late Breaking Abstract LO12; .33811356 [Google Scholar]
- 129.Geretti AM GE, Lacombe K, Balabanska R, Attley G, Teixeira P, Luangsay S, Triyatni M, Lu S, Lin S et al. :: LIVER DIRECTED TARGETING OF PD-L1 WITH R07191863, A LOCKED NUCLEIC ACID, IN CHRONIC HEPATITIS B: FIRST REPORT OF PHASE 1 TOLERABILITY, PHARMACOKINETICS, AND PHARMACODYNAMICS. Hepatology 2021, 74:54: Late breaking abstract LP29. [Google Scholar]
- 130.Tan E, Gakhar N, Kirtane K: TCR gene-engineered cell therapy for solid tumors. Best Pract Res Clin Haematol 2021, 34(3):101285. [DOI] [PubMed] [Google Scholar]
- 131.Depil S, Duchateau P, Grupp SA, Mufti G, Poirot L: ‘Off-the-shelf’ allogeneic CAR T cells: development and challenges. Nat Rev Drug Discov 2020, 19(3):185–199. [DOI] [PubMed] [Google Scholar]
- 132.Lau GK, Lok AS, Liang RH, Lai CL, Chiu EK, Lau YL, Lam SK: Clearance of hepatitis B surface antigen after bone marrow transplantation: role of adoptive immunity transfer. Hepatology 1997, 25(6):1497–1501. [DOI] [PubMed] [Google Scholar]
- 133.Kruse RL, Shum T, Tashiro H, Barzi M, Yi Z, Whitten-Bauer C, Legras X, Bissig-Choisat B, Garaigorta U, Gottschalk S et al. : HBsAg-redirected T cells exhibit antiviral activity in HBV-infected human liver chimeric mice. Cytotherapy 2018, 20(5):697–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Gehring AJ, Xue SA, Ho ZZ, Teoh D, Ruedl C, Chia A, Koh S, Lim SG, Maini MK, Stauss H et al. : Engineering virus-specific T cells that target HBV infected hepatocytes and hepatocellular carcinoma cell lines. J Hepatol 2011, 55(1):103–110. [DOI] [PubMed] [Google Scholar]
- 135.Fergusson JR, Wallace Z, Connolly MM, Woon AP, Suckling RJ, Hine DW, Barber C, Bunjobpol W, Choi BS, Crespillo S et al. : Immune-Mobilizing Monoclonal T Cell Receptors Mediate Specific and Rapid Elimination of Hepatitis B-Infected Cells. Hepatology 2020, 72(5):1528–1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Qasim W, Brunetto M, Gehring AJ, Xue SA, Schurich A, Khakpoor A, Zhan H, Ciccorossi P, Gilmour K, Cavallone D et al. : Immunotherapy of HCC metastases with autologous T cell receptor redirected T cells, targeting HBsAg in a liver transplant patient. J Hepatol 2015, 62(2):486–491. [DOI] [PubMed] [Google Scholar]
- 137.Meng F, Zhao J, Tan AT, Hu W, Wang SY, Jin J, Wu J, Li Y, Shi L, Fu JL et al. : Immunotherapy of HBV-related advanced hepatocellular carcinoma with short-term HBV-specific TCR expressed T cells: results of dose escalation, phase I trial. Hepatol Int 2021, 15(6):1402–1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Zoulim F, Fournier C, Habersetzer F, Sprinzl M, Pol S, Coffin CS, Leroy V, Ma M, Wedemeyer H, Lohse AW et al. : Safety and immunogenicity of the therapeutic vaccine TG1050 in chronic hepatitis B patients: a phase 1b placebo-controlled trial. Hum Vaccin Immunother 2020, 16(2):388–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Chinnakannan SK, Cargill TN, Donnison TA, Ansari MA, Sebastian S, Lee LN, Hutchings C, Klenerman P, Maini MK, Evans T et al. : The Design and Development of a Multi-HBV Antigen Encoded in Chimpanzee Adenoviral and Modified Vaccinia Ankara Viral Vectors; A Novel Therapeutic Vaccine Strategy against HBV. Vaccines (Basel) 2020, 8(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Michler T, Kosinska AD, Festag J, Bunse T, Su J, Ringelhan M, Imhof H, Grimm D, Steiger K, Mogler C et al. : Knockdown of Virus Antigen Expression Increases Therapeutic Vaccine Efficacy in High-Titer Hepatitis B Virus Carrier Mice. Gastroenterology 2020, 158(6):1762–1775 e1769. [DOI] [PubMed] [Google Scholar]
- 141.Akbar SMF, Al Mahtab M, Aguilar JC, Yoshida O, Khan S, Penton E, Gerardo GN, Hiasa Y: The Safety and Efficacy of a Therapeutic Vaccine for Chronic Hepatitis B: A Follow-Up Study of Phase III Clinical Trial. Vaccines 2022, 10(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Ma H, Lim TH, Leerapun A, Weltman M, Jia J, Lim YS, Tangkijvanich P, Sukeepaisarnjaroen W, Ji Y, Le Bert N et al. : Therapeutic vaccine BRII-179 restores HBV-specific immune responses in patients with chronic HBV in a phase Ib/IIa study. JHEP Rep 2021, 3(6):100361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Yuen MF AT JI, Brunetto M, Janssen HLA et al. : Efficacy and Safety of the siRNA JNJ-3989 and/or the Capsid Assembly Modulator JNJ-6379 for the Treatment of Chronic Hepatitis B Virus Infection : Results From the Phase 2b REEF-1 Study Hepatology 2021, 74:17: Late Breaking Abstract LO10. [Google Scholar]
- 144.Chang TT, Lai CL, Kew Yoon S, Lee SS, Coelho HS, Carrilho FJ, Poordad F, Halota W, Horsmans Y, Tsai N et al. : Entecavir treatment for up to 5 years in patients with hepatitis B e antigen-positive chronic hepatitis B. Hepatology 2010, 51(2):422–430. [DOI] [PubMed] [Google Scholar]
- 145.Schulze A, Gripon P, Urban S: Hepatitis B virus infection initiates with a large surface protein-dependent binding to heparan sulfate proteoglycans. Hepatology 2007, 46(6):1759–1768. [DOI] [PubMed] [Google Scholar]
- 146.Yan H, Zhong G, Xu G, He W, Jing Z, Gao Z, Huang Y, Qi Y, Peng B, Wang H et al. : Sodium taurocholate cotransporting polypeptide is a functional receptor for human hepatitis B and D virus. Elife 2012, 1:e00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


